INTRODUCTION:
An estimated 15%–29% of patients report new gastrointestinal (GI) symptoms after coronavirus-19 disease (COVID-19) while 4%–31% report new depressive symptoms. These symptoms may be secondary to gut microbiome tryptophan metabolism and 5-hydroxytryptamine (5-HT)-based signaling.
METHODS:
This study used specimens from 2 patient cohorts: (i) fecal samples from patients with acute COVID-19 who participated in a randomized controlled trial testing prebiotic fiber and (ii) blood samples from patients with acute COVID-19. Six months after recovering from COVID-19, both cohorts answered questions related to GI symptoms and anxiety or depression. Microbiome composition and function, focusing on tryptophan metabolism-associated pathways, and plasma 5-HT were assessed.
RESULTS:
In the first cohort (n = 13), gut microbiome L-tryptophan biosynthesis during acute COVID-19 was decreased among those who developed more severe GI symptoms (2.0-fold lower log activity comparing those with the most severe GI symptoms vs those with no symptoms, P = 0.06). All tryptophan pathways showed decreased activity among those with more GI symptoms. The same pathways were also decreased in those with the most severe mental health symptoms after COVID-19. In an untargeted analysis, 5 additional metabolic pathways significantly differed based on subsequent development of GI symptoms. In the second cohort (n = 39), plasma 5-HT concentration at the time of COVID-19 was increased 5.1-fold in those with GI symptoms alone compared with those with mental health symptoms alone (P = 0.02).
DISCUSSION:
Acute gut microbiome-mediated reduction in 5-HT signaling may contribute to long-term GI and mental health symptoms after COVID-19. Future studies should explore modification of 5-HT signaling to reduce post-COVID symptoms.
INTRODUCTION
Gastrointestinal (GI) symptoms are a common sequela of acute coronavirus-19 disease (COVID-19), with surveys suggesting a 15%–29% prevalence of at least 1 GI symptom at 6 months after infection (1–3). The development of chronic GI symptoms after infection is not unique to COVID-19, and post-COVID GI symptoms may be understood using the framework of postinfection irritable bowel syndrome (PI-IBS) (4–7). Approximately 10% of patients meet criteria for IBS 1 year after diagnosis with infectious enteritis (8). The cause of PI-IBS is unknown but may be related to changes in serotonergic signaling and serotonin (5-hydroxytryptamine [5-HT]) metabolism (9). Anxiety and depression are established risk factors for the development of PI-IBS (4,8), and 5-HT is a key enteric neurotransmitter, with roles in activating peristalsis and secretory functions (10,11).
The gut microbiota influences 5-HT through the production and metabolism of tryptophan, the rate-limiting precursor of 5-HT (12–15). Postprandial 5-HT concentrations have been associated with IBS subtypes (16), and patients with IBS can have decreased nocturnal levels of tryptophan metabolites (17). Furthermore, acute tryptophan depletion induces IBS-C-like symptoms and brain patterns on functional MRI in healthy controls, supporting the role of 5-HT as a modulator of the brain-gut microbiome axis (18). Patients with PI-IBS have increased mucosal 5-HT containing enterochromaffin cells (EC) and also differ from healthy controls in microbiome composition (19,20).
It is uncertain whether GI symptoms that arise after COVID-19 are similar to the symptoms that arise after PI-IBS from non-COVID infectious causes. There is a mechanistic rationale for considering COVID-19 as a cause of PI-IBS. The severe acute respiratory syndrome coronavirus 2 virus that causes COVID-19 actively replicates within the GI mucosa (21,22). Acute COVID-19 alters the gut microbiome (23), although it is unclear whether these alterations are caused by the receipt of antibiotics and other medical interventions or by severe acute respiratory syndrome coronavirus 2 infection per se (24). At the same time, pandemic-related stress and anxiety (25,26)—established risk factors for IBS—are also crucial determinants of who gets long-term GI symptoms after COVID-19 (2,3).
Anxiety and depression are relatively common after COVID-19, with the frequency of depression ranging from 4% to 31% and anxiety 6%–63% (27,28) A prospective cohort of patients with long COVID-19 symptoms found a high prevalence of depression (15%) and posttraumatic stress disorder (22%) (29).
This study investigated the hypothesis that alterations in gut microbial tryptophan metabolism during COVID-19 lead to decreased 5-HT signaling and post-COVID GI symptoms. We sought to assay both longitudinal fecal microbial samples and plasma samples for 5-HT; however, we did not have access to stool and blood samples from a single cohort. Instead, we combined data from 2 similar COVID-19 cohorts, one which provided serial fecal samples and another which provided blood samples. Both cohorts answered clinical questions related to GI and mental health symptoms at baseline and 6 months after diagnosis of COVID-19.
METHODS
Complete methods appear in the Methods Supplementary File (see Supplementary Digital Content 3, http://links.lww.com/CTG/A873).
Cohort 1: The gut microbiome and post-COVID GI symptoms
Study population.
This was a substudy conducted within a parent double-blinded randomized controlled trial with a 1:1:1 assignment of patients to placebo, inulin 16 g/d, or inulin 32 g/d given in 2 divided doses for 7 days (NCT03865706). At the start of the COVID-19 pandemic, the parent trial was paused and patients with moderate severity COVID-19 were randomly assigned the trial intervention. Patients aged 18 years and older were included if they reported that they were free from chronic GI symptoms at baseline and required hospitalization, but not intensive care unit-level care, for COVID-19 before June 1, 2020, and received broad-spectrum antibiotics within 24 hours before enrollment. Both the parent study and this substudy were approved by the Columbia University Irving Medical Center Institutional Review Board.
Biosamples and sequencing.
Patients donated deep rectal swabs immediately before the study intervention (day 0) and subsequently at days 3, 7, 14, and 30 or until hospital discharge. At the end of this study, samples were sequenced for the V4 hypervariable region of the 16S ribosomal ribonucleic acid (RNA) gene using a previously described protocol (30). The PICRUSt2 pipeline was used with 16S data to estimate the abundance of functional pathways including those for tryptophan biosynthesis (31).
Measurement of post-COVID GI and mental health symptoms.
Six months after COVID-19 diagnosis, patients were contacted by telephone and asked questions evaluating Rome IV criteria for IBS. All patients completed the IBS Severity Scoring System (IBS-SSS), although none met formal IBS criteria (32). The IBS-SSS was selected rather than competing instruments because it has been highly validated in non-COVID cohorts, and it was used to classify GI symptoms as none/mild (IBS-SSS <50, which was approximately the median in the cohort), mild/moderate (IBS-SSS 50–200), or moderate/severe (>200, which was the 90th percentile in the cohort). Combined symptoms of anxiety/depression were reported using the EQ-5D-5L (33) as a 4-point Likert scale (no symptoms, mild, moderate, or severe) because none of the respondents were in the most severe level 5 category.
Outcomes and statistical approach.
The primary outcome was differences in tryptophan metabolism between patients with different levels of severity of GI and/or self-reported anxiety/depression, assessed using phylogenetic investigation of communities by reconstruction of unobserved states results. Tryptophan metabolism was compared across categories using a repeated-measures mixed model. Unsupervised analyses were also performed for differences in microbiome composition or function across groups; to enhance rigor, features were first tested for false discovery rate-adjusted differences (P < 0.05) and hits were then retested using the repeated-measures mixed model.
Cohort 2: Plasma serotonin and post-COVID GI symptoms
Patient population.
Patients older than 18 years were included if they had mild-moderate COVID-19 between April and November 2020 and enrolled in a COVID-19 longitudinal cohort. From within this cohort, 40 patients were randomly selected, including 20 patients with and 20 patients without GI symptoms 6 months after COVID-19 (not matched).
Measurement of 5-HT.
Biobanked plasma was retrieved and tested for the absolute concentration of 5-HT (in pg/mL) using liquid chromatography-mass spectrometry with a spike-in internal standard (34).
Measurement of post-COVID GI and mental health symptoms.
Six months after COVID-19, patients were asked by an electronic survey to indicate the presence of COVID-related GI symptoms including diarrhea, constipation, abdominal pain and, if present, rate symptoms on a scale from 1 to 5 (very mild, mild, moderate, severe, very severe). On the same Likert scale, they were asked to rate anxiety or sadness.
Outcomes and statistical approach.
The primary outcome was 5-HT concentrations, compared in patients with vs without any GI symptoms, in patients with vs without self-reported anxiety or sadness, and in a factorial manner. The Mann-Whitney U test was used to assess for differences between any 2 groups and the Kruskal-Wallis test for differences between more than 2 groups.
RESULTS
Cohort 1: The gut microbiome in those with and without post-COVID GI symptoms
Patient characteristics.
Thirteen patients with moderate severity COVID-19 were randomized to placebo, inulin 16 g/d, or inulin 32 g/d. At the time of enrollment, all patients were hospitalized but not intubated and all received broad-spectrum antibiotics within 24 hours before enrollment. Additional patient characteristics are given in Table 1. Six months later, they completed the IBS-SSS with a median score of 45 (interquartile range [IQR] 2–90). None of the patients met Rome IV criteria for IBS.
Table 1.
Cohort 1 characteristics at baseline and during COVID-19 treatment of 13 patients treated with prebiotic inulin for moderate severity COVID-19
| Characteristics | n (proportion) |
| Age (yr, median/IQR) | 58 (50–64) |
| Sex | |
| Male | 6 (46%) |
| Female | 7 (54%) |
| Race | |
| White | 9 (69%) |
| Black | 3 (23%) |
| Asian | 1 (8%) |
| Ethnicity | |
| Hispanic | 6 (46%) |
| Non-Hispanic | 7 (54%) |
| Days hospitalized with COVID-19 (median/IQR) | 5 (3–12) |
| COVID-19 treatmentsa | |
| Supplemental oxygen | 13 (100%) |
| Steroids | 3 (23%) |
| Remdesivir | 1 (8%) |
| Antibioticsb | |
| Any antibiotic | 13 (100%) |
| Ceftriaxone | 8 (62%) |
| Piperacillin-tazobactam | 5 (38%) |
| Doxycycline | 3 (23%) |
| Azithromycin | 4 (31%) |
| Vancomycin | 4 (31%) |
| Discharge on supplemental oxygen | 4 (31%) |
| Psychotropic medications | |
| Selective serotonin reuptake inhibitor | 0 (0%) |
| Tricyclic antidepressant | 0 (0%) |
| Serotonin-norepinephrine reuptake inhibitor (duloxetine) | 1 (8%) |
COVID-19, coronavirus-19 disease; IQR, interquartile relationship.
One subject received both convalescent plasma and ivermectin; a different subject received hydroxychloroquine.
Antibiotics received within 24 hours before enrollment. Ten of 13 patients received multiple antibiotics. In addition to the antibiotics listed, 1 subject received levofloxacin and another received cephalexin.
Microbiome composition/function.
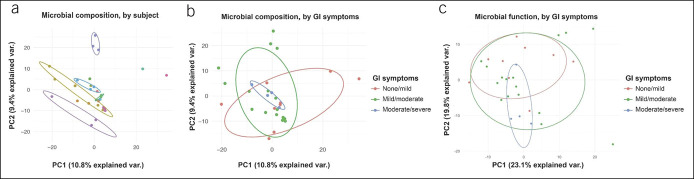
A median of 2 fecal samples per patient were collected over 3 days (IQR 0–7 days). On principal coordinate analysis, there was a high degree of stability within patients over time (i.e., patients patients continued to resemble themselves longitudinally; Figure 1a). Samples were then partitioned based on IBS-SSS, assessed 6 months after COVID-19 diagnosis. There were minimal differences between categories based on IBS-SSS in microbial composition (Figure 1b) or in microbial function (Figure 1c).
Figure 1.
Cohort 1: principal coordinate plots of gut microbiome composition and function during acute COVID-19. Principal coordinate plots from 16S sequencing results based on OTU read count from samples gathered during acute COVID-19. (a) Microbial composition, organized by subject (each individual patient has a unique color) at 6 months of follow-up after COVID-19. (b) Microbial composition, organized by GI symptoms at 6 months of follow-up. (c) Microbial function, imputed from 16S sequencing results and organized by GI symptoms at 6 months of follow-up. GI symptoms were classified based on IBS-SSS points. COVID-19, coronavirus-19 disease; GI, gastrointestinal; IBS-SSS, Irritable Bowel Syndrome Severity Scoring System; OTU, operational taxonomic unit.
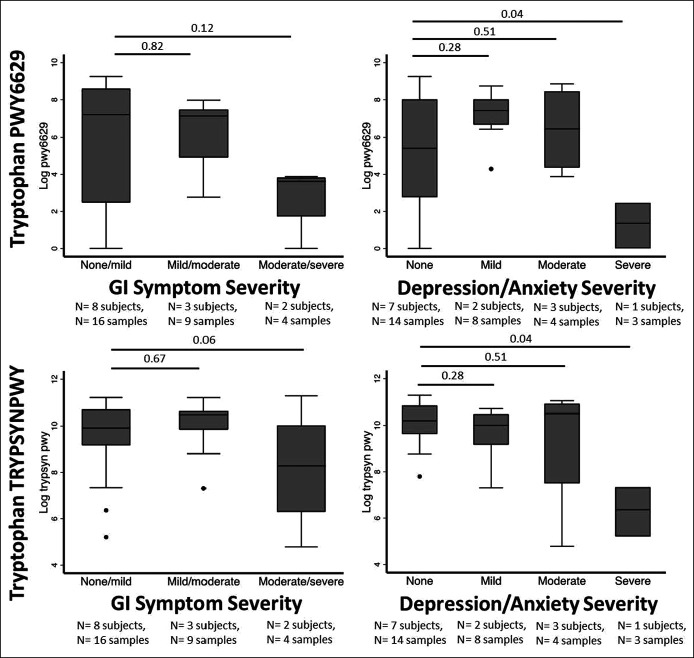
Tryptophan metabolism.
Tryptophan is the rate-limiting precursor of 5-HT and is metabolized by the gut microbiome. The 6 major metabolic pathways related to tryptophan were designated a priori for analysis (BioCyC ID numbers 6629, TRPSYN, NADSYN, 6505, 5651, and 5655). Of these, only PWY-6629 and TRPSYN-PWY (the 2 major superpathways for L-tryptophan biosynthesis) were represented in >50% of samples and were analyzed. In both pathways, decreased tryptophan biosynthesis was observed among those with increased GI symptoms (Figure 2a). The patient with the most severe symptoms (IBS-SSS > 200) had diarrhea predominantly, but further subtyping of symptoms could not be performed because of the limited number of patients within categories.
Figure 2.
Cohort 1: Gut microbiome tryptophan metabolism during acute COVID-19, stratified based on GI and depression/anxiety symptoms at 6 months of follow-up. GI and depression/anxiety symptoms were classified in 13 patients at 6 months of follow-up after COVID-19. Tryptophan metabolism was imputed from 16S sequencing results from longitudinal samples gathered for up to 30 days after the diagnosis of COVID-19. GI symptoms were classified based on IBS-SSS points and depression/anxiety symptoms based on responses to EQ-5D-5L, which characterizes depression/anxiety on a 4-point Likert scale (none, mild, moderate, and severe). P values are for a repeated-measures mixed model incorporating between-patients and within-patients effects. COVID-19, coronavirus-19 disease; GI, gastrointestinal; IBS-SSS, Irritable Bowel Syndrome Severity Scoring System.
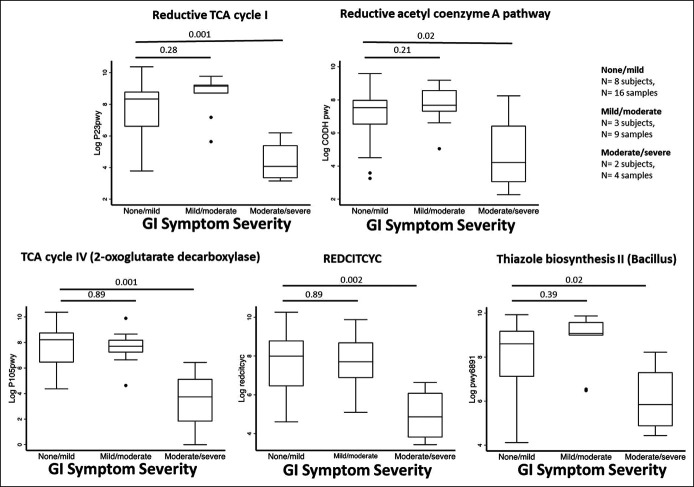
Our previous work suggested an interaction between GI and mental health symptoms (3,35) We, therefore, examined gut microbiome tryptophan biosynthesis next based on the severity of self-reported anxiety/depression. Decreased activity within the tryptophan biosynthesis pathway was correlated with an increased severity of mental health symptoms (Figure 2b; P < 0.05 in both pathways). When GI and mental health symptoms were classified in a factorial manner, a similar pattern was seen (see Figure S1, Supplementary Digital Content 2, http://links.lww.com/CTG/A872). In an untargeted analysis, there were no differential compositional features based on IBS groups but there were 5 differential metabolic pathways, all related to the tricarboxylic acid (TCA) cycle (Figure 3). All of these showed significant differences in tryptophan biosynthesis activity between the groups with the least and most GI symptoms.
