Abstract
Background
To assess treatment response, objective measures are superior to clinical improvement in Crohn’s disease [CD]. Intestinal ultrasound [IUS] is an attractive, non-invasive alternative to endoscopy, demonstrating early transmural changes after treatment initiation. Therefore, we investigated IUS and contrast-enhanced ultrasound [CEUS] to predict [early] endoscopic treatment response.
Methods
Consecutive patients with endoscopically active CD, starting anti-TNFα therapy, were included. Clinical, biochemical, IUS, and CEUS parameters at baseline [T0], after 4–8 weeks [T1] and 12–34 weeks [T2] were collected. The most severely inflamed segment at endoscopy (highest segmental Simplified Endoscopic Score for Crohn’s Disease [SES-CD]) and IUS (highest segmental bowel wall thickness [BWT]) was identified. At T2, endoscopic response [decrease in SES-CD ≥ 50%] and remission [SES-CD = 0] were scored.
Results
A total of 40 patients were included: 14 reached endoscopic remission and 17 endoscopic response. At T1 (3.1 mm [1.9–4.2] vs 5.3 mm [3.8–6.9], p = 0.005) and T2 (2.0 mm [1.8–3.1] vs 5.1 [3.0–6.3] mm, p = 0.002) BWT was lower in patients with endoscopic remission. At T1 and T2, 18% (area under the receiver operating curve [AUROC]: 0.77; odds ratio [OR]: 10.80, p = 0.012) and 29% [AUROC: 0.833; OR: 37.50, p = 0.006] BWT decrease predicted endoscopic response, respectively. To determine endoscopic remission, BWT 3.2 mm was most accurate [AUROC: 0.94; OR: 39.42, p < 0.0001] at T2. In addition, absence of colour Doppler signal [OR: 13.76, p = 0.03] and the CEUS parameter wash-out rate [OR: 0.76, p = 0.019] improved the prediction model.
Conclusions
Reduction in BWT, already after 4–8 weeks of follow-up, predicted endoscopic response and remission. CEUS parameters were of limited value. Furthermore, we have provided accurate cut-offs for BWT reflecting endoscopic response and remission at different time points.
Keywords: Non-invasive imaging, inflammatory bowel disease, close monitoring, transmural healing
1. Introduction
Crohn’s disease [CD] is a chronic inflammatory disease that can affect the complete gastrointestinal [GI] tract. It is characterised by a relapsing-remitting pattern, often with an onset in adolescence.1 In the treatment of CD, close monitoring in a treat-to-target setting is a key principle to prevent relapse and complications.2 Although the presence of clinical symptoms might reflect active inflammation, clinical scoring indices show poor correlation with the true state of disease activity; hence, other objective measures are needed.2
Endoscopy has become the gold standard to objectify active inflammation.1,2 However, it is invasive, expensive, and not without risks.3,4 Consequently, it is an unattractive tool for frequent monitoring. Alternatively, non-invasive biochemical markers such as C-reactive protein [CRP] and faecal calprotectin [FCP] are being used and are theoretically attractive. However, they lack ability to determine disease location, severity, and extent of disease activity, and are not always accurate.5,6
Intestinal ultrasound [IUS] is a promising, non-invasive, cross-sectional imaging technique that has a low cost and high accessibility. Previous studies showed high accuracy for IUS to detect disease activity, severity, and extent when compared with endoscopy or magnetic resonance imaging [MRI].7–9 Furthermore, reliability is high among different operators.10 Predominantly bowel wall thickness [BWT] combined with colour Doppler signal [CDS] indicates presence of disease activity in most patients. Multiple cross-sectional studies have confirmed these findings.8,11 So far, studies assessing the capability of IUS to measure change [ie. responsiveness] after initiation of treatment in CD using IUS are limited, particularly with endoscopy as the reference standard.
In addition to B-mode and Doppler parameters, contrast-enhanced ultrasound [CEUS] has been investigated.12–15 Inflammation in CD leads to increased microvessel density and a local dysregulation of blood flow in the GI wall.16–18 This causes changes in the bowel wall perfusion, which can be quantified with CEUS. Previous studies have shown a role for CEUS in determining disease activity at endoscopy and furthermore in predicting endoscopic response and remission in an early phase.12–14 However, data are conflicting and limited.
In this study, we aimed to investigate conventional IUS and CEUS parameters to predict endoscopic treatment response and remission early after treatment initiation. In addition, we aimed to determine cut-off values for IUS and CEUS parameters to reflect endoscopic endpoints.
2. Materials and Methods
2.1. Study design
This was a single-centre, longitudinal, prospective, cohort study. Patients ≥ 18 years of age with active CD at endoscopy (Simple Endoscopic Score for Crohn’s Disease [SES-CD] ≥ 3 in at least one segment), starting treatment with TNFα inhibitors [adalimumab or infliximab], were eligible for inclusion.
Patients were excluded when there was no endoscopy performed at the start of treatment or when treatment was changed between baseline endoscopy and IUS examination. Previous TNF-α inhibitor use, pregnancy, obesity (body mass index [BMI] > 35 kg/m2), chronic obstructive lung disease, unstable heart disease, ongoing gastroenteritis, or a previous allergic reaction to SonoVue or its components, were also exclusion criteria. In addition, patients were excluded when there was no thickened bowel segment at IUS or endoscopy did not show at least aphthous ulcers. All patients were informed and gave informed consent. This study was approved by the medical ethical committee of the Amsterdam University Medical Center [MEC2015_359].
2.2. Procedures
Medical history and demographics were collected at baseline. At start of treatment [T0], after 4–8 weeks [T1], and after 12–34 weeks [T2], the Harvey–Bradshaw Index [HBI] score, C-reactive protein [CRP], albumin, haemoglobin, leukocyte count, thrombocyte count, and faecal calprotectin [FCP] levels were collected and IUS with CEUS was performed. T1 was considered an early time point. At T0 and T2, a complete ileocolonoscopy was performed. IUS/CEUS and endoscopy were performed on separate days, avoiding oedema or bowel preparation as a consequence of endoscopy. At T0, anti-TNFα treatment was also initiated.
2.3. Objectives
The primary objective was to study the difference in BWT at T1 between patients with endoscopic remission and no remission at T2. Secondary objectives at T1 and T2 were differences in BWT, other IUS parameters, and CEUS parameters, between patients reaching endoscopic remission or response and no remission or response, respectively. Furthermore, we aimed to determine accurate cut-off values at T1 and T2 for IUS and CEUS parameters, to predict or determine endoscopic remission and response.
2.4. Intestinal ultrasound measurements
All the IUS examinations were performed by one of three trained ultrasonographers [KN, SB, and FV] using an Epiq 5G ultrasound scanner [Philips, The Netherlands] with a C5-1 convex and L12-5 linear probe. Frequency, focus, and gain settings were optimised to get the best images of the patient. For CDS, the L12-5 transducer was used with a velocity scale of 5 cm/s for registration of the slow flow in the GI wall. The terminal ileum [TI] and large intestines were scanned by following their course from the TI in the right lower quadrant to the rectum, and the small intestine was examined by scanning systematically through the nine sectors of the abdomen. Images and cine-loops of pathological segments were stored per segment. At the time of IUS, the sonographer was blinded to biochemical and endoscopic disease activity information.
