Abstract
Antibody responses to Acinetobacter (five strains), Pseudomonas aeruginosa, Escherichia coli, myelin basic protein (MBP), and neurofilaments were measured in sera from 26 multiple sclerosis (MS) patients, 20 patients with cerebrovascular accidents (CVA), 10 patients with viral encephalitis, and 25 healthy blood donors. In MS patients, elevated levels of antibodies against all strains of Acinetobacter tested were present, as well as antibodies against P. aeruginosa, MBP, and neurofilaments, but not antibodies to E. coli, compared to the CVA group and controls. The myelin-Acinetobacter-neurofilament antibody index appears to distinguish MS patients from patients with CVAs or healthy controls. The relevance of such antibodies to the neuropathology of MS requires further evaluation.
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, affecting approximately 80,000 individuals in the United Kingdom and 400,000 in the United States. It is generally thought to be an autoimmune disease that is triggered by an infectious agent, possibly through a molecular mimicry mechanism of induction (1), similar to the situation occurring in the pathology of rheumatic fever (8).
A number of different microbial sequences, including Pseudomonas peptides, are capable of activating myelin basic protein (MBP)-specific T-cell clones from MS patients (19), thereby indicating that several microorganisms may carry mimicry epitopes of myelin. This could explain the difficulty of linking the immunopathogenesis of MS to a single microbial agent, suggesting that several such bacteria could be involved in initiating the autoimmune process (6).
It was decided to investigate MS patients for possible immune responses to pathogens that carry molecular mimicry sequences resembling brain components, such as Acinetobacter species and Pseudomonas aeruginosa. We have identified an amino acid sequence similarity between the encephalitogenic peptide of bovine MBP (5) and a sequence present in 4-carboxymuconolactone decarboxylase of Acinetobacter calcoaceticus (3). A similar sequence was also found in γ-carboxymuconolactone decarboxylase of P. aeruginosa (Swissprot).
The aims of this study were to determine whether MS patients have elevated levels of antibodies to five different strains of Acinetobacter as well as to P. aeruginosa, Escherichia coli, MBP, and neurofilaments, compared to cerebrovascular-accident (CVA) patients and control healthy blood donors. A further study was carried out, incorporating an inflammatory non-MS disease control, viral encephalitis, and a new set of control subjects, to assess whether MS patients have elevated levels of immunoglobulin G (IgG) antibodies to A. calcoaceticus 16904.
MATERIALS AND METHODS
Serum samples.
Sera from 26 MS patients (nine males and 17 females; mean age, 42 years; range, 29 to 55 years) were obtained from the Institute of Neurology at the Hospital for Nervous Diseases, London, United Kingdom. Diagnosis was made according to the Poser criteria (13). Benign MS patients are designated by infrequent exacerbation with full recovery. Relapsing remitting MS patients are those patients who have more frequent exacerbations followed by partial or complete remission. Serum samples were obtained from relapsing remitting patients during remission and exacerbation. Secondary progressive MS patients are defined as patients who continue to deteriorate without remission following an initial relapsing remitting course of disease. Primary progressive MS is defined as continuous deterioration without remission from the onset of disease. The clinical features of the MS patients are summarized in Table 1. In addition, serum samples were obtained from 20 patients in the Department of Geriatric Medicine at University College Hospital, who had suffered from a unilateral hemiplegia due to a CVA (10 males and 10 females; mean age, 80.5 years; range, 69 to 94 years) and 10 patients with viral encephalitis (eight males and two females; mean age, 38 years; range, 3 to 66 years) attending the National Hospital for Neurology and Neurosurgery. Sera from 25 subjects attending the London Blood Donor services were used as healthy controls (12 males and 13 females; mean age, 40.6 years; range, 22 to 67 years). A further set of sera from 29 healthy control subjects attending the London Blood Donor services was used in the viral encephalitis study (15 males and 14 females; mean age, 43 years; range, 19 to 66 years).
TABLE 1.