Figure 3.
Cohort 1: Gut microbiome metabolic pathways that significantly differed during acute COVID-19 based on GI symptoms at 6 months of follow-up. The differential pathways are shown based on GI symptoms that were identified in an untargeted analysis of all pathways using 16S sequencing results. GI symptoms were classified based on IBS-SSS points. P values are for a repeated-measures mixed model incorporating between-patients and within-patients effects. COVID-19, coronavirus-19 disease; GI, gastrointestinal; IBS-SSS, Irritable Bowel Syndrome Severity Scoring System; TCA, tricarboxylic acid.
Inulin intervention.
Patients received a median of 7 doses (IQR 2–14 doses) of placebo, low-dose inulin, or high-dose inulin. There were no adverse events related to inulin. Looking across intervention groups, the median fiber intake was 0 g for placebo, 56 g for low-dose inulin, and 116 g for high-dose inulin over a median of 3 days. There were no differences between intervention groups in tryptophan metabolic pathways (see Figure S2, Supplementary Digital Content 2, http://links.lww.com/CTG/A872), IBS-SSS (see Figure S3, Supplementary Digital Content 2, http://links.lww.com/CTG/A872), or mental health symptoms (data not shown). There were no false discovery rate-adjusted differential features based on the intervention group.
Cohort 2: Plasma serotonin in those with and without post-COVID GI symptoms
Patient characteristics.
Of 1,783 patients in the COVID-19 longitudinal cohort, 749 (42%) completed the 6-month follow-up survey. From these 749 patients, 40 were randomly selected, and plasma 5-HT was successfully quantified in 39 of them (28 women and 11 men) using samples banked at the time of COVID-19 diagnosis. Seven patients (18%) were hospitalized for COVID-19. The median age was 47 years (IQR 39–59 years) (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A871).
Post-COVID GI symptoms.
Forty-nine percent of the cohort had new symptoms self-perceived to be COVID-related. Diarrhea was the most common post-COVID GI symptom, reported by 28% of the cohort. Constipation was reported by 10% and abdominal pain by 15%.
5-HT concentration and post-COVID GI symptoms.
The median plasma 5-HT concentration was 25,023 pg/mL (IQR 11,286–37,313 pg/mL) at the time of COVID-19. There was no difference in 5-HT concentrations comparing those with or without any post-COVID GI symptoms or those with or without specific GI symptoms, including diarrhea, constipation, or abdominal pain (Table 2).
Table 2.
Cohort 2 plasma 5-HT concentrations at the time of COVID-19, stratified by GI and mental health symptoms at 6 mo of follow-up
| n (%) | Median 5-HT concentration in pg/mL (IQR) | P valuea | |
| Post-COVID GI symptoms | |||
| Any GI symptoms | 19 (49%) | 24,962 (17,744–31,154) | 0.91 |
| No GI symptoms | 20 (51%) | 26,199 (6,969–93,297) | |
| Specific GI symptoms | |||
| Diarrhea | 11 (28%) | 24,962 (11,286–29,989) | 0.47 |
| No diarrhea | 28 (72%) | 25,158 (11,454–93,297) | |
| Constipation | 4 (10%) | 23,817 (18,451–29,233) | 0.93 |
| No constipation | 35 (90%) | 25,023 (10,806–40,918) | |
| Abdominal pain | 6 (15%) | 25,127 (19,672–28,476) | 0.88 |
| No abdominal pain | 33 (85%) | 25,023 (10,806–40,918) | |
| Post-COVID sadness or anxiety | |||
| Sadness | 12 (31%) | 19,415 (5,182–26,617) | 0.028 |
| No sadness | 27 (69%) | 27,637 (12,102–93,790) | |
| Anxiety | 20 (51%) | 19,415 (6,030–26,751) | <0.01 |
| No anxiety | 19 (49%) | 32,553 (21,399–93,790) | |
| Sadness or anxiety | 21 (54%) | 19,158 (6,365–25,292) | <0.01 |
| No sadness or anxiety | 18 (46%) | 32,953 (22,199–93,790) | |
| Post-COVID GI and mental health symptoms | |||
| Neither GI nor mental health symptoms | 13 (33%) | 32,553 (22,199–93,790) | 0.02 |
| Mental health but no GI symptoms | 7 (18%) | 6,365 (4,648–25,023) | |
| GI but no mental health symptoms | 5 (13%) | 33,354 (31,154–40,918) | |
| Both GI and mental health symptoms | 14 (36%) | 21,371 (14,442–28,210) |
5-HT, serotonin; COVID-19, coronavirus-19 disease; GI, gastrointestinal; IQR, interquartile range.
