Abstract
The presence of memory lymphocytes in non-lymphoid tissues reflects prior immunological experience and can provide non-specific defense against infection. Here, we used a mouse cohousing approach to examine the effect of prior immunological experience on Salmonella and Chlamydia infection. As expected, co-housing of “dirty mice” with SPF laboratory mice increased the frequency of effector memory T cells (TEM) in laboratory mice and enhanced protection against systemic Listeria infection. In contrast, the course of systemic infection with Salmonella and mucosal infection with Chlamydia was largely unaffected by co-housing, despite enhanced frequencies of memory T cells. Thus, co-housing of laboratory mice reliably increases the proportion of memory T cells in circulation, but can have variable effects on pathogen clearance.
Introduction
The immunological landscape of nonlymphoid tissues (NLT) from mice housed in specific pathogen free (SPF) conditions resembles that of a neonate human (1). These naïve tissues are devoid of most lymphocytes, including memory T cells (1), reflecting the fact that naïve lymphocytes largely restrict their movement to lymphoid tissues and blood before activation (2). In marked contrast, NLT samples from pet shop mice, previously infected SPF mice, or adult humans, are usually replete with memory lymphocytes generated during prior immunological events, such as past infections (1, 3–5). These memory lymphocyte populations are capable of accelerating immune protection against pathogens in an antigen non-specific manner (1). A mouse model has been established that allows investigation of this non-specific protection where genetically identical SPF mice share housing for a period of time with pet shop or feral mice, allowing laboratory mice to be exposed to a “dirty” environment and generate a more “experienced” immune system (1, 6). Previous studies show that co-housing of laboratory mice with “dirty” mice caused an increase in memory lymphocyte populations and enhanced control of systemic infection with Listeria monocytogenes and Plasmodium berghei (1, 6). However, it is not yet clear whether similar protection extends to other infections, especially intracellular bacteria that require clearance by CD4 T cells (7, 8). Our laboratory has previously shown that non-cognate activation of antigen-experienced CD4 T cells enhanced clearance of intracellular bacteria (9, 10), suggesting that effector memory CD4 T cells circulating through blood and tissues might potentially contribute to early non-specific defense. While the mechanism of this non-cognate activation of CD4 T cell remains under investigation, it is clear that a variety of inflammatory cytokines provoke the production of IFN-γ to restrict growth of intracellular pathogens (11). Thus, the increase in memory CD4 T cells observed in an “experienced” immune system might potentially provide substantial non-specific protection against intra-macrophage pathogens.
Chlamydia trachomatis (Ct) is an obligate intracellular gram-negative bacterium that causes a local infection of the upper reproductive tract in humans and mice (12). The adult human female reproductive tract (FRT) is replete with lymphocytes and contains memory lymphoid structures that might enhance clearance of genital tract infections such as Ct (1, 13, 14). In the current study, we sought to determine whether increasing the immunological experience of laboratory mice (SPF) would generate a mouse model that was more relevant to human Chlamydia infection. To answer this question, we examined infection with mouse-adapted Chlamydia muridarum, a model that is commonly used to study Chlamydia infection of the genital tract (15–17). Previous work from our laboratory has demonstrated that increased numbers of non-specific memory T cells in CCR7-deficient mice correlated with enhanced resistance to Chlamydia muridarum infection (18). Therefore, we hypothesized that increasing non-specific memory lymphocyte populations in SPF mice using a co-housing strategy would similarly enhance clearance of Chlamydia muridarum. In this study, we report that co-housing of “dirty mice” with SPF laboratory mice increased the frequency of effector memory T cells (TEM) in laboratory mice and enhanced protection against systemic Listeria infection. However, we did not detect any alteration in the course of genital Chlamydia infection or systemic Salmonella infection, demonstrating that this co-housing approach has variable effects on the clearance of intracellular pathogens.
Materials and Methods
Mice
C57BL/6 mice 6 weeks old were purchased from the Jackson Laboratory and housed in specific pathogen-free or conventional conditions. “Dirty” mice were purchased from Kasch’s Kritters (Citrus Heights, CA), Petco (Davis, CA), Pet Supplies Plus (Woodland, CA), or were caught in traps at UC Davis. Laboratory mice were conventionally housed with “dirty” mice at a 3 to 1 ratio for approximately 2 months before analysis. All experimental time points mentioned therefore relate to the beginning of this co-housing experience. Co-housed C57BL/6 mice were age-matched with regular SPF mice in each experiment. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Bacterial strains and infection of mice
Salmonella enterica serovar Typhimurium (BRD509 aroA mutant) was injected intravenously to induce systemic bacterial infection in mice, as previously described (19). STm was grown in LB broth at 37°C overnight and 5 × 105 CFU injected intravenously (IV) in a 200ul volume of PBS. Listeria Monocytogenes strain LM10403s was injected IV to induce systemic bacterial infection in mice. LM was grown in Brain-Heart Infusion media at 37°C overnight to an OD600 of 0.8 and 4.5 × 104 CFU injected in 200ul of PBS. Chlamydia muridarum was purchased from ATCC and propagated in HeLa cells as previously described (20). Chlamydia infections were delivered at a dose of 105 IFU in 5ul of SPG buffer intravaginally to induce an upper reproductive tract infection.
Bacterial enumeration
Spleens and livers were harvested from STm-infected mice, homogenized in a known quantity of PBS, before serial dilutions were plated on MacConkey agar plates and incubated overnight at 37°C. The following day, bacteria were enumerated on plates and the total CFU/organ calculated. For Listeria-infected mice, bacteria were similarly enumerated in the liver of infected mice using BHI plates. For Chlamydia infection experiments, mice were swabbed at regular intervals every 3–4 days and calcium alginate swabs agitated in 500ul of SPG buffer with 2 glass beads. These supernatants were frozen at −80°C until the end of experiment for analysis. Frozen swabs from all time points were thawed, serially diluted, and plated on a HeLa cell monolayer, centrifuged for 1hr at 37°C and incubated for approximately 16–20 hours in media and cycloheximide. After this incubation period, cells were washed and fixed with 100% methanol for 15 min. After fixation, HeLa cells were stained with Chlamydia hyperimmune serum for 45–60 min, washed and then incubated with secondary FITC-conjugated goat anti-mouse IgG antibody and washed again. Chlamydia inclusions were enumerated under a fluorescent microscope and the total IFU/swab calculated, as previously described (21).
