Abstract
Clinical studies have shown positive associations among sustained and intense inflammatory responses and the incidence of bacterial infections. Patients presenting with acute respiratory distress syndrome (ARDS) and high levels of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), and IL-6, have increased risk for developing nosocomial infections attributable to organisms such as Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter spp., compared to those patients with lower levels. Our previous in vitro studies have demonstrated that these bacterial strains exhibit enhanced growth extracellularly when supplemented with high concentrations of pure recombinant TNF-α, IL-1β, or IL-6. In addition, we have shown that the intracellular milieu of phagocytic cells that are exposed to supraoptimal concentrations of TNF-α, IL-1β, and IL-6 or lipopolysaccharide (LPS) favors survival and replication of ingested bacteria. Therefore, we hypothesized that under conditions of intense inflammation the host's micromilieu favors bacterial infections by exposing phagocytic cells to protracted high levels of inflammatory cytokines. Our clinical studies have shown that methylprednisolone is capable of reducing the levels of TNF-α, IL-1β, and IL-6 in ARDS patients. Hence, we designed a series of in vitro experiments to test whether human monocytic cells (U937 cells) that are activated with high concentrations of LPS, which upregulate the release of proinflammatory cytokines from these phagocytic cells, would effectively kill or restrict bacterial survival and replication after exposure to methylprednisolone. Fresh isolates of S. aureus, P. aeruginosa, and Acinetobacter were used in our studies. Our results indicate that, compared with the control, stimulation of U937 cells with 100-ng/ml, 1.0-μg/ml, 5.0-μg/ml, or 10.0-μg/ml concentrations of LPS enhanced the intracellular survival and replication of all three species of bacteria significantly (for all, P = 0.0001). Stimulation with ≤10.0 ng of LPS generally resulted in efficient killing of the ingested bacteria. Interestingly, when exposed to graded concentrations of methylprednisolone, U937 cells that had been stimulated with 10.0 μg of LPS were able to suppress bacterial replication efficiently in a concentration-dependent manner. Significant reduction in numbers of CFU was observed at ≥150 μg of methylprednisolone per ml (P values were 0.032, 0.008, and 0.009 for S. aureus, P. aeruginosa, and Acinetobacter, respectively). We have also shown that steady-state mRNA levels of TNF-α, IL-1β, and IL-6 in LPS-activated cells were reduced by treatment of such cells with methylprednisolone, in a concentration-dependent manner. The effective dose of methylprednisolone was 175 mg, a value that appeared to be independent of priming level of LPS and type of mRNA. We therefore postulate that a U-shaped relationship exists between the level of expression of TNF-α, IL-1β, and IL-6 within the phagocytic cells and their abilities to suppress active survival and replication of phagocytized bacteria.
The inflammatory reaction is part of the innate immune response of the host to an infectious or noninfectious assault. The most proximal expression of such a response is the elaboration of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), and IL-6. When present at optimal concentrations, these biologically active molecules recruit both specific and nonspecific immune cells—nonlymphoid leukocytes and lymphocytes—to the site of assault and activate them, thereby helping to eradicate the assault and to restore homeostasis. Whereas optimal levels of these peptides are important for a successful defense, at progressively higher concentrations they mediate proportionately stronger local and finally systemic responses (systemic inflammation), with predominantly destructive rather than protective effects on the host (4). Reduction in the effective concentration of proinflammatory mediators is an important component in the resolution of inflammation (27).
Acute respiratory distress syndrome (ARDS) is a frequent form of hypoxemic respiratory failure caused by excessive systemic inflammation, with an associated mortality of 40 to 60% (15). We have previously investigated the longitudinal relationship between pulmonary and circulatory proinflammatory TNF-α, IL-1β, and IL-6 levels, infections, and outcome in patients with (sepsis-induced) ARDS (9, 19, 22). We reported that at the onset of ARDS and over time, nonsurvivors (n = 17) had significantly (P < 0.001) higher plasma TNF-α, IL-1β, and IL-6 levels than survivors (n = 17) did (19). The rate of nosocomial infection per day of mechanical ventilation was 1% in survivors and 8% in nonsurvivors (9). Moreover, none of the proven (n = 36) or suspected (n = 55) nosocomial infections caused either a transient or a sustained increase in plasma TNF-α, IL-1β, IL-6, and IL-8 levels above preinfection values (9). The findings of these studies (9, 19, 22) suggested that final outcome in patients with ARDS is related to the magnitude and duration of the host inflammatory response and that nosocomial infections might be an epiphenomenon of prolonged intense inflammation.
Until recently, very little was known of the ability of bacteria to interfere with or to utilize extracellular cytokines secreted by the host cells or intracellular cytokines within phagocytic cells. Recent reports have shown that certain bacteria have receptors for the cytokines IL-1β and TNF-α and that exposure of bacteria to these cytokines enhanced their growth (12, 25, 29) and virulence (16). Furthermore, studies have reported enhancement of bacterial growth in the presence of cytokines for Escherichia coli (IL-1β [25] gamma interferon [10], IL-2, and granulocyte-macrophage colony-stimulating factor [7]), Staphylococcus aureus (IL-4 [11]), Legionella pneumophila (IL-10 [24]), and Mycobacterium avium (IL-3, granulocyte-macrophage colony-stimulating factor [6, 28], and IL-6 [6, 28]).