The Mann-Whitney U test was used for comparison of any 2 groups; the Kruskal-Wallis test was used for comparison of any 3 or more groups.
5-HT concentration and post-COVID mental health symptoms.
Next, we explored whether a correlation existed between 5-HT concentrations and self-reported anxiety or sadness (2,36). Six months after the initial COVID-19 diagnosis, 31% of patients reported sadness and 51% reported anxiety, with 54% reporting either symptom. The median 5-HT concentration was significantly lower among those who reported sadness (19,415 vs 27,637 pg/mL, P = 0.028), anxiety (19,415 vs 32,553 pg/mL, P < 0.01), or either symptom (19,158 vs 32,953 pg/mL, P < 0.01; Table 2) compared with those who did not. The 5-HT concentration was also lower in 3 patients who reported pre-COVID mental health symptoms compared with those who did not, although this difference was not significant (5,695 vs 26,334 pg/mL, P = 0.07). Patients were then categorized into 4 groups according to the presence of both post-COVID anxiety or sadness and post-COVID GI symptoms in a factorial manner. Plasma 5-HT concentrations were highest among those without symptoms, lowest among those with anxiety or sadness only, and intermediate among those with mixed GI and anxiety or sadness symptoms (Kruskal-Wallis test for a difference between groups P = 0.02, Table 2 and Figure 4).
Figure 4.
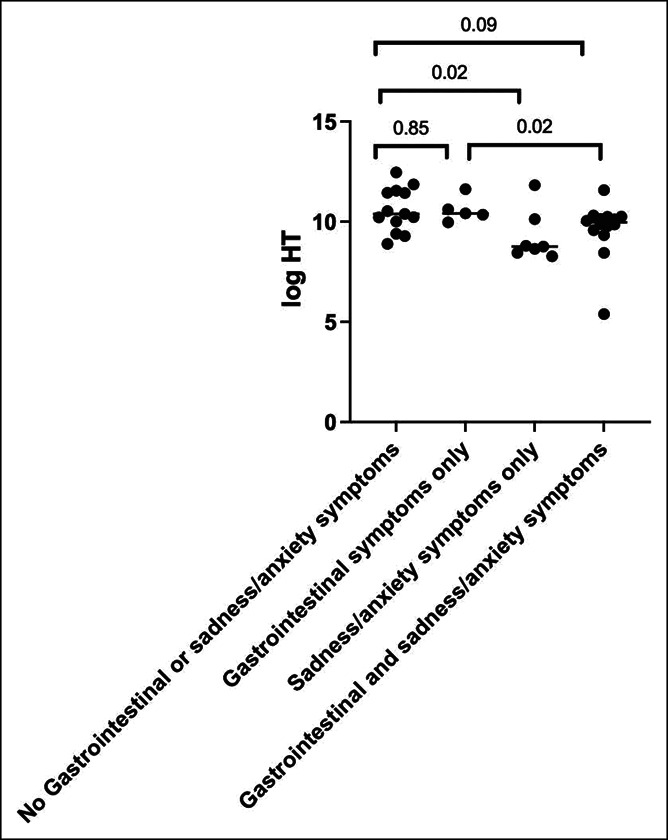
Cohort 2: Plasma 5-HT concentrations during acute COVID-19, stratified based on GI and sadness/anxiety symptoms at 6 months of follow-up. The Mann-Whitney U P value is shown for a difference between groups. 5-HT, serotonin; COVID-19, coronavirus-19 disease.
DISCUSSION
New GI symptoms are common after COVID-19 (3,37,38). The mechanisms underlying these symptoms are unknown, and 1 hypothesis likens the problem to PI-IBS. It has been postulated in PI-IBS that the gut microbiome alteration leads to persistent changes in the host—perhaps mediated through serotonergic signaling—to cause IBS-like symptoms. This study interrogated a 5-HT-based explanatory mechanism for post-COVID GI symptoms. Two distinct cohorts were used. Within the first cohort, which provided stool samples, there was decreased microbial tryptophan biosynthesis based on the subsequent development of GI symptoms. Within the second cohort, which provided blood samples, there was a decrease in plasma 5-HT concentrations associated with the subsequent development of sadness or anxiety and GI symptoms (but not GI symptoms alone). In both cohorts, there were significant differences in tryptophan biosynthesis or 5-HT concentrations when comparing patients with neither, either, or both GI and mental health symptoms, suggesting an interaction between these symptoms, which is consistent both with prior research and with the established relationship between IBS and mental health (20,39,40). Overall, this study provides preliminary evidence for potential biological links between COVID-19 and long-term disorders of gut-brain interactions such as IBS. We view these results as a first step which may help delineate areas for future research.
Our study found significant differences in microbial tryptophan biosynthesis—prespecified as the outcome of interest—comparing those with or without new GI symptoms 6 months after COVID-19. Some of these differences were large in magnitude. Previous studies have also found decreased serum tryptophan metabolites among patients with COVID-19 which may be unsurprising because COVID-19 is an inflammatory state and shunting of tryptophan toward its main metabolite, kynurenine, is a feature of systemic inflammatory response syndrome (41). In an untargeted analysis of serum metabolites, Thomas et al. identified decreased tryptophan production as the most prominent feature distinguishing patients with acute COVID-19 from controls (42). In a different cohort, the ratio of kynurenine to tryptophan was highest among those with acute COVID-19, middling in acutely ill controls, and lowest in healthy controls (43). Using serial samples, Ansone et al. (44) found that serum L-tryptophan levels were depleted during acute COVID-19 and recovered only 47% during 40 days of follow-up. Our results suggesting that there may be reduced gut microbial tryptophan biosynthesis in patients with more severe post-COVID GI and anxiety/depression is novel yet mesh with previous studies.
