Abstract
The mutual mitigation of selenium and mercury toxicity is particularly interesting, especially for humans. Mercury is widely recognized as a pan-toxic element; all forms are toxic to all organisms. Less well known is that selenium in excess is toxic as well. The high affinity between these elements influences their bioavailability and toxicity. In this paper we use selected species from Barnegat and Delaware Bays in New Jersey to examine variations in levels of selenium and mercury, and selenium:mercury molar ratios between and within species. We report on species ranging from horseshoe crab eggs (Limulus polyphemus) a keystone species of the food chain, to several fish species, to fish-eating birds. Sampling began in the 1970s for some species and in the 1990s for others. We found no clear time trends in mercury levels in horseshoe crab eggs, but selenium levels declined at first, then remained steady after the mid-1990s. Concentrations of mercury and selenium in blood of migrant shorebirds directly reflected levels in horseshoe crab eggs (their food at stopover). Levels of mercury in eggs of common terns (Sterna hirundo) varied over time, and may have declined slightly since the mid-2000s; selenium levels also varied temporally, and declined somewhat. There were variations in mercury and selenium levels in commercial, recreational, and subsistence fish as a function of species, season, and size (a surrogate for age). Selenium:mercury molar ratios also varied as a function of species, year, season, and size in fish. While mercury levels increased with size within individual fish species, selenium levels remained the same or declined. Thus selenium:mercury molar ratios declined with size in fish, reducing the potential of selenium to ameliorate mercury toxicity in consumers. Mercury levels in fish examined were higher in early summer and late fall, and lower in the summer, while selenium stayed relatively similar; thus selenium:mercury molar ratios were lower in early summer and late fall than in mid-summer. We discuss the importance of temporal trends in biomonitoring projects, variations in levels of mercury, selenium and the molar ratios as a function of several variables, and the influence of these on risks to predators and humans eating the fish, and the eggs of gulls, terns. Our data suggests that variability limits the utility of the selenium:mercury molar ratio for fish consumption advisories and for risk management.
Keywords: Mercury, Selenium, Selenium:mercury molar ratio, Fish consumption, Horseshoe crabs, Fish, Birds, Humans
Introduction
Changing global temperatures, changes in sea levels, increasing human populations, and chronic and acute exposure to contaminants have led society and some governments to address these issues at multiple levels. Promulgation of laws, regulations, and advisories require data to support these measures (ESA 1973: Fairbrother 2009; Evers et al. 2011; Piersma et al. 2016). Concentrations of contaminants in a wide range of organisms is key to understanding the risk to humans and the environment. Site specific information is required on indicator species that represent different levels of the food chain. Understanding contaminant patterns requires examining several indicators, for several years. However, biological monitoring requires careful selection of indicators that provide data on whether there are changes in exposure, temporal or spatial trends, and whether there are spatial or species differences that reflect exposure leading to adverse health effects (Burger 2006; Egwumah et al. 2017; He et al. 2019). This led us to take a food web approach for biota living in estuaries.
Examining yearly variations or trends in contaminant levels is not only time-consuming, but requires funding commitment, and the ability to analyze levels year after year. Monitoring studies are difficult to fund, but provide some of the most useful data for understanding whether the risks to biota or human are increasing or decreasing. There are few studies in birds and other biota that provide temporal patterns for 20 years or more (Weseloh et al. 2011; Burger and Gochfeld 2016). Food webs can be used to examine contaminant fate and transfer through an ecosystem. Yet often eco-toxicologists examine contaminant levels in organisms without considering the complex food webs that surround their target species of concern. While it is time consuming and complicated, examining contaminant levels with a food web structure provides a more realistic picture of risk to organisms, including humans. It is particularly time-consuming when developing long-term data sets of contaminant levels in indicator species. In some cases (e.g. lead, cadmium, ATSDR 1996, 2007; Raymond et al. 2014), contaminant levels have decreased in the environment over the last few decades, requiring that the temporal patterns of data collection are critical to determining risk.
Concentrations of a few contaminants vary seasonally (Eagles-Smith et al. 2009a, b), and as a function of size (e.g. age in some species) in fish, birds, and other animals (Lange et al. 1994; Bidone et al. 1997; Burger and Gochfeld 2000, 2016). Generally contaminant concentrations in tissues of species increase with age, although this is not always the case (Evers et al. 2005; Seewagen 2010). To understand the levels of contaminants in biota, and associated potential health effects, it is essential to identify the factors that affect these levels. Understanding exposure routes, contaminants levels, and potential health effects is especially required for species that are declining, are of special concern, are consumed by people, or that are used for cultural or medicinal practices.
There are two types of trophic webs: 1) food and energy-based webs that are designed to examine the patterns of consumption at different trophic levels (DeVault et al. 2003; Montevecchi 2008), and 2) contaminant webs based on trophic level relationships (Braune et al. 2015; Rolfhus et al. 2011; Burger and Gochfeld 2016). The latter are designed to understand the fate and transfer of contaminants as they move up the food chain (Gerwing et al. 2016; Elnoder et al. 2018). Data from contaminant webs provide the information needed to conduct risk assessments for humans and other biota. Such data, combined with temporal trends in metal levels indicates whether risk is increasing or decreasing over time.
In this paper we use selected species to test whether selenium, mercury, and selenium:mercury ratios vary among different species, years, and seasons. We examine: 1) yearly trends in horseshoe crab (Limulus polyphemus) eggs from 1993 to 2019, 2) yearly trends in levels of selenium and mercury in common tern (Sterna hirundo) eggs from 1971 to 2019, 3) differences in levels in small prey fish eaten by terns, 4) levels in commercial/recreational fish, 5) seasonal patterns in level of selenium and mercury in striped bass (Morone saxatilis), bluefish (Pomatomus saltatrix) and American eel (Angulia rostrata), and 6) the relationship between levels of selenium and mercury in horseshoe eggs and the blood of shorebirds during the period when they are consuming only these eggs on Delaware Bay (spring stopover). The species of shorebirds examined include red knot (Calidris canutus rufa), semipalmated sandpipers (Calidris pusilla), sanderling (Calidris alba), and turnstone (Arenaria interpres).
We concentrate on selenium and mercury because although mercury is one of the most toxic environmental pollutants (Eisler 1987; Furness and Rainbow 1990; Mason 2014), selenium is seldom examined. Further, each potentially mitigates the toxic effects of the other (ATSDR 1996; Eisler 2000; Ralston and Raymond 2018). The molar ratio gives an indication of the protective benefits of selenium against mercury poisoning. Ratios greater than 1:1 are hypothesized to protect against mercury toxicity on the assumption that all the mercury will be bound up by selenium Raymond and Ralston 2004; Ralston and Raymond 2001, 2018). The actual molar level that would confer protection against mercury toxicity, however, is not known (Squadrone et al. 2015; Burger and Gochfeld 2011; Gochfeld and Burger 2020, this issue).
We present data on horseshoe crab eggs because they are a keystone prey of the food chain in Delaware Bay, and are eaten by fish, eels, shorebirds and gulls (Burger and Gochfeld 2016, Fig. 1). The fish-eating common tern represents a top trophic level, and our egg contaminant data are the longest, continually monitored study of mercury and selenium in birds in the U.S. Fish are good species to examine mercury and selenium levels because as they age they increase in size, usually making size and indication of age. Striped bass and bluefish, also top predators, may show size and seasonal differences in concentrations in edible muscle (and selenium:mercury molar ratios) that are relevant to human consumers. Migrant shorebirds foraging on eggs of horseshoe crab eggs represent mid-level consumers that feed entirely on horseshoe crab eggs when in Delaware Bay (Tsipoura and Burger 1999; Novic et al. 2015; Mizrahi et al. 2012) although elsewhere (and at other times of the year) they eat mainly small invertebrates (Baker et al. 2013). The eggs of horseshoe crab are also eaten by other birds (e.g. gulls) and fish including American Eel (Burger and Gochfeld 2016). The food webs in Delaware Bay and Barnegat Bay are obviously much more complicated and diverse than presented in figure 1 (Burger and Gochfeld 2016); this figure only represents the species discussed in this paper. Our intent in this paper is to bring together data on temporal patterns of contaminant exposure in a number of indicator species, that indicate changes (or lack thereof) in exposure to mercury and selenium. While earlier papers have provided some of the temporal data, these figures represent additional years and/or additional species that are synthesized to show the importance of biomonitoring.
Figure 1.
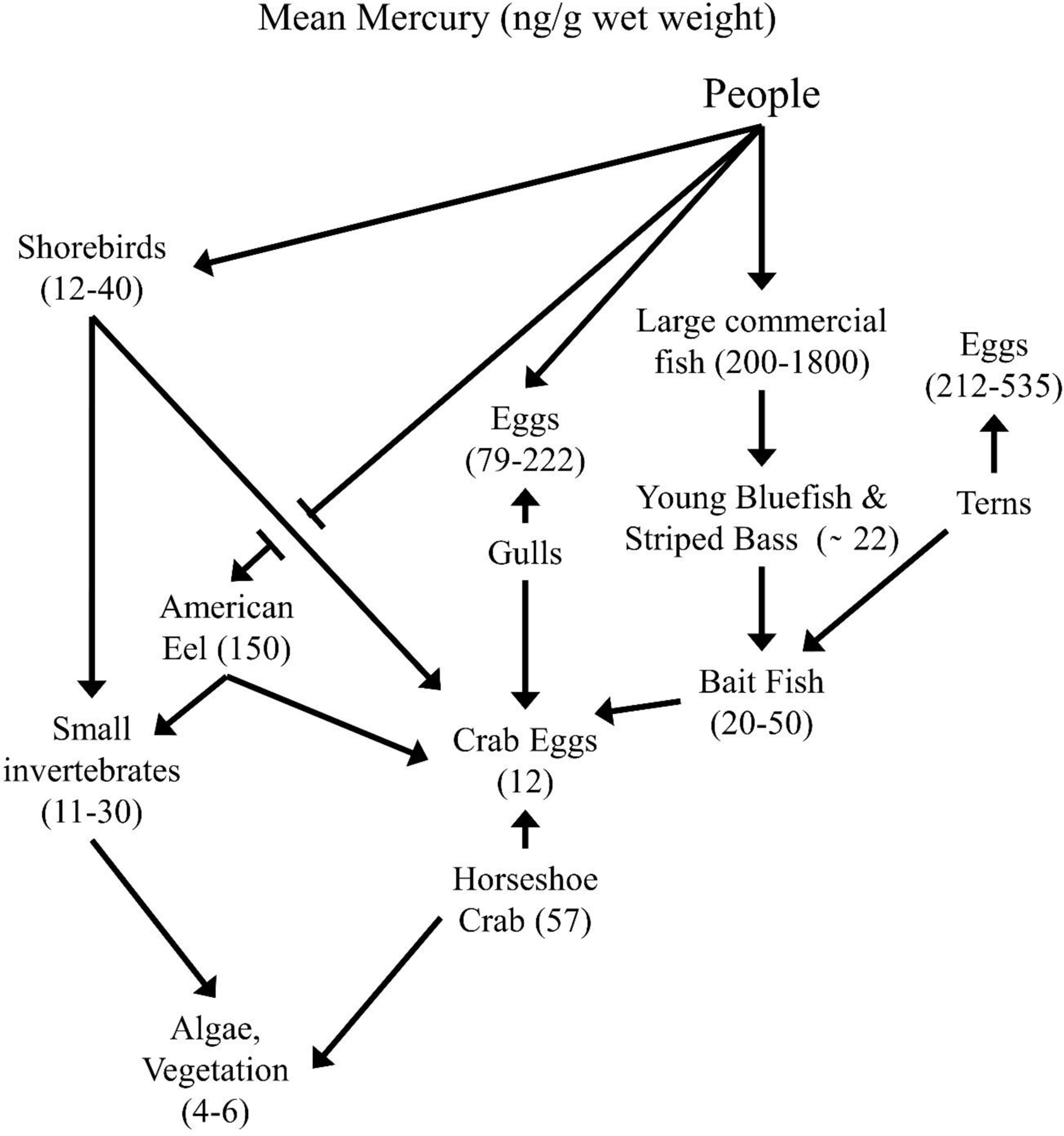
Simplified food web for Delaware and Barnegat Bays (New Jersey), illustrating the species discussed in this paper to examine mercury and selenium levels, and selenium:mercury molar ratios in biota.