MS patient clinical data
| Laboratory no. | Sexa | Age (yr) | Diagnosisb | EDSSc | Disease duration (yr) |
|---|---|---|---|---|---|
| MS 006 | M | 53 | B | 3.0 | 20 |
| MS 028 | F | 54 | B | NAd | NA |
| MS 033 | F | NA | B | NA | NA |
| MS 051 | F | 55 | B | 1.0 | 25 |
| MS 054 | F | 49 | B | 3.0 | 17 |
| MS 080 | F | 49 | B | 1.0 | 20 |
| MS 007 | F | 38 | RR | 2.5 | 10 |
| MS 042 | F | 31 | RR | 3.5 | NA |
| MS 084 | F | 50 | RR | 5.5 | 8 |
| MS 095 | F | 32 | RR | 6.5 | 3 |
| MS 017 | F | 53 | ARR | 4.0 | 10 |
| MS 019 | F | 29 | ARR | 4.0 | 1 |
| MS 030 | F | 31 | ARR | 4.0 | 7 |
| MS 037 | F | 34 | ARR | 5.0 | 7 |
| MS 087 | M | 40 | Transitional | 5.0 | 9 |
| MS 016 | M | 49 | PP | 5.5 | 5 |
| MS 056 | M | 41 | PP | 8.0 | 1 |
| MS 062 | M | 31 | PP | 7.0 | 10 |
| MS 065 | M | 43 | PP | 8.0 | 8 |
| MS 091 | M | 47 | PP | 7.5 | 23 |
| MS 092 | F | 44 | PP | 8.5 | 6 |
| MS 102 | F | 47 | PP | 8.0 | 26 |
| MS 020 | M | 31 | 2P | 8.0 | 5 |
| MS 040 | F | 33 | 2P | 8.0 | 2 |
| MS 055 | M | 51 | 2P | 8.0 | 10 |
| MS 104 | F | 38 | 2P | 6.5 | 4 |
Abbreviations: M, male; F, female.
Abbreviations: B, benign; RR, relapsing remitting; ARR, acute relapsing remitting; PP, primary progressive, 2P, secondary progressive.
EDSS, expanded disability status scale.
NA, not available.
Preparation of bacteria.
Cultures of Acinetobacter sp. strain 11171, Acinetobacter sp. strain 19004, Acinetobacter junii 17908, Acinetobacter lwoffii 5866 and Acinetobacter radioresistens (sp12) were provided by the Public Health Laboratory, Nottingham, United Kingdom. A. calcoaceticus (NCIMB 16904) was obtained from the National Collections of Industrial and Murine Bacteria Ltd. (Aberdeen, Scotland). The Department of Microbiology at King's College London provided P. aeruginosa (NCTC 8203) and E. coli (NCTC 9002).
Cultures were grown in 1-liter flasks on an orbital shaker for 2 days at 30°C for all Acinetobacter strains and at 37°C for P. aeruginosa and E. coli in 200 ml of nutrient broth (25 g/liter; Oxoid). Flasks were inoculated with two loopfuls of starter culture and left shaking for 6 h at 37°C. Batch culture cells were harvested by centrifugation at 4,000 rpm for 20 min at less than 10°C (six 250-ml aliquots; Beckman JA-20 rotor). Pellets of cells were washed three times in 0.15 M phosphate-buffered saline (PBS) (pH 7.4) and finally resuspended in 10 ml of PBS. For the enzyme-linked immunosorbent assay (ELISA) a stock solution of the bacterial suspension was prepared by diluting in 0.05 M carbonate buffer (pH 9.6) to give an optical density (OD) reading of 0.25 at 540 nm on a spectrophotometer (Dynatech MR606), which had previously been determined as the optimum concentration (15). For sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, the resuspended pellet was ultrasonicated at an amplitude of 12 μm with 30-s bursts and 60-s rest periods (seven cycles). The protein content of the sonicated samples was measured using Bradford's protein assay (2). The sample was then diluted in sample buffer (0.0625 M Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 0.001% bromophenol blue) to a protein concentration of 1 μg/μl and heated at 100°C for 3 min.
ELISA.
