Abstract
The systemic host response to microbial infection involves clinical signs and symptoms of infection, including fever and elevated white blood cell (WBC) counts. In addition, inflammatory mediators are released, including activated complement product C3a, interleukin 6 (IL-6), and the acute-phase reactant secretory phospholipase A2 (sPLA2). To compare the value of the latter with the former in predicting (the degree of) microbial infection at the bedside, we determined clinical variables and took blood samples daily for 3 consecutive days in 300 patients with a new fever (>38.0°C rectally or >38.3°C axillary). Microbiological culture results for 7 days after inclusion were collected. Patients were divided into clinical and microbial categories: those without and with a clinical focus of infection and those with negative cultures, with positive local cultures or specific stains for fungal (n = 13) or tuberculous infections (n = 1), and with positive blood cultures, including one patient with malaria parasitemia. The area under the curve (AUC) of the receiver operating characteristic (ROC) for prediction of positive cultures was 0.60 (P < 0.005) for peak temperature and 0.59 (P < 0.01) for peak WBC count, 0.60 (P < 0.005) for peak C3a, 0.63 (P < 0.001) for peak IL-6, and 0.61 (P < 0.001) for peak sPLA2. The AUC under the ROC curve for prediction of positive blood cultures was 0.68 (P < 0.001) for peak temperature and 0.56 for peak WBC count (P < 0.05). The AUC for peak C3a was 0.69, that for peak IL-6 was 0.70, and that for sPLA2 was 0.67 (for all, P < 0.001). The degree of microbial invasion is thus a major determinant of the clinical and inflammatory host response in patients with fever. Moreover, circulating inflammatory mediators such as C3a and IL-6 may help to predict positive blood cultures, together with clinical signs and symptoms of the host response to microbial infection, even before culture results are available. This may help in the designing of entry criteria for therapeutic intervention studies.
Fever is considered to be clinical evidence for inflammation, mostly caused by microbial infection, at least in patients (22). The criteria of systemic inflammatory response syndrome (SIRS), which may predict microbial infection, are fever and elevated white blood cell (WBC) counts, while the SIRS criteria tachypnea and tachycardia may be nonspecific and too sensitive for microbial infection (2–5, 33). Hence, authors have searched, with varying success, for other objective predictors of the host response to microbial infection (2–5, 33). This may help to identify patients who may benefit most from immunomodulating therapies of sepsis, trials that have failed so far in heterogeneous patient populations with sepsis (1). Prediction of microbial bloodstream infection at an early stage would be particularly helpful since this may carry a mortality rate of 30% or more, if treated inappropriately or too late (21, 33).
The host response to microbial infection is thought to be mediated by the release of inflammatory substances that may help the host to eradicate the invading organisms. During a local infection and host response, the release may be confined to the affected tissue, while a systemic infection and host response may be associated with release of inflammatory mediators into the bloodstream (10, 19, 31). Therefore, circulating inflammatory mediators have been suggested to be predictive for a systemic microbial infection and for a poor outcome, in patients with fever (5). In surgical and emergency departments and in critically ill febrile patients and neonates, relatively small studies indeed have suggested some predictive value for systemic microbial infection and its severity of C3a, a product of complement activation; of interleukin 6 (IL-6), the alarm cytokine, as an indicator of an activated host defense; and of the acute-phase reactant secretory phospholipase A2 (sPLA2), believed to be produced in response to IL-6 in the liver, among others (8, 9, 13, 20, 26–29). The factors may be superior to the commonly used acute-phase reactant C1-reactive protein (CRP) in predicting microbial infection (9, 13, 20, 26, 27). Moreover, systemically elevated mediators of the primary nonspecific host response to microbial infection, including circulating activated complement products, cytokines (IL-6) and sPLA2, are of prognostic value, during established sepsis and septic shock (6–8, 11, 12, 14–18, 23–25, 29, 30, 32).
To compare the relative value of circulating inflammatory variables versus clinical variables of the systemic host response in predicting microbial infection locally and in the bloodstream, at an early stage when results of microbiological studies are not yet available, we evaluated a large cohort of febrile patients in whom circulating levels of C3a, IL-6, and sPLA2 were measured serially. We have chosen these factors because of interrelationships in the primary nonspecific host response to microbial infection (12, 14, 32).
MATERIALS AND METHODS
During a 1-year period a total of 300 consecutive patients with newly onset fever (body temperature of ≥38.0°C axillary or ≥38.3°C rectal), admitted to the Department of Internal Medicine in a university hospital, were included in the study (4, 5). Two hundred eighty-four patients had SIRS and 200 had sepsis, i.e., SIRS and a clinical focus of infection, but of the SIRS criteria involved, only temperature and WBC counts contributed to prediction of positive (blood) cultures (4, 5). The study had been approved by the local committee on ethics. All patients or their closest relatives gave informed consent before inclusion. Exclusion criteria were pregnancy; use of cytokines potentially interfering with host defense, e.g., administration of gamma interferon and IL-2; treatment for malignant hematological disease; shock; and a life expectancy of less than 24 h.
