Abstract
Background
Rotarix (GlaxoSmithKline) oral rotavirus vaccine is licensed as 2 doses in the first 6 months of life. In settings with high child mortality rates, clinical protection conferred by 2 doses of Rotarix is reduced. We assessed vaccine immune response when an additional dose of Rotarix was given to Australian Aboriginal children 6 to <12 months old.
Methods
ORVAC is a 2-stage, double-blind, randomized, placebo-controlled trial. Australian Aboriginal children 6 to <12 months old who had received 1 or 2 prior doses of Rotarix rotavirus vaccine were randomized 1:1 to receive an additional dose of Rotarix or matched placebo. The primary immunological end point was seroresponse defined as an anti-rotavirus immunoglobulin A level ≥20 AU/mL, 28–56 days after the additional dose of Rotarix or placebo.
Results
Between March 2018 and August 2020, a total of 253 infants were enrolled. Of these, 178 infants (70%) had analyzable serological results after follow-up; 89 were randomized to receive Rotarix, and 89 to receive placebo. The proportion with seroresponse was 85% after Rotarix compared with 72% after placebo. There were no occurrences of intussusception or any serious adverse events.
Conclusions
An additional dose of Rotarix administered to Australian Aboriginal infants 6 to <12 months old increased the proportion with a vaccine seroresponse.
Clinical Trials Registration
Keywords: Aboriginal, clinical trial, Immunogenicity, Rotarix, rotavirus vaccine
We randomized Australian Aboriginal infants 6 to ≤12 months old to receive 3 versus 2 doses of Rotarix rotavirus vaccine. A third dose increased the proportion with vaccine seroresponse.
Before the introduction of vaccines, it was estimated that rotavirus was responsible for the deaths of >500 000 young children every year, predominantly in low-resource settings [1]. Since 2006, >00 countries have implemented rotavirus vaccines in their national immunization programs, substantially reducing the number of gastroenteritis-related hospitalizations and deaths [2]. However, rotavirus vaccine effectiveness (VE) is variable, being highest in settings with low child mortality rates (VE, 83%–85%) and lowest in settings with high child mortality rates (VE, 45%–58%), where there is also evidence of waning effectiveness after age 12 months[3].
In Australia, introduction of rotavirus vaccines was followed by a 71% decline in rotavirus hospitalizations in children <5 years old [4]. However, for 1–4-year-old Aboriginal and Torres Strait Islander children (hereafter respectfully referred to as Aboriginal children), rotavirus hospitalizations fell by only 8% in the same period [4]. Despite achievement of >70% vaccine coverage, rotavirus hospitalizations among Aboriginal children living in rural and remote central and northern Australia remained >20 times higher than for non-Aboriginal children living in other states and territories of Australia [4]. During 2 separate G2P[4] genotype rotavirus epidemics in the Northern Territory (NT), rotavirus VE was estimated (albeit imprecisely) to be as low as 20% (odds ratio, 0.81 [95% confidence interval [CI], .32–2.05] and 0.79 [.46–1.34]), with evidence of little or no protection after age 12 months [5, 6].
Controlled trials in Africa suggest that administering 3 rather than 2 doses of Rotarix might provide more sustained protection against severe rotavirus gastroenteritis in those settings [7, 8]. These studies observed a trend toward higher clinical efficacy in the second year of life with 3-dose versus 2-dose schedules, albeit with wide and overlapping confidence intervals. In South Africa, vaccine efficacy against severe rotavirus gastroenteritis in the second year of life was 3% (95% CI, −43% to 82%) in children vaccinated with 2 doses and 76% (−143% to 100%) in the 3-dose group [7]. In Malawi, vaccine efficacy in the second year of life was 3% (95% CI, −101% to 53%) in the 2-dose group and 33% (−47% to 71%) in the 3-dose group [8]. However, each of these studies gave all vaccine doses in an accelerated schedule and before infants were 6 months old, which aligns with the period when persisting maternally derived anti-rotavirus immunoglobulin (Ig) G antibodies might diminish IgA vaccine responses [9].
In Australia, Rotarix (GlaxoSmithKline) oral rotavirus vaccine is administered in a 2-dose schedule, with dose 1 scheduled at age 2 months (6–14 weeks) and dose 2 at age 4 months (10–24 weeks). We hypothesized that for regional and remote Aboriginal infants, administering an additional Rotarix dose after age 6 months might improve immune response and thereby extend protection against rotavirus gastroenteritis into the second year of life.
