Abstract
Polyneuropathy Organomegaly, Endocrinopathy, Monoclonal protein and Skin changes syndrome is a rare multisystem condition with a range of manifestations which are often overlooked as trivial comorbidities, until their whole triggers the possibility of the diagnosis. The diagnosis is typically delayed by 12–16 months, by which time patients can be severely disabled. There are no established consensus guidelines. We provide clinicians a comprehensive blueprint for managing POEMS from diagnostic suspicion through the work-up, selection of therapy, follow-up, and treatment of relapse based on published evidence and our large single-center experience. A multidisciplinary approach is essential including expert hematologists, neurologists, histopathologists, radiologists, and neurophysiologists. The aim of treatment is to eradicate the underlying plasma cell dyscrasia, but there are limited trial data to guide treatment decisions. Supportive care considerations include management of endocrinopathy, neuropathy, thrombosis, and infection. Response assessment is centered on clinical, neuropathy, hematological, vascular endothelial growth factor, and radiological criteria. Future clinical trials are welcomed in this setting where evidence is limited.
INTRODUCTION
Polyneuropathy Organomegaly, Endocrinopathy, Monoclonal protein and Skin changes (POEMS) syndrome is a rare multisystem condition of undefined pathogenesis. Patients present most often to neurologists or hematologists with progressive peripheral neuropathy and evidence a of monoclonal plasma cell disorder. There are a range of systemic manifestations which are noted but often overlooked as trivial comorbidities. The diagnosis is typically delayed by 12–16 months, by which time patients can be severely disabled and bed or wheelchair-bound with established neuropathy.1 Delayed or misdiagnosis, which has also been noted in specialist centres2 is also financially burdensome for healthcare systems.3
Treatment, in the main, improves functionality and leads to encouraging rates of progression-free survival. Furthermore dramatic improvements in overall survival have been achieved in the last 20 years.4–6 Knowledge of this treatment responsiveness should motivate clinical teams to complete diagnostic tests without delay and identify this condition promptly before irreversible changes occur.
There are no established treatment guidelines for POEMS syndrome. Effective management requires targeting the underlying plasma cell clone in parallel with proactive management of comorbid risks and POEMS-induced physiological impairments. Response assessment requires experienced interpretation of multiple clinical and investigation modalities.
The aim of this framework is to provide clinicians a blueprint for managing POEMS from the moment of diagnostic suspicion through the work-up, selection of therapy, follow-up, and treatment of relapse. We describe the clinical symptoms, diagnostic work-up, treatment, and response assessment.
FRAMEWORK FOR DIAGNOSIS AND MANAGEMENT
POEMS syndrome has a discernible homogeneity despite its multiplicity of features as the frequent occurrence of so many of the multisystem features together as described in the diagnostic criteria is so recognizable.7,8 Table 1 outlines diagnostic criteria. Clinical features include a lambda light-chain-restricted plasma cell dyscrasia, progressive inflammatory conduction slowing (demyelinating) neuropathy with early and predominantly lower limb axonal loss, characteristic skin changes, frequent thromboembolic events, vasculopathy, erythrocytosis, thrombocytosis, respiratory, and cardiac insufficiency. Experienced hematologists, neurologists, radiologists, histopathologists, and neurophysiologists must differentiate POEMS from more common conditions.
Table 1.
Diagnostic Criteria for POEMS Syndrome
| Criteria | |
|---|---|
| Mandatory major criteria—both required | Polyneuropathy, typically conduction slowing (“demyelinating”) |
| Monoclonal plasma cell disorder, typically lambda light-chain restricted | |
| Other major criteria—one required | Castleman disease |
| Sclerotic bone lesions | |
| Elevated VEGF | |
| Minor criteria—one required | Organomegaly |
| • Splenomegaly, hepatomegaly, lymphadenopathy | |
| Extravascular volume overload | |
| • Peripheral edema, ascites, pleural effusions | |
| Endocrinopathy | |
| • Adrenal, pituitary, gonadal, parathyroid | |
| • Thyroid and pancreatic (nondiagnostic) | |
| Skin changes | |
| • Hyperpigmentation, hypertrichosis, glomeruloid hemangiomata, plethora, acrocyanosis, flushing, white nails | |
| Papilledema | |
| Thrombocytosis or polycythemia | |
CLINICAL SYMPTOMS: IDENTIFYING POEMS SYNDROME IN THE CLINIC
Sensorimotor neuropathy is the most common initial symptom of POEMS syndrome. This typically starts distally, in the feet and progresses proximally in a length-dependent fashion; the hands only become affected once neuropathy reaches the knees.1 Deterioration may occur in days-to-weeks in some and weeks-to-months in others. Patients initially complain of a cramping, tight sensation in the calves initially as if they have “run too far” and later, bilateral, hyperesthetic and dysaesthetic neuropathic pain. Weakness is striking in its distal selectivity (below the ankles and the intrinsic hand muscles) and differs from chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) in its lack of proximal weakness for the earlier course. Early increased tendency to trip over feet may be due to footdrop. The distal, ataxic sensory neuropathy associated with IgM anti-MAG antibodies is entirely different. Indeed, the clinical neuropathy of POEMS is almost entirely clinically discernible from all other paraprotein-associated neuropathies by its clinical and electrophysiological features.
These clinical features alongside a monoclonal plasma cell disorder—almost always lambda light-chain restricted—or unexplained polycythemia or thrombocytosis should prompt investigation for other POEMS features. Peripheral edema is found in most1 and may present as ascites (7%–54%), pleural (3%–43%), and pericardial effusions (1%–64%). This is often poorly responsive to diuretics as the excess fluid is not within the vascular compartment. Papilledema, sometimes with characteristic subretinal hemorrhages, and without raised intracranial pressure is present in 30%–40% of patients.1 Stroke is a recognized feature,9 and noninfiltrative, noninfective pachymeningitis caused by meningeal fluid leak has been recently reported in 70% of cases.10
Skin lesions are common (>70%). Glomerular hemangiomata are a pathognomonic finding, but sclerodermoid changes of the digits (5%–43%), temperature-sensitive blue/purple discoloration of the feet (acrocyanosis 19%), leukonychia, clubbing (5%–49%), hyperpigmentation of the skin (46%–93%), and new growth of excess bodily hair (26%–74%) are frequent and readily visible.1,11–13
Other features suggestive of POEMS include fatigue, unexplained weight loss, enlarged lymph nodes or organomegaly, unprovoked venous and/or arterial thrombosis and features of gonadal failure (erectile dysfunction, early menopausal symptoms, infertility). Diabetes and thyroid disease are common in the general population and although potentially associated are not included in the diagnostic algorithm because they lack any diagnostic differentiation.
Examination may reveal lower limb symmetrical distal wasting and weakness, particularly of ankle dorsiflexion and intrinsic foot muscles, initially full strength at the knees and elbows, and more proximally; severely affected rapidly progressive or untreated patients may have proximal weakness at the knees and hips. Upper limb involvement also starts distally with wasting and weakness of intrinsic hand muscles and finger extensors with preservation of other muscles in the earlier stages. Sensory loss is variable; most commonly vibration and pin prick modalities are contiguously affected to the same level and are more severely affected than joint position sensation. Pin sensation is frequently hyperesthetic.