Flow Cytometry
Blood was collected by cheek bleed, one and two months after the start of co-housing, ACK lysed, and washed with FACS buffer. Spleens were harvested and homogenized through a filter into a single cell suspension, ACK lysed, and washed with FACs buffer. Cell suspensions were incubated in Fc block for 10–30 min and then further washed before staining. Cells were stained using antibodies resuspended in Fc block for 30 min at room temperature. Antibodies used in the experiments included: CD11b PE, CD11c PE, B220 PE, F4/80 PE, CD8 PerCP eFluor 710, CD4 FITC, CD44 PE-Cy7, CD4 APC, CD62L FITC. All stained cells were subsequently fixed and samples analyzed using a BD Fortessa flow cytometer.
Statistical Analysis
All statistical analyses were conducted using GraphPad Prism version 8.
Results
Cohousing with dirty mice results in a higher frequency of memory CD4 T cells
“Dirty” mice for co-housing were obtained from a local pet shop vendor and assessed for pathogen exposure. Serological, culture, parasitology, and PCR analysis of up to 9 pet shop mice uncovered a wide variety of pathogen exposures in this cohort of animals (Suppl. Table 1). Previous work had noted an increased frequency of memory T cells in mice previously exposed to a non-SPF environment (22). Thus, we initially examined the frequency of CD44+ memory T cells in “pet shop mice”, compared to inbred C57BL/6 mice housed in our SPF facilities. In the blood of pet shop, around 10% of CD4 T cells expressed high levels of CD44, compared to 5% of CD4 T cells in the blood of SPF C57BL/6 mice (Fig. 1). Similarly, the spleen of pet shop mice contained approximately 30% CD4+CD44+ cells while SPF mouse spleens had less than 10% CD4+CD44+ cells (Fig. 1). Surprisingly, there was no significant differences noted in the frequency of CD8+CD44+ T cells in the blood and spleen of this particular batch of pet shop mice when compared to SPF mice (Fig. 1). Thus, consistent with a previous report, mice obtained from a pet shop displayed a higher frequency of memory T cells in circulation and this correlates with prior pathogen exposure.
Figure 1: Increased memory T cells in blood and spleens pet shop mice.
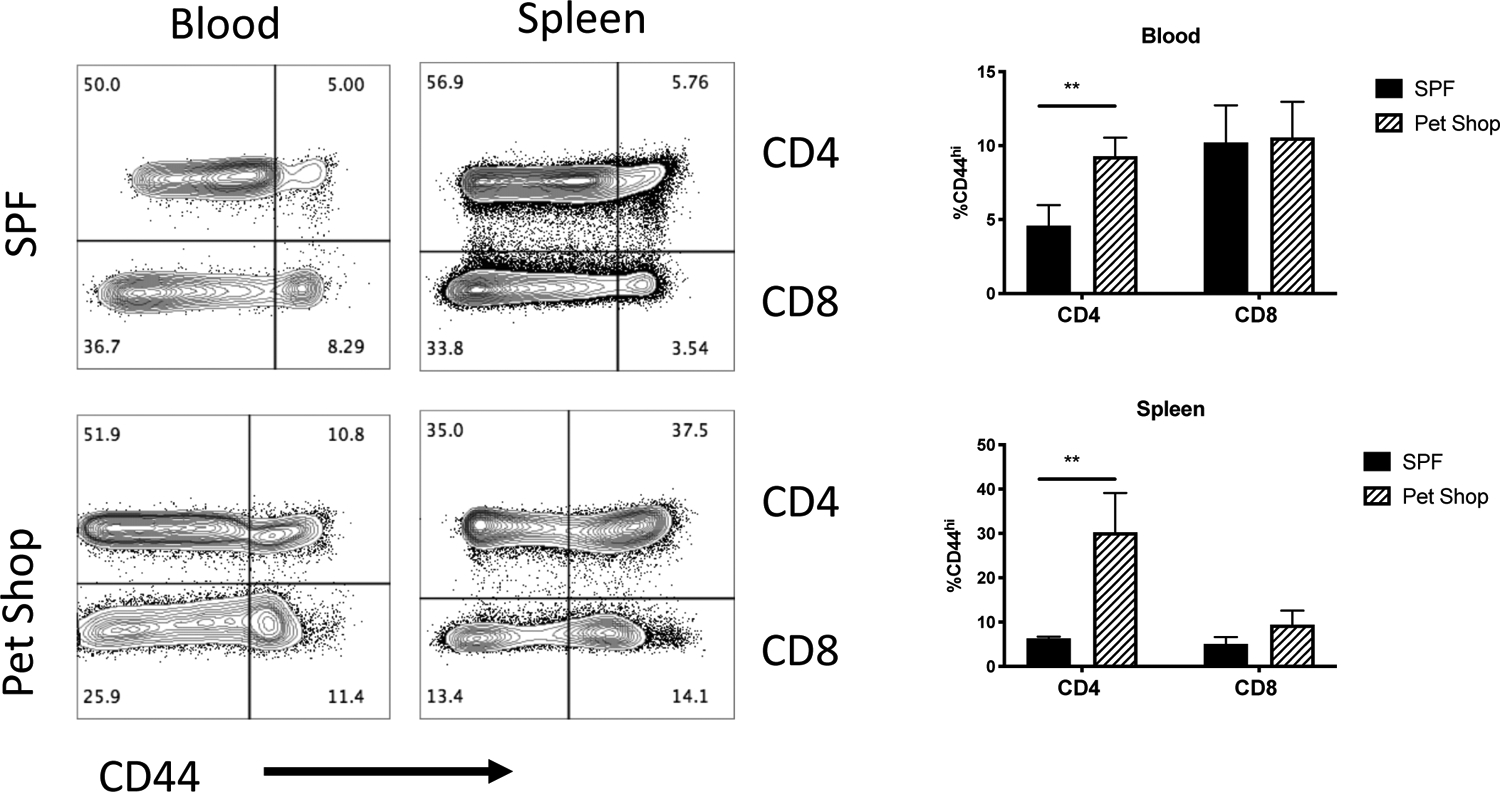
Memory CD4 and CD8 T cell populations were analyzed by flow cytometry in the blood and spleens of SPF C57BL/6 mice and pet shop mice. Plots show antigen experienced CD44hi CD4 T cells and CD4-negative lymphocytes. Data are similar to other experiments where CD8 staining was used. Antigen-experienced T cells were measured, quantified, and differences assessed by t-test. N=4 mice per group, **p < 0.01.
