Abstract
Background:
This study’s purposes were to characterize detection rates of several sexually-transmitted infection (STI) agents and describe the effect additional specimen source and analyte screening has on STI detection within a cohort of young MSM and transgender women.
Methods:
Within a sixteen-month interval, 1966 encounters involved dual urine and rectal swab submissions assessed by commercial transcription-mediated amplification (TMA)-based assays for Chlamydia trachomatis and Neisseria gonorrhoeae and by off-label TMA-based Trichomonas vaginalis and Mycoplasma genitalium testing. Identification of STI carriers utilized algorithms involving FDA-cleared screening methods, laboratory-modified testing for extra-urogenital C. trachomatis and N. gonorrhoeae, and laboratory-developed tests for T. vaginalis and M. genitalium.
Results:
FDA-indicated urine C. trachomatis and N. gonorrhoeae screening revealed 39 encounters (2.0%) yielding one or both agents. Via C. trachomatis and N. gonorrhoeae screening that included rectal swab analysis, 264 encounters (13.4%) yielded evidence of either (140 C. trachomatis; 88 N. gonorrhoeae) or both (36 participants) infections. Detection rates for C. trachomatis and N. gonorrhoeae were 1.4% and 0.6% for urine screening and 8.2% and 6.2% for rectal screening respectively. Off-label screening identified 413 additional encounters with STI (5 T. vaginalis; 396 M. genitalium; 12 with both). 81.9% of these identifications were generated from analysis of rectal swabs (4 participants with T. vaginalis; 323 participants with M. genitalium; 12 with both). Overall detection rates of T. vaginalis (0.2% urine; 1.3% rectal) and M. genitalium (9.1% urine; 21.5% rectal) were variable.
Conclusion:
Additive analyte testing, including extra-urogenital collections, contributes to comprehensive STI screening within a high-risk demographic.
Keywords: RADAR, MSM, Rectal swab, Mycoplasma genitalium, Trichomonas vaginalis
SHORT SUMMARY
Additive, non-FDA-cleared specimen (rectal swab) and analyte (Trichomonas vaginalis and Mycoplasma genitalium) screening identified seventeen-fold more STI carriers in a cohort of young MSM and transgender women.
INTRODUCTION
Men who have sex with men (MSM) and transgender women are at increased risk for acquisition of sexually-transmitted infection (STI), with the rectal mucosa being particularly susceptible to infection by a number of bacterial pathogens.1 Several factors may be responsible for the increased risk of STI among this population including a greater number of sexual partners, increased use of illicit substances, and declining rates of condom use.2 Among transgender women specifically, past research has observed that those who have sex with men often engage in receptive anal intercourse, increasing risk of STI acquisition when compared with insertive sexual partners.3 In turn, the higher prevalence of STI among these high-risk populations increases risk for HIV acquisition.4 More specifically, both rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections have been associated with increased risk of HIV seroconversion among MSM.5 Further, MSM who were newly diagnosed with HIV infection were more likely to be asymptomatically infected with either C. trachomatis or N. gonorrhoeae than those who were HIV-seronegative.6
In its most recent STI guidelines, the United States Centers for Disease Control and Prevention (CDC) recommended annual screening of urethral STI (urine testing cited as a preferred approach) for sexually-active MSM who participate in insertive anal intercourse.1 Screening for rectal STI is also recommended for MSM who have engaged in receptive anal intercourse within the past year. While preferred assays within the guidelines for both C. trachomatis and N. gonorrhoeae detection are those based on nucleic acid amplification testing (NAAT),1 such commercialized testing assays have not traditionally possessed United States Food and Drug Administration (FDA) indications for extra-urogenital testing. Laboratories with sufficient resources and technical expertise have the capability to bring laboratory-developed tests or laboratory-modified assays onto their respective testing platforms7 to assist their clinician and community partners in following CDC STI screening guidelines. One investigation of two commercial NAAT assays for off-label detection of STI agents from rectal sources8 revealed sensitivity values of ≥ 93.1% and ≥ 94.7% for detection of N. gonorrhoeae and C. trachomatis, respectively. Corresponding culture sensitivity indices were 41.4% and 21.1%.