ELISAs were carried out as previously described (9). Briefly, aliquots of 200 μl of bacterial suspension or bovine MBP or neurofilaments (25 μg/ml; Sigma), diluted in a 0.05 M carbonate buffer (pH 9.6), were adsorbed onto a 96-well flat-bottom polystyrene microELISA plate (Dynatech) overnight at 4°C. Plates were washed three times for 5 min in PBS containing 0.05% (vol/vol) Tween 20 (Sigma) (washing and incubation buffer) and were blocked with PBS containing 0.1% bovine serum albumin (Sigma) for 1 h at 37°C. The washing procedure was repeated, and 200 μl of test or control serum, diluted 1 in 200 in incubation buffer, was added to the wells in duplicate and incubated for 1 h at 37°C. Plates were washed three times in washing buffer, and 200 μl of IgM, IgG, or IgA rabbit anti-human conjugate with horseradish peroxidase (Dako), diluted 1 in 500 in incubation buffer, was added and incubated for 1 h at 37°C. The washing procedure was repeated, and 200 μl of substrate solution—2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (0.5 mg/ml; Sigma) in citrate phosphate buffer, pH 4.1, containing 0.98 mM H2O2 (Sigma)—was added to each well. The plates were developed in the dark, at room temperature, for 25 min. The reaction was stopped by addition of 100 μl of sodium fluoride (2 mg/ml; Sigma) solution. Absorbances were measured on a microtiter plate reader (Dynatech MR606) at 630 nm. All studies were carried out under code, in that the tester did not know which sera were test or control.
Triplicate ELISA studies measuring total immunoglobulins (IgG, IgM, and IgA) against A. radioresistens, Acinetobacter sp. strain 11171 and P. aeruginosa were carried out in serial doubling dilutions using a high- and low-titer MS serum and a control sample (M39).
Statistical analysis.
The mean OD units of control groups (CVA and healthy blood donors) were compared with the mean OD of the 26 MS patients, using a one-tail Student's t test, and 95% confidence limits of control groups were calculated. Pearson's correlation coefficient (r) was also calculated using the statistical package Prism3.0 (GraphPad Software).
Western blot analysis.
Electrophoresis was performed according to the method of Laemmli (10). Briefly, prepared samples (10 μl) and a set of molecular weight markers (5 μl) (RPN800; Amersham) were loaded and run on a 10% SDS-polyacrylamide gel at 100 V for 2 h in electrolyte buffer (0.025 M Tris, 0.2 M glycine, and 0.1% SDS, pH 8.3) and the gel was transferred to nitrocellulose membrane at 30 V for 16 h in transfer buffer (20 mM Tris, 0.15 M glycine, pH 8.3, containing 20% [vol/vol] methanol) (Protean III cell; Bio-Rad Laboratories). All incubation steps were carried out at room temperature with gentle shaking. After transfer the membrane was cut into individual strips and incubated in 2% Marvel–Tris-buffered saline (TBS) for 2 h. The strips were washed five times for 5 min in TBS-Tween and then individually incubated with patient or control serum for 2 h. Serum was diluted at 1 in 200 in 2% Marvel–TBS. The strips were then washed as before and incubated for 2 h with rabbit anti-human immunoglobulin (IgG, IgM, and IgA) conjugate with horseradish peroxidase (Dako), diluted 1 in 1,000 in 2% Marvel–TBS. Strips were washed and developed using a chemiluminescent detection assay (Amersham).
RESULTS
IgA anti-Acinetobacter antibodies.
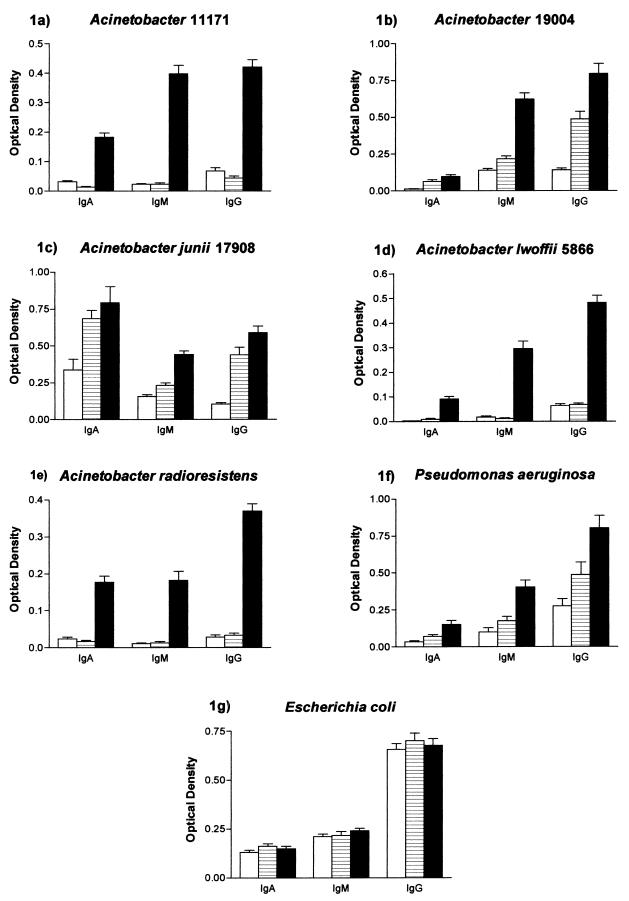
Levels of IgA antibodies to Acinetobacter sp. strain 11171 (P < 0.0001), Acinetobacter sp. strain 19004 (P < 0.0001), A. junii 17908 (P < 0.01), A. lwoffii 5866 (P < 0.0001), and A. radioresistens (P < 0.0001) in MS patients were significantly higher than those in the healthy control group.