Next, we examined whether close contact between these two groups of mice would be sufficient to modify the memory compartment of SPF C57BL/6 mice. We co-housed female SPF mice with female pet shop mice in a 3:1 ratio for two months to provide sufficient time to normalize environmental factors. Blood was sampled from C57BL/6 mice at one and two month time points after co-housing and analyzed by flow cytometry for to determine effector memory (TEM) CD4 and CD8 T cell (CD44hi CD62L−) frequencies. After one month of co-housing, a substantial increase in CD4 and CD8 TEM was observed in the blood of co-housed SPF mice, although this was still substantially lower than pet shop mice (Fig. 2, left panel). After 2 months of co-housing the frequency of CD4 and CD8 TEM cells in co-housed C57BL/6 mice remained higher than SPF C57BL/6 mice (Fig. 2, right panel). Thus, co-housing with locally sourced pet shop mice is sufficient to increase the percentage of TEM in the blood of SPF laboratory mice, as previously reported (1).
Figure 2: Co-housing causes an increase in circulating effector memory T cells.
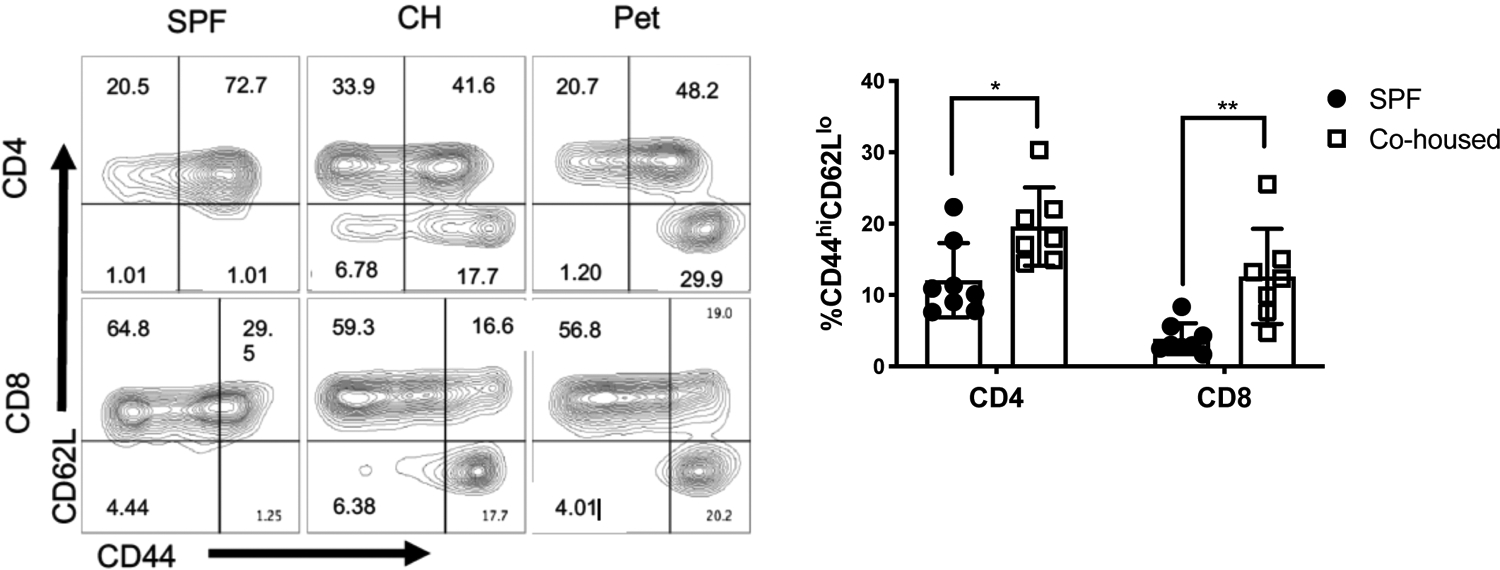
Female C57BL/6 mice were co-housed with female pet shop mice and then memory T cells assessed by flow cytometry after 1 month of co-housing (left panel) or 2 months of cohousing (right panel). Mean frequencies were compared by t-test. N=7 mice per group, *p < 0.05, **p < 0.01.
Co-housing with pet shop mice induces a higher TEM frequency than feral mice
Although co-housing with pet shop mice increased the frequency of memory T cells in laboratory mice, the magnitude of this effect appeared somewhat lower than a previous report (1). In considering possible variables, we first examined whether the type of mouse used for co-housing was a factor in this process. The capacity of feeder (snake food) mice, non-feeder (fancy) mice, or feral mice (captured locally) on memory T cell transition after co-housing was examined. Female feeder, fancy, and feral mice were individually co-housed with female SPF laboratory mice for two months and assessed for memory T cell frequency. Interestingly, co-housing C57BL/6 mice with feral mice only caused a modest increase in CD4 TEM in the blood, perhaps indicating that this particular cohort of feral mice had fewer transmissible agents. In contrast, both feeder and fancy mice induced greater increases in TEM frequency across CD4 and CD8 T cell populations (Fig. 3). The CD4 TEM frequency was comparable in laboratory mice co-housed with either source of pet shop mice, resulting in a memory frequency of around 20–25% (Fig. 2 and 3). However, it should be noted that none of the dirty mice used in our study were able to induce TEM population changes of the magnitude that was reported previously (1), perhaps reflecting geographical differences in the animal sources or other uncontrolled variables. Magnitude aside, the basic pattern of changes observed in our study is consistent with the idea that co-housing of SPF mice with dirty mice causes expansion of memory T cell populations in laboratory mice.
Figure 3: Mice source influences memory cell development in co-housed SPF mice.
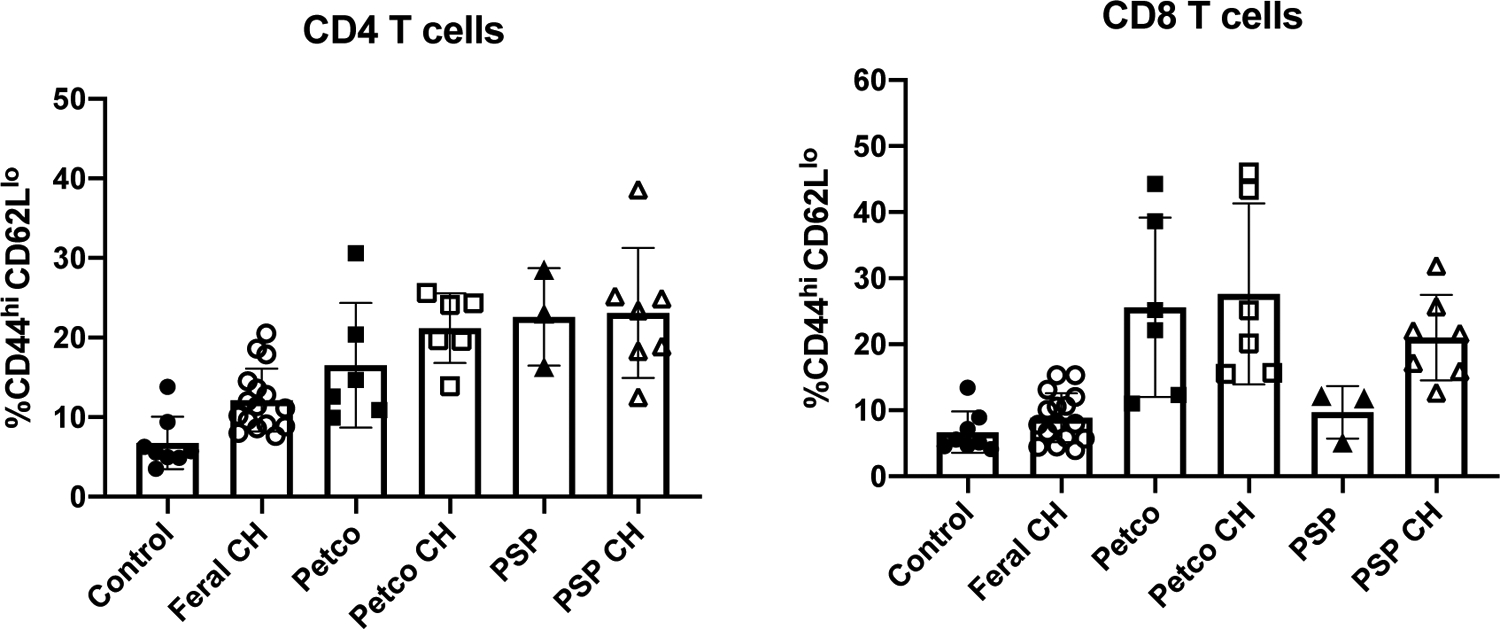
Female C57BL/6 mice were co-housed with female feral mice (Feral), feeder mice (Petco), or fancy mice (PSP). Immediately after two months of cohousing (CH), SPF, co-housed, or “dirty” mice were assessed for memory cell development in peripheral blood by flow cytometry. N= 3–15 mice per group.
Co-housing with “dirty” mice protects against systemic Listeria, but not Salmonella, infection
It was previously shown that co-housing SPF mice with pet shop mice enhanced non-specific immunity against systemic L. monocytogenes and P. berghei infection, two infections that predominantly depend on CD8 T cells for clearance (23–25). To confirm that our co-housing model was similarly protective, after 2 months of co-housing, we challenged co-housed laboratory mice IV with 4.5 × 104 CFU of L. monocytogenes and examined bacterial burdens. C57BL/6 mice that had been co-housed with pet store mice displayed a significantly lower burden of Listeria in the liver, compared to age-matched non-cohoused mice (Fig. 4A). Again, these data are consistent with a previous study showing that co-housing with “dirty” mice enhances immunity to Listeria challenge (1).
Figure 4: Co-housing with “dirty” mice provides protection against systemic Listeria monocytogenes, but not Salmonella Typhimurium infection.
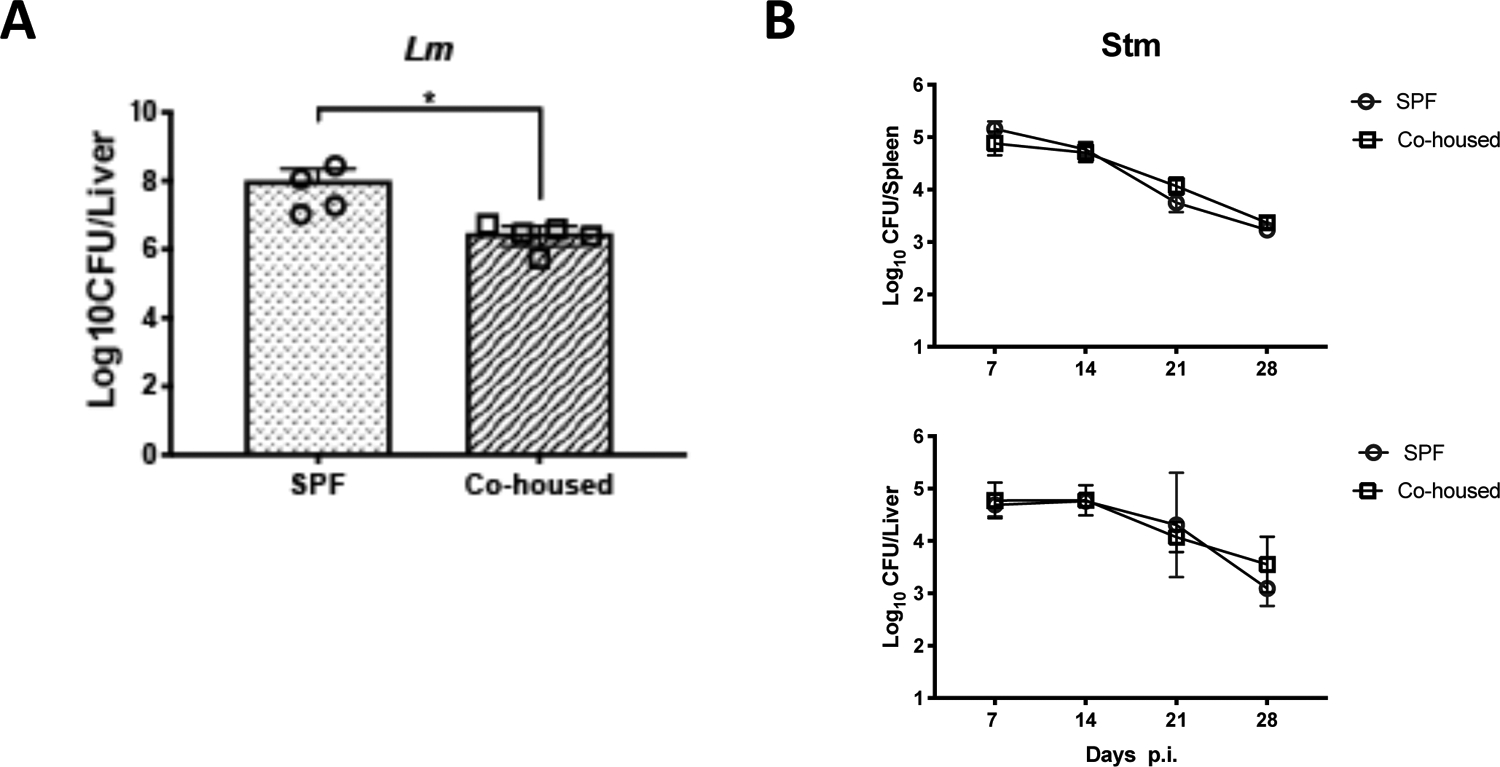
Female C57BL/6 mice were co-housed for two months with female “dirty” mice before being infected with intracellular pathogens. Co-housed mice or naïve C57BL/6 mice were infected intravenously with 4.5 × 104 CFU of (A) L.m. or (B) 5 × 105 CFU of Stm. (A) At 3 days post-infection, L.m. bacterial loads were measured in the livers. Bar graph shows Log10 CFUs. (B). At 7, 14, 21, and 28 days post infection Stm bacterial loads were measured in spleens and livers. Line graphs show Log10 CFUs at each time point. N=3–5 mice per time point. Significance was determined with a Mann-Whitney test (A) or an ANOVA (B). *p < 0.05.
We next tested whether this observation of non-specific protection extended to an intra-macrophage pathogen, Salmonella enterica serover Typhimurium (STm), that requires CD4 T cells for clearance (26, 27). Co-housed and non-cohoused C57BL/6 mice were infected with 5 × 105 CFU STm and euthanized at one-week intervals over the course of infection. Bacterial burdens were assessed in the spleen and liver of infected mice since these are the primary sites of systemic Salmonella replication (28). However, no difference in bacterial loads was detected in the spleen or liver of co-housed or non-cohoused mice at any of the time-points assessed (Fig. 4B). Thus, an increase in CD4 TEM through the process of co-housing with “dirty” mice did not substantially affect the outcome of systemic STm infection in laboratory mice.
