Abstract
SGLT2 (sodium-glucose cotransporter 2) inhibitors produce a distinctive pattern of benefits on the evolution and progression of cardiomyopathy and nephropathy, which is characterized by a reduction in oxidative and endoplasmic reticulum stress, restoration of mitochondrial health and enhanced mitochondrial biogenesis, a decrease in proinflammatory and profibrotic pathways, and preservation of cellular and organ integrity and viability. A substantial body of evidence indicates that this characteristic pattern of responses can be explained by the action of SGLT2 inhibitors to promote cellular housekeeping by enhancing autophagic flux, an effect that may be related to the action of these drugs to produce simultaneous upregulation of nutrient deprivation signaling and downregulation of nutrient surplus signaling, as manifested by an increase in the expression and activity of AMPK (adenosine monophosphate–activated protein kinase), SIRT1 (sirtuin 1), SIRT3 (sirtuin 3), SIRT6 (sirtuin 6), and PGC1-α (peroxisome proliferator–activated receptor γ coactivator 1-α) and decreased activation of mTOR (mammalian target of rapamycin). The distinctive pattern of cardioprotective and renoprotective effects of SGLT2 inhibitors is abolished by specific inhibition or knockdown of autophagy, AMPK, and sirtuins. In the clinical setting, the pattern of differentially increased proteins identified in proteomics analyses of blood collected in randomized trials is consistent with these findings. Clinical studies have also shown that SGLT2 inhibitors promote gluconeogenesis, ketogenesis, and erythrocytosis and reduce uricemia, the hallmarks of nutrient deprivation signaling and the principal statistical mediators of the ability of SGLT2 inhibitors to reduce the risk of heart failure and serious renal events. The action of SGLT2 inhibitors to augment autophagic flux is seen in isolated cells and tissues that do not express SGLT2 and are not exposed to changes in environmental glucose or ketones and may be related to an ability of these drugs to bind directly to sirtuins or mTOR. Changes in renal or cardiovascular physiology or metabolism cannot explain the benefits of SGLT2 inhibitors either experimentally or clinically. The direct molecular effects of SGLT2 inhibitors in isolated cells are consistent with the concept that SGLT2 acts as a nutrient surplus sensor, and thus, its inhibition causes enhanced nutrient deprivation signaling and its attendant cytoprotective effects, which can be abolished by specific inhibition or knockdown of AMPK, sirtuins, and autophagic flux.
Keywords: autophagy, heart failure, sirtuins, sodium-glucose transporter 2 inhibitors, TOR serine-threonine kinases
SGLT2 (sodium-glucose cotransporter 2) inhibitors slow the evolution and progression of heart failure (HF), whether they are administered to high-risk patients without symptoms or to symptomatic patients across a broad range of ejection fractions. In 12 large-scale trials involving >70 000 patients, long-term SGLT2 inhibition produced a consistent 20% to 25% reduction in the combined risk of cardiovascular death or hospitalizations for HF.1 SGLT2 inhibitors are now considered 1 of the 4 foundational drugs for the management of HF in patients with a reduced ejection fraction.
Potential Actions of SGLT2 Inhibitors as Nephrocentric Neurohormonal Antagonists and as Osmotic Diuretics
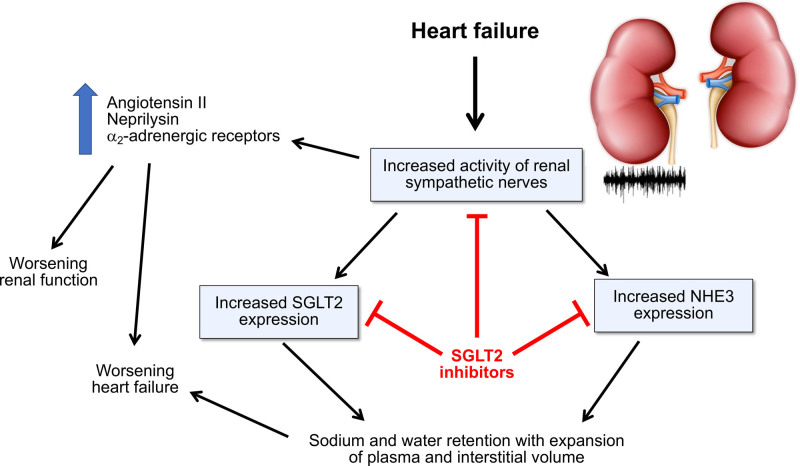
The other foundational drugs for HF—angiotensin receptor neprilysin inhibitors, β-blockers, and mineralocorticoid receptor antagonists—interfere with a neurohormonal mechanism whose activation leads to deleterious effects on the myocardium (ie, angiotensin II, catecholamines, aldosterone, and neprilysin). However, an important neurohormonal mechanism that is not fully antagonized by current foundational drugs is the marked increase in sympathetic nerve traffic to the kidneys. Renal sympathetic tone is strikingly enhanced in experimental and clinical HF and adversely influences prognosis.2,3 An increase in renal sympathetic nerve traffic adversely affects the heart by increasing the release of angiotensin II and neprilysin from the kidneys, both acting to promote adverse cardiac remodeling and fibrosis as well as sodium retention and volume expansion.4,5 Enhanced renal sympathetic nerve traffic has also been implicated in the progression of chronic kidney disease, in part related to the stimulation of intrarenal angiotensin II and in part related to the activation of α-2 adrenoreceptors, which may play a role in the pathogenesis of renal interstitial inflammation and fibrosis.6,7 Renal denervation produces favorable effects in experimental HF and renal disease and improves cardiac function in the clinical setting.4–9 Of note, although they are generally characterized as inhibitors of the sympathetic nervous system, β-blockers do not reduce renal sympathetic nerve traffic.10
Potential Antagonism of Renal Sympathetic Hyperactivity by SGLT2 Inhibitors
Enhanced renal sympathetic nerve traffic would be expected to promote sodium retention by the kidney. The antinatriuretic effect of renal sympathetic activation is particularly pronounced in the proximal renal tubule11 and proximal tubular hyperreabsorption of sodium is abrogated by renal denervation.12 The interplay of NHE3 (sodium-hydrogen exchanger isoform 3) and SGLT2 plays a key role in the reabsorption of sodium in the proximal tubule, and increases in renal sympathetic nerve traffic augments the expression of both NHE3 and SGLT2,13,14 accounting for their increased activity in HF.14–16
Most diuretics do not address sodium reabsorption in the proximal renal tubule. It is therefore noteworthy that SGLT2 inhibitors interfere with both SGLT2 and NHE3 in the proximal tubule16,17 and this action yields short-term increases in the fractional excretion of sodium.18 HF potentiates the natriuretic effects of SGLT2 inhibition (as would be expected by upregulation of SGLT2 and NHE3 in this disorder)14,16 and renal NHE3 inhibition by SGLT2 inhibitors promotes euvolemia in rats with HF.16 In addition, SGLT2 inhibition attenuates renal sympathetic activity and reduces renal norepinephrine content in states of experimental nutrient excess.19,20 Renal denervation attenuates the magnitude of the responses to SGLT2 inhibition in the kidney in animals with (but not without) HF.14 Taken collectively, these observations position SGLT2 inhibitors as functional antagonists of renal sympathetic nerve hyperactivity in HF. However, any postulated sympatholytic action is likely to be highly selective for the renal nerves, because SGLT2 inhibition does not reduce cardiac sympathetic activity21 and has not been shown to decrease central sympathetic outflow.
Potential Benefits of SGLT2 Inhibitors Acting as Osmotic Diuretics
The possibility that SGLT2 inhibitors might treat HF by functioning as antagonists of renal sympathetic nerve hyperactivity or by ameliorating sodium retention is appealing (Figure 1). However, it has been difficult to show that SGLT2 inhibitors exert important natriuretic effects in patients with HF. In this setting, SGLT2 inhibition exerts a short-term osmotic diuretic effect with little increase in urinary sodium excretion,22,23 suggesting that any increase in urine volume is related to augmented glucose (and not sodium) excretion. However, early increases in urinary water excretion are not sustained because of the activation of adaptive mechanisms that decrease free water clearance,24 explaining why long-term SGLT2 inhibition does not change serum sodium concentration.25
Figure 1.
Proposed framework by which SGLT2 (sodium-glucose cotransporter 2) inhibitors might exert cardioprotective and nephroprotective effects by acting to mute renal sympathetic nerve activity and promote natriuresis and osmotic diuresis. NHE3 indicates sodium-hydrogen exchanger isoform 3.
Additional lines of evidence raise further doubts that a sustained diuretic effect can account for the benefits of SGLT2 inhibitors in HF. SGLT2 inhibitors are not more effective in patients with HF who have volume overload, as compared with those who are euvolemic.25 Although these drugs produce a modest decrease in body weight, this appears to be related to the urinary caloric loss and the shrinkage of fat depots rather than to changes in fluid status26,27; changes in body weight are poorly correlated with changes in natriuretic peptides.25 The increase in hematocrit seen in clinical trials is a delayed effect that is related to erythrocytosis rather than hemoconcentration.28 Although SGLT2 inhibitors can decrease plasma volume, this represents a compensatory mechanism that is triggered by the increase in red blood cell mass29; if plasma volume were not reduced, erythrocytosis would lead to intolerable hypervolemia.
It has been proposed that the osmotic diuretic effect of SGLT2 inhibitors leads primarily to decreases in interstitial fluid (rather than plasma volume), but this hypothesis has been on the basis of modeling rather than direct measurements.30 Indeed, at a time when the diuretic effects of SGLT2 inhibitors are most prominent (ie, during the first weeks of treatment), changes in cardiac filling pressures and natriuretic peptides are very small.31–33 A transient diuresis cannot explain the meaningful changes in cardiac geometry34 or the striking reduction in the risk of major adverse renal events seen in patients with HF with a reduced ejection fraction.35 Any improvement stemming from an osmotic diuretic action of these drugs would be expected to be potentiated in individuals with diabetes (who exhibit the most marked glycosuria), but patients with HF are not more likely to respond to SGLT2 inhibitors if they are diabetic.35,36 Conversely, preexisting renal impairment would be expected to attenuate the glycosuric and osmotic diuretic actions of SGLT2 inhibitors; however, chronic kidney disease does not diminish the effect of SGLT2 inhibitors to reduce hospitalizations for HF.26,37
Enthusiasm for the hypothesis that SGLT2 inhibitors act primarily as nephrocentric neurohormonal antagonists or as diuretics has been driven by the belief that the efficacy of these drugs should be linked to their actions within the kidney, because SGLT2 is expressed primarily in the proximal renal tubule. A diuretic effect of SGLT2 inhibitors may underlie some of the short-term benefits of these drugs, and the enhanced distal delivery of sodium may also promote the exchange of sodium for potassium, thus enhancing kaliuresis and mitigating the risk of hyperkalemia.38 Aside from these actions, renal sympatholysis or increases in urinary volume do not appear to contribute importantly to the long-term ability of SGLT2 inhibitors to reduce the risk of HF or renal events.
Potential Importance of Mitigation of Glomerular Hypertension by SGLT2 Inhibitors
Glomerular hyperfiltration has long been regarded as the mechanism driving the progression of kidney disease in patients with diabetes. The benefits of angiotensin receptor blockers and mineralocorticoid receptor antagonists on the evolution of diabetic nephropathy have been attributed to their effect to decrease intraglomerular pressures. However, the role of glomerular hyperfiltration in the progression of kidney disease in HF has been questioned. Angiotensin-converting enzyme inhibitors and mineralocorticoid receptor antagonists do not slow the loss of nephrons in patients with HF.39,40 Furthermore, neprilysin inhibition reduces the risk of major renal events in patients with HF, even though the afferent arteriolar vasodilation produced by enhanced cyclic guanosine monophosphate signaling increases intraglomerular pressures and albuminuria.41
Doubts about the role of glomerular hyperfiltration in the progression of renal disease in patients with HF have clouded efforts to understand the nephroprotective effects of SGLT2 inhibitors in this disorder. By inhibiting proximal tubular sodium hyperreabsorption, SGLT2 inhibitors can rapidly reduce intraglomerular pressures, although this may not be related to enhanced tubuloglomerular feedback.42 SGLT2 inhibition produces an immediate decrease in glomerular filtration rate in patients with HF.35 The magnitude of the early decline in glomerular filtration is attenuated in patients with renal impairment43; however, the ability of SGLT2 inhibitors to slow the progression of renal disease is not diminished.37 It is therefore noteworthy that experimental knockout of SGLT2 is sufficient to attenuate glomerular hyperfiltration, but it does not prevent renal injury, inflammation, or fibrosis in experimental diabetes or ischemia.44,45 An action of SGLT2 inhibitors to reduce intraglomerular pressures may not be relevant to their renoprotective effects in HF.
Potential Actions of SGLT2 Inhibitors to Promote Energy Substrate Delivery
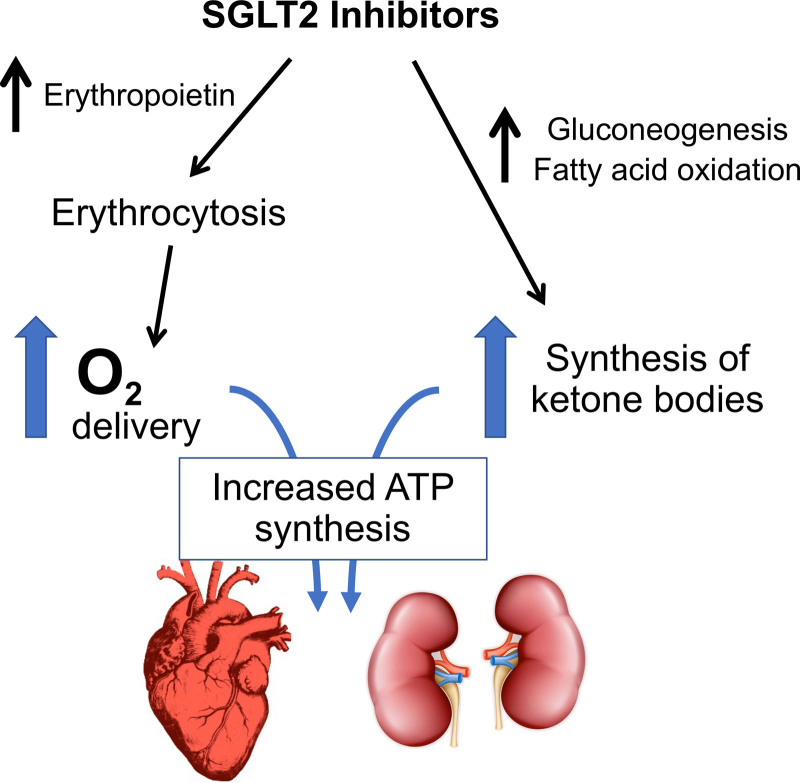
In the absence of a nephrocentric explanation for the benefits of SGLT2 inhibitors, investigators have focused on 2 prominent physiologic features of these drugs: ketogenesis and erythrocytosis. Both ketonemia and an increased red blood cell mass might augment delivery of efficient fuels and oxygen, both of which may have favorable effects on the energy state of hearts and kidneys under stress (Figure 2).
Figure 2.
Proposed framework by which SGLT2 (sodium-glucose cotransporter 2) inhibitors might act to increase delivery of substrates that could lead to enhanced synthesis of ATP (adenosine triphosphate).