In the context of our clinical observations (9) and the experimental work described above, we hypothesized that cytokines secreted by the host during ARDS may indeed favor the growth of bacteria and explain the association between exaggerated and protracted systemic inflammation and the frequent development of nosocomial infections. To test this hypothesis, we conducted in vitro studies evaluating the extracellular and intracellular growth response of three clinically relevant bacteria—S. aureus, Pseudomonas aeruginosa, and Acinetobacter—in response to graded concentrations of proinflammatory cytokines TNF-α, IL-1β, and IL-6 (13, 21). In these studies, we identified a U-shaped response of bacterial growth to proinflammatory cytokines. When the tested bacteria were exposed in vitro to a lower concentration of TNF-α, IL-1β, or IL-6, similar to the values in plasma of ARDS survivors (19), extracellular and intracellular bacterial growth was not promoted and human monocytic cells were efficient in killing the ingested bacteria (13, 21). In contrast, when bacteria were exposed to higher concentrations of these of proinflammatory cytokines, similar to the values in plasma of ARDS nonsurvivors (19), intracellular and extracellular bacterial growth was enhanced in a dose-dependent manner (13, 21).
Recently, we completed a randomized, double-blind, placebo-controlled trial showing significant physiological and survival benefit when prolonged methylprednisolone treatment was administered to ARDS patients failing to improve after 1 week of mechanical ventilation (18). Similar to results of a previous uncontrolled study (20), improvement during methylprednisolone administration was associated with a significant reduction in plasma TNF-α, IL-1β, and IL-6 levels (23).
In the present study, we tested the hypothesis that methylprednisolone can decrease cytokine-mediated enhancement of in vitro bacterial growth and that LPS-stimulated monocytic cells that are impaired in their abilities to kill ingested bacteria would regain their abilities to suppress the survival and/or replication of internalized bacteria when such cells are exposed to adequate concentrations of methylprednisolone.
MATERIALS AND METHODS
Bacteria.
Fresh clinical isolates of S. aureus, P. aeruginosa, and Acinetobacter were obtained from bronchoalveolar lavage fluid or peripheral blood of patients admitted to the University of Tennessee Bowld Hospital, Memphis. All the bacterial isolates were tested for susceptibility to a gentamicin (100 μg/ml), streptomycin (100 μg/ml), and penicillin (100 U/ml) combination using standard in vitro antibiotic susceptibility testing techniques. These fresh isolates of bacteria were grown in 3 ml of RPMI medium without serum or antibiotics (Life Technologies, Bethesda, Md.) at 37°C for 8 h. The bacterial cultures were washed and resuspended in 1 ml of RPMI medium without antibiotics to a concentration of 105 CFU/ml.
Maintenance of U937 cells.
Human monocytic cell line U937 was obtained from the American Type Culture Collection (ATCC; Rockville, Md.). These cells were maintained in RPMI medium with 10% fetal calf serum, 100 U of penicillin per ml and 100 μg of streptomycin (Life Technologies) per ml. Prior to each experiment, cells were centrifuged, resuspended in RPMI containing 2% fetal bovine serum without antibiotics, and seeded into 12-well tissue culture plates (Costar, Cambridge, Mass.) to a concentration of 2 × 106 cells/ml. RPMI medium lacks complex organic materials that may be present in a conventional bacteriological medium and does not interfere with the biological activities of the tested cytokines.
Priming of U937 cells with graded concentrations of LPS.
Replicates of U937 cells (2 × 106 cells/ml) were exposed to various amounts (0, 10, and 100 ng and 1.0, 5.0, and 10.0 μg) of lipopolysaccharide (LPS) purified from E. coli strain K235 (Sigma Chemicals, St. Louis, Mo.). Cells were then incubated for 6 h at 37°C in a humid atmosphere of 5% CO2.
Exposure of LPS-primed U937 cells to graded concentrations of methylprednisolone.
U937 cells (2 × 106 cells/ml) primed with 10.0 μg of LPS (the concentration of LPS shown to give maximal intracellular bacterial survival and replication) were exposed to various amounts (0, 25, 50, 75, 100, 150, and 250 μg) of methylprednisolone sodium succinate (Pharmacia-Upjohn Company, Kalamazoo, Mich.) and incubated at 37°C in a humidified atmosphere of 5% CO2 for 4 to 6 h.
Cell viability as assessed by trypan blue dye exclusion.
The viability of cells was assessed by suspending the isolated cells in 0.04% trypan blue and counting the viable cells under a light microscope. Viabilities of the cells were 70 to 80% in both control and experimental samples (microscopic data not shown; no photographs taken).
Bacterial infection of LPS-primed U937 cells.
Intracellular bacterial survival and replication of the tested bacteria were investigated under two conditions: (i) U937 cells primed with graded concentrations of LPS and (ii) LPS-primed U937 cells exposed to graded concentrations of methylprednisolone. Before these experiments were started, the maintenance or growth culture media were removed from the cell sheets and fresh media without fetal bovine serum or antibiotics were added. These cells (2 × 106 cells/ml) were mixed with 4 × 106 CFU of the above-mentioned bacterial species (S. aureus, P. aeruginosa, and Acinetobacter spp.) per ml and incubated at 37°C in a humidified atmosphere of 5% CO2 for 2 h with intermittent shaking. The extracellular bacteria were killed and removed by treating the respective cultures with a mixture of 200 μg of streptomycin, 200 U of penicillin, and 200 μg of gentamicin (Life Technologies) per ml. The monocytic cells containing internalized bacteria were then washed to remove antibiotics as well as killed extracellular bacteria and most probably any adherent bacteria. The cells were then resuspended in antibiotic-and serum-free RPMI medium and incubated for 6 to 8 h at 37°C in a humid atmosphere of 5% CO2.
Estimation of bacterial CFU.
After the specified incubation, the cells and bacteria were spun down. The pellets were suspended in 1.0 ml of sterile distilled water and sonicated to disrupt the U937 cells without affecting the viability of the bacteria. The lysates were then diluted 10-fold in RPMI-Dulbecco modified Eagle medium without antibiotics or serum. Serially diluted lysates were then plated on Luria-Bertani agar (Difco, Detroit, Mich.) plates and incubated at 37°C for 18 h. The bacterial colonies were counted, and the results were expressed as CFU per milliliter of lysate.