Recent studies have documented significant detection of C. trachomatis- and N. gonorrhoeae-specific nucleic acid from extra-urogenital specimens collected from STI clinic populations and from other at-risk demographics.9,10 The urogenital parasite Trichomonas vaginalis and the cell wall-devoid bacterium Mycoplasma genitalium have been found to be more commonly-identified agents of STI than previously considered. NAAT technologies have advanced to the point where men can now be screened for these agents, with the assistance of laboratory-modified testing.11,12 However, few reports have documented the prevalence of these agents in the young MSM and transgender women demographics. We hereby provide preliminary insight into the utility of T. vaginalis and M. genitalium screening of both urogenital and rectal swab specimens within this high-risk cohort.
MATERIALS AND METHODS
Cohort eligibility.
RADAR is a cohort study (current n=1,132) examining HIV risk factors, substance use, and relationship patterns among young MSM and transgender women in the Chicago, Illinois region. The study’s multilevel design focuses on individual, dyadic, network and biologic factors that may be associated with HIV. Participants are followed through the developmental period of late adolescence to early adulthood, which is a critical period of initiation and acceleration of sexual behavior and substance use. This cohort and methods of recruitment have been described previously and were selected in order to achieve the multiple cohort, accelerated longitudinal study design. Eligibility requirements at time of participant enrollment include: ages 16–29 years; male assignment at birth; English speaking; and, report of a sexual encounter with a man in the previous year or identification as gay, bisexual, or transgender. The overall sample is also augmented by recruitment of serious partners of RADAR cohort members who meet eligibility criteria, “serious” being self-defined by the participant. Participant interviews included both self-reported and interviewer-administered sections. Data collection began in February 2015 with participants being followed for 4.5 years and study visits occurring every six months. This investigation received Institutional Review Board approval through Northwestern University. Informed consent was obtained from all study participants.
Collection of participant data and provision of specimens.
Data utilized in these analyses were collected from March 2018 through June 2019, as screening for T. vaginalis and M. genitalium was added to the protocol during this time. Participants were asked to provide 10–15 mL of first-void urine (subsequently aliquoted to an Aptima Urine Specimen Collection Kit [Hologic, Incorporated; San Diego, CA] at the study site) and to self-collect a rectal swab specimen using the Aptima Multitest Swab Specimen Collection Kit (Hologic). All specimens were maintained at 2–30°C prior to test performance. All specimens were tested within 2–14 days of collection.
Molecular assays.
Simultaneous detection of C. trachomatis-specific 23S ribosomal (r)RNA and N. gonorrhoeae-specific 16S rRNA from separate first-void urine and rectal swab specimens occurred by FDA-indicated and off-label utilizations, respectively, of Aptima Combo 2 (Hologic). Detection of T. vaginalis 18S rRNA from both specimen sources occurred by off-label utilization of Aptima Trichomonas vaginalis (Hologic). Detection of M. genitalium 16S rRNA was facilitated by a laboratory-developed test utilizing an analyte-specific reagent provided by Hologic. Accuracy of the aforementioned transcription-mediated amplification (TMA)-based assays for off-label detection of T. vaginalis rRNA and for detection of M. genitalium rRNA has previously been demonstrated.10,13 All testing was performed via direct tube sampling on the Panther automated system (Hologic). The analyzer provided qualitative interpretive data from C. trachomatis, N. gonorrhoeae, and T. vaginalis testing. Relative light unit values ≥ 50,000 generated from M. genitalium TMA were interpreted positive for M. genitalium rRNA detection.14
Data analysis.
Frequency and proportion of detectable STI results were examined for each STI agent and specimen source. In order to account for within-subject correlation produced through repeated STI testing, generalized estimating equations (GEE) using a binary distribution were used to compute 95% confidence intervals and to test for statistical significance in proportions between STI agents and specimen sources. An a priori decision was made to examine statistical significance using a two-tailed test with an alpha level of 0.05. In instances where missing data was present (<1.0%), observations were excluded from analyses.
RESULTS
Demographic characteristics associated with patient encounters.