At inclusion, data on variables that could affect culture results and levels of inflammatory mediators were recorded, including age, gender, known infection with human immunodeficiency virus or presence of AIDS, known malignancies, known diabetes mellitus, prior treatment with immunocompromising (cytostatic and steroidal) drugs and antibiotics, and the estimated time elapsed between onset of fever and inclusion. We further estimated the time elapsed between hospital admission and onset of fever. Patients admitted into the hospital more than 72 h before onset of fever were considered to suffer from nosocomial fever. A potential focus was searched for by the treating physician on the basis of clinical examination and appropriate imaging techniques, and a clinical focus of infection was diagnosed according to the results. Patients were monitored until day 28 for outcome, and patients discharged within this period were considered to be survivors. We only considered death potentially related to the febrile episode, thereby excluding five patients in whom fever had resolved and who ultimately died from apnea following aspiration (n = 1), cardiac arrest in metastatic malignant disease (n = 2), cardiogenic shock following cardiomyopathy (n = 1), and respiratory insufficiency following cervical cord dissection (n = 1).
Microbial cultures.
At least two blood samples for culture were obtained by venipuncture at inclusion. Supplementary blood for culture was collected when clinically indicated. Local specimens for culture were collected, depending on the clinical focus of infection. All local and blood culture results during a follow-up period of 7 days after inclusion were recorded. Blood for culture was processed using delayed vial entry bottles for aerobic and anaerobic cultures and Bactec 9120 and 9240 automatic analyzers (Becton Dickinson, Erembodegem, Belgium). Bottles were incubated for a maximum of 7 days. If the analyzers showed growth, Gram stains were prepared and identification and sensitivity cultures were processed. Blood cultures containing Staphylococcus epidermidis were considered contaminated if only one bottle revealed growth and there were no indwelling vascular catheters. Local specimens were processed using standardized procedures, including Gram staining, culture, and sensitivity tests, and specific stains were performed, if indicated, to document fungal (n = 13) or tuberculous infections (n = 1). Positive local microbiological results were thought to reflect infection as opposed to colonization if the treating physician decided to prescribe or continue antimicrobial therapy based on these results.
Blood sampling.
We obtained at inclusion and daily thereafter for two consecutive days (days 1, 2, and 3) blood samples for determination of WBC counts (Sysmec [Kolbe, Japan] SE 9000 analyzer). The samples for mediator levels were collected in tubes containing soybean trypsin inhibitor (final concentration, 100 μg/ml), EDTA (10 mmol/liter), and benzamidine (10 mmol/liter), to prevent in vitro activation. All tubes were centrifuged for 10 min at 2,750 rpm (Rotixo 120R; Hettich Zentrifugen, Tuttlingen, Germany), and the plasma was stored immediately at −70°C until assays were performed. To determine normal levels in plasma, blood was obtained from healthy laboratory personnel under similar conditions.
Assays.
Complement activation in the patients was measured by assessing circulating levels of C3a with a radioimmunoassay as described before (17). In this test binding of 125I-labeled C3 to anti-C3a antibodies immobilized onto a solid phase (Sepharose) was inhibited by C3a present in plasma samples. To prevent interference by native C3, plasma samples were first precipitated with 11% (wt/vol) polyethylene glycol 6000, after which the supernatant was tested. Results were expressed as nanomoles per liter. Normal values in healthy volunteers are less than or equal to 5 nmol/liter. The lower limit of detection is 1 nmol/liter. IL-6 in plasma was measured by an enzyme-linked immunosorbent assay (ELISA) as described before (21). Briefly, the ELISA plates were coated with the monoclonal antibody CLB. IL-6 and plasma samples were incubated on these plates. After a washing procedure, bound IL-6 was detected by incubation with biotinylated sheep antibodies against IL-6, after which plates were incubated with streptavidin-polymerized horseradish peroxidase. Finally, the plates were developed with 3,5,3′,5′-tetramethyl benzidine. Results were related to a dose-response curve obtained with recombinant human IL-6 and expressed as picograms per milliliter. Normal values in healthy volunteers are less than 10 pg/ml. The lower limit of detection is 3 pg/ml. Antigen levels of secretory nonpancreatic type II (sPLA2) were assessed with help of an ELISA. Two different monoclonal antibodies against human sPLA2 were used as capture and detection antibodies, respectively. Results were compared with those obtained with culture medium from HepG2 cells stimulated with human IL-6, as this medium contains significant amounts of sPLA2. The amount of sPLA2 in this medium was assessed by comparison with purified recombinant human sPLA2. The lower limit of detection was 0.1 ng/ml. Normal values are <5 ng/ml.
Statistical analysis.