Co-housing with “dirty” mice has no effect on localized reproductive tract infection
Although co-housing with “dirty” mice did not affect the replication of systemic CD4-dependent pathogen, it was of interest to determine whether there would be a greater effect on Chlamydia infection, since pathogen replication is largely localized to the female reproductive tract (29, 30). Laboratory mice were co-housed with feral or pet shop mice for 2 months before intravaginal infection with 105 IFU of Chlamydia muridarum. Infected mice were vaginally swabbed every 3–4 days over the month-long infection period and no significant differences was detected in bacterial shedding between these mice and non-cohoused age-matched cohorts (Fig. 5). We reasoned that his lack of effect on an FRT infection, could be due to the inability to transfer sexually transmitted infections during the co-housing period, since all co-housed mice were female. To examine this particular variable, we co-housed female laboratory mice with male or female pet shop mice for 2 months and all mice were rested for an additional month to ensure that none of the animals were pregnant before challenge infection. However, no significant differences were detected in the course of Chlamydia infection between these differently cohoused mice. Thus, prior co-housing with male mice did not substantially alter the course of Chlamydia infection in female laboratory mice. Taken together, the co-housing of laboratory mice with pet shop or feral mice expands the CD4 memory T cell pool but does not affect the replication of Chlamydia in the FRT.
Figure 5: Co-housing with feral or pet shop mice does not influence mucosal infection with Chlamydia muridaurm.
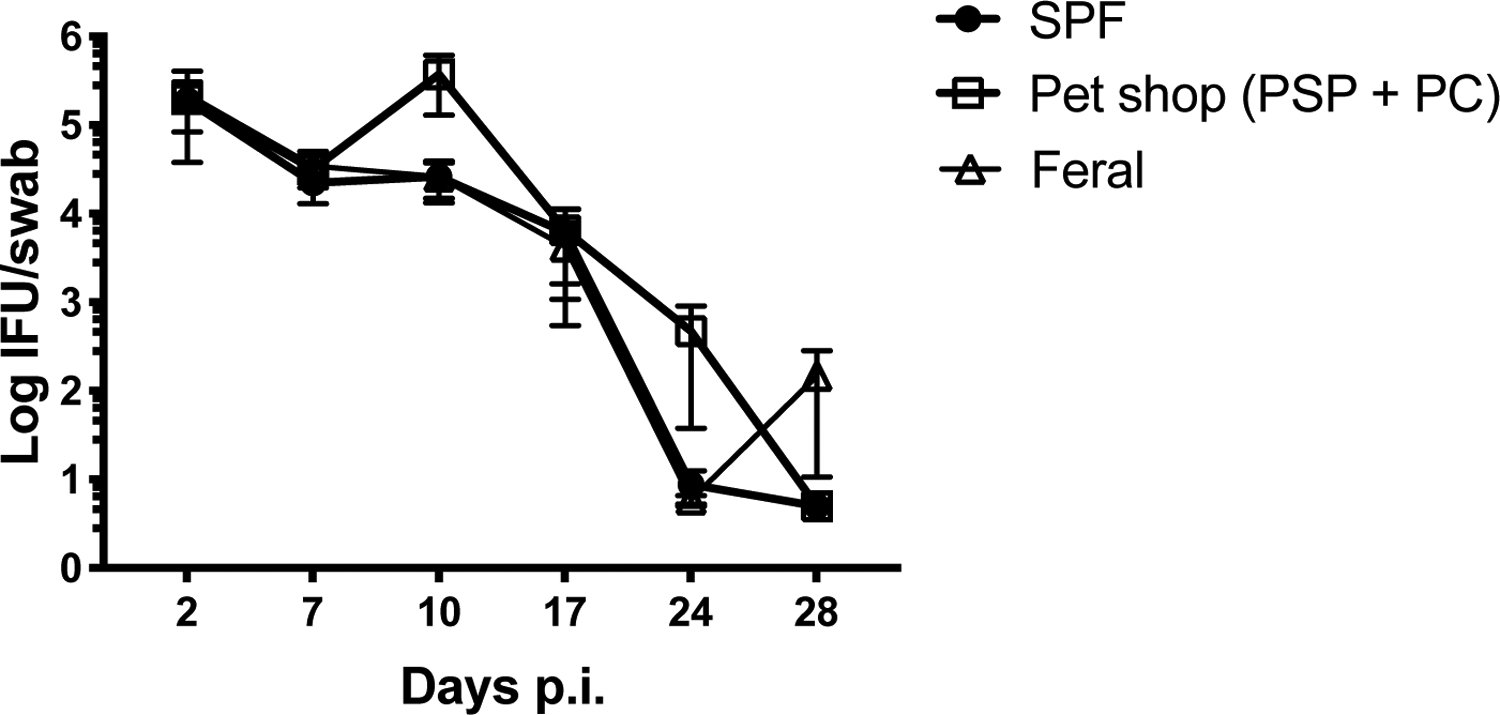
Immediately after two months of co-housing with female pet shop or feral mice, co-housed female C57BL/6 mice or naïve C57BL/6 mice were vaginally challenged with 105 IFU Chlamydia muridarum. Bacterial shedding was measured at multiple time points using vaginal swabs. N=4–8 mice per group. Significance was determined using a Two-way Repeated Measures ANOVA.
Discussion
Given the large number of uncontrolled variables during mouse studies, experimental approaches to limit their influence on complex mechanistic questions are often implemented, including age- and sex-matching, in-breeding, diet and environmental standardization, and specific pathogen free (SPF) housing. Although these operating practices are designed to limit the effect of uncontrolled variables on experimental studies, they also have the potential to introduce new variables that complicate data interpretation. SPF housing controls were developed to limit animal exposure to unknown infectious agents and thus enhance reproducibility, but it has only recently been appreciated that SPF mice do not develop an immune system that is comparable to an adult human or wild mouse (1, 3). This is evident by a low number of memory T cells and lack of ectopic lymphoid structures, when compared to wild mice and adult humans (1, 13, 14, 18, 31, 32). Diminished memory lymphocyte frequencies in SPF laboratory mice is likely due to minimal exposure to the typical pathogens or microbiota that would commonly activate and develop a normal lymphocyte repertoire (1). Since the study of outbred wild mice is challenging using current reagents, a new animal model has been developed that provides genetically controlled in-bred mice with appropriate maturation of the immune system (1, 6, 33). This model involves co-housing of SPF laboratory mice with feral or pet store mice that typically have murine pathogens, and are often referred to as “dirty” mice (1, 34). Co-housing with “dirty” mice therefore provides laboratory mice with limited exposure to a range of typical pathogens that promotes the development of an antigen-experienced immune system that is arguably more reflective of an adult human (1, 34). Indeed, this exposure period has been shown to increase the overall frequency of memory lymphocytes in mice and confer non-specific protection against systemic pathogens (1, 6, 33).
The female reproductive tract is a mucosal tissue exposed to the outside environment that frequently comes into contact with pathogens. Indeed, sexually transmitted infections such as Chlamydia trachomatis, are common among young women (35), but there is limited understanding of why some women control this infection better than others (36). Human studies of Chlamydia infection in women show the existence of memory lymphocyte clusters in the genital tract that are thought to play a role in fighting Chlamydia infections (32). This hypothesis is supported by a recent study demonstrating that CCR7-deficient mice display increased non-specific memory CD4 T cells and display increased capacity to control Chlamydia infections, compared to wildtype mice (18). Given this observation and the fact that CD4 T cells are required for primary clearance of Chlamydia infection (29, 30), we hypothesized that boosting of memory CD4 T cell frequencies in laboratory mice via co-housing could enhance protection against Chlamydia infection.
Indeed, we have been able to confirm previous results that co-housing induces enhanced frequencies of circulating CD4 and CD8 memory T cells in laboratory mice. This co-housing approach allowed enhanced control of Listeria monocytogenes infection in laboratory mice, as previously reported (1). Thus, our study confirms that co-housing laboratory mice with “dirty mice” allows maturation of T cell memory responses and correlates with natural resistance to Listeria infection. However, the magnitude of memory cell generation in our co-housing experiments is somewhat lower than previously reported (1), and similarly Listeria clearance was much less robust. While there could be many reasons for these differences, it would seem most likely that the magnitude change is driven by environmental differences in the source of dirty mice used for co-housing. While it may be tempting to speculate that a pet shop mouse from Sacramento has fewer pathogens, it is also possible that the opposite is true. Indeed, during our study we noted that many co-housed C57BL/6 mice displayed lack of grooming and sluggishness, suggesting overt pathogen exposure. While a previous study also documented similar illness, mice in this study fully recovered after 6 weeks (1, 34), while these effects persisted in our cohorts. Analysis of common pathogens present in pet shop mice from Sacramento did not uncover obvious differences to those previously reported from Minnesota. Furthermore, the use of feeder or fancy mice was not a primary driver of obvious differences in our study, although it was notable that feral mice were less able to drive enhanced memory development. It would obviously be of interest to directly compare pet shop mice derived from these different geographical locations head to head, however restrictions in shipping these animals between facilities precludes such investigation. However, it would seem likely that the quantity or quality of pathogens or the microbiota between these two different animal sources is the driver of differential effects. Although we are currently unable to identify the source of this variability, it would be important to define this in more detail before embarking on long-term studies of environmental effects on memory responses.