RNA extraction.
Total cellular RNA was isolated from U937 cells stimulated with various amounts of LPS (100 ng, 1.0 μg, 5.0 μg, and 10.0 μg) and treated with various amounts (0, 25, 50, 75, 100, 150, and 250 μg) of methylprednisolone, using a modified procedure previously described (14). Briefly, the cells were harvested, washed in sterile normal phosphate-buffered saline, and lysed using Trizol reagent (Life Technologies). Total cellular RNA was extracted from the cell lysates with chloroform (Sigma Chemicals) followed by ethanol (Sigma Chemicals) precipitation. The RNA was stored as dry pellets or as aliquots of aqueous solutions at −80°C until used.
RT.
Reverse transcription (RT) reactions were performed in accordance with a procedure described by Kanangat et al. (14). Five micrograms of total cellular RNA was reverse transcribed using avian myeloblastosis virus reverse transcriptase and oligo(dT)18 primer (Promega Corporation, Madison, Wis.). In addition to avian myeloblastosis virus reverse transcriptase and oligo(dT)18 primer, the reaction mixture consisted of 5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100, 2 mM deoxynucleoside triphosphate, and 40 U of ribonuclease inhibitor (Promega). The mixture was incubated for 15 min at ambient temperature and for an additional 90 min at 42°C and then heated at 99°C for 5 min and cooled on ice.
PCR.
PCR was done using a previously described method (14). Five microliters of the RT mixture (cDNA) was used in a 25.0-μl PCR mixture for qualitative detection of β-actin (used as a control for RNA isolation and RT efficiency) and mRNA of TNF-α, IL-1β, and IL-6. All these reactions were done in separate tubes to avoid possible competition among different target and primer pairs. The reaction mixture consisted of 1.5 to 2.5 mM MgCl2, 0.1% Triton X-100, 125 μM (each) dATP, dCTP, dGTP, and dTTP, 50 mM Tris HCl (pH 8.3), and 1.0 U of Taq DNA polymerase (Life Technologies). The conditions for PCR consisted of denaturing at 94°C for 90 s and annealing at 55°C for 60 s followed by extension at 72°C for 120 s. These cycles were repeated 35 times for each message mentioned above. The primers were used at a concentration of 15 pmol per reaction. PCR products were analyzed on a 2.5% gel (Life Technologies), stained with ethidium bromide (Sigma Chemicals), and photographed. The intensities of the bands were measured using the Alpha Imager 2000 documentation and analysis system (Alpha Inotech Corporation, San Leandro, Calif.).
Repetition of experiments.
The experiments evaluating intracellular survival and replication of bacteria within U937 cells primed with graded concentrations of LPS were run in triplicate. The experiments assessing the intracellular survival and replication of bacteria in LPS-primed U937 cells exposed to graded concentrations of methylprednisolone were run in duplicate. The experiments with U937 cells exposed to 10.0 μg of LPS and 250 μg of methylprednisolone were done in triplicate. The quantification of steady-state mRNA levels of TNF-α, IL-1β, and IL-6 were done in duplicate.
Statistical analysis.
For U937 cells primed with graded concentrations of LPS, intracellular survival and replication (106 CFU/ml) were analyzed with two-way analysis of variance (ANOVA); the main effects of bacterial type (df = 6), as well as the interaction effects (df = 12), were included in the model. Within each bacterial type, the following seven preplanned comparisons were made: response to each concentration of LPS compared to the control and response to 1.0 ng compared to 10 ng.
For LPS-primed U937 cells exposed to graded concentrations of methylprednisolone, intracellular survival and replication (106 CFU/ml) were analyzed with two-way ANOVA. Each bacterial type was analyzed separately. The two treatments of the U937 cells were (i) priming with 10 μg of LPS alone and (ii) priming with 10 μg of LPS and graded concentrations of methylprednisolone. Statistical models included the main effects of treatment (df = 1) and dose of methylprednisolone (df = 5), as well as the interaction effects. Within each type of bacteria, six preplanned contrasts were made; these were contrasts between the two treatments at the same dose of methylprednisolone. For a separate experiment in which bacterial survival and replication was measured in U937 cells primed with 10 μg of LPS with or without exposure to 250 μg of methylprednisolone, intracellular survival and replication (106 CFU/ml) were analyzed with independent sample t tests for equal or unequal variances.
For U937 monocytic cells primed with four levels of LPS and exposed to seven levels of methylprednisolone, mRNA expression of TNF-α, IL-1β, or IL-6 within each level of LPS was analyzed with simple linear regression with expression of mRNA (units of intensity) as the dependent variable and methylprednisolone as the independent variable. For each regression model (i.e., for each type of mRNA expression at each level of LPS), the effective dose of methylprednisolone was defined as the level of methylprednisolone associated with a 50% reduction in mRNA expression. The value for mRNA expression that corresponded to a 50% reduction was empirically derived according to the following formula: median maximum expression/2. Then, each value was substituted in the appropriate regression equation and the effective dose determined by solving for the unknown level of methylprednisolone. Because of the assumptions necessary for linear regression, such values are assumed to be invariate. For each cytokine, effective doses were averaged over levels of LPS. Because these average effective doses were remarkably similar, the effective dose was estimated by averaging over both levels of LPS and type of cytokine.
RESULTS
Bacterial survival and replication in U937 cells primed with graded concentrations of LPS.