Patients were classified into groups depending on presence of clinical focus of infection and culture results: no positive cultures; positive local cultures only, including specific stains for fungal (n = 13) or tuberculous (n = 1) infections; and positive blood cultures, including one patient with malaria parasitemia. These groups were considered to reflect the degree of microbial invasion. Groups were compared for daily and peak values among the daily plasma levels of circulating variables, using Kruskal-Wallis tests. The χ2 test with Yates' correction was used for binomial variables. The Spearman rank correlation coefficient rs (if above 0.35 or below −0.35) was used to describe relations between continuous variables. Receiver operating characteristic (ROC) curves, plotting sensitivity versus 1 − specificity, were made to evaluate the diagnostic performance for cultures of the clinical variables and inflammatory mediators at various cutoff points. An area under the curve (AUC) closer to 1 indicates greater diagnostic power, while an AUC of 0.5 denotes no diagnostic potential. The optimum cutoff value for maximum sensitivity and specificity was calculated. Multiple logistic regression (forward stepwise method, on the basis of likelihood ratio) was used to evaluate the contribution of inflammatory mediators to clinical variables in the prediction of microbiologically confirmed infection. The Hosmer Lemeshow test was used to verify adequate calibration, as indicated by a P value of >0.05. Sensitivity, specificity, positive and negative predictive values, and likelihood ratio were calculated according to standard formulae. Values of continuous variables were expressed as median and range. For graphic presentation, data were summarized by mean and standard deviations. A P value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics.
Table 1 describes patient characteristics. Of the 300 patients included, 212 had a clinical focus of infection and 133 (44%) had positive cultures. In the 53 patients with positive blood cultures, 27 had positive local cultures. The presence of a clinical focus predicted positive cultures (P < 0.005), since only 27 patients with the latter did not have a clinical infection focus. The presence of a clinical focus of infection also predicted positive blood cultures (P < 0.005), since only seven bacteremic patients did not have a clinical infection focus. The mortality was higher when a clinical focus was present and cultures proved positive. Subgroups according to clinical focus of infection and culture results were comparable, except for younger age in patients without a clinical focus of infection but with positive blood cultures and except for the relative predominance of women and presence of diabetes mellitus among patients with a clinical focus of infection and positive blood cultures. The time elapsed between onset of fever and inclusion was somewhat shorter among patients with a clinical focus of infection who later proved to have positive blood cultures. Table 2 describes clinical foci and associated microorganisms. It is shown that most patients with negative cultures had respiratory and gastrointestinal infections. Most patients with positive local cultures had respiratory and urinary tract infections with gram-negative organisms, and most patients with bacteremia had skin or subcutaneous infections with gram-positive organisms.
TABLE 1.
Patient characteristicsa
| Parameter | No clinical focus of infection
|
Clinical focus of infection
|
||||||
|---|---|---|---|---|---|---|---|---|
| Negative culture (n = 61) | Positive local culture (n = 20) | Positive blood culture (n = 7) | KW Pd | Negative culture (n = 106) | Positive local culture (n = 60) | Positive blood culture (n = 46) | KW Pd | |
| Age (yr)b | 59 (17–89) | 62 (27–89) | 32 (26–56) | <0.005 | 64 (18–92) | 66 (17–97) | 69 (21–92) | NS |
| Genderc | 34/27 | 9/11 | 3/4 | NS | 59/47 | 34/36 | 14/32 | <0.01 |
| HIV and/or AIDS | 3 (5) | 1 (5) | 1 (14) | NS | 9 (8) | 5 (8) | NS | |
| Malignancy | 14 (23) | 6 (30) | 3 (43) | NS | 25 (24) | 6 (10) | 11 (24) | NS |
| Diabetes mellitus | 5 (8) | 1 (5) | NS | 15 (11) | 9 (15) | 14 (30) | <0.05 | |
| Immunocompromising drugs | ||||||||
| Cytostatics | 5 (8) | 3 (15) | 2 (20) | NS | 8 (8) | 2 (3) | 4 (11) | NS |
| Steroids | 8 (13) | 2 (10) | NS | 7 (7) | 2 (3) | 6 (13) | NS | |
| Prior antibiotics | 10 (19) | 5 (33) | NS | 27 (26) | 15 (26) | 10 (22) | NS | |
| Nosocomial fever | 22 (26) | 6 (30) | 1 (14) | NS | 25 (24) | 12 (20) | 17 (37) | NS |
| Time to inclusion (h)b | 13 (1–575) | 7 (1–1,226) | 16 (3–64) | NS | 10 (5–382) | 25 (0–275) | 7 (0–198) | <0.05 |
| Mortality | 6 (10) | 2 (10) | 2 (28) | NS | 2 (2) | 9 (15) | 6 (13) | <0.005 |
Unless otherwise specified, results are presented as number (percent). Abbreviations: HIV, human immunodeficiency virus; KW, Kruskal-Wallis test; NS, not significant.
Range shown in parentheses.
Number of males/number of females.
P < 0.005 for presence or absence of a clinical focus of infection versus culture results.
TABLE 2.
Clinical focus of infection and potentially causative organism
| Culture focus | No. of patients with:
|
|||||
|---|---|---|---|---|---|---|
| Negative culture | Gram positive | Gram negative | Polybacterial | Fungus | Tuberculosis | |
| Negative culture (n = 167) | ||||||
| Respiratory tract | 60 | |||||
| Urinary tract | 12 | |||||
| Skin | 4 | |||||
| Gastrointestinal tract | 22 | |||||
| Bone and joint | 1 | |||||
| Catheter | 4 | |||||
| Miscellaneous | 3 | |||||
| Positive local culture (n = 80) | ||||||
| Respiratory tract | 3 | 16 | 7 | 9 | ||
| Urinary tract | 1 | 15 | 3 | 3 | ||
| Skin | 6 | 3 | 6 | 1 | 1 | |
| Gastrointestinal tract | 1 | 2 | ||||
| Bone and joint | 1 | |||||
| Miscellaneous | 1 | |||||
| Positive blood culture (n = 53) | ||||||
| Respiratory tract | 4 | |||||
| Urinary tract | 2 | 8 | ||||
| Skin | 11 | 2 | 2 | |||
| Gastrointestinal tract | 1 | 9 | 1 | |||
| Bone and joint | 2 | |||||
| Heart and catheter | 5 | 1 | ||||
| Miscellaneous | 1 | 1 | ||||
Clinical variables.
