Abstract
Purpose of review
The current review combines past findings with recent advances in our understanding of the homeostatic response to potassium imbalance.
Recent findings
Following the ingestion of a dietary potassium load, a combination of extrarenal and renal mechanisms act to maintain extracellular K+ within a tight window. Through hormonal regulation and direct K+ sensing, the nephron is ideally suited to respond to wide shifts in external K+ balance. Current evidence indicates that dietary K+ loading triggers a coordinated kaliuretic response that appears to involve voltage-dependent changes in sodium transport across multiple nephron segments, including the proximal tubule, medullary loop of Henle, and distal tubule. Inhibition of sodium transport in these segments would accomplish the final goal of enhancing distal NaCl delivery, luminal flow, and K+ secretion in the aldosterone sensitive distal nephron (ASDN).
Summary
Ongoing research seeks to define the relationship between potassium and volume homeostasis by elucidating pathways that couple renal K+ sensing and tubular function during the potassium stress response.
Keywords: aldosterone, distal nephron, epithelial transport, potassium, sodium
INTRODUCTION
Living organisms are perpetually challenged by potassium stress. In humans, shifts in extracellular K+ that are significant enough to cause frank hyperkalemia or hypokalemia are associated with increased morbidity and mortality [1,2]. The prevalence of hyperkalemia is as high as 50% in the chronic kidney disease population [3] and is a common cause of emergency department visits and inpatient hospital admissions. After kidney transplantation, hyperkalemia can persist, with a reported incidence of 44% in patients on calcineurin inhibitors [4]. Hypokalemia commonly occurs in the setting of diuretic use and gastrointestinal losses, and potassium deficiency has been associated with hypertension and progression of chronic kidney disease [1,5–7]. Furthermore, hyperkalemia and hypokalemia occur in up to 30% of hospitalized patients [8,9].
Potassium is the predominant intracellular cation; over 90% of the total body K+ is located intracellularly. Thus, only a small percentage of potassium in the body is readily accessible to dietary and renal/gastrointestinal control. Assuming normal extracellular fluid (ECF) volume of 14 L in a 70-kg adult man, the total ECF pool of potassium is very small at 70 mmol (14 L × 5 mmol/l), representing only 2% of total body K+. Compare that with the ECF pool of sodium, which is ~2000 mmol (14 L × 142 mmol/l) [10,11]. Current nutritional guidelines recommend a daily intake of potassium of 120 mmol/day (4.7 g/day) [12,13], an amount that is ~2 times the total ECF pool. Surprisingly, only 2% of US adults meet the recommendations for dietary potassium, with a majority of adults consuming a mere 60–100 mmol K+/day [14]. This amount of potassium is in stark contrast to the typical dietary potassium intake of our ancestors: the typical ‘Paleolithic’ diet consisted of a daily potassium load of up to 400 mmol K+/day [15]. Despite these large fluctuations in potassium intake, we evolved to maintain extracellular potassium levels within a very precise range of 3.5–5 mmol/L.
In this review, we will integrate insights into how the body handles a potassium load, with a particular emphasis on renal tubular mechanisms. Traditionally, the collecting duct has been viewed as the primary site for regulated K+ handling, although the weight of evidence suggests that other nephron segments also participate in the renal response to hyperkalemia. Based on recent and long-established discoveries, we propose that the entire nephron functions as a voltage-responsive potassium handling machine; one that intertwines K+ transport mechanisms with those classically associated with sodium and volume homeostasis.
THE HUMAN RESPONSE TO A DIETARY POTASSIUM LOAD
Contemporary ‘Western’ diets are sodium-rich and contain considerably less potassium (60–100 mmol K+/day) than the Paleolithic diets of our ancestors (400 mmol K+/day) [15,16]. Nevertheless, the potassium content that is absorbed during a meal can approach the total ECF potassium content, which averages 50–70 mmol. Thus, even a nourishing meal could be considered to induce a stress upon the organism that requires immediate intervention. The consumption of a dietary K+ load is associated with an integrated response that involves multiple organs, including the gut, kidney, and the muscle and liver.
Extrarenal mechanisms
Upon the ingestion of a potassium load, K+ is efficiently and rapidly absorbed in the small intestine. Generally, 90% of a dietary potassium bolus will enter the portal circulation, whereas 10% is subject to fecal excretion [16]. The proximal colon is a site of potassium absorption, whereas the distal colon mediates K+ secretion, a process that can be markedly enhanced by oral potassium binder resins such as sodium polystyrene sulfonate (Kayexalate), or newer agents such as patiromer and sodium zirconium cyclosilicate [17]. Chronic dietary K+ loading enhances the expression of large conductance Maxi-K (BK, slo1) channels along the apical membrane of the distal colon, indicating a mechanism by which this colonic segment can be converted from a potassium absorptive to secretory epithelium [18]. This process may be involved in colonic potassium adaptation when renal function is reduced, such as in chronic or end-stage kidney disease [19].
The body relies on transcellular K+ shifts to rapidly control the extracellular potassium concentration and prevent life-threatening hyperkalemia. This is because an ingested potassium load is rapidly taken up into the circulation but can take hours to be excreted. Potassium absorption into the portal circulation triggers the release of insulin, which facilitates transcellular shifts in the liver and muscle to regulate internal K+ balance [20]. Other factors that control internal K+ balance include catecholamines and pH [20,21]. Although the major reservoir for K+ resides in the muscle, it was recently proposed that the liver may serve to rapidly absorb a dietary potassium bolus and buffer wide shifts in extracellular K+. This may be due to the biosynthesis of lactate that follows the concomitant insulin-dependent absorption of glucose during a meal; the fall in pH associated with hepatocyte lactate production would theoretically trigger H+/K+exchange from the portal circulation [21].
The renal tubular response to potassium loading
Though extrarenal mechanisms are important for potassium homeostasis, the kidney plays a central role in the regulation of external K+ balance. The goal of renal potassium handling is to match potassium intake with potassium output to maintain serum potassium levels between 3.5 and 5 mmol/L, and this can occur within just a few hours [22]. This precise regulation requires the coordinated action of multiple cell types, acting across multiple nephron segments. During acute and chronic potassium loading, the nephron undergoes a coordinated response that ultimately enhances K+ secretion in the aldosterone-sensitive distal nephron (ASDN), a collection of tubular segments that includes the late portion of the distal convoluted tubule (DCT), the connecting tubule (CNT), and cortical collecting duct (CCD).
Proximal tubule
Potassium is freely filtered by the glomerulus, and 60–70% of the filtered potassium load is reabsorbed in the proximal tubule. The bulk of potassium reabsorption in this nephron segment occurs via para-cellular reabsorption proportional to sodium and water flux. Basolateral potassium channels are coupled with the Na–K–ATPase to recycle K+ and drive the activity of the pump [23]. Further the along the proximal tubule, the luminal voltage becomes slightly more positive, which favors potassium reabsorption via diffusion [24]. As tubular fluid enters the corticomedullary region of the descending loop of Henle, potassium enters the prourine via passive diffusion due to the high interstitial concentration of K+. [25].
The proximal tubule is also the major site of bulk sodium reabsorption in the kidney, a process that appears to be inversely dependent on extracellular potassium. In 1972, Brandis et al. [26] showed that potassium infusion decreases the rate of proximal tubule NaCl reabsorption in rodents, a process that likely serves to enhance distal sodium delivery and facilitate potassium secretion in the ASDN. This was supported by a recently published mathematical model of the rat nephron that included contributions from the medullary vasculature [27▪]. During simulations of mild hyperkalemia (K+ 5.5 mmol/L), this model found that increasing peritubular (K+) diminished transcellular sodium reabsorption in the proximal tubule, which contributed substantially to net K+ excretion. The effect could be explained by voltage-dependent effects on the proximal tubule basolateral membrane: hyperkalemia-induced depolarization of the basolateral membrane induced intracellular Cl− and HCO3− retention, cytosolic alkalinization, and suppression of the luminal sodium–hydrogen exchanger (NHE3) (Fig. 1a).
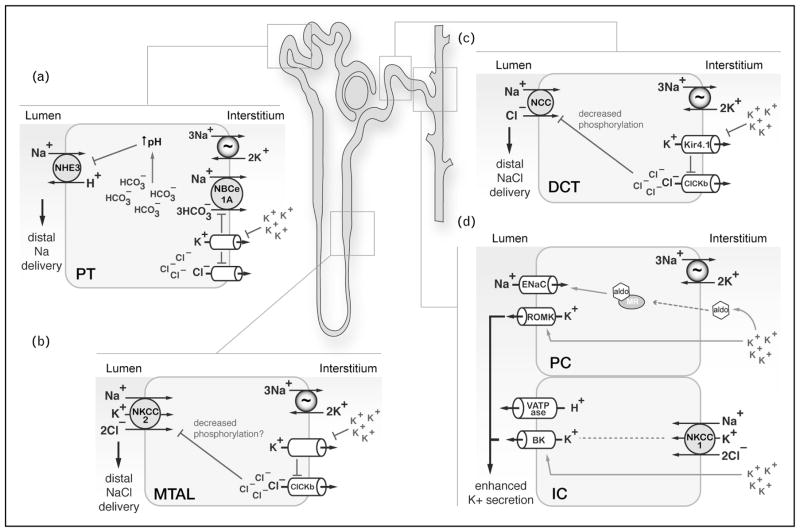
FIGURE 1.