A diagnosis of CIDP that is unresponsive to treatments such as intravenous immunoglobulin or plasma exchange should also trigger a search for POEMS syndrome1,14,15 to avoid delay with further unnecessary and expensive therapies before a diagnosis of is POEMS considered.3 Corticosteroids may induce partial and temporary benefit but can also suppress diagnostic features such as the serum vascular endothelial growth factor (VEGF) and delay the diagnosis.
DIAGNOSTIC WORK-UP
We regard POEMS syndrome as a hematological/neurological emergency and complete diagnostic testing as soon as possible to enable rapid institution of therapy. Diagnostic resource use is high, but each day’s delay impacts on the patient’s performance status that can add weeks or months to the recovery phase and has an adverse effect on prognosis and residual disability. A parallel multidisciplinary approach is advantageous and efficient. Table 2 summarizes a minimum set of tests in the diagnostic work-up of patients at diagnosis and relapse.
Table 2.
Diagnostic Work-up for Suspected POEMS Syndrome (Diagnosis and Relapse)
| Diagnostic Work-up for Suspected POEMS Syndrome and at Suspected Relapse |
|---|
| Full blood count with peripheral blood film |
| Renal and liver panel, electrolytes including calcium |
| Serum immunoglobulins |
| Serum (or plasma) VEGF |
| Routine urinalysis and 24-h urine collection for proteinuria |
| Testosterone, estradiol, luteinizing hormone, follicle-stimulating hormone, glucose, glycosylated hemoglobin, thyroid profile, prolactin, cortisol, parathyroid hormone; and adrenocorticotrophic hormone |
| Demonstration of a monoclonal plasma cell disorder |
| • Serum and urine electrophoresis, immunofixation, and serum-free light chains |
| • Bone marrow aspirate and trephine biopsy, demonstration of clonal plasma cell infiltration |
| • Consider bone biopsy from FDG-avid osteosclerotic lesion, demonstrating clonal plasma cells |
| Nerve conduction studies and EMG |
| Whole body FDG PET-CT |
| Electrocardiogram and echocardiogram |
| Pulmonary function tests |
CT = computed tomography; EMG = electromyography; FDG = fluorodeoxyglucose; PET = positron emission tomography; POEMS = Polyneuropathy Organomegaly, Endocrinopathy, M-protein and Skin changes; VEGF = vascular endothelial growth factor.
The objective of the diagnostic work-up is to confirm the diagnosis, establish a biometric baseline and commence treatment promptly. Recommended minimum testing is eloquently described in detail elsewhere.7
Monoclonal plasma cell disorder
A monoclonal plasma cell disorder evidenced as a lambda-restricted plasma cell dyscrasia in the blood, urine, bone marrow, or bone lesions should be sought.1,7 Protein electrophoresis and immunofixation (IF) of the serum and urine are essential. Serum-free light-chain (SFLC) assays are not an acceptable substitute as polyclonal plasma cell activation may normalize the SFLC ratio and may not raise the SFLC levels above “normal” values. When present, the serum monoclonal protein (M-protein) is usually less than 10 g/L and myeloma-defining features such as hypercalcemia, renal impairment, or pathological fractures are usually absent.7
A bone marrow biopsy should be reviewed by a specialized hematopathologist to identify the high-frequency characteristic features. POEMS bone lesions identified radiologically should be avoided when taking a biopsy, but solitary fluorodeoxyglucose (FDG)-avid lesions on positron emission tomography (PET) scanning may be a solitary plasmacytoma amenable to radiotherapy especially in the absence of bone marrow involvement (see below). Megakaryocyte hyperplasia or clustering, with mono- and hypolobated nuclei may be seen in the bone marrow trephine samples, with driver mutations associated with myeloproliferative neoplasms absent.16 Nonlesional iliac crest marrow plasma cell infiltration usually comprises <10% infiltrate. Plasma cell immunophenotype is consistent with other plasma cell neoplasms.16 Data regarding the cytogenetic and molecular profile of POEMS are scant, but the few studies performed show abnormalities seen in other plasma cell neoplasms.17,18
The monoclonal plasma cell disorder may be limited to one or more solitary plasmacytomata which can be present in osteolytic or osteosclerotic bone lesions. In such instances, computed tomography (CT)-PET targeting and CT image-guided lesion biopsy to obtain histological confirmation is essential. Light-chain restriction by immunohistochemistry of bone biopsies may be unsuccessful as there are frequently low levels of clonal cells. In such cases, the diagnosis is confirmed based on clinical and laboratory features, albeit that there must be evidence of a monoclonal plasma cell disorder by some methodology.
Fewer than 30% of patients with POEMS with a documented plasma cell disorder also have evidence of Castleman disease.1,13 If lymphadenopathy is present, histology may show changes in keeping with angiofollicular lymph node hyperplasia, or Castleman disease with or without evidence of a plasma cell clone.11,19 Idiopathic multicentric Castleman disease is a polyclonal lymphoproliferative disorder of benign lymphocytes, plasma cells and vessels. These features may overlap with those seen in POEMS syndrome including lymphadenopathy, hepatosplenomegaly, effusions, hypoalbuminaemia, and polyclonal hypergammaglobulinemia. Thorough examination of diagnostic material including lymph node biopsy by an expert hematopathologist is essential alongside hematological and clinical parameters to distinguish idiopathic multicentric Castleman disease from POEMS-associated Castleman disease.20,21
Vascular endothelial growth factor
VEGF is a key biomarker in diagnosis and disease monitoring,22 available for testing in specialist laboratories. VEGF is a potent proinflammatory angiogenic cytokine which is significantly raised in POEMS syndrome compared to other inflammatory neuropathies.22–24 Serum VEGF levels in POEMS syndrome have a median value of approximately 4000 pg/mL. Serum VEGF concentrations are approximately 10 times higher than plasma in health and disease, as serum contains additional VEGF released by ex vivo platelet activation during clotting. Either plasma or serum VEGF can be used in making the POEMS diagnosis with equal accuracy24; clinicians should confirm which samples should be sent to their laboratory and ensure results are correctly presented and interpreted. In conjunction with a conduction slowing (demyelinating) neuropathy and a lambda light-chain monoclonal plasma cell disorder, a serum VEGF >1000 pg/mL has a sensitivity of 100% and specificity of 93% for a diagnosis of POEMS. Hypoxia and iron deficiency (with or without anemia) can lead to false-positive results, with levels up to 2000 pg/mL, but rarely as high as POEMS-positive samples.22 Falsely negative (suppressed) VEGF levels may be found in patients recently treated with steroids for incorrectly diagnosed CIDP. At present no other cytokine analysis has revealed additional diagnostic utility.
Bone lesions
Bone lesions are present in most patients with POEMS and may be sclerotic, lytic, or mixed. The presence of sclerotic bone lesions is one of 5 major diagnostic criteria for POEMS (see Table 1). Bone lesions may range from densely sclerotic, lytic with sclerotic rim to a soap bubble appearance. Most are seen in the axial skeletal and are <1 cm, explaining the superior sensitivity of CT over plain films.11,25,26
Osteosclerotic lesions in POEMS can be confused with bone islands, cysts, fibromas, fibrous dysplasia and sclerotic metastases.7 Some authors believe that the ring-like appearance of some of the lesions in POEMS can be distinctive. Purely lytic lesions were reported in 4.2% in a study of 102 patients using plain radiographs.11 However, plain radiographic skeletal survey is not recommended because of its poor sensitivity. CT with bone window displays is more sensitive for the detection of relevant bone lesions than a plain radiographic skeletal survey27 and since this can be combined with PET scanning it is the radiological examination of first choice and increasingly utilized. Expert review is important to identify and characterize significant skeletal lesions.
FDG PET-CT has demonstrated variable FDG uptake in focal bone lesions seen on the CT component of the studies. Increased FDG uptake is more frequent in lytic lesions or mixed sclerotic/lytic lesions, with higher uptake intensity in the former. The highest FDG-avid lesions should be targeted for diagnostic biopsy where necessary, in particular where the bone marrow demonstrates no identifiable systemic involvement. Assessment of lymphadenopathy, organomegaly, and fluid overload should also be performed.28,29
Brain and spinal cord
White matter disease is no more common than in the comparable population of other inflammatory neuropathies. Arterial and venous central thromboembolic events are occasionally the presenting feature of POEMS. We do not routinely perform CNS imaging at baseline unless clinically indicated as above. However if imaging is performed, asymptomatic pachymeningeal thickening may be seen in 70% of gadolinium enhanced magnetic resonance imaging (MRI) brain images in POEMS when compared with CIDP where it does not occur,10 likely due to fluid leak into the meninges. Thickening, high signal, and enhancement of the nerves in the lumbosacral or brachial plexus is common but not diagnostically useful in distinguishing POEMS from its main differential, CIDP.
Bone density
As part of the work-up of gonadal failure, prolonged steroid use or severely impaired mobility, a dual-energy x-ray absorptiometry (DEXA) scan is a useful adjunct to initial assessments to guide the need for appropriate measures such as bisphosphonate therapy, or in the case of gonadal insufficiency hormone replacement therapy.
Cardiopulmonary work-up
Pericardial effusion and signs of pulmonary arterial hypertension are nonspecific, but their identification can support a diagnosis of POEMS over CIDP. Pulmonary hypertension has been shown to be a prognostic survival marker in POEMS.30
We routinely use a sniff nasal inspiratory pressure (SNIP) device to assess inspiratory muscle strength. If pressures are lower than the expected normal range, we initiate comprehensive pulmonary function tests for spirometry, gas transfer, lung volumes, and mouth pressures and jointly manage patients with respiratory specialists to optimize treatment options and outcomes.
Nerve conduction tests/EMG for characterization of the neuropathy
Nerve conduction studies must be performed to confirm the electrophysiological features of the neuropathy. POEMS neurophysiology fulfills electrophysiological criteria for CIDP in 70% of cases,14 and thus, conclusions in neurophysiological reports can be misleading if it is assumed that conduction slowing is always representative of “demyelination” and “equates to” CIDP; it does not. Identification of distinguishing features separating POEMS from CIDP with monoclonal gammopathy are crucial and described elsewhere.15,31–33 In summary, the electrophysiology in POEMS is almost pathognomonic. Significant conduction slowing is seen in the upper limbs, with profound axonal loss in the lower limbs (potentials often absent and florid denervation in electromyography [EMG]) and the sensory nerve action potentials are always small if not altogether absent. There is no block or dispersion, little or no patchiness and homogenous slowing proximally to distally in the nerves with slowing. The neuropathy associated with Castleman disease-associated POEMS tends to be less severe, mostly sensory and may be subclinical or even absent.
Endocrinopathy
Endocrine abnormalities are a minor criterion for POEMS but crucial to patient morbidity. As such, consultation with an endocrinologist is essential. Diabetes mellitus and thyroid abnormalities are excluded from diagnostic criteria given their high prevalence in the general population. Other endocrinopathy is a common feature in POEMS, present in up to 68% of the patients at diagnosis.1 The pathogenesis of the endocrinopathy is currently unknown and some endocrinopathy might be treatment- or disease severity-related. The presentation of endocrine abnormalities is variable, including: (1) excess of hormonal production (hyperprolactinemia, increase of IGF-1, hyperparathyroidism)34; (2) primary or secondary insufficiency (hypogonadism, adrenal insufficiency, hypothyroidism and hypoparathyroidism); and (3) abnormal glucose metabolism (type 2 diabetes mellitus and impaired glucose tolerance).35 The most common endocrine abnormality is hypogonadism, followed by hyperprolactinemia, hypothyroidism, abnormal glucose metabolism, adrenal insufficiency, and high IGF-1 levels 1.34–36 The prevalence of endocrinopathy increases during the disease, occurring in up to 92% of patients.34 Therefore, it is essential to regularly and systematically assess for endocrine disease to detect new endocrinopathies.
Some cases with pituitary disease, including acromegaly,37 pituitary macroadenoma38 and empty sella,34 have been described in POEMS; however, the low frequency of these cases precludes a definite causative link. Gynecomastia is a common clinical finding,6,7 but not always associated with hyperprolactinemia or hypogonadism.7
Elevation of the IGF-1 has been described in several patients, but the significance of this is unclear and only one patient so far had abnormal findings on a growth hormone suppression test, suggestive of abnormal growth hormone dynamics.34,37 Patients with POEMS should be systemically assessed for endocrinopathy as resolution of endocrine abnormalities at the end of follow-up may be seen.
OPTIMIZATION OF MULTISYSTEM INSUFFICIENCIES TO MITIGATE CLINICAL RISK
Several prognostic factors have been identified from the evaluation of numerous patient cohorts, response criteria proposed and a prognostic nomogram developed to stratify high and low relapse risk cases.1 Age, serum albumin <32 g/L, pulmonary hypertension, the presence of pleural effusions and reduced glomerular filtration rate have been identified as pretreatment contributors to poor outcome.39
Table 3 summarizes key areas for optimization of performance status which should occur in parallel with targeted treatment. A multidisciplinary approach is required. Respiratory involvement can occur from neuromuscular weakness, fluid leak, pulmonary hypertension, or thromboembolism. Pulmonary function tests using SNIP testing and oxygen saturations at each visit on the background of a DLCO as baseline is recommended as a minimum. Fluid management is crucial, including the use of diuretics where fluid leak is compromising care, although patients may be poorly responsive to diuretics due to third space losses. In male patients with hypogonadism, before starting testosterone replacement, the clinician should recognize that polycythemia can be part of POEMS itself. Since testosterone replacement can exacerbate polycythemia, patients on testosterone replacement should be risk assessed and monitored with full blood count with hemoglobin 3 monthly. Erectile dysfunction may respond to testosterone replacement, phosphodiesterase type 5 inhibitors or vacuum pumps. Patients should be reviewed to address fertility status and consider fertility preservation before therapy. This can include sperm or egg/embryo cryopreservation, and in the case of radiotherapy to the pelvic field, ovarian shielding or transposition.
Table 3.