We also found differences based on GI symptoms in 5 untargeted microbial metabolic pathways. Four of the 5 differentially expressed pathways were directly related to aerobic respiration through the TCA cycle (i.e., production of adenosine triphosphate [ATP]); the fifth pathway was related to thiazole biosynthesis of thiamine (vitamin B1), an essential cofactor for the pyruvate dehydrogenase multienzyme complex, which oxidizes glucose into the TCA cycle. The TCA cycle is closely associated with serotonin synthesis (45–47), and all the pathways showed the same directionality (decreased activity among those with more GI symptoms). Liu et al. (23) recently reported on 106 patients with COVID-19 and identified 32 metabolic pathways that differed based on postacute COVID-19 syndrome (primarily fatigue). Interestingly, all the pathways identified in this study were associated with ATP production, which mediates intestinal injury in animal models (48). This may be a fruitful area for future studies.
Serotonin is produced in intestinal EC from tryptophan and may be elevated or decreased among patients with postinfection IBS compared with controls (11,49–52). In this study, we did not have blood samples from the same cohort that provided gut microbiome samples, so to address the question of plasma 5-HT concentrations, we gathered blood samples from a separate cohort of patients who similarly completed the 6-month follow-up after COVID-19 infection. Most of bodily 5-HT is synthesized within the intestinal epithelium and is stored within platelets. It is thus conceivable that plasma 5-HT concentrations reflect the local levels produced by EC cells (53,54). Plasma 5-HT concentrations have been reported to be increased in patients with acute COVID-19 presenting with diarrhea, but there have been no previous studies evaluating the association between 5-HT and chronic post-COVID GI symptoms (55).
Our analysis showed that 5-HT concentrations at the time of acute COVID-19 were lower in patients who later reported COVID-related sadness or anxiety; there was evidence of an interaction between sadness/anxiety and GI symptoms, and the lowest 5-HT concentrations were observed in those with COVID-related sadness/anxiety but not GI symptoms. Only 8% of patients reported significant mental health symptoms before COVID-19, compared with 54% 6 months after COVID-19, mirroring other studies which show a large increase in the mental health burden after recovery from COVID-19 (29,56,57). It is unclear whether these new symptoms are due to the virus itself, the sequelae of hospitalization, the toll of other coexisting long COVID-19 symptoms, or nonmedical stressors related to the pandemic. It is likely that mental health symptoms are both a cause and an effect of chronic GI symptoms after COVID-19 infection, and the observation that patients with the lowest 5-HT concentrations had highly increased odds of reporting new anxiety or sadness 6 months later supports the role of 5-HT in mediating symptoms after COVID-19 infection. The efficacy of serotonin-modulating medications for treating COVID-related depression, anxiety, or IBS should be further investigated.
This study has several key strengths. COVID-19 disease severity and antibiotic administration (in the microbiome cohort) were homogeneous. In previous studies, these 2 factors—disease severity and receipt of antibiotics—have been the main drivers of observed microbiome differences (24,58). This study was longitudinal, with follow-up after 6 months and, in the cohort providing fecal samples, gathered serial samples from individual patients. It interrogated a specific hypothesis, derived from prior literature on postinfection IBS, and tested this hypothesis in multiple ways (i.e., using both stool and blood samples).
This study also has limitations. The sample size was small, especially in the cohort providing fecal samples. 5-HT concentrations were measured at the time of COVID-19 diagnosis and were not obtained postprandially (which is when differences between IBS phenotypes and controls are most apparent), and there may be significant differences in serotonin signaling that become apparent in the months after COVID-19. The severity of GI symptoms in cohort 1 was assessed using IBS-SSS, but none of the patients met Rome criteria for IBS, and most had mild symptoms. Using the standard IBS-SSS would have meant that almost all our patients were rated as mild, leaving no groups to compare. Instead, we used the approximate median IBS-SSS score (45) to divide the cohort in half and then selected the 90th percentile of the IBS-SSS score (200) to define (relatively) severe symptoms (IBS-SSS >200). Another limitation arises from the fact that 2 different cohorts were initially designed as separate studies, resulting in different methods for assessing self-reported mental health symptoms in both cohorts because the primary authors did not have input on the questionnaire for cohort 2. Therefore, cohort 2 assessed self-reported sadness and anxiety separately while cohort 1 assessed combined symptoms of anxiety/depression together. These self-reported symptoms do not necessarily indicate the presence of a clinical psychiatric diagnosis. Furthermore, the specific timing and use of psychotropic medications during the 6 months after COVID-19 infection were unknown, which may affect the severity of such symptoms. Finally, all the patients were unvaccinated patients from the first wave of the pandemic, and their results may not be generalizable to later waves of patients.
In sum, this prospective study of patients with mild-to-moderate severity COVID-19 found decreased fecal microbial tryptophan biosynthesis and plasma 5-HT concentrations during acute COVID-19 that associated with GI symptoms 6 months later. Grouped symptoms of anxiety/depression interacted with gut microbiome tryptophan biosynthesis, and grouped symptoms of anxiety/sadness interacted with plasma 5-HT concentration. These findings are preliminary and derived from a relatively small number of patients. Nonetheless, they may help pave the way for longitudinal studies that address the role of the brain-gut axis and specifically whether serotonergic signaling mediates long COVID-19 GI and mental health-related symptomatology. Additional studies to compare serotonin levels in a larger cohort of patients meeting criteria for IBS and patients who were vs those who were not clinically diagnosed with depression and/or anxiety after COVID-19 infection, rather than relying on self-reported symptoms alone, would provide valuable additional information.
CONFLICTS OF INTEREST
Guarantor of the article: Daniel E. Freedberg, MD, MS.
Specific author contributions: J.W.B.: investigation, analysis, writing original draft, and review and editing. Y.S.: investigation, data analysis, methodology, and review and editing. L.P.: methodology and review and editing. K.G.M.: conceptualization, analysis, and review and editing. M.S.V.E.: conceptualization, analysis, and review and editing. S.O.B.: investigation and review and editing. M.W.: analysis and review and editing. J.A.A.: conceptualization, analysis, and review and editing. H.H.W.: conceptualization, analysis, and review and editing. L.C.: conceptualization, analysis, and review and editing. D.E.F.: conceptualization, investigation, analysis, methodology, writing original draft, and review and editing. All authors approved the final draft of this manuscript.
Financial support: This work was funded by a 2022 Clinical Trial Award from the American College of Gastroenterology and by a Columbia University Division of Digestive and Liver Diseases Research Center Pilot Award. Dr. Margolis was funded in part by NIH NIDDK 1RO1DK130518-01, NIH NIDDK RO1DK126644, NIHNINDS4RO1NS015547, DoD PR160365, the NASPGHAN Takeda Innovator Award, and the Seidenberg Family Foundation. Dr Wang was funded in part by the Hirschl Research Scientist Award and Burroughs Welcome Fund PATH (1,016,691). Dr Chang was funded in part by NIH grants U54 DK123755 and SPARC OT2OD024899. Dr. Freedberg was funded in part by a Columbia University Irving Scholar Award and by the Department of Defense PR181960.
Potential competing interests: We have no relevant conflicts to disclose.
Study Highlights.
WHAT IS KNOWN
✓ COVID-19 is associated with persistent gastrointestinal and mental health symptoms in a subset of patients.
✓ The mechanism of these symptoms is unknown.
WHAT IS NEW HERE
✓ Gut microbiome tryptophan biosynthesis pathways were reduced in patients who reported more severe gastrointestinal symptoms after COVID-19.
✓ The same pathways were reduced in patients who reported more severe mental health symptoms.
✓ Plasma 5-HT concentrations at the time of COVID-19 were significantly reduced in patients who later reported new mental health symptoms.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A871, http://links.lww.com/CTG/A872, http://links.lww.com/CTG/A873
Contributor Information
Yiwei Sun, Email: ys3235@cumc.columbia.edu.
Lawrence Purpura, Email: lp2745@cumc.columbia.edu.
Kara Gross Margolis, Email: kjg2133@cumc.columbia.edu.
Mitchell S.V. Elkind, Email: mse13@cumc.columbia.edu.
Sheila O'Byrne, Email: so2017@cumc.columbia.edu.
Milton Wainberg, Email: mlw35@cumc.columbia.edu.
Julian A. Abrams, Email: ja660@cumc.columbia.edu.
Harris H. Wang, Email: hw2429@cumc.columbia.edu.
Lin Chang, Email: linchang@mednet.ucla.edu.
Daniel E. Freedberg, Email: def2004@cumc.columbia.edu.
REFERENCES
- 1.Yusuf F, Fahriani M, Mamada SS, et al. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: A systematic review and meta-analysis. F1000Res 2021;10:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackett JW, Li J, Jodorkovsky D, Freedberg DE. Prevalence and risk factors for gastrointestinal symptoms after recovery from COVID-19. Neurogastroenterol Motil 2022;34:e14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackett JW, Wainberg M, Elkind MSV, Freedberg DE. Potential long coronavirus disease 2019 gastrointestinal symptoms 6 Months after coronavirus infection are associated with mental health symptoms. Gastroenterology 2022;162:648–50 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Card T, Enck P, Barbara G, et al. Post-infectious IBS: Defining its clinical features and prognosis using an internet-based survey. United Eur Gastroenterol J 2018;6:1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiller R. Postinfectious functional dyspepsia and postinfectious irritable bowel syndrome: Different symptoms but similar risk factors. Gastroenterology 2010;138:1660–3. [DOI] [PubMed] [Google Scholar]
- 6.Wouters MM, Van Wanrooy S, Nguyen A, et al. Psychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritis. Gut 2016;65:1279–88. [DOI] [PubMed] [Google Scholar]
- 7.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol 2003;98:1578–83. [DOI] [PubMed] [Google Scholar]
- 8.Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: A systematic review and meta-analysis. Gastroenterology 2017;152:1042–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YY, Annamalai C, Rao SSC. Post-infectious irritable bowel syndrome. Curr Gastroenterol Rep 2017;19:56. [DOI] [PubMed] [Google Scholar]
- 10.Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol 2004;141:1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon MD, Tack J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007;132:397–414. [DOI] [PubMed] [Google Scholar]
- 12.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun 2018;9:3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reigstad CS, Salmonson CE, Rainey JF, III, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 2015;29:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Villalobos-Hernandez EC, Pradhananga S, et al. Deoxycholic acid activates colonic afferent nerves via 5-HT3 receptor-dependent and -independent mechanisms. Am J Physiol Gastrointest Liver Physiol 2019;317:G275–84. [DOI] [PubMed] [Google Scholar]
- 15.Priyadarshini M, Navarro G, Reiman DJ, et al. Gestational insulin resistance is mediated by the gut microbiome-indoleamine 2,3-dioxygenase axis. Gastroenterology 2022;162:1675–89.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: Role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil 2012;18:258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burr RL, Gu H, Cain K, et al. Tryptophan metabolites in irritable bowel syndrome: An overnight time-course study. J Neurogastroenterol Motil 2019;25:551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labus JS, Mayer EA, Jarcho J, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut 2011;60:1196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalanka-Tuovinen J, Salojarvi J, Salonen A, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014;63:1737–45. [DOI] [PubMed] [Google Scholar]