The renal response to hyperkalemia involves multiple nephron segments. Summary of proposed transport events occurring in various sections of the nephron during dietary potassium loading. Current evidence suggests that in the proximal tubule, medullary thick ascending limb, and distal convoluted tubule, hyperkalemia-induced depolarization events at the basolateral membrane diminish transcellular sodium reabsorption, enhancing distal Na+ delivery. (a) A mathematical model [27▪] suggests that depolarization suppresses bicarbonate reabsorption via electrogenic sodium bicarbonate cotransporter 1A (NBCe1A), resulting in cytosolic alkalinization and reduced sodium–hydrogen exchanger activity. (b) In the medullary thick ascending limb, increased interstitial [K+] caused by enhanced medullary recycling diminishes sodium reabsorption, likely via NKCC2. It has been proposed that this process is also linked to basolateral membrane depolarization, which may downregulate NKCC2 activity via dephosphorylation, though this remains to be tested empirically. (c) In the distal convoluted tubule, hyperkalemia depolarization downregulates Kir4.1 activity, which increases intracellular chloride levels, attenuating sodium chloride cotransporter phosphorylation and activity. (d) Blockade of sodium transport in these regions of the nephron enhance NaCl delivery to principal cells and intercalated cells of the connecting tubule and cortical collecting duct, where voltage dependent K+ secretion via epithelial sodium channel and renal outer medullary K+ channel and flow dependent K+ secretion via BK channels occur, respectively. The enhanced K+ secretion in the connecting tubule and cortical collecting duct occurs through both aldosterone-dependent and aldosterone-independent processes.
Loop of Henle
Micropuncture experiments conducted in the early 1980s revealed that under conditions of potassium loading, the concentration of tubular K+ at the base of the loop of Henle exceeds 100% of the filtered K+ load [28]. This indicates that potassium undergoes active recycling in the renal medulla. The recycling circuit consists of luminal K+ absorption in the medullary collecting duct, extrusion into the medullary interstitium, and secretion back into the tubule via the thin limbs of the loop of Henle and pars recta [29,30]. Due to this absorptive process, the renal medulla can accumulate K+ up to a concentration of 50 mmol/L [31].
The physiological reasons for this interstitial buildup of potassium are not entirely clear. However, it does appear to participate in the renal response to hyperkalemia. Potassium recycling is enhanced during both acute and chronic K+ loading [28,31], and this has been associated with reduced NaCl reabsorption in the medullary thick ascending limb (MTAL) of the loop of Henle [32]. NaCl reabsorption in the thick ascending limb (TAL) is mediated by NKCC2 (SLC12A1, the loop diuretic-sensitive electroneutral Na-K-2Cl cotransporter). In 1982, Stokes [32] proposed that the reduced medullary Na transport during hyperkalemia is due to increased intracellular chloride concentrations caused by depolarization of the MTAL basolateral membrane. It was reasoned that this would enhance sodium chloride delivery and tubular flow to the ASDN, to enhance renal K+ secretion (Fig. 1).
Though Stokes originally advanced this theory of hyperkalemia-induced natriuresis 35 years ago, it remains an attractive model today. Indeed, in recent years, it has become apparent that intracellular chloride ([Cl−]i) regulates the activation status of NKCC2 and other SLC12 family members through phosphorylation. This process is mediated by The With-No-Lysine (WNK), Ste20/SpS1 related proline alanine-rich kinase (SPAK; STK39) and oxidative stress responsive kinase 1 (OSR1; OXSR1) pathway, a chloride-responsive serine–threonine kinase cascade [33]. WNK kinases are the chloride sensing kinases that mediate this effect. A rise in [Cl−]i causes chloride ions to bind to a pocket in the WNK kinase domain. This pocket is positioned close to the activation loop which when occupied, shuts off WNK kinase activity [34]. This inactivates SPAK and OSR1, two downstream effector kinases that directly phosphorylate NKCC2 at sites that increase its activity [35]. The WNK–SPAK/OSR1 pathway is strongly expressed in the loop of Henle; thus, through inhibitory effects on the WNK kinases, an increase in [Cl−]i could potentially block the activity of NKCC2 in the MTAL and enhance distal NaCl delivery to the ASDN (Fig. 1b). Alternatively, decreased NaCl reabsorption in the MTAL could be linked to acute NKCC2 dephosphorylation during hyperkalemia; a recent study identified calcineurin (PP2B) as a phosphatase that inhibits NKCC2 activity in vivo [36].
Distal convoluted tubule
Only 5–10% of the filtered potassium reaches the distal nephron, yet this region precisely regulates potassium balance. The DCT can be divided into early and late segments, termed the DCT1 and DCT2, respectively. Both of these segments express the thiazide-sensitive sodium chloride cotransporter (NCC, SLC12A3), an electroneutral cotransporter that is structurally similar to NKCC2, and is also activated by WNK–SPAK/OSR1 dependent phosphorylation [37]. The DCT1 is aldosterone-insensitive despite containing mineralocorticoid receptors, because it lacks the enzyme 11-β hydroxysteroid dehydrogenase 2 (11-βHSD2), which confers aldosterone specificity [38]. In contrast to the early portion, the DCT2 expresses 11-βHSD2 and is sensitive to aldosterone, making it the most proximal portion of the ASDN [38,39]. Consistent with this, the DCT2 expresses other aldosterone-responsive genes, including the epithelial sodium channel (ENaC) [40] and the renal outer medullary K+ channel (ROMK, Kir1.1) [41]. ENaC-mediated sodium transport is electrogenic; this leads to an increasingly negative lumen that favors voltage-dependent potassium secretion via ROMK [40].
Recent studies indicate that the DCT senses and responds to changes in extracellular [K+] via the basolateral inwardly rectifying K+ channels Kir4.1 and Kir5.1 [42▪,43]. Potassium depletion causes basolateral hyperpolarization and chloride efflux out of the cell. This leads to activation of DCT-specific WNK kinases by the mechanisms described above. Similar to NKCC2, the activated WNK–SPAK/OSR1 cascade phosphorylates and activates the thiazide-sensitive NCC, increasing sodium reabsorption in the DCT [44–47]. Conversely, potassium loading has been shown to depolarize the DCT and decrease NCC-mediated NaCl reabsorption [22,48–50]. This effect is associated with a decrease in NCC phosphorylation, either through direct dephosphorylation by phosphatases such as calcineurin [51], or via chloride-dependent inhibition of WNK–SPAK signaling [46,52] (Fig. 1c). Similar to the previously described effects in the proximal tubule and MTAL, hyperkalemia-mediated downregulation of NCC would be expected to enhance tubular NaCl delivery to the CNT and CCD.
Recent evidence clearly indicates that NCC-mediated NaCl reabsorption is suppressed during the renal response to hyperkalemia. However, the selective blockade of NaCl reabsorption in the DCT per se may not generate a sufficient natriuresis to cause enhanced distal K+ secretion acutely. In an interesting and provocative study by Hunter et al. [53], acute NCC inhibition with hydrochlorothiazide (HCTZ) induced a natriuresis as expected, but potassium excretion was not enhanced. Moreover, though administration of the ENaC-specific inhibitor benzamil did induce its own separate natriuretic effect, treatment with HCTZ following benzamil preadministration did not augment the natriuretic effect of thiazides. This suggests that the distal NaCl delivery caused by acute administration of HCTZ was not limited by enhanced ENaC activity. Thus, the data suggest that at least in the short term, HCTZ administration did not provide an acute supply of sodium to the collecting duct to drive ENaC activity and ROMK-mediated K+ secretion. The authors proposed that chronic/structural adaptations in the distal nephron may account for the kaliuretic effect of thiazides. A recent study in mice expressing overactive NCC in DCT1 suggested that the converse may also be true. In these mice, which express a constitutively active form of SPAK in DCT1, chronically high NCC activity induced long-term remodeling of the collecting duct to limit K+ secretion [54▪▪]. Collectively, these recent studies suggest that the DCT may do more than simply ‘meter’ the delivery of sodium chloride to the CNT and CCD to control renal K+ secretion, as it may also induce more chronic structural adaptations in these downstream nephron segments.
Connecting tubule and cortical collecting duct
Potassium secretion in the CNT and CCD is primarily dependent on the aldosterone-regulated proteins ENaC and ROMK. The CNT and CCD have different embryonic origins, yet both exhibit similar aldosterone-dependent mechanisms for potassium secretion. Several studies suggest that the late DCT and CNT are the main site of potassium secretion in vivo [40,55,56]; however, as these segments are difficult to isolate for ex-vivo studies, the CCD has classically been used as a model to understand distal K+ secretion.
The CNT and CCD are heterogeneously populated with principal cells and intercalated cells, interspersed in a mosaic pattern [57]. Principal cells secrete potassium via an electrogenic gradient created by Na, K–ATPase in the basolateral membrane driving luminal sodium reabsorption via the apical ENaC, which stimulates potassium efflux into the lumen via ROMK. ROMK is considered to be the major potassium-secretory pathway due to its high open probability and increased surface expression in the setting of potassium excess and aldosterone secretion [24,58]. Under basal conditions, potassium secretion appears to be almost entirely dependent upon the gradient established by ENaC; however, with extended K+ loading, there is upregulation of a sodium-independent mechanism for the secretion of potassium involving kaliuretic factors [58–61] (Fig. 1). ENaC is strongly regulated by aldosterone, a hormone whose secretion is enhanced during hyperkalemia (Fig. 1d). Aldosterone stimulates ENaC activity through a variety of mechanisms [62], but one of the most well appreciated involves the serum and glucocorticoid regulated kinase, (SGK1). SGK1 is an aldosterone-inducible kinase that enhances ENaC surface expression by inhibiting Nedd4–2, an E3 ubiquitin ligase that suppresses ENaC activity at baseline. A recent study found that Nedd4–2 knockout mice become profoundly hypokalemic during prolonged dietary potassium restriction, due to ENaC disinhibition [63▪▪]. This suggests an essential role for Nedd4–2 in limiting ENaC activity during chronic hypokalemia, to prevent aberrant voltage dependent K+ secretion. Conversely, when SGK1 knockout mice were placed on a high K+ diet, they developed severe hyperkalemia, suggesting an important role for this kinase in the aldosterone-induced enhancement of ENaC activity during hyperkalemic stress [64].