Potential Role of Ketone Bodies Acting as a Fuel for the Heart and Kidneys
By promoting glycosuria, SGLT2 inhibitors trigger a state of starvation mimicry that is characterized by the production of ketone bodies—particularly β-hydroxybutyrate—in the liver.46 Although the infusion of large doses of β-hydroxybutyrate exerts positive inotropic and chronotropic effects,47 SGLT2 inhibition does not produce these hemodynamic effects in patients or in isolated cardiomyocytes.33,34,48 Furthermore, circulating ketone bodies are increased in patients with HF,49 and the failing heart already uses ketone bodies as a preferred fuel.50 In patients without diabetes, the degree of ketonemia is muted,36,51 but the HF benefits are not.35
Furthermore, under experimental conditions, SGLT2 inhibitors have not consistently enhanced ketone body consumption by the myocardium,52–54 and increases in ATP (adenosine triphosphate) production seen after SGLT2 inhibition are not related to increased ketone body metabolism.52 Augmentation of ketone body oxidation is not beneficial in experimental HF,55 and clinically, ketogenesis after SGLT2 inhibition does not consistently yield increases in myocardial ATP.54,56 Similarly, the nephroprotective actions of SGLT2 inhibitors cannot be directly linked to ketogenesis-related increases in ATP.57
Potential Role of Erythrocytosis to Increase Tissue Oxygen Delivery
It has been hypothesized that the increased tubular workload related to increased distal sodium delivery after SGLT2 inhibition might produce renal medullary hypoxia,58 thus triggering the synthesis of erythropoietin and an increase in hemoglobin. However, magnetic resonance imaging has not discerned evidence for the induction or alleviation of renal hypoxia in patients treated with SGLT2 inhibitors,59 suggesting that the increase in erythropoietin is likely related to an effect on hypoxia-inducible factors.60
Might the increase in red blood cell mass improve tissue oxygenation and thereby reduce HF or renal events? Oxygen utilization is not typically impaired in hemodynamically stressed hearts, but oxygen delivery still might be important in patients with coronary artery disease. However, the benefits of SGLT2 inhibitors in HF are not enhanced by the presence of ischemic heart disease.35 Furthermore, when red blood cell mass is increased by means of erythropoietin-mimetic agents in large-scale trials, the risk of major HF events or of progression of renal disease is not diminished, even though patients were anemic at the start of treatment.61,62 Therefore, the totality of evidence does not suggest that the cardioprotective or renoprotective effects of SGLT2 inhibitors can be explained by enhanced delivery of energy substrates.
Potential Actions of SGLT2 Inhibitors to Inhibit Sodium-Hydrogen Exchange
The function of sodium-glucose cotransporters and sodium-hydrogen exchangers are closely intertwined in several organs. The actions of SGLT1 (sodium-glucose cotransporter 1) and NHE3 are linked in the intestinal mucosa63 and SGLT2 and NHE3 are colocalized in the proximal renal tubule,64 such that inhibition of SGLT2 also impairs the actions of NHE3.16,17 It is therefore possible that SGLT2 inhibitors might interfere with other sodium-hydrogen exchangers (eg, NHE1 [sodium-hydrogen exchanger isoform 1]). Experimental activation of NHE1 in the heart can promote hypertrophy, cell death, and the development of HF,65 making it an attractive therapeutic target.
Baartscheer and colleagues66,67 first proposed that SGLT2 inhibitors might function as NHE1 antagonists in the heart; they postulated that these drugs could exert direct benefits on cardiomyocytes, independent of renal glycosuria or any interaction with SGLT2, because SGLT2 is not expressed in the myocardium. Incubation of rat cardiomyocytes with SGLT2 inhibitors delayed pH recovery after an ammonium ion (NH4+) pulse and reduced intracellular sodium concentrations, actions consistent with NHE1 inhibition, and the investigators reported a docking site for SGLT2 inhibitors on the NHE1 protein. The effects on cardiomyocytes were attenuated by cariporide, a canonical NHE1 antagonist.
Despite these observations, a role for NHE1 inhibition in mediating the cardiovascular benefits of SGLT2 inhibitors remains in doubt. Chung et al.69 reported no effect of SGLT2 inhibitors to delay pH recovery after a NH4+ pulse or to reduce intracellular sodium concentrations, thus challenging the findings of Baartscheer and colleagues.66,67 The published interchange between the 2 groups revealed that the data of Chung et al.69 were derived from a larger sample size.68,69 Osaka et al.70 claimed that SGLT2 inhibition inhibited NHE1, but the effect was substantially less than that seen with cariporide. Li et al.48 failed to show meaningful docking of empagliflozin to NHE1 and reported that cariporide did not mimic the action of the SGLT2 inhibitor on isolated cardiomyocytes. Therefore, if there is an effect of SGLT2 inhibitors to blunt increases in intracellular sodium, it may be secondary to their effects to reduce cellular stress or to an action on the late sodium current.60,71
Actions of SGLT2 Inhibitors to Promote Nutrient Deprivation Signaling and Autophagy to Reduce Cellular Stress and Promote Cellular Survival
The key clinical biomarkers of the action of SGLT2 inhibitors—ketogenesis and erythrocytosis—reflect the typical responses to nutrient and oxygen deprivation.60 SGLT2 inhibitors also reduce serum uric acid, an indicator of oxidative stress in patients with HF.72 How are these 3 biomarkers related to each other? Enhanced nutrient and oxygen deprivation signaling can promote erythropoietin synthesis and reduces oxidative stress in cardiomyocytes and renal parenchymal cells.60 It is therefore noteworthy that mediation analyses of large-scale outcomes trials in patients with type 2 diabetes have consistently identified increases in hemoglobin and decreases in uric acid as statistical determinants of the ability of SGLT2 inhibitors to reduce the risk of HF hospitalizations and major adverse renal events.73–75 These clinical findings, taken together with a wealth of data from experimental studies, have led to the hypothesis that the cardiorenal benefits of SGLT2 inhibitors are related to the activation of nutrient deprivation signaling, with its attendant effects to promote autophagy and mitochondrial health, reduce the generation of reactive oxygen species, mute inflammation and fibrosis, and enhance the viability of cardiomyocytes and renal parenchymal cells.60 Although this response may be triggered by an action of SGLT2 inhibitors to induce a state of starvation mimicry secondary to the urinary loss of calories,76 numerous studies published during the past 2 years have shown that the adaptive cellular reprogramming produced by these drugs is seen in isolated cell cultures, indicating that SGLT2 inhibitors have direct glycosuria-independent actions to reduce cellular stress and promote cellular survival.
Nutrient Sensors and Cellular Signaling in the Heart and Kidney in Health and Disease
SGLT2 protein functions as an energy sensor, discerning an excess nutrient state when there is excessive glucose in the proximal renal tubules, and changes in SGLT2 parallel changes in other energy sensors in response to shifts in environmental nutrients.77 When faced with changes in extracellular glucose and amino acids, the interplay of several master switches adapts the cell to promote its growth or survival. These include mTOR (mammalian target of rapamycin); sirtuins (SIRT1 [sirtuin 1], SIRT3 [sirtuin 3], and SIRT6 [sirtuin 6]); and AMPK (adenosine monophosphate–activated protein kinase; Figure 3).
Figure 3.
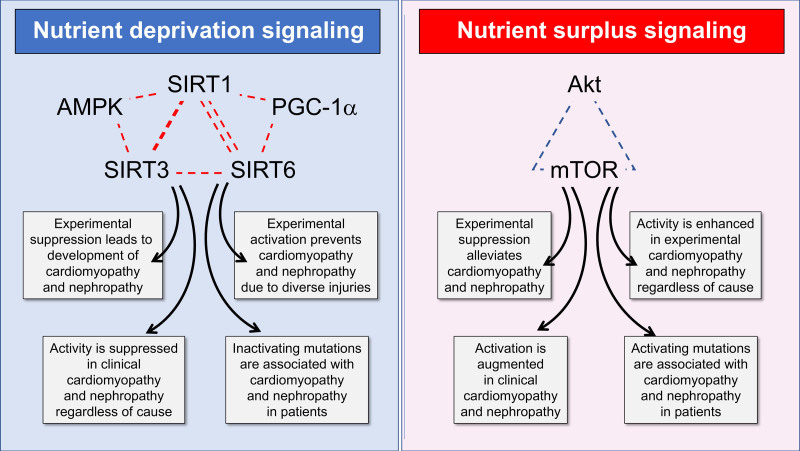
Effect of nutrient deprivation and nutrient surplus signaling on the evolution and progression of cardiomyopathy and nephropathy in experimental and clinical settings. Akt indicates protein kinase B; AMPK, adenosine monophosphate–activated protein kinase; mTOR, mammalian target of rapamycin; PGC-1α, peroxisome proliferator–activated receptor γ coactivator 1-α; SIRT1, sirtuin 1; SIRT3, sirtuin 3; and SIRT6, sirtuin 6.
Mammalian Target of Rapamycin
mTOR is a serine/threonine protein kinase that is activated by a surplus of environmental amino acids and functions to promote cell growth and proliferation. mTOR exists in 2 complexes—mTOR complex 1 (mTORC1) and mTOR complex 2—and Akt (protein kinase B) potentiates the activation of mTORC1, which is preferentially inhibited by rapamycin. Akt/mTORC1 signaling influences hundreds of downstream effectors to promote anabolic pathways, driving the mitochondrial production of reactive oxygen species to facilitate cellular replication, proinflammatory pathways, and innate immunity, and enhancing the expression of the senescence-associated secretory phenotype that is essential to the cellular disposal required for effective organ growth.78 The action of mTOR activity to promote anabolic pathways is required for cardiomyocyte replication during fetal development and adaptive hypertrophy during acute massive pressure overload,79,80 but it leads to maladaptive cardiac remodeling when activated in hearts that are injured in adulthood. In experimental models, cardiac-specific overactivation of the Akt/mTOR pathway induces HF,81 whereas suppression of Akt or mTOR signaling ameliorates the development of cardiomyopathy.82,83 Similarly, Akt/mTOR signaling is hyperactivated in the kidney in nutrient surplus states, thus triggering proinflammatory pathways and promoting renal injury,84–86 whereas inhibition of mTOR can ameliorate fibrosis and the development of chronic kidney disease.86,87
In the clinical setting, patients with dilated cardiomyopathy show aberrant myocardial activation of mTORC1, the intensity of which is associated with the severity of cardiac fibrosis and a poor prognosis.88 Activation of Akt in the human myocardium characterizes the transition from well-compensated left ventricular hypertrophy to decompensated HF.89 mTOR-activating sequence variations can cause clinical cardiomyopathy and kidney tubulopathy90 and renal mTORC1 activation is associated with disease activity and prognosis in patients with nondiabetic kidney disease.91
Sirtuin 1 and Other Sirtuins
Sirtuins are a family of redox-sensitive nicotinamide adenine dinucleotide (NAD)–dependent deacetylases that catalyze the posttranslational modification of hundreds of proteins that are involved in metabolism and cellular homeostasis. They serve as a redox rheostat and represent the primary cellular response to glucose deprivation. SIRT1, SIRT3, and SIRT6 share similar functions, with SIRT1 and SIRT6 being localized to the nucleus and SIRT3 to the mitochondria. Cardiac-specific deletion or suppression of SIRT1, SIRT3, and SIRT6 augments production of reactive oxygen species, enhances endoplasmic reticulum stress, and sensitizes the heart to injury, leading to cardiomyopathy.92–94 Conversely, SIRT1, SIRT3, and SIRT6 enrichment or activation alleviates oxidative and endoplasmic reticulum stress, disposes of dysfunctional mitochondria and promotes mitochondrial biogenesis, mitigates cell senescence and death, ameliorates fibrosis and remodeling, and preserves cardiac function.95–98 Deficiencies in SIRT1, SIRT3, and SIRT6 exacerbate glomerular injury and glomerulosclerosis after renal stress,99–101 and SIRT1, SIRT3, and SIRT6 activation is accompanied by reduction in oxidative stress, tubulointerstitial inflammation, and fibrosis; amelioration of glomerular and tubular damage; and maintenance of renal function.102–104 The adaptive effects of sirtuins on organellar health and cellular stress are potentiated by its downstream effector (eg, PGC-1α [peroxisome proliferator–activated receptor γ coactivator 1-α]), which plays a key role in mitochondrial biogenesis, and PGC-1α has been implicated in the pathogenesis of experimental cardiomyopathy and nephropathy.105,106
In the clinical setting, patients with cardiomyopathy show suppressed expression of SIRT1, SIRT3, and PGC-1α107,108 and point sequence variations in the genes for SIRT3 and PGC-1α.109,110 Loss-of-function polymorphisms in the SIRT1 gene increase the risk of chronic kidney disease in patients with type 2 diabetes.111 In patients with diabetes, the glomerular expression of SIRT6 is suppressed101; serum SIRT1 levels are decreased, with a decline that parallels the development of albuminuria112; and there is an inverse relationship between the expression of SIRT1 and SGLT2 in the human diabetic kidney.77
Adenosine Monophosphate–Activated Protein Kinase
AMPK is a serine/threonine kinase that discerns the balance between cytosolic levels of ATP and AMP (adenosine monophosphate). AMPK is activated when the ATP-to-AMP ratio is low and phosphorylates downstream proteins that promote increased catabolism and decrease anabolism, thereby augmenting ATP production.199 Knockout or suppression of AMPK diminishes the heart’s ability to respond to stress, promotes cardiac aging, and leads to cardiomyopathy.113,114 Activation of AMPK ameliorates oxidative stress, mitochondrial dysfunction, proinflammatory pathways, fibrosis, and apoptosis and preserves ventricular function during cardiac stresses produced by ischemia, diabetes, pressure overload, or cardiotoxic agents.115–117 AMPK activity is impaired in the diabetic kidney, which contributes to the development of nephropathy.118 Activation of AMPK promotes glomerular health and podocyte survival and reduces tubular injury, apoptosis, and fibrosis triggered by metabolic stresses, ischemia, and nephrotoxic drugs.119–121
In the clinical setting, patients with sequence variations in the PPKAG2 gene (which codes for an AMPK subunit) develop cardiomyopathy with atrial and ventricular arrhythmias.122 Sequence variants of PPKAG2 are also linked to accelerated decline in glomerular filtration rate and the development of kidney disease in the general population and in patients with diabetes.123,124
These 3 master regulators—mTOR, sirtuins, and AMPK—modulate changes in cellular biology that allow organisms to adapt to environmental opportunities and challenges. When nutrients are plentiful, organisms prioritize the use of fuels to expand cell mass and mTOR signaling is central to this process. In contrast, when nutrients are depleted, organisms mute anabolic pathways and adopt a sheltered set of biological conditions to preserve the structural and functional integrity of existing cells; the sirtuins, PGC-1α, and AMPK are critical to this response. The set point for the interplay of these master switches is determined by the level of nutrients and the redox state.125
Of note, SIRT1/PGC-1α and Akt/mTOR pathways are highly interconnected at a molecular level. SIRT1 and PGC-1α can negatively regulate the transcription of Akt and directly interfere with the actions of Akt and mTOR.126,127 Conversely, upregulation of Akt leads to suppression of PGC-1α, whereas inhibition of mTOR by rapamycin or Akt downregulation leads to activation of SIRT1 and PGC-1α.128,129 The effect of AMPK to promote NAD+ leads to SIRT1 activation130 and AMPK can activate PGC-1α by phosphorylation.131 Conversely, AMPK can inhibit mTOR by an action on Akt as well as through a direct effect on the mTORC1 complex.132
Action of Nutrient Sensors to Influence Cellular Stress by Modulation of Autophagy
The interplay of the actions of the mTOR, sirtuin, and AMPK master switches to reduce cellular stress and promote cellular survival may be primarily mediated by their actions to influence the cellular housekeeping process of autophagy. Autophagy is an evolutionarily conserved intracellular degradative pathway, which involves the encircling of unwanted or dangerous cellular components by a double-membrane vesicle (the autophagosome), whose fusion with the lysosome allows degradative enzymes to destroy the contents of the vesicle.133,134 The primordial stimulus to autophagy is nutrient deprivation, and when activated nonselectively, autophagy allows for recycling of cellular constituents, which generates ATP for energy-starved cells.135 However, autophagic flux also can be activated selectively in response to a broad range of cellular stresses, including oxidative and endoplasmic reticulum stress. The most important sources of oxidative stress are dysfunctional mitochondria and peroxisomes and their clearance by autophagy is referred to as mitophagy and pexophagy. Endoplasmic reticulum stress is typically caused by the accumulation of misfolded proteins or glucose or fatty acid intermediates that may result from a variety of cellular injuries. Autophagic clearance of these damaged cellular constituents markedly reduces cellular stress and autophagic flux also directly acts to mitigate proinflammatory and profibrotic responses.136 The overall effect of enhanced autophagic flux is to preserve cellular integrity and prevent apoptotic cell death, thus maintaining and restoring organ structure and function.