We designed a 2-stage randomized controlled clinical trial to determine both the immunological effect (stage 1) and clinical protection (stage 2) of administering an additional dose of oral rotavirus vaccine to NT Aboriginal children 6 to <12 months old. Here we report the immunological results of stage 1.
METHODS
Study Design
ORVAC is a 2-stage, double-blind, randomized, placebo-controlled, bayesian clinical trial evaluating the immunological and clinical effectiveness of administering an additional dose of Rotarix oral rotavirus vaccine to Aboriginal infants aged 6 to <12 months. ORVAC stage 1 was conducted in regional urban and remote locations of the tropical north and arid center of Australia’s NT. Remote areas of the NT encompass some of the most socially disadvantaged regions in Australia [10]. Approximately 3600 infants are born in the NT each year, approximately one-third of whom are Aboriginal [11].
Both the study protocol and statistical analysis plan have been published elsewhere [12, 13]. Approvals were obtained from the NT Department of Health and Menzies School of Health Research Human Research Ethics Committee (no. 2016–2658), and the Central Australian Human Research Ethics Committee (no. 16–426). The protocol is registered on ClinicalTrials.gov (NCT02941107).
Participants
Potentially eligible infants were identified after birth from participating hospitals and from client lists at remote community health centers. Written informed consent was obtained from the legally responsible caregiver of participating infants (hereafter “parent”). In keeping with the pragmatic objectives, all NT Aboriginal children aged 6 to <12 months who had received 1 or 2 prior doses of Rotarix met the inclusion criteria for enrollment. Criteria for exclusion (contraindication to vaccine) and for deferred enrollment (acute diarrheal or systemic febrile illness, or receipt of Rotarix in the preceding 28 days) were based on national guidelines [14].
Randomization and Blinding
Randomization of eligible participants was undertaken by contiguous allocation from a computer-generated random sequence, within 2 strata (regional urban or remote) to receive either Rotarix or placebo in a 1:1 ratio. Rotarix is a human monovalent oral live-attenuated vaccine comprising a G1P[8] strain, the most common human disease–causing serotype worldwide. The placebo (produced by Optima Ovest) was a clear flavored solution used as a pharmaceutical excipient that, once prepared by an unblinded study nurse, was identical in appearance to the active vaccine product. Rotarix and placebo were drawn into identical syringes and delivered by oral administration.
Procedures
After consent and eligibility assessment, a baseline blood sample of 1.2 mL was collected to measure anti-rotavirus serum IgA levels before administration of Rotarix or placebo, and a follow-up 1.2-mL sample was collected 28–55 days afterward. Medical record and hospital admission reviews and/or attempted telephone contact of the guardians were performed 14–21 days after administration of Rotarix or placebo to ascertain any adverse events.
Laboratory procedures for measuring serum IgA to rotavirus have been detailed elsewhere [12, 15, 16]. In brief, specific rotavirus IgA antibodies were measured by enzyme-linked immunoassays using rabbit anti-rotavirus polyclonal antisera as the coating antibody to capture a rotavirus lysate (G1P8) strain. Concentrations of rotavirus-specific IgA were measured in patient serum samples using a reference standard having been assigned a concentration of 1000 arbitrary units (AU)/mL.
Outcomes
The primary immunological end point was vaccine seroresponse, defined as a serum anti-rotavirus IgA level ≥20 AU/mL, measured approximately 1 month (28–55 days) after Rotarix or placebo. The secondary immunological end point was the change in anti-rotavirus serum IgA titer 1 month (28–55 days) after the additional dose of Rotarix or placebo. Safety end points were the occurrence of intussusception (fulfilling Brighton criteria) [17] or any serious adverse event within the first 28 days after administration of Rotarix or placebo.
Statistical Methods
All analyses occurred within a bayesian inferential framework. Analysis of the immunological end point was undertaken as specified in the published statistical analysis plan [13].The study design and operating characteristics, including sample size, number and timing of interim analyses, power and false positive rate were calibrated via prestudy simulations. In brief, stage 1 was designed to have 90% power to detect a differential increase in the proportion with IgA seroresponse of 5% in the placebo arm versus 15% in the Rotarix arm, with a maximum of 250 infants with immunological results. In the null scenario (no increase in seroresponse), the risk of a false-positive conclusion was estimated to be 1.1%.