Renal intercalated cells are essential regulators of acid–base homeostasis; yet, more recent work has illuminated their role in regulating potassium balance [65]. The most abundant intercalated cell is the Type A pendrin-lacking cells, which secrete protons into the urine. Type A cells contain an apical H, K–ATPase and under conditions of potassium depletion they can transport potassium into the cell in exchange for proton secretion [65–67]. Type A cells also apically express the large conductance BK (Maxi K; slo1) channels that secrete potassium in response to increased flow and calcium activation. For years, the BK channel’s physiologic relevance in potassium secretion was overlooked due to its exceedingly low open probability [68,69]. However, the observation that infants with loss-of-function ROMK mutations (type II Bartter syndrome) develop postnatal hypokalemia (and not persistent hyperkalemia as would be expected), helped establish the importance of BK mediated potassium secretion [70]. BK channels are expressed in principal cells and intercalated cells; however, the intercalated cell appears to be ideally poised to facilitate BK-mediated K+ secretion. The apical membranes of intercalated cells are depolarized (−36 mV) as compared with principal cells (−82 mV), a voltage that favors the BK channel open state [68,71]. Consistent with this, the density of pore-forming BK channel subunits is higher in intercalated cells than principal cells, and BK channel activity is greater in intercalated cells [68]. Recent studies have shown that dietary K+ loading enhances BK channel expression and activity in intercalated cells [72▪] (Fig. 1), an effect that is more pronounced in animals given an alkaline high K+ diet, and is absent in animals lacking accessory BK channel subunits that are specifically expressed in intercalated cells [73].
In states of potassium excess where apical BK channel expression is increased [67], potassium secretion relies on the gradient established by the apical VATPase, which leads to a decrease in intra-cellular sodium via basolateral anion exchanger isoform 4. This allows for basolateral entry of sodium, potassium, and chloride via NKCC1 (SLC12A2), an electroneutral Na–K–2Cl cotransporter that is closely related to NKCC2 and NCC [58,68]. Current evidence suggests that NKCC1 is the primary pathway for basolateral K+ transport into the intercalated cell (Fig. 1). Consistent with this, application of the NKCC1 inhibitor bumetanide to the bath of isolated perfused collecting ducts completely inhibited flow-induced potassium secretion by BK channels [74]. As NKCC1 is activated by SPAK and OSR1 [33], it seems likely that the WNK–SPAK/OSR1 signaling pathway plays an important role in mediating transcellular K+ secretion via NKCC1 and BK channels. Indeed, recent studies have found that kinase active WNK1 is robustly expressed in intercalated cells in rabbits treated with a high K+ diet [72▪], and that this WNK kinase can activate BK channels [75]. Additional studies are needed to determine the relevance of this pathway for flow-induced K+ secretion in intercalated cells of the kidney.
CONCLUSION
Hyperkalemia is a life-threatening stress that requires correction via a multiorgan homeostatic mechanism, that includes K+ sensing and adaptation in the gut, internal cellular shifts, and renal mechanisms. In the kidney, hyperkalemia induces a coordinated response among multiple tubular segments. Current evidence suggests that voltage-dependent attenuation of sodium chloride reabsorption in the proximal tubule, loop, and DCT may enhance tubular flow and facilitate the delivery of solute to the ASDN, where K+ is secreted via BK and ROMK channels. In many of these cell types, it is possible that overlapping signaling systems are involved, such as the WNK–SPAK/OSR1 pathway. Future studies will further define how these signaling networks, the physiologic stimuli that activate them, and the effector pathways that they influence, coordinate the renal response to potassium stress.
KEY POINTS.
Hyperkalemia is a physiologic stress that requires internal correction via cellular shifts, and external correction via adaptive mechanisms in the gut and kidney.
In the proximal tubule, medullary thick ascending limb, and distal convoluted tubule, hyperkalemia results in voltage-dependent inhibition of sodium reabsorption, enhancing sodium delivery to the ASDN, likely to augment K+ secretion.
The With-No-Lysine (WNK), Ste20/SpS1 related proline alanine-rich kinase (SPAK) and oxidative stress responsive kinase 1 (OSR1) pathway is regulated by changes in extracellular K+, a process that controls distal sodium delivery and long-term remodeling of the ASDN.
Potassium secretion in the ASDN is controlled by the coordinated action of epithelial sodium channel and renal outer medullary K+ channel in principal cells, and by flow activated BK channels in intercalated cells.
Acknowledgments
The authors would like to acknowledge Tom Kleyman, Lisa Satlin, and Alan Weinstein for helpful discussions.