From March 2018 to June 2019, 1983 patient encounters among 888 unique participants within the RADAR cohort study involved collection of rectal swab and/or first-void urine specimens for molecular detection of C. trachomatis, N. gonorrhoeae, T. vaginalis, and M. genitalium. The mean age of participants at their first visit within this time period was 23.6 ± 3.1 years. 89.0% of the participants were young MSM participants with the remainder being young transgender women (Table 1). Race/ethnicity distribution included 34.5% black, 23.5% white, and 31.5% Latinx. An overall HIV-seropositive rate of 21.3% was documented, which included participants who were known positive at baseline and those who seroconverted during the course of the study. HIV point-of-care (POC) testing was conducted for all HIV-negative participants with confirmatory testing performed on all reactive results following CDC guidelines. 15 STI transmission-risk sexual practices described among participants included 46.2% being engaged in insertive condomless anal/vaginal sex and 46.3% involved in receptive condomless anal sex (Table 1).
TABLE 1:
Selected demographics associated with participants in the RADAR cohort study at their first screening for Trichomonas vaginalis and Mycoplasma genitalium.
| n (%) | |
|---|---|
|
| |
| Gender | |
| Cisgender man | 790 (89.0) |
| Transgender woman | 57 (6.4) |
| Other gender | 41 (4.6) |
| Race/ethnicity | |
| Black | 306 (34.5) |
| Hispanic/Latinx | 280 (31.5) |
| White | 209 (23.5) |
| Other | 93 (10.5) |
| HIV serostatus | |
| Positive | 189 (21.3) |
| Negative | 699 (78.7) |
| Condomless insertive anal/vaginal sex in the past 6 months | |
| No | 478 (53.8) |
| Yes | 410 (46.2) |
| Condomless receptive anal sex in the past 6 months | |
| No | 477 (53.7) |
| Yes | 411 (46.3) |
Detection rates of STI pathogens from first-void urine and rectal swab specimens.
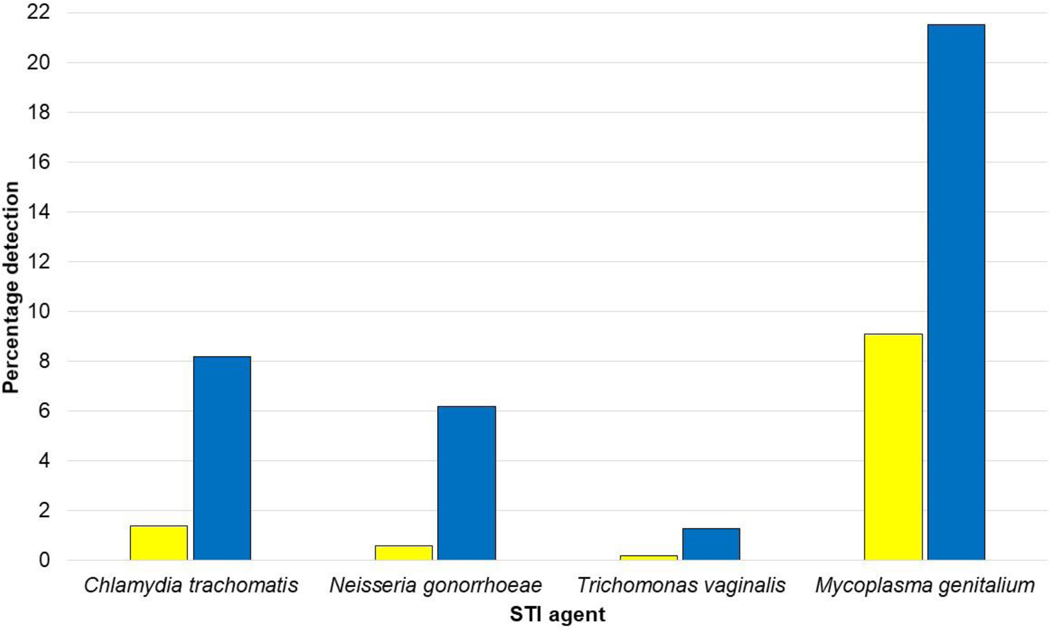
From 1977 encounters in which urine specimens were collected, 28 (1.4%; 95% CI: 0.9%, 2.0%) yielded detectable C. trachomatis rRNA and 12 (0.6%; 95% CI: 0.2%, 1.0%) were positive for N. gonorrhoeae rRNA (Fig. 1). The urine detection rate for T. vaginalis rRNA (0.2%; 95% CI: 0.0%, 0.5%) was similar to that for N. gonorrhoeae; however, M. genitalium rRNA was detected from 179 (9.1%; 95% CI: 7.3%, 10.9%) urine specimens. From 1972 encounters in which rectal swab specimens were collected, rRNA detection rates of 8.2% (95% CI: 6.9%, 9.5%) and 6.2% (95% CI: 5.0%, 7.4%) were observed for C. trachomatis and N. gonorrhoeae, respectively (Fig. 1). T. vaginalis rRNA was detected from 26 (1.3%; 95% CI: 0.7%, 1.9%) rectal swab specimens. In contrast, 423 (21.5%; 95% CI: 19.1%, 23.8%) rectal swab specimens yielded detectable M. genitalium rRNA (Fig. 1). This percentage exceeded those of other STI agent rectal swab detections (P < 0.0001). All increases in rectal swab detection rates per analyte when compared to first-void urine specimens were significant (P < 0.01). Multi-site detection rates for C. trachomatis, N. gonorrhoeae, T. vaginalis and M. genitalium were 0.6% (95% CI: 0.3%, 1.0%), 0.5% (95% CI: 0.2%, 0.8%), 0.2% (95% CI: 0.0%, 0.4%) and 4.2% (95% CI: 3.1%, 5.3%).