Levels of IgA to Acinetobacter sp. strain 11171 (P < 0.0001), A. lwoffii 5866 (P < 0.0001), and A. radioresistens (P < 0.0001) were also shown to be significantly elevated in MS patients compared to CVA patients. No significant difference between MS and CVA patients was seen for IgA antibodies to either Acinetobacter sp. strain 19004 or Acinetobacter sp. strain 17908.
Significantly elevated levels of IgA to Acinetobacter sp. strain 19004 (P < 0.0001) and A. junii 17908 (P < 0.001) were observed in CVA patients compared to controls. Levels of IgA antibody to Acinetobacter sp. strain 11171 were shown to be significantly higher in controls (P < 0.001) than in CVA patients. No other significant differences were seen in the levels of IgA antibodies to A. lwoffii 5866 or A. radioresistens between the control and CVA groups (Fig. 1a to e).
FIG. 1.
Levels of IgA, IgM, and IgG antibodies (mean + standard error [error bars]) to Acinetobacter sp. strain 11171 (a), Acinetobacter sp. strain 19004 (b), A. junii 17908 (c), A. lwoffii 5866 (d), A. radioresistens (e), P. aeruginosa (f), and E. coli (g), in sera from 26 MS patients, 20 CVA patients, and 25 healthy blood donors. Symbols: □, controls; ▤, CVA; ▪, MS.
Furthermore, when patients were divided into different MS groups, secondary progressive patients were shown to have significantly elevated levels of IgA antibodies to Acinetobacter sp. strain 11171 compared to relapsing remitting MS (P < 0.001), benign MS (P < 0.02), acute relapsing remitting MS (P < 0.02), and primary progressive MS (P < 0.05) patients. Primary progressive patients were also shown to have significantly elevated levels of IgA to Acinetobacter sp. strain 11171 compared to relapsing remitting patients (P < 0.01) (see Fig. 3a).
FIG. 3.
Levels of IgA, IgM, and IgG antibody (mean + standard error [error bars]) to Acinetobacter sp. strain 11171 (a), A. radioresistens (b), MBP (c), and neurofilaments (d) in sera from 26 MS patients, 20 CVA patients, and 25 healthy blood donors.
IgG anti-Acinetobacter antibodies.
Levels of IgG antibodies to Acinetobacter sp. strain 11171 (P < 0.0001), Acinetobacter sp. strain 19004 (P < 0.0001), A. junii 17908 (P < 0.0001), A. lwoffii 5866 (P < 0.0001), and A. radioresistens (P < 0.0001) were significantly elevated in MS patients compared to healthy controls.
IgG antibodies to Acinetobacter sp. strain 11171 (P < 0.0001), Acinetobacter sp. strain 19004 (P < 0.01), A. junii 17908 (P < 0.05), A. lwoffii 5866 (P < 0.0001), and A. radioresistens (P < 0.0001) were significantly higher in MS patients than in CVA patients.
Significantly elevated levels of IgG antibodies to Acinetobacter sp. strain 19004 (P < 0.0001) and A. junii 17908 (P < 0.0001) were found in CVA patients compared to healthy controls. However, there was no significant difference in the level of IgG antibodies to Acinetobacter sp. strain 11171, A. lwoffii 5866, and A. radioresistens between the CVA patients and healthy controls (Fig. 1a to e).
A further study, measuring IgG antibody levels to A. calcoaceticus 16904 demonstrated a significant elevation in sera from MS patients compared to levels in sera from viral encephalitis patients (P < 0.02) and healthy blood donor controls (P < 0.05) (Fig. 2).
FIG. 2.