Financial support and sponsorship
The work was supported by National Institutes of Health Grants R01DK098145 (A.R.S), and P30DK79307 (to the Pittsburgh Center for Kidney Research). C.R.B.-S. is supported by NIH T32DK061296.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Hayes J, Kalantar-Zadeh K, Lu JL, et al. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120:c8–c16. doi: 10.1159/000329511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Chang AR, McAdams DeMarco MA, et al. Serum potassium, mortality, and kidney outcomes in the atherosclerosis risk in communities study. Mayo Clin Proc. 2016;91:1403–1412. doi: 10.1016/j.mayocp.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarafidis PA, Blacklock R, Wood E, et al. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin J Am Soc Nephrol. 2012;7:1234–1241. doi: 10.2215/CJN.01150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan B, Wang Z, Abecassis MM, et al. Frequency of hyperkalemia in recipients of simultaneous pancreas and kidney transplants with bladder drainage. Transplantation. 1996;62:1174–1175. doi: 10.1097/00007890-199610270-00025. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee M, Wingo CS, McDonough AA, et al. Narrative review: evolving concepts in potassium homeostasis and hypokalemia. Ann Intern Med. 2009;150:619–625. doi: 10.7326/0003-4819-150-9-200905050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki S-I, Haneda M, Koya D, et al. Urinary potassium excretion and renal and cardiovascular complications in patients with type 2 diabetes and normal renal function. Clin J Am Soc Nephrol. 2015;10:2152–2158. doi: 10.2215/CJN.00980115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, McFann K, Chonchol M, et al. Association between dietary sodium and potassium intake with chronic kidney disease in US adults: a cross-sectional study. Am J Nephrol. 2013;37:526–533. doi: 10.1159/000351178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paice BJ, Paterson KR, Onyanga-Omara F, et al. Record linkage study of hypokalaemia in hospitalized patients. Postgrad Med J. 1986;62:187–191. doi: 10.1136/pgmj.62.725.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shemer J, Modan M, Ezra D, Cabili S. Incidence of hyperkalemia in hospitalized patients. Isr J Med Sci. 1983;19:659–661. [PubMed] [Google Scholar]
- 10.Cheng C-J, Kuo E, Huang C-L. Extracellular potassium homeostasis: insights from hypokalemic periodic paralysis. Semin Nephrol. 2013;33:237–247. doi: 10.1016/j.semnephrol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev. 2015;95:297–340. doi: 10.1152/physrev.00011.2014. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Agriculture and U.S. Department of Health and Human Services. 2015–2020 dietary guidelines for Americans. Washington, D.C: U.S. Department of Agriculture and U.S. Department of Health and Human Services; 2016. [Google Scholar]
- 13.Institute of Medicine. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, D.C: National Academies Press; 2005. [Google Scholar]
- 14.Cogswell ME, Zhang Z, Carriquiry AL, et al. Sodium and potassium intakes among US adults: NHANES. Am J Clin Nutr. 2012;96:647–657. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebastian A, Frassetto LA, Sellmeyer DE, Morris RC. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin Nephrol. 2006;26:447–453. doi: 10.1016/j.semnephrol.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology. 1994;107:548–571. doi: 10.1016/0016-5085(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 17.Chaitman M, Dixit D, Bridgeman MB. Potassium-binding agents for the clinical management of hyperkalemia. P T. 2016;41:43–50. [PMC free article] [PubMed] [Google Scholar]
- 18.Perry MD, Rajendran VM, MacLennan KA, Sandle GI. Segmental differences in upregulated apical potassium channels in mammalian colon during potassium adaptation. Am J Physiol Gastrointest Liver Physiol. 2016;311:G785–G793. doi: 10.1152/ajpgi.00181.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batlle D, Boobés K, Manjee KG. The colon as the potassium target: entering the colonic age of hyperkalemia treatment? EBioMedicine. 2015;2:1562–1563. doi: 10.1016/j.ebiom.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clausen T. Hormonal and pharmacological modification of plasma potassium homeostasis. Fundam Clin Pharmacol. 2010;24:595–605. doi: 10.1111/j.1472-8206.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheema-Dhadli S, Chong C-K, Kamel KS, Halperin ML. An acute infusion of lactic acid lowers the concentration of potassium in arterial plasma by inducing a shift of potassium into cells of the liver in fed rats. Nephron Physiol. 2012;120:7–15. doi: 10.1159/000336321. [DOI] [PubMed] [Google Scholar]
- 22.Rengarajan S, Lee DH, Oh YT, et al. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol. 2014;306:F1059–F1068. doi: 10.1152/ajprenal.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton KL, Devor DC. Basolateral membrane K+ channels in renal epithelial cells. Am J Physiol Renal Physiol. 2012;302:F1069–F1081. doi: 10.1152/ajprenal.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10:1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandis M, Keyes J, Windhager EE. Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol. 1972;222:421–427. doi: 10.1152/ajplegacy.1972.222.2.421. [DOI] [PubMed] [Google Scholar]
- 27▪.Weinstein AM. A mathematical model of the rat kidney: K+-induced natriuresis. Am J Physiol Renal Physiol. 2017;312:F925–F950. doi: 10.1152/ajprenal.00536.2016. This study presented a new expanded mathematical model of the nephron that incorporated contributions from the medullary vasculature. During simulations of mild hyperkalemia, the model predicted that the bulk of K+-induced natriuresis is derived from the proximal tubule, not more distal segments of the nephron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arrascue JF, Dobyan DC, Jamison RL. Potassium recycling in the renal medulla: effects of acute potassium chloride administration to rats fed a potassium-free diet. Kidney Int. 1981;20:348–352. doi: 10.1038/ki.1981.145. [DOI] [PubMed] [Google Scholar]
- 29.Rocha AS, Kokko JP. Membrane characteristics regulating potassium transport out of the isolated perfused descending limb of Henle. Kidney Int. 1973;4:326–330. doi: 10.1038/ki.1973.124. [DOI] [PubMed] [Google Scholar]
- 30.Wasserstein AG, Agus ZS. Potassium secretion in the rabbit proximal straight tubule. Am J Physiol. 1983;245:F167–F174. doi: 10.1152/ajprenal.1983.245.2.F167. [DOI] [PubMed] [Google Scholar]
- 31.Battilana CA, Dobyan DC, Lacy FB, et al. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest. 1978;62:1093–1103. doi: 10.1172/JCI109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest. 1982;70:219–229. doi: 10.1172/JCI110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alessi DR, Zhang J, Khanna A, et al. The WNK–SPAK/OSR1 pathway: master regulator of cation–chloride cotransporters. Sci Signal. 2014;7:re3. doi: 10.1126/scisignal.2005365. [DOI] [PubMed] [Google Scholar]
- 34.Piala AT, Moon TM, Akella R, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7:ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson C, Sakamoto K, de los Heros P, et al. Regulation of the NKCC2 ion cotransporter by SPAK–OSR1-dependent and -independent pathways. J Cell Sci. 2011;124:789–800. doi: 10.1242/jcs.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blankenstein KI, Borschewski A, Labes R, et al. Calcineurin inhibitor cyclosporine A activates renal Na–K–Cl cotransporters via local and systemic mechanisms. Am J Physiol Renal Physiol. 2017;312:F489–F501. doi: 10.1152/ajprenal.00575.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson C, Rafiqi FH, Karlsson HKR, et al. Activation of the thiazide-sensitive Na+–Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 38.Bostanjoglo M, Reeves WB, Reilly RF, et al. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na–Cl cotransporter expression by distal tubules. J Am Soc Nephrol. 1998;9:1347–1358. doi: 10.1681/ASN.V981347. [DOI] [PubMed] [Google Scholar]
- 39.Poulsen SB, Christensen BM. Long-term aldosterone administration increases renal Na+–Cl− cotransporter abundance in late distal convoluted tubule. Am J Physiol Renal Physiol. 2016. Epub ahead of print. [DOI] [PubMed]
- 40.Wade JB, Fang L, Coleman RA, et al. Differential regulation of ROMK (Kir1. 1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol. 2011;300:F1385–F1393. doi: 10.1152/ajprenal.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanya AR, Ellison DH. Distal convoluted tubule. Clin J Am Soc Nephrol. 2014;9:2147–2163. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪.Cuevas CA, Su X-T, Wang M-X, et al. Potassium sensing by renal distal tubules requires Kir4. 1. J Am Soc Nephrol. 2017;28:1814–1825. doi: 10.1681/ASN.2016090935. This study employed a tubule specific knockout model to verify that sodium chloride cotransporter (NCC) activity is dependent on Kir4.1, and that potassium-dependent regulation of NCC requires this basolateral K+ channel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellison DH, Terker AS. Why your mother was right: how potassium intake reduces blood pressure. Trans Am Clin Climatol Assoc. 2015;126:46–55. [PMC free article] [PubMed] [Google Scholar]
- 44.Castañeda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, et al. Modulation of NCC activity by low and high K(+) intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol. 2014;306:F1507–F1519. doi: 10.1152/ajprenal.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terker AS, Zhang C, McCormick JA, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terker AS, Zhang C, Erspamer KJ, et al. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016;89:127–134. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol. 2010;299:F890–F897. doi: 10.1152/ajprenal.00323.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorensen MV, Grossmann S, Roesinger M, et al. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 49.van der Lubbe N, Moes AD, Rosenbaek LL, et al. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+–Cl− cotransporter. Am J Physiol Renal Physiol. 2013;305:F1177–F1188. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 50.Vallon V, Schroth J, Lang F, et al. Expression and phosphorylation of the Na+–Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoorn EJ, Walsh SB, McCormick JA, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011;17:1304–1309. doi: 10.1038/nm.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Penton D, Czogalla J, Wengi A, et al. Extracellular K(+) rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl(−)-dependent and independent mechanisms. J Physiol (Lond) 2016;594:6319–6331. doi: 10.1113/JP272504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunter RW, Craigie E, Homer NZM, et al. Acute inhibition of NCC does not activate distal electrogenic Na+ reabsorption or kaliuresis. Am J Physiol Renal Physiol. 2014;306:F457–F467. doi: 10.1152/ajprenal.00339.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Grimm PR, Coleman R, Delpire E, Welling PA. Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016090948. [Epub ahead of print]. This study found that mice expressing a constitutively kinase active form of SPAK in distal convoluted tubule (DCT)1 exhibited hypertension and hyperkalemia due to hyperactivity of NCC. Interestingly, the mice developed a reduction in connecting tubule mass, suggesting the presence of a remodeling process that involves coupling between the early DCT and the ASDN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frindt G, Shah A, Edvinsson J, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol. 2009;296:F347–F354. doi: 10.1152/ajprenal.90527.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol. 2004;287:F593–F601. doi: 10.1152/ajprenal.00454.2003. [DOI] [PubMed] [Google Scholar]