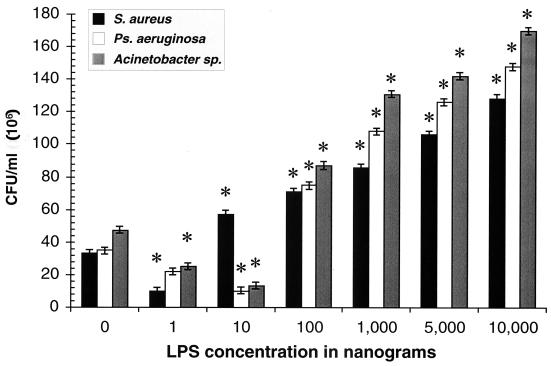
Figure 1 shows the intracellular survival and replication of S. aureus, P. aeruginosa, and Acinetobacter spp. within U937 cells exposed to graded concentrations of LPS. For all three species of bacteria, concentration-dependent responses were observed, with reductions in intracellular survival and replication at lower concentrations of LPS and enhancements at higher concentrations (≥100 ng of LPS per ml) except S. aureus, for which the lowest enhancing concentration was 10 ng of LPS per ml. However, at priming concentration of 1 ng of LPS per ml, reductions in intracellular survival and replication in comparison to the control (i.e., no LPS) were observed for all three bacterial species (P values, 0.0001, 0.02, and 0.0001, respectively).
FIG. 1.
Intracellular bacterial survival/replication of S. aureus, P. aeruginosa, and Acinetobacter in U937 cells primed with graded concentrations of LPS. Concentration-dependent responses were observed, with reductions in intracellular bacterial survival and replication at lower concentrations of LPS (1.0 and 10.0 ng/ml, except for S. aureus) and enhancements in intracellular bacterial survival and replication at higher concentrations (≥100 ng/ml, except for S. aureus at ≥10.0 ng/ml). Standard errors were estimated by the square root of the mean square error from ANOVA divided by the square root of 3. Each P value is probability that the difference between the mean intracellular survival and replication for cells primed with a particular concentration of LPS and the mean of the control cells (i.e., no LPS) is zero, given that the null hypothesis is true. ∗, P = 0.0001;
Bacterial survival and replication in LPS-primed U937 cells treated with graded concentrations of methylprednisolone.
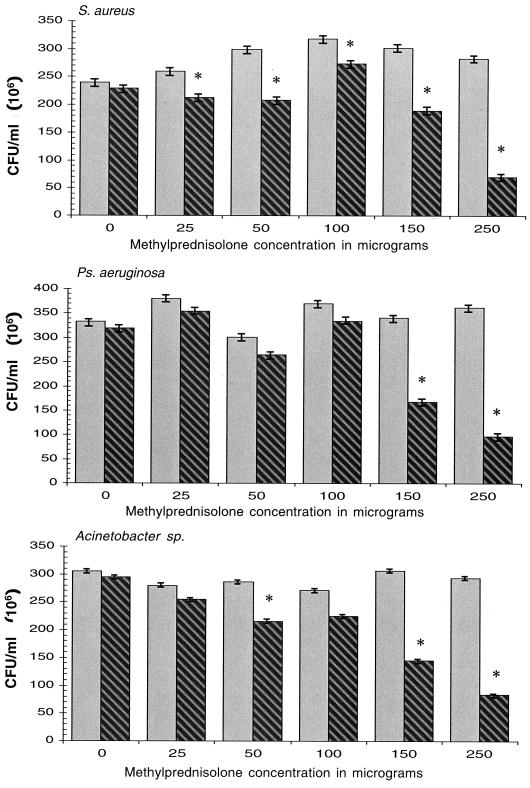
Figure 2 shows the intracellular survival and replication (106 CFU/ml) of S. aureus, P. aeruginosa, and Acinetobacter in U937 cells primed with 10 μg of LPS per ml (the concentration of LPS associated with the highest intracellular bacterial survival and replication) and then exposed to graded concentrations of methylprednisolone (0, 25, 50, 75, 100, 150, and 250 μg per ml). Although intracellular survival and replication were significantly reduced for S. aureus and Acinetobacter by lower doses of methylprednisolone, concentration-dependent responses were observed for all three bacterial species at concentrations of methylprednisolone of 150 and 250 μg/ml (P = 0.001).
FIG. 2.
Intracellular bacterial survival and replication of S. aureus, P. aeruginosa, and Acinetobacter in U937 cells primed with 10 μg of LPS per ml and then exposed to graded concentrations of methylprednisolone. (These experiments were done in duplicate.) Gray bars, U937 monocytic cells primed with LPS alone; hatched bars, U937 cells primed with LPS and then exposed to methylprednisolone (0, 25, 50, 75, 100, 150, and 250 μg per ml). Standard errors for each bacterial species were estimated from the square root of the mean square error from ANOVA divided by the square root of 2, and the P values reflect the probability that the the mean intracellular bacterial survival and replication of primed U937 cells with exposure to methylprednisolone is equal to the mean of primed U937 cells without exposure ∗, P = 0.0001.
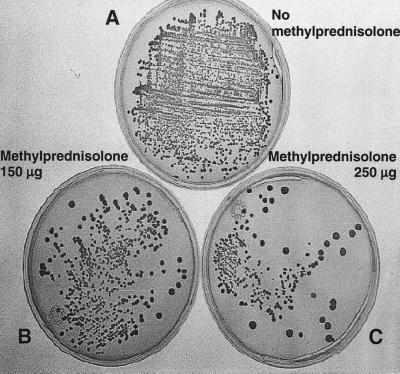
Figure 3 shows the concentration-dependent effect of methylprednisolone on the survival and replication of ingested S. aureus, P. aeruginosa, and Acinetobacter within LPS-primed (10 μg/ml) U937 cells. Because the maximal effects were detected with 250 mg of methylprednisolone per ml, the experiments on intracellular bacterial survival and replication within U937 cells exposed to 10 μg of LPS per ml and subsequently treated with 250 μg of methylprednisolone per ml were replicated three times. The data presented in Table 1 show significant reductions in the survival and replication of all three bacterial species in cells treated with 250 μg of methylprednisolone per ml compared with specimens not treated with methylprednisolone.