As prespecified in the protocol, the first scheduled analysis occurred after 70 infants had full immunogenicity results available, and subsequent analyses occurred after the enrollment of every 50th infant until 250 infants were enrolled. At each scheduled analysis, we used a logistic regression model to estimate the log-odds of seroresponse by treatment group (Rotarix vs placebo). We used the posterior predictive distribution to evaluate prespecified adaptation rules based on a decision threshold for expected success. We prespecified that enrollment into the immunogenicity study (stage 1) would stop before 250 enrollments if the predicted probability of expected success was >90% or futility was evident with >85% probability. Prespecified sensitivity analyses were performed, adjusting for locality and number of prior doses of Rotarix.
Analyses were implemented with R software, version 4.02, using Stan interfaced by RStan (version 2.21.2). The analyses and safety data were regularly reviewed by an independent Data Safety and Monitoring Committee.
RESULTS
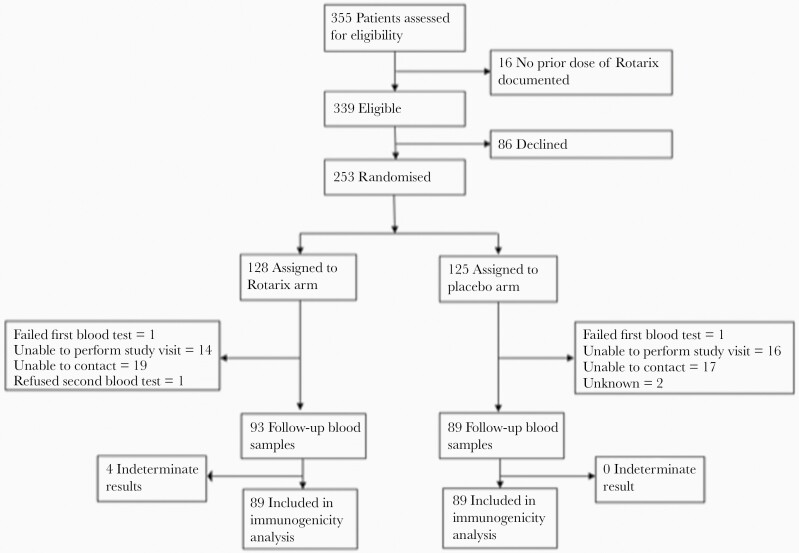
Enrollment occurred between 25 March 2018 and 28 August 2020. The prespecified criterion for early stopping of stage 1 was not met at any scheduled analysis, and a total of 253 infants were enrolled. Of these, 64 (25%) were from regional urban centers (Darwin or Alice Springs) and the other 189 (75%) resided in remote communities; 241 (95%) were verified as having received 2 doses of Rotarix ≥28 days before enrollment. Of the first 250 infants enrolled, 3 were replaced: 1 declined baseline blood collection, 1 had multiple unsuccessful attempts at blood collection, and 1 infant moved away from the Northern Territory before follow-up and was withdrawn.
Of the 253 randomized infants, 128 were assigned to receive Rotarix and 125 to placebo; 120 and 121, respectively, had analyzable IgA results at baseline (Figure 1). The baseline anti-rotavirus IgA concentration was ≥20 AU/mL in 83 of 120 (69%) in the Rotarix arm and 92 of 121 (76%) in the placebo arm; the median baseline IgA concentrations were 59.6 (interquartile range [IQR], 17.8–151.0) AU/mL in the Rotarix group and 93.1 (21.2–164.0) AU/mL in the placebo group (Table 1). The median times between the last dose of Rotarix in the primary series and receipt of the additional dose of Rotarix or placebo were 19.3 (IQR, 12.4–27.1) and 20.1 (14.0–26.2) weeks, respectively.
Figure 1.
Trial profile. Flow diagram shows the progress of participants through ORVAC stage 1.
Table 1.