IgG antibody levels (mean + standard error [error bars]) to A. calcoaceticus 16904 and E. coli in sera from 26 MS patients, 10 viral encephalitis patients, and 29 healthy blood donors.
IgM anti-Acinetobacter antibodies.
Levels of IgM antibodies to Acinetobacter sp. strain 11171 (P < 0.0001), Acinetobacter sp. strain 19004 (P < 0.0001), A. junii 17908 (P < 0.0001), A. lwoffii 5866 (P < 0.0001), and A. radioresistens (P < 0.0001) were significantly higher in MS patients than in the healthy control group.
Levels of IgM antibody to Acinetobacter sp. strain 11171 (P < 0.0001), Acinetobacter sp. strain 19004 (P < 0.0001), A. junii 17908 (P < 0.0001), A. lwoffii 5866 (P < 0.0001), and A. radioresistens (P < 0.0001) were also shown to be significantly elevated in MS patients compared to CVA patients.
Significantly elevated levels of IgM antibodies to Acinetobacter sp. strain 19004 (P < 0.001) and Acinetobacter sp. strain 17908 (P < 0.001) were found in CVA patients compared to healthy controls. No significant differences were seen in the level of IgM antibodies to Acinetobacter sp. strain 11171, A. lwoffii 5866, and A. radioresistens between the CVA and control groups (Fig. 1a to e).
Antibodies to P. aeruginosa
Levels of IgA antibodies to Pseudomonas were shown to be significantly higher in MS patients than in the control group (P < 0.001) and CVA patients (P < 0.05). There was also a significant elevation in the level of IgA anti-Pseudomonas antibodies in the CVA group (P < 0.05) compared to controls.
Elevated levels of IgG antibodies to Pseudomonas were also observed in MS sera compared to controls (P < 0.0001) and CVA patients (P < 0.05). Furthermore, there was also a significant elevation in the level of IgG antibodies to Pseudomonas (P < 0.05) in the CVA group compared to controls.
IgM antibody levels to Pseudomonas were also shown to be elevated in MS patients compared to controls (P < 0.0001) and CVA patients (P < 0.001). There was no significant difference in the level of IgM antibodies to Pseudomonas between the two control groups (Fig. 1f).
Antibodies to E. coli.
No significant differences in the levels of IgA, IgM, or IgG anti-E. coli antibodies were observed between any of the groups tested (Fig. 1g and 2).
Antibodies to brain components. (i) MBP.
Significantly elevated levels of IgA to MBP were observed in MS sera compared to controls (P < 0.0001) and CVA sera (P < 0.001). However, no significant difference was observed between the control and CVA group, when measuring IgA antibody levels.
Increased levels of anti-MBP IgG antibodies were also seen in MS sera compared to controls (P < 0.0001) and CVA patients (P < 0.01), and MS patients showed significantly higher levels of IgM antibodies to MBP than those seen in controls (P < 0.0001) and CVA patients (P < 0.0001). There were also increased levels of anti-MBP IgM and IgG antibodies in CVA patients compared to controls (P < 0.0001) (Fig. 3c).
(ii) Neurofilaments.
Levels of IgA antibody to neurofilaments were shown to be significantly higher in MS patients than in controls (P < 0.01) and CVA patients (P < 0.0001). No significant difference between the control and CVA groups was seen in the level of antineurofilament IgA antibodies.
MS patients also showed significantly elevated IgG antineurofilament antibodies compared to the controls (P < 0.0001) and CVA patients (P < 0.01). The level of IgG antibodies was significantly increased in CVA patients compared to controls (P < 0.0001).
Significantly elevated levels of IgM to neurofilaments were observed in MS sera compared to those from healthy controls (P < 0.0001) and CVA patients (P < 0.0001). There was no significant difference between the control and CVA groups in the levels of antineurofilament IgM antibodies (Fig. 3d).
No significant differences in the levels of IgA, IgM, or IgG antibodies to A. radioresistens, A. lwoffii 5866, A. junii 17908, Acinetobacter sp. strain 19004, P. aeruginosa, MBP and neurofilaments were found between the different groups of MS patients (Fig. 3).
Measurement of serial dilutions.
Levels of total immunoglobulin (IgG, IgM, and IgA) to A. radioresistens (Fig. 4a), Acinetobacter sp. strain 11171 (Fig. 4b), and P. aeruginosa (Fig. 4c) are shown for a high- and low-titer MS serum and a control sample (M39).