Figure 1.
Detection rates of sexually-transmitted agent-specific rRNA from primary urine (yellow bars) and rectal swab (blue bars) specimens from 1983 participant encounters in the RADAR cohort study.
Effects of additive specimen source on detection of C. trachomatis and N. gonorrhoeae rRNA.
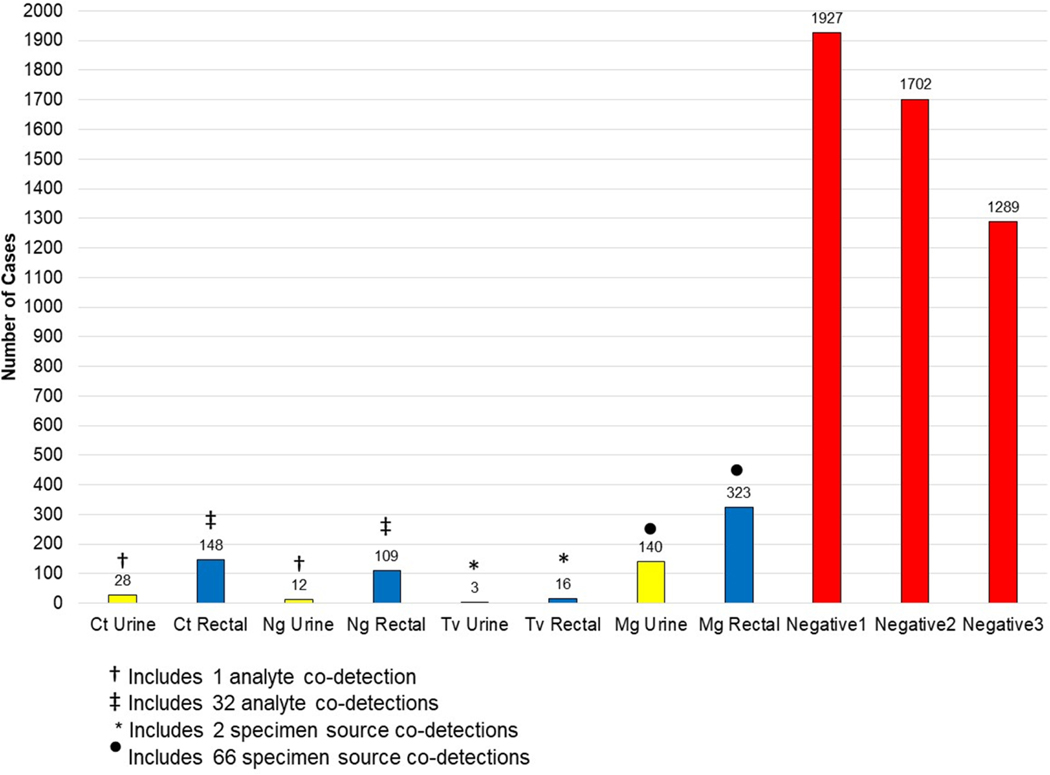
1966 (99.1%) of participant encounters involved the collection of both first-void urine and rectal swab specimens. Utilization of FDA-indicated NAAT for C. trachomatis and N. gonorrhoeae detection (urine testing only) would have resulted in 39 encounters with documented STI detection (Fig. 2). One urine specimen yielded co-detection of C. trachomatis and N. gonorrhoeae rRNA. Additive off-label rectal swab testing increased the frequency of C. trachomatis and/or N. gonorrhoeae rRNA detections to 13.4% (95% CI: 11.7%, 15.2%; n = 264 encounters) of the testing sample. 32 co-detections of C. trachomatis and N. gonorrhoeae rRNA were observed from rectal swab testing. The number of negative patient encounters decreased from 1927 to 1702 with the addition of off-label rectal swab NAAT (Fig. 2).