At least 3 months after IUS examination and blinded to all other patient data and each other’s data, two raters [SB: 5 years of IUS experience and FV: 3 years of IUS experience] independently scored all IUS parameters per segment [Table 1] using a DICOM-viewer (RadiAnt DICOM Viewer [Software]. Version 2016). As there is a current lack of consensus per parameter, measurements and definitions were based on current literature and were discussed in a study team consensus meeting before the scoring procedures started.10,19 Following individual parameters, presence or absence of disease activity was scored per rater. In addition, the most severely affected segment was defined as the segment with the highest BWT and was also independently determined by the two raters. Before start of the study there was agreement among all investigators to use the data from the second reader to correlate, determine, and predict endoscopic outcomes.
Table 1.
Intestinal ultrasound parameters
| IUS parameter | Technique/categories | Pathological |
|---|---|---|
| Bowel wall thickness | [2 x longitudinal plane + 2 × cross-sectional plane]/4 | ≥3.0 mm |
| Colour Doppler signal [modified Limberg score10] |
1: absent 2: small spots [single vessels] within the wall 3: long stretches within the wall 4: long stretches extending into the mesentery 5: measurement failed |
Category 3 or 4 |
| Wall layer stratification | 1: preserved 2:focal loss [< 3 cm extent] 3: extensive loss [≥ 3 cm extent] 4: measurement failed |
Category 2 or 3 |
| Presence of inflammatory fat | 1: absent;2: uncertain 3: present |
Category 3 |
| Presence of enlarged lymph nodes [≥5 mm in shortest axis] | 1: absent 2:uncertain 3: present |
Category 3 |
| Motility in terminal ileum | 1: present 2: uncertain 3: absent |
Category 3 |
| Colonic haustrations | 1: preserved 2: uncertain 3: loss |
Category 3 |
IUS, intestinal ultrasound.
2.5. Contrast-enhanced ultrasound measurements
The L12-5 transducer was used together with contrast specific pre-sets on an Epiq 5G ultrasound scanner which were equal for all patients. The most affected [ie. thickest] bowel segment was chosen for CEUS measurements. At T1 and T2, CEUS measurements were performed in the same segment, also when BWT normalised. The mechanical index [MI] was set as close to 0.05 by adjusting power and depth, and the focus region was set just below the area of interest. The gain was kept constant during the study; 2.4 mL of contrast agent [Sonovue, Bracco, Milan, Italy] with 10 mL 0.9% saline were administered via a venous catheter with a diameter of at least 1.1 mm, in the left elbow vein. Immediately after administration, a cine-loop was recorded for 90 s. This procedure was performed twice in the same segment.
At post-processing, all CEUS cine-loops were analysed by one ultrasonographer [FV] with VueBox [Bracco, Milan, Italy]. The complete bowel with surrounding mesentery was delineated using the peritoneum as delineation border. Then, motion compensation was applied using the peak-enhancement [PE] slide as reference standard. Subsequently, images with > 1 cm motion out of the delineation area were omitted. The cine-loop with least omitted images was used in further processing and analysis. Then, four regions of interest [ROI] were drawn with ROI1 encompassing the complete anterior wall, ROI2 the submucosa, ROI3 a single vessel in mucosa or submucosa, and ROI 4 mucosa and submucosa [Supplementary Figure 1a, b]. All ROIs [except ROI3] had to be over 0.5 cm2 surface and at least one ROI had to reach a quality of fit ≥ 85%.20
Subsequently, data on peak enhancement [PE], wash-in area under the curve [WiAUC], rise time [RT], mean transit time [MTT], time to peak [TTP], wash-in rate [WiR], wash-in perfusion index [WiPi], wash-out area under the curve [WoAUC], wash-in and wash-out area under the curve [WiWoAUC], fall time [FT], and wash-out rate [WoR] were collected both as linear and as log converted data (decibel [dB] or seconds [s]). To assess inter-observer agreement for CEUS measurements, 30 CEUS cine-loops were randomly selected and similarly rated by a second reader [KN].
2.6. Ileocolonoscopy
The patients were also scheduled for ileocolonoscopy at T = 0 and T = 2. All examinations were performed by trained gastroenterologists. The interval between the first ileocolonoscopy and IUS was always shorter than 12 weeks without changes in treatment between the procedures. The ileocolonoscopies were directly scored per segment for SES-CD. In addition, the most affected segment was determined as the segment with the highest SES-CD score. Segmental endoscopic remission was defined as SES-CD = 0, segmental endoscopic treatment response was defined as a decrease of SES-CD ≥ 50%, and complete endoscopic remission was defined as SES-CD = 0 in all segments. The performing gastroenterologist was blinded for the results of IUS.
2.7. Statistics
Statistical analysis was performed with SPSS Statistics for Windows, version 26 [IBM Corp., Armonk, NY, USA]. All normally distributed data were reported in mean ± standard deviation [SD] and non-normally distributed in median and interquartile range [IQR]. Mann–Whitney U tests were used to compare continuous non-parametric variables, chi square tests for dichotomous variables, and Wilcoxon rank tests or McNemar tests for paired samples. Area under the receiver operating characteristic curve [AUROC] was used to determine accuracy, sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]. Logistic regression was used to determine odds ratios and for univariable and multivariable analysis using forward selection. Spearman correlation coefficient was used to determine correlation with 0.00–0.09, 0.10–0.39, 0.40–0.69, 0.70–0.89, 0.90–1.00 considered as negligible, weak, moderate, strong, very strong correlation, respectively. Inter-observer agreement was assessed with intra-class correlation coefficient [ICC], weighted kappa [κ], and Cohen’s kappa [κ] for continuous, ordinal, and dichotomous outcomes.21,22 For ICC, a value below 0.50 was considered as poor, 0.50-0.75 as moderate, 0.75-0.90 as substantial, and 0.90–1.00 as strong agreement. Kappa statistics 0.0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00 were considered as slight, fair, moderate, substantial, and perfect agreement, respectively. For ICC, a p-value of 0.05 was used to determine significance.
3. Results
3.1. Baseline and follow-up characteristics
From April 2016 to March 2020 we included 40 consecutive CD patients with active disease and starting anti-TNFα treatment. Baseline characteristics are shown in Table 2. Follow-up is demonstrated in Supplementary Figure 2. In total, 23 patients underwent a second endoscopy at T2 (median: 22 weeks [IQR: 19-26.5]) to evaluate treatment response. In further analysis, all patients who underwent surgery between T0 and T2 were considered as non-responders.
Table 2.
Baseline characteristics.
| Baseline | n = 40 |
|---|---|
| Female; n [%] | 20 [50.0%] |
| Age at inclusion; median [range], years | 33 [18–68] |
| Disease duration in median years [IQR] | 3.88 [1–14.25] |
| Montreal classification in CD patients | |
| A1 [<16 years] | 5 [12.5%] |
| A2 [17–40 years] A3 [>40 years] |
27 [67.5%] 8 [20.0%] |
| L1 [ileum] | 17 [42.5%] |
| L2 [colon] | 9 [22.5%] |
| L3 [ileocolonic] | 14 [35.0%] |
| B1 [non stricturing, non-penetrating] | 16 [40.0%] |
| B2 [stricturing] | 12 [30.0%] |
| B3 [penetrating] | 12 [30.0%] |
| P [perianal disease] | 11 [27.5%] |
| Previous surgical resection at time of IUS | |
| ICR and ileal re-resections | 15 [37.5%] |
| [Partial] colonic resection | 5 [12.5%] |
| Medication use in medical history | |
| Biologics [infliximab, adalimumab, vedolizumab, ustekinumab] | 15 [37.5%] |
| Immunomodulators [thiopurines/methotrexate] | 20 [50.0%] |
| Corticosteroids | 30 [75.0%] |
| Aminosalicylates | 8 [20.0%] |
| Medication use at inclusion | |
| Corticosteroids | 6 [15.0%] |
| Aminosalicylates | 1 [2.5%] |
| Immunomodulators [thiopurines/methotrexate] | 25 [62.5%] |
| Medication after inclusion | |
| Infliximab | 28 [70.0%] |
| Adalimumab | 12 [30.0%] |
| Clinical and biochemical parameters in median [IQR] | |
| Harvey–Bradshaw Index | 5.0 [3.0–8.0] |
| C-reactive protein in mg/L | 8.25 [2.43–30.08] |
| Haemoglobin in mmol/L | 8.10 [7.40–8.88] |
| Leukocyte count in 109/L | 7.65 [5.95–10.75] |
| Platelet count 1012/L | 340.0 [269.25–405.25] |
| Albumin in g/L | 41.0 [37.75–-44.25] |
| Faecal calprotectin in µg/g | 688.0 [382.0–-1810.50] |
| Intestinal ultrasound parameters | |
| Most severely affected segment | |
| Sigmoid colon | 5 [12.5%] |
| Descending colon | 4 [10.0%] |
| Transverse colon | 2 [5.0%] |
| Ascending colon | 4 [10.0%] |
| Terminal ileum | 25 [62.5%] |
| Bowel wall thickness in mm [median and IQR] | |
| Colour Doppler signal | |
| No signal | 5.21 [4.60–6.84] |
| Single vessel | 1 [2.5%] |
| Stretches within the wall | 5 [12.5%] |
| Stretches in the wall and mesentery | 21 [52.5%] |
| Measurement failed | 10 [25.0%] |
| Loss of stratification | 3 [7.5%] |
| Preserved | |
| Focal loss [< 3 cm] | 19 [47.5%] |
| Extensive loss [≥ 3 cm] | 11 [27.5%] |
| Measurement failed | 7 [17.5%] |
| Presence of inflammatory fat | 3 [7.5%] |
| Not present | 8 [20.0%] |
| Uncertain | 6 [15.0%] |
| Present | 26 [65.0%] |
| Presence of lymph nodes [> 5 mm in shortest axis] | |
| Present | 8 [20.0%] |
| Uncertain | 3 [7.5%] |
| Not present | 29 [72.5%] |
| Motility in terminal ileum [n = 25] | |
| Present | 3 [12.5%] |
| Uncertain | 3 [12.5%] |
| Absent | 19 [79.2%] |
| Colonic haustrations [n = 15] | |
| Loss | 11 [73.3%] |
| Preserved | 4 [26.7%] |
| Endoscopic parameters | |
| Most severely affected segment | |
| Rectum Sigmoid colon |
1 [2.5%] 6 [15.0%] |
| Descending colon | 2 [5.0%] |
| Transverse colon | 2 [5.0%] |
| Ascending colon | 3 [7.5%] |
| Terminal ileum | 26 [65.0%] |
| Total SES-CD score [median and IQR] | 9.0 [5.25–15.00] |
| SES-CD of most affected segment [median and IQR] | 6.50 [3.25–8.00] |
IQR, interquartile range; ICR, ileocecal resection; SES-CD, Simple Endoscopic Score-Crohn’s Disease; IUS, intestinal ultrasound.
At baseline endoscopy, the worst segment was the TI in 26/40 and a colonic segment in 14/40 patients, respectively. At baseline IUS, the TI was the worst segment in 25/40 patients and a colonic segment in 15/40 patients [Table 2]. Per segment analysis [sigmoid, descending, transverse, ascending colon, and TI] showed strong correlation between endoscopic and IUS presence or absence of disease [ρ = 0.81, p < 0.0001]. BWT was higher in patients with disease activity in the TI than in the colon [5.8 ± 1.5 mm vs 4.9 ± 1.2 mm, p = 0.04]. There was a mean of 38 ± 38 days between baseline ileocolonoscopy and IUS without change in treatment.
At T2, 14/40 [35.0%] (14/23 [60.9%]) patients had complete endoscopic remission in all segments and 17/40 [42.5%] (17/23 [73.9%]) had endoscopic response. Patients with a colonic segment as the most severely affected, were more likely to have endoscopic response [OR: 2.59, 95% CI: 1.43-4.70, p = 0.004] and remission [OR: 3.07, 95% CI: 1.44-6.52, p = 0.006] at T2 as compared with patients with disease activity in the TI. There was a mean of 39 ± 21 days between ileocolonoscopy and IUS at T2. None of the patients changed treatment between the last endoscopy and last IUS.
3.2. IUS parameters
3.2.1. Correlation with SES-CD
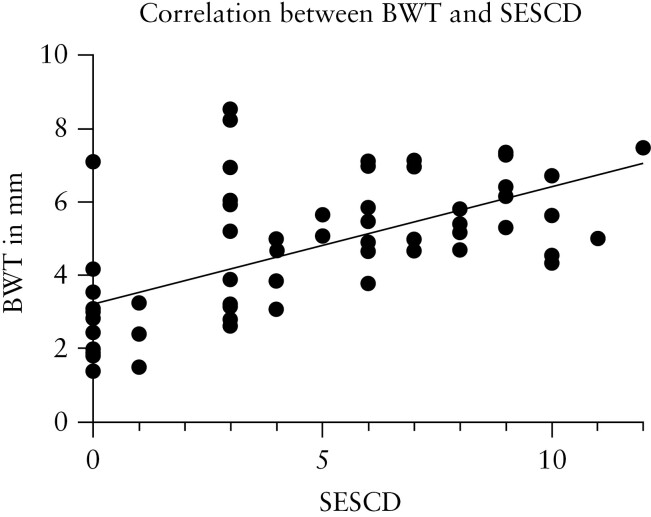
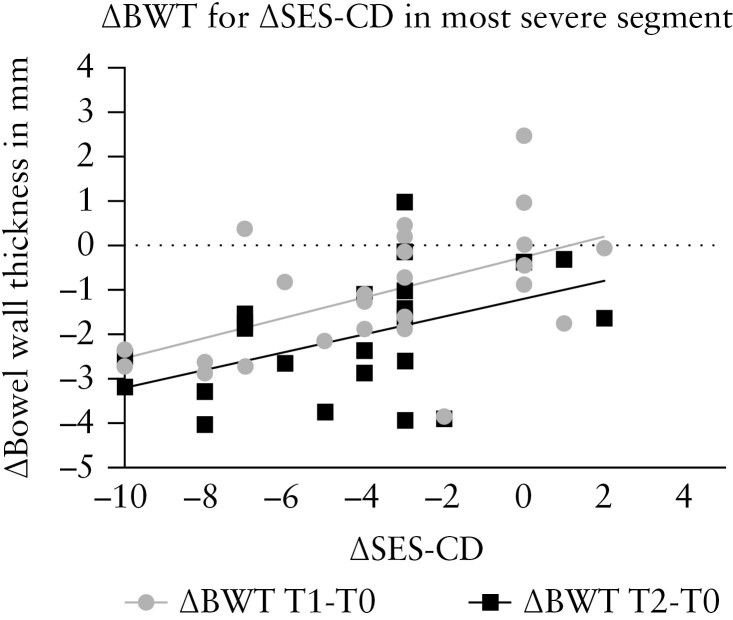
There was moderate to strong correlation for BWT [ρ = 0.61, p < 0.0001] [Figure 1], CDS [ρ = 0.73, p < 0.0001], loss of motility [ρ = 0.50, p = 0.001], presence of inflammatory fat [ρ = 0.58, p < 0.0001], and loss of haustrations [ρ = 0.48, p = 0.031] with presence of disease activity at endoscopy [≥SESCD 1] at both T0 and T2. In addition, there was weak correlation for loss of wall layer stratification [WLS] [ρ = 0.34, p = 0.007]. ΔBWT at T1 [ρ = 0.54, p = 0.003] and T2 [ρ = 0.47, p = 0.025] correlated moderately with ΔSES-CD for the most severe segment [Figure 2].