FIG. 4.
Serial doubling dilutions (mean ± standard error [error bars]) of high- and low-titer MS sera and a control sample against A. radioresistens (a), Acinetobacter sp. strain 11171 (b), and P. aeruginosa (c).
For each antigen tested, the high-titer and low-titer MS serum reacted with a dilution of up to 1/6,400 and 1/3,200, respectively, whereas the control serum gave lower readings. The coefficient of variation was less than 10% for all ELISA tests carried out.
Western blot analysis.
The specificity of the serum antibodies was evaluated by Western blotting using A. radioresistens, Acinetobacter sp. strain 11171, and P. aeruginosa antigens. The high-titer MS serum (MS080) showed prominent reactivity to a 15- to 28.6-kDa region of both A. radioresistens and Acinetobacter sp. strain 11171, and a further band of reactivity can be seen with a 9.1-kDa protein of Acinetobacter sp. strain 11171. The control serum (M39) showed much weaker reactivity to proteins of Acinetobacter sp. Reactivity to a number of proteins of P. aeruginosa can be seen in both the MS and control patients. The MS patient demonstrated prominent reactivity to a 78-kDa protein and an 8.5-kDa protein of P. aeruginosa (Fig. 5).
FIG. 5.
Immunoblotting antigenic bands developed with serum samples from an MS patient (MS080) (MS) and a control healthy blood donor (M39) (Con) against A. radioresistens (lanes A and B), Acinetobacter sp. strain 11171 (lanes C and D), and P. aeruginosa (lanes E and F).
Correlation coefficient analysis.
The correlation coefficient (r) was calculated between all strains of Acinetobacter tested and P. aeruginosa. There was a significant positive correlation between the IgM levels of A. junii 17908 and P. aeruginosa (r = +0.831; P < 0.0001). A significant positive correlation was also seen between the IgM levels of Acinetobacter sp. strain 19004 and P. aeruginosa (r = +0.819; P < 0.0001). This was also the case for levels of IgA antibodies between these two organisms (r = +0.407; P < 0.01). Both Acinetobacter sp. strain 19004 and A. junii 17908 also showed significant positive correlations with both MBP and neurofilaments, with regards to IgM and IgG levels (P < 0.0001). A significant positive correlation was seen between the IgM levels of P. aeruginosa and both MBP (r = +0.851; P < 0.0001) and neurofilaments (r = +0.693; P < 0.0001). A significant positive correlation was also seen between the IgA (r = +0.399; P < 0.05), IgM (r = +0.663; P < 0.001) and IgG (r = +0.772; P < 0.0001) levels of MBP and neurofilaments.
MAN index.
The myelin-Acinetobacter-neurofilament (MAN) index was calculated using the results from the IgG antibody assays. The formula used was as follows: (MBP OD × 10) (Acinetobacter strain OD/Pseudomonas OD × 10) (neurofilament OD × 10). All MS patients had values above the 99.9% confidence limit of the controls when the MAN index was calculated using A. lwoffii 5866, Acinetobacter sp. strain 11171, A. radioresistens, Acinetobacter sp. strain 19004, and A. junii 17908. Only 88.5% of MS patients were shown to have a MAN index above the 99.9% confidence limit, when calculating with P. aeruginosa antibodies. In all cases only one control was shown to lie above the 99.9% confidence limit. Seven CVA patients were shown to have values above the 99.9% confidence limit when the MAN index was calculated using Acinetobacter sp. strain 11171. For all other strains of Acinetobacter and P. aeruginosa more than 12 CVA patients had MAN indices above the 99.9% confidence limit. No significant differences were seen in the MAN indices between the different MS groups. Interestingly, when calculating the MAN index using A. radioresistens all MS patients could be distinguished from the two control groups. When removing the Acinetobacter parameter, the MAN index could distinguish MS patients from healthy controls but not from the CVA group (Fig. 6). When the MAN index was calculated with IgA and IgM antibodies, the differences between the MS patients and control groups were not as significant (data not shown).
FIG. 6.
MAN index, calculated as follows: (MBP OD × 10) (Acinetobacter OD/Pseudomonas OD × 10) (neurofilament OD × 10) expressed in log10, in sera from 26 MS patients (B, benign; RR, relapsing remitting; ARR, acute relapsing remitting; 2P, secondary progressive; PP, primary progressive; Trans, transitional), 20 CVA patients, and 25 healthy blood donors. The dashed line represents the 99.9% confidence limit of controls.