FIG. 3.
Intracellular survival and replication of S. aureus in U937 cells primed with 10 μg of LPS per ml and then exposed to methylprednisolone at 0, 150, and 250 μg per ml. Note the reductions in intracellular survival and replication with methylprednisolone.
TABLE 1.
Reduced survival and replication of bacteria in LPS-activated U937 cells exposed to methylprednisolone at 250 μg/ml compared to controls
| Organism | 106 CFU/ml (mean ± SDa)
|
Pb | |
|---|---|---|---|
| Without methylprednisolone | With methylprednisolone | ||
| S. aureus | 275 ± 62 | 82 ± 7 | 0.033 |
| P. aeruginosa | 381 ± 53 | 104 ± 11 | 0.012 |
| Acinetobacter | 375 ± 71 | 127 ± 30 | 0.006 |
Values are means for three experiments.
Each P value is the probability that the mean of the group treated with methylprednisolone is equal to the mean of the control group, given that the null hypothesis is true. Independent sample t tests for equal or unequal variants were used.
Expression of TNF-α, IL-1β, and IL-6 in LPS-primed U937 cells exposed to graded concentrations of methylprednisolone.
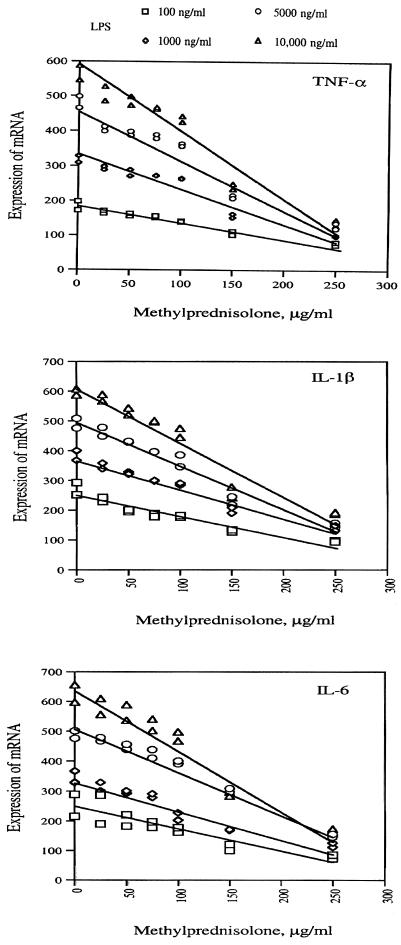
Figure 4 shows the regression of mRNA levels of TNF-α, IL-1β, and IL-6 in U937 cells primed with four different concentrations of LPS (100 ng, 1.0 μg, 5 μg, and 10 μg per ml) on graded concentrations of methylprednisolone (0, 25, 50, 75, 100, 150, and 250 μg per ml). Table 2 depicts parameter estimates from regression of mRNA levels of TNF-α, IL-1β, and IL-6 in U937 cells primed with four different concentrations of LPS (100 ng, 1.0 μg, 5 μg, and 10 μg per ml) on graded concentrations of methylprednisolone (0, 25, 50, 75, 100, 150, and 250 μg per ml). Based on these data, the estimated effective dose of methylprednisolone (defined as the dose associated with a 50% reduction in mRNA) was 175 μg/ml. The effective dose of methylprednisolone appeared to be independent of LPS and the type of proinflammatory cytokine mRNA. Interestingly, this estimate and the estimates obtained from each regression equation (Table 2) were all within the range of methylprednisolone concentrations (150 to 250 μg/ml) at which significant reductions were observed in survival and replication of all three types of bacteria (Fig. 2).
FIG. 4.
Regression of concentration of methylprednisolone on steady-state mRNA levels of TNF-α, IL-1β, and IL-6 in U937 cells primed with LPS. U937 cells (2 × 106/ml) were primed with LPS and exposed to graded concentrations of methylprednisolone for 4 to 6 hs. The results are expressed as the ratio of the mRNA for a particular cytokine to mRNA for β-actin.
TABLE 2.
Parameter estimates from regression of ratios of mRNA for TNF-α, IL-1β, and IL-6 to mRNA for β-actin obtained from LPS-stimulated U937 cells on level of methylprednisolone (MP)
| Cytokine | LPS concn (ng/ml) | Cytokine/β-actin ratio
|
Regression equationc | Effective dose of MP (μg/ml)d | ||
|---|---|---|---|---|---|---|
| Minimuma | Maximuma | 50% reductionb | ||||
| TNF-α | 100 | 77.0 | 185.0 | 92.50 | 181.73 − 0.44(MP) | 203 |
| 1,000 | 99.5 | 318.5 | 159.25 | 326.02 − 0.92(MP) | 181 | |
| 5,000 | 129.0 | 481.5 | 240.75 | 468.06 − 1.40(MP) | 162 | |
| 10,000 | 137.5 | 566.0 | 283.00 | 570.84 − 1.79(MP) | 161 | |
| Mean | 177 | |||||
| IL-1β | 100 | 96.0 | 272.5 | 136.25 | 247.97 − 0.67(MP) | 167 |
| 1,000 | 140.0 | 384.5 | 192.25 | 376.37 − 1.00(MP) | 184 | |
| 5,000 | 150.0 | 392.5 | 246.25 | 497.26 − 1.46(MP) | 172 | |
| 10,000 | 189.5 | 597.0 | 298.50 | 612.94 − 1.81(MP) | 174 | |
| Mean | 174 | |||||
| IL-6 | 100 | 79.5 | 252.5 | 126.25 | 245.03 − 0.73(MP) | 163 |
| 1,000 | 119.0 | 348.0 | 174.00 | 338.86 − 0.96(MP) | 172 | |
| 5,000 | 151.5 | 488.5 | 244.25 | 512.30 − 1.39(MP) | 193 | |
| 10,000 | 168.5 | 625.5 | 312.75 | 643.36 − 1.95(MP) | 170 | |
| Mean | 175 | |||||
| Overall mean | 175 | |||||
| Overall median | 172 | |||||
Median of duplicate measurements.