Methods
As part of long-terms study of contaminants in the bays of New Jersey, we have sampled species representing different nodes in estuarine food webs. We were particularly interested in temporal trends and interspecific differences in mercury and selenium from Delaware Bay and Barnegat Bay (Fig. 1, after Burger and Gochfeld 2016). Under appropriate state and federal permits we collected invertebrates, fish, birds and bird eggs. All methods were conducted with permits from New Jersey and the federal government, under the animal care committee of Rutgers University (permit 92–036, renewed every three years), and in accordance with the guidelines of the Ornithological Council (www.nmnh.si.edu/BIRDNET/GuideToUse) that consider animal welfare.
We used a variety of means to collect samples. Clutches of horseshoe crab eggs were scooped from nests on spawning beaches, pooling eggs from different clutches (Burger and Tsipoura 2014; Burger et al. 2017; this study; 8–12 clutches of egg/year). Bait fish were collected by seine in Barnegat Bay (Able and Fahay 2014)(>25–85/species. Edible fish were collected from fishermen (Burger 2009; Burger and Gochfeld 2011; Gochfeld et al 2012)(20–258/species). American eels were collected in traps in shallow water about 30 m from horseshoe crab spawning beaches (this study, n = 8). Bird eggs were collected by hand, removing only one egg from a clutch to reduce any effects on reproductive success (Burger and Gochfeld 2016, this study)(>15/species/year). Shorebirds were captured by cannon net or mistnets. Shorebird blood was taken from wing veins into heparinized capillary tubes and refrigerated (Tsipoura et al. 2017; Burger et al. 2019, this study), a technique that has no obvious effect on the reproductive success of birds (Orzechowski 2019).
All samples were prepared and analyzed in laboratories of the Environmental and Occupational Health Sciences Institute (EOHSI) at Rutgers University. Further, all samples were analyzed in EOHSI by the same methods, assuring consistency over the collection period. Horseshoe crab eggs were blended and digested. Small fish were analyzed whole and larger edible muscle samples were analyzed individually. Bird eggs were analyzed individually, homogenizing the yolk and albumin. In all cases, the figures/tables presented here include much new data and analyses not previously published.
Separate laboratories were used for dissection, digestions, and then chemical analysis. Before each use, containers and other equipment were washed and rinsed (10 % HNO solution, deionized water). Bird eggs were individually homogenized and dried to a constant weight, while feathers were alternately washed with acetone and deionized water, and air dried. Samples were then digested in 70% TraceMetal grade nitric acid (Fisher Chemical) in a microwave (MDS 2000 CEM). We analyzed total mercury using a Perkin Elmer FIMS-100 mercury analyzer, and selenium using a Perkin Elmer 5100 flameless atomic absorption spectrometer (Zeeman correction). The instrument detection limits were 0.02 ng/g for selenium and 0.2 ng/g for mercury. Metal levels in bird eggs are expressed as ng/g (ng/g, dry weight; later converted to wet weight for comparison to the published literature). Horseshoe crab eggs and fish tissue are expressed in ng/g (ng/g, wet weight). Standard reference material (from NIST), spiked specimens, and blanks were determined along with samples to evaluate analytical control and accuracy. Certified Reference Material (CRM) DORM-2, “Dogfish Muscle Certified Reference Material for Trace Metals” from the National Institute of Standards and Technology (NIST), was used for cold vapor atomic absorption spectroscopy (Hg). DORM-2 Standard Reference Material (SRM) 1640, Trace Metals in Natural Water” from the National Institute of Standards and Technology (NIST), was used for Zeeman graphite furnace atomic absorption spectroscopy (selenium) quality control evaluation. Recoveries ranged from 85 % to 115 %. Further descriptions of methods, instrumentation, and QA/QC ca be located in Burger and Gochfeld (2011, 2018), Burger et al. (2017, 2019), and other references noted in figure legends.
Results
Yearly patterns
There were no clear yearly trends in levels of mercury in the eggs of horseshoe crabs (although 1994 level was significantly higher), but selenium levels declined for several years and remained level from the late-1990s to 2019, Fig. 3). Both mercury (chi-squre=23.0 p<0.0001) and selenium (chi-squre=59.0 p<0.0001) decrease significantly from the 1990’s to the 2010’s. The levels of selenium were high relative to mercury, resulting in no consistent temporal pattern in the selenium:mercury molar ratio. However, the ratio were all well above 50 in all years.
Figure 3.
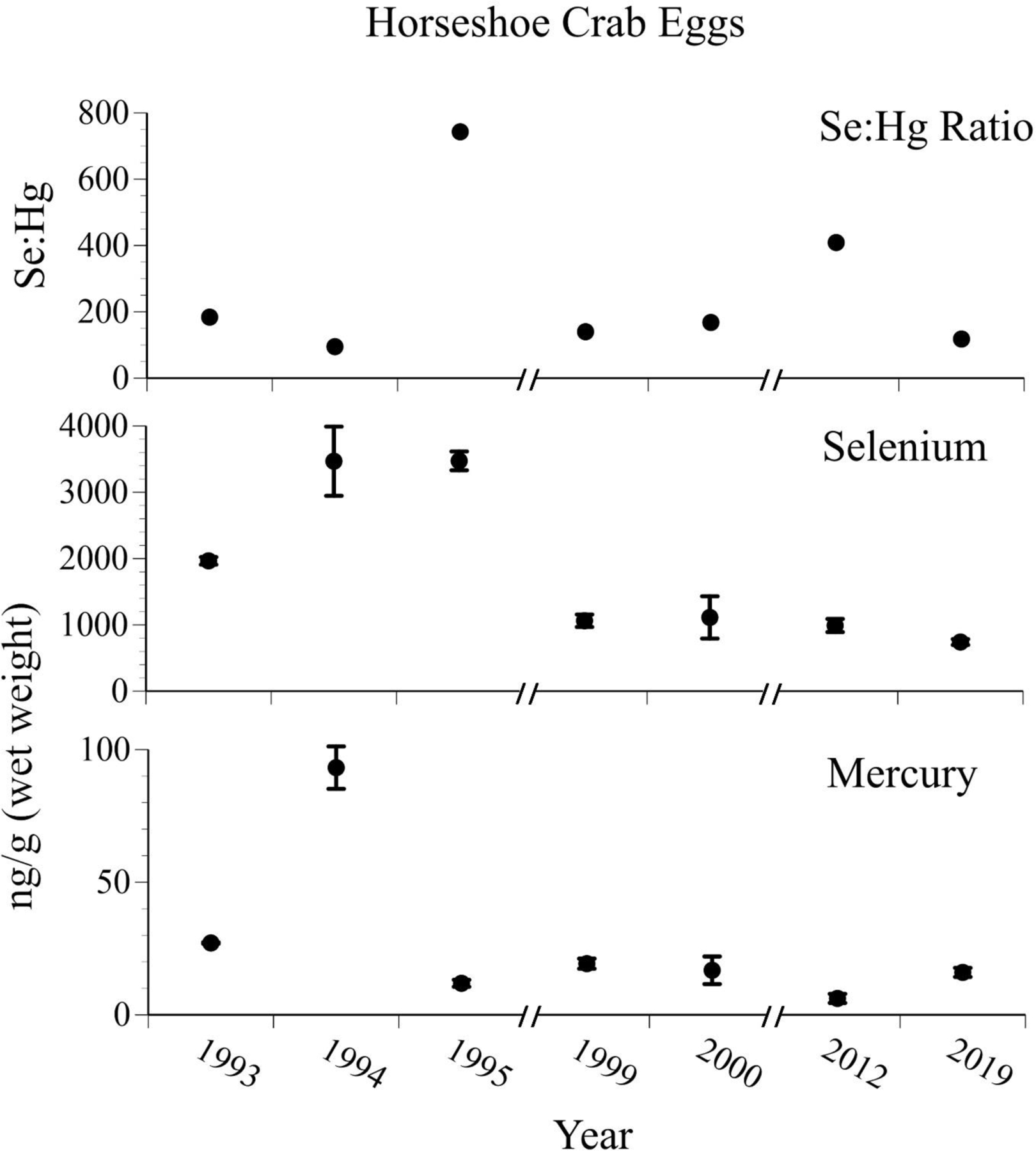
Mean ± SE levels (ng/g, wet weight) of selenium, mercury, and selenium:mercury molar ratios in eggs of horseshoe crabs collected from Delaware Bay from 1993 – 2019. Crab eggs are one of key bases of food webs in east coast beaches and estuaries (after Burger et al. 2014, unpubl. data).
The mercury levels in eggs of common terns varied over time, and may have declined slightly since the mid-2000s (Kendall tau correlation coefficient −0.1 p=0.001), and selenium levels have varied markedly, without showing a significant trend (Fig. 4). Again, mercury was higher in 1994 and 1999 than in the other years, perhaps due to heavy rains and flooding bring higher levels of mercury into the estuarine system. The selenium:mercury molar ratios varied over the years, mainly tracking selenium levels. However, the selenium:mercury molar ratios for eggs of common terns were much lower than those in horseshoe crab eggs by two orders of magnitude (and in one year was below a 1:1 ratio).
Figure 4.
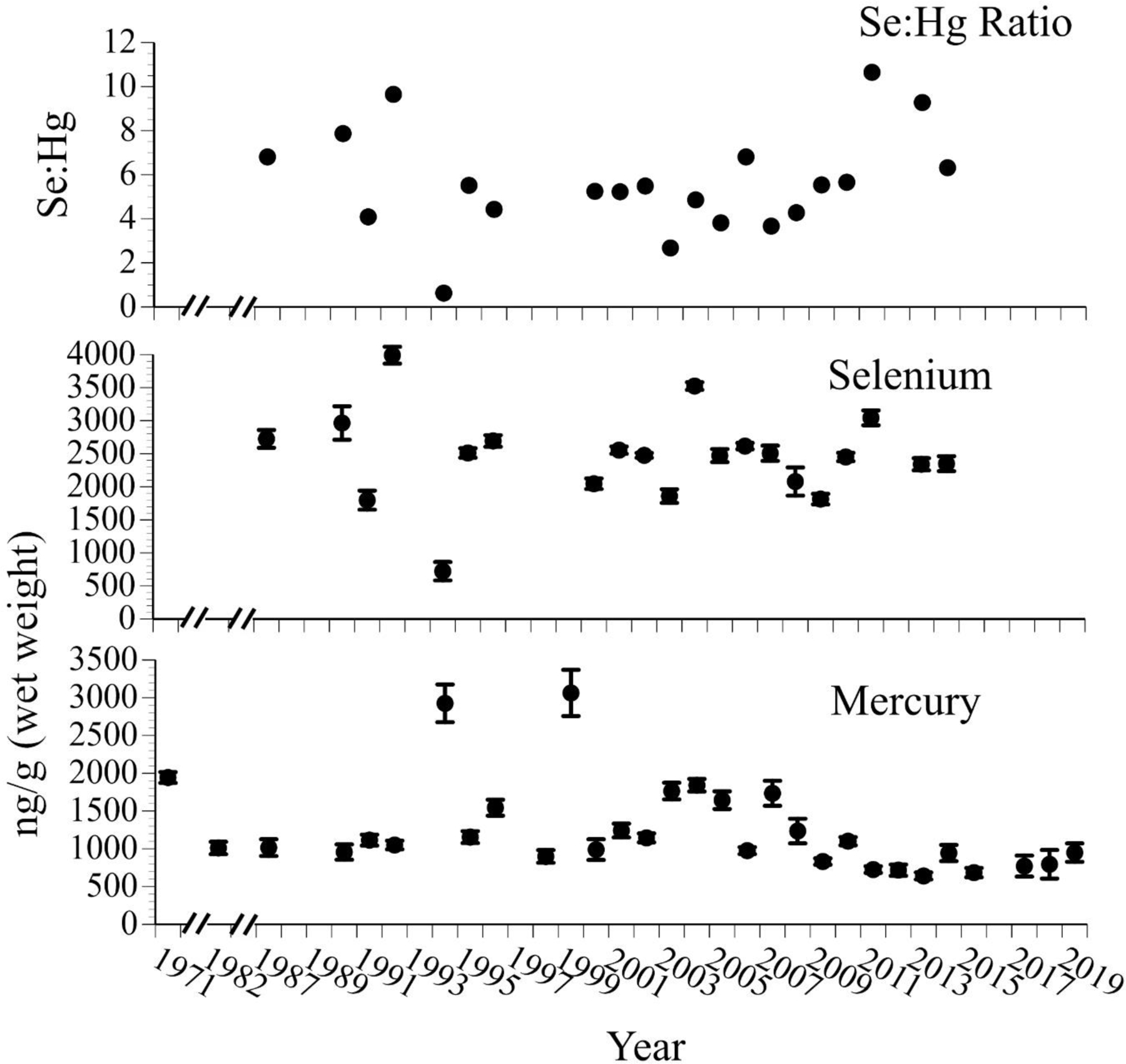
Mean ± SE levels (ng/g, wet weight, n=8–20) of selenium, mercury, and selenium:mercury molar ratios in eggs of common terns collected from nests on Barnegat Bay islands from 1971 to 2019. Common terns prey on small bait fish, and tern eggs are eaten by many predators, including people in some countries (after Burger and Gochfeld 2016, unpubl. data).