DISCUSSION
Elevated levels of IgA, IgM, and IgG directed against Acinetobacter sp. strain 11171, A. lwoffii 5866, and A. radioresistens were found in the sera from MS patients compared to CVA patients or healthy controls. A similar observation was seen for Acinetobacter sp. strain 19004 and Acinetobacter sp. strain 17908, although there were no significant differences in the levels of IgA antibodies between the MS and CVA groups. A significant increase in levels of IgG to A. calcoaceticus 10694 was also shown in MS patients compared to healthy controls and viral encephalitis patients. Primary and secondary progressive MS patients tended to have higher levels of IgA antibodies to Acinetobacter sp. strain 11171 compared to other MS groups.
Molecular mimicry appears to be involved in MS (1, 7), but efforts to identify an etiological agent have not been successful. Seven viral and one bacterial peptides have been found to activate three of seven T-cell clones specific to MBP (amino acids 85 to 99) isolated from MS patients. The bacterial peptide identified was from phosphomannomutase protein in P. aeruginosa (19). In this study we have looked at potential respiratory pathogens, including Acinetobacter and P. aeruginosa, which carry sequences that mimic brain components. An amino acid sequence homology between a known encephalitogenic myelin peptide (5) and the enzyme 4-carboxymuconolactone decarboxylase has been found in both Acinetobacter spp. and P. aeruginosa (3).
Autoantibodies to neuronal components, including MBP (17), myelin oligodendrocyte protein (14), myelin-associated glycoprotein (16), proteolipid protein (18), and neurofilaments (11) have previously been reported in MS. IgG antimyelin antibodies, but to a lesser extent IgM and IgA, have also previously been demonstrated in 88% of sera from healthy controls by indirect immunofluorescence assays (4). Increased levels of IgA, IgM, and IgG to both bovine MBP and neurofilaments acting as heterospecific antigens were observed in MS patients compared to controls and the CVA group. There is 92% homology between bovine and human MBP, but almost complete amino acid identity over the most immunogenic epitopes (5). If the presence of anti-MBP and antineurofilament autoantibodies occurs as a result of previous brain damage, antibodies to these brain components should also be found in CVA patients as well as MS patients. However, the results of this study indicate that the MS sera but not the CVA sera had significantly elevated levels of these autoantibodies compared to controls. The pathology of MS is usually thought of as being confined to the white matter, but the observation that autoantibodies to neurofilaments were seen in MS patients and not controls indicates that gray matter of the brain may also be involved in the disease. This confirms previous observations indicating the presence of gray matter pathology in MS patients using magnetic resonance imaging (12) and studies demonstrating the presence of antibodies to the 69-kDa neurofilament protein (11).
We have used our results from the ELISAs measuring IgG antibodies against MBP, Acinetobacter, P. aeruginosa, and neurofilaments to obtain a MAN antibody index. This parameter made it possible for us to identify all the MS patients, and therefore it could offer the possibility of a diagnostic test, but prospective studies will be required to establish the validity of the MAN index.
The agent responsible for autoantibodies in MS is still unclear, but this study does show that MS patients have probably been exposed to Acinetobacter spp. and P. aeruginosa. It is likely that infection with these microorganisms, which carry epitopes resembling brain antigens, could lead to activation of auto-reactive cells and production of autoantibodies, which may cause tissue damage and thus lead to the development of a neurological autoimmune disease, such as MS. However, it must be considered that in the ELISA we are detecting anti-MBP antibodies that are cross-reacting with Acinetobacter spp. Longitudinal studies are necessary to determine whether exposure to Acinetobacter antigens precedes or follows pathological changes. Further work is required to evaluate the role, if any, of Acinetobacter spp. and P. aeruginosa in MS.
ACKNOWLEDGMENTS
We thank K. Towner of the Public Health Laboratories, for providing cultures of Acinetobacter.
This work was supported by the Trustees of the Middlesex Hospital, Ministry of Agriculture, Fisheries, and Food grant CSA 5115 and U.S. Friends of King's College London.