Figure 2.
Detection of Chlamydia trachomatis (Ct)-, Neisseria gonorrhoeae (Ng)-, Trichomonas vaginalis (Tv)-, and Mycoplasma genitalium (Mg)-specific rRNA from a 1966-encounter cohort. Data are presented in terms of FDA-indicated testing of first-void urine specimens (urine specimens testing negative for C. trachomatis and N. gonorrhoeae are indicated by the bar labeled “Negative1”); followed by additive testing of rectal specimens for C. trachomatis- and N. gonorrhoeae-specific rRNA (urine and rectal specimens testing negative for C. trachomatis and N. gonorrhoeae are indicated by the bar labeled “Negative2”); and followed by additive analysis of all specimens for T. vaginalis- and M. genitalium-specific rRNA (specimens testing negative for all eight analyte permutations are indicated by the bar labeled “Negative3”).
Detection of T. vaginalis and M. genitalium rRNA from encounters yielding negative screening results for both C. trachomatis and N. gonorrhoeae.
Addition of off-label and laboratory-developed NAAT resulted in nominal and substantial increases in T. vaginalis and M. genitalium detection, respectively (Fig. 2). From encounters that screened negative for urogenital and extra-urogenital C. trachomatis and N. gonorrhoeae, T. vaginalis rRNA was detected from a urine specimen at one encounter, from a rectal swab specimen during 14 encounters, and from both specimen sources during two encounters. These positive results were duplicated upon repeat analysis.
In contrast, from encounters that screened negative for urogenital and extra-urogenital C. trachomatis, N. gonorrhoeae, and T. vaginalis, M. genitalium rRNA was detected from urine specimens in 74 instances, from rectal swab specimens during 256 encounters, and from both specimen sources during 66 encounters. In total, addition of M. genitalium rRNA detection resulted in 409 (24.0%; 95% CI: 21.4%, 26.7%) positive patient encounters out of 1702 encounters without detectable C. trachomatis or N. gonorrhoeae rRNA.
Addition of T. vaginalis and M. genitalium analytes to the comprehensive STI screen resulted in a 34.4% (95% CI: 31.7%, 37.3%) positive participant encounter rate. This value represented a 156% increase over the level of C. trachomatis and/or N. gonorrhoeae rRNA detection, even when factoring extra-urogenital testing. The number of participant encounters negative for the four STI agents decreased from the original value of 1927 to 1289 (Fig 2).
M. genitalium rRNA co-detection data.
M. genitalium rRNA was co-detected in 15 of the 26 rectal swab specimens with detectable T. vaginalis rRNA. In addition, M. genitalium rRNA was co-detected in 53 rectal swab specimens with detectable C. trachomatis rRNA; in 43 rectal swab specimens with detectable N. gonorrhoeae rRNA; in six urine specimens with detectable C. trachomatis rRNA; and, in five urine specimens with detectable N. gonorrhoeae rRNA (data not illustrated).
DISCUSSION
NAAT has become a reference method for laboratory detection of non-ulcerative STI agents. However, at the inception of this study, commercial assays did not possess FDA indications for performance on extra-urogenital specimens, such as rectal swab specimens. Laboratory-developed assays or laboratory-modified tests are, therefore, alternative means for detection of C. trachomatis and N. gonorrhoeae nucleic acid from at-risk demographics. When developing these assays, the potential effect of endogenous specimen inhibitors of nucleic acid amplification on assay performance must be considered. With respect to analysis of rectal swab specimens, inhibitory agents endogenous to feces16 and commensal enteric Gram-negative bacteria17 may be encountered. Despite the potential of encountering these endogenous specimen inhibitors during the analysis of rectal swab specimens in our investigation, we report significant detection rates of N. gonorrhoeae- (6.2)%, C. trachomatis- (8.2%), and M. genitalium-specific (21.5%) nucleic acid from this specimen source. Analogous detection rates of the aforementioned STI agents from first-void urine were 0.6%, 1.4% and 9.1% respectively.
One component of the commercial amplification assay utilized in this study is a mechanism in which magnetic-linked oligonucleotide sequences specific for the pathogen being assayed are allowed to hybridize to target nucleic acid prior to the amplification process. This system of target capture essentially sequesters hybridized target nucleic acid to the side of a reaction tube (in the presence of a magnetic field) while a vacuum aspiration system washes the contents of the reaction tube, effectively removing endogenous agents of inhibition prior to the initiation of amplification. The target capture paradigm contributes to enhanced analytic sensitivity of the system. Using first-void urine specimens as an example, substances noted to inhibit C. trachomatis nucleic acid amplification have included hemoglobin, urine crystals, iron, urine nitrites, and phosphate.18,19 One first-generation commercial amplification assay revealed an 11.9% rate of amplification inhibition from 388 urine specimens.20 One subsequent study21 revealed an inhibition rate of 0.3% from urine specimens when using a second-generation commercial amplification assay employing target capture.
In addition to the mitigation of endogenous inhibitory factors via target capture, laboratory detection of STI agents from extra-urogenital specimens (particularly rectal swab specimens) is facilitated by RNA amplification assays. Schachter et al.22 reported 44.4% sensitivity of one commercial DNA amplification assay for detection of N. gonorrhoeae nucleic acid from rectal swab specimens when compared to commercial RNA amplification. Commercial RNA amplification was also 14.8% more sensitive than a second format of DNA amplification in detection of N. gonorrhoeae nucleic acid from rectal swab specimens. Ota et al.8 reported 64.7% sensitivity of DNA amplification for detection of C. trachomatis nucleic acid from rectal swab specimens when compared to second-generation RNA amplification. Furthermore, RNA amplification was 30% more sensitive than a second format of DNA amplification testing in detection of C. trachomatis nucleic acid from rectal swab specimens. These data parallel studies that have demonstrated increased analytic sensitivity of commercial RNA amplification over that of DNA amplification for detection of STI agents from female and male urogenital specimens.19,21,23 Such differences may be explained by the inherent multiplicity of rRNA target in living organisms, including pathogens, when compared to single-copy DNA or plasmid DNA target sequences.
While T. vaginalis has been associated with non-gonococcal urethritis in males and has been potentially linked to prostate carcinoma,11 few reports discuss its detection in male rectal swab specimens. A 2005–2006 study of MSM in San Francisco24 reported three positive commercial T. vaginalis TMA results from 500 rectal swabs. Only one of these positive results was duplicated by repeat TMA analysis; the three swabs failed to generate a positive result by research PCR. In another United States study, Cosentino et al.25 documented a nearly 9% TMA detection rate of T. vaginalis rRNA from rectal swabs obtained from women and only a 0.9% detection rate on those collected from males. These two positive male rectal swab specimen results were duplicated through the use of an alternative TMA primer set. In a region of Africa severely affected by the HIV/AIDS epidemic, the detection rate of T. vaginalis by rectal swab PCR was 2.1%.26 Data recently published from South Africa27 describe seven MSM with positive T. vaginalis PCR results from rectal swab specimens. Two of these patients presented with symptoms of proctitis, although one of these individuals had concomitant detection of N. gonorrhoeae and M. genitalium DNA from a urethral swab specimen. All 26 rectal swab specimen detections of T. vaginalis rRNA in the presented study were confirmed by repeat testing; however, symptomatic status of participants in this investigation was not ascertained. Additional studies of this cohort can assist in investigations of both the clinical significance of rectal T. vaginalis rRNA detection and the potential cost-benefit of site-specific screening for this STI agent. Of particular importance would be the characterization of confirmed T. vaginalis proctitis and its delineation from potential deposit contamination in the context of recent receptive anal sex.