Autophagic flux is driven by the interplay of nutrient deprivation and surplus sensors. The formation of the autophagosome is initiated by the concerted interaction of the ULK1 (Unc-51-like kinase 1) complex with the Beclin 1–PI3KC3 (class III phosphatidylinositol 3-kinase) complex.134,135 AMPK activates ULK1, but ULK1 phosphorylation by mTORC1 prevents its activation. Beclin 1 is activated when deacetylated by SIRT1137 and TLR9 (Toll-like receptor 9) interacts with Beclin 1 to regulate the assembly of the PI3KC3 complex.138 Elucidation of the numerous influences of mTOR, sirtuins, and AMPK on the components of the autophagic machinery is beyond the scope of this review.
The adaptive actions of autophagy are particularly important in the heart and kidneys. Both the heart and kidneys are replete with mitochondria and peroxisomes, which underlie their enormous capacity to consume oxygen and generate reactive oxygen species. Amelioration of oxidative and endoplasmic reticulum stress is particularly important to cardiomyocytes, because nonproliferating cells cannot replace cells that have died. Chronic HF and chronic kidney disease is characterized by the accumulation of intracellular debris and deleterious metabolic intermediates, a marked increase in oxidative stress, and the activation of proinflammatory and profibrotic signals.60 Autophagic flux represents a major adaptive and restorative response; impairment of autophagy dramatically heightens the likelihood that an injurious event will lead to cardiomyopathy and nephropathy.139,140 Although excessive stimulation of autophagy may exert deleterious effects on the heart (particularly after acutely imposed massive stresses in the absence of HF),141,142 measured augmentation of selective autophagy protects the heart against pressure overload, hypoxia, and injury produced by cardiotoxic agents,143,144 and shields podocytes and renal tubular cells from damage caused by diabetes, ischemia, and nephrotoxic drugs.145,146
Autophagic vacuoles are increased in patients who show reverse cardiac remodeling or receive mechanical unloading,147,148 whereas persistent impairment in autophagy flux in the failing human heart is a poor prognostic sign.149,150 Similarly, autophagic function is blunted in renal tubular cells of patients with diabetic nephropathy151 and loss of autophagosomes is a marker of chronic graft dysfunction in renal transplant patients.152 By contrast, increased autophagosome density in podocytes presages the preservation of glomerular function and a reduced risk of progression in patients with kidney disease.153,154
Effects of SGLT2 Inhibitors on Nutrient Deprivation and Surplus Signaling, Autophagic Flux, and Cellular Stress in Experimental Cardiomyopathy and Nephropathy
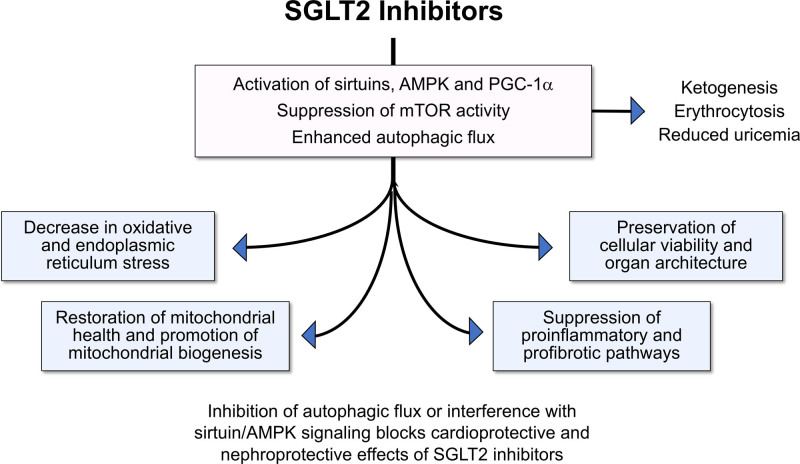
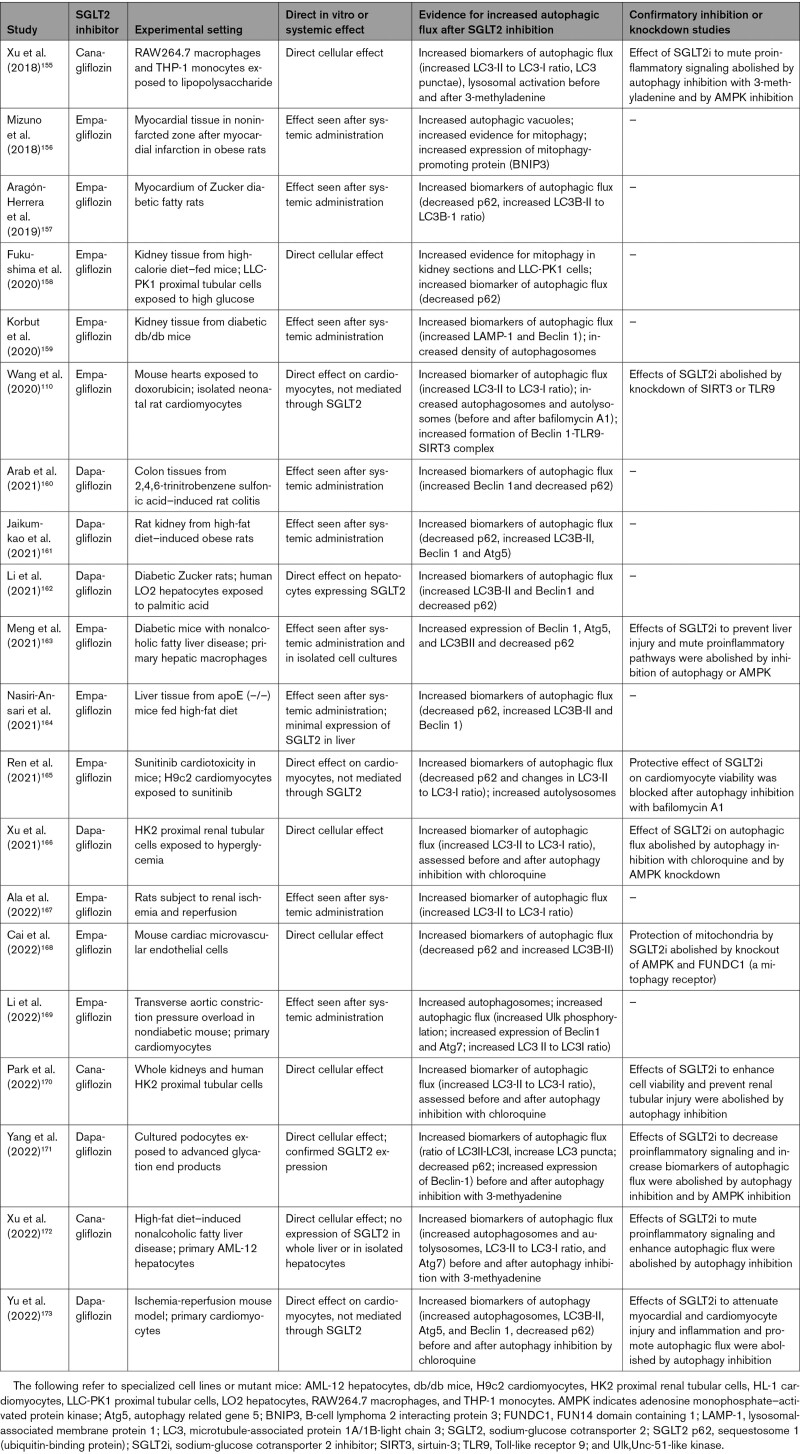
SGLT2 inhibitors produce a highly distinctive pattern of favorable effects on the evolution and progression of cardiomyopathy and nephropathy in experimental animals (Figure 4). First, SGLT2 inhibitors consistently enhance autophagic flux (including mitophagy) in the heart and kidney; the action to augment autophagy is seen in tissue harvested from chronically treated animals as well as in isolated cell cultures perfused with SGLT2 inhibitors (Table 1).155–173 Second, SGLT2 inhibitors mute the generation of reactive oxygen species, enhance antioxidant mechanisms, and mitigate endoplasmic reticulum stress.165,167,169,174–176 Third, SGLT2 inhibitors accelerate the disposal of injured mitochondria, restore healthy mitochondrial function, and promote mitochondrial biogenesis167,169; the increase in mitochondrial mass is a characteristic feature of the action of these drugs on electron microscopy.156 Fourth, SGLT2 inhibitors reduce inflammation and fibrosis by interfering with the activation of NF-κB (nuclear factor kappa B) and the NLRP3 (NLR family pyrin domain containing 3) inflammasome, thus reducing the synthesis of proinflammatory cytokines; they also mute profibrotic pathways, fibroblast proliferation, and the deposition of collagen.177,178 Fifth, SGLT2 inhibitors preserve cellular functions and integrity, prevent the loss of cells by apoptosis and senescence, and act to maintain normal tissue architecture, leading to the prevention of adverse structural remodeling and the restoration of organ function.34,48,54,167,179 This characteristic pattern of cellular and tissue responses has been observed with diverse SGLT2 inhibitors in experimental cardiomyopathy and nephropathy, regardless of the triggering mechanism.
Figure 4.
Proposed framework by which SGLT2 (sodium-glucose cotransporter 2) inhibitors can modulate nutrient deprivation signaling and thereby enhance autophagic flux and reduce cellular stress. AMPK indicates adenosine monophosphate–activated protein kinase; mTOR, mammalian target of rapamycin; and PGC-1α, peroxisome proliferator–activated receptor γ coactivator 1-α.
Table 1.
Experimental Studies on the Effect of SGLT2 Inhibitors on Autophagic Flux
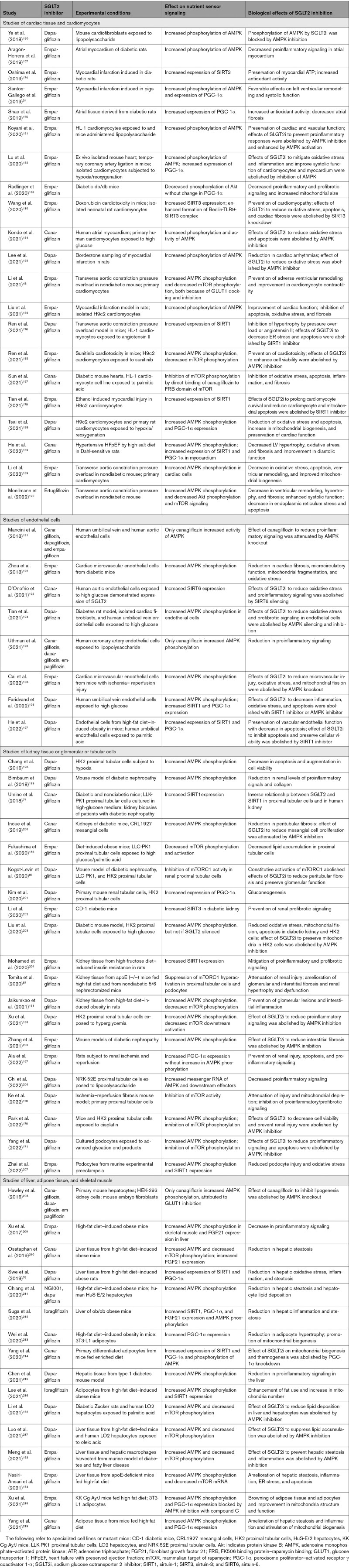
The distinctive pattern of cellular and tissue benefits of SGLT2 inhibitors in the heart and kidney is consistently accompanied by simultaneous upregulation of nutrient deprivation signaling and downregulation of nutrient surplus signaling. The effect of SGLT2 inhibitors to reduce oxidative stress, enhance mitochondrial integrity, mute inflammatory pathways, and preserve cell viability is paralleled by an increase in expression or activity of AMPK, SIRT1, SIRT3, SIRT6, and PGC-1α and decreased activation of mTOR in diverse tissues under stress, particularly in experimentally induced cardiomyopathy and nephropathy (Figure 4 and Table 2).48,54,57,76,77,87,110,157,158,161–171,174–219 The increase in AMPK and autophagic flux also explains the increase in ATP production that is seen in the heart and kidneys, both experimentally and clinically, after SGLT2 inhibition.52,54,57,181,220
Table 2.
Experimental Studies on the Effect of SGLT2 Inhibitors on Nutrient Deprivation and Surplus Signaling
When the concept of SGLT2-enhanced nutrient deprivation signaling and autophagic flux was first proposed,60 there were only scattered supportive observations. However, during the past 2 years, nearly 60 additional studies have been published, using a broad range of experimental conditions and methods, and all have confirmed the original hypothesis (Tables 1 and 2). Changes in nutrient deprivation and surplus signaling produced by SGLT2 inhibitors have been noted both in harvested whole organs and in isolated cell cultures and have been seen in tissues that express SGLT2 (eg, proximal renal tubules) and in those that do not express SGLT2 (eg, heart). They also have been demonstrated in the endothelium, liver, and adipose tissue. Most importantly, in a large proportion of these reports, when the effects of SGLT2 inhibitors to promote activation of AMPK, SIRT1, SIRT3, and SIRT6 were attenuated by specific pharmacologic inhibition or knockdown of nutrient deprivation signaling pathways or when their ability to promote autophagy has been genetically or pharmacologically blocked, the beneficial effects of SGLT2 inhibitors to mute oxidative stress, promote mitochondrial health, attenuate inflammation and fibrosis, and maintain cell viability and organ integrity have been abolished (Tables 1 and 2). These experimental observations, taken together, provide compelling evidence that the cardioprotective and nephroprotective effects of SGLT2 inhibitors can be explained by their actions to modulate nutrient and energy sensors and their downstream effect to promote autophagy.
Are nutrient deprivation pathways activated when SGLT2 inhibitors are used in the clinical setting? AMPK, SIRT1, SIRT3, and PGC-1α are the cornerstone of the body’s response to starvation, and thus, their synthesis in the liver is enhanced when there is substantial loss of calories in the urine (as with SGLT2 inhibition).76,210 After the induction of glycosuria, the activation of SIRT1, SIRT6, and PGC-1α supports blood glucose by promoting gluconeogenesis76,221—a distinctive metabolic signature of SGLT2 inhibitors (demonstrated clinically), which limits their antihyperglycemic response.201,222 Activation of AMPK and PGC-1α promotes fatty acid oxidation and increased synthesis of SIRT1 and SIRT3 stimulates 3-hydroxy-3-methylglutaryl CoA synthase, the rate-limiting enzyme for ketone body formation.223 As a result, AMPK, SIRT1, SIRT3, and PGC-1α act in concert to promote ketogenesis in the liver and the clinical finding of ketogenesis during starvation and after SGLT2 inhibition reflects enhanced nutrient deprivation signaling. In addition, SIRT1 activates HIF-2α (hypoxia-inducible factor 2α),224 the master regulator of the production of erythropoietin and stimulus to erythrocytosis. The action of SIRT1 to reduce oxidative stress can also contribute to the decrease in uric acid seen in patients treated with SGLT2 inhibitors72 and SIRT1 can directly promote uric acid secretion.225 The simultaneous finding of ketogenesis, erythrocytosis, and reduced uricemia in clinical trials with SGLT2 inhibitors suggests that nutrient deprivation signaling is upregulated in patients who are treated with these drugs. Increased AMPK and SIRT1 activity may also directly enhance tubuloglomerular feedback,226,227 thereby linking changes in these energy sensors to the effect of SGLT2 inhibitors to rapidly reduce glomerular filtration rate in the clinical setting.35
A recent proteomics analysis of blood samples collected from >1100 patients with HF before and after treatment with placebo or empagliflozin identified a limited number of proteins whose serum levels were significantly changed as a result of SGLT2 inhibition.228 Of the 21 differentially increased proteins with known effects on the heart and kidneys, 6 were linked to the promotion of autophagy in the heart, kidneys, or endothelium, and 4 proteins (IGF-1 [insulin-like growth factor binding protein 1], TfR1 [transferrin receptor protein 1], erythropoietin, and AFABP [adipocyte fatty acid binding protein 4]) are known to be upregulated by enhanced SIRT1 signaling.224,229–231 The latter proteins were also noted to be differentially increased by Ferrannini et al.232 in a second proteomics analysis of blood collected from patients with type 2 diabetes. Neither of the 2 proteomics analyses performed direct measurements of SIRT1, SIRT3, SIRT6, PGC-1α, or phosphorylated AMPK, but it is not clear that changes in blood levels of these proteins have the capacity to reflect the importance of their intracellular actions accurately.