Optimization of Performance Status
| System | Effects | Tests | Interventions |
|---|---|---|---|
| Respiratory | Neuromuscular weakness, fluid leak, pulmonary hypertension, thromboembolism | Pulmonary function tests, eg, SNIP testing and oxygen saturation at clinic visits | CPAP/BiPAP may be required |
| Cardiovascular | Fluid overload | ECG, troponin, NT-pro-BNP Consider cardiac MRI and global longitudinal strain if symptomatic |
Diuretics and urinary catheterization may be required |
| Gastrointestinal | Neuromuscular weakness may compromise swallow | Speech and language assessment Nutritional assessment |
Nasogastric or nasojejunal feeding may be necessary |
| Endocrine | Hypogonadism Reduced bone density |
Testosterone/estrogen levels | Hormone replacement may be needed |
| Fertility | Subfertility post treatment Erectile dysfunction |
As guided by fertility specialists | Offer fertility preservation prior to chemoimmunotherapy or radiotherapy to pelvic field, ovarian shielding or transplantation |
| Pain | Neuropathic pain Bone pain |
Nil | Consider neuropathic pain medication such as gabapentin, tricyclics |
| Physiotherapy, occupational therapy and orthotics | Deformities | Nil | In-bed or in-chair exercises Mobility aids and adaptations Orthotics to consider: AFO Anterior splints (eg, MatrixMax and ToeOff) Lesser foot drop (Dyanstab Boa or AequiSplints) Swedish AFO Tendon transfer |
| Psychosocial support | Depression, anxiety | Nil | Clinical nurse specialist input Signposting counseling and psychological support Online support groups including www.smartpatients.com |
AFO = ankle foot orthoses; BiPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure; ECG = electrocardiogram; ECG = electrocardiogram; MRI = magnetic resonance imaging; NT-pro-BNP = N-terminal pro–brain; SNIP = sniff nasal inspiratory pressure.
Most patients with POEMS syndrome respond favorably to treatment, but neurological improvement may not commence for 2 years after treatment and may continue for years. Chronic and irreversible functional impairment following treatment is common and requires ongoing monitoring and support. Some is preventable and early provision of occupational and physiotherapy support to maintain passive and active movement, reduce tendon contractures and pain and prevent complications of immobility is invaluable. Provision of in-bed or in-chair exercises, mobility aids, and adaptations have to adjust to the changing needs of the patient. Ankle foot orthoses (AFO) are important to aiding mobility in those with severe ankle weakness. Because patients often have normal proximal strength, anterior splints such as MatrixMax and ToeOff can provide significant stability and mobility gains. In lesser foot drop, Dynastab Boa or AequiSplints work very well. In some cases, Swedish AFO are necessary. However, patients should be assessed by experienced and versatile orthotists in conjunction with their physiotherapy needs. In some cases, when sufficient recovery of power has been regained, tendon transfer operations may obviate the need for AFO.
Clinical nurse specialists or equivalent should act as a keyworker and signpost patients and family members to sources of information and support. Psychological support is crucial in POEMS syndrome, where patients commonly struggle to understand the complexities of the diagnosis, its pathogenesis and management. The rarity of the syndrome means patients will not have heard of POEMS before and will not encounter another patient in a similar situation.
TREATMENT
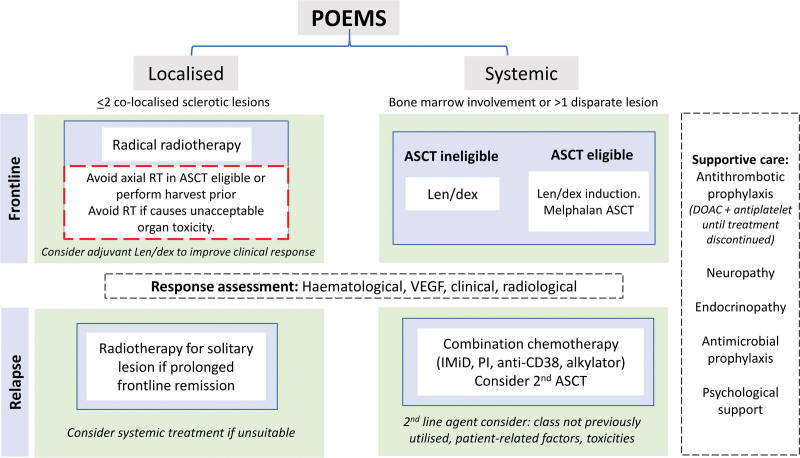
The aim of treatment is to eradicate the underlying plasma cell dyscrasia, but there are limited trial data to guide treatment decisions. Therapeutic options are broadly adapted from those used in multiple myeloma and solitary plasmacytoma. Patients are likely to gain the best outcome from access to the full gamut of myeloma therapies. The attainment of complete hematological (CRH) and VEGF responses (CRVEGF) is associated with improved progression-free and overall survival so are key considerations.40 Decision-making of the choice of therapy may be broadly categorized into disease-related factors (localized or systemic presentation of POEMS); patient-related factors (fitness for intensive treatments and comorbidities, eg, cardiac, respiratory and thrombotic risk) relevant specific to treatment side effects; patient preferences including route of administration [oral, subcutaneous (SC) or intravenous (IV)] and local funding arrangements for drugs. A suggested treatment algorithm is outlined in Figure 1. A detailed review of the current available data of treatment options has been published elsewhere.41
Figure 1.
Treatment algorithm. DOAC = direct oral anticoagulants; IMid = immunomodulatory drugs; len/dex = lenalidomaide dexamethasone; PI = proteasome inhibitor; SFLC = serum-free light chains; SPEP = serum protein electrophoresis; uBJP = urine Bence-Jones protein. Adapted from Khwaja et al.41
Frontline therapy
Localized disease
POEMS may be driven by an isolated osteosclerotic bone lesion and in those cases without bone marrow involvement, involved-field radiotherapy (RT) can provide definitive disease control. To try maximize response rates and times, local RT may be supplemented at diagnosis with a short course of adjuvant chemoimmunotherapy initiated immediately at diagnosis, with additional cycles after completion of RT. We avoid significant RT dose to the axial skeleton for transplant eligible patients as this may compromise future peripheral blood stem cell harvest. For patients whose primary lesion is in the pelvis or sacrum pre-emptive stem cell harvest and storage prior to commencing RT should be considered and if appropriate performed.
Systemic disease
Systemic disease (involvement of the bone marrow by a monoclonal plasma cell disorder) requires chemotherapy. The choice of therapy depends on transplant eligibility. For those eligible, frontline induction and autologous stem cell transplantation (ASCT) consolidation is the preferred approach with impressive durable outcomes replicated in several large studies. Hematological response rates may be significantly upgraded after ASCT, alongside performance status. Neurological improvement may be seen as early as 6 months after ASCT but continues to improve for 2 years.4 While there are no standardized eligibility criteria for ASCT, we consider adequate organ function and functional status to be most important, in preference to pre-ASCT disease response alone.
Pre-ASCT induction has been shown to improve performance status, reduce risk of peri-ASCT complications, and hasten time to disease response.40,42,43 Melphalan is avoided during induction due to its potential risk of myelosuppression and compromise of stem cell harvest. Our standard practice is to employ induction lenalidomide/dexamethasone which has the most evidence supporting its use,40,44 and we aim to harvest stem cells after 4 cycles of treatment. We favor a lower dose of lenalidomide of 15 mg rather than 25 mg (days 1–21 of a 28-day cycle), particularly for frailer patients, together with dexamethasone weekly as a means of reducing treatment toxicity.
Standard dose melphalan ASCT conditioning is given at 200 mg/m2 and only dose reduced to 140 mg/m2 in the context of renal impairment or significant comorbidities.
Bortezomib is an effective treatment in POEMS but concerns about neurotoxicity have limited its use historically. We reserve its use for the relapse or refractory setting, given weekly rather than twice weekly and SC rather than IV to reduce the risk of peripheral neuropathy. The anti-CD38 monoclonal antibody, daratumumab, is another attractive option, but with limited data to support its use.45
VEGF inhibitors such as bevacizumab have a theoretical rationale, but been associated with increased mortality and are not used.46
Some patients reach a diagnosis of POEMS via the Castleman disease route with biopsy-proven evidence of Castleman disease and usually little or no neuropathy. Such patients may have received a Castleman disease-approved agent such as siltuximab; however, this treatment is not recommended in the POEMS setting,21 in which siltuximab is seldom effective and the priority is to target the underlying plasma cell clone.