Baseline Demographic Characteristics, Prior Vaccine Doses, and Seropositivity for the Randomized Population
| Characteristic | Infants by Vaccine Group, No. (%)a | |
|---|---|---|
| Rotarix (n = 128) | Placebo (n = 125) | |
| Male sex | 64 (50.0) | 67 (53.6) |
| Age, median (IQR), mo | 8.5 (6.9–10.3) | 8.7 (7.3–10.3) |
| Indigenous status | ||
| Aboriginal | 123 (96.1) | 121 (96.8) |
| Torres Strait Islander | 5 (3.9) | 2 (1.6) |
| Aboriginal and Torres Strait Islander | 0 (0) | 2 (1.6) |
| Usual location | ||
| Regional urban | 31 (24.2) | 33 (26.4) |
| Remote | 97 (75.8) | 92 (73.6) |
| Breastfed | ||
| Exclusively | 10 (7.8) | 8 (6.4) |
| Partially | 89 (69.5) | 97 (77.6) |
| Not breastfed | 29 (22.7) | 20 (16.0) |
| Weight, median (IQR), k | 8.5 (7.7–9·4) | 8.5 (7.8–9.1) |
| MUAC, median (IQR), mm | 145 (140–155) | 144 (140–155) |
| Preenrollment Rotarix doses | ||
| 1st Dose | 128 (100) | 125 (100) |
| Age at 1st dose, median (IQR), wk | 6.6 (6.2–7.1) | 6.7 (6.3–7.6) |
| 2nd Dose | 119 (93·0) | 122 (97·6) |
| Age at 2nd dose, median (IQR), wk | 17.9 (17.3–18.8) | 17.7 (17.4–18.4) |
| Seroresponse at baseline(IgA ≥20 AU/mL) | ||
| Yes | 83 (64.8) | 92 (73.6) |
| No | 37 (28.9) | 29 (23.2) |
| Missing | 8 (6.2) | 4 (3.2) |
| IgA concentration at baseline, median (IQR), AU/mL | 59.6 (17.8–151.0) | 93.1 (21.2–164.0) |
Abbreviations: AU, arbitrary units; IgA, immunoglobulin A; IQR, interquartile range; MUAC, mid-upper arm circumference.
Data represent no. (%) of infants unless otherwise specified.
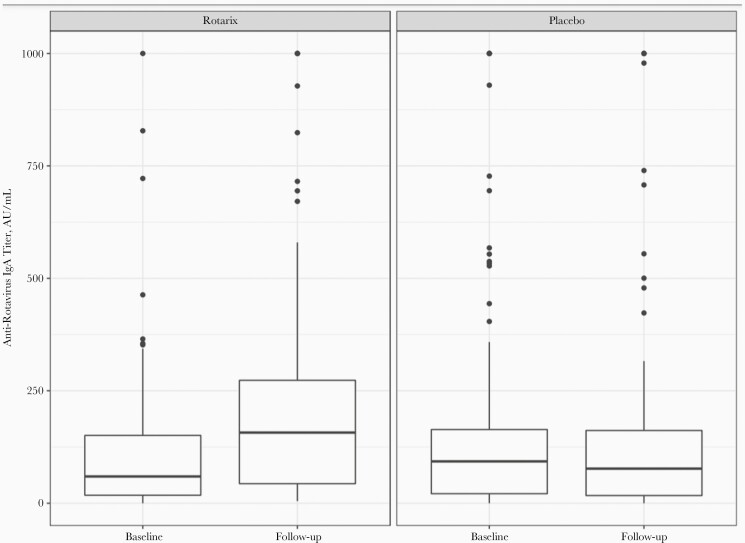
Of the 253 infants randomized, 178 (70%) were successfully followed up after 1 month and had analyzable immunological results, 89 in the Rotarix and 89 in the placebo arm. In the Rotarix arm, 76 of 89 (85%) had evidence of vaccine seroresponse (serum IgA ≥20 AU/mL) 1 month afterward. In the placebo arm, 64 of 89 (72%) had evidence of vaccine seroresponse 1 month afterward. The median IgA concentrations were 157.1 (IQR, 43.5–273.3) AU/mL in the Rotarix arm and 76.9 (17.1–161.8) AU/mL in the placebo arm (Figure 2); the geometric mean concentrations were 118.0 and 57.6 AU/mL in the Rotarix and placebo arms, respectively. Of those infants with paired serum samples who were seronegative at baseline, 18 of 28 (64%) infants administered Rotarix became seropositive, compared with 2 of 25 (8%) administered placebo.
Figure 2.
Change in anti-rotavirus immunoglobulin A (IgA) titer from baseline to 28–56 days after the additional dose of Rotarix or placebo. Abbreviation: AU, arbitrary units.
The odds ratio for seroresponse at follow-up in the Rotarix versus the placebo arm was 2.5 (95% credible interval, 1.1–5.0), with a 99% probability of a higher proportion with seroresponse in the Rotarix than in the placebo arm. The odds ratios for seroresponse were similar after adjustment for baseline differences in remoteness (2.44 [95% credible interval, 1.09–4.85]) and number of prior Rotarix doses (2.48 [1.09 –4.92]).
Neither of the prespecified stopping rules were met at the nominate decision threshold. However, at the final stage 1 analysis, the predicted probability of expected success with complete data was 89% (90% was the prespecified threshold for stopping).