Table 3 describes the differences in clinical variables according to microbiological test results in patients with a clinical focus: the temperature was higher and leukocytosis was greater in patients with a clinical focus and positive blood cultures. There were no differences in initial or peak temperature and WBC counts among microbiological groups in patients without a clinical focus of infection.
TABLE 3.
Clinical variables when clinical focus of infection present
| Variable | Median (range)
|
KW Pa | ||
|---|---|---|---|---|
| Negative culture (n = 106) | Positive local culture (n = 60) | Positive blood culture (n = 46) | ||
| Temp (°C) | ||||
| Initial | 38.9 (38.0–40.7) | 38.9 (38.2–41.3) | 39.1 (38.4–40.5) | NS |
| Peak | 39.1 (38.3–40.7) | 39.2 (38.3–41.3) | 39.6 (38.4–40.8) | <0.001 |
| WBC counts (109/liter) | ||||
| Initial | 12.0 (0.3–42.3) | 14.3 (0.3–32.2) | 14.7 (2.6–38.4) | <0.001 |
| Peak | 12.4 (0.4–43.1) | 14.7 (0.4–41.9) | 16.9 (2.9–38.4) | <0.001 |
KW, Kruskal-Wallis test.
Inflammatory mediators.
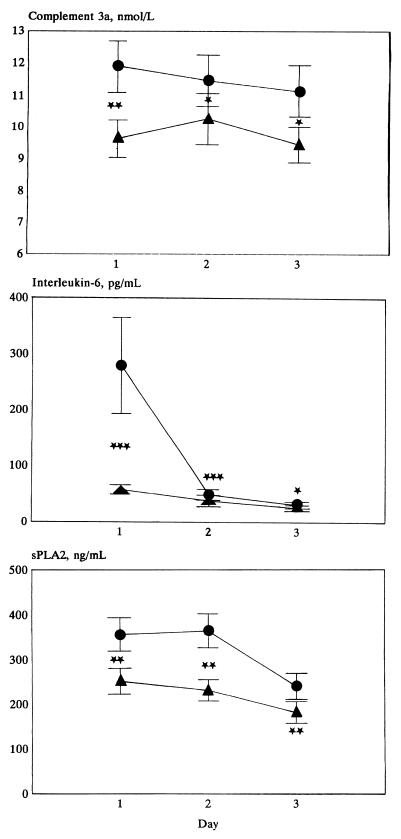
Figure 1 describes the daily course and Table 4 describes the initial and peak levels of the circulating inflammatory mediators according to the subgroups of the study. Peak levels of C3a in plasma were above normal (>5 nmol/liter) in 279 (93%) patients, peak IL-6 plasma levels were above normal (>10 pg/ml) in 245 (82%) patients, and peak sPLA2 levels were supranormal (>5 ng/ml) in 294 of 300 (98%) of patients. The sensitivity for prediction of positive blood cultures varied between 97 and 100%, at specificities varying between 2 and 21%. Both in patients without and in those with a clinical infection focus, the C3a and IL-6 levels were higher when cultures, particularly those of blood, later proved positive. Daily and peak levels of inflammatory mediators did not differ according to the focus of infection or to the Gram stain result of the associated microorganism. Furthermore, the daily or peak levels of mediators did not differ among patients with bacteremia with pure gram-positive (n = 26) or gram-negative (n = 23) bacteria.
FIG. 1.
Course of mean levels (error bars, standard deviations) in plasma of inflammatory mediators, complement C3a, IL-6, and sPLA2, in patients without (n = 167) (▴) and with (n = 133) (●) positive (local and blood) cultures, at inclusion (day 1) and daily for two days thereafter (days 2 and 3). Symbols for comparison between groups: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005.
TABLE 4.
Initial and peak inflammatory mediator levels in plasma
| Mediator | No clinical focus of infection
|
KW Pa | Clinical focus of infection
|
KW P | ||||
|---|---|---|---|---|---|---|---|---|
| Negative culture (n = 61) | Positive local culture (n = 20) | Positive blood culture (n = 7) | Negative culture (n = 106) | Positive local culture (n = 60) | Positive blood culture (n = 46) | |||
| Complement 3a (nmol/liter) | ||||||||
| Initial | 7.5 (2.4–23) | 7.1 (3.2–52) | 15.0 (8.6–21) | <0.05 | 8.2 (2.3–68) | 8.9 (2.2–21) | 10.0 (3.5–52) | <0.05 |
| Peak | 8.8 (3.0–45) | 8.9 (4.8–52) | 18.0 (11.0–54) | <0.005 | 9.5 (3.6–101) | 11.0 (4.1–36) | 15.0 (4.8–59) | <0.01 |
| IL-6 (pg/ml) | ||||||||
| Initial | 18 (2.4–906) | 23 (4–775) | 130 (21–825) | <0.005 | 26 (1.4–418) | 30 (2–3,502) | 56 (4–8,952) | <0.01 |
| Peak | 26 (3.1–1,168) | 26 (6.9–775) | 130 (27–825) | <0.01 | 29 (1.9–630) | 33 (4–3,205) | 72 (4–8,952) | <0.001 |
| Secretory phospholipase A (ng/ml) | ||||||||
| Initial | 99 (1–1,500) | 171 (2–1,500) | 252 (12–1,500) | <0.05 | ||||
| Peak | 178 (2–1,760) | 262 (5–1,250) | 413 (18–1,250) | <0.01 | ||||
KW, Kruskal-Wallis test.