Species and Size differences
Species differences in levels of both mercury and selenium occur in a wide range of fish species (as there are in other related groups of organisms). The prey base of small fish available to predators such as larger fish, gulls and terns in both bays is diverse. Mercury levels are generally low in small prey fish; average levels in bay anchovy (Anchoa mitchilli) were the highest, and averaged 100 ng/g (0.1 mg/g, Fig. 5). Mean selenium levels were higher than mean mercury levels, but generally correlated to mercury levels, except for round herring (Spratelloiees gracilias). We have no explanation for the higher levels of selenium in round herring, but suggest it bears examination. Selenium levels were highest in round herring and bay anchovy, Fig. 5). Selenium:mercury molar ratios generally tracked selenium levels. The low variances indicate low variation in levels over the years (not surprising, given the small size and short lifespan).
Figure 5.
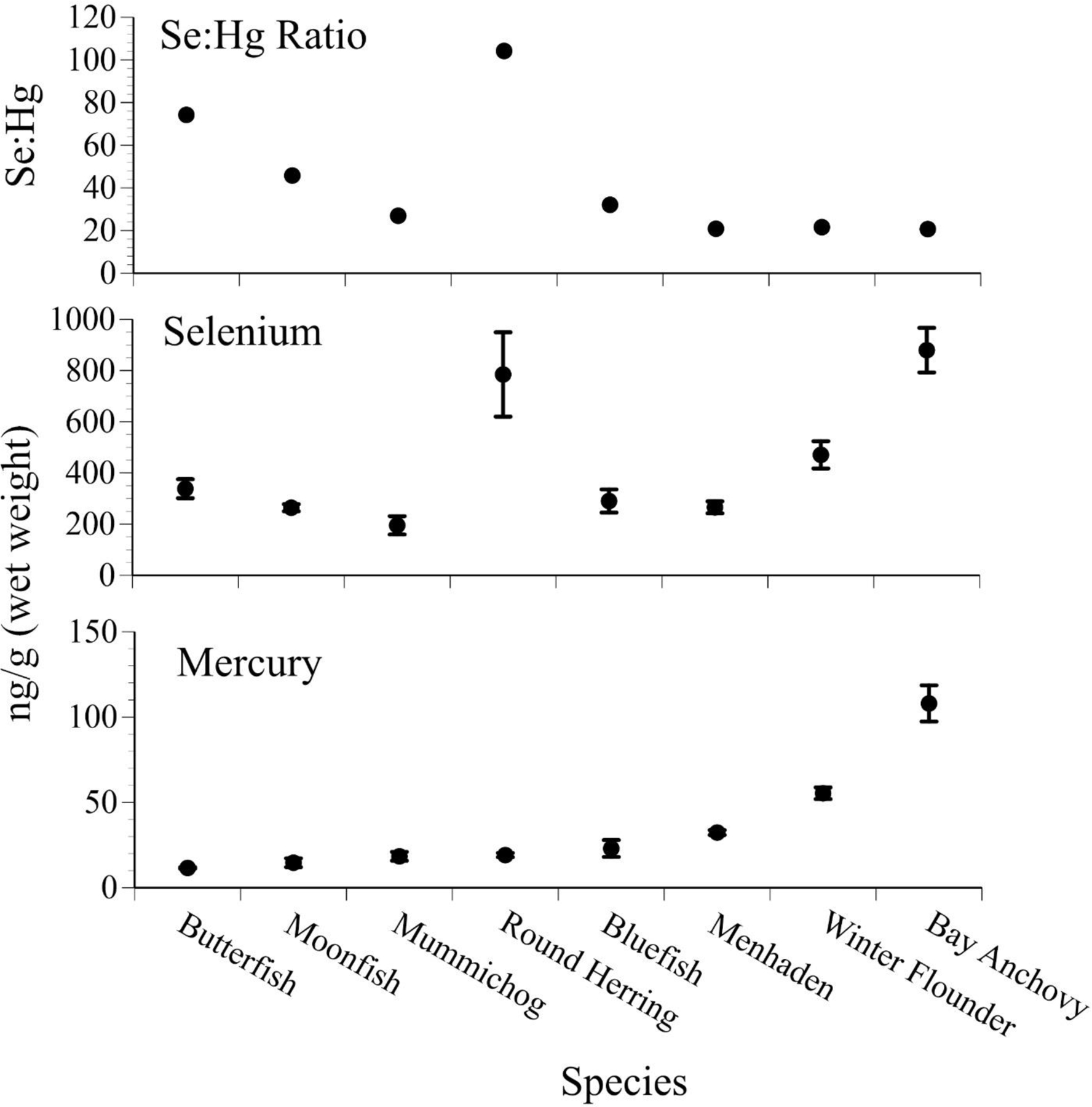
Mean ± SE levels (ng/g, wet weight, n=25–88) of selenium, mercury, and selenium:mercury molar ratios in several small “bait” fish species collected from Barnegat Bay that are prey for common terns and other birds, as well as for larger fish (after Burger and Gochfeld 2016, unpubl. data). The prey fish were brought back to the nest to feed chicks (2001–2014; small SE indicates little variation over the years).
Similarly, levels of mercury varied significantly in medium-sized saltwater fish (caught commercially and recreationally by people)(Fig. 6). The levels of selenium in these fish, however, did not track mercury levels, and thus the relationship between selenium and mercury is not predictable. The selenium:mercury molar ratios increased as the denominator, mercury, decreased (Fig. 6).
Figure 6.
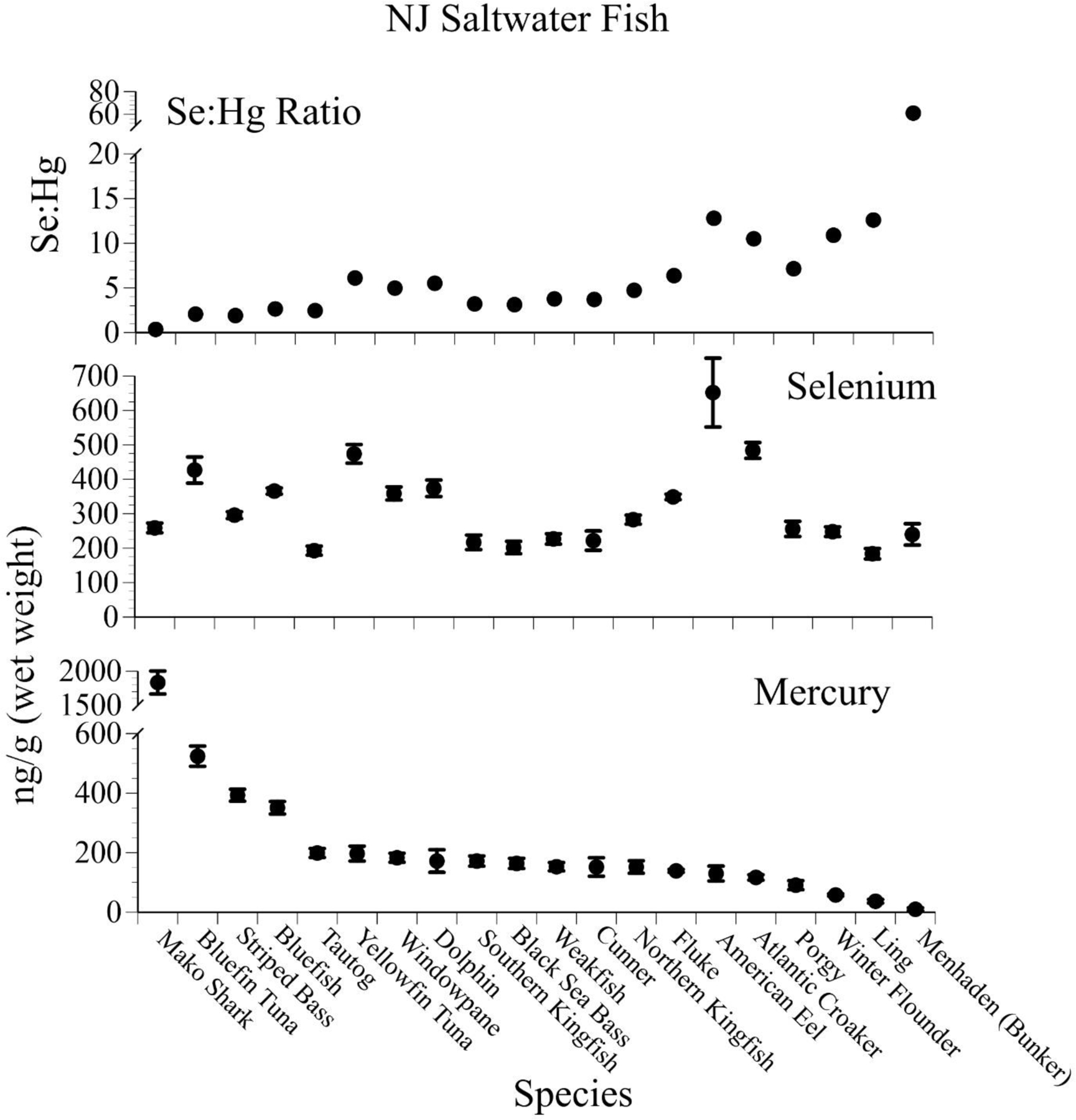
Mean ± SE levels (ng/g, wet weight, n=20–258) of selenium, mercury, and selenium:mercury molar ratios in several fish species that were collected from people fishing recreationally along the Jersey shore (after Burger and Gochfeld 2011, 2013, unpubl data).
For bluefish and striped bass, significant differences in mercury levels occurred as a function of size, but size and metal relationships were seldom examined for selenium (Fig. 7). Mercury levels increased for both species, and selenium either remained similar (bluefish) or declined slightly with size. The selenium:mercury molar ratios decreased with size of the fish, and for some species was below 1:1.
Figure 7.
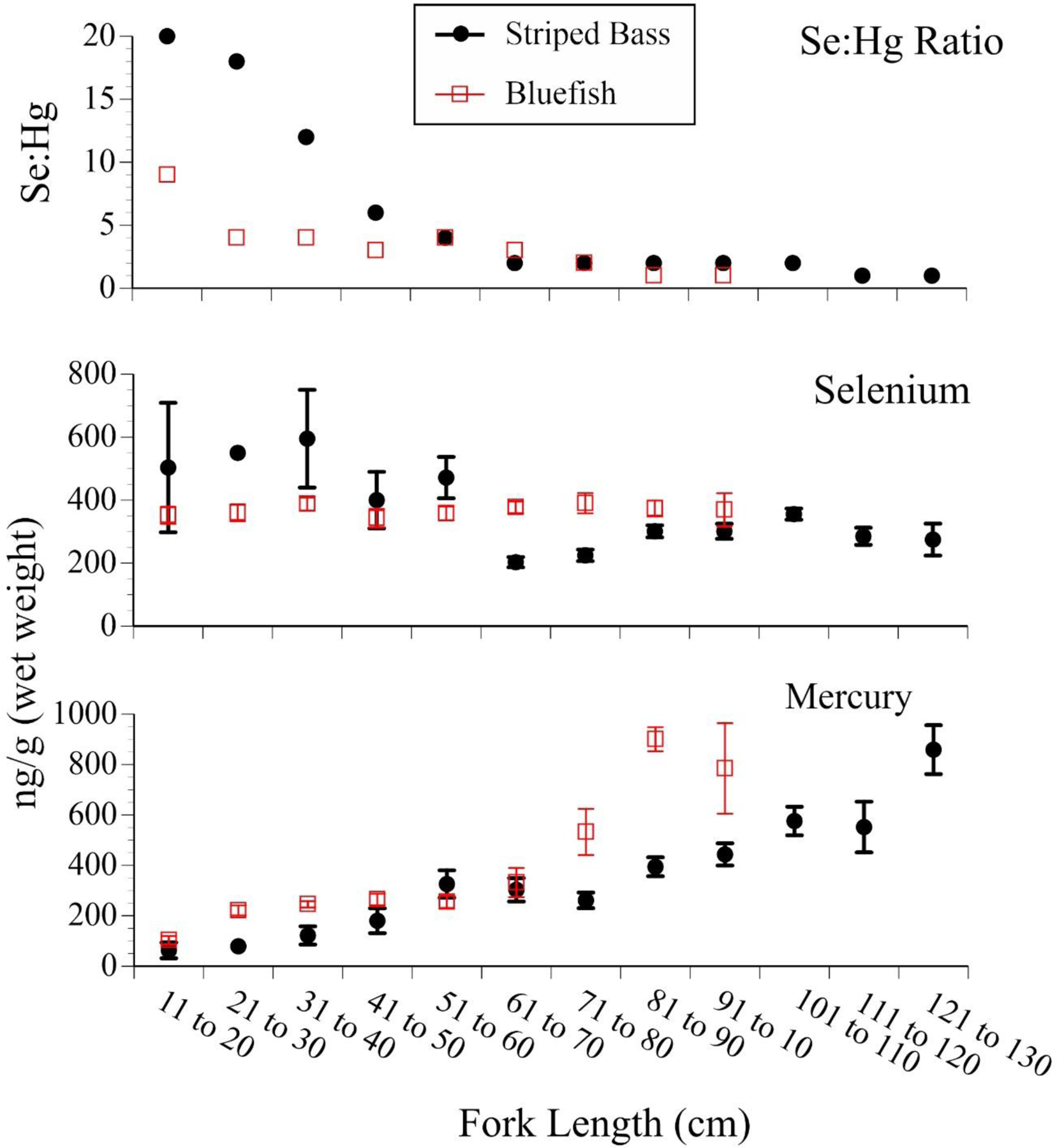
Mean ± SE levels (ng/g, wet weight) of selenium, mercury, and selenium:mercury molar ratios in bluefish (n=206) and striped bass (n=178) collected from people fishing recreationally along the New Jersey coast, as a function of size (which relates to age) (after Burger 2009; Gochfeld et al. 2012, unpubl. data).
Seasonal patterns
In 2002–2004 we analyzed separate monthly samples for striped bass and bluefish. In the same-size commercial fish, mercury levels in muscle of bluefish and striped bass varied seasonally, with mercury levels being higher in early summer and late fall, and lower in the same-sized fish collected in the summer (Fig. 8). Selenium levels, however, were similar throughout the season, or were higher in late summer. This results in selenium:mercury molar ratios being significantly higher in late summer than in early summer or late fall (Fig. 8).
Figure 8.
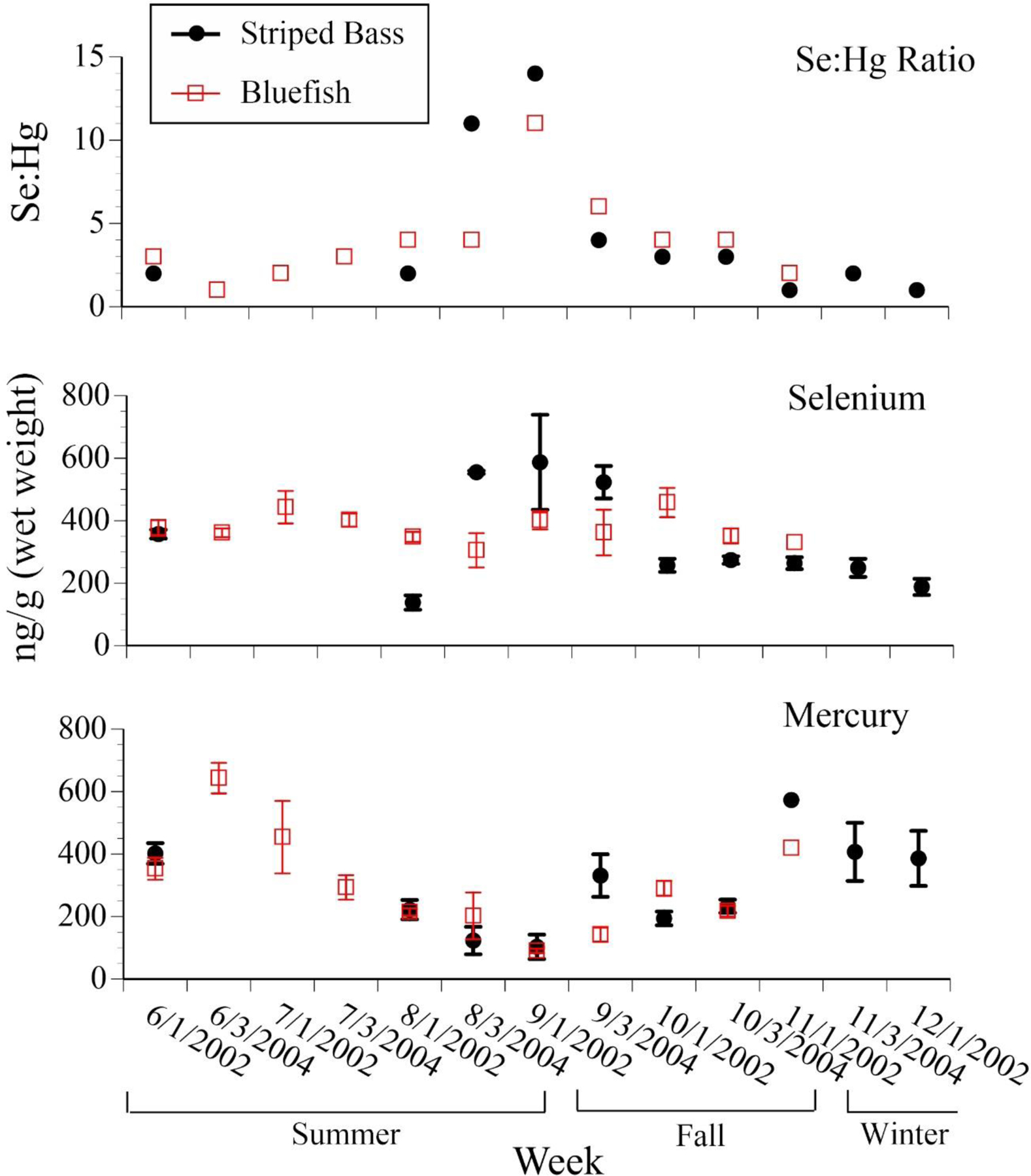
Mean ± SE levels (ng/g, wet weight) of selenium, mercury, and selenium:mercury molar ratios in bluefish and striped bass collected from people fishing recreationally along the Jersey coast, as a function of season (after Burger 2009; Gochfeld et al. 2012, unpubl. data)..
Food chain effects
There was a significant relationship between the levels of selenium and mercury in horseshoe crab eggs, and in blood of shorebirds (Table 1). Thus, when shorebirds are eating horseshoe crab eggs, their blood levels of mercury and selenium reflect crab egg levels. The levels in the blood are then transferred to other internal tissues of shorebirds.
Table 1.
Mercury (ng/g, wet weight), selenium, and Se:Hg molar ratios in horseshoe crab eggs and shorebird blood collected from Delaware Bay collected (2011 and 2012).
| Sample Size | Mercury Mean ± SE | Selenium Mean ± SE | Hg nmol/g wet wt. | Se nmol/g wet wt. | (Mean Ratio) Se:Hg | |
|---|---|---|---|---|---|---|
| Horseshoe Crab Eggs | 20a | 12.6 ± 2.4 | 990 ± 99 | 0.06 | 12.5 | 199.6 |
| Red Knot | 30 | 16.2 ± 3.1 | 5873 ± 573 | 0.08 | 74.4 | 921.0 |
| Semipalmated Sandpiper | 38 | 12.7 ± 3.3 | 4422 ± 470 | 0.06 | 56.0 | 884.5 |
| Sanderling | 20 | 24.8 ± 5.3 | 14500 ± 2306 | 0.12 | 183.6 | 1485 |
| Turnstone | 32 | 39.9 ± 6.7 | 6294 ± 785 | 0.20 | 79.7 | 400.7 |
a = from 20 clutches for this period.
The levels of mercury and selenium in tern and gull eggs (reflective of their foods) varied (Table 2). Levels of mercury in eggs (relevant to consumption by predators and people) of Forster’s tern were as high as 535 ng/g (= 0.5 mg/g), while those of laughing gulls were only 82 ng/g (= 0.08 mg/g).
Table 2.
Mercury, selenium and SE:Hg ratios in bird eggs collected from Barnegat Bay (2010 through 2019). Both wet and dry weights are given for comparison. The % eggs above 0.3 mg/g (EPA mercury criterion) and 0.5 mg/g (WHO standard) are given to indicate risk to human consumption. All years combined.
| Laughing Gull | Herring Gull | Common Tern | Great Black-backed Gull | Forster’s Tern | |
|---|---|---|---|---|---|
| Food Items | Omnivore (Horseshoe Crab Eggs) | Omnivore (Scavenger) | Predator Small fish (ocean & bay) | Predator & Omnivore | Predator Small fish (bay & marsh pools) |
| n=25 | n=44 | n=177 | n=4 | n=35 | |
| Mercury (Dry Wt) | 334 ± 28 | 305 ± 41 | 866 ± 28 | 835 ± 194 | 2300 ± 106 |
| Selenium (Dry Wt.) | 1770 ± 79 | 1490 ± 67 | 2790 ± 78 | 2140 ± 73 | |
| Mercury (Wet Wt) | 82 ± 7 | 79 ± 10 | 212 ± 7 | 222 ± 52 | 535 ± 25 |
| Selenium (Wet Wt.) | 432 ± 19 | 369 ± 17 | 625 ± 13 | 498 ± 17 | |
| Hg nmol/g wet wt. | 0.4 | 0.4 | 1.1 | 1.1 | 2.7 |
| Se nmol/g wet wt. | 5.5 | 4.7 | 7.9 | 6.3 | |
| Se:Hg Molar Ratio | 13.5 | 11.9 | 7.5 | 2.4 | |
| % Hg above 0.3 mg/g (ww) | 0% | 2% | 14% | 25% | 94% |
| % Hg above 0.5 mg/g (ww) | 0% | 0% | 1% | 0% | 52% |
| % Hg above 1.0 mg/g (ww) | 0% | 0% | 0% | 0% | 0% |
Discussion
The importance of biomonitoring for yearly variations
In our view, a successful bioindicator must indicate something about the environment, not just the levels of a contaminant in an organism at a particular time (Burger 1993, 2006; Egwumah et al. 2017). Simply reporting levels is not sufficient to “state” that this is a bioindicator – they must be an indicator of something of interest or importance. Initially, many bioindicator studies were aimed at determining whether levels of a contaminant in biota living in a potentially polluted environment were indicative of local exposures, whether levels were higher than in a control site, and whether levels were in a range to cause adverse effects. This is still the case (Ohlendorf et al 1989; Burger and Gochfeld 2000; Ohlendorf 2011; Abdullah et al. 2015; Bakker et al. 2016a,b; He et al. 2019). Bioindicator studies can be aimed at determining appropriate sampling methods (Rutkowska et al. 2018; Lasters et al. 2019; Zabala et al. 2019), maternal transfer of contaminants to offspring (Ackerman et al. 2016; Bakkar et al. 2016a,b), and whether a particular non-invasive tissue can serve as an “indicator” of internal levels. For example, blood may be a good indicator of either current local exposure or foods (e.g. this study), or of internal tissue levels (e.g. Berglund 2018), or for examining movement of contaminants through food webs (Abassi et al. 2015; Burger and Gochfeld 2016).