Recent literature has documented several reports of rectal M. genitalium incidence being variable on the basis of geography and patient demographics. In Europe, a 4.8% rectal swab M. genitalium detection rate by PCR was reported from 165 MSM.28 An Australian study reported an 8.9% M. genitalium commercial PCR detection rate from rectal swab analysis of a cohort of symptomatic and asymptomatic MSM.29 In the United States, Dionne-Odom et al.30 reported M. genitalium urine and rectal swab detection rates of 10.8% and 6.4%, respectively, by PCR in a cohort of 157 HIV-seropositive MSM. Cosentino et al.25 documented an 11.1% detection rate of M. genitalium rRNA from male rectal swab specimens by commercial TMA. From a United States high-prevalence STI community, M. genitalium rRNA rectal swab and first-void urine detection rates of 5.8% and 6.6%, respectively, were derived from commercial TMA.10 Age and race distribution, symptomatic/asymptomatic status, and HIV seropositivity status of that cohort were not determined. In the context of these cited studies, the substantial rates of both M. genitalium urine (9.1%) and rectal swab (21.5%) rRNA detection in the current study merit further investigation. Possible factors include the limited and young age-based enrollment criteria of the cohort and the preponderance of condomless transmission risk behaviors (Table 1). Moreover, potential M. genitalium associations with HIV acquisition and transmission, as have been demonstrated with other STI agents,4–6 cannot be ignored in the context of clinical and public health.
Even in light of our meaningful findings, our study should be considered in the context of its limitations. First, we were lacking both symptom and treatment data, thus we cannot be sure whether detected infectious were persistent and untreated or if these were recurrent infections. Second, participants were able to contribute multiple encounters to the data. Finally, this sample was a community sample rather than a probability sample and, as such, findings may not generalize to the larger population of young men who have sex with men, particularly those outside an urban environment.
In conclusion, procurement of (and the capability of technically-competent molecular diagnostics laboratories to test) off-label specimen sources, particularly rectal swab specimens, enhances the overall identification of male STI carrier status in high-risk demographics. [FDA clearance of rectal swab screening was granted to the commercial C. trachomatis/N. gonorrhoeae TMA assay of interest in mid-2019.] We also demonstrate that additional T. vaginalis and, particularly, M. genitalium analysis of multiple specimen sources assists in the comprehensive screening of a high-risk demographic. Additional studies are warranted to not only elucidate the elevated first-void urine and rectal swab M. genitalium rRNA detection rates in this cohort but to also to understand morbidity and optimal therapies for rectal M. genitalium infections and to determine financial and disease transmission impacts of these findings on the potential paradigm of inclusive STI screening algorithms.
ACKNOWLEDGMENTS
The authors wish to thank Justin Franz, Peter Cleary, and Michael Pulte for assistance at the RADAR study sites and all of the research participants. The RADAR study was supported by grants from the National Institute on Drug Abuse (U01DA036939, PI: Mustanski; F32DA046313, PI: Morgan). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Source of Funding:
E. Munson has received honoraria and travel grants from Hologic, Incorporated. B. Mustanski has received consulting fees from Hologic, Incorporated. For the remaining authors, none were declared. This work was funded by grants from the National Institute on Drug Abuse (U01DA036939; F32DA046313). Hologic, Incorporated provided multi-test swab and urine collection kits, as well as testing reagents for the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Conflict of Interest: The sponsor had no involvement in the conduct of the research.
REFERENCES
- 1.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 2.Swann G, Newcomb ME, Crosby S, et al. Historical and developmental changes in condom use among young men who have sex with men using a multiple-cohort, accelerated longitudinal design. Arch Sex Behav 2019; 48:1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva-Santisteban A, Raymond HF, Salazar X, et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS Behav 2012; 16:872–881. [DOI] [PubMed] [Google Scholar]
- 4.Jones J, Weiss K, Mermin J, et al. Proportion of incident human immunodeficiency virus cases among men who have sex with men attributable to gonorrhea and chlamydia: a modeling analysis. Sex Transm Dis 2019; 46:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010; 53:537–543. [DOI] [PubMed] [Google Scholar]
- 6.Scott KC, Philip S, Ahrens K, et al. High prevalence of gonococcal and chlamydial infection in men who have sex with men with newly diagnosed HIV infection: an opportunity for same-day presumptive treatment. J Acquir Immune Defic Syndr 2008; 48:109–112. [DOI] [PubMed] [Google Scholar]
- 7.Burd EM. Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev 2010; 23:550–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ota KV, Tamari IE, Smieja M, et al. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima Combo 2 assay and culture. Sex Transm Infect 2009; 85:182–1856. [DOI] [PubMed] [Google Scholar]
- 9.Mustanski B, Parsons JT, Sullivan PS, et al. Biomedical and behavioral outcomes of Keep It Up!: an eHealth HIV prevention program RCT. Am J Prev Med 2018; 55:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecular assessment: a retrospective analysis. J Clin Microbiol 2017; 55:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meites E, Gaydos CA, Hobbs MM, et al. A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin Infect Dis 2015; 61(suppl 8):S837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbiol 2017; 55:2894–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munson KL, Napierala M, Munson E, et al. Screening of male patients for Trichomonas vaginalis with transcription-mediated amplification in a community with a high prevalence of sexually transmitted infection. J Clin Microbiol 2013; 51:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wroblewski JK, Manhart LE, Dickey KA, et al. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J Clin Microbiol 2006; 44:3306–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Laboratory testing for the diagnosis of HIV infection : Updated Recommendations. In:2014. [Google Scholar]
- 16.Monteiro L, Bonnemaison D, Vekris A, et al. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J Clin Microbiol 1997; 35:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JW, Goodwin PH. Extraction of genomic DNA from extracellular polysaccharide-synthesizing Gram-negative bacteria. Biotechniques 1995; 18:418–422. [PubMed] [Google Scholar]
- 18.Rosenstraus M, Wang Z, Chang SY, et al. An internal control for routine diagnostic PCR: design, properties, and effect on clinical performance. J Clin Microbiol 1998; 36:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda-Dantsuji Y, Konomi I, Nagayama A. In vitro assessment of the APTIMA Combo 2 assay for the detection of Chlamydia trachomatis using highly purified elementary bodies. J Med Microbiol 2005; 54(Pt 4):357–360. [DOI] [PubMed] [Google Scholar]
- 20.Mahony J, Chong S, Jang D, et al. Urine specimens from pregnant and nonpregnant women inhibitory to amplification of Chlamydia trachomatis nucleic acid by PCR, ligase chain reaction, and transcription-mediated amplification: identification of urinary substances associated with inhibition and removal of inhibitory activity. J Clin Microbiol 1998; 36:3122–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chernesky M, Jang D, Luinstra K, et al. High analytical sensitivity and low rates of inhibition may contribute to detection of Chlamydia trachomatis in significantly more women by the APTIMA Combo 2 assay. J Clin Microbiol 2006; 44:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35:637–642. [DOI] [PubMed] [Google Scholar]
- 23.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200:188.e1-e7. [DOI] [PubMed] [Google Scholar]
- 24.Francis SC, Kent CK, Klausner JD, et al. Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005–2006. Sex Transm Dis 2008; 35:797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosentino LA, Campbell T, Jett A, et al. Use of nucleic acid amplification testing for diagnosis of anorectal sexually transmitted infections. J Clin Microbiol 2012; 50:2005–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuylsteke B, Semde G, Sika L, et al. High prevalence of HIV and sexually transmitted infections among male sex workers in Abidjan, Cote d’Ivoire: need for services tailored to their needs. Sex Transm Infect 2012; 88:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman CM, Fritz L, Radebe O, et al. Rectal Trichomonas vaginalis infection in South African men who have sex with men. Int J STD AIDS 2018; doi: 10.1177/0956462418788418. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Foschi C, Gaspari V, Sgubbi P. Sexually transmitted rectal infections in a cohort of men having sex with men. J Med Microbiol 2018; 67:1050–1057. [DOI] [PubMed] [Google Scholar]
- 29.Couldwell DL, Jalocon D, Power M, et al. Mycoplasma genitalium: high prevalence of resistance to macrolides and frequent anorectal infection in men who have sex with men in western Sydney. Sex Transm Infect 2018; 94:406–410. [DOI] [PubMed] [Google Scholar]
- 30.Dionne-Odom J, Geisler WM, Aaron KJ, et al. High prevalence of multidrug-resistant Mycoplasma genitalium in human immunodeficiency virus-infected men who have sex with men in Alabama. Clin Infect Dis 2018; 66:796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]