Predictive value for positive local and blood cultures.
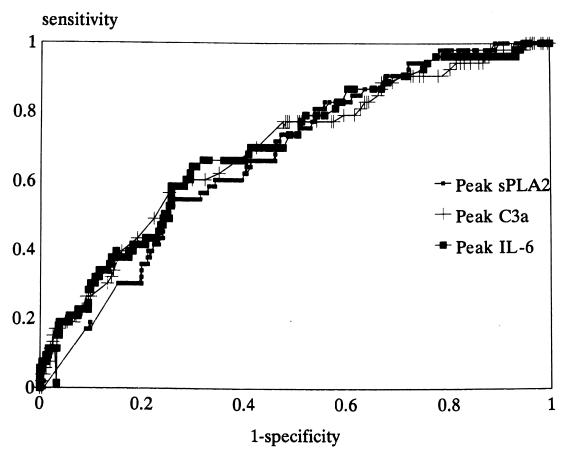
The AUC for the ROC curve for prediction of positive local and blood cultures was 0.60 for peak temperature (P < 0.005) and 0.59 for peak WBC (P < 0.01). For peak C3a the AUC was 0.60 (P < 0.005), for peak IL-6 it was 0.63 (P < 0.001), and for peak sPLA2 it was 0.61 (P < 0.001). The AUC of the ROC curve for prediction of positive blood cultures for peak temperature was 0.68 (P < 0.001), while for peak WBC count it was 0.56 (P < 0.05) and lower than those for peak IL-6 and peak C3a (P < 0.05). Indeed, the AUC of the ROC curve for peak C3a was 0.69, that for peak IL-6 was 0.70, and that for sPLA2 was 0.67 (Fig. 2) (for all, P < 0.001). This indicates that the predictive values of clinical variables and mediator levels were higher for prediction of positive blood cultures than for prediction of positive local cultures and that mediator levels were superior to some clinical variables in this respect. The predictive value of optimum cutoff values of clinical and mediator variables for positive blood cultures is shown in Table 5. The presence of a clinical focus of infection had a high positive predictive value, while the clinical and inflammatory variables had similar, relatively low positive but high negative predictive values.
FIG. 2.
ROC curves of mediator concentrations in plasma for positive blood cultures. The AUC for peak C3a was 0.69, that for peak IL-6 was 0.70, and that for peak sPLA2 was 0.67 (for all, P < 0.001).
TABLE 5.
Diagnostic value for positive blood cultures of clinical and inflammatory variables
| Parameter | Clinical variables
|
Inflammatory mediators
|
||||
|---|---|---|---|---|---|---|
| Clinical infection | Peak temp | Peak WBC count | Peak C3a | Peak IL-6 | Peak sPLA2 | |
| Optimum cutoff value | 39.7 | 16.4 × 109/liter | 14 nmol/liter | 54 pg/ml | 368 ng/ml | |
| Sensitivity (%) | 22 | 48 | 45 | 57 | 64 | 55 |
| Specificity (%) | 92 | 83 | 78 | 75 | 71 | 74 |
| Positive predictive value (%) | 87 | 37 | 31 | 33 | 32 | 30 |
| Negative predictive value (%) | 67 | 88 | 87 | 89 | 90 | 88 |
| Likelihood ratio | 2.75 | 2.79 | 2.12 | 2.26 | 2.20 | 2.05 |
Correlations and logistic regression.
Correlations on days 1, 2, and 3 between IL-6 and sPLA2 were 0.43, 0.44, and 0.44 (P < 0.001), respectively. There were no correlations above 0.35 or below −0.35 for clinical variables versus mediators or between mediators on either day of the study.
Multiple logistic regression was done to evaluate the contribution of mediator levels to clinical variables in predicting positive (blood) cultures. If combined with clinical signs (presence of a clinical focus of infection, peak temperature and WBC counts [for all, P < 0.05]), the peak C3a, IL-6, and sPLA2 did not contribute to prediction of a positive culture. However, if combined with clinical signs (presence of a clinical focus of infection and peak temperature [for both, P < 0.05]) both peak C3a and IL-6 independently contributed (P < 0.05) to prediction of positive blood cultures (81% correct; sensitivity, 17%; specificity, 98%; positive predictive value, 60%; negative predictive value, 85%; Hosmer Lemeshow P = 0.83; X2 = 4.3; df = 8), while peak WBC count and sPLA2 appeared not to contribute independently. Otherwise, the latter model had an area under the ROC curve of 0.76. This indicates that the inflammatory mediators may contribute to clinical signs and symptoms in predicting positive blood cultures, even before results of the latter are reported.