Having biomonitoring data for several species and ecosystems is critical for understanding the health and well-being of our environment, particularly when faced with global population changes, sea level rise, and climate change. There are literally hundreds of papers that use the word monitoring or biomonitoring in a range of biota (see the journal Ecological Indicators), but most have only a very few years, and almost none have data that span 20 years or more, especially in the United States for birds. However, museum specimens can be used to examine long time series of mercury contamination using avian feathers (Movalli 2017). We provide data on mercury, selenium, and selenium:mercury molar ratios for the eggs of common terns from 1971 to 2019, and for horseshoe crab eggs from 1993 to 2019. These data indicate that levels of mercury and selenium have varied over the years, but there is no clear temporal patterns. These data are important because, unlike cadmium and lead that have declined dramatically in the last 50 years (ASTDR 2007; Ettinger et al. 2020), mercury does not show clear decreases (despite decreasing use of mercury and heightened U.S. regulatory standards). Mercury may not have decreased over time in the United States due to the failure to control emissions from coal-burning power plants, as well as the global transport of mercury from increased emissions from new coal-burning plants in China and elsewhere (Mason et al. 2000; Huang et al. 2017). Selenium is also released from coal-burning plants (Mason et al. 2000). However, recent relaxing of some U.S. standards may have an impact, and continued biomonitoring is required. Elsewhere, the concentrations of some metals in shorebirds have declined temporally (Mason 2014; Vallius 2013). Selenium is also released from coal combustion (Mason et al. 2000), metal smelting and refineries, mining operations, and semi-conductor manufacturers, ATSDR 1996).
Yearly or at least long-term biomonitoring is needed to detect and confirm trends in pollutants. Trends inferred from short-term studies can be misleading. For example, selecting data from any two years in the Common Tern egg time series we presented could show either an increasing or a decreasing trend (see Fig.4) Further, having data from nearly every year (e.g. common tern eggs) shows that there is yearly variation, although no consistent pattern. The high levels of mercury in some years could be due to increased rainfall resulting in increased metal inputs from rivers and streams into bays and estuaries, increased storm frequency or intensity that results in re-suspension of contaminants, and/or shifts in foods eaten by the terns. Without time series of data on mercury and selenium levels it is difficult to determine whether high (or low) levels are representative of the situation, and whether there are ecosystem (and human health) concerns because of high levels.
A time series of levels of mercury and selenium in horseshoe crab eggs “indicates” whether levels have changed over time, what the levels are, and allows for a determination of whether they provide a threat to the organisms themselves, or to their predators, including people. Horseshoe crab eggs form one of the main bases for Northeastern US estuaries, and thus determining levels is critical for understanding relationships in the food web. In the present study, the levels in eggs provide an indication of changes over time, levels that might provide a risk to the species laying them (e.g. horseshoe crabs), and to the species that eat them (fish and shorebirds eating horseshoe crab eggs). The horseshoe eggs are food for many invertebrates, fish, and birds in the U.S. east coast bays and estuaries.
Finally, one advantage of having long time series of mercury and selenium levels is the ability to note when a level is out of line with the general pattern, as occurred in 1994 for mercury in horseshoe crab eggs (Fig. 1) and in 1994 and 1999 for common tern eggs. It may be that in 1994 there was higher mercury in atmospheric deposition (or more rain in that season, accounting for greater deposition), or more terrestrial runoff from cities or industrialized areas. Whatever the reason, it illustrates that there can be years with high mercury levels that do not reflect the trend (but if only those years were sampled, it would present in in accurate picture f the overall trens).
The food web and potential effects on biota
The effects of trophic level on the bioaccumulation of mercury (and other contaminant) have often been discussed (Sarkka et al. 1978; Wiemeyer et al. 1986; Burger and Gochfeld 1996, 2016). Species that are on a high trophic level generally have higher levels of mercury than lower levels (Mason et al. 2000; Baeyens et al. 2003; Cameiro et al. 2016). Yet, one difficult problems is to show the relationship between exposure and levels in consumers, largely because most species (including humans) consume a range of different food items in different quantities (Rolfhus et al. 2011; Mendoza-Carranza et al. 2016; Burger et al. 2019). Migrant shorebirds foraging in Delaware Bay in May are an exception. There, for their 2–3 week refueling stopover, they feed exclusively on horseshoe crab eggs (Tsipoura and Burger 1999). The data presented in this paper indicate a clear, positive relationship between mercury and selenium levels in eggs of horseshoe crabs and in the blood of shorebirds that forage exclusively on these eggs on stopover on Delaware Bay (Tsipoura and Burger 1999). It is unusual for birds to be feeding on one prey item during a 2–3 week period, allowing for this evaluation. At other times of the year the diet of shorebirds is more varied (e.g. Baker et al. 2004). The data, therefore, show a clear relationship between exposure levels and mercury and selenium, and what is circulating in their blood. Thereafter, however, metals are deposited in other organs, which may then accumulate higher levels than the levels found in blood (Burger et al. 2015; Tsipoura et al. 2017). Mercury levels in liver of migrant semipalmated sandpipers averaged 260 ng/g compared to 13 ng/g in blood and 430 ng/g in feathers (Burger et al. 2014).
Worldwide, shorebird populations in general are decreasing: 4 species we studied are declining at a greater rate than many other shorebird species (Morrison et al. 2001; Baker et al. 2004; Andres et al. 2013). We suggest that it is critical for conservation to understand whether the levels of either mercury or selenium are such that they could cause negative effects. The mean levels of mercury in shorebirds reported herein (mean of 13 – 39 ng/g), however, were lower than those known to cause negative effects (700 – 2000 ng/g effects level, depending upon species, Evers et al. 2008, 2011).
Similarly, common terns nesting in Barnegat Bay have declined sharply in the last 40 years (Burger and Gochfeld 2016). The declines are mainly due to habitat degradation and loss of nesting islands from rising sea level and increased devastating storms (Burger and Gochfeld 2016). However, the potential importance of contaminants should be considered. The heavy metals in eggs of common terns represent local exposure since females arrive on the colony for weeks before they begin to lay eggs (Burger and Gochfeld 1991). Thus they can serve as bioindicators of local exposure, as well as being indicators of potential effects on developing embryos, and of potential effects on predators that eat them (Burger and Gochfeld 2016). Compared to blood, there are more data on the levels of mercury in eggs that can cause adverse effects. However, levels between 500 and 1500 ng/g (wet weight) can cause adverse effects on embryonic development (Wolfe et al. 1998; Heinz et al. 2009), depending upon species. Heinz et al. (2009) determined that the developing embryo of common tern was intermediate in vulnerability compared to other species of waterbirds.
Mercury, particularly methylmercury is the element known to cause the greatest ill effects in vertebrates (Wolfe et al. 1998; ATSDR, 1999, 2013). Mercury effects have been demonstrated in wild birds (Jackson et al. 2011) and other biota (Eisler 1987; Evers et al. 2011). In birds, the reproductive and neurobehavioral toxic effects of methylmercury have been demonstrated in the laboratory (Heinz 1979; Spalding et al., 2000; Wiener et al. 2003) and under wild conditions (Frederick et al. 2002; Frederick and Jaysena 2010; Whitney and Cristol 2018). In developing embryos, mercury binds to the sulfhydryl (-SH) group on the tubulin protein, leading to catastrophic disassembly of microtubules, including those that form the cell spindle that guides chromosome separation during cell division (Weis and Weis 1977; Graff et al. 1993). Blood levels of 2 µg/ml (2 mg/g) can reduce reproductive success in common loons (Gavia immer) (Evers et al. 2008, 2011), and levels of 0.7 µg/g (mg/g = 700 ng/g), and result in negative effects in Carolina wren (Thryothorus ludovicianus) (Jackson et al. 2011). These data suggest that birds eating small insects at the lower mercury levels (0.7 mg/g = 700 ng/g) can be impacted. Thus, Perkins et al. (2016) suggested that breeding shorebirds in Alaska might suffer adverse effects from mercury. Blood mercury levels in migrant shorebirds in Delaware Bay averaged less than 100 ng/g (0.1 mg/g), suggesting no adverse effects. However, over time, mercury is sequestered in internal tissues and in feathers, where the levels are considerably higher. In semipalmated sandpipers, for example, the mean mercury levels in other tissues were higher in liver (mean of 260 ng/g [or 0.26 mg/g], Burger et al. 2014) and feathers (mean of 353 or 507, depending upon sampling time, Tsipoura et al. 2017).
Unlike mercury, selenium is an essential nutrient that is regulated in the body. Selenium deficiency is rare, but selenium toxicity has been documented. For humans there is a narrow range of tolerance between too little and too much (ATSDR 1996; Eisler 2000; Vinceti et al. 2001). Thus, selenium protects cells from oxidative stress, while oxidative damage occurs with high levels of selenium (Hoffman 2002). The adverse effects of selenium in waterbirds were first described by Ohlendorf (Ohlendorf et al.1986, 1989, Ohlendorf 2011) at Kesterson Reservoir, California. In aquatic systems, high selenium levels can be toxic to fish (Muscatello et al. 2006), as well as birds (Heinz 1996). The difficulty is that there are far fewer studies that even examine levels of selenium in wild biota, and almost none that examine the relationship between selenium levels in tissues and observed toxic effects. The potential ameliorating effects of mercury and selenium on their respective toxicities is discussed briefly below. While no specific threshold value for selenium toxicity has been identified for fish or birds, selenium toxicity does occur (Ohlendorf 2011). The prey fish examined in this paper are all species that are eaten by predators, such as terns and gulls, bluefish, striped bass, and other higher-trophic level predators. Similarly, the larger fish are highly prized by humans including by recreational fisher folks, and by people who buy them in markets (Burger et al. 2004, Burger and Gochfeld 2012).
Implications for humans
People do not eat horseshoe crab eggs, but they do consume fish, birds and bird eggs. Prior to the Migratory Bird Treaty Act (1918), the eggs of shorebirds, terns and others were avidly consumed in the United States and this practice continues today in many parts of the world, even where nominal protection is provided. Commercial shorebird hunting was popular in North America into the late 1890s, and still occur on wintering grounds in South America (Wege et al. 2014), accounting for the decline in other species. Further, recent ethnic immigrants in the northeast eat terns and gull eggs during the nesting season. Thus humans have potential exposure to toxics in birds and their eggs. Of course, fish are an important part of people’s diets throughout the world.
The data presented in this paper have implications for understanding potential health effects for humans and other biota in at least four ways: 1) they can serve as bioindicators of changes in mercury and selenium levels in our environment, and suggest possible exposure from consumption, 2) mercury can cause both lethal and non-lethal effects at high levels in humans and other biota (ATSDR 1999, 2013; Evers et al. 2011), 3) selenium can cause both lethal and non-lethal effects at high or low levels (Ohlendorf et al. 1989; ATSDR 1996; Heinz 1996; Eisler 2000 ), and 4) selenium can potentially mitigate the toxic effects of mercury, (Raymond and Ralston 2004; Ralston and Raymond 2001, 2018) and vice-versa. These are important for understanding the risks from toxic exposure for species at different nodes on the food web. The importance of long-term studies for understanding environmental exposure was discussed above under bioindicators.
Mercury has long been recognized as toxic to humans and other biota, and has no known beneficial effect, in contrast to selenium, which is an essential trace element (ATSDR 1996, 1999; Fairweather-Tait et al. 2011). The importance of mercury in aquatic systems was recognized by the U.S. Environmental Protection Agency when they established an ambient freshwater mercury fish criteria as 0.3 mg/g in freshwater fish (EPA 2001), based partly on protecting human consumers of fish. Selenium is of interest for human risk assessment because there is a narrow acceptable level: both high and low levels are harmful (Vinceti et al. 2001). The data presented on mercury levels in this paper indicate that mercury levels in some recreational and commercial fish are in the toxic range, both as mean levels for species, and in the number of individuals within a species that exceed 0.3 mg/g. Similarly, the mercury levels in some of the eggs of birds examined in this study were above 0.5 mg/g (the WHO 2004 guidance for fish consumption).
In humans, selenium can have adverse effects if levels are both too low, and too high; it is usually regulated in the body in a narrow range (ATSDR 1996, Fairweather-Tait et al. 2011). Selenium is incorporated into selenoproteins that have several effects from antioxidants, to anti-inflammatory, to production of active thyroid hormones (Rayman 2012). A deficiency in selenium does exist in some places in the world (Khan and Wang 2009; Rayman 2012, see below). Excess, however, is also deleterious because it can affect thyroid function, impair natural killer cells, and lead to gastrointestinal disturbances and hepatotoxicity (Vinceti et al. 2001, Fairweather-Tait et al. 2011).
Our intent was not to examine the positive and negative effects of selenium, but rather to explore factors that affect differences in selenium levels in the food chain. Our data indicate that mercury varies by fish species, fish size, and season. Mercury levels were higher in larger fish and other species that are high on the trophic web (e.g. top level predators). Our findings also indicated that selenium varies by organism, whether birds or fish, but unlike mercury, selenium did not vary significantly by season or by fish size. These factors have implications for risk of mercury toxicity both to the organisms, as well as to predators that consume them. The lack of predictable trends in selenium levels (e.g. no increase with age and size), and the variations over time, suggest that in biota, as well as humans, there is less predictability of the potential for harm from selenium, especially for consumers of these organisms. Further it suggests the need for more research because selenium is usually considered to be regulated, and thus should be less variable among and within organisms (ATSDR 1996; Heinz 1996; Eisler 2000; Hoffman 2002) – this bears further examination.