Mechanism of Cellular Benefits of SGLT2 Inhibitors in Organs Without SGLT2 Expression
How can SGLT2 inhibitors promote nutrient deprivation signaling and autophagy with their attendant favorable effects on cellular stress, homeostasis, and survival in tissues that do not express SGLT2? There are 3 possibilities: glycosuric caloric loss, ketogenesis-induced autophagic flux, and direct nutrient-independent cellular effects.
Glycosuric Caloric Loss
SGLT2 inhibitors induce a substantial loss of calories in the urine and the resulting glucose depletion can stimulate a system-wide upregulation of nutrient deprivation signaling in organs throughout the body, a shift that does not depend on the presence or absence of SGLT2 in a specific tissue.76,210 Because the action of SGLT2 inhibitors to promote gluconeogenesis through SIRT1 limits the reduction in blood glucose, glycosuria serves to shift the set point between SIRT1 signaling and glycemia, and as a result, the upregulation of nutrient deprivation signaling with SGLT2 inhibitors can occur even when blood glucose is only modestly diminished.76 This hypothesis may explain the reported relationship between the magnitude of glycosuria and increases in erythropoietin in patients treated with these drugs.233 However, this hypothesis is difficult to reconcile with the fact that chronic kidney disease meaningfully impairs the glycosuric response to SGLT2 inhibitors, but it does not diminish the ability of these drugs to reduce HF and major renal events.26,37
Ketone Body–Induced Nutrient Deprivation Signaling and Autophagic Flux
SGLT2 inhibitors induce a state of starvation mimicry characterized by ketogenesis46,51 and the delivery of ketone bodies to organs can exert a direct effect to increase AMPK and decrease mTOR phosphorylation, to increase the expression of PGC-1α, and to promote autophagic flux.57,234–238 Potentially acting through these mediators, ketonemia has been shown to reduce oxidative stress, mute cellular inflammation, enhance mitochondrial biogenesis, and preserve cardiac and renal function after diverse stresses.57,234–240 Ketone body supplementation inhibits phenylephrine-induced hypertrophy in experiments238 and interruption of ketogenesis negates the benefits of SGLT2 inhibitors on the kidney.57 These observations suggest that the ketonemia seen after SGLT2 inhibitors use may exert cardioprotective and nephroprotective effects as a result of a direct action to stimulate nutrient deprivation signaling rather than because of an ability to serve as an efficient fuel for the generation of ATP. However, the presence of diabetes attenuates the magnitude of ketonemia, but it does not influence the cardiorenal benefits of SGLT2 inhibitors.35,36,51 Furthermore, as noted in Tables 1 and 2, SGLT2 inhibitors exert direct benefits on the heart and kidney in isolated cell preparations, which are not subject to fluctuations in environmental ketone bodies.
Direct Glucose- and Ketone-Independent Cellular Effects
SGLT2 inhibitors exert direct cellular effects that are independent of changes in glucose induced by loss of glucose by the kidney or ketone body production in the liver. Numerous studies (Tables 1 and 2) have shown that SGLT2 inhibitors can enhance nutrient deprivation signaling, promote autophagy, maintain mitochondrial health, mute cellular stresses, and mitigate proinflammatory and profibrotic pathways in isolated organs and in cell cultures—experimental conditions where the level of environmental glucose or ketone bodies is held constant. Studies demonstrating the direct effects of these drugs on isolated cardiomyocytes, podocytes, and proximal renal tubular cells have also established that the cardioprotective and renoprotective benefits of these drugs are not dependent on changes in cardiovascular physiology (eg, changes in blood pressure or renal sympathetic tone); on changes proximal renal tubular function (glycosuria or natriuresis); or on changes in the level of a blood or plasma constituent (eg, hemoglobin, glucose, uric acid, or ketones).
How do SGLT2 inhibitors exert direct effects on cardiac and renal parenchymal cells to preserve homeostasis and survival? Many studies have been performed in cells that express SGLT2, either clinically or experimentally (eg, proximal renal tubular cells, endothelial cells, and hepatocytes), but SGLT2 inhibitors exert direct cytoprotective effects in cardiomyocytes that do not express SGLT2 (Tables 1 and 2). It is possible that the concentrations of SGLT2 inhibitors used in certain experiments might interfere with other glucose transporters. Kondo et al.184 suggested that the cardioprotection produced by canagliflozin was related to SGLT1 inhibition,241 but empagliflozin (which does not inhibit SGLT1) produces cytoprotective effects on isolated cardiomyocytes. Li et al.48 have proposed that empagliflozin can dock with GLUT1 (glucose transporter 1) and GLUT4 (glucose transporter 4) receptors to inhibit the cellular uptake of glucose, which can trigger the nutrient deprivation signaling and autophagy. An inhibitory effect of SGLT2 inhibitors on GLUT receptors has been proposed by others,208,242 but has yet to be confirmed. Potential effects of SGLT2 inhibitors on ion transport67,71 or Ca/calmodulin-dependent kinase243 cannot explain their effects on autophagy or nutrient deprivation signaling.244
Because SGLT2 functions as a nutrient surplus sensor, it is noteworthy that there exists an inverse relationship between SGLT2 and nutrient deprivation signaling in individual cells.77 This observation raises the possibility that SGLT2 inhibitors might bind directly to nutrient sensors to influence their function. Sun et al.187 reported that canagliflozin binds to mTOR in the same structural domain used by rapamycin. Ying et al.245 demonstrated that phloretin (an inhibitor of SGLT1 and SGLT2) ameliorates cardiomyocyte injury by upregulating SIRT1 and showed that phloretin docks directly with SIRT1 to form a stable complex. Wang et al.110 proposed that empagliflozin acts directly on SIRT3 to promote the formation of a complex of SIRT3, Beclin 1, and TLR9, which enhances autophagic flux. In that study, experimental knockout of either SIRT3 or TLR9 abolished the ability of SGLT2 inhibition to protect the heart from injury by doxorubicin. Wang et al.110 identified 3 patients with dilated cardiomyopathy who underwent myocardial biopsy before and after treatment with empagliflozin for 28 days. Two patients without a SIRT3 variation exhibited the expected enhancement of mitochondrial respiration and expression of TLR9 after treatment with the SGLT2 inhibitor; however, these changes were not seen in 1 patient who had a loss-of-function variation in SIRT3 in the myocardium.110
Conclusions
SGLT2 inhibitors produce a distinctive pattern of favorable effects on the evolution and progression of cardiomyopathy and nephropathy, which is characterized by a reduction in oxidative and endoplasmic reticulum stress, restoration of healthy mitochondrial function and enhanced mitochondrial biogenesis, a decrease in proinflammatory and profibrotic pathways, preservation of cellular integrity and viability, and maintenance of normal organ structure and function. A substantial body of evidence indicates that this characteristic pattern of responses can be explained by the action of SGLT2 inhibitors to promote cellular housekeeping by autophagic flux, a process that is triggered by the effect of these drugs to produce simultaneous upregulation of nutrient deprivation signaling and downregulation of nutrient surplus signaling, as manifested by an increase in the expression and activity of AMPK, SIRT1, SIRT3, SIRT6, and PGC-1α and decreased activation of mTOR in diverse tissues under stress. The distinctive pattern of cardioprotective and renoprotective effects of SGLT2 inhibitors is abolished by specific inhibition or knockdown of autophagy, AMPK, and sirtuins. In the clinical setting, proteomics analyses of blood collected from participants in randomized trials show a pattern of differentially increased proteins that is consistent with enhanced autophagy and SIRT1 activity. Patients treated with SGLT2 inhibitors exhibit augmented gluconeogenesis, ketogenesis, and erythrocytosis and reduced uricemia, the hallmarks of nutrient deprivation signaling and the principal statistical mediators of the ability of SGLT2 inhibitors to reduce the risk of HF hospitalizations and serious renal events in large-scale trials. The action of SGLT2 inhibitor to promote autophagic flux is seen in isolated cells and tissues that do not express SGLT2 and are not exposed to changes in environmental glucose or ketones and may be related to an ability of these drugs to bind directly to sirtuins or mTOR. Changes in renal or cardiovascular physiology, energy use, or metabolism cannot explain the benefits of SGLT2 inhibitors. Substantial experimental work on the molecular and cellular actions of these drugs, when taken together with highly supportive observations in the clinical setting, has provided critical insights into the mechanisms that underlie their remarkable ability to preserve cardiac and renal function in patients whose hearts and kidneys are under stress.
Article Information
Sources of Funding
None.
Disclosures
Dr Packer has served as a consultant for AbbVie, Altimmune, Amarin, Amgen, Ardelyx, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Moderna, Novartis, Reata, Relypsa, and Salamandra. The author reports no current or planned financial relationships related to SGLT2 inhibitors or neprilysin inhibition.
Nonstandard Abbreviations and Acronyms
- AFABP
- adipocyte fatty acid binding protein 4
- Akt
- protein kinase B
- AMP
- adenosine monophosphate
- AMPK
- adenosine monophosphate–activated protein kinase
- ATP
- adenosine triphosphate
- GLUT1
- glucose transporter 1
- GLUT4
- glucose transporter 4
- HF
- heart failure
- HIF-2α
- hypoxia-inducible factor 2α
- IGF-1
- insulin-like growth factor binding protein 1
- mTOR
- mammalian target of rapamycin
- mTORC1
- mammalian target of rapamycin complex 1
- NAD
- nicotinamide adenine dinucleotide
- NF-κB
- nuclear factor kappa B
- NH4+
- ammonium ion
- NHE1
- sodium-hydrogen exchanger isoform 1
- NHE3
- sodium-hydrogen exchanger isoform 3
- NLRP3
- NLR family pyrin domain containing 3
- PGC-1α
- peroxisome proliferator–activated receptor γ coactivator 1α
- PI3KC3
- class III phosphatidylinositol 3-kinase
- SGLT1
- sodium-glucose cotransporter 1
- SGLT2
- sodium-glucose cotransporter 2
- SIRT1
- sirtuin 1
- SIRT3
- sirtuin 3
- SIRT6
- sirtuin 6
- TfR1
- transferrin receptor protein 1
- TLR9
- Toll-like receptor 9
- ULK1
- Unc-51-like kinase 1
Circulation is available at www.ahajournals.org/journal/circ
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Sources of Funding and Disclosures, see page 1398.
References
- 1.Giugliano D, Longo M, Scappaticcio L, Bellastella G, Maiorino MI, Esposito K. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta-analysis of 11 CVOTs. Cardiovasc Diabetol. 2021;20:236. doi: 10.1186/s12933-021-01430-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlap ME, Kinugawa T, Sica DA, Thames MD. Cardiopulmonary baroreflex control of renal sympathetic nerve activity is impaired in dogs with left ventricular dysfunction. J Card Fail. 2019;25:819–827. doi: 10.1016/j.cardfail.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 3.Petersson M, Friberg P, Eisenhofer G, Lambert G, Rundqvist B. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J. 2005;26:906–913. doi: 10.1093/eurheartj/ehi184 [DOI] [PubMed] [Google Scholar]
- 4.Sharp TE, Polhemus DJ, Li Z, Spaletra P, Jenkins JS, Reilly JP, White CJ, Kapusta DR, Lefer DJ, Goodchild TT. Renal denervation prevents heart failure progression via inhibition of the renin-angiotensin system. J Am Coll Cardiol. 2018;72:2609–2621. doi: 10.1016/j.jacc.2018.08.2186 [DOI] [PubMed] [Google Scholar]
- 5.Polhemus DJ, Trivedi RK, Gao J, Li Z, Scarborough AL, Goodchild TT, Varner KJ, Xia H, Smart FW, Kapusta DR, et al. Renal sympathetic denervation protects the failing heart via inhibition of neprilysin activity in the kidney. J Am Coll Cardiol. 2017;70:2139–2153. doi: 10.1016/j.jacc.2017.08.056 [DOI] [PubMed] [Google Scholar]
- 6.Jang HS, Kim J, Padanilam BJ. Renal sympathetic nerve activation via α2-adrenergic receptors in chronic kidney disease progression. Kidney Res Clin Pract. 2019;38:6–14. doi: 10.23876/j.krcp.18.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Deng Y, Liu L, Zhang C, Cai Y, Zhang T, Han M, Xu G. Renal sympathetic denervation ameliorates renal fibrosis via inhibition of cellular senescence. Front Immunol. 2022;12:823935. doi: 10.3389/fimmu.2021.823935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N. Effects of catheter-based renal denervation on heart failure with reduced ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2022;27:29–36. doi: 10.1007/s10741-020-09974-4 [DOI] [PubMed] [Google Scholar]
- 9.Li J, He Q, Wu W, Li Q, Huang R, Pan X, Lai W. Role of the renal sympathetic nerves in renal sodium/potassium handling and renal damage in spontaneously hypertensive rats. Exp Ther Med. 2016;12:2547–2553. doi: 10.3892/etm.2016.3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majcherczyk S, Mikulski A, Sjölander M, Thorén P. Increase of renal sympathetic nerve activity by metoprolol or propranolol in conscious spontaneously hypertensive rats. Br J Pharmacol. 1987;91:711–714. doi: 10.1111/j.1476-5381.1987.tb11267.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pontes RB, Girardi AC, Nishi EE, Campos RR, Bergamaschi CT. Crosstalk between the renal sympathetic nerve and intrarenal angiotensin II modulates proximal tubular sodium reabsorption. Exp Physiol. 2015;100:502–506. doi: 10.1113/EP085075 [DOI] [PubMed] [Google Scholar]
- 12.Boer PA, Morelli JM, Figueiredo JF, Gontijo JA. Early altered renal sodium handling determined by lithium clearance in spontaneously hypertensive rats (SHR): role of renal nerves. Life Sci. 2005;76:1805–1815. doi: 10.1016/j.lfs.2004.09.029 [DOI] [PubMed] [Google Scholar]
- 13.Healy V, Thompson C, Johns EJ. The adrenergic regulation of proximal tubular Na⁺/H⁺ exchanger 3 in the rat. Acta Physiol (Oxf). 2014;210:678–689. doi: 10.1111/apha.12181 [DOI] [PubMed] [Google Scholar]
- 14.Katsurada K, Nandi SS, Sharma NM, Patel KP. Enhanced expression and function of renal SGLT2 (sodium-glucose cotransporter 2) in heart failure: role of renal nerves. Circ Heart Fail. 2021;14:e008365. doi: 10.1161/CIRCHEARTFAILURE.121.008365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue BH, dos Santos L, Pessoa TD, Antonio EL, Pacheco BP, Savignano FA, Carraro-Lacroix LR, Tucci PJ, Malnic G, Girardi AC. Increased NHE3 abundance and transport activity in renal proximal tubule of rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2012;302:R166–R174. doi: 10.1152/ajpregu.00127.2011 [DOI] [PubMed] [Google Scholar]
- 16.Borges-Júnior FA, Silva Dos Santos D, Benetti A, Polidoro JZ, Wisnivesky ACT, Crajoinas RO, Antônio EL, Jensen L, Caramelli B, Malnic G, et al. Empagliflozin inhibits proximal tubule NHE3 activity, preserves GFR, and restores euvolemia in nondiabetic rats with induced heart failure. J Am Soc Nephrol. 2021;32:1616–1629. doi: 10.1681/ASN.2020071029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, Song P, Freeman B, Kim YC, Soleimani M, et al. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol. 2020;319:F712–F728. doi: 10.1152/ajprenal.00264.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142:1028–1039. doi: 10.1161/CIRCULATIONAHA.120.045691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gueguen C, Burke SL, Barzel B, Eikelis N, Watson AMD, Jha JC, Jackson KL, Sata Y, Lim K, Lambert GW, et al. Empagliflozin modulates renal sympathetic and heart rate baroreflexes in a rabbit model of diabetes. Diabetologia. 2020;63:1424–1434. doi: 10.1007/s00125-020-05145-0 [DOI] [PubMed] [Google Scholar]
- 20.Herat LY, Magno AL, Rudnicka C, Hricova J, Carnagarin R, Ward NC, Arcambal A, Kiuchi MG, Head GA, Schlaich MP, et al. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci. 2020;5:169–179. doi: 10.1016/j.jacbts.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, Iwasaki YK, Yamamoto T, Takano H, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19:148. doi: 10.1186/s12933-020-01127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boorsma EM, Beusekamp JC, Ter Maaten JM, Figarska SM, Danser AHJ, van Veldhuisen DJ, van der Meer P, Heerspink HJL, Damman K, Voors AA. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail. 2021;23:68–78. doi: 10.1002/ejhf.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE-CHF Trial. Circulation. 2020;142:1713–1724. doi: 10.1161/CIRCULATIONAHA.120.048739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholtes RA, Muskiet MHA, van Baar MJB, Hesp AC, Greasley PJ, Hammarstedt A, Karlsson C, Hallow KM, Danser AHJ, Heerspink HJL, et al. The adaptive renal response for volume homeostasis during 2 weeks of dapagliflozin treatment in people with type 2 diabetes and preserved renal function on a sodium-controlled diet. Kidney Int Rep. 2022;7:1084–1092. doi: 10.1016/j.ekir.2022.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Sattar N, Brueckmann M, Jamal W, Cotton D, et al. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR-Reduced Trial. J Am Coll Cardiol. 2021;77:1381–1392. doi: 10.1016/j.jacc.2021.01.033 [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–1735. doi: 10.2337/dc15-0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neeland IJ, McGuire DK, Chilton R, Crowe S, Lund SS, Woerle HJ, Broedl UC, Johansen OE. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13:119–126. doi: 10.1177/1479164115616901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Jüni P, et al. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2020;141:704–707. doi: 10.1161/CIRCULATIONAHA.119.044235 [DOI] [PubMed] [Google Scholar]
- 29.Schmidt WFJ, Wachsmuth N, Jimenez J, Soria R. Hemoglobin mass and blood volume in patients with altitude-related polycythemia. Front Physiol. 2022;13:867108. doi: 10.3389/fphys.2022.867108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. doi: 10.1111/dom.13126 [DOI] [PubMed] [Google Scholar]
- 31.Januzzi JL, Zannad F, Anker SD, Butler J, Filippatos G, Pocock SJ, Ferreira JP, Sattar N, Verma S, Vedin O, Schnee J, et al. Prognostic importance of NT-proBNP and effect of empagliflozin in the EMPEROR-Reduced trial. J Am Coll Cardiol. 2021;78:1321–1332. doi: 10.1016/j.jacc.2021.07.046 [DOI] [PubMed] [Google Scholar]
- 32.Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, Lamba S, Bhatt K, Brush J, Civitello A, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: Results From the EMBRACE-HF Trial. Circulation. 2021;143:1673–1686. doi: 10.1161/CIRCULATIONAHA.120.052503 [DOI] [PubMed] [Google Scholar]
- 33.Omar M, Jensen J, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, Möller S, Ali M, Gustafsson F, Køber L, et al. Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2020;76:2740–2751. doi: 10.1016/j.jacc.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 34.Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143:516–525. doi: 10.1161/CIRCULATIONAHA.120.052186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 36.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356 [DOI] [PubMed] [Google Scholar]
- 37.Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Hauske SJ, Brueckmann M, Pfarr E, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation. 2021;143:310–321. doi: 10.1161/CIRCULATIONAHA.120.051685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen L, Kristensen SL, Bengtsson O, Böhm M, de Boer RA, Docherty KF, Inzucchi SE, Katova T, Køber L, Kosiborod MN, et al. Dapagliflozin in HFrEF patients treated with mineralocorticoid receptor antagonists: an analysis of DAPA-HF. JACC Heart Fail. 2021;9:254–264. doi: 10.1016/j.jchf.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 39.McCallum W, Tighiouart H, Ku E, Salem D, Sarnak MJ. Trends in kidney function outcomes following RAAS inhibition in patients with heart failure with reduced ejection fraction. Am J Kidney Dis. 2020;75:21–29. doi: 10.1053/j.ajkd.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaduganathan M, Ferreira JP, Rossignol P, Neuen B, Claggett BL, Pfeffer MA, McMurray JJV, Pitt B, Zannad F, Solomon SD. Effects of steroidal mineralocorticoid receptor antagonists on acute and chronic estimated glomerular filtration rate slopes in patients with chronic heart failure [published online July 22, 2022]. Eur J Heart Fail. doi: 10.1002/ejhf.2635 [DOI] [PubMed] [Google Scholar]
- 41.Mc Causland FR, Lefkowitz MP, Claggett B, Packer M, Senni M, Gori M, Jhund PS, McGrath MM, Rouleau JL, Shi V, et al. Angiotensin-neprilysin inhibition and renal outcomes across the spectrum of ejection fraction in heart failure [published online January 5, 2022]. Eur J Heart Fail. doi: 10.1002/ejhf.2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, Bozovic A, Danser AHJ, Geurts F, Hoorn EJ, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020;97:202–212. doi: 10.1016/j.kint.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 43.Adamson C, Docherty KF, Heerspink HJL, de Boer RA, Damman K, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Petrie MC, et al. Initial decline (“dip”) in estimated glomerular filtration rate following initiation of dapagliflozin in patients with heart failure and reduced ejection fraction: Insights from DAPA-HF. Circulation. 2022;146:438–449. doi: 10.1161/CIRCULATIONAHA.121.058910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304:F156–F167. doi: 10.1152/ajprenal.00409.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nespoux J, Patel R, Zhang H, Huang W, Freeman B, Sanders PW, Kim YC, Vallon V. Gene knockout of the Na+-glucose cotransporter SGLT2 in a murine model of acute kidney injury induced by ischemia-reperfusion. Am J Physiol Renal Physiol. 2020;318:F1100–F1112. doi: 10.1152/ajprenal.00607.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herring RA, Shojaee-Moradie F, Stevenage M, Parsons I, Jackson N, Mendis J, Middleton B, Umpleby AM, Fielding BA, Davies M, et al. The SGLT2 inhibitor dapagliflozin increases the oxidation of ingested fatty acids to ketones in type 2 diabetes. Diabetes Care. 2022;45:1408–1415. doi: 10.2337/dc21-2043 [DOI] [PubMed] [Google Scholar]
- 47.Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–2141. doi: 10.1161/CIRCULATIONAHA.118.036459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Lu Q, Qiu Y, do Carmo JM, Wang Z, da Silva AA, Mouton A, Omoto ACM, Hall ME, Li J, et al. Direct cardiac actions of the sodium glucose co-transporter 2 inhibitor empagliflozin improve myocardial oxidative phosphorylation and attenuate pressure-overload heart failure. J Am Heart Assoc. 2021;10:e018298. doi: 10.1161/JAHA.120.018298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, Härkönen M. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8 [DOI] [PubMed] [Google Scholar]
- 50.Voros G, Ector J, Garweg C, Droogne W, Van Cleemput J, Peersman N, Vermeersch P, Janssens S. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ Heart Fail. 2018;11:e004953. doi: 10.1161/CIRCHEARTFAILURE.118.004953 [DOI] [PubMed] [Google Scholar]
- 51.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Barsotti E, Clerico A, Muscelli E. Renal handling of ketones in response to sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2017;40:771–776. doi: 10.2337/dc16-2724 [DOI] [PubMed] [Google Scholar]
- 52.Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, Dyck JE, Uddin GM, Oudit GY, Mayoux E, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3:575–587. doi: 10.1016/j.jacbts.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdurrachim D, Teo XQ, Woo CC, Chan WX, Lalic J, Lam CSP, Lee PTH. Empagliflozin reduces myocardial ketone utilization while preserving glucose utilization in diabetic hypertensive heart disease: a hyperpolarized 13 C magnetic resonance spectroscopy study. Diabetes Obes Metab. 2019;21:357–365. doi: 10.1111/dom.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. doi: 10.1016/j.jacc.2019.01.056 [DOI] [PubMed] [Google Scholar]
- 55.Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, Xiao H, Yu H, Zheng Y, Liang Y, et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res. 2021;128:232–245. doi: 10.1161/CIRCRESAHA.120.317933 [DOI] [PubMed] [Google Scholar]
- 56.Gaborit B, Ancel P, Abdullah AE, Maurice F, Abdesselam I, Calen A, Soghomonian A, Houssays M, Varlet I, Eisinger M, et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovasc Diabetol. 2021;20:57. doi: 10.1186/s12933-021-01237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomita I, Kume S, Sugahara S, Osawa N, Yamahara K, Yasuda-Yamahara M, Takeda N, Chin-Kanasaki M, Kaneko T, Mayoux E, et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab. 2020;32:404–419.e6. doi: 10.1016/j.cmet.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 58.Hare GMT, Zhang Y, Chin K, Thai K, Jacobs E, Cazorla-Bak MP, Nghiem L, Wilson DF, Vinogradov SA, Connelly KA, et al. Impact of sodium glucose linked cotransporter-2 inhibition on renal microvascular oxygen tension in a rodent model of diabetes mellitus. Physiol Rep. 2021;9:e14890. doi: 10.14814/phy2.14890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanchi A, Burnier M, Muller ME, Ghajarzadeh-Wurzner A, Maillard M, Loncle N, Milani B, Dufour N, Bonny O, Pruijm M. Acute and chronic effects of SGLT2 inhibitor empagliflozin on renal oxygenation and blood pressure control in nondiabetic normotensive subjects: a randomized, placebo-controlled trial. J Am Heart Assoc. 2020;9:e016173. doi: 10.1161/JAHA.119.016173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Packer M. Role of deranged energy deprivation signaling in the pathogenesis of cardiac and renal disease in states of perceived nutrient overabundance. Circulation. 2020;141:2095–2105. doi: 10.1161/CIRCULATIONAHA.119.045561 [DOI] [PubMed] [Google Scholar]
- 61.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O’Connor C, Pfeffer MA, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865 [DOI] [PubMed] [Google Scholar]
- 62.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845 [DOI] [PubMed] [Google Scholar]
- 63.Chan LKY, Wang Y, Ng EKW, Leung PS. Na+/H+ exchanger 3 blockade ameliorates type 2 diabetes mellitus via inhibition of sodium-glucose co-transporter 1-mediated glucose absorption in the small intestine. Diabetes Obes Metab. 2018;20:709–717. doi: 10.1111/dom.13151 [DOI] [PubMed] [Google Scholar]