Figure 1.
Correlation between SES-CD score and BWT in the most severely affected segment [r = 0.61, p < 0.0001]. SES-CD, Simple Endoscopic Score-Crohn’s Disease; BWT, bowel wall thickness.
Figure 2.
Correlation between ΔBWT (T1 [ρ = 0.54, p = 0.003] and T2 [ρ = 0.47, p = 0.025]) and ΔSES-CD for the most severe segment. SES-CD, Simple Endoscopic Score-Crohn’s Disease; BWT, bowel wall thickness.
3.2.2. Bowel wall thickness
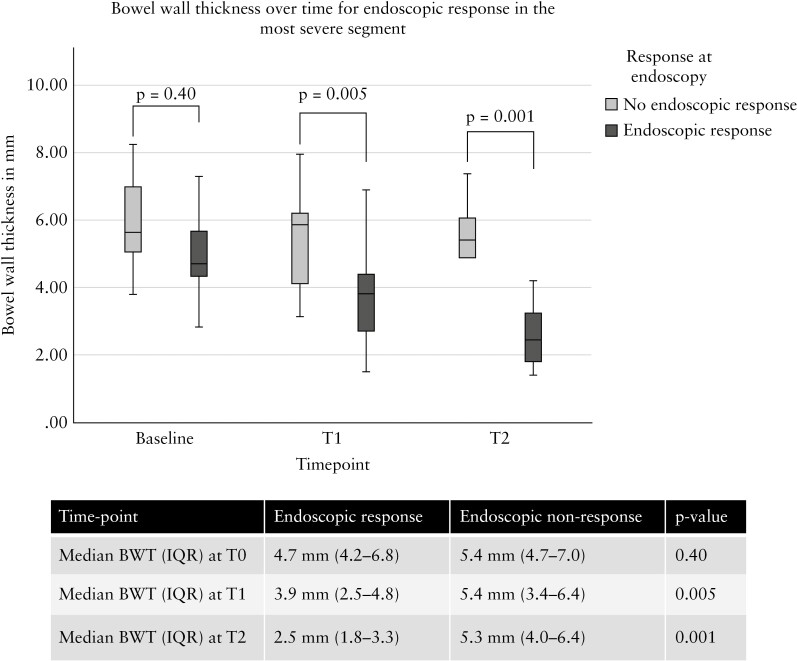
Patients with endoscopic response had a significantly lower BWT at T1 and T2 compared with patients without endoscopic response [Figure 3]. Similarly for endoscopic remission, BWT was lower at T1 (3.1 mm [1.9–4.2] vs 5.3 mm [3.8–6.9], p = 0.005) and T2 (2.0 mm [1.8–3.1] vs 5.1 [3.0–6.3] mm, p = 0.002). In addition, decrease in BWT at T1 was significantly different between endoscopic responders versus non-responders (ΔBWT: -1.7 mm [-2.6– -0.2] vs -0.1 mm [-1.1– -0.7], p = 0.012), respectively. At T2, BWT decreased further compared with T0 and was significantly different between endoscopic responders and non-responders (ΔBWT: -2.5 mm [-3.3– -1.4] vs -0.7 [-1.5– -0.2], p = 0.035), respectively.
Figure 3.
Decrease in BWT in the most severely affected segment at IUS according to endoscopic response and non-response at T1 and T2. BWT, bowel wall thickness; IUS, intestinal ultrasound; T, time.
At T2, a BWT cut-off value of 3.2 mm was most accurate to predict endoscopic remission [AUROC: 0.940, 95% CI: 0.862-1.000, p < 0.0001] with 70% sensitivity, 85% specificity, PPV: 87%, and NPV: 63%. For prediction of endoscopic response a decrease in BWT from baseline expressed in percentage was most accurate. At T1, an 18% decrease in BWT predicted endoscopic response [AUROC: 0.765, 95% CI: 0.580-0.949, p = 0.02] with 82% sensitivity, 71% specificity, PPV: 64%, NPV: 86%. A 29% decrease in BWT at T2 predicted endoscopic response [AUROC: 0.833, 95% CI: 0.626-1.000, p = 0.017] with 83% sensitivity, 88% specificity, PPV: 93%, NPV: 71%.
3.2.3. Other parameters
When there was endoscopic response, presence of hyperaemia [CDS 3 or 4] decreased significantly at T1 [T0: 87% vs T1: 35.5%, p = 0.004] and T2 [T0: 87% vs T2: 6%, p < 0.0001] [Supplementary Figure 3a]. A decrease of 1 point in CDS score at T1 [OR: 2.89, 95% CI: 1.054-7.907, p = 0.039] and T2 [OR: 5.44, 95% CI: 1.258-23.478, p = 0.023] was associated with endoscopic response. WLS normalised more frequently at T2 when there was endoscopic response [T0: 55% vs T2: 12%, p = 0.016] but not at T1 [T0: 53% vs T1: 29%, p = 0.289]. However, a normalisation of WLS at T1 [OR: 4.91, 95% CI: 0.496-48.622, p = 0.174] or T2 [OR: 3.56, 95% CI: 0.326-38.777, p = 0.30], respectively, could not predict endoscopic response at T2.
Presence of lymph nodes did not decrease significantly when there was endoscopic response at T1 [T0: 19% vs T1: 19%, p = 1.00] or at T2 [T0: 19% vs T2: 6.3%, p = 0.08].
Presence of inflammatory fat did decrease with a trend towards significance at T1 [T0: 82% vs T1: 44%, p = 0.07] and significantly at T2 [T0: 82% vs T2: 6%, p < 0.0001], respectively [Supplementary Figure 3b]. There was no significant change in presence of inflammatory fat when there was no endoscopic response.
In patients with disease activity in the TI, motility returned at T2 [absence of motility T0: 88% vs T2: 10%, p = 0.03] but not at T1 [absence of motility T0: 88% vs T1: 45%, p = 0.25]. When there was no endoscopic response at T2, there was no significant change in motility [T0: 85%, T1: 89%, T2: 75%, p = ns].