Supportive care at front line
Up to 40% of patients have arterial and venous thrombosis47,48 at presentation or during active treatment which is likely due to underlying disease, patient immobility from neurological impairment as well as treatment including immunomodulatory drugs (IMiDs) and steroids. The greatest risk is before treatment initiation and during active disease.47 Although there is no prospective data to guide prevention decisions, we give prophylactic anticoagulation (with a direct oral anticoagulant as this is preferable and pragmatic over subcutaneous low molecular heparin) alongside antiplatelet therapy (eg, clopidogrel 75 mg or aspirin 75mg) until clinical remission and the VEGF is <1000 pg/mL after careful assessment of bleeding risk. Prophylaxis against infection during and after therapy should be offered as per local practice guidelines. Bisphosphonates are not routinely required, but where greater age, steroid use or hormonal failure are present should be considered. Ongoing optimization of physiological deficits is important; appropriate management of endocrine imbalances, rehabilitation input and thromboprophylaxis will help to keep the patient well and functioning pending observation. Physiotherapy and occupational therapy can also help to optimize cardiorespiratory and physical function.
Relapsed disease
There is even fewer data to guide the optimal treatment strategy in the relapse setting. The choice of agent may be based on many factors including duration of initial response, potential toxicity of therapy, patient frailty and preference. Preference may be given to a class of drug not previously employed to overcome resistant clones. It is reasonable to use drugs with proven efficacy at relapse including re-treatment with the same agent if a prolonged remission was achieved, taking into account the considerations listed above, as well as responses and tolerability of previous treatments. In this context a second ASCT can also be considered in selected patients.49 Small series show alkylators or anthracyclines have CRH/VGPRH rates of 43% and proteasome inhibitors/IMiD-based therapy alone had lower responses (<10% CRH/VGPRH).50 There is limited data on combination of proteasome inhibitor-IMiD regimens.
Active trials in frontline and relapse include the use of ixazomib lenalidomide and dexamethasone (NCT02921893), daratumumab and lenalidomide (NCT04396496), and CD19/BCMA CAR-T in refractory POEMS (NCT05263817). Future clinical trials are welcomed in this setting where evidence is limited.
ASSESSING PROGNOSIS
Assessing prognosis in POEMS syndrome at the outset is multifaceted. For most patients, POEMS syndrome is not a life-limiting disease. Although a clonal plasma cell disorder, its natural history is not one of progressive accumulation of the clonal population; rather progressive morbidity can significantly impair quality of life and well-being due to physiological impairments and disability. In terms of risk to life, progressive POEMS syndrome can result in severe cardiorespiratory compromise in some patients which can result in early mortality.39 Such patients benefit from early and aggressive management in an intensive care setting if needed, pending physiological improvements in response to therapy. In our experience, neuromuscular weakness resulting in poor respiratory effort, refractory anasarca accompanied by hypoalbuminemia and failure to gain weight after institution of therapy are poor prognostic features in practice. Early involvement by experts and transfer to a specialist center is advisable.
Prognosis may be significantly better for patient with solitary bone lesions51 which can be irradiated to cure. Survival is independent of the number of bone lesions, but patients with limited sclerotic lesions that can be treated with radiation to the dominant bone lesions52 achieve better outcomes.
Failure to achieve hematological or VEGF response suggests persistence of pathological drivers in POEMS syndrome. Normalization of VEGF at 6 months after treatment has been found to correlate with prolonged relapse-free survival and better clinical outcomes.53 The lack of clearance of serum M-protein and eradication of clonal plasma cells from the bone marrow following ASCT is also associated with an approximately 30% incidence of relapse at 5 years.54 Higher rates of progression or relapse following frontline therapy has also been observed in patients of higher age and those with a presentation serum albumin lower than 32 g/L.4,6
As yet, knowledge of these poor prognostic features does not guide alternative management strategies such as using maintenance or additional lines of therapy. As always, a balance needs to be struck between achieving CR and the inevitable immunosuppression and risk of second primary malignancies with additional therapy, especially given the low inherently malignant behavior of POEMS syndrome.
A prognostic nomogram has been created for POEMS syndrome in a Japanese cohort and cross validated in the United States, but may need further exploration before utilized in routine practice.8
ASSESSING RESPONSE TO TREATMENT AND MONITORING STRATEGIES
Universal response criteria following treatment have not been established, but published adapted criteria tend to be used to measure and record response to treatment (Table 4)1,55 using five categories: clinical, neuropathy, hematological, VEGF, and radiological. Those on chemoimmunotherapy also require assessment of treatment tolerability. We monitor VEGF and M-protein levels with each treatment cycle to correlate with clinical changes. Short-term neurological deterioration before subsequent improvement is experienced by many patients due to the rigors of the treatment and should not in isolation be considered evidence of treatment failure. Endocrine status may also be destabilized by therapy and need recalibration.34
Table 4.
| Clinical response |
|---|
| Based on patient and physician qualitative reports of constitutional symptoms and systemic features |
| • Clinical improvement (Ci) |
| • Clinical progression (Cp) |
| • Mixed clinical response (Cm) |
| • Clinical stability (Cs) |
| Neuropathy response |
| Defined by clinical examination, neurophysiology, i-RODS (POEMS-RODS in future), modified Rankin Score56 and ONLS.57 Progression defined as worsening of functional ability by an increase in i-RODS of>4 or ONLS by ≥1 |
| Hematological response |
| • Complete response (CRH)—Negative bone marrow (if originally involved) and negative immunofixation of serum and urine. |
| • Very good partial response (VGPRH)—90% reduction in the M-protein or immunofixation positive only, provided M-protein ≥5 g/L at baseline. |
| • Partial response (PRH)—90% reduction in M-protein or immunofixation positive, provided baseline M-protein ≥10g/L. |
| • No response—Less than PRH. |
| • Progression—reemergence of M-protein in serum and/or urine or an increase of ≥25% from posttreatment nadir, but the M-protein must be >5g/L. |
| VEGF response |
| • Complete response (CRVEGF)—Normalization of VEGF (<771 pg/mL) |
| • Partial response (PRVEGF)—Decrease of ≥50% (baseline must be ≥2000 pg/mL) |
| • No response (NRVEGF)—Less than a PRVEGF |
| • Progression—Persistent (≥2 recordings) VEGF elevation ≥771pg/ml from a previously normal result or a persistent rise in VEGF of >50% from posttreatment nadir (if <CRVEGF achieved) |
| Radiological response |
| • Complete radiologic response (CRR)—Resolution of FDG-avidity |
| • Partial radiologic response (PRR)—FDG-avidity improved by ≥50% |
| • No radiologic response—Less than 50% reduction in FDG-avidity |
| • Progression—30% increase in sum of SUVmax from lowest level, but must be at least 4 SUVmax OR the appearance of a new FDG-avid lesion |
FDG = fluorodeoxyglucose; ONLS = Overall Neuropathy Limitation Score; POEMS = Polyneuropathy Organomegaly, Endocrinopathy, M-protein and Skin changes; VEGF = vascular endothelial growth factor.