- 64.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol. 2014;25:2028–2039. doi: 10.1681/ASN.2013060588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura TY, Iwata Y, Arai Y, Komamura K, Wakabayashi S. Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ Res. 2008;103:891–899. doi: 10.1161/CIRCRESAHA.108.175141 [DOI] [PubMed] [Google Scholar]
- 66.Baartscheer A, Schumacher CA, Wüst RC, Fiolet JW, Stienen GJ, Coronel R, Zuurbier CJ. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60:568–573. doi: 10.1007/s00125-016-4134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia. 2018;61:722–726. doi: 10.1007/s00125-017-4509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung YJ, Park KC, Tokar S, Eykyn TR, Fuller W, Pavlovic D, Swietach P, Shattock MJ. Off-target effects of sodium-glucose co-transporter 2 blockers: empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+]i in the heart. Cardiovasc Res. 2021;117:2794–2806. doi: 10.1093/cvr/cvaa323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung YJ, Park KC, Tokar S, Eykyn TR, Fuller W, Pavlovic D, Swietach P, Shattock MJ. SGLT2 inhibitors and the cardiac Na+/H+ exchanger-1: the plot thickens. Cardiovasc Res. 2021;117:2702–2704. doi: 10.1093/cvr/cvab184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osaka N, Mori Y, Terasaki M, Hiromura M, Saito T, Yashima H, Shiraga Y, Kawakami R, Ohara M, Fukui T, et al. Luseogliflozin inhibits high glucose-induced TGF-β2 expression in mouse cardiomyocytes by suppressing NHE-1 activity. J Int Med Res. 2022;50:3000605221097490. doi: 10.1177/03000605221097490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Philippaert K, Kalyaanamoorthy S, Fatehi M, Long W, Soni S, Byrne NJ, Barr A, Singh J, Wong J, Palechuk T, et al. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation. 2021;143:2188–2204. doi: 10.1161/CIRCULATIONAHA.121.053350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Packer M. Uric acid is a biomarker of oxidative stress in the failing heart: lessons learned from trials with allopurinol and SGLT2 inhibitors. J Card Fail. 2020;26:977–984. doi: 10.1016/j.cardfail.2020.08.015 [DOI] [PubMed] [Google Scholar]
- 73.Fitchett D, Inzucchi SE, Zinman B, Wanner C, Schumacher M, Schmoor C, Ohneberg K, Ofstad AP, Salsali A, George JT, et al. Mediators of the improvement in heart failure outcomes with empagliflozin in the EMPA-REG OUTCOME trial. ESC Heart Fail. 2021;8:4517–4527. doi: 10.1002/ehf2.13615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J, Neal B, Perkovic V, de Zeeuw D, Neuen BL, Arnott C, Simpson R, Oh R, Mahaffey KW, Heerspink HJL. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int. 2020;98:769–777. doi: 10.1016/j.kint.2020.04.051 [DOI] [PubMed] [Google Scholar]
- 75.Li J, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, de Zeeuw D, Vercruysse F, Shaw W, Matthews DR, et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail. 2020;8:57–66. doi: 10.1016/j.jchf.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 76.Swe MT, Thongnak L, Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Lungkaphin A. Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin Sci (Lond). 2019;133:2415–2430. doi: 10.1042/CS20190863 [DOI] [PubMed] [Google Scholar]
- 77.Umino H, Hasegawa K, Minakuchi H, Muraoka H, Kawaguchi T, Kanda T, Tokuyama H, Wakino S, Itoh H. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep. 2018;8:6791. doi: 10.1038/s41598-018-25054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su M, Chen Z, Wang C, Song L, Zou Y, Zhang L, Hui R, Wang J. Cardiac-specific overexpression of miR-222 induces heart failure and inhibits autophagy in mice. Cell Physiol Biochem. 2016;39:1503–1511. doi: 10.1159/000447853 [DOI] [PubMed] [Google Scholar]
- 82.Gao G, Chen W, Yan M, Liu J, Luo H, Wang C, Yang P. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int J Mol Med. 2020;45:195–209. doi: 10.3892/ijmm.2019.4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sang HQ, Jiang ZM, Zhao QP, Xin F. MicroRNA-133a improves the cardiac function and fibrosis through inhibiting Akt in heart failure rats. Biomed Pharmacother. 2015;71:185–189. doi: 10.1016/j.biopha.2015.02.030 [DOI] [PubMed] [Google Scholar]
- 84.Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Haneda M, et al. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol. 2013;24:1769–1781. doi: 10.1681/ASN.2012111080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121:2181–2196. doi: 10.1172/JCI44771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mori H, Inoki K, Masutani K, Wakabayashi Y, Komai K, Nakagawa R, Guan KL, Yoshimura A. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun. 2009;384:471–475. doi: 10.1016/j.bbrc.2009.04.136 [DOI] [PubMed] [Google Scholar]
- 87.Kogot-Levin A, Hinden L, Riahi Y, Israeli T, Tirosh B, Cerasi E, Mizrachi EB, Tam J, Mosenzon O, Leibowitz G. Proximal tubule mTORC1 is a central player in the pathophysiology of diabetic nephropathy and its correction by SGLT2 inhibitors. Cell Rep. 2020;32:107954. doi: 10.1016/j.celrep.2020.107954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yano T, Shimoshige S, Miki T, Tanno M, Mochizuki A, Fujito T, Yuda S, Muranaka A, Ogasawara M, Hashimoto A, et al. Clinical impact of myocardial mTORC1 activation in nonischemic dilated cardiomyopathy. J Mol Cell Cardiol. 2016;91:6–9. doi: 10.1016/j.yjmcc.2015.12.022 [DOI] [PubMed] [Google Scholar]
- 89.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670 [DOI] [PubMed] [Google Scholar]
- 90.Schlingmann KP, Jouret F, Shen K, Nigam A, Arjona FJ, Dafinger C, Houillier P, Jones DP, Kleinerüschkamp F, Oh J, et al. mTOR-activating mutations in RRAGD are causative for kidney tubulopathy and cardiomyopathy. J Am Soc Nephrol. 2021;32:2885–2899. doi: 10.1681/ASN.2021030333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mao Z, Tan Y, Tao J, Li L, Wang H, Yu F, Perl A, Zhao M. Renal mTORC1 activation is associated with disease activity and prognosis in lupus nephritis. Rheumatology (Oxford). 2022;18:keac037. doi: 10.1093/rheumatology/keac037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanz MN, Grimbert L, Moulin M, Gressette M, Rucker-Martin C, Lemaire C, Mericskay M, Veksler V, Ventura-Clapier R, Garnier A, et al. Inducible cardiac-specific deletion of Sirt1 in male mice reveals progressive cardiac dysfunction and sensitization of the heart to pressure overload. Int J Mol Sci. 2019;20:5005. doi: 10.3390/ijms20205005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo X, Yan F, Li J, Zhang C, Su H, Bu P. SIRT3 ablation deteriorates obesity-related cardiac remodeling by modulating ROS-NF-κB-MCP-1 signaling pathway. J Cardiovasc Pharmacol. 2020;76:296–304. doi: 10.1097/FJC.0000000000000877 [DOI] [PubMed] [Google Scholar]
- 94.Huang Y, Zhang J, Xu D, Peng Y, Jin Y, Zhang L. SIRT6-specific inhibitor OSS-128167 exacerbates diabetic cardiomyopathy by aggravating inflammation and oxidative stress. Mol Med Rep. 2021;23:367. doi: 10.3892/mmr.2021.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yuan YP, Ma ZG, Zhang X, Xu SC, Zeng XF, Yang Z, Deng W, Tang QZ. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol. 2018;114:38–47. doi: 10.1016/j.yjmcc.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 96.Bugyei-Twum A, Ford C, Civitarese R, Seegobin J, Advani SL, Desjardins JF, Kabir G, Zhang Y, Mitchell M, Switzer J, et al. Sirtuin 1 activation attenuates cardiac fibrosis in a rodent pressure overload model by modifying Smad2/3 transactivation. Cardiovasc Res. 2018;114:1629–1641. doi: 10.1093/cvr/cvy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomczyk MM, Cheung KG, Xiang B, Tamanna N, Fonseca Teixeira AL, Agarwal P, Kereliuk SM, Spicer V, Lin L, Treberg J, et al. Mitochondrial sirtuin-3 (SIRT3) prevents doxorubicin-induced dilated cardiomyopathy by modulating protein acetylation and oxidative stress. Circ Heart Fail. 2022;15:e008547. doi: 10.1161/CIRCHEARTFAILURE.121.008547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu S, Lan J, Li L, Wang X, Tong M, Fu L, Zhang Y, Xu J, Chen X, Chen H, et al. Sirt6 protects cardiomyocytes against doxorubicin-induced cardiotoxicity by inhibiting P53/Fas-dependent cell death and augmenting endogenous antioxidant defense mechanisms [published online October 28, 2021]. Cell Biol Toxicol. doi: 10.1007/s10565-021-09649-2 [DOI] [PubMed] [Google Scholar]
- 99.Chuang PY, Xu J, Dai Y, Jia F, Mallipattu SK, Yacoub R, Gu L, Premsrirut PK, He JC. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am J Pathol. 2014;184:1940–1956. doi: 10.1016/j.ajpath.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng X, Su H, He X, Chen JX, Zeng H. SIRT3 deficiency sensitizes angiotensin-II-induced renal fibrosis. Cells. 2020;9:2510. doi: 10.3390/cells9112510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang W, Liu H, Zhu S, Woodson M, Liu R, Tilton RG, Miller JD, Zhang W. Sirt6 deficiency results in progression of glomerular injury in the kidney. Aging (Albany NY). 2017;9:1069–1083. doi: 10.18632/aging.101214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang T, Chi Y, Kang Y, Lu H, Niu H, Liu W, Li Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J Cell Physiol. 2019;234:5033–5043. doi: 10.1002/jcp.27306 [DOI] [PubMed] [Google Scholar]
- 103.Locatelli M, Zoja C, Zanchi C, Corna D, Villa S, Bolognini S, Novelli R, Perico L, Remuzzi G, Benigni A, et al. Manipulating Sirtuin 3 pathway ameliorates renal damage in experimental diabetes. Sci Rep. 2020;10:8418. doi: 10.1038/s41598-020-65423-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan Y, Yang Q, Yang Y, Gao Z, Ma Y, Zhang L, Liang W, Ding G. Sirt6 suppresses high glucose-induced mitochondrial dysfunction and apoptosis in podocytes through AMPK activation. Int J Biol Sci. 2019;15:701–713. doi: 10.7150/ijbs.29323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clark AJ, Parikh SM. Targeting energy pathways in kidney disease: the roles of sirtuins, AMPK, and PGC1α. Kidney Int. 2021;99:828–840. doi: 10.1016/j.kint.2020.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kärkkäinen O, Tuomainen T, Mutikainen M, Lehtonen M, Ruas JL, Hanhineva K, Tavi P. Heart specific PGC-1α deletion identifies metabolome of cardiac restricted metabolic heart failure. Cardiovasc Res. 2019;115:107–118. doi: 10.1093/cvr/cvy155 [DOI] [PubMed] [Google Scholar]
- 107.Akkafa F, Halil Altiparmak I, Erkus ME, Aksoy N, Kaya C, Ozer A, Sezen H, Oztuzcu S, Koyuncu I, Umurhan B. Reduced SIRT1 expression correlates with enhanced oxidative stress in compensated and decompensated heart failure. Redox Biol. 2015;6:169–173. doi: 10.1016/j.redox.2015.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang S, Fu C, Wang H, Shi Y, Xu X, Chen J, Song X, Sun K, Wang J, Fan X, et al. Polymorphisms of the peroxisome proliferator-activated receptor-gamma coactivator-1alpha gene are associated with hypertrophic cardiomyopathy and not with hypertension hypertrophy. Clin Chem Lab Med. 2007;45:962–967. doi: 10.1515/CCLM.2007.189 [DOI] [PubMed] [Google Scholar]
- 110.Wang CY, Chen CC, Lin MH, Su HT, Ho MY, Yeh JK, Tsai ML, Hsieh IC, Wen MS. TLR9 binding to Beclin 1 and mitochondrial SIRT3 by a sodium-glucose co-transporter 2 inhibitor protects the heart from doxorubicin toxicity. Biology (Basel). 2020;9:369. doi: 10.3390/biology9110369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao Y, Wei J, Hou X, Liu H, Guo F, Zhou Y, Zhang Y, Qu Y, Gu J, Zhou Y, et al. SIRT1 rs10823108 and FOXO1 rs17446614 responsible for genetic susceptibility to diabetic nephropathy. Sci Rep. 2017;7:10285. doi: 10.1038/s41598-017-10612-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shao Y, Ren H, Lv C, Ma X, Wu C, Wang Q. Changes of serum Mir-217 and the correlation with the severity in type 2 diabetes patients with different stages of diabetic kidney disease. Endocrine. 2017;55:130–138. doi: 10.1007/s12020-016-1069-4 [DOI] [PubMed] [Google Scholar]
- 113.Tokarska-Schlattner M, Kay L, Perret P, Isola R, Attia S, Lamarche F, Tellier C, Cottet-Rousselle C, Uneisi A, Hininger-Favier I, et al. Role of cardiac AMP-activated protein kinase in a non-pathological setting: evidence from cardiomyocyte-specific, inducible AMP-activated protein kinase α1α2-knockout mice. Front Cell Dev Biol. 2021;9:731015. doi: 10.3389/fcell.2021.731015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sung MM, Zordoky BN, Bujak AL, Lally JS, Fung D, Young ME, Horman S, Miller EJ, Light PE, Kemp BE, et al. AMPK deficiency in cardiac muscle results in dilated cardiomyopathy in the absence of changes in energy metabolism. Cardiovasc Res. 2015;107:235–245. doi: 10.1093/cvr/cvv166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu D, Ma Z, Di S, Yang Y, Yang J, Xu L, Reiter RJ, Qiao S, Yuan J. AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic Biol Med. 2018;129:59–72. doi: 10.1016/j.freeradbiomed.2018.08.032 [DOI] [PubMed] [Google Scholar]
- 116.Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P, Mao X, Huang K, Xie Z, Zou MH. Hyperglycemia-driven inhibition of AMP-activated protein kinase α2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation. 2019;139:1913–1936. doi: 10.1161/CIRCULATIONAHA.118.033552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nam DH, Kim E, Benham A, Park HK, Soibam B, Taffet GE, Kaelber JT, Suh JH, Taegtmeyer H, Entman ML, et al. Transient activation of AMPK preceding left ventricular pressure overload reduces adverse remodeling and preserves left ventricular function. FASEB J. 2019;33:711–721. doi: 10.1096/fj.201800602R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–4899. doi: 10.1172/JCI66218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Banu K, Lin Q, Basgen JM, Planoutene M, Wei C, Reghuvaran AC, Tian X, Shi H, Garzon F, Garzia A, et al. AMPK mediates regulation of glomerular volume and podocyte survival. JCI Insight. 2021;6:e150004. doi: 10.1172/jci.insight.150004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lim JH, Kim HW, Kim MY, Kim TW, Kim EN, Kim Y, Chung S, Kim YS, Choi BS, Kim YS, et al. Cinacalcet-mediated activation of the CaMKKβ-LKB1-AMPK pathway attenuates diabetic nephropathy in db/db mice by modulation of apoptosis and autophagy. Cell Death Dis. 2018;9:270. doi: 10.1038/s41419-018-0324-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsogbadrakh B, Ryu H, Ju KD, Lee J, Yun S, Yu KS, Kim HJ, Ahn C, Oh KH. AICAR, an AMPK activator, protects against cisplatin-induced acute kidney injury through the JAK/STAT/SOCS pathway. Biochem Biophys Res Commun. 2019;509:680–686. doi: 10.1016/j.bbrc.2018.12.159 [DOI] [PubMed] [Google Scholar]
- 122.Lopez-Sainz A, Dominguez F, Lopes LR, Ochoa JP, Barriales-Villa R, Climent V, Linschoten M, Tiron C, Chiriatti C, Marques N, et al. Clinical features and natural history of PRKAG2 variant cardiac glycogenosis. J Am Coll Cardiol. 2020;76:186–197. doi: 10.1016/j.jacc.2020.05.029 [DOI] [PubMed] [Google Scholar]