When there was endoscopic response, colonic haustrations normalised significantly at T1 [absence of haustrations at T0: 78% vs absence of haustrations at T1: 14%, p = 0.031] and at T2 [absence of haustrations at T0: 78% vs absence of haustrations at T2: 0%, p = 0.016]. When there was no endoscopic response, there was no normalisation of colonic haustrations [T0: 75%, T1: 66%, T2: 75%, p = ns]].
3.2.4. Contrast-enhanced ultrasound
Totals of 40, 32, and 23 patients underwent CEUS at T0, T1, and T2, respectively. In three patients at T1 and six patients at T2, CEUS measurements were of low quality of fit due to a normal BWT [n = 7] or present motility in the TI [n = 2] and were excluded from further analysis. For the 17 patients at T2 with a second endoscopy and valid CEUS measurements available, 12 [71%] patients had endoscopic response and eight [47%] patients were in endoscopic remission.
CEUS was analysed for ROI 1, 2, and 3. ROI 4 measurements were omitted from further analysis as air in the lumen was often within the ROI, resulting in uninterpretable data or an ROI matching ROI 2. Quality of fit [QoF] at baseline was high for ROI1 (median: 93.81% [84.93–96.17]), ROI2 (median: 88.21% [80.68–91.97]), and ROI3 (median: 93.06% [80.19–95.16]). At T1 and T2, QoF for all ROIs did not significantly differ with regards to baseline [data not shown]. At T1, QoF for ROI 3 was lower when there was endoscopic response compared with no endoscopic response at T2 (median QoF ROI 3: 73.11% [47.28–84.94] vs 86.38% [81.59–93.47], p = 0.012).
At T1, none of the logarithmic [Supplementary Table 1a–c] or linear data [Supplementary Table 2a–c] could predict endoscopic remission. For ROI1, percentage decrease of WoR at T1 was significantly different when there was endoscopic response compared with non-response at T2 (median ΔT0–T1: -32.55% [-44.74– -1.90] vs -1.28% [-12.58–49.53], p = 0.04]. At T2, decrease in percentage for PE, WiR, WiPi, and WoR was significantly more pronounced in endoscopic responders [Supplementary Figure 4]. For ROI2, ROI3, and the other CEUS parameters, there was no significant change distinguishing endoscopic responders from non-responders at T1 or T2. Also, for the linear data at T1 or T2 there was no significant change with regards to baseline for all three ROIs [data not shown].
3.2.5. Clinical and biochemical response
HBI, ΔHBI, FCP, and ΔFCP values were significantly different between endoscopic responders and non-responders at T1 and T2 [Supplementary Table 3]. Corresponding AUROC and cut-off values for FCP are demonstrated in Supplementary Table 4. All other [changes in] biochemical parameters were not significantly different between endoscopic responders and non-responders.
3.2.6. Multivariable regression for endoscopic remission and response with conventional IUS and CEUS parameters, HBI, and FCP
When BWT was dichotomised with a cut-off value of 3.2 mm, endoscopic remission was shown [OR for BWT ≤ 3.2 mm: 39.42, 95% CI: 7.67-202.57, p < 0.0001] and normalisation of CDS [CDS < 3] significantly improved the model [OR: 13.76, 95% CI: 1.28-147.78, p = 0.03]. Adding the other IUS parameters, FCP or HBI did not significantly improve the model [Supplementary Table 5]. In addition, BWT decrease of 18% and 29% at T1 [OR: 10.80, 95% CI: 1.69-68.94, p = 0.012] and T2 [OR: 37.50, 95% CI: 2.77-507.48, p = 0.006] predicted endoscopic response, respectively. The other IUS parameters, FCP, and HBI did not significantly improve the model to predict endoscopic response.
When combined with BWT, WoR significantly improved the model to predict endoscopic remission at T2 [WoR per 1-dB increase: OR: 0.76, 95% CI: 0.60-0.96, p = 0.019]. All other CEUS parameters did not improve the model. In addition, at T1, none of the absolute CEUS values nor changes in CEUS parameters at T1 or T2 contributed to the model with BWT to predict endoscopic response [Supplementary Table 5].
3.2.7. Inter-observer agreement per segment
There was perfect agreement on the most affected segment between the two raters [κ = 0.81, 95% CI: 0.68-0.93, p < 0.0001]. Per segment agreement on presence of disease activity is demonstrated in Supplementary Table 6. For BWT, there was strong agreement for sigmoid colon [ICC: 0.979, 95% CI: 0.938-0.993, p < 0.0001], ascending colon [ICC: 0.971, 95% CI: 0.855-0.994, p < 0.0001] and TI [ICC: 0.953, 95% CI: 0.917-0.973, p < 0.0001]. There was substantial and moderate agreement for descending [ICC: 0.884, 95% CI: 0.669-0.960, p < 0.0001] and transverse colon [ICC: 0.697, 95% CI: 0.006-0.907, p = 0.024], respectively. Agreement per parameter and per segment is demonstrated in Supplementary Table 7.
3.2.8. Inter-observer agreement for CEUS
For CEUS measurements there was moderate to strong agreement [Supplementary Table 8]. Particularly, WoR had strong agreement [ICC: 0.943, 95% CI: 0.875-0.974, p < 0.0001]. For the other ROIs [single vessel and submucosa] there was moderate to strong agreement [data not shown].
4. Discussion
IUS, particularly BWT and CDS, showed moderate to strong correlation with the SES-CD in the most severely affected segment. In addition, BWT and CDS were responsive and decreased in patients with endoscopic response [Figures 4 and 5]. After 4–8 weeks after treatment initiation, BWT showed already a significant decrease, with 18% thereby predicting endoscopic response with high accuracy [OR: 10.80, 95% CI: 1.69-68.94, p = 0.012]. Accuracy increases after 12–34 weeks with a BWT decrease of 29% being most accurate to determine endoscopic response [OR: 37.50, 95% CI: 2.77-507.48, p = 0.006] and a cut-off value of 3.2 mm most accurate to reflect endoscopic remission [OR: 39.42, 95% CI: 7.67-202.57, p < 0.0001]. Although other IUS parameters and CEUS parameters also decrease when there is endoscopic response, they are of limited merit in predicting and determining endoscopic outcomes in addition to BWT and CDS. Furthermore, inter-observer agreement for both conventional IUS parameters and CEUS parameters was good to perfect, indicating a high reliability for IUS and CEUS in clinical practice.
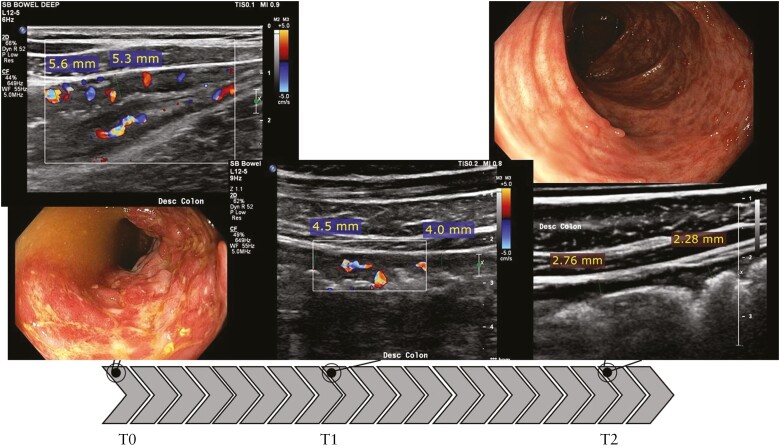
Figure 4.