In the period between randomization and 28 days afterward, there were 3 serious adverse events (hospitalization for pneumonia, bronchiolitis and Salmonella enteritis); none were attributed to Rotarix or placebo, and there were no cases of intussusception or death. The treatment assignment of infants remains concealed to allow blinded follow-up of their clinical outcomes, with safety outcomes to be reported by study arm at the completion of ORVAC stage 2.
DISCUSSION
Administering an additional dose of Rotarix vaccine at age 6 to <12 months increased the proportion of Aboriginal infants with evidence of anti-rotavirus IgA seroresponse by 16% (from 69% to 85%). There was no increase in seroresponse among placebo recipients, demonstrating that the increase in anti-rotavirus serum IgA observed in Rotarix recipients is unlikely to be attributable to natural infection. ORVAC was purposely pragmatic, being conducted under real-world conditions and with exclusion criteria largely limited to medical contraindications to vaccination. Three-quarters of enrolled infants resided in remote locations.
These results are consistent with evidence from trials in Bangladesh and Mali where rotavirus vaccines were coadministered with measles-rubella vaccine at age 9 months and with measles, yellow fever, and meningococcal vaccines at age 9–11 months, respectively [18, 19]. In the former trial anti-rotavirus IgA seroresponses increased from 45% to 75% after an additional dose of RotaTeq rotavirus vaccine [18], and in the latter trial the seroresponse increased from 53% to 70% after an additional dose of Rotarix [19]. Taken together, these data suggest that scheduling an additional Rotarix dose after age 6 months is likely to have an appreciable effect on seroresponse rates across a range of high-burden settings.
Reduced vaccine protection has been described for a number of oral vaccines in low- and middle-income settings [20], but the reasons are not well understood. For oral rotavirus vaccines, several possible factors have been proposed, including high levels of maternal-derived vaccine neutralizing anti-rotavirus antibodies, poor nutrition, intestinal microbiota dysbiosis and environmental enteropathy, high prevalence of comorbid infections, and prevalent genetic determinants of poor vaccine responses or increased susceptibility to different rotavirus genotypes [21]. The mortality rate in children <5 years old is several-fold higher among remote Australian Aboriginal infants than among non-Aboriginal infants [22] but is low compared with most low- and middle- income settings. While environmental enteropathy, food insecurity, and iron deficiency affect many remote Aboriginal children [23], the middle upper arm circumferences of participants in our study suggest that protein-calorie malnutrition was not prevalent.
The mechanism of immunological protection against rotavirus infection and immune responses to vaccination remain incompletely understood but likely involve both humoral and cellular mechanisms [24]. Anti-rotavirus serum IgA is widely used as an immunological correlate of clinical protection at the population level [25]; lower anti-rotavirus IgA seroresponses to rotavirus vaccines are observed in settings where reduced clinical protection against rotavirus disease is also observed. A systematic review reported that rates of IgA seroresponse to rotavirus vaccine corresponded with a country’s child mortality rate, 53% (95% CI, 41%–64%) in countries with a high under-age-5 mortality rate compared with 74% (61%–84%) in those with a medium rate, and 87% (78%–92%) in those with a low rate [26]. Similarly, a postimmunization anti-rotavirus IgA geometric mean concentration <90 IU/mL at a population level has been correlated with reduced vaccine efficacy in that population [26]. In our study, an additional dose of Rotarix vaccine increased the probability of seroresponse from 71% to 85%, and the geometric mean concentration of anti-rotavirus IgA from 58 to 118 AU/mL.
While high serum anti-rotavirus IgA vaccine seroresponse has been correlated with high population efficacy of oral rotavirus vaccines, it is not a perfect serological correlate of protection [24]; it is therefore important to evaluate whether the improved anti-rotavirus seroresponses observed in this study translate into improved real-world clinical protection against rotavirus disease. ORVAC is designed as a bayesian seamless 2-stage vaccine clinical trial, in which extension to enable evaluation of clinical protection (stage 2) is predicated on first demonstrating an acceptable vaccine immune response (stage 1). Subject to resourcing, ORVAC stage 2 plans to enroll up to 750 additional infants to determine whether scheduling an additional dose of Rotarix vaccine at age 6 to <12 months results in improved protection against acute gastroenteritis/diarrhea illness up to age 3 years.
Rotashield, a rhesus-derived rotavirus vaccine licensed in the 1990s caused intussusception in a small number of infants after their first dose of vaccine, with the highest risk in infants receiving their first dose after age 3 months[27]. As a consequence, current rotavirus vaccines were tested and licensed for use in early infancy only, although postlicensure studies have found no increase in risk of intussusception [28], and the World Health Organization advises that children in high-burden settings may receive rotavirus vaccine up to age 24 months [27]. The baseline incidence of intussusception among NT Aboriginal children is 16 per 100 000 live births, several fold lower than the baseline rate in other Australian children [29]. We found no cases of intussusception in our study, although an increased risk could not be excluded. In Australia, Rotarix is not recommended after age 24 weeks, in line with its licensure.
Logistically, the most feasible age to schedule a third dose of Rotarix in our setting is 6 months, coinciding with the third scheduled dose of the diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine. High rates of delayed vaccination have been previously reported among Aboriginal children living in rural and remote settings [30], although most infants (>95%) enrolled in our study had received 2 Rotarix doses before enrollment, and most of these doses had been given on time (Table 1). We pragmatically allowed enrollment and administration of Rotarix/placebo to occur at any age from 6 to <12 months; the median age at enrollment into our study was 8.5 (IQR, 6.9–10.3) months. This age distribution likely reflects the large proportion of infants enrolled from very remote settings where enrollment visits were infrequent, but it also covers the broad age range in which children in our setting currently receive their third DTaP dose, scheduled at age 6 months. Only 62% of Australian Aboriginal children receive their third DTaP dose before age 7 months (compared with 81% of non-Indigenous children), approximately 18% at age 7–9 months, and approximately 10% at age 9–12 months[31]. We note that the age distribution of enrollment in our study also covers the existing 9-month schedule point for measles vaccination used in the Expanded Program on Immunization.
Randomization was used to ensure exchangeability of infants in the Rotarix and placebo arms. The 2 arms were well matched on most baseline factors, except that infants in the Rotarix arm were less likely than those in the placebo arm to be breastfed and less likely to have evidence of a seroresponse at baseline (69% vs 76%). Almost one-third of infants (29%) in our study could not contribute follow-up specimens to the immunological analysis. This was largely owing to restrictions on travel by study staff to remote communities because of dangerous weather (monsoonal storms), cultural grieving (“sorry business”), and restrictions imposed by jurisdictional and institutional authorities to prevent COVID-19 transmission. The proportions of Rotarix and placebo recipients with missing outcome data were similar, and while blinding ensures that selection bias is unlikely, it is possible that rates of seroresponse were different in those with missing immunological outcome data.
Adaptive designs are increasingly used for prelicensure therapeutic trials and may also have value in vaccine trials, especially in low-resource settings [32, 33]. Adaptive designs require upfront investment of resources into statistical modelling and simulations [33], but they might enable more judicious investment of field resources for recruitment and follow-up and may expedite translation of positive trial findings into clinical practice or refocusing on alternative strategies if trial results are negative. In addition, when superiority or futility of an intervention is already clear, the ability to stop trials early is ethical because it minimizes the burden and risks of study participation [32]; these were motivating factors in the design choice for ORVAC in this vulnerable and highly researched population.
In conclusion, administration of an additional dose of Rotarix vaccine to Australian Aboriginal children aged 6 to <12 months increased the proportion of children with evidence of a vaccine seroresponse by approximately 16%. If it can be demonstrated that this increase translates into improved protection against gastroenteritis, the additional dose could be a highly viable strategy to further decrease the burden of diarrheal disease among young children in this and other high-burden settings.
Notes
Acknowledgments. The study investigators acknowledge the valuable contribution of Aboriginal Elder, Ada Parry, Aboriginal members of the Steering Committee, Dennis Bonney (MBBS, FRACP), Olivia O’Donoghue (MBBS, FRACGP), and Simone Raye (MBBS, FRACGP), and the Australian First Nations Reference Group for Child Health at Menzies School of Health Research and the Kulunga Aboriginal Research Development Unit at the Telethon Kids Institute. We also acknowledge the valuable contribution of the independent Data Safety Monitoring Committee and Ross Andrews’ (PhD, MAE, MPH, DipAppSc) contribution to the design and implementation of the ORVAC trial. Finally, we sincerely thank Monica McNeal (MS) and her team at Cincinnati Children’s Hospital for enabling training in the rotavirus immunoglobulin A enzyme-linked immunosorbent assay and for generous sharing of specialist reagents; this permitted the collected blood samples to remain in Australia, fulfilling the request from the local Australian First Nations Reference Group for Child Health.