Potential mitigating effects of selenium on mercury toxicity
Our emphasis in this paper was on showing how mercury and selenium levels vary over time (yearly and seasonally), space, species size, and species, as well as with variations with trophic position. It is widely recognized that selenium can partly mitigate the negative effects of mercury in fish, but how much protection is uncertain as is the location of the protection----is it in the fish tissue or in the fish dinner or in the consumer? There is considerable controversy about the degree of this protection, what molar ratio(s) are protective, and whether there are mercury levels that no amount of selenium can mitigate (Burger and Gochfeld 2013). Mercury and selenium can each modify the toxicity of the other, and have a high mutual affinity and (Komsta-Szumska et al. 1983; Beyrouty and Chan, 2006; Khan and Wang 2009). The relationships are complex. Mercury toxicity is associated with its high affinity to bind to sulfur, resulting in a strong binding to cysteine residues in proteins and enzymes that disrupts their function. However, by binding selenium, mercury can create a selenium deficiency, leading Ralston and others to identify mercury poisoning with the syndrome of selenium deficiency as (Ralston et al. 2007; Khan and Wang 2009; Peterson et al. 2009a,b). This selenium deficiency could account at least for the oxidative stress caused by mercury, for example by modulating the activity of selenium-dependent enzymes (i.e. glutathione peroxidase, Pinheiro et al. 2009). That selenium can ameliorate some of the negative toxic effects of mercury has been shown in some field studies. The selenium levels and the ratio were also associated with metallothionein induction in free-ranging fish (Sormo et al. 2011; Mulder et al. 2012), another potential modulator of mercury toxicity.
Ralston and others (Ralston 2009; Ralston and Raymond 2018) have suggested that selenium can ameliorate the toxic effect of mercury if the selenium/mercury molar ratio > 1, leaving no free mercury to cause ill effects. That is, one component of mercury toxicity is that it interferes with essential functions of selenium, which is one component of mercury toxicity. In contrast, excess mercury can also lead to selenium deficiency through binding (Watanabe et al. 1999). There are many papers that now examine the levels of mercury and selenium in fish, for example, and compute the molar ratios. The implication in most papers is that if the selenium:mercury molar ratio is greater than 1, consumers (biota and humans) are protected from mercury toxicity. And some authors state that if the selenium:molar ratio is above 1, the mercury levels in fish do not pose a risk to public health (Hoang et al. 2017; Ulusoy et al. 2019), even though the mean levels of mercury are above 0.5 mg/g. The acceptance of a 1:1 has led to shortening the selenium:mercury molar ratio to a “health benefit value”; if the ratio is 5:1, the health benefit value is 5. The use of the molar ratios, and of the health benefit value, in human health risk assessment, is becoming more widespread (Polak-Juszczak 2015). However, we caution that this is based on theory and presumption that all mercury will be captured by selenium, and elsewhere (Gochfeld and Burger 2020, this issue) we show that this assumption, though widely repeated and appealing is not correct. For example, the selenium/mercury molar ratio for both bluefish and striped bass varied over the fishing season, and was sometimes close to or below 1 – suggesting that selenium is not mitigating the toxic effects of mercury.
One very important aspect to bear in mind is that mercury toxicity occurs with high levels of mercury even in the presence of high levels of selenium, and high levels of selenium can cause toxicity (Vinceti et al. 2001; Pinheiro et al. 2009; Ohlendorf 2011; Fairweather-Tait et al. 2011; Rayman 2012; Mulder et al. 2012). Further, we found that the selenium:mercury ratio decreases with size in top-level fish predatory fish (e.g. bluefish, striped bass). This was due to increases in mercury levels that were not accompanied by increases in selenium; this relationship was also reported by Wang et al. (2018) for some fish. This means that with increasing fish size, the level of mercury increases, but any protective benefit of the selenium:mercury molar ratio decreases. Further, in localities with selenium deficiencies, piscivores have low selenium:mercury molar ratios, presumably lead to higher risk for organisms eating them (Donald 2016). We further suggest that an equivalent selenium/mercury molar ratio may not be protective in all organs (e.g. liver vs kidney vs muscle vs brain, Burger and Gochfeld 2013).
Both laboratory and field observations and experiments are going to be critical to determine the exact relationship between mercury and selenium, and the selenium:mercury molar ratios. However, we caution that there are several unanswered questions relating to the relative level of each element in different tissues, the relative selenium:mercury molar ratio in each tissue over time, and the ameliorating effects of each element on the other. Further, since the sensitivity to each element clearly varies by animal species (Heinz et al. 2009 and many other studies), levels of both selenium and mercury vary within species, the relationship between these molar ratios varies within species (Burger and Gochfeld 2011; Cusack et al. 2017; Azad et al. 2019), making is difficult to determine if there are ameliorating effects. That is, some species have a lower threshold of response for mercury, and show more severe teratogenic effects than other species when given the same dose (Heinz et al. 2009). This suggests that given the same selenium:mercury molar ratios, different animal species will derive different benefits from selenium. There is still a lively discussion among eco-toxicologists (and human health professionals) about whether the selenium:mercury molar ratio for protection is 1:1, 2:1, or some other ratio (and how this varies among and within species, Reyes-Avila et al. 2019; Azad et al. 2019). Thus, for risk assessment and management of the consumption of seafood, the variability in selenium:mercury molar ratio makes it difficult to develop a clear message (Cusack et al. 2015). Further, in some fish, mercury exceeds acceptable health standards even though the selenium:mercury molar ratio is above 1 (Squadrone et al. 2015). For human exposure considerations, however, we suggest that erring on the side of consuming only foods that are below the known toxic levels of each may be more prudent and conservative than relying on the possible ameliorating effects of a molar ratio over 1.
Conclusions
Whether there have been declines in mercury and selenium concentrations in the New Jersey estuarine biota over the last forty years is unclear – but it is clear that mercury varies with a great many internal (age, size, gender) and external (season, year, location) factors. This makes continuing to biomonitor the levels of mercury and selenium in biota where time series data exists, is very important. Understanding how mercury and selenium vary, both among and within species is also critical for understanding the threats organisms face, and those threats faced by their predators, including humans. While it may be convenient to examine human exposure from consuming fish or other seafood by analyzing mercury and selenium levels at one point in time (year or season), in one place, for only a few species, this is not going to capture the complexity of variations in mercury, selenium, and selenium:mercury molar ratios. If we are to understand exposure and risk to biota and humans, understanding this complexity is essential, particularly before risk assessors and risk managers use the selenium:mercury molar ratio in communication to regulators or the public. It is also critical to reduce mercury use overall, and human exposure, which supports the Minamata Convention (2013), an international agreement signed by 140+ countries, pledging to end uses of mercury and the international trade in mercury-containing objects (Stankiewicz 2020).
Figure 2.
Map of showing Barnegat Bay and Delaware Bay in New Jersey, United States (after Burger and Gochfeld 2016).
Highlights.
Mercury, selenium, and selenium:mercury ratios were examined in crab eggs, fish and birds from New Jersey (USA).
There were no clear yearly patterns in mercury or selenium levels in crab (1993–2019) or tern eggs (1971–2019).
Selenium and mercury varied by species in small prey fish and commercial/recreational fish, leading to differences in selenium:mercury molar ratios.
Mercury levels increased with fish size and season, while selenium did not.
Risk from consumption of crab and tern eggs, and fish varied for predators and people, limiting the utility of the ratio in risk communication and risk management.
Acknowledgments
We particularly thank F. Lesser for his help in Barnegat Bay for 40+ years. We also thank Mandy Dey, Larry Niles, Nellie Tsipoura, Mark Peck and Stephanie Feigin. The Endangered and Nongame Species Program of NJ Department of Environmental Protection, and the U.S. Fish & Wildlife Service provided permits, and the Rutgers University IUCAC for protocol approval (E97–017, three-year renewals). We thank Carlos Lodeiro, José Luis Capelo and the 3rd PTIM Conference for inviting us and supporting our travel.
Funding:
This research was funded by the USDA (Hatch Multistate Project 1008906, through the New Jersey Agriculture and Extension Service, Hatch NJ12233 and W3045, W4045), NIEHS Center of Excellence (NIH-NIEHS P30ES005022), NFWF, CWFNJ, Rutgers University, and Tiko Fund. We thank Carlos Lodeiro, José Luis Capelo and the 3rd PTIM Conference for inviting us and supporting our travel.
Footnotes
Declarations
Ethical Approval: All samples and data collected in this paper were gathered by the authors under the appropriate ethical standards of the University and under appropriate Institutional Review Board protocols (92–036, 97–017).
Consent to Participate: No consent to participate is required because no human participants were involved.
Consent to Publish: Not applicable.
Competing Interests: The authors have no competing interests or financial interests.