The main thrust of this particular investigation was to determine whether pet shop co-housing could enhance protective responses against a bacterial infection that primarily requires CD4 T cells for clearance. However, we found no effect of prior co-housing on the course of systemic Salmonella Typhimurium or localized Chlamydia muridarum infection. The absence of effects on Chlamydia FRT infection was independent of whether female laboratory mice were previously housed with female or male pet shop mice. Overall, we did not detect any major influence of prior co-housing on the protective immune response to two CD4 T cell-dependent pathogens. While it remains possible that such effects would emerge if there had been a greater effect on memory cell formation during our co-housing, the fact that we could replicate protective effects on Listeria infection makes it likely that CD4-dependent pathogens are simply less affected by non-specific memory expansion.
Overall, it is hard to deny that using a pet-shop co-housing model is a useful way to model human disease since the maturation of the immune system more accurately reflects the condition of an adult human. However, our experience is that this model displays considerable heterogeneity in driving memory formation, most likely due to the source of dirty mice causing “normalization”. Furthermore, this limited study also suggests that the pre-exposure memory pool may have differential effects on pathogens that require CD8 versus CD4 T cells for clearance. Efforts to standardize systems across different laboratories and examine a wider variety of potential pathogens would be a prudent strategy.
Supplementary Material
Figure 6: Co-housing with male or female dirty mice does not influence mucosal infection with Chlamydia muridaurm.
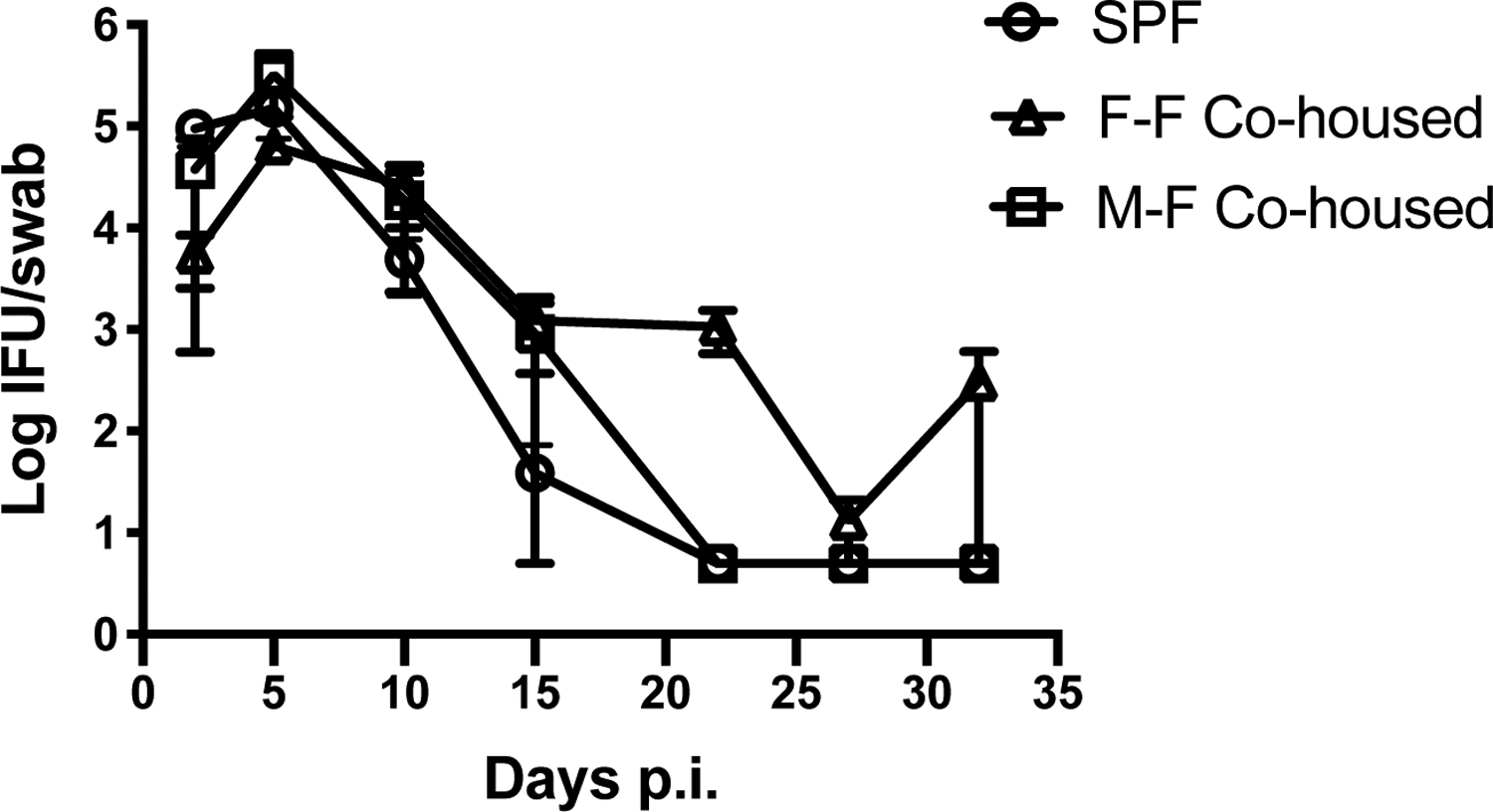
Female C57BL/6 mice were co-housed for two months with either male or female pet shop mice. After this co-housing period, all mice were separated and rested for an additional month. Naïve C57BL/6 and co-housed mice were vaginally challenged with 105 IFU Chlamydia muridarum. Bacterial shedding was measured at multiple time points using vaginal swabs. N=4–5 mice per group. Significance was determined using a Two-way Repeated Measures ANOVA.
Acknowledgments
We would like to thank the Bales and Foley laboratories at UC Davis for assistance in acquiring feral mice. We would also like to acknowledge the openness, invaluable assistance, and helpful advice of the members of the Masopust and Jameson laboratories at the University of Minnesota when we initiated these studies at UC Davis.