The goal of treatment in POEMS syndrome is to suppress the underlying plasma cell clone, but although in some cases it is possible, complete disease suppression (CRH) is not always achievable particularly in frail patients. Nevertheless, most patients will derive meaningful clinical benefit from treatment. We perform disease evaluation at six months or sooner if there are concerns about an inadequate response. This comprises evaluation of the underlying plasma cell clone and secondary organ dysfunction, including repeat physical and neurological examination and disease markers. Notably, clinical response to therapy is variable, especially in regard to neurological improvement; thus even stabilization of clinical decline can be regarded as an early sign of success. Improvement in well-being, reduction in edema, weight gain and better energy levels are also encouraging.
An outline of our monitoring approach is summarized in Table 5. Early detection of relapse and institution of therapy are important to avoid accumulation of further disability but relapses are often slow to develop and there is often time to consider what treatment to initiate, and when. A multidisciplinary approach is essential to diagnosis of relapse considering multiple domains of relapse over time. Frequent monitoring poses a significant time cost both for the patient, clinician, and health service. A pragmatic approach we adopt is for full assessment and evaluation at a specialist POEMS center for unstable or complex patients and shared care between the specialist and local center for those who following a steady clinical course.
Table 5.
Our Approach to Monitoring and Assessing Disease
| Stage of Treatment | Assessment Goals | Intervention | Timing |
|---|---|---|---|
| During treatment | Treatment tolerance Treatment response |
Physical and neurological examination Neurological outcome measures |
3 mo and if any new clinical symptoms reported |
| Toxicity (infections, neuropathy, organ function | FBC, SPEP ± SFLC (if informative) | With each treatment cycle | |
| Anticoagulation plan | Review anticoagulation and antiplatelet therapy | With each treatment cycle and adjust according to VEGF reduction/ platelet count | |
| Endocrinopathy | Renal, liver and bone profile. Hepatitis B DNA PCR if prior exposure | With each treatment cycle | |
| Ongoing response assessment | Endocrine profilea | Adjust steroid replacement (Hydrocortisone) dose for hypoadrenalism according to concurrent therapeutic steroids (dexamethasone) Tailor to individual requirement (3–6 mo) |
|
| VEGF | With each treatment cycle | ||
| Imaging | Not routinely indicated for treatment surveillance; consider appropriate imaging for new symptoms Consider DEXA scan for bone density if hypogonadal or hypoadrenal, on long-term steroids or severe impaired mobility |
||
| Categorical response assessment | Overall assessment of treatment response (3–6 mo following frontline therapy) | Physical and neurological examination (including appropriate neurological outcome measure testing) | |
| FBC, SPEP, SFLC, serum immunoglobulins, renal, liver and bone profile | |||
| Endocrine profilea | |||
| VEGF | |||
| Pulmonary function tests, echocardiogram | If significant cardiopulmonary dysfunction before treatment or ongoing | ||
| Nerve conduction studies/EMG | If evidence of further neurological deterioration on assessment | ||
| Imaging (PET-CT/MRI) | Depending on clinical circumstances (FDG-avidity may persist months to years) | ||
| Bone marrow biopsy | If previous positive findings AND ambiguity about categorical response | ||
| Posttreatment | Monitor for disease relapse Monitoring of late effects/ treatment toxicity: skeletal, infections, secondary malignancy (particularly post-ASCT) Neuropathy Endocrinopathy Health education (diet, physical activity, weight control) |
||
| Physical and neurological examination Neurological outcome measures |
CR: 6–12 mo <CR: 3–6 mo |
||
| FBC, SPEP, SFLC, Serum immunoglobulins, Renal, liver and bone profile | |||
| Endocrine profilea | |||
| VEGF | |||
| Pulmonary function tests, echocardiogram | Consider annually | ||
| Nerve conduction studies/EMG | Depending on clinical symptoms | ||
| Imaging (PET-CT/MRI) | Low threshold for reimaging if new clinical symptoms arise | ||
| Imaging (DEXA) | Consider as per osteoporosis guidelines if hypogonadal or prolonged corticosteroid exposure | ||
| Bone marrow biopsy | Before any new treatment consideration | ||
Endocrine profile: testosterone, estradiol; fasting glucose, glycosylated hemoglobin; thyroid-stimulating hormone, parathyroid hormone; luteinizing hormone, follicle-stimulating hormone, and adrenocorticotrophic hormone.
ASCT = autologous stem cell transplantation; DEXA = dual-energy x-ray absorptiometry; EMG = electromypgraphy; FBC = full blood count; MRI = magnetic resonance imaging; PET = positron emission tomography; SFLC = serum-free light chains; SPEP = serum protein electrophoresis; VEGF = vascular endothelial growth factor.
Clinical response
Clinical response is subjective. It is determined by the clinician and patient regarding the degree of improvement or worsening of symptoms following treatment, tailored to the patient’s individual phenotype. Respiratory function tests and echocardiography may be carried out at the 6-month evaluation point, or sooner if clinically indicated. We have established a joint clinical pathway with cardiology and respiratory specialists with agreed clinical goals and prompt intervention as needed. There may be discordance between clinical improvement and VEGF levels. In our opinion, clinical improvement takes precedence.
Neurological response
A neurological examination should be supplemented by quantitative outcome measures of neurological disability. The inflammatory neuropathy Rasch-built overall disability scale (i-RODS)58 is a 24-question scale which is simple and quick to administer and easy to interpret for non-neurologists. While not validated in POEMS, a decrease of more than 4 points may indicate a deterioration in neuropathy59 and should prompt further assessment (eg, repeat nerve conduction studies). A new, disease-specific POEMS-RODS will be published very shortly. Other neurological outcome measures such as the overall neuropathy limitation score57 and the MRC Sum Score may also be completed during specialist clinic follow-up, although like the i-RODS these are not validated in POEMS. Neurological improvement is typically slow, often starting only 2 years after disease suppression and continuing for 3–5 years more. This improvement is slower than in other inflammatory neuropathies and in isolation should not be considered evidence of treatment failure. Neurological recovery is often incomplete especially in those most severely affected at diagnosis.1,2 Nerve conduction studies improve slowly and in our experience, they can remain relatively unchanged despite improvements in strength and sensation. Nerve conduction studies do, however, tend to deteriorate in line with worsening neuropathy and thus we repeat these if there is worsening of neurological symptoms or examination.
Hematological response
Disease response assessment is more challenging in those with a very small or absent detectable clone at baseline and is dependent on testing sensitivity. Patients without a monoclonal protein response may still demonstrate significant clinical improvements. Resolution of polycythemia and thrombocytosis is also expected in line with other improvements following treatment and failure of these to improve could indicate treatment resistance.
VEGF response
VEGF levels may fall within a few months of treatment,60 with responses often quicker following ASCT and lenalidomide compared to melphalan40 and thalidomide.53 Small fluctuations in VEGF levels are common during long-term monitoring. Changes in VEGF within the normal range (<771 pg/mL) should not prompt a change in treatment. Similarly, a rise in VEGF > 1000 pg/mL without any clinical deterioration may not be significant—if there is uncertainty, it should be repeated a few months later with concomitant investigation for other conditions that can elevate VEGF including iron deficiency, hypoxia, and vasculitis.22
Radiological response
Patients who originally had FDG-avidity on PET-CT should be considered for repeat imaging at a minimum of 1 year posttreatment initiation. Ongoing FDG-avidity may take many months or even years to resolve even in radiation fields.1 If clinical status and VEGF are improving, persistent FDG-avidity should be monitored rather than retreated and repeated PET imaging if the patient is well with complete response is unnecessary. However, new unexplained symptoms suggestive of POEMS or reminiscent of previous symptoms warrant repeat imaging.