- 20.Barbara G, Grover M, Bercik P, et al. Rome foundation working team report on post-infection irritable bowel syndrome. Gastroenterology 2019;156:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020;158:1831–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian Q, Fan L, Liu W, et al. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis 2021;73:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Mak JWY, Su Q, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022;71:544–52. [DOI] [PubMed] [Google Scholar]
- 24.Yeoh YK, Zuo T, Lui GCY, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021;70:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakov R, Dimitrova-Yurukova D, Snegarova V, et al. Increased prevalence of gastrointestinal symptoms and disorders of gut-brain interaction during the COVID-19 pandemic: An internet-based survey. Neurogastroenterol Motil 2022;34:e14197. [DOI] [PubMed] [Google Scholar]
- 26.Gubatan J, Zikos T, Spear Bishop E, et al. Gastrointestinal symptoms and healthcare utilization have increased among patients with functional gastrointestinal and motility disorders during the COVID-19 pandemic. Neurogastroenterol Motil 2022;34:e14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renaud-Charest O, Lui LMW, Eskander S, et al. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J Psychiatr Res 2021;144:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanbehzadeh S, Tavahomi M, Zanjari N, et al. Physical and mental health complications post-COVID-19: Scoping review. J Psychosom Res 2021;147:110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyzar EJ, Purpura LJ, Shah J, et al. Anxiety, depression, insomnia, and trauma-related symptoms following COVID-19 infection at long-term follow-up. Brain Behav Immun Health 2021;16:100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji BW, Sheth RU, Dixit PD, et al. Quantifying spatiotemporal variability and noise in absolute microbiota abundances using replicate sampling. Nat Methods 2019;16:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douglas GM, Maffei VJ, Zaneveld JR, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 2020;38:685–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 33.Stolk E, Ludwig K, Rand K, et al. Overview, update, and lessons learned from the international EQ-5D-5L valuation work: Version 2 of the EQ-5D-5L valuation protocol. Value Health 2019;22:23–30. [DOI] [PubMed] [Google Scholar]
- 34.Virag D, Kiraly M, Drahos L, et al. Development, validation and application of LC-MS/MS method for quantification of amino acids, kynurenine and serotonin in human plasma. J Pharm Biomed Anal 2020;180:113018. [DOI] [PubMed] [Google Scholar]
- 35.Blackett JW, Li J, Jodorkovsky D, Freedberg DE. Prevalence and risk factors for gastrointestinal symptoms after recovery from COVID-19. Neurogastroenterol Motil 2022;34:e14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021;397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 2021;93:1013–22. [DOI] [PubMed] [Google Scholar]
- 38.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker CH, Naliboff BD, Shih W, et al. Negative events during adulthood are associated with symptom severity and altered stress response in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2019;17:2245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michaelis S, Zelzer S, Schnedl WJ, et al. Assessment of tryptophan and kynurenine as prognostic markers in patients with SARS-CoV-2. Clin Chim Acta 2022;525:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas T, Stefanoni D, Reisz JA, et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020;5:140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lionetto L, Ulivieri M, Capi M, et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochim Biophys Acta Mol Basis Dis 2021;1867:166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ansone L, Briviba M, Silamikelis I, et al. Amino acid metabolism is significantly altered at the time of admission in hospital for severe COVID-19 patients: Findings from longitudinal targeted metabolomics analysis. Microbiol Spectr 2021;9:e0033821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baraldo G, Etemad S, Weiss AKH, et al. Modulation of serotonin signaling by the putative oxaloacetate decarboxylase FAHD-1 in Caenorhabditis elegans. PLoS One 2019;14:e0220434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weng R, Shen S, Tian Y, et al. Metabolomics approach reveals integrated metabolic network associated with serotonin deficiency. Sci Rep 2015;5:11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyu Y, Weaver KJ, Shaukat HA, et al. Drosophila serotonin 2A receptor signaling coordinates central metabolic processes to modulate aging in response to nutrient choice. Elife 2021;10:e59399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grubisic V, Perez-Medina AL, Fried DE, et al. NTPDase1 and -2 are expressed by distinct cellular compartments in the mouse colon and differentially impact colonic physiology and function after DSS colitis. Am J Physiol Gastrointest Liver Physiol 2019;317:G314–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol 1996;270:G778–82. [DOI] [PubMed] [Google Scholar]
- 49.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 2003;125:1651–9. [DOI] [PubMed] [Google Scholar]
- 51.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol 2005;3:349–57. [DOI] [PubMed] [Google Scholar]
- 52.Del Colle A, Israelyan N, Gross Margolis K. Novel aspects of enteric serotonergic signaling in health and brain-gut disease. Am J Physiol Gastrointest Liver Physiol 2020;318:G130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Israelyan N, Margolis KG. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol Res 2018;132:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ha S, Jin B, Clemmensen B, et al. Serotonin is elevated in COVID-19-associated diarrhoea. Gut 2021;70:2015–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with covid-19: Cohort study. BMJ 2022;376:e068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8:416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren Z, Wang H, Cui G, et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut 2021;70:1253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X, Zhang W, Guo M, et al. Integrated analysis of gut microbiome and host immune responses in COVID-19. Front Med 2022;16:263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.