Disclaimer. N. C. was affiliated with the National Institute for Health Research (NIHR) Health Protection Research Unit in Gastrointestinal Infections at University of Liverpool, in partnership with Public Health England and in collaboration with the University of Warwick, and is based at the University of Liverpool. The views expressed are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care, or Public Health England. The National Health Medical Research Council had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Financial support. This work was supported by the National Health Medical Research Council (NHMRC) (1086952). B.F.M. is supported by an NHMRC Postgraduate Scholarship (1134095), a RACP P&CHD NHMRC Scholarship and a Douglas and Lola Douglas Scholarship in Medical Science, Australian Academy of Science. T.S. is supported by an NHMRC Career Development Fellowship (1111657). M.D. is supported by a Clinician Scientist Research Fellowship from the Murdosch Children's Research Institute.
Contributor Information
Bianca F Middleton, Global and Tropical Health Division, Menzies School of Health Research, Charles Darwin University, Darwin, Australia.
Margie Danchin, Vaccine Uptake Group, Murdoch Children’s Research Institute, Melbourne, Australia; Department of Paediatrics, University of Melbourne, Melbourne, Australia; Department of General Medicine, Royal Children’s Hospital, Melbourne, Australia.
Mark A Jones, Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Perth, Australia; School of Public Health, University of Sydney, Sydney, Australia.
Amanda J Leach, Child Health Division, Menzies School of Health Research, Charles Darwin University, Darwin, Australia.
Nigel Cunliffe, Clinical Infection, Microbiology and Immunology, University of Liverpool, Liverpool, United Kingdom.
Carl D Kirkwood, Enteric and Diarrheal Diseases, Bill and Melinda Gates Foundation, Seattle, USA.
Jonathan Carapetis, Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Perth, Australia; Centre for Child Health Research, University of Western Australia, Perth, Australia.
Sarah Gallagher, Global and Tropical Health Division, Menzies School of Health Research, Charles Darwin University, Darwin, Australia.
Lea-Ann Kirkham, Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Perth, Australia; Centre for Child Health Research, University of Western Australia, Perth, Australia.
Caitlyn Granland, Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Perth, Australia.
Monica McNeal, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, USA; Division of Infectious Disease, Cincinnati Children’s Hospital Medical Centre, Cincinnati, USA.
Julie A Marsh, Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Perth, Australia.
Claire S Waddington, Department of Medicine, University of Cambridge School of Clinical Medicine, Cambridge, United Kingdom.
Thomas L Snelling, Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Perth, Australia; School of Public Health, University of Sydney, Sydney, Australia; School of Public Health, Curtin University, Perth, Australia.
References
- 1. Parashar UD, Burton A, Lanata C, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:S9–S15. [DOI] [PubMed] [Google Scholar]
- 2. Burnett E, Parashar UD, Tate JE.. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2016-2019. J Infect Dis 2020; 222:1731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burnett E, Parashar UD, Tate JE.. Real-world effectiveness of rotavirus vaccines, 2006-19: a literature review and meta-analysis. Lancet Glob Health 2020; 8:e1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dey A, Wang H, Menzies R, et al. Changes in hospitalisations for acute gastroenteritis in Australia after the national rotavirus vaccination program. Med J Aust 2012; 197:453–7. [DOI] [PubMed] [Google Scholar]
- 5. Snelling TL, Andrews RM, Kirkwood CD, et al. Case-control evaluation of the effectiveness of the G1P[8] human rotavirus vaccine during an outbreak of rotavirus G2P[4] infection in central Australia. Clin Infect Dis 2011; 52:191–9. [DOI] [PubMed] [Google Scholar]
- 6. Middleton BF, Danchin M, Quinn H, et al. Retrospective case-control study of 2017 G2P[4] rotavirus epidemic in rural and remote Australia. Pathogens 2020; 9:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madhi SA, Kirsten M, Louw C, et al. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine 2012; 30:A44–51. [DOI] [PubMed] [Google Scholar]
- 8. Cunliffe NA, Witte D, Ngwira BM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine 2012; 30:A36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moon SS, Groome MJ, Velasquez DE, et al. Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin Infect Dis 2016; 62:157–65. [DOI] [PubMed] [Google Scholar]