Response on IUS and paired colonoscopy in the most severely affected segment [descending colon]. BWT decreases with 22% at T1 from 5.5 mm [T0] to 4.3 mm [T1]. At T2, BWT decreased further to 2.6 mm or 53% with regards to baseline. Also colour Doppler signal showed improvement over time and colonic haustrations returned. SES-CD = 7 at baseline, with deep ulcerations present. At T2, SES-CD = 0 with no ulcers present; presence of pseudopolyps and mucosal scar tissue. SES-CD, Simple Endoscopic Score-Crohn’s Disease; BWT, bowel wall thickness; IUS, intestinal ultrasound; T, time.
Figure 5.
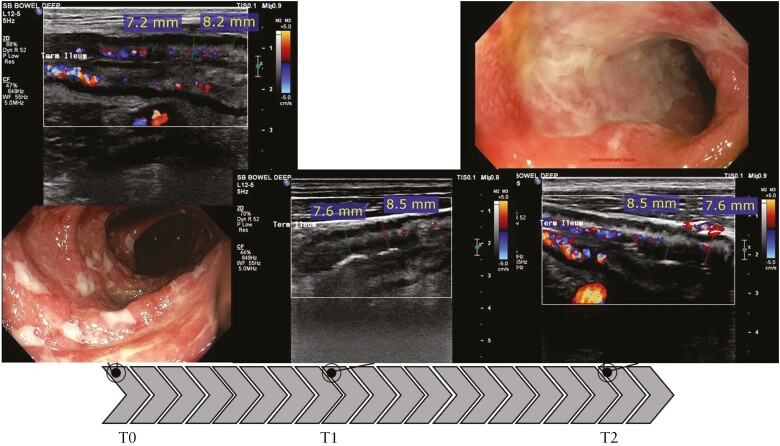
No response on IUS and paired colonoscopy in the most severely affected segment [terminal ileum]. BWT shows no improvement with 7.7 mm at baseline, 8.1 mm at T1, and 8.1 mm at T2. Colour Doppler signal improves at T1 but deteriorates at T2 and is similar to baseline. SES-CD = 10 in the neoterminal ileum at baseline. At T2, SES-CD = 9, no endoscopic response. SES-CD, Simple Endoscopic Score-Crohn’s Disease; BWT, bowel wall thickness; IUS, intestinal ultrasound; T, time.
In this study, we have demonstrated high accuracy for IUS, and specifically BWT, to predict endoscopic response and remission early after initiation of anti-TNFα treatment. Previous studies have demonstrated the value of IUS to measure treatment response.12,23–32 The largest study was conducted by Kucharzik et al. and showed IUS to detect response to treatment in a large cohort of CD patients clinically responding to anti-inflammatory treatment.29 Most IUS parameters normalised within the first 3 months of treatment, which is in concordance with our findings. However, a robust reference standard was not used. In our study, predominantly BWT, CDS, and inflammatory fat were discriminative in an early phase and are perhaps the most responsive to improvement and healing of the wall, which has also been demonstrated in recent studies.12,23,25–27,30,32 Moreover, these parameters were often pathological at baseline in most patients, whereas WLS and presence of lymph nodes were less frequently seen and, accordingly, also less responsive to change during treatment.
Previous studies have investigated IUS response according to endoscopic response and remission.23–27,30,32,33 Although these studies found favourable outcomes for patients reaching transmural healing in addition to mucosal healing, definitions for transmural healing varied and were not validated. Therefore, in our cohort, we decided to investigate which IUS parameters best reflect endoscopic response and remission. To our knowledge, this is first study presenting accurate cut-off values and decrease in percentages for BWT indicating endoscopic remission and response in the corresponding segments, respectively. Intriguingly, a BWT cut-off value of 3.2 mm reflected endoscopic remission in both the TI and the colon accurately. Whereas in diagnosing CD a cut-off value of 2.0 mm [especially in the TI] or 3.0 mm, is not uncommon,8,11 this might be too stringent to determine endoscopic remission after anti-inflammatory treatment. Moreover, fibrosis or scar tissue might result in a thickened bowel wall.34 However, a certain proportion of patients reached a cut-off value < 2.0 mm or 3.0 mm. Consequently, even in patients with a BWT < 3.2 mm, there might be room for improvement. Future research should elucidate this.
IUS is also sensitive enough to demonstrate endoscopic response. A 29% decrease of BWT at T2 reflected endoscopic treatment response. A recent study defining treatment response according to SES-CD score and the Crohn’s Disease Activity Index found similar findings after 12 weeks of treatment with anti-TNFα.19 Another study defined IUS response as a decrease of 25% in BWT and found moderate correlation with endoscopic response after 16 weeks of treatment with ustekinumab, according to the SES-CD score in the corresponding segment.28 In our cohort, a decrease of 18% at T1 could already reflect endoscopic treatment response in an early phase. In addition the 12–34 weeks’ time frame at T2 could have biased our results with potential lower percentage decrease for patients undergoing second endoscopy early in this time frame. To overcome this bias, future research should confirm this statement in prospective designs with larger patient cohorts and one time point instead of a time frame.
Most CEUS parameters significantly reflected endoscopic disease activity, in line with previous studies.12,31,35,36 In our cohort, only percentage change in WoR could significantly predict endoscopic response and non-response, whereas previous studies also showed other parameters to reflect endoscopic response.12,19 Quaia et al. found a significant percentage change in most CEUS parameters after 6 weeks of treatment, between endoscopic responders and non-responders after 14 weeks of treatment. Similarly, Laterza et al. found a percentage change for almost all evaluated CEUS parameters already after 2 to 6 weeks of treatment in endoscopic responders after 12 weeks. In contrast to these previous studies, we had to exclude patients due to normalisation of the bowel wall or return of motility resulting in poor CEUS cine-loops when most of these patients were endoscopic responders. Consequently, change in CEUS parameters during treatment within the endoscopic responding group might be underestimated. Although we reached high inter-observer agreements for most CEUS parameters in a subset of randomly selected patients, CEUS might become less feasible when BWT normalises.
Whereas BWT is the most important parameter indicating endoscopic remission, CDS or WoR added significantly to the model reflecting endoscopic remission. Two recent studies using consensus panels found a combination of BWT with CDS accurately to reflect endoscopic disease activity and endoscopic response.10,37 In addition, a recent and partly validated scoring index with endoscopy as reference standard incorporated both BWT and CDS.38 In clinical practice, patients with a normal BWT but increased CDS are less likely to have reached complete endoscopic remission for the specific segment. Whether this should lead to dose escalation or change of treatment is unclear and is a subject for future studies. Potentially, WoR could fulfil a similar role as CDS. Although promising and reproducible, measuring WoR is more difficult and time-consuming than CDS, as the patient needs contrast administration and CEUS cine-loops need post-processing. Especially in a point-of-care setting, this is less attractive. In addition, a recent study found similar accuracy for a model with BWT and CDS compared with a model with BWT, CDS, and CEUS parameters.31 Since CDS is a reliable parameter to score,10 CDS deserves recommendation over WoR to be incorporated in future scoring indices and definitions for transmural response or healing. Consequently, the role for CEUS in the treatment follow-up and response assessment is in its current state limited. More practical on-board methods, including reliable scaling, correction of the arterial input function, and immediate availability of the measurements, could pave the way to broader utility of CEUS.39, 40
In addition, we have shown that next to BWT, decrease in FCP accurately detects endoscopic response in an early phase. However, early absolute measurements for FCP could not predict endoscopic response or remission. Moreover, FCP is subject to other circumstances which could lead to false-positive or -negative results when compared with endoscopic outcomes.41, 42 In our cohort, we have demonstrated that both absolute measurements and change in BWT measurements reflect endoscopic disease activity at a later stage. Consequently, BWT is not inferior to FCP and additionally informs on disease extent. Incorporating FCP in an IUS parameter-based prediction model did not significantly improve the model to predict endoscopic response or remission.