50% reduction = (median maximum expression)/2.
Ŷ = α̂ − β̂(level of MP), where Ŷ is the ratio of mRNA for a particular cytokine, α̂ is the intercept, and β̂ is the regression coefficient.
After the value of a 50% reduction was substituted for Ŷ, the effective dose was obtained by solving for the unknown level of MP.
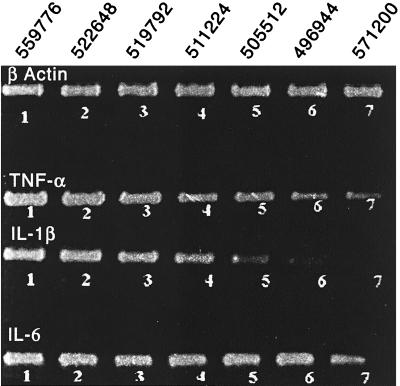
Figure 5 is an ethidium bromide-stained gel photograph showing the reductions in the intensities of specific bands of TNF-α, IL-1β, and IL-6 (a reflection of their respective steady-state mRNA levels) in LPS-primed (10 μg/ml) U937 cells treated with graded concentrations of methylprednisolone. The values are related to the levels of β-actin, which according to the densitometric analysis of the bands do not differ appreciably from experiment to experiment. Therefore, we assume that there are no biologically important numbers of cell deaths associated with the manipulations with LPS and methylprednisolone in vitro.
FIG. 5.
Steady-state mRNA levels of TNF-α, IL-1β, and IL-6 in U937 cells primed with 10 μg of LPS and exposed to graded concentrations of methylprednisolone. U937 cells (2 × 106/ml) were primed with LPS at 10 μg and exposed to graded concentrations of methylprednisolone (0, 25, 50, 75, 100, 150, and 250 μg per ml [bands 1 through 7, respectively]) for 4 to 6 h. The cells were then harvested, and the total cellular RNA was extracted. The cellular mRNAs were then reverse transcribed using oligo(dT)18 primers, and the steady-state level of TNF-α, IL-1β, and IL-6 mRNAs were measured by semiquantitative PCR, taking β-actin mRNA levels as the internal control for total mRNA extraction and general efficiency of the reverse transcription. The three-dimensional measurement of intensities of the bands (visualized by ethidium bromide staining) was obtained using the Alpha Inotech 2000 imaging system. The values related to the levels of β-actin do not differ appreciably from experiment to experiment and are presented above the gel.
DISCUSSION
In the present study, we found that the intracellular survival and replication of S. aureus, P. aeruginosa, and Acinetobacter in U937 monocytic cells was affected by the degree of cell activation obtained with exposure to increasing concentrations of LPS. Activation of phagocytic cells with high concentrations of LPS (≥100 ng/ml) significantly enhanced intracellular bacterial survival and replication, while stimulation with lower concentrations of LPS (1.0 or 10.0 ng/ml) effectively suppressed bacterial survival and replication (Fig. 1), depending on the type of bacteria. The impairment in intracellular bacterial killing coincided with an increased expression of proinflammatory cytokines (Fig. 4A). Exposure of LPS-stimulated cells (10.0 μg/ml) to graded concentrations of methylprednisolone resulted in appreciable dose-dependent reductions in the survival and replication of internalized bacteria (Fig. 2 and 3 and Table 1). Replication of all three bacterial species was significantly reduced in LPS-activated cells treated with 250 μg of methylprednisolone per ml (Table 1). Methylprednisolone treatment of LPS-activated U937 cells resulted in appreciable reductions in the steady state levels of mRNA for TNF-α, IL-1β, and IL-6 (Fig. 4 and Table 2). The effective dose for reducing transcription of TNF-α, IL-1β, and IL-6 was 175 μg/ml, a value that coincided with levels that reduced bacterial intracellular survival and replication in a manner independent of the priming dose of LPS (Table 2).
Our investigation utilized in vitro studies wherein potentially phagocytic cells were tested in microenvironments that mimic selected aspects of in vivo inflammation. Certain technical aspects of the protocol require clarification. We have not quantified the phagocytic indices under the different experimental conditions because our design provided appropriate controls. We eliminated the extracellular bacteria with appropriate antibiotic combinations. However, we did not add antibiotics to the final step of the culture process. Our intent was to quantify the survival and replication of the bacteria engulfed by the monocytic cells. We recognize that some cells may have died during the process and released bacteria to the external medium. The external medium was likely rich in cytokines and might have favored the survival and replication of bacteria. Because antibiotics in the extracellular medium would have affected survival of the released bacteria, at this step we avoided any inclusion of antibiotics in the extracellular medium.