- 10. Zhao Y, You J, Wright J, et al. Health inequity in the Northern Territory, Australia. Int J Equity Health 2013; 12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Australian Bureau of Statistics. Births, Australia 2019. 2020. https://www.abs.gov.au/statistics/people/population/births-australia/latest-release. Accessed 18 August 2021. [Google Scholar]
- 12. Middleton BF, Jones MA, Waddington CS, et al. The ORVAC trial protocol: a phase IV, double-blind, randomised, placebo-controlled clinical trial of a third scheduled dose of Rotarix rotavirus vaccine in Australian Indigenous infants to improve protection against gastroenteritis. BMJ Open 2019; 9:e032549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones MA, Graves T, Middleton B, et al. The ORVAC trial: a phase IV, double-blind, randomised, placebo-controlled clinical trial of a third scheduled dose of Rotarix rotavirus vaccine in Australian Indigenous infants to improve protection against gastroenteritis: a statistical analysis plan. Trials 2020; 21:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Australian Technical Advisory Group on Immunisation (ATAGI). Australian immunisation handbook. Canberra, Australia: Australian Government Department of Health, 2018. [Google Scholar]
- 15. Ward RL, Bernstein DI, Shukla R, et al. Protection of adults rechallenged with a human rotavirus. J Infect Dis 1990; 161:440–5. [DOI] [PubMed] [Google Scholar]
- 16. Bernstein DI, Smith VE, Sherwood JR, et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89-12. Vaccine 1998; 16:381–7. [DOI] [PubMed] [Google Scholar]
- 17. Bines JE, Kohl KS, Forster J, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004; 22:569–74. [DOI] [PubMed] [Google Scholar]
- 18. Haidara FC, Tapia MD, Sow SO, et al. Evaluation of a booster dose of pentavalent rotavirus vaccine coadministered with measles, yellow fever, and meningitis A vaccines in 9-month-old Malian infants. J Infect Dis 2018; 218:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaman K, Fleming JA, Victor JC, et al. Noninterference of rotavirus vaccine with measles-rubella vaccine at 9 months of age and improvements in antirotavirus immunity: a randomized trial. J Infect Dis 2016; 213:1686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parker EP, Ramani S, Lopman BA, et al. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol 2018; 13:97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Velasquez DE, Parashar U, Jiang B.. Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: causes and contributing factors. Expert Rev Vaccines 2018; 17:145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Australian Institute of Health and Welfare. Australia’s health 2018. Australia’s health series no. 16. AUS 221. Canberra, Australia: Australian Institute of Health and Welfare,2018. [Google Scholar]
- 23. Tonkin E, Kennedy D, Hanieh S, et al. Dietary intake of Aboriginal Australian children aged 6-36 months in a remote community: a cross-sectional study. Nutr J 2020; 19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clarke E, Desselberger U.. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol 2015; 8:1–17. [DOI] [PubMed] [Google Scholar]
- 25. Cheuvart B, Neuzil KM, Steele AD, et al. Association of serum anti-rotavirus immunoglobulin A antibody seropositivity and protection against severe rotavirus gastroenteritis: analysis of clinical trials of human rotavirus vaccine. Hum Vaccin Immunother 2014; 10:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel M, Glass RI, Jiang B, et al. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208:284–94. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization. Rotavirus vaccines: WHO position paper - February 2013. Weekly Epidemiological Record 2021; 88:49–64. [Google Scholar]
- 28. Lu HL, Ding Y, Goyal H, et al. Association between rotavirus vaccination and risk of intussusception among neonates and infants: a systematic review and meta-analysis. JAMA Netw Open 2019; 2:e1912458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Webby RJ, Bines JE, Barnes GL, et al. Intussusception in the Northern Territory: the incidence is low in Aboriginal and Torres Strait Islander children. J Paediatr Child Health 2006; 42:235–9; discussion 227–8. [DOI] [PubMed] [Google Scholar]
- 30. Moore HC, Fathima P, Gidding HF, et al. Assessment of on-time vaccination coverage in population subgroups: a record linkage cohort study. Vaccine 2018; 36:4062–9. [DOI] [PubMed] [Google Scholar]
- 31. Ioannides S, Beard F, Larter N, et al. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander People, Australia, 2011-2015. Commun Dis Intell (2018) 2019; 43:81. [PubMed] [Google Scholar]
- 32. Park JJH, Ford N, Xavier D, et al. Randomised trials at the level of the individual. Lancet Glob Health 2021; 9:e691–700. [DOI] [PubMed] [Google Scholar]
- 33. Liu M, Li Q, Lin J, et al. Innovative trial designs and analyses for vaccine clinical development. Contemp Clin Trials 2021; 100:106225. [DOI] [PMC free article] [PubMed] [Google Scholar]