REFERENCES
- 1.Albert L J, Inman R D. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341:2068–2074. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Ebringer A, Pirt S J, Wilson C, Cunningham P, Thorpe C, Ettelaie C. Bovine spongiform encephalopathy: is it an autoimmune disease due to bacteria showing molecular mimicry with brain antigens? Environ Health Perspect. 1997;105:1172–1174. doi: 10.1289/ehp.971051172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edgington T S, Dalessio D J. The assessment by immunofluorescence methods of humoral anti-myelin antibodies in man. J Immunol. 1979;105:248–255. [PubMed] [Google Scholar]
- 5.Eylar E H, Caccam J, Jackson J J. Experimental allergic encephalomyelitis: synthesis of disease-inducing site of the basic protein. Science. 1970;168:1220–1223. doi: 10.1126/science.168.3936.1220. [DOI] [PubMed] [Google Scholar]
- 6.Fazakerley J E, Webb H E. Multiple sclerosis, viruses and glycolipids. Nat Lett. 1986;321:386. doi: 10.1038/321386a0. [DOI] [PubMed] [Google Scholar]
- 7.Gran B, Hemmer B, Vergelli M, McFarland H F, Martin R. Molecular mimicry and multiple sclerosis: degenerate T-cell recognition and the induction of autoimmunity. Ann Neurol. 1999;45:559–567. doi: 10.1002/1531-8249(199905)45:5<559::AID-ANA3>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan M H, Meyeserian M. An immunological cross-reaction between group A streptococcal cells and human heart tissue. Lancet. 1962;i:706–710. doi: 10.1016/s0140-6736(62)91653-7. [DOI] [PubMed] [Google Scholar]
- 9.Khalafpour S, Ebringer A, Abuljadayel I, Corbett M. Antibodies to Klebsiella and Proteus micro-organisms in ankylosing spondylitis and rheumatoid arthritis patients measured by ELISA. Br J Rheumatol. 1988;27(Suppl.):86–89. doi: 10.1093/rheumatology/xxvii.suppl_2.86. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Newcombe J, Gahan S, Cuzner M L. Serum antibodies against central nervous system proteins in human demyelinating disease. Clin Exp Immunol. 1985;59:383–390. [PMC free article] [PubMed] [Google Scholar]
- 12.Newcombe J, Hawkins C P, Henderson C L, Patel H A, Woodroofe M N, Hayes G M, Cuzner M L, MacManus D, Du Boulay E P G H, McDonald W I. Histopathology of multiple sclerosis lesions detected by magnetic resonance imaging in unfixed postmortem central nervous system tissue. Brain. 1991;114:1013–1023. doi: 10.1093/brain/114.2.1013. [DOI] [PubMed] [Google Scholar]
- 13.Poser C M, Paty D W, Scheinberg L, McDonald W I, Davis F A, Ebers G C, Johnson K P, Sibley W A, Silberberg D H, Toutellotte W W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 14.Reindl M, Linington C, Brehm U, Egg R, Dilitz E, Deisenhammer F, Poewe W, Berger T. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain. 1999;122:2047–2056. doi: 10.1093/brain/122.11.2047. [DOI] [PubMed] [Google Scholar]
- 15.Tiwana H, Wilson C, Cunningham P, Binder A, Ebringer A. Antibodies to four Gram-negative bacteria in rheumatoid arthritis which share sequences with the rheumatoid arthritis susceptibility motif. Br J Rheumatol. 1996;35:592–594. doi: 10.1093/rheumatology/35.6.592. [DOI] [PubMed] [Google Scholar]
- 16.Wajgt A, Gorney M. CSF antibodies to myelin basic protein and to myelin associated glycoprotein in multiple sclerosis: evidence of the intrathecal production of antibodies. Acta Neurol Scand. 1983;68:337–343. doi: 10.1111/j.1600-0404.1983.tb04841.x. [DOI] [PubMed] [Google Scholar]
- 17.Warren K G, Catz I. A correlation between cerebrospinal fluid myelin basic protein and anti-myelin basic protein in multiple sclerosis patients. Ann Neurol. 1987;21:183–189. doi: 10.1002/ana.410210211. [DOI] [PubMed] [Google Scholar]
- 18.Warren K G, Catz I, Johnson E, Mielke B. Anti-myelin basic protein and anti-proteolipid protein specific forms of multiple sclerosis. Ann Neurol. 1994;35:280–289. doi: 10.1002/ana.410350307. [DOI] [PubMed] [Google Scholar]
- 19.Wucherpfennig K W, Strominger J L. Molecular mimicry in T cell mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]