DISCUSSION
This study suggests that, in febrile patients, the degree of microbial invasion determines the height of the systemic host response, which is better reflected by circulating inflammatory mediators, in particular C3a and IL-6, than by commonly used clinical variables, such as fever and WBC counts. Conversely, circulating mediators may help predict later-reported positive blood cultures better than or as well as some simple clinical variables. This is important, since bacteremia may worsen patient outcome and early appropriate empirical antibiotic therapy may save lives (21, 33).
The frequencies of clinical foci of infection (71%) and of positive blood cultures (18%) in our study agree with previous reports (33). The latter also indicate that the prediction of bacteremia on the basis of clinical variables is hard (3–5, 9, 13, 33). For instance, other authors described that the area under the ROC curve of multivariate models of clinical variables was about 0.70 and commonly lower (3–5, 33). That clinical variables may only poorly relate to inflammatory mediators such as IL-6 has been suggested before (8, 9, 24, 28, 29). Hence, the clinical signs of an inflammatory host response may relate to inflammatory mediators other than those studied here, including perhaps tumor necrosis factor and IL-1. The many recent anti-inflammatory intervention studies for improving outcome from sepsis have failed, partly because of inclusion of noninfected patients and the too-late inclusion of patients already in severe sepsis and shock (1, 25). Nevertheless, there was a trend for improved survival in patients with markedly elevated IL-6 concentrations at baseline (25). Our study suggests that inflammatory cytokines are already released in febrile patients before positive reports of microbiological cultures and development of severe septic shock. Hence, IL-6 levels, if available at an early stage, might help to stratify patients in future studies for treatment of microbial infection at an early stage.
The local host response to microbial infection may include mechanisms to compartmentalize infection and prevent systemic spread, and may thereby contribute to survival. Release of inflammatory mediators into the circulation reflects a systemic response that may, as supported by our study, be associated with systemic spread of the microbial infection. In patients with bacterial meningitis or pneumonia, inflammatory mediators are elevated in cerebrospinal fluid and bronchoalveolar lavage, respectively, whereas a rise in blood levels is frequently associated with bacteremia (10, 19, 31). Furthermore, blood levels of several inflammatory mediators are elevated during sepsis, particularly when associated with shock and a poor outcome, but an association with positive blood cultures has hardly been found or stressed before (6–8, 15, 23, 24, 27–30). In contrast, in patients in emergency departments suspected of a microbial infection, circulating inflammatory mediators may be better predictive of subsequently documented microbial infection, particularly bacteremia, than a set of clinical signs (28, 29), in line with our study of hospitalized patients. In febrile neutropenic patients, elevated circulating IL-6 (and IL-8) also predicted microbial infection (9). The level of IL-6 in plasma may predict sepsis in neonates better than clinical signs, circulating IL-1 receptor antagonist, and CRP (20). In critically ill surgical patients, circulating IL-6, and to a lesser extent CRP, had predictive value for nosocomial infection (13). In critically ill patients with clinical signs of an inflammatory response, circulating complement activation product C3a and IL-6 (and procalcitonin) were predictive of positive local and/or blood cultures (27).
The systemic levels of the inflammatory mediators have been suggested to relate to patient morbidity and mortality during sepsis and shock (6–8, 11, 12, 16, 17, 23, 24, 29, 30, 32). We chose to determine levels of C3a in plasma because complement activation is thought to play a central role in the pathogenesis of severe sepsis and septic shock through, among others, activation of neutrophils (17). Levels of IL-6 in plasma were measured because this cytokine is thought to have a central signaling function in the inflammatory response to microbial infection, i.e., induction of the acute-phase reaction. An elevated level of IL-6 in plasma has been suggested to predict shock and outcome in patients with established sepsis (6–8, 11, 18, 24, 29). The levels of other cytokines, such as tumor necrosis factor or IL-1, were not determined because of poorer predictive value than of IL-6 for the presence, course, and outcome of severe microbial infections, among others related to the often transient nature of their release and the potential to induce natural inhibitors (6–8, 15, 20, 23, 24, 31). The value of proximal versus distal mediators in the inflammatory network, including neutrophil degranulation products, procalcitonin, and CRP, remains controversial, but studies suggest somewhat better predictive power of the proximal mediators (5, 9, 13, 20, 26, 27, 30). In any case, the concentration in blood of the distal mediator sPLA2 was of somewhat less predictive value than the proximal mediators C3a and IL-6 in our febrile patients, but the value of the latter may be similar to those of neutrophilic products and procalcitonin (5).
The relation between sPLA2 and IL-6 may indicate a common source and stimulus for release, including production by macrophages and neutrophils triggered by bacterial products. Alternatively, sPLA2 may originate from the liver upon stimulation by IL-6, and others have found a fair relation of plasma levels with CRP, also originating from the liver following IL-6 stimulation, during sepsis (8, 32). Therefore, sPLA2 can also be regarded as an acute-phase reactant. sPLA2 contributes to release of eicosanoids and platelet activating factor, by acting on cell membranes and thereby contributing to tissue damage, and may activate primed neutrophils, but its activity may be inhibited by C3a (14). Alternatively, sPLA2 and CRP may localize together on damaged plasma membranes, thereby activating complement, so that the elevated C3a level in febrile patients with microbial infection does not necessarily indicate complement activation directly by microbial products (32). The sPLA2 concentration in blood, nevertheless, related more to IL-6 than to C3a in our patients.