- 123.Gorski M, Jung B, Li Y, Matias-Garcia PR, Wuttke M, Coassin S, Thio CHL, Kleber ME, Winkler TW, Wanner V, et al. Meta-analysis uncovers genome-wide significant variants for rapid kidney function decline. Kidney Int. 2021;99:926–939. doi: 10.1016/j.kint.2020.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Zuydam NR, Ahlqvist E, Sandholm N, Deshmukh H, Rayner NW, Abdalla M, Ladenvall C, Ziemek D, Fauman E, Robertson NR, et al. A genome-wide association study of diabetic kidney disease in subjects with type 2 diabetes. Diabetes. 2018;67:1414–1427. doi: 10.2337/db17-0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meijles DN, Zoumpoulidou G, Markou T, Rostron KA, Patel R, Lay K, Handa BS, Wong B, Sugden PH, Clerk A. The cardiomyocyte “redox rheostat”: redox signalling via the AMPK-mTOR axis and regulation of gene and protein expression balancing survival and death. J Mol Cell Cardiol. 2019;129:118–129. doi: 10.1016/j.yjmcc.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ Res. 2014;114:368–378. doi: 10.1161/CIRCRESAHA.113.300536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng H, Fu Y, Huang Y, Zheng X, Yu W, Wang W. mTOR signaling promotes foam cell formation and inhibits foam cell egress through suppressing the SIRT1 signaling pathway. Mol Med Rep. 2017;16:3315–3323. doi: 10.3892/mmr.2017.7032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Y, Li X, He Z, Chen W, Lu J. Rapamycin attenuates palmitate-induced lipid aggregation by up-regulating SIRT-1 signaling in AML12 hepatocytes. Pharmazie. 2016;71:733–737. doi: 10.1691/ph.2016.6695 [DOI] [PubMed] [Google Scholar]
- 130.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of Raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564–1576. doi: 10.1056/NEJMra2022774 [DOI] [PubMed] [Google Scholar]
- 134.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;159;1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu Y, Wan W. Acetylation in the regulation of autophagy. Autophagy. 2022;18:1–9. doi: 10.1080/15548627.2022.2062112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu Y, Nguyen PT, Wang X, Zhao Y, Meacham CE, Zou Z, Bordieanu B, Johanns M, Vertommen D, Wijshake T, et al. TLR9 and beclin 1 crosstalk regulates muscle AMPK activation in exercise. Nature. 2020;578:605–609. doi: 10.1038/s41586-020-1992-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gatica D, Chiong M, Lavandero S, Klionsky DJ. The role of autophagy in cardiovascular pathology. Cardiovasc Res. 2022;118:934–950. doi: 10.1093/cvr/cvab158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen XC, Li ZH, Yang C, Tang JX, Lan HY, Liu HF. Lysosome depletion-triggered autophagy impairment in progressive kidney injury. Kidney Dis (Basel). 2021;7:254–267. doi: 10.1159/000515035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sciarretta S, Maejima Y, Zablocki D, Sadoshima J. The role of autophagy in the heart. Annu Rev Physiol. 2018;80:1–26. doi: 10.1146/annurev-physiol-021317-121427 [DOI] [PubMed] [Google Scholar]
- 143.Nah J, Shirakabe A, Mukai R, Zhai P, Sung EA, Ivessa A, Mizushima W, Nakada Y, Saito T, Hu C, et al. Ulk1-dependent alternative mitophagy plays a protective role during pressure overload in the heart. Cardiovasc Res. 2022;9:cvac003. doi: 10.1093/cvr/cvac003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ghosh R, Gillaspie JJ, Campbell KS, Symons JD, Boudina S, Pattison JS. Chaperone mediated autophagy protects cardiomyocytes against hypoxic-cell death [published online May 18, 2022]. Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00369.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Han YC, Tang SQ, Liu YT, Li AM, Zhan M, Yang M, Song N, Zhang W, Wu XQ, Peng CH, et al. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 2021;12:925. doi: 10.1038/s41419-021-04184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yuan L, Yuan Y, Liu F, Li L, Liu J, Chen Y, Cheng J, Lu Y. PGC-1α alleviates mitochondrial dysfunction via TFEB-mediated autophagy in cisplatin-induced acute kidney injury. Aging (Albany NY). 2021;13:8421–8439. doi: 10.18632/aging.202653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kanamori H, Yoshida A, Naruse G, Endo S, Minatoguchi S, Watanabe T, Kawaguchi T, Tanaka T, Yamada Y, Takasugi N, et al. Impact of autophagy on prognosis of patients with dilated cardiomyopathy. J Am Coll Cardiol. 2022;79:789–801. doi: 10.1016/j.jacc.2021.11.059 [DOI] [PubMed] [Google Scholar]
- 148.Nandi SS, Duryee MJ, Shahshahan HR, Thiele GM, Anderson DR, Mishra PK. Induction of autophagy markers is associated with attenuation of miR-133a in diabetic heart failure patients undergoing mechanical unloading. Am J Transl Res. 2015;7:683–696. [PMC free article] [PubMed] [Google Scholar]
- 149.Yan H, Du D, Wang C, Tian M. Downregulation of autophagy-related circular RNA (ACR) is correlated with poor survival of patients with chronic heart failure. Bioengineered. 2022;13:13141–13149. doi: 10.1080/21655979.2022.2059862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saito T, Asai K, Sato S, Hayashi M, Adachi A, Sasaki Y, Takano H, Mizuno K, Shimizu W. Autophagic vacuoles in cardiomyocytes of dilated cardiomyopathy with initially decompensated heart failure predict improved prognosis. Autophagy. 2016;12:579–587. doi: 10.1080/15548627.2016.1145326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu WJ, Shen TT, Chen RH, Wu HL, Wang YJ, Deng JK, Chen QH, Pan Q, Huang Fu CM, Tao JL, et al. Autophagy-lysosome pathway in renal tubular epithelial cells is disrupted by advanced glycation end products in diabetic nephropathy. J Biol Chem. 2015;290:20499–20510. doi: 10.1074/jbc.M115.666354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gui Z, Suo C, Wang Z, Zheng M, Fei S, Chen H, Sun L, Han Z, Tao J, Ju X, et al. Impaired ATG16L-dependent autophagy promotes renal interstitial fibrosis in chronic renal graft dysfunction through inducing EndMT by NF-κB signal pathway. Front Immunol. 2021;12:650424. doi: 10.3389/fimmu.2021.650424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeng C, Fan Y, Wu J, Shi S, Chen Z, Zhong Y, Zhang C, Zen K, Liu Z. Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. J Pathol. 2014;234:203–213. doi: 10.1002/path.4382 [DOI] [PubMed] [Google Scholar]
- 154.da Silva CA, Monteiro MLGdR, Araújo LS, Urzedo MG, Rocha LB, Dos Reis MA, Machado JR. In situ evaluation of podocytes in patients with focal segmental glomerulosclerosis and minimal change disease. PLoS One. 2020;15:e0241745. doi: 10.1371/journal.pone.0241745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xu C, Wang W, Zhong J, Lei F, Xu N, Zhang Y, Xie W. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem Pharmacol. 2018;152:45–59. doi: 10.1016/j.bcp.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 156.Mizuno M, Kuno A, Yano T, Miki T, Oshima H, Sato T, Nakata K, Kimura Y, Tanno M, Miura T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6:e13741. doi: 10.14814/phy2.13741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aragón-Herrera A, Feijóo-Bandín S, Otero Santiago M, Barral L, Campos-Toimil M, Gil-Longo J, Costa Pereira TM, García-Caballero T, Rodríguez-Segade S, Rodríguez J, et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol. 2019;170:113677. doi: 10.1016/j.bcp.2019.113677 [DOI] [PubMed] [Google Scholar]
- 158.Fukushima K, Kitamura S, Tsuji K, Sang Y, Wada J. Sodium glucose co-transporter 2 inhibitor ameliorates autophagic flux impairment on renal proximal tubular cells in obesity mice. Int J Mol Sci. 2020;21:4054. doi: 10.3390/ijms21114054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Korbut AI, Taskaeva IS, Bgatova NP, Muraleva NA, Orlov NB, Dashkin MV, Khotskina AS, Zavyalov EL, Konenkov VI, Klein T, et al. SGLT2 inhibitor empagliflozin and dpp4 inhibitor linagliptin reactivate glomerular autophagy in db/db mice, a model of type 2 diabetes. Int J Mol Sci. 2020;21:2987. doi: 10.3390/ijms21082987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arab HH, Al-Shorbagy MY, Saad MA. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem Biol Interact. 2021;335:109368. doi: 10.1016/j.cbi.2021.109368 [DOI] [PubMed] [Google Scholar]
- 161.Jaikumkao K, Promsan S, Thongnak L, Swe MT, Tapanya M, Htun KT, Kothan S, Intachai N, Lungkaphin A. Dapagliflozin ameliorates pancreatic injury and activates kidney autophagy by modulating the AMPK/mTOR signaling pathway in obese rats. J Cell Physiol. 2021;236:6424–6440. doi: 10.1002/jcp.30316 [DOI] [PubMed] [Google Scholar]
- 162.Li L, Li Q, Huang W, Han Y, Tan H, An M, Xiang Q, Zhou R, Yang L, Cheng Y. Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front Pharmacol. 2021;12:589273. doi: 10.3389/fphar.2021.589273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meng Z, Liu X, Li T, Fang T, Cheng Y, Han L, Sun B, Chen L. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int Immunopharmacol. 2021;94:107492. doi: 10.1016/j.intimp.2021.107492 [DOI] [PubMed] [Google Scholar]
- 164.Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, et al. Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in high fat diet fed ApoE(-/-) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci. 2021;22:818. doi: 10.3390/ijms22020818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ren C, Sun K, Zhang Y, Hu Y, Hu B, Zhao J, He Z, Ding R, Wang W, Liang C. Sodium-glucose cotransporter-2 inhibitor empagliflozin ameliorates sunitinib-induced cardiac dysfunction via regulation of AMPK-mTOR signaling pathway-mediated autophagy. Front Pharmacol. 2021;12:664181. doi: 10.3389/fphar.2021.664181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu J, Kitada M, Ogura Y, Liu H, Koya D. Dapagliflozin restores impaired autophagy and suppresses inflammation in high glucose-treated HK-2 cells. Cells. 2021;10:1457. doi: 10.3390/cells10061457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ala M, Khoshdel MRF, Dehpour AR. Empagliflozin enhances autophagy, mitochondrial biogenesis, and antioxidant defense and ameliorates renal ischemia/reperfusion in nondiabetic rats. Oxid Med Cell Longev. 2022;2022:1197061. doi: 10.1155/2022/1197061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cai C, Guo Z, Chang X, Li Z, Wu F, He J, Cao T, Wang K, Shi N, Zhou H, et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKα1/ULK1/FUNDC1/mitophagy pathway. Redox Biol. 2022;52:102288. doi: 10.1016/j.redox.2022.102288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li X, Flynn ER, do Carmo JM, Wang Z, da Silva AA, Mouton AJ, Omoto ACM, Hall ME, Hall JE. Direct cardiac actions of sodium-glucose cotransporter 2 inhibition improve mitochondrial function and attenuate oxidative stress in pressure overload-induced heart failure. Front Cardiovasc Med. 2022;9:859253. doi: 10.3389/fcvm.2022.859253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Park CH, Lee B, Han M, Rhee WJ, Kwak MS, Yoo TH, Shin JS. Canagliflozin protects against cisplatin-induced acute kidney injury by AMPK-mediated autophagy in renal proximal tubular cells. Cell Death Discov. 2022;8:12. doi: 10.1038/s41420-021-00801-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang L, Liang B, Li J, Zhang X, Chen H, Sun J, Zhang Z. Dapagliflozin alleviates advanced glycation end product induced podocyte injury through AMPK/mTOR mediated autophagy pathway. Cell Signal. 2022;90:110206. doi: 10.1016/j.cellsig.2021.110206 [DOI] [PubMed] [Google Scholar]
- 172.Xu Z, Hu W, Wang B, Xu T, Wang J, Wei D. Canagliflozin ameliorates nonalcoholic fatty liver disease by regulating lipid metabolism and inhibiting inflammation through induction of autophagy. Yonsei Med J. 2022;63:619–631. doi: 10.3349/ymj.2022.63.7.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yu YW, Que JQ, Liu S, Huang KY, Qian L, Weng YB, Rong FN, Wang L, Zhou YY, Xue YJ, et al. Sodium-glucose co-transporter-2 inhibitor of dapagliflozin attenuates myocardial ischemia/reperfusion injury by limiting NLRP3 inflammasome activation and modulating autophagy. Front Cardiovasc Med. 2022;8:768214. doi: 10.3389/fcvm.2021.768214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oshima H, Miki T, Kuno A, Mizuno M, Sato T, Tanno M, Yano T, Nakata K, Kimura Y, Abe K, et al. Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther. 2019;368:524–534. doi: 10.1124/jpet.118.253666 [DOI] [PubMed] [Google Scholar]
- 175.Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. doi: 10.1186/s12933-019-0964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ren FF, Xie ZY, Jiang YN, Guan X, Chen QY, Lai TF, Li L. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress [published online December 1, 2021]. Acta Pharmacol Sin. doi: 10.1038/s41401-021-00805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, Paccone A, Altucci L, Conte M, Canale ML, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20:150. doi: 10.1186/s12933-021-01346-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ke Q, Shi C, Lv Y, Wang L, Luo J, Jiang L, Yang J, Zhou Y. SGLT2 inhibitor counteracts NLRP3 inflammasome via tubular metabolite itaconate in fibrosis kidney. FASEB J. 2022;36:e22078. doi: 10.1096/fj.202100909RR [DOI] [PubMed] [Google Scholar]
- 179.Tian G, Yu Y, Deng H, Yang L, Shi X, Yu B. Empagliflozin alleviates ethanol-induced cardiomyocyte injury through inhibition of mitochondrial apoptosis via a SIRT1/PTEN/Akt pathway. Clin Exp Pharmacol Physiol. 2021;48:837–845. doi: 10.1111/1440-1681.13470 [DOI] [PubMed] [Google Scholar]
- 180.Ye Y, Jia X, Bajaj M, Birnbaum Y. Dapagliflozin attenuates Na+/H+ exchanger-1 in cardiofibroblasts via AMPK activation. Cardiovasc Drugs Ther. 2018;32:553–558. doi: 10.1007/s10557-018-6837-3 [DOI] [PubMed] [Google Scholar]
- 181.Koyani CN, Plastira I, Sourij H, Hallström S, Schmidt A, Rainer PP, Bugger H, Frank S, Malle E, von Lewinski D. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol Res. 2020;158:104870. doi: 10.1016/j.phrs.2020.104870 [DOI] [PubMed] [Google Scholar]
- 182.Lu Q, Liu J, Li X, Sun X, Zhang J, Ren D, Tong N, Li J. Empagliflozin attenuates ischemia and reperfusion injury through LKB1/AMPK signaling pathway. Mol Cell Endocrinol. 2020;501:110642. doi: 10.1016/j.mce.2019.110642 [DOI] [PubMed] [Google Scholar]
- 183.Radlinger B, Hornsteiner F, Folie S, Salvenmoser W, Haubner BJ, Schuetz T, Haas S, Ress C, Adolph TE, Salzmann K, et al. Cardioprotective effects of short-term empagliflozin treatment in db/db mice. Sci Rep. 2020;10:19686. doi: 10.1038/s41598-020-76698-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kondo H, Akoumianakis I, Badi I, Akawi N, Kotanidis CP, Polkinghorne M, Stadiotti I, Sommariva E, Antonopoulos AS, Carena MC, et al. Effects of canagliflozin on human myocardial redox signalling: clinical implications. Eur Heart J. 2021;42:4947–4960. doi: 10.1093/eurheartj/ehab420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee CC, Chen WT, Chen SY, Lee TM. Dapagliflozin attenuates arrhythmic vulnerabilities by regulating connexin43 expression via the AMPK pathway in post-infarcted rat hearts. Biochem Pharmacol. 2021;192:114674. doi: 10.1016/j.bcp.2021.114674 [DOI] [PubMed] [Google Scholar]
- 186.Liu Y, Wu M, Xu J, Xu B, Kang L. Empagliflozin prevents from early cardiac injury post myocardial infarction in non-diabetic mice. Eur J Pharm Sci. 2021;161:105788. doi: 10.1016/j.ejps.2021.105788 [DOI] [PubMed] [Google Scholar]
- 187.Sun P, Wang Y, Ding Y, Luo J, Zhong J, Xu N, Zhang Y, Xie W. Canagliflozin attenuates lipotoxicity in cardiomyocytes and protects diabetic mouse hearts by inhibiting the mTOR/HIF-1α pathway. iScience. 2021;24:102521. doi: 10.1016/j.isci.2021.102521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tsai KL, Hsieh PL, Chou WC, Cheng HC, Huang YT, Chan SH. Dapagliflozin attenuates hypoxia/reoxygenation-caused cardiac dysfunction and oxidative damage through modulation of AMPK. Cell Biosci. 2021;11:44. doi: 10.1186/s13578-021-00547-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.He L, Ma S, Zuo Q, Zhang G, Wang Z, Zhang T, Zhai J, Guo Y. An effective sodium-dependent glucose transporter 2 inhibition, canagliflozin, prevents development of hypertensive heart failure in dahl salt-sensitive rats. Front Pharmacol. 2022;13:856386. doi: 10.3389/fphar.2022.856386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Moellmann J, Mann PA, Kappel BA, Kahles F, Klinkhammer BM, Boor P, Kramann R, Ghesquiere B, Lebherz C, Marx N, et al. The sodium-glucose co-transporter-2 inhibitor ertugliflozin modifies the signature of cardiac substrate metabolism and reduces cardiac mTOR signalling, endoplasmic reticulum stress and apoptosis [published online July 8, 2022]. Diabetes Obes Metab. doi: 10.1111/dom.14814 [DOI] [PubMed] [Google Scholar]