The results of our in vitro experiments indicate that methylprednisolone does not impair the ability of activated monocytes to phagocytize and kill ingested bacteria. To the contrary, we found that methylprednisolone restored the intracellular killing capacity of phagocytic cells primed with high concentrations of LPS. In methylprednisolone-treated cells, the reduction in intracellular bacterial survival and replication in these cells coincided (Table 1) with a reduction in the expression of TNF-α, IL-1β, and IL-6 (Fig. 4; Table 2). Hence we presume that bacterial survival and replication within phagocytic cells is in part associated with the cytokines expressed by such cells. How exactly the cells lose their killing functions without any apparent impairment in the phagocytic abilities is not known. It is likely that once bacteria are internalized and are under selective pressure they adapt to an otherwise hostile microenvironment by switching on novel genes' expression, which enables them to utilize cytokines as growth factors. It has previously been shown that the ability of fresh bacterial isolates (obtained from infected patients) to respond to proinflammatory cytokines is lost after several in vitro passages on a bacteriological medium without added cytokines (21). LPS activation of monocytes may also induce the expression of a variety of small mRNA molecules, which are thought to be converted into biologically active low-molecular-weight proteins (26). The low-molecular-weight mRNA molecules may code for proteins or small polypeptides that become growth factors for certain bacteria, or bacteria may acquire the ability to utilize those peptides under selective pressure.
It is unclear how bacteria may use cytokines for their growth, since bacteria are prokaryotes without a defined nucleus and cytokines are intended to work on well-defined eukaryotic cells with consequent signal transduction events. However, in a host milieu, bacteria may adapt to eukaryotic cellular processes (8). Although the subsequent sequence of intracellular events has not been delineated, it is possible that bacteria might use cytokines through receptor-mediated, signal transduction-induced activities that would require the presence of biochemical processes akin to those seen in eukaryotic cells; cytokines may act on bacteria through a signaling process similar to that of eukaryotes but involving different biochemical pathways; or bacteria may break down cytokines into biologically active fragments that are transported across the bacterial cell membranes and act on specific gene transcription and translation. In-depth studies of LPS activation and bacterial infections at the cellular and molecular levels are necessary to investigate the foregoing speculations.
Our results suggest that glucocorticoids may have the capacity to restore the intracellular killing functions of phagocytic cells impaired by excessive activation. By showing that methylprednisolone can reduce, in a dose-dependent-manner, the mRNA expression of TNF-α, IL-1β, and IL-6 and the intracellular bacterial growth of the tested bacteria, we provide in vitro experimental data suggesting a cause-and-effect relationship between excessive inflammation and bacterial growth. Finally, in the present study, we have assumed that the effects of methylprednisolone on intracellular bacterial growth are mediated by affecting monocyte activation and their ability to produce proinflammatory cytokines. However, we cannot exclude the possibility that methylprednisolone may have an additional direct effect on bacterial structural integrity and replication potential. In this regard, we have observed a direct detectable bactericidal effect of methylprednisolone on the tested bacteria (as demonstrated in culture by significant reductions in numbers of CFU per milliliter and by electron microscopy by morphological changes) at concentrations of methylprednisolone of ≥600 μg/ml, a value that is 2.5 times the highest concentration used in this study (unpublished data). Additionally, when we removed methylprednisolone from the culture medium of LPS-activated cells before and after adding bacteria, we did not observe any significant difference in numbers of CFU of bacteria from the cell culture (unpublished data).
Overall, our findings indicate that excessive systemic inflammation not only leads to inappropriate tissue damage and maladaptive tissue repair (17) but may also create an “acquired” host environment that favors the growth of bacteria. We have provided in vitro experimental evidence to suggest a possible cause-and-effect relationship between excessive inflammation (TNF-α, IL-1β, and IL-6) and enhanced bacterial survival. We and others have previously shown that in patients with life-threatening systemic inflammation, prolonged glucocorticoid treatment has to be sustained to decrease circulating levels of inflammatory mediators (3, 20) and to improve organ function (1, 2, 5, 18). This experimental work suggests that under such conditions, administration of exogenous glucocorticoids may also restore the host's ability to counteract infections.
ACKNOWLEDGMENTS
This study was supported by the Assisi Foundation of Memphis and the Baptist Memorial Health Care Foundation and in part by the University of Tennessee Medical Group (UTMG), Memphis.
We thank Vivian Gomez for creating the figures and Gail Spake for revision of the manuscript.