- 57.Muto S. Potassium transport in the mammalian collecting duct. Physiol Rev. 2001;81:85–116. doi: 10.1152/physrev.2001.81.1.85. [DOI] [PubMed] [Google Scholar]
- 58.Welling PA. Roles and regulation of renal K channels. Annu Rev Physiol. 2016;78:415–435. doi: 10.1146/annurev-physiol-021115-105423. [DOI] [PubMed] [Google Scholar]
- 59.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol. 2009;297:F389–F396. doi: 10.1152/ajprenal.90528.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rutledge JC, Rabinowitz L. Kaliuretic regulatory factors in the rat. Am J Physiol. 1987;253:F1182–F1196. doi: 10.1152/ajprenal.1987.253.6.F1182. [DOI] [PubMed] [Google Scholar]
- 61.Nesterov V, Dahlmann A, Krueger B, et al. Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Renal Physiol. 2012;303:F1289–F1299. doi: 10.1152/ajprenal.00247.2012. [DOI] [PubMed] [Google Scholar]
- 62.Soundararajan R, Lu M, Pearce D. Organization of the ENaC-regulatory machinery. Crit Rev Biochem Mol Biol. 2012;47:349–359. doi: 10.3109/10409238.2012.678285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63▪▪.Al-Qusairi L, Basquin D, Roy A, et al. Renal tubular ubiquitin-protein ligase NEDD4-2 is required for renal adaptation during long-term potassium depletion. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016070732. [Epub ahead of print]. Though numerous prior in-vitro studies suggested a role for Nedd4-2 in epithelial sodium channel (ENaC) inhibition, analyses of Nedd4-2 mice on high-salt diets failed to confirm this effect in vivo. This study found that when Nedd4-2 knockout mice were placed on a low-potassium diet, the mice became profoundly hypokalemic due to ENaC overactivity. Thus, the study confirmed the in-vivo relevance of Nedd4-2 ENaC interactions and uncovered why this interaction developed during the course of evolution: to prevent life-threatening hypokalemia caused by excessive ENaC activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Qusairi L, Basquin D, Roy A, et al. Renal tubular SGK1 deficiency causes impaired K+ excretion via loss of regulation of NEDD4-2/WNK1 and ENaC. Am J Physiol Renal Physiol. 2016;311:F330–F342. doi: 10.1152/ajprenal.00002.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy A, Al-bataineh MM, Pastor-Soler NM. Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol. 2015;10:305–324. doi: 10.2215/CJN.08880914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gumz ML, Lynch IJ, Greenlee MM, et al. The renal H+–K+–ATPases: physiology, regulation, and structure. Am J Physiol Renal Physiol. 2010;298:F12–21. doi: 10.1152/ajprenal.90723.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Najjar F, Zhou H, Morimoto T, et al. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol. 2005;289:F922–F932. doi: 10.1152/ajprenal.00057.2005. [DOI] [PubMed] [Google Scholar]
- 68.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol. 2007;292:F966–F973. doi: 10.1152/ajprenal.00191.2006. [DOI] [PubMed] [Google Scholar]
- 69.Carrisoza-Gaytán R, Carattino MD, Kleyman TR, Satlin LM. An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron. Am J Physiol Cell Physiol. 2016;310:C243–C259. doi: 10.1152/ajpcell.00328.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simon DB, Karet FE, Rodriguez-Soriano J, et al. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 71.Muto S, Giebisch G, Sansom S. An acute increase of peritubular K stimulates K transport through cell pathways of CCT. Am J Physiol. 1988;255:F108–F114. doi: 10.1152/ajprenal.1988.255.1.F108. [DOI] [PubMed] [Google Scholar]
- 72▪.Webb TN, Carrisoza-Gaytán R, Montalbetti N, et al. Cell-specific regulation of L-WNK1 by dietary K. Am J Physiol Renal Physiol. 2016;310:F15–F26. doi: 10.1152/ajprenal.00226.2015. This study found that with-no-lysine (amino acid =K) (WNK)1 activates BK channels in cell culture and is upregulated in intercalated cells during dietary K+ loading. This is intriguing, as hyperkalemia is generally considered to be a mechanism that inhibits WNK kinase activity. These data suggest that WNK1 is upregulated during hyperkalemia to activate BK channels via a unique intercalated cell-specific mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cornelius RJ, Wen D, Li H, et al. Low Na, high K diet and the role of aldosterone in BK-mediated K excretion. PLoS One. 2015;10:e0115515. doi: 10.1371/journal.pone.0115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu W, Schreck C, Coleman RA, et al. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol. 2011;301:F1088–F1097. doi: 10.1152/ajprenal.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Song X, Shi Y, et al. WNK1 activates large-conductance Ca2+-activated K+ channels through modulation of ERK1/2 signaling. J Am Soc Nephrol. 2015;26:844–854. doi: 10.1681/ASN.2014020186. [DOI] [PMC free article] [PubMed] [Google Scholar]