Our study has a few limitations. Some patients did not reach T2 because of surgery, worsening disease, or loss to follow-up. Although this is suboptimal for the analysis, our results truly reflect clinical practice which might therefore also be a strength of this study. Also, time between IUS and endoscopy was in some patients suboptimal. We generally performed IUS and CEUS at anti-TNFα administration, explaining the delay between endoscopy and IUS/CEUS. However, this reflects the use of IUS in a point-of-care setting and probably limited generalisability of our results. Furthermore, we scored IUS and CEUS cine-loops and images per segment after the patient visit, which might have resulted in a certain bias for inter-observer agreement. IUS is operator-dependent and ideally inter-observer agreement is scored in a real-time setting with blinded operators. However, our scoring methods approach a clinical trial setting with central reading, and we have demonstrated a feasible and reliable process using still images and cine-loops to score IUS and CEUS parameters. Future studies should also incorporate central reading for endoscopy, especially when IUS parameters or a score are validated.
Our study also has several strengths. Sonographer and gastroenterologists were blinded to the other examinations. In addition, we used a validated and robust endoscopic reference standard and have shown good correlation with IUS and CEUS parameters. Also, we did not predefine IUS response or remission but showed changes on IUS and demonstrated cut-off values for BWT according to endoscopic changes.
In conclusion, we have demonstrated IUS response to anti-TNFα treatment according to endoscopic treatment response and remission. As endoscopy is still the gold standard but invasive, IUS, and especially decrease of BWT in percentages, has potential to determine endoscopic response in most patients for both the TI and colon. In addition, this is feasible already after 4–8 weeks. The additional value of performing [early] CEUS in this perspective was limited in our study. Definition, standardisation, and validation of transmural healing and transmural response should be a next step in incorporation of IUS in research and clinical practice.
Supplementary Material
Contributor Information
F de Voogd, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands; Amsterdam Gastroenterology Endocrinology Metabolism Research Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
S Bots, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
K Gecse, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
O H Gilja, National Centre of Ultrasound in Gastroenterology, Haukeland University Hospital, Bergen, Norway; Department of Clinical Medicine, University of Bergen, Bergen, Norway.
G D’Haens, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
K Nylund, National Centre of Ultrasound in Gastroenterology, Haukeland University Hospital, Bergen, Norway; Department of Clinical Medicine, University of Bergen, Bergen, Norway.
Funding
This research received no funding from any public, commercial, or non-profit organisation.
Conflict of interest
FV received speaker or honoraria fees from AbbVie and Janssen. SB has served as speaker for Abbvie, Merck, Sharp & Dome, Takeda, Jansen Cilag, Pfizer, and Tillotts. KG has received grants from Pfizer and Celltrion; consultancy fees from AbbVie, Arena Pharmaceuticals, Galapagos, Gilead, Immunic Therapeutics, Janssen Pharmaceuticals, Novartis, Pfizer, Samsung Bioepis, and Takeda; and speaker’s honoraria from Celltrion, Ferring, Janssen Pharmaceuticals, Novartis, Pfizer, Samsung Bioepis, Takeda, and Tillotts. OHG has received speaker’s honoraria from AbbVie, Bracco, Almirall, GE Healthcare, Takeda AS, Meda AS, Ferring AS, Allergan, and Janssen-Cilag; and has served as consultant for Bracco, GE Healthcare, Takeda, and Samsung. GD has served as adviser for Abbvie, Ablynx, Amakem, AM Pharma, Avaxia, Biogen, Bristol Meiers Squibb, Boerhinger Ingelheim, Celgene, Celltrion, Cosmo, Covidien, Ferring, Dr FALK Pharma, Engene, Galapagos, Gilead, Glaxo Smith Kline, Hospira, Immunic, Johnson and Johnson, Lycera, Medimetrics, Millenium/Takeda, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Novonordisk, Pfizer, Prometheus laboratories/Nestle, Protagonist, Receptos, Robarts Clinical Trials, Salix, Sandoz, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant, and Vifor; and received speaker fees from Abbvie, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Shire, Millenium/Takeda, Tillotts, and Vifor. KN has received speaker honoraria from Takeda and Janssen.
Author Contributions
FV: patient selection, data acquisition, data interpretation, writing first draft of the manuscript, and final approval of the manuscript. SB: study design, patient selection, data acquisition, data interpretation, writing first draft of the manuscript, and final approval of the manuscript. KG: patient selection, revising and final approval of the manuscript. OG: study design, revising and final approval of the manuscript. GD: study design, patient selection, revising and final approval of the manuscript. KN: study design, patient selection, data acquisition, data interpretation, writing first draft of the manuscript. and final approval of the manuscript.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Maaser C, Sturm A, Vavricka SR, et al. . ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64. [DOI] [PubMed] [Google Scholar]
- 2. Turner D, Ricciuto A, Lewis A, et al. . STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE] initiative of the International Organization for the Study of IBD [IOIBD]: determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. [DOI] [PubMed] [Google Scholar]
- 3. Buisson A, Gonzalez F, Poullenot F, Nancey S, Sollellis E, Fumery M, et al. . Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. InflammBowel Dis 2017;23:1425–33. [DOI] [PubMed] [Google Scholar]
- 4. Terheggen G, Lanyi B, Schanz S, et al. . Safety, feasibility, and tolerability of ileocolonoscopy in inflammatory bowel disease. Endoscopy 2008;40:656–63 [DOI] [PubMed] [Google Scholar]
- 5. Gecse KB, Brandse JF, Van Wilpe S, et al. . Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol 2015;50:841–7. [DOI] [PubMed] [Google Scholar]
- 6. Schoepfer AM, Beglinger C, Straumann A, et al. . Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease [SES-CD] than CRP, blood leukocytes, and the CDAI. Am Coll Gastroenterol 2010;105:162–9. [DOI] [PubMed] [Google Scholar]
- 7. Dong J, Wang H, Zhao J, et al. . Ultrasound as a diagnostic tool in detecting active Crohn’s disease: a meta-analysis of prospective studies. Eur Radiol 2014;24:26–33. [DOI] [PubMed] [Google Scholar]
- 8. Goodsall TM, Nguyen TM, Parker CE, et al. . Systematic review: gastrointestinal ultrasound scoring indices for inflammatory bowel disease. J Crohns Colitis 2021;15:125–42. [DOI] [PubMed] [Google Scholar]
- 9. Panes J, Bouzas R, Chaparro M, et al. . Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125–45. [DOI] [PubMed] [Google Scholar]