References
- 1.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, and Masopust D. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masopust D, and Soerens AG. 2019. Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 37: 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masopust D, Sivula CP, and Jameson SC. 2017. Of Mice, Dirty Mice, and Men: Using Mice To Understand Human Immunology. J Immunol 199: 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thome JJC, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, and Farber DL. 2014. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159: 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reese TA, Bi K, Kambal A, Filali-Mouhim A, Beura LK, Burger MC, Pulendran B, Sekaly RP, Jameson SC, Masopust D, Haining WN, and Virgin HW. 2016. Sequential Infection with Common Pathogens Promotes Human-like Immune Gene Expression and Altered Vaccine Response. Cell Host Microbe 19: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beura LK, Fares-Frederickson NJ, Steinert EM, Scott MC, Thompson EA, Fraser KA, Schenkel JM, Vezys V, and Masopust D. 2019. CD4(+) resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J Exp Med 216: 1214–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham OH, and McSorley SJ. 2015. Protective host immune responses to Salmonella infection. Future Microbiol 10: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labuda JC, and McSorley SJ. 2018. Diversity in the T cell response to Chlamydia-sum are better than one. Immunol Lett 202: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell H, Pham OH, Li LX, Atif SM, Lee SJ, Ravesloot MM, Stolfi JL, Nuccio SP, Broz P, Monack DM, Baumler AJ, and McSorley SJ. 2014. Toll-like Receptor and Inflammasome Signals Converge to Amplify the Innate Bactericidal Capacity of T Helper 1 Cells. Immunity 40: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham OH, O’Donnell H, Al-Shamkhani A, Kerrinnes T, Tsolis RM, and McSorley SJ. 2017. T cell expression of IL-18R and DR3 is essential for non-cognate stimulation of Th1 cells and optimal clearance of intracellular bacteria. PLoS pathogens 13: e1006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell H, and McSorley SJ. 2014. Salmonella as a model for non-cognate Th1 cell stimulation. Front Immunol 5: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunham RC, and Rey-Ladino J. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nature Reviews Immunology 5: 149–161. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RM, and Brunham RC. 2016. Tissue-Resident T Cells as the Central Paradigm of Chlamydia Immunity. Infection and Immunity 84: 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiviat NB, Wølner-Hanssen P, Eschenbach DA, Wasserheit JN, Paavonen JA, Bell TA, Critchlow CW, Stamm WE, Moore DE, and Holmes KK. 1990. Endometrial Histopathology in Patients with Culture-proved Upper Genital Tract Infection and Laparoscopically Diagnosed Acute Salpingitis. The American Journal of Surgical Pathology 14. [DOI] [PubMed] [Google Scholar]
- 15.Farris CM, and Morrison RP. 2011. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infection and immunity 79: 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poston TB, and Darville T. 2018. Chlamydia trachomatis: Protective Adaptive Responses and Prospects for a Vaccine. Curr Top Microbiol Immunol 412: 217–237. [DOI] [PubMed] [Google Scholar]
- 17.Li LX, and McSorley SJ. 2015. A re-evaluation of the role of B cells in protective immunity to Chlamydia infection. Immunol Lett 164: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L-X, Labuda JC, Imai DM, Griffey SM, and McSorley SJ. 2017. CCR7 Deficiency Allows Accelerated Clearance of Chlamydia from the Female Reproductive Tract. The Journal of Immunology 199: 2547–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan A, Nanton M, Griffin A, and McSorley SJ. 2009. Culling of activated CD4 T cells during typhoid is driven by Salmonella virulence genes. Journal of immunology 182: 7838–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L-X, and McSorley SJ. 2013. B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Chlamydia muridarum Genital Tract Infection. PLoS Pathogens 9: e1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labuda JC, Pham OH, Depew CE, Fong KD, Lee BS, Rixon JA, and McSorley SJ. 2021. Circulating immunity protects the female reproductive tract from Chlamydia infection. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, and Masopust D. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladel CH, Flesch IE, Arnoldi J, and Kaufmann SH. 1994. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol 153: 3116–3122. [PubMed] [Google Scholar]
- 24.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, and Nussenzweig V. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330: 664–666. [DOI] [PubMed] [Google Scholar]
- 25.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, and Good MF. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A 85: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labuda JC, Depew CE, Pham OH, Benoun JM, Ramirez NA, and McSorley SJ. 2019. Unexpected Role of CD8 T Cells in Accelerated Clearance of Salmonella enterica Serovar Typhimurium from H-2 Congenic mice. Infection and immunity 87: e00588–00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donnell H, Pham OH, Li L.-x., Atif SM, Lee S-J, Ravesloot MM, Stolfi JL, Nuccio S-P, Broz P, Monack DM, Baumler AJ, and McSorley SJ. 2014. Toll-like receptor and inflammasome signals converge to amplify the innate bactericidal capacity of T helper 1 cells. Immunity 40: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benoun JM, Labuda JC, and McSorley SJ. 2016. Collateral Damage: Detrimental Effect of Antibiotics on the Development of Protective Immune Memory. mBio 7: e01520–01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison RP, Feilzer K, and Tumas DB. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infection and Immunity 63: 4661–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison SG, Farris CM, Sturdevant GL, Whitmire WM, and Morrison RP. 2011. Murine Chlamydia trachomatis genital infection is unaltered by depletion of CD4+ T cells and diminished adaptive immunity. J Infect Dis 203: 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iijima N, and Iwasaki A. 2014. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science (New York, N.Y.) 346: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiviat NB, Paavonen JA, Wølner-Hanssen P, Critchlow CW, Stamm WE, Douglas J, Eschenbach DA, Corey LA, and Holmes KK. Histopathology of endocervical infection caused by Chlamydia trachomatis, herpes simplex virus, Trichomonas vaginalis, and Neisseria gonorrhoeae. Human Pathology 21: 831–837. [DOI] [PubMed] [Google Scholar]
- 33.Beura LK, Wijeyesinghe S, Thompson EA, Macchietto MG, Rosato PC, Pierson MJ, Schenkel JM, Mitchell JS, Vezys V, Fife BT, Shen S, and Masopust D. 2018. T Cells in Nonlymphoid Tissues Give Rise to Lymph-Node-Resident Memory T Cells. Immunity 48: 327–338.e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton SE, Badovinac VP, Beura LK, Pierson M, Jameson SC, Masopust D, and Griffith TS. 2020. New Insights into the Immune System Using Dirty Mice. The Journal of Immunology 205: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prevention., C. f. D. C. a. 2019. Sexually Transmitted Disease Surveillance 2018. Atlanta: U.S. Department of Health and Human Services; DOI: 10.15620/cdc.79370. [DOI] [Google Scholar]
- 36.Geisler WM, Lensing SY, Press CG, and Hook EW 3rd. 2013. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. The Journal of infectious diseases 207: 1850–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