- 191.Mancini SJ, Boyd D, Katwan OJ, Strembitska A, Almabrouk TA, Kennedy S, Palmer TM, Salt IP. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep. 2018;8:5276. doi: 10.1038/s41598-018-23420-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. doi: 10.1016/j.redox.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.D’Onofrio N, Sardu C, Trotta MC, Scisciola L, Turriziani F, Ferraraccio F, Panarese I, Petrella L, Fanelli M, Modugno P, et al. Sodium-glucose co-transporter2 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of sodium-glucose co-transporter2 inhibitor treatment. Mol Metab. 2021;54:101337. doi: 10.1016/j.molmet.2021.101337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tian J, Zhang M, Suo M, Liu D, Wang X, Liu M, Pan J, Jin T, An F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J Cell Mol Med. 2021;25:7642–7659. doi: 10.1111/jcmm.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Uthman L, Kuschma M, Römer G, Boomsma M, Kessler J, Hermanides J, Hollmann MW, Preckel B, Zuurbier CJ, Weber NC. Novel anti-inflammatory effects of canagliflozin involving hexokinase II in lipopolysaccharide-stimulated human coronary artery endothelial cells. Cardiovasc Drugs Ther. 2021;35:1083–1094. doi: 10.1007/s10557-020-07083-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Faridvand Y, Kazemzadeh H, Vahedian V, Mirzajanzadeh P, Nejabati HR, Safaie N, Maroufi NF, Pezeshkian M, Nouri M, Jodati A. Dapagliflozin attenuates high glucose-induced endothelial cell apoptosis and inflammation through AMPK/SIRT1 activation. Clin Exp Pharmacol Physiol. 2022;49:643–651. doi: 10.1111/1440-1681.13638 [DOI] [PubMed] [Google Scholar]
- 197.He L, Li Y, Zhang D, Song H, Xu D, Song Z. Dapagliflozin improves endothelial cell dysfunction by regulating mitochondrial production via the SIRT1/PGC-1α pathway in obese mice. Biochem Biophys Res Commun. 2022;615:123–130. doi: 10.1016/j.bbrc.2022.05.022 [DOI] [PubMed] [Google Scholar]
- 198.Chang YK, Choi H, Jeong JY, Na KR, Lee KW, Lim BJ, Choi DE. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One. 2016;11:e0158810. doi: 10.1371/journal.pone.0158810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Birnbaum Y, Bajaj M, Yang HC, Ye Y. Combined SGLT2 and DPP4 inhibition reduces the activation of the NLRP3/ASC inflammasome and attenuates the development of diabetic nephropathy in mice with type 2 diabetes. Cardiovasc Drugs Ther. 2018;32:135–145. doi: 10.1007/s10557-018-6778-x [DOI] [PubMed] [Google Scholar]
- 200.Inoue MK, Matsunaga Y, Nakatsu Y, Yamamotoya T, Ueda K, Kushiyama A, Sakoda H, Fujishiro M, Ono H, Iwashita M, et al. Possible involvement of normalized Pin1 expression level and AMPK activation in the molecular mechanisms underlying renal protective effects of SGLT2 inhibitors in mice. Diabetol Metab Syndr. 2019;11:57. doi: 10.1186/s13098-019-0454-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim JH, Ko HY, Wang HJ, Lee H, Yun M, Kang ES. Effect of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on gluconeogenesis in proximal renal tubules. Diabetes Obes Metab. 2020;22:373–382. doi: 10.1111/dom.13905 [DOI] [PubMed] [Google Scholar]
- 202.Li J, Liu H, Takagi S, Nitta K, Kitada M, Srivastava SP, Takagaki Y, Kanasaki K, Koya D. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight. 2020;5:e129034. doi: 10.1172/jci.insight.129034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu X, Xu C, Xu L, Li X, Sun H, Xue M, Li T, Yu X, Sun B, Chen L. Empagliflozin improves diabetic renal tubular injury by alleviating mitochondrial fission via AMPK/SP1/PGAM5 pathway. Metabolism. 2020;111:154334. doi: 10.1016/j.metabol.2020.154334 [DOI] [PubMed] [Google Scholar]
- 204.Mohamed HE, Asker ME, Keshawy MM, Hasan RA, Mahmoud YK. Inhibition of tumor necrosis factor-α enhanced the antifibrotic effect of empagliflozin in an animal model with renal insulin resistance. Mol Cell Biochem. 2020;466:45–54. doi: 10.1007/s11010-020-03686-x [DOI] [PubMed] [Google Scholar]
- 205.Zhang Z, Ni L, Zhang L, Zha D, Hu C, Zhang L, Feng H, Wei X, Wu X. Empagliflozin regulates the AdipoR1/p-AMPK/p-ACC pathway to alleviate lipid deposition in diabetic nephropathy. Diabetes Metab Syndr Obes. 2021;14:227–240. doi: 10.2147/DMSO.S289712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chi PJ, Lee CJ, Hsieh YJ, Lu CW, Hsu BG. Dapagliflozin ameliorates lipopolysaccharide related acute kidney injury in mice with streptozotocin-induced diabetes mellitus. Int J Med Sci. 2022;19:729–739. doi: 10.7150/ijms.69031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhai R, Liu Y, Tong J, Yu Y, Yang L, Gu Y, Niu J. Empagliflozin ameliorates preeclampsia and reduces postpartum susceptibility to adriamycin in a mouse model induced by angiotensin receptor agonistic autoantibodies. Front Pharmacol. 2022;13:826792. doi: 10.3389/fphar.2022.826792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65:2784–2794. doi: 10.2337/db16-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, Mayoux E, Kaneko S, Ota T. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–149. doi: 10.1016/j.ebiom.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, Dreyfuss JM, Pan H, Tangcharoenpaisan Y, Morningstar J, et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight. 2019;4:e123130. doi: 10.1172/jci.insight.123130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chiang H, Lee JC, Huang HC, Huang H, Liu HK, Huang C. Delayed intervention with a novel SGLT2 inhibitor NGI001 suppresses diet-induced metabolic dysfunction and non-alcoholic fatty liver disease in mice. Br J Pharmacol. 2020;177:239–253. doi: 10.1111/bph.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Suga T, Sato K, Ohyama T, Matsui S, Kobayashi T, Tojima H, Horiguchi N, Yamazaki Y, Kakizaki S, Nishikido A, et al. Ipragliflozin-induced improvement of liver steatosis in obese mice may involve sirtuin signaling. World J Hepatol. 2020;12:350–362. doi: 10.4254/wjh.v12.i7.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wei D, Liao L, Wang H, Zhang W, Wang T, Xu Z. Canagliflozin ameliorates obesity by improving mitochondrial function and fatty acid oxidation via PPARα in vivo and in vitro. Life Sci. 2020;247:117414. doi: 10.1016/j.lfs.2020.117414 [DOI] [PubMed] [Google Scholar]
- 214.Yang X, Liu Q, Li Y, Tang Q, Wu T, Chen L, Pu S, Zhao Y, Zhang G, Huang C, et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte. 2020;9:484–494. doi: 10.1080/21623945.2020.1807850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen H, Birnbaum Y, Ye R, Yang HC, Bajaj M, Ye Y. SGLT2 inhibition by dapagliflozin attenuates diabetic ketoacidosis in mice with type-1 diabetes [published online August 27, 2021]. Cardiovasc Drugs Ther. doi: 10.1007/s10557-021-07243-6 [DOI] [PubMed] [Google Scholar]
- 216.Lee JY, Lee M, Lee JY, Bae J, Shin E, Lee YH, Lee BW, Kang ES, Cha BS. Ipragliflozin, an SGLT2 inhibitor, ameliorates high-fat diet-induced metabolic changes by upregulating energy expenditure through activation of the AMPK/ SIRT1 pathway. Diabetes Metab J. 2021;45:921–932. doi: 10.4093/dmj.2020.0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Luo J, Sun P, Wang Y, Chen Y, Niu Y, Ding Y, Xu N, Zhang Y, Xie W. Dapagliflozin attenuates steatosis in livers of high-fat diet-induced mice and oleic acid-treated L02 cells via regulating AMPK/mTOR pathway. Eur J Pharmacol. 2021;907:174304. doi: 10.1016/j.ejphar.2021.174304 [DOI] [PubMed] [Google Scholar]
- 218.Xu L, Xu C, Liu X, Li X, Li T, Yu X, Xue M, Yang J, Kosmas CE, Moris D, et al. Empagliflozin induces white adipocyte browning and modulates mitochondrial dynamics in KK Cg-Ay/J mice and mouse adipocytes. Front Physiol. 2021;12:745058. doi: 10.3389/fphys.2021.745058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yang X, Liu Q, Li Y, Ding Y, Zhao Y, Tang Q, Wu T, Chen L, Pu S, Cheng S, et al. Inhibition of the sodium-glucose co-transporter SGLT2 by canagliflozin ameliorates diet-induced obesity by increasing intra-adipose sympathetic innervation. Br J Pharmacol. 2021;178:1756–1771. doi: 10.1111/bph.15381 [DOI] [PubMed] [Google Scholar]
- 220.Thirunavukarasu S, Jex N, Chowdhary A, Hassan IU, Straw S, Craven TP, Gorecka M, Broadbent D, Swoboda P, Witte KK, et al. Empagliflozin treatment is associated with improvements in cardiac energetics and function and reductions in myocardial cellular volume in patients with type 2 diabetes. Diabetes. 2021;70:2810–2822. doi: 10.2337/db21-0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354 [DOI] [PubMed] [Google Scholar]
- 222.Wolf P, Fellinger P, Pfleger L, Beiglböck H, Krumpolec P, Barbieri C, Gastaldelli A, Harreiter J, Metz M, Scherer T, et al. Gluconeogenesis, but not glycogenolysis, contributes to the increase in endogenous glucose production by SGLT-2 inhibition. Diabetes Care. 2021;44:541–548. doi: 10.2337/dc20-1983 [DOI] [PubMed] [Google Scholar]
- 223.Hirschey MD, Shimazu T, Capra JA, Pollard KS, Verdin E. SIRT1 and SIRT3 deacetylate homologous substrates: AceCS1,2 and HMGCS1,2. Aging (Albany NY). 2011;3:635–642. doi: 10.18632/aging.100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen R, Xu M, Hogg RT, Li J, Little B, Gerard RD, Garcia JA. The acetylase/deacetylase couple CREB-binding protein/Sirtuin 1 controls hypoxia-inducible factor 2 signaling. J Biol Chem. 2012;287:30800–30811. doi: 10.1074/jbc.M111.244780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang J, Zhu XX, Liu L, Xue Y, Yang X, Zou HJ. SIRT1 prevents hyperuricemia via the PGC-1α/PPARγ-ABCG2 pathway. Endocrine. 2016;53:443–452. doi: 10.1007/s12020-016-0896-7 [DOI] [PubMed] [Google Scholar]
- 226.Huang DY, Gao H, Boini KM, Osswald H, Nürnberg B, Lang F. In vivo stimulation of AMP-activated protein kinase enhanced tubuloglomerular feedback but reduced tubular sodium transport during high dietary NaCl intake. Pflugers Arch. 2010;460:187–196. doi: 10.1007/s00424-010-0803-7 [DOI] [PubMed] [Google Scholar]
- 227.Hasegawa K. Novel tubular-glomerular interplay in diabetic kidney disease mediated by sirtuin 1, nicotinamide mononucleotide, and nicotinamide adenine dinucleotide: Oshima Award Address 2017. Clin Exp Nephrol. 2019;23:987–994. doi: 10.1007/s10157-019-01719-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zannad F, Ferreira JP, Butler J, Filippatos G, Januzzi JL, Sumin M, Zwick M, Saadati M, Pocock SJ, Sattar N, et al. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights from the EMPEROR program [published online August 26, 2022]. Eur Heart J. doi: 10.1093/eurheartj/ehac495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gan L, Han Y, Bastianetto S, Dumont Y, Unterman TG, Quirion R. FoxO-dependent and -independent mechanisms mediate SirT1 effects on IGFBP-1 gene expression. Biochem Biophys Res Commun. 2005;337:1092–1096. doi: 10.1016/j.bbrc.2005.09.169 [DOI] [PubMed] [Google Scholar]
- 230.Crane FL, Navas P, Low H, Sun IL, de Cabo R. Sirtuin activation: a role for plasma membrane in the cell growth puzzle. J Gerontol A Biol Sci Med Sci. 2013;68:368–370. doi: 10.1093/gerona/gls184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Josephrajan A, Hertzel AV, Bohm EK, McBurney MW, Imai SI, Mashek DG, Kim DH, Bernlohr DA. Unconventional secretion of adipocyte fatty acid binding protein 4 is mediated by autophagic proteins in a sirtuin-1-dependent manner. Diabetes. 2019;68:1767–1777. doi: 10.2337/db18-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ferrannini E, Murthy AC, Lee YH, Muscelli E, Weiss S, Ostroff RM, Sattar N, Williams SA, Ganz P. Mechanisms of sodium-glucose cotransporter 2 inhibition: insights from large-scale proteomics. Diabetes Care. 2020;43:2183–2189. doi: 10.2337/dc20-0456 [DOI] [PubMed] [Google Scholar]
- 233.Thiele K, Rau M, Hartmann NK, Möllmann J, Jankowski J, Böhm M, Keszei AP, Marx N, Lehrke M. Effects of empagliflozin on erythropoiesis in patients with type 2 diabetes: data from a randomized, placebo-controlled study. Diabetes Obes Metab. 2021;23:2814–2818. doi: 10.1111/dom.14517 [DOI] [PubMed] [Google Scholar]
- 234.Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, Chung KW, Kim SM, Im DS, Chung HY. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7:66444–66454. doi: 10.18632/oncotarget.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jung J, Park WY, Kim YJ, Kim M, Choe M, Jin K, Seo JH, Ha E. 3-hydroxybutyrate ameliorates the progression of diabetic nephropathy. Antioxidants (Basel). 2022;11:381. doi: 10.3390/antiox11020381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yu Y, Yu Y, Zhang Y, Zhang Z, An W, Zhao X. Treatment with D-β-hydroxybutyrate protects heart from ischemia/reperfusion injury in mice. Eur J Pharmacol. 2018;829:121–128. doi: 10.1016/j.ejphar.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 237.Kim DH, Park MH, Ha S, Bang EJ, Lee Y, Lee AK, Lee J, Yu BP, Chung HY. Anti-inflammatory action of β-hydroxybutyrate via modulation of PGC-1α and FoxO1, mimicking calorie restriction. Aging (Albany NY). 2019;11:1283–1304. doi: 10.18632/aging.101838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nakamura M, Odanovic N, Nakada Y, Dohi S, Zhai P, Ivessa A, Yang Z, Abdellatif M, Sadoshima J. Dietary carbohydrates restriction inhibits the development of cardiac hypertrophy and heart failure. Cardiovasc Res. 2021;117:2365–2376. doi: 10.1093/cvr/cvaa298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mikami D, Kobayashi M, Uwada J, Yazawa T, Kamiyama K, Nishimori K, Nishikawa Y, Morikawa Y, Yokoi S, Takahashi N, et al. β-Hydroxybutyrate, a ketone body, reduces the cytotoxic effect of cisplatin via activation of HDAC5 in human renal cortical epithelial cells. Life Sci. 2019;222:125–132. doi: 10.1016/j.lfs.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 240.Byrne NJ, Soni S, Takahara S, Ferdaoussi M, Al Batran R, Darwesh AM, Levasseur JL, Beker D, Vos DY, Schmidt MA, et al. Chronically elevating circulating ketones can reduce cardiac inflammation and blunt the development of heart failure. Circ Heart Fail. 2020;13:e006573. doi: 10.1161/CIRCHEARTFAILURE.119.006573 [DOI] [PubMed] [Google Scholar]
- 241.Sokolov V, Yakovleva T, Chu L, Tang W, Greasley PJ, Johansson S, Peskov K, Helmlinger G, Boulton DW, Penland RC. Differentiating the sodium-glucose cotransporter 1 inhibition capacity of canagliflozin vs. dapagliflozin and empagliflozin using quantitative systems pharmacology modeling. CPT Pharmacometrics Syst Pharmacol. 2020;9:222–229. doi: 10.1002/psp4.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, Du F, Liu Y, Xu J, Conway B, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One. 2012;7:e30555. doi: 10.1371/journal.pone.0030555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Sag CM, Lebek S, Tarnowski D, Reinders J, Perbellini F, et al. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail. 2018;5:642–648. doi: 10.1002/ehf2.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hu Y-X, Han X-S, Jing Q. Ca(2+) ion and autophagy. Adv Exp Med Biol. 2019;1206:151–166. doi: 10.1007/978-981-15-0602-4_7 [DOI] [PubMed] [Google Scholar]
- 245.Ying Y, Jiang C, Zhang M, Jin J, Ge S, Wang X. Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging (Albany NY). 2019;11:2822–2835. doi: 10.18632/aging.101954 [DOI] [PMC free article] [PubMed] [Google Scholar]