- 10. Novak KL, Nylund K, Maaser C, et al. . Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A reliability and inter-rater variability study on intestinal ultrasonography in Crohn’s disease. J Crohns Colitis 2021;15:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bots S, Nylund K, Löwenberg M, Gecse K, Gilja OH, D’Haens G.. Ultrasound for assessing disease activity in IBD patients: a systematic review of activity scores. J Crohns Colitis 2018;12:920–9. [DOI] [PubMed] [Google Scholar]
- 12. Quaia E, Gennari AG, Cova MA.. Early predictors of the long-term response to therapy in patients with Crohn disease derived from a time-intensity curve analysis after microbubble contrast agent injection. J Ultrasound Med 2019;38:947–58. [Google Scholar]
- 13. Ripollés T, Martínez MJ, Paredes JM, Blanc E, Flors L, Delgado F.. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 2009;253:241–8. [DOI] [PubMed] [Google Scholar]
- 14. Saevik F, Nylund K, Hausken T, Ødegaard S, Gilja OH.. Bowel perfusion measured with dynamic contrast-enhanced ultrasound predicts treatment outcome in patients with Crohn’s disease. Inflamm Bowel Dis 2014;20:2029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piscaglia F, Nolsøe C, Dietrich CA, et al. . The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound [CEUS]: update 2011 on non-hepatic applications. Eur J Ultrasound 2012;33:33–59. [DOI] [PubMed] [Google Scholar]
- 16. Maconi G, Parente F, Bollani S, et al. . Factors affecting splanchnic haemodynamics in Crohn’s disease: a prospective controlled study using Doppler ultrasound. Gut 1998;43:645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danese S, Sans M, De La Motte C, et al. . Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 2006;130:2060–73. [DOI] [PubMed] [Google Scholar]
- 18. Hatoum OA, Binion DG, Otterson MF, Gutterman DD.. Acquired microvascular dysfunction in inflammatory bowel disease: loss of nitric oxide-mediated vasodilation. Gastroenterology 2003;125:58–69. [DOI] [PubMed] [Google Scholar]
- 19. Laterza L, Ainora ME, Garcovich M, et al. . Bowel contrast-enhanced ultrasound perfusion imaging in the evaluation of Crohn’s disease patients undergoing anti-TNFα therapy. Dig Liver Dis 2021;53:729–37. [DOI] [PubMed] [Google Scholar]
- 20. Wilkens R, Peters DA, Nielsen AH, Hovgaard VP, Glerup H, Krogh K.. Dynamic contrast-enhanced magnetic resonance enterography and dynamic contrast-enhanced ultrasonography in Crohn’s disease: an observational comparison study. Ultrasound Int Open 2017;3:E13–E24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koo TK, Li MY.. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J ChiropractMed 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landis JR, Koch GG.. The measurement of observer agreement for categorical data. Biometrics 1977:159–74. [PubMed] [Google Scholar]
- 23. Calabrese E, Rispo A, Zorzi F, et al. . Ultrasonography tight control and monitoring in Crohn’s disease during different biological therapies: a multicenter study. Clin Gastroenterol Hepatol 2022;20:e711–22. [DOI] [PubMed] [Google Scholar]
- 24. Castiglione F, Imperatore N, Testa A, et al. . One-year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther 2019;49:1026–39. [DOI] [PubMed] [Google Scholar]
- 25. Castiglione F, Mainenti P, Testa A, et al. . Cross-sectional evaluation of transmural healing in patients with Crohn’s disease on maintenance treatment with anti-TNF alpha agents. Dig Liver Dis 2017;49:484–9. [DOI] [PubMed] [Google Scholar]
- 26. Castiglione F, Testa A, Rea M, et al. . Transmural healing evaluated by bowel sonography in patients with Crohn’s disease on maintenance treatment with biologics. Inflamm Bowel Dis 2013;19:1928–34. [DOI] [PubMed] [Google Scholar]
- 27. Helwig U, Fischer I, Hammer L, et al. . Transmural response and transmural healing defined by intestinal ultrasound: new potential therapeutic targets? J Crohns Colitis 2022;16:57–67. [DOI] [PubMed] [Google Scholar]
- 28. Kucharzik T, Wilkens R, Maconi G, et al. . Intestinal ultrasound response and transmural healing after ustekinumab induction in Crohn’s disease: Week 16 interim analysis of the STARDUST trial substudy. Zeitschrift für Gastroenterologie 2020;58:P04. [Google Scholar]
- 29. Kucharzik T, Wittig BM, Helwig U, et al. . Use of intestinal ultrasound to monitor Crohn’s disease activity. Clin Gastroenterol Hepatol 2017;15:535–42. e2. [DOI] [PubMed] [Google Scholar]
- 30. Moreno N, Ripollés T, Paredes JM, et al. . Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis 2014;8:1079–87. [DOI] [PubMed] [Google Scholar]
- 31. Ripollés T, Poza J, Suarez Ferrer C, Martínez-Pérez MJ, Martín-Algíbez A, de las Heras Paez B.. Evaluation of Crohn’s disease activity: development of an ultrasound score in a multicenter study. Inflamm Bowel Dis 2021;27:145–54. [DOI] [PubMed] [Google Scholar]
- 32. Zorzi F, Ghosh S, Chiaramonte C, et al. . Response assessed by ultrasonography as target of biological treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2020;18:2030–7. [DOI] [PubMed] [Google Scholar]
- 33. Chen J-M, He L-W, Yan T, et al. . Oral exclusive enteral nutrition induces mucosal and transmural healing in patients with Crohn’s disease. Gastroenterol Rep 2019;7:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bettenworth D, Bokemeyer A, Baker M, et al. . Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut 2019;68:1115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Franco A, Di Veronica A, Armuzzi A, et al. . Ileal Crohn disease: mural microvascularity quantified with contrast-enhanced US correlates with disease activity. Radiology 2012;262:680–8. [DOI] [PubMed] [Google Scholar]
- 36. Serafin Z, Białecki M, Białecka A, Sconfienza LM, Kłopocka M.. Contrast-enhanced ultrasound for detection of Crohn’s disease activity: systematic review and meta-analysis. J Crohns Colitis 2016;10:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodsall TM, Jairath V, Feagan BG, et al. . Standardisation of intestinal ultrasound scoring in clinical trials for luminal Crohn’s disease. Aliment Pharmacol Ther 2021;53:873–86. [DOI] [PubMed] [Google Scholar]
- 38. Sævik F, Eriksen R, Eide GE, Gilja OH, Nylund K.. Development and validation of a simple ultrasound activity score for Crohn’s disease. J Crohns Colitis 2021;15:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jirik R, Nylund K, Gilja OH, et al. . Ultrasound perfusion analysis combining bolus-tracking and burst-replenishment. IEEE Trans UltrasonFerroelectr Freq Control 2013;60:310–9. [DOI] [PubMed] [Google Scholar]
- 40. Medellin A, Merrill C, Wilson SR.. Role of contrast-enhanced ultrasound in evaluation of the bowel. Abdom Radiol 2018;43:918–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cremer A, Ku J, Amininejad L, et al. . Variability of faecal calprotectin in inflammatory bowel disease patients: an observational case-control study. J Crohns Colitis 2019;13:1372–9. [DOI] [PubMed] [Google Scholar]
- 42. Du L, Foshaug R, Huang VW, Kroeker KI, et al. . Within-stool and within-day sample variability of fecal calprotectin in patients with inflammatory bowel disease. J Clin Gastroenterol 2018;52:235–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.