REFERENCES
- 1.Bollaert P E, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–650. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Briegel J, Haller M, Forst H, Schelling G, Kuprat G, Hemmer B, Peter K. Effect of hydrocortisone on reversal of hyperdynamic septic shock: a randomized, double-blind, placebo-controlled, single-center study. Shock. 1997;7:165. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann G E, Buchler M, Uhl W, Peter K. Low-dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. The Phospholipase A2 Study Group. Clin Investig. 1994;72:782–787. doi: 10.1007/BF00180547. [DOI] [PubMed] [Google Scholar]
- 4.Cerami A. Inflammatory cytokines. Clin Immunol Immunopathol. 1992;62:S3–S10. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 5.Chawla K, Kupfer Y, Goldman I, Tessler S. Hydrocortisone reverses refractory septic shock. Crit Care Med. 1999;27:A33. [Google Scholar]
- 6.Denis M. Growth of Mycobacterium avium in human monocytes: identification of cytokines which reduce and enhance intracellular microbial growth. Eur J Immunol. 1991;21:391–395. doi: 10.1002/eji.1830210221. [DOI] [PubMed] [Google Scholar]
- 7.Denis M, Campbell D, Gregg E O. Interleukin-2 and granulocyte-macrophage colony-stimulating factor stimulate growth of a virulent strain of Escherichia coli. Infect Immun. 1991;59:1853–1856. doi: 10.1128/iai.59.5.1853-1856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkow S. Perspectives series: host/pathogen interactions. Invasion and intracellular sorting of bacteria: searching for bacterial genes expressed during host/pathogen interactions. J Clin Invest. 1997;100:239–243. doi: 10.1172/JCI119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Headley A S, Tolley E, Meduri G U. Infections and the inflammatory response in acute respiratory distress syndrome. Chest. 1997;111:1306–1321. doi: 10.1378/chest.111.5.1306. [DOI] [PubMed] [Google Scholar]
- 10.Hogan J S, Todhunter D A, Smith K L, Schoenberger P S, Sordillo L M. Growth responses of coliform bacteria to recombinant bovine cytokines. J Dairy Sci. 1993;76:978–982. doi: 10.3168/jds.S0022-0302(93)77425-1. [DOI] [PubMed] [Google Scholar]
- 11.Hultgren O, Kopf M, Tarkowski A. Staphylococcus aureus-induced septic arthritis and septic death is decreased in IL-4-deficient mice: role of IL-4 as promoter for bacterial growth. J Immunol. 1998;160:5082–5087. [PubMed] [Google Scholar]
- 12.Kanangat S, Bronze M S, Meduri G U, Postlethwaite A, Stentz F, Tolley E, Schaberg D. Enhanced extracellular growth of Staphylococcus aureus in the presence of selected linear peptide fragments of human interleukin (IL)-1beta and IL-1 receptor antagonist. J Infect Dis. 2001;183:65–69. doi: 10.1086/317645. [DOI] [PubMed] [Google Scholar]
- 13.Kanangat S, Meduri G U, Tolley E A, Patterson D R, Meduri C U, Pak C, Griffin J P, Bronze M S, Schaberg D R. Effects of cytokines and endotoxin on the intracellular growth of bacteria. Infect Immun. 1999;67:2834–2840. doi: 10.1128/iai.67.6.2834-2840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanangat S, Thomas J, Gangappa S, Babu J S, Rouse B T. Herpes simplex virus type 1-mediated up-regulation of IL-12 (p40) mRNA expression. Implications in immunopathogenesis and protection. J Immunol. 1996;156:1110–1116. [PubMed] [Google Scholar]
- 15.Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald R D, Koc D, Schneider B, Hammerle A F, Steltzer H. The acute respiratory distress syndrome: definitions, severity and clinical outcome. An analysis of 101 clinical investigations. Intensive Care Med. 1996;22:519–529. doi: 10.1007/BF01708091. [DOI] [PubMed] [Google Scholar]
- 16.Luo G, Niesel D W, Shaban R A, Grimm E A, Klimpel G R. Tumor necrosis factor alpha binding to bacteria: evidence for a high-affinity receptor and alteration of bacterial virulence properties. Infect Immun. 1993;61:830–835. doi: 10.1128/iai.61.3.830-835.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meduri G U. The role of the host defence response in the progression and outcome of ARDS: pathophysiological correlations and response to glucocorticoid treatment. Eur Respir J. 1996;9:2650–2670. doi: 10.1183/09031936.96.09122650. [DOI] [PubMed] [Google Scholar]
- 18.Meduri G U, Headley S, Carson S, Umberger R, Kelso T, Tolley E. Prolonged methylprednisolone treatment improves lung function and outcome of unresolving ARDS. A randomized, double-blind, placebo-controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 19.Meduri G U, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 20.Meduri G U, Headley S, Tolley E, Shelby M, Stentz F, Postlethwaite A. Plasma and BAL cytokine response to corticosteroid rescue treatment in late ARDS. Chest. 1995;108:1315–1325. doi: 10.1378/chest.108.5.1315. [DOI] [PubMed] [Google Scholar]
- 21.Meduri G U, Kanangat S, Stefan J, Tolley E, Schaberg S. Cytokines IL-1beta, IL-6, and TNF-alpha enhance in vitro growth of bacteria. Am J Respir Crit Care Med. 1999;160:961–967. doi: 10.1164/ajrccm.160.3.9807080. [DOI] [PubMed] [Google Scholar]
- 22.Meduri G U, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 23.Meduri G U, Tolley E A, Chrousos G P, Stentz F. Prolonged glucocorticoid (GC) treatment suppresses systemic inflammation in patients with unresolving ARDS: evidence for inadequate endogenous GC secretion and inflammation-induced cell resistance to GCS. Am J Respir Crit Care Med. 2001;163:A139. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- 24.Park D R, Skerrett S J. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ: differential responses of blood monocytes and alveolar macrophages. J Immunol. 1996;157:2528–2538. [PubMed] [Google Scholar]
- 25.Porat R, Clark B D, Wolff S M, Dinarello C A. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991;254:430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro R A, Flores C A, Cunha F Q, Ferreira S H, De Lucca F L. Partial characterization of the RNA from LPS-stimulated macrophages that induces the release of chemotactic cytokines by resident macrophages. Mol Cell Biochem. 1995;148:105–113. doi: 10.1007/BF00928147. [DOI] [PubMed] [Google Scholar]
- 27.Rossi A G, Haslett C. Inflammation, cell injury, and apoptosis. In: Said S I, editor. Proinflammatory and anti-inflammatory peptides. Vol. 112. New York, N.Y: Marcel Dekker, Inc; 1998. pp. 9–24. [Google Scholar]
- 28.Shiratsuchi H, Johnson J L, Ellner J J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991;146:3165–3170. [PubMed] [Google Scholar]
- 29.Zav'yalov V P, Chernovskaya T V, Navolotskaya E V, Karlyshev A V, MacIntyre S, Vasiliev A M, Abramov V M. Specific high affinity binding of human interleukin 1 beta by Caf1A usher protein of Yersinia pestis. FEBS Lett. 1995;371:65–68. doi: 10.1016/0014-5793(95)00878-d. [DOI] [PubMed] [Google Scholar]