Availability of data and materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- Abbasi NA, Jaspers VLBJ, Chaudhry MJI, Ali S, Malik RN (2015) Influence of taxa, trophic level, and location on bioaccumulation of toxic metals in bird’s feathers: a preliminary biomonitoring study using multiple bird species from Pakistan. Chemosph 120:527–537 [DOI] [PubMed] [Google Scholar]
- Abdullah M, Fasola M, Muhammad A, Malik SA, Bostan N, Bokhari H, Kamran MA, Shafqat MN, Alamdar A, Khan M, Ali N, Musstjab SA, Eqani AS (2015) Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: a case study from severely contaminated areas. Chemosph 119:553–361 [DOI] [PubMed] [Google Scholar]
- Able KW Fahay MP (2014) The first year in the life of estuarine fishes in the Middle Atlantic Bight Rutgers, Univ Press: New Brunswick, NJ [Google Scholar]
- Ackerman JT, Eagles-Smeith CA, Herzog MP, Hartman CA (2016) Maternal transfer of contaminants in birds: mercury and selenium concentrations in parents and their eggs. Environ Poll 210:145–154 [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR)(1996) Toxicological profile for selenium Agency for Toxic Substances and Disease Registry, US Public Health Service. Atlanta, Georgia [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR)(1999). Toxicological profile for mercury Agency for Toxic Substances and Disease Registry, US Public Health Service: Atlanta, Georgia [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR)(2007) Toxicological profile for lead Agency for Toxic Substances and Disease Registry, US Public Health Service. Atlanta, Georgia [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR)(2013) Addentum to the Toxicological Profile for Mercury (Alkyl and Dialkyl compounds) Agency for Toxic Substances and Disease Registry, US Public Health Service: Atlanta, Ga [Google Scholar]
- Andres BA, Smith PA Morrison RG, Gratto-Trevor CL, Brown SC, Friis CA (2013) Population estimates of North American shorebirds. Wader Study Group Bull 119:178–194 [Google Scholar]
- Azad AM, Frantzen S, Bank MS, Nilsen BM, Duinker A, Madsen L, Maage A (2019) Effects of geography and species variation on selenium and mercury molar ratios in Northeast Atlantic marine fish communities. Sci Total Envir 652:1482–1496 [DOI] [PubMed] [Google Scholar]
- Baeyens W, Leenmakers M, Papina T, Saprykin A, Brion N, Noyen J, DeGieter M, Elskens M (2003) Bioconcentration and biomagnification of mercury and methylmercury in North Sea Scheldt Estuay fish. Arch Environ Contam Toxicol 45:498–508 [DOI] [PubMed] [Google Scholar]
- Baker AJ, Gonzalez PM, Piersma T, Niles LJ, deLima I, Nascimento S, Atkinson PW, Collins P, Clark NA, Minton CDT, Peck MK, Gates S (2004) Rapid population decline in red knots: fitness consequences of refuelling rates and late arrival in Delaware Bay. Proc Royal Society, London 271:875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A, Gonzalez P, Morrison RIG, Harrington BA (2013) Red Knot (Calidris canutus). In The Birds of North America Online Cornell Lab of Ornithology, ed. Poole A Ithaca, NY, America: Online. http://bna.birds.cornell.edu.bnaproxy.birds.cornell.edu/bna/species/563 (accessed January 3, 2020) [Google Scholar]
- Bakker A, Dutton J, Santangelo N (2016a) Metal accumulation in horseshoe crab (Limulus polyphemus) eggs, embryos, and larvae from potentially contaminated public beaches. Wild Environ Med 27:430–431 [Google Scholar]
- Bakkar A, Dutton J, Sclafani M, Santangelo N (2016b) Environmental exposure of Atlantic horseshoe crab (Limulus polyphemus) early life stages to essential trace elements. Sci Total Environ 572:804–812 [DOI] [PubMed] [Google Scholar]
- Berglund AMM (2018) Evaluating blood and excrement as bioindicators for metal accumulation in birds. Environ Pollut 233:1198–1206 [DOI] [PubMed] [Google Scholar]
- Beyrouty P, Chan HM (2006) Co-consumption of selenium and vitamin E altered the reproductive and developmental toxicity of methylmercury in rats. Neurotoxicol Teratol 28:49–58 [DOI] [PubMed] [Google Scholar]
- Bidone ED, Castilhos ZC, Santos TJS, Souza TMC, Lacerda LD (1997) Fish contamination and human exposure to mercury in Tartarugalzinho River, Northern Amazon, Brazil: a screening approach. Water Air Soil Pollut 97:9–15 [Google Scholar]
- Braune BM, Gaston AJ, Hobson KA, Gilchrist HG, Mallory ML (2015) Changes in trophic position affect rates of contaminant decline in two seabird colonies in the Canadian Arctic. Ecotoxicol Environ Safety 115:7–13 [DOI] [PubMed] [Google Scholar]
- Burger J (1993) Metals in avian feathers: Bioindicators of environmental pollution. Rev. Environ Toxicol 5:197–306 [Google Scholar]
- Burger J (2006) Bioindicators: a review of their use in the environmental literature 1970 – 2005. Environ Bioindicat 1:136–144 [Google Scholar]
- Burger J (2009) Risk to consumers from mercury in bluefish (Pomatomus saltatrix) from New Jersey: size, season and geographical effects. Environ Res 109:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M (1991) The common tern: its breeding biology and behavior Columbia University Press, New York. 401 pp [Google Scholar]
- Burger J, Gochfeld M (1996) Heavy metals and selenium levels in birds at Agassiz National Wildlife Refuge, Minnesota: food chain differences. Environ Monit Asses 43:267–282 [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M (2000) Metals in albatross feathers from Midway Atoll: influence of species, age, and nest location. Environ Res 82:207–221 [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M (2011) Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci Total Environ 409:1418–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M (2012) Selenium and mercury molar ratios in saltwater fish from New Jersey: individual and species variability complicates use in human health risk consumption advisories. Environ Res 114:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M (2013) Selenium/mercury molar ratios in freshwater, marine, and commercial fish from the USA: variation, risk, and health management. Rev Environ Health 28:129–143 [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M (2016) Habitat, population dynamics, and metal levels in colonial waterbirds: A food chain approach CRC Press, Boca Raton, FL, USA [Google Scholar]
- Burger J, Tsipoura N (2014) Metals in horseshoe crab eggs from Delaware Bay, USA: temporal patterns from 1993 to 2012. Environ Monit Assess 186:6947–6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Tsipoura N, Gochfeld M (2017) Metals levels in blood of three species of shorebirds during stopover reflect levels their food, Horseshoe Crab eggs. Toxics 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Stern AH, Dixon C, Jeitner C, Shukla S, Burke S, Gochfeld M (2004) Fish availability in supermarkets and fish markets in New Jersey. Sci Total Environ 333:89–97 [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Niles L, Dey A, Jeitner C, Pittfield T, Tsipoura N (2014). Metals in tissues of migrant semipalmated sandpipers (Calidris pusilla) from Delware Bay, New Jersey. Environ Res 133: 362–370 [DOI] [PubMed] [Google Scholar]
- Burger J, Tsipoura N, Niles LJ, Dey A, Mizrahi D (2015). Mercury, lead, cadmium, arsenic, chromium and selenium in feathers of shorebirds during migration through Delaware Bay, New Jersey: comparing the 1990s and 2011/2012. Toxics 3:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Tsipoura N, Niles L, Dey A, Jeitner C, Gochfeld M (2019) Heavy metals in biota in Delaware Bay, NJ: developing a food web approach to contaminants. Toxics 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameiro M, Colaco B, Colaco J, Faustino-Rocha AI, Colaco A, Lavin S, Oliveira PA (2016) Biomontoring of metals and metalloids with raptors from Portugal and Spain: a review. Environ Rev 24:63–83 [Google Scholar]
- Cusack LK, Eagles-Smith C, Harding AK, Kile M, Stone D (2017) Selenium:mercury molar ratios in freshwater fish in the Columbia River Basin: potential application for specific fish consumption advisories. Biol Trace Elem Res 178:136–146 [DOI] [PubMed] [Google Scholar]
- DeVault TL, Rhodes OE Jr, Shivik JA (2003) Scavenging by vertebrates: behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 102:225–34 [Google Scholar]
- Donald DB (2016) Relationship for mercury and selenium in muscle and ova of gravid freshwater fish. Environ Monit Assess 188, # 582. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Ackerman JT, Yee T, Adelsbach TL (2009a) Mercury demethylation in waterbird livers: dose-response thresholds and differences among species. Environ Toxicol Chem 28:568–577 [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Ackerman JT, De La Cruz SE, Takekawa JY (2009b) Mercury bioaccumulation and risk to three waterbird foraging guilds is influenced by foraging ecology and breeding stage. Environ 157:1993–2002 [DOI] [PubMed] [Google Scholar]
- Egwumah FA, Egwumah PO, Edet DI (2017) Paramount roles of wild birds as bioindicators of contamination. Intl J Avian Widl Biol 2:194–200 [Google Scholar]
- Eisler R (1987) Mercury Hazards to Fish, Wildlife and Invertebrates: a Synoptic Review U. S. Fish &Wildlife Service, Biol. Rep. 85 (1.10): Washington DC [Google Scholar]
- Eisler R (2000) Selenium. In. Handbook of Chemical Risk Assessment: Health Hazards to Humans, Plants and Animals Vol. 1. CRC Press, Boca Raton, FL [Google Scholar]
- Elnoder LD, MacLeod CK, Coughanowr C (2018) Metal and isotope analysis of bird feathers in a contaminated estuary reveals bioaccumulation, biomagnificaiton, and potential toxic effects. Arch Environ. Contam Toxicol 75:96–110 [DOI] [PubMed] [Google Scholar]
- Endangered Species Act (ESA)(1973) Public Law 93–205, as amended, 16USC 1513 et seq
- Environmental Protection Agency (EPA)(2001) Freshwater criterion for Fish http://www.epa.gov/fedrgstr/EPA-WATER/2001/January/Day-08/w217.htm.
- Ettinger AS, Egan KB, Homa DM, Brown MJ (2020) Blood lead levels in U.S. women of childbearing age, 1976–2016. Environ Health Persp 10.1289/EHP5926 [DOI] [PMC free article] [PubMed]
- Evers DC, Burgess NM, Champoux L, Hoskins B, Major A, Goodale WM, Taylor RJ, Poppenga R, Daigle T (2005) Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicol 14:93–221 [DOI] [PubMed] [Google Scholar]
- Evers DC, Savoy LJ, DeSorba CR, Yates DE, Hanson W, Taylor KM et al. (2008) Adverse effects from environmental mercury loads on breeding common terns. Ecotoxic 17:69–81 [DOI] [PubMed] [Google Scholar]
- Evers DC, Wiener JG, Basu N, Bodaly RA, Morrison HA, Williams KA (2011) Mercury in the Great Lakes region: bioaccumulation, spatiotemporal patterns, ecological risks, and policy. Ecotoxicol 20:1487–1499 [DOI] [PubMed] [Google Scholar]
- Fairbrother A (2009) Federal environmental legislation in the US for protection of wildlife and regulation of environmental contaminants. Ecotoxicol 18:784–790 [DOI] [PubMed] [Google Scholar]
- Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R (2011) Selenium in human health and diseases. Antioxid Redox Signal 14:1337–1338 [DOI] [PubMed] [Google Scholar]
- Frederick P, Jayasena N (2010) Altered pairing behaviour and reproductive success in White Ibises exposed to environmentally relevant concentrations of methylmercury. Proc Royal Soc Biol Sci 282:1851–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick PC, Spalding MG, Dusek R (2002) Wading birds as bioindicators of mercury contamination in Florida, USA: annual and geographic variation. Environ Toxicol Chem 21:163–167 [PubMed] [Google Scholar]
- Furness RW, Rainbow PS (1990) Heavy metals in the Marine Environment CRC Press; Boca Raton, FL., USA [Google Scholar]
- Gerwing TG, Kim JH, Hamilton DJ, Barbeau MA, Addison JA (2016) Diet reconstruction using next-generation sequencing increases the known ecosystem usage by a shorebird. Auk Ornithol Advan 133:168–177 [Google Scholar]
- Gochfeld M, Burger J (2020). Mercury toxicity: interactions with sulphur and selenium. Environ Sci Pollut Res Submitted for special issue.