Endocrine response
Patients with known endocrinopathy should undergo regular clinical and biochemical review of endocrine function, particularly as dysfunction can have major effects on bone health and quality of life. Chronically established endocrine dysfunction should be managed appropriately. Hormone replacement therapy may be needed for premature ovarian failure, especially following high-dose chemotherapy. Persistent erectile dysfunction is likely to be multifactorial, and review in an andrology clinic is worth considering.
Skin response
Skin changes improve in most patients over months61 and should be assessed regularly. Purple discoloration of the extremities may persist even after successful treatment and may not signify treatment failure. Careful attention to skin and nail care of the feet is important especially if there is numbness. Similarly, AFO should be reviewed regularly to ensure appropriate fitting and avoid the development of foot ulcers. Recrudescence or increased numbers of glomeruloid hemangiomata (driven by VEGF levels) warrant monitoring in the context of other clinical features and the VEGF level over a timescale of months before action is taken. Especially large pedunculated hemangiomata can be removed by cryotherapy.
CONCLUSIONS
POEMS syndrome is the archetypal monoclonal gammopathy of clinical significance. The driver clone can produce severe physiological impairments but with early diagnosis and effective treatment, there is much to gain to restore the patient back to functionality and independence. We have highlighted an approach which we hope will improve outcomes by early diagnosis and rapid appropriate interventions. The advancement in therapies for plasma cell disorders bodes well for patients affected by POEMS syndrome, but strenuous efforts are needed to build on clinical evidence through dedicated clinical trials and multidisciplinary cooperation. We welcome international collaboration in this rare disorder to achieve this. Above all, careful attention to the patient’s history and thorough clinical examination is paramount as the diagnosis of usually to be found therein.
AUTHOR CONTRIBUTIONS
SDS, JS, SK, and MPL conceived and planned the manuscript. SDS, JK, SK, RK, DS, and JS wrote the manuscript. All authors reviewed the manuscript.
DISCLOSURES
SDS reports speaker fees and research funding from Janssen, BeiGene and Sanofi. MPL reports speaker fees from BeiGene and Sanofi. Conference Travel fees from CSL Behring. Ad hoc consulting fees from AstraZeneca, Roche, UCB Pharma, and Polyneuron pharmaceuticals. All the other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Keddie S, Foldes D, Caimari F, et al. Clinical characteristics, risk factors, and outcomes of POEMS syndrome: a longitudinal cohort study. Neurology. 2020;95:e268–e279. [DOI] [PubMed] [Google Scholar]
- 2.Karam C, Moshe-Lilie O, Chahin N, et al. Diagnosis of paraproteinemic neuropathy: room for improvement. J Neurol Sci. 2020;415:116902. [DOI] [PubMed] [Google Scholar]
- 3.Marsh ES, Keddie S, Terris-Prestholt F, et al. Early VEGF testing in inflammatory neuropathy avoids POEMS syndrome misdiagnosis and associated costs. J Neurol Neurosurg Psychiatry. 2021;92:172–176. [DOI] [PubMed] [Google Scholar]
- 4.Ohwada C, Sakaida E, Kawajiri-Manako C, et al. Long-term evaluation of physical improvement and survival of autologous stem cell transplantation in POEMS syndrome. Blood. 1312;018:2173–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook G, Iacobelli S, van Biezen A, et al. High-dose therapy and autologous stem cell transplantation in patients with POEMS syndrome: a retrospective study of the Plasma Cell Disorder sub-committee of the Chronic Malignancy Working Party of the European Society for Blood & Marrow Transplantation. Haematologica. 2017;102:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kourelis TV, Buadi FK, Kumar SK, et al. Long-term outcome of patients with POEMS syndrome: an update of the Mayo Clinic experience. Am J Hematol. 2016;91:585–589. [DOI] [PubMed] [Google Scholar]
- 7.Dispenzieri A. POEMS syndrome: 2021 update on diagnosis, risk-stratification, and management. Am J Hematol. 2021;96:872–888. [DOI] [PubMed] [Google Scholar]
- 8.Suichi T, Misawa S, Sato Y, et al. Proposal of new clinical diagnostic criteria for POEMS syndrome. J Neurol Neurosurg Psychiatry. 2019;90:133–137. [DOI] [PubMed] [Google Scholar]
- 9.Dupont SA, Dispenzieri A, Mauermann ML, et al. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73:1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziff OJ, Hoskote C, Keddie S, et al. Frequent central nervous system, pachymeningeal and plexus MRI changes in POEMS syndrome. J Neurol. 2019;266:1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanishi T, Sobue I, Toyokura Y, et al. The Crow-Fukase syndrome: a study of 102 cases in Japan. Neurology. 1984;34:712–720. [DOI] [PubMed] [Google Scholar]
- 12.Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101:2496–2506. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zhang W, Jiao L, et al. Combination of melphalan and dexamethasone for patients with newly diagnosed POEMS syndrome. Blood. 2011;117:6445–6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83:476–479. [DOI] [PubMed] [Google Scholar]
- 15.Mauermann ML, Sorenson EJ, Dispenzieri A, et al. Uniform demyelination and more severe axonal loss distinguish POEMS syndrome from CIDP. J Neurol Neurosurg Psychiatry. 2012;83:480–486. [DOI] [PubMed] [Google Scholar]
- 16.Dao LN, Hanson CA, Dispenzieri A, et al. Bone marrow histopathology in POEMS syndrome: a distinctive combination of plasma cell, lymphoid, and myeloid findings in 87 patients. Blood. 2011;117:6438–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang WY, Shen KN, Duan MH, et al. 14q32 translocations and 13q14 deletions are common cytogenetic abnormalities in POEMS syndrome. Eur J Haematol. 2013;91:490–496. [DOI] [PubMed] [Google Scholar]
- 18.Bryce AH, Ketterling RP, Gertz MA, et al. A novel report of cig-FISH and cytogenetics in POEMS syndrome. Am J Hematol. 2008;83:840–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni GB, Mahadevan A, Taly AB, et al. Clinicopathological profile of polyneuropathy, organomegaly, endocrinopathy, M protein and skin changes (POEMS) syndrome. J Clin Neurosci. 2011;18:356–360. [DOI] [PubMed] [Google Scholar]
- 20.Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129:1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lomas OC, Streetly M, Pratt G, et al. The management of Castleman disease. Br J Haematol. 2021;195:328–337. [DOI] [PubMed] [Google Scholar]
- 22.Pihan M, Keddie S, D’Sa S, et al. Raised VEGF: high sensitivity and specificity in the diagnosis of POEMS syndrome. Neurol Neuroimmunol Neuroinflamm. 2018;5:e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobile-Orazio E, Terenghi F, Giannotta C, et al. Serum VEGF levels in POEMS syndrome and in immune-mediated neuropathies. Neurology. 2009;72:1024–1026. [DOI] [PubMed] [Google Scholar]
- 24.D’Souza A, Hayman SR, Buadi F, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood. 2011;118:4663–4665. [DOI] [PubMed] [Google Scholar]
- 25.Glazebrook K, Guerra Bonilla FL, Johnson A, et al. Computed tomography assessment of bone lesions in patients with POEMS syndrome. Eur Radiol. 2015;25:497–504. [DOI] [PubMed] [Google Scholar]
- 26.Shibuya K, Misawa S, Horikoshi T, et al. Detection of bone lesions by CT in POEMS syndrome. Intern Med. 2011;50:1393–1396. [DOI] [PubMed] [Google Scholar]
- 27.Shi X, Hu S, Luo X, et al. CT characteristics in 24 patients with POEMS syndrome. Acta Radiol. 2016;57:51–57. [DOI] [PubMed] [Google Scholar]
- 28.Albertí MA, Martinez-Yélamos S, Fernandez A, et al. 18F-FDG PET/CT in the evaluation of POEMS syndrome. Eur J Radiol. 2010;76:180–182. [DOI] [PubMed] [Google Scholar]
- 29.Pan Q, Li J, Li F, et al. Characterizing POEMS Syndrome with 18F-FDG PET/CT. J Nucl Med. 2015;56:1334–1337. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Tian Z, Zheng HY, et al. Pulmonary hypertension in POEMS syndrome. Haematologica. 2013;98:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung JY, Kuwabara S, Ogawara K, et al. Patterns of nerve conduction abnormalities in POEMS syndrome. Muscle Nerve. 2002;26:189–193. [DOI] [PubMed] [Google Scholar]
- 32.Koike H, Iijima M, Mori K, et al. Neuropathic pain correlates with myelinated fibre loss and cytokine profile in POEMS syndrome. J Neurol Neurosurg Psychiatry. 2008;79:1171–1179. [DOI] [PubMed] [Google Scholar]
- 33.Liu M, Zou Z, Guan Y, et al. Motor nerve conduction study and muscle strength in newly diagnosed POEMS syndrome. Muscle Nerve. 2015;51:19–23. [DOI] [PubMed] [Google Scholar]
- 34.Caimari F, Keddie S, Lunn MP, et al. Prevalence and course of endocrinopathy in POEMS syndrome. J Clin Endocrinol Metab. 2019;104:2140–2146. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi GY, Basu R, Dispenzieri A, et al. Endocrinopathy in POEMS syndrome: the Mayo Clinic experience. Mayo Clin Proc. 2007;82:836–842. [DOI] [PubMed] [Google Scholar]
- 36.Shi X, Hu S, Yu X, et al. Clinicopathologic analysis of POEMS syndrome and related diseases. Clin Lymphoma Myeloma Leuk. 2015;15:e15–e21. [DOI] [PubMed] [Google Scholar]
- 37.Murphy PT, Ahmed N, Hassan HT. Chronic myeloid leukemia and acromegaly in POEMS syndrome. Leuk Res. 2002;26:1135–1137. [DOI] [PubMed] [Google Scholar]
- 38.Bruno C, Fleck JD, Cavaghan MK. Pituitary macroadenoma in a patient with POEMS syndrome in conjunction with Castleman disease: first report of a case and review of related literature. Endocr Pract. 2010;16:97–101. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Huang XF, Cai QQ, et al. Prognostic study for overall survival in patients with newly diagnosed POEMS syndrome. Leukemia. 2017;31:100–106. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H, Huang XF, Gao XM, et al. What is the best first-line treatment for POEMS syndrome: autologous transplantation, melphalan and dexamethasone, or lenalidomide and dexamethasone? Leukemia. 2019;33:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khwaja J, D'Sa S, Lunn MP, Sive J. Evidence-based medical treatment of POEMS syndrome. Br J Haematol. 2022. Aug 7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Duan MH, Wang C, et al. Impact of pretransplant induction therapy on autologous stem cell transplantation for patients with newly diagnosed POEMS syndrome. Leukemia. 2017;31:1375–1381. [DOI] [PubMed] [Google Scholar]
- 43.D’Souza A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012;120:56–62. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Huang XF, Cai QQ, et al. A prospective phase II study of low dose lenalidomide plus dexamethasone in patients with newly diagnosed polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome. Am J Hematol. 2018;93:803–809. [DOI] [PubMed] [Google Scholar]
- 45.Khwaja J, Keh R, Smyth D, Lunn MP, D’Sa S, Sive J. Daratumumab–bortezomib–dexamethasone use in relapsed POEMS syndrome. EJHaem. 2022;3:1021–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sekiguchi Y, Misawa S, Shibuya K, et al. Ambiguous effects of anti-VEGF monoclonal antibody (bevacizumab) for POEMS syndrome. J Neurol Neurosurg Psychiatry. 2013;84:1346–1348. [DOI] [PubMed] [Google Scholar]
- 47.Sayar Z, Weatherill A, Keddie S, et al. High rates of venous and arterial thrombotic events in patients with POEMS syndrome: results from the UCLH (UK) POEMS Registry. Blood Adv. 2020;4:2139–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellors PW, Kourelis T, Go RS, et al. Characteristics and risk factors for thrombosis in POEMS syndrome: a retrospective evaluation of 230 patients. Am J Hematol. 2022;97:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shibamiya A, Ohwada C, Ishii A, et al. Successful second autologous stem-cell transplantation for patients with relapsed and refractory POEMS syndrome. Bone Marrow Transplant. 2021;56:517–520. [DOI] [PubMed] [Google Scholar]
- 50.Jurczyszyn A, Castillo JJ, Olszewska-Szopa M, et al. POEMS syndrome: real world experience in diagnosis and systemic therapy - 108 patients multicenter analysis. Clin Lymphoma Myeloma Leuk. 2022;22:297–304. [DOI] [PubMed] [Google Scholar]
- 51.Soubrier MJ, Dubost JJ, Sauvezie BJ. POEMS syndrome: a study of 25 cases and a review of the literature. French Study Group on POEMS syndrome. Am J Med. 1994;97:543–553. [DOI] [PubMed] [Google Scholar]
- 52.Dispenzieri A. POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:591–601. [DOI] [PubMed] [Google Scholar]
- 53.Misawa S, Sato Y, Katayama K, et al. Safety and efficacy of thalidomide in patients with POEMS syndrome: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016;15:1129–1137. [DOI] [PubMed] [Google Scholar]
- 54.Tomkins O, Keddie S, Lunn MP, et al. High-dose therapy and autologous transplantation for POEMS Syndrome: effective, but how to optimise? Br J Haematol. 2019;186:e178–ee81. [DOI] [PubMed] [Google Scholar]
- 55.Humeniuk MS, Gertz MA, Lacy MQ, et al. Outcomes of patients with POEMS syndrome treated initially with radiation. Blood. 2013;122:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 57.Graham RC, Hughes RA. A modified peripheral neuropathy scale: the overall neuropathy limitations scale. J Neurol Neurosurg Psychiatry. 2006;77:973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Nes SI, Vanhoutte EK, van Doorn PA, et al. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology. 2011;76:337–345. [DOI] [PubMed] [Google Scholar]
- 59.Draak TH, Vanhoutte EK, van Nes SI, et al. Changing outcome in inflammatory neuropathies: rasch-comparative responsiveness. Neurology. 2014;83:2124–2132. [DOI] [PubMed] [Google Scholar]
- 60.Kuwabara S, Misawa S, Kanai K, et al. Neurologic improvement after peripheral blood stem cell transplantation in POEMS syndrome. Neurology. 2008;71:1691–1695. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y, Zhang S, Yang L, et al. Skin responses in newly diagnosed polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome after therapy with low-dose lenalidomide plus dexamethasone. Front Immunol. 2021;12:681360. [DOI] [PMC free article] [PubMed] [Google Scholar]