That the inflammatory mediator levels were also elevated above normal in some febrile patients without microbial infection can be caused by an underestimation of the rate of microbial infections following prior antibiotic therapy, even though the latter was not more common in patients with negative microbiological test results. Nevertheless, positive blood cultures were found more often when the interval between onset of fever and inclusion was relatively short. Furthermore, the predictive values reported in this study do not suggest that taking specimens for culture in febrile patients can be omitted, even though, at sensitivities of supranormal values exceeding 97%, normal values of the mediators missed at maximum 2 of 53 (4%) cases of positive blood cultures. Our study also does not solve the question of whether it is safe to withhold antibiotic treatment in patients with normal levels of mediators.
In conclusion, our study suggests that the systemic host response to infection is a marker of the degree of microbial invasion, rather than the focus and type of microbial infection, at an early stage in patients with fever. Circulating complement activation products and IL-6 may help to predict microbial bloodstream infection, together with clinical signs of the inflammatory host response such as fever and leukocytosis. A rapid bedside test may thus help guide the choice of therapies, even before culture results are available, in future studies.
ACKNOWLEDGMENT
Financial support was provided by grant number 28-2275 from the Dutch “Het Praeventiefonds.”
REFERENCES
- 1.Abraham E. Why immunomodulatory therapies have not worked in sepsis. Intens Care Med. 1999;25:556–566. doi: 10.1007/s001340050903. [DOI] [PubMed] [Google Scholar]
- 2.American College of Chest Physicians-Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–875. [PubMed] [Google Scholar]
- 3.Bates D W, Sands K, Miller E, Lanken P N, Hibberd P L, Graman P S, Schwarts J S, Kahn K, Snydman D R, Parsonnet J, Moore R, Black E, Jonhson B L, Jha A R, Platt R. Predicting bacteremia in patients with sepsis syndrome. J Infect Dis. 1997;176:1538–1551. doi: 10.1086/514153. [DOI] [PubMed] [Google Scholar]
- 4.Bossink A W J, Groeneveld A B J, Hack C E, Thijs L G. The clinical host response to microbial infection in medical patients with fever. Chest. 1999;116:380–390. doi: 10.1378/chest.116.2.380. [DOI] [PubMed] [Google Scholar]
- 5.Bossink A W J, Groeneveld A B J, Hack C E, Thijs L G. Prediction of microbial infection and mortality in medical patients with fever: plasma procalcitonin, neutrophilic elastase-α1-antitrypsin, and lactoferrin compared with clinical variables. Clin Infect Dis. 1999;29:398–407. doi: 10.1086/520222. [DOI] [PubMed] [Google Scholar]
- 6.Calandra T, Gerain J, Heumann D, Baumgartner J-D, Glauser M P. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines. Am J Med. 1991;91:23–29. doi: 10.1016/0002-9343(91)90069-a. [DOI] [PubMed] [Google Scholar]
- 7.Casey L C, Balk R A, Bone R C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bont E S J M, Vellenga E, Swaanenburg J C J M, Fidler V, Visser-van Brummen P J, Kamps W A. Plasma IL-8 and IL-6 levels can be used to define a group with low risk of septicaemia among cancer patients with fever and neutropenia. Br J Haematol. 1999;107:375–380. doi: 10.1046/j.1365-2141.1999.01707.x. [DOI] [PubMed] [Google Scholar]
- 10.Dehoux M S, Boutten A, Ostinelli J, Seta N, Dombret M C, Crestani B, Deschenes M, Trouillet J L, Aubier M. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1994;150:710–716. doi: 10.1164/ajrccm.150.3.8087341. [DOI] [PubMed] [Google Scholar]
- 11.Dofferhoff A S M, Bom V J J, de Vries-Hospers H G, van Ingen J, van der Meer J, Hazenberg B P C, Mulder P O M, Weits J. Patterns of cytokines, plasma endotoxin, plasminogen activator inhibitor, and acute-phase proteins during the treatment of severe sepsis in humans. Crit Care Med. 1992;20:185–192. doi: 10.1097/00003246-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Endo S, Inada K, Nakae H. Plasma levels of type II phospholipase A2 and cytokines in patients with sepsis. Res Commun Mol Pathol Pharmacol. 1995;90:413–421. [PubMed] [Google Scholar]
- 13.Fassbender K, Pargger H, Müller W, Zimmerli W. Interleukin-6 and acute-phase protein concentrations in surgical intensive care unit patients: diagnostic signs in nosocomial infection. Crit Care Med. 1993;21:1175–1180. doi: 10.1097/00003246-199308000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Gijon M A, Pérez C, Méndez E, Sánchez Crespo M. Phospholipase A2 from plasma of patients with septic shock is associated with high-density lipoproteins and C3 anaphylatoxin: some implications for its functional role. Biochem J. 1995;306:167–175. doi: 10.1042/bj3060167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gogos C A, Drosou E, Bassaris H P, Skoutelis A. Pro- versus antiinflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 16.Guidet B, Piot O, Masliah J, Barakett V, Maury E, Bereziat G, Offenstadt G. Secretory non-pancreatic phospholipase A2 in severe sepsis: relation to endotoxin, cytokines and thromboxane B2. Infection. 1996;24:103–108. doi: 10.1007/BF01713312. [DOI] [PubMed] [Google Scholar]
- 17.Hack C E, Nuijens J H, Felt-Bersma R J F, Schreuder W O, Eerenberg-Belmer A J M, Paardekoper J, Bronsveld W, Thijs L G. Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am J Med. 1989;86:20–26. doi: 10.1016/0002-9343(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 18.Hack C E, de Groot E R, Felt-Bersma R J F, Nuijens J H, Strack van Schijndel R J M, Eerenberg-Belmer A J M, Thijs L G, Aarden L A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- 19.Kragsbjerg P, Jones I, Vikerfors T, Holmberg H. Diagnostic values of blood cytokine concentrations in acute pneumonia. Thorax. 1995;50:1253–1257. doi: 10.1136/thx.50.12.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Küster H, Weiss M, Willeitner A E, Detlieffsen S, Jeremias I, Zbojan J, Geiger R, Lipowsky G, Simbruner G. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet. 1998;352:1271–1277. doi: 10.1016/S0140-6736(98)08148-3. [DOI] [PubMed] [Google Scholar]
- 21.Leibovici L, Samra A, Konigsberger H, Drucker M, Ashkenazi S, Pitlik S D. Long-term survival following bacteremia or fungemia. JAMA. 1995;274:807–812. [PubMed] [Google Scholar]
- 22.McGowan J E, Rose R C, Jacobs N F, Schaberg D R, Haley R W. Fever in hospitalized patients. With special reference to the medical service. Am J Med. 1987;82:580–586. doi: 10.1016/0002-9343(87)90103-3. [DOI] [PubMed] [Google Scholar]
- 23.Pinsky M R, Vincent J-L, Deviere J, Alegre M, Kahn R J, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 24.Presterl E, Staudinger T, Pettermann M, Lassnigg A, Burgmann H, Winkler S, Frass M, Graninger W. Cytokine profile and correlation to the APACHE III and MPM II scores in patients with sepsis. Am J Respir Crit Care Med. 1997;156:825–832. doi: 10.1164/ajrccm.156.3.9607131. [DOI] [PubMed] [Google Scholar]
- 25.Reinhart K, Wiegand-Löhnert C, Grimminger F, Kaul M, Withington S, Treacher D, Eckart J, Willatts S, Bouza C, Krausch D, Stockenhuber F, Eiselstein J, Daum L, Kempeni J. Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment MAK 195F, in patients with sepsis and septic shock: a multicenter, randomized, placebo-controlled, dose-ranging study. Crit Care Med. 1996;24:733–742. doi: 10.1097/00003246-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Rintala E S, Nevalainen T J. Group II phospholipase A2 in sera of febrile patients with microbiologically or clinically documented infections. Clin Infect Dis. 1993;17:864–870. doi: 10.1093/clinids/17.5.864. [DOI] [PubMed] [Google Scholar]
- 27.Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Köhl J. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med. 2000;28:2793–2798. doi: 10.1097/00003246-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Takala A, Jousela I, Olkkola K T, Jansson S-E, Leirisalo-Repo M, Takkunen O, Repo H. Systemic inflammatory response syndrome without systemic inflammation in acutely ill patients admitted to hospital in a medical emergency. Clin Sci. 1999;96:287–295. [PubMed] [Google Scholar]
- 29.Terregino C A, Lopez B L, Karras D J, Killian A J, Arnold G K. Endogenous mediators in emergency department patients with presumed sepsis: are levels associated with progression to severe sepsis and death? Ann Emerg Med. 2000;35:26–34. doi: 10.1016/s0196-0644(00)70101-6. l. [DOI] [PubMed] [Google Scholar]
- 30.Uhl W, Beger H G, Hoffman G, Hanisch E, Schild A, Waydhas C, Entholzner E, Müller K, Kellerman W, Vogeser M, Zügel M, Busch E W, Büchler M W. A multicenter study of phospholipase A2 in patients in intensive care units. J Am Coll Surg. 1995;180:323–331. [PubMed] [Google Scholar]
- 31.Van Deuren M, van der Ven-Jongekrijg J, Bartelink A K, van Dalen R, Sauerwein R W, van der Meer J W. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–439. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 32.Wolbink G-J, Bossink A W J, Groeneveld A B J, de Groot M C M, Thijs L G, Hack C E. Complement activation in patients with sepsis is in part mediated by C-reactive protein. J Infect Dis. 1998;177:81–87. doi: 10.1086/513803. [DOI] [PubMed] [Google Scholar]
- 33.Yehezkeli Y, Subah S, Elhanan G, Raz R, Porter A, Regev A, Leibovici L. Two rules for early prediction of bacteremia: testing in a university and a community hospital. J Gen Intern Med. 1996;11:98–103. doi: 10.1007/BF02599585. [DOI] [PubMed] [Google Scholar]