- Gochfeld M, Burger J, Jeitner C, Donio M, Pittfield T (2012) Seasonal, locational and size variations in mercury and selenium levels in striped bass (Morone saxatilis) from New Jersey. Environ Res 112:8–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff RD, Philbert MA, Lowndes HE, Reuhl KR. 1993. The effect of glutathione depletion on methyl mercury-induced microtubule disassembly in cultured embryonal carcinoma cells. Toxicol Appl Pharmacol 120(1):20–28. [DOI] [PubMed] [Google Scholar]
- He C, Su T, Liu S, Jiang A, Goodale E, Qui G (2019) Heavy metals, arsenic, and selenium concentrations in bird feathers from a region in southern China impacted by intensive mining of nonferrous metals. Environ Toxicol 39:371–380 [DOI] [PubMed] [Google Scholar]
- Heinz GH (1979) Methylmercury: reproductive and behavioral effects on three generations of mallard ducks. J Wildl Manage 43:394–401 [Google Scholar]
- Heinz GH (1996) Selenium in birds. In: Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations, Beyer WM; Heinz WM (editors). Lewis, Boca Raton, FL, CRC Press, pp 447–458 [Google Scholar]
- Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA (2009) Species differences in the sensitivity of avian embryos to methylmercury. Arch Environ Cont Toxicol 56:129–138. [DOI] [PubMed] [Google Scholar]
- Hoang VAT, Sakamoto M, Yamamoto M (2017) Mercury and selenium levels, and their molar ratios in several species of commercial shrimp in Japan regarding the health risk of methymercury exposure. J Tox Sci 42:509–517 [DOI] [PubMed] [Google Scholar]
- Hoffman DJ (2002) Role of selenium toxicity and oxidative stress in aquatic birds. Aquatic Toxicol 57:11–26 [DOI] [PubMed] [Google Scholar]
- Huang Y, Deng M, Li T, Japenga J, Chen Q, Yang X, He Z (2017) Anthropogenic mercury emissions from 1980 to 2012 in China. Environ Pollut 226:230–239 [DOI] [PubMed] [Google Scholar]
- Jackson AK, Evers DC, Matthew A, Etterson MA, Condon AN, Folsom SB, Detweiler J, Schmerfeld J, Cristol DS (2011) Mercury exposure affects the reproductive success of a free-living terrestrial songbird, the Carolina Wren (Thryothorus ludovicianus). Auk 128:759–769. [Google Scholar]
- Khan MAK, Wang F (2009) Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem 28:1569–1577 [DOI] [PubMed] [Google Scholar]
- Komsta-Szumska E, Reuhl KR, Miller DR. (1983) The effect of methylmercury on the distribution and excretion of selenium by the guinea pig. Arch Toxicol 54:303–310. [DOI] [PubMed] [Google Scholar]
- Lange TR, Royals HE, Connor LL (1994) Mercury accumulation in largemouth bass (Micropterus salmoides) in a Florida Lake. Arch Environ Contam Toxicol 27:466–499 [DOI] [PubMed] [Google Scholar]
- Lasters R, Groffen T, Lopez-Anita A, Bervoets L, Eens M (2019) variations in PFFA concentrations and egg parameters throughout the egg-laying sequence in a free-living songbird (the great tit, Parus major): implications for biomonitoring studies. Environ Poll 246:237–248 [DOI] [PubMed] [Google Scholar]
- Mason RP (2014) Mercury Concentrations in Fish from Tidal Waters of the Chesapeake Bay Final Report to Maryland Department of Natural Resources; http://www.dnr.state.md.us/irc/docs/00006644.pdf. [Google Scholar]
- Mason RP, Laport J-M, Andres S (2000) Factors controlling the bioaccumulation of mercury, methymercury, arsenic, selenium, and cadmium in freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297 [DOI] [PubMed] [Google Scholar]
- Mendoza-Carranza MM, Sepulveda-Lozada A, Dias-Rerreira C, Geissen V (2016) Distribution and bioconcentration of heavy metals in a tropical aquatic food web: a case study of a tropical estuarine lagoon in SE Mexico. Environ Pollut 210:155–165 [DOI] [PubMed] [Google Scholar]
- Mizrahi DS, Peters KA, Hodgetts PA (2012) Energetic condition of Semipalmated and Least Sandpipers during northbound migration staging periods in Delaware Bay. Waterbirds 35:135–145 [Google Scholar]
- Montevecchi WA (2008) Binary dietary responses of Northern Gannets (Sula bassana) indicate changing food web and oceanographic conditions. Mar Ecol Press Ser 352:213–220 [Google Scholar]
- Morrison REG, Aubrey Y, Butler RW, Beyersbergen GW, Donaldson GM, Gratto-Trevor CL, Hicklin PW, Johnson WH, Ross RK (2001) Declines in North American shorebird populations. Wader Study Group Bull 94:37–42 [Google Scholar]
- Movalli P, Bode P, Dekker R, Fornasari L, van der Mije S, Yosef R (2017) Restrospective biomonitoring of mercury and other elements in museum feathers of common kestrel Falco tinnunculus using instrumental neutron activation analysis (INAA). Environ Sci Pollut Res 24:25986–26006 [DOI] [PubMed] [Google Scholar]
- Mulder PJ, Lie E, Eggen GS, Ciesielski TM, Berg T, Skaare JU, Jenssen BM, Sormo EG (2012) Mercury in molar excess of selenium interferes with thyroid hormone function in free-ranging freshwater fish. Environ Sci Tech 46:9027–9037 [DOI] [PubMed] [Google Scholar]
- Muscatello JR, Bennett PM, Himbeault KT, Belknap AM, Janz DM (2006) Larval deformities associated with selenium accumulation in northern pike (Esox Lucius) exposed to metal mining effluent. Environ Sci Technol 40:6506–6502. [DOI] [PubMed] [Google Scholar]
- Novcic I, Mizrahi DS, Veit RR, Symondson WO (2015) Molecular analysis of the value of Horseshoe Crab eggs to migrating shorebirds. Avian Biol Res 8:210–220 [Google Scholar]
- Ohlendorf HM (2011) Selenium, salty water, and deformed birds. Pp. 325–357 In Elliot JE, Bishop CA, Morrissey CA (eds). Wildlife Ecotoxicology: Emerging Topics in Ecotoxicology New York, Springer [Google Scholar]
- Ohlendorf H, Hothem RL, Bunck CM, Aldrich TW, Moore JR (1986) Relationship between selenium concentrations and avian reproduction. Trans 51st North American Wildl Res Conf 51:330–342 [Google Scholar]
- Ohlendorf HM, Hothem RL, Welsh D (1989) Nest success, cause-specific nest failure, and hatchability of aquatic birds at selenium-contaminated Kesterson Resevoir and a reference site. Condor 91:787–796. [Google Scholar]
- Orzechowski SCM, Shipley JR, Pegan TM, Winkler DW (2019) Negligible effects of blood sampling on reproductive performance and return rates of tree swallows. J Field Ornithol 90:2–138 [Google Scholar]
- Perkins M, Ferguson L, Lanctot RB, Stenhouse IJ, Kendall S, Brown S, Gates HR, Hall JO, Regan K, Evers DC (2016) Mercury exposure and risk in breeding and staging Alaskan shorebirds. Condor 118:571–582 [Google Scholar]
- Peterson SA, Ralston NVC, Peck DV, Van Sickle J, Robertson JD, Spate VL, Morris JS (2009a) How might selenium moderate the toxic effects of mercury in stream fish in western US? Environ Sci Technol 43:3919–3915. [DOI] [PubMed] [Google Scholar]
- Peterson SA, Ralston NVC, Wranger PD, Oldfield JE, Mosher WD (2009b) selenium and mercury interactions with emphasis on fish tissue. Environ Bioindic 4:318–334 [Google Scholar]
- Piersma T, Lok T, Chen Y, Hassell CJ, Yang HY, Boyle A, Slaymaker M, Chan YC (2016) Melville DS; Zhang Z-W; Ma Z. Simultaneous declines in summer survival of three shorebird species signals in a flyway at risk. J Appl Ecol 53:479–490 [Google Scholar]
- Pinheiro MCN, Nascimento JLM, Silveira LCD, Rocha JBT, Aschner M (2009) Mercury and selenium – a review on aspects related to the health of human populations in the Amazon. Environ Bioindic 4:222–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak-Juszczak L (2015) Selenium and mercury molar ratios in commercial fish from the Baltic Sea: additional risk assessment criterion for mercury exposure. Food Control 50:881–888 [Google Scholar]
- Ralston NV, Raymond LJ (2001) Dietary selenium’s protective effects against methylmercury toxicity. Toxicol 278:112–123 [DOI] [PubMed] [Google Scholar]
- Ralston NV (2009) Introduction to 2nd issue on special topic: Selenium and mercury as interactive environmental indicators. Environ Bioind 4:286–290 [Google Scholar]
- Ralston NV, Raymond L (2018) Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. BBA – General Subjects 10.1016/j.bbagen.2018.05.009 [DOI] [PubMed]
- Ralston NVC, Blackwell L, Raymond LJ (2007) Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol Trace Elem Res 119:255–268 [DOI] [PubMed] [Google Scholar]
- Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268 www.lancet.com [DOI] [PubMed] [Google Scholar]
- Raymond J, Wheeler W, Brown MJ (2014) Lead screening and prevalence of blood levels in children aged 1–2 years-child blood lead surveillance system, United States, 2002–2012 and National Health and Nutrition Examination Survey, United States, 1999–2010. MMWR Suppl 63:36–42 [PubMed] [Google Scholar]
- Raymond L, Ralston NVC (2004) Mercury:selenium interactions and health implications. Seychelles Med Dental J 17:72–77 [DOI] [PubMed] [Google Scholar]
- Reyes-Avila AD, Laws EQ, Hermann AD, DeLaune RD, Blanchard TP (2019) Mercury and selenium levels, and Se:Hg molar rations in freshwater fish from Louisiana. J Environ Sci Health 54:238–245 [DOI] [PubMed] [Google Scholar]
- Rolfhus KR, Hall BD, Monson BA, Paterson MJ, Jeremiason JD (2011) Assessment of mercury bioaccumulation within the pelagic food web of lakes in the western Great Lakes region. Ecotoxicol 20:1520–1529 [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Plotka-Wasylka J, Lubinska-Szczgel M, Rozanska A, Mozejko-Ciesielska J, Namiesnik J (2018) Birds’ feathers – suitable samples for determination of environmental pollutants. Trends Anal. Chem 109:97–115 [Google Scholar]
- Sarkka J, Hattula L, Paasivirta J, Janatuinen J (1978) Mercury and chlorinated hydrocarbons in the food chain of lake Paijanne, Finland. Hol Ecol 1:326–332 [Google Scholar]
- Seewagen CL (2010) Threats of environmental mercury to birds: Knowledge gaps and priorities for future research. Bird Conser Intern 20:112–123 [Google Scholar]
- Sormo EG, Ciesielski TM, Overjordet IB, Lierhagen S, Eggen GS, Berg T, Jenssen BM (2011) Selenium moderates mercury toxicity in free-ranging freshwater fish. Environ Sci Tech 45:6561–666 [DOI] [PubMed] [Google Scholar]
- Spalding MG, Frederick PC, McGill HC, Bouton SN, Richwy LJ, Schumacher IM, Blackmore SGM, Harrison J (2000) Histologic, neurologic, and immunologic effects of methylmercury on appetite and hunting behavior juvenile Great Egrets (Ardea albus). Environ Toxicol Chem 18:1934–1939 [Google Scholar]
- Squadrone S, Benedetto A, Brizio P, Prearo M, Abete MC (2015) Mercury and selenium in European catfish (Silurus glanis) from Northern Italian rivers: can molar ratio be a predictive factor for mercury toxicity in a top predator? Chemosphere 119:24–30 [DOI] [PubMed] [Google Scholar]
- Stankiewicz M (2020). Minamata Convention on Mercury marks three years of protecting human health and the environment https://www.unenvironment.org/news-and-stories/story/minamata-convention-mercury-marks-three-years-protecting-human-health-and-the-environment
- Tsipoura N, Burger J (1999) Shorebird diet during spring migration stop-over on Delaware Bay. Condor 101:635–644 [Google Scholar]
- Tsipoura N, Burger J, Niles L, Dey A, Gochfeld M, Peck M, Mizrahi D (2017) Metal levels in shorebird feathers and blood during migration through Delaware Bay. Arch Environ Contam Toxicol 72:562–574 [DOI] [PubMed] [Google Scholar]
- Ulusoy S, Mol S, Karakulak F-S, Kahraman AE (2019) Selenium-mercury balance in commercial fish species from Turkish waters. Biol Trace Elem Res 119:207–213. [DOI] [PubMed] [Google Scholar]
- Vallius H (2013) Heavy metal concentrations in sediment cores from the northern Baltic Sea: Declines over the last two decades. Mar Pollut Bull 79:359–364 [DOI] [PubMed] [Google Scholar]
- Vinceti M, Wei ET, Malagoli C. Bergomi M, Vivoli G (2001) Adverse health effects of selenium in humans. Rev Environ Health 16:233–251 [DOI] [PubMed] [Google Scholar]
- Wang X, Wu L, Sun J, Wei Y, Zhou Y, Yuan L, Liu X (2018) Mercury concentrations and se:hg molar ratios in flying fish (Exocoetus volitans) and squid (Uroteuthis chinensis). Bull Environ Contam Toxicol 101:42–48 [DOI] [PubMed] [Google Scholar]
- Watanabe C, Yin K, Kasanuma Y, Satoh H (1999) In utero exposure to methymercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol Teratol 21:83–88 [DOI] [PubMed] [Google Scholar]
- Wege DC, Birke W, Reed ET (2014) Migratory shorebirds in Barbados: hunting, management and conservation http://shorebirdconservationtrust.files.wordpress.com/2-13/oi/shorebird-hunting-barbados_wege-et-al-2014.pdf
- Weis P, Weis JS. 1977. Methylmercury teratogenesis in the killifish, Fundulus heteroclitus. Teratology 1:317–325 [DOI] [PubMed] [Google Scholar]
- Wege DC, birke W, Reed. ET 2014. Migratory shorebirds in Barbados: hunting, management and conservatio http://shorebirdconservationtrust.files.wordpress.com/2-13/oi/shorebird-hunting-barbados_wege-et-al-2014.pdf
- Weseloh DVC, Moore DJ, Hebert CD, de Solla SR, Braune BM, McGoldrick DJ (2011) current concentrations and spatial and temporal trends in mercury in Great Lakes Herring Gull eggs, 1974–2009. Ecotoxic 20:1644–1658 [DOI] [PubMed] [Google Scholar]
- Whitney MC, Cristol DA (2018) Impacts of sublethal exposure on birds: A detailed review. Rev Environ Contam Toxicol 244:113–163 [DOI] [PubMed] [Google Scholar]
- Wiemeyer SN, Jerek RM, Moore JE (1986) Environmental contaminants in surrogates, food, and feathers of California condors (Gymnogyps californianus). Environ Monit Asses 6:91–11 [DOI] [PubMed] [Google Scholar]
- Wiener JC, Krabbenhoft DP, Heinz GH, Scheuhammer M (2003) Ecotoxicology of mercury. In: Hoffman DJ; Rattner BA; Burton GA Jr; Cairns J Jr (eds). Handbook of Ecotoxicology Lewis Publ. Boca Raton FL [Google Scholar]
- Wolfe M, Schwarzbach S, Sulaiman RS (1998) Effects of mercury on wildlife: A comprehensive review. Environ Toxicol Chem 17:146–160 [Google Scholar]
- World Health Organization (WHO)(2004) Guidance for identifying populations at risk from mercury exposure UNEP/WHO, Geneva, Switzerland [Google Scholar]
- Zabala J, Meade AM, Frederick P (2019) Variation in nestling feather mercury concentrations at individual, brood, and breeding colony levels: implications for sampling mercury in birds. Sci Total Environ 671:617–621 [DOI] [PubMed] [Google Scholar]


