Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system in which peripheral blood monocytes play an important role. We have previously reported that patients with chronic progressive MS (CPMS) have significantly increased numbers of circulating monocytes which express the urokinase plasminogen activator receptor (uPAR). In the present study, we examined the expression of uPAR on monocytes in patients with relapsing-remitting multiple sclerosis (RRMS) not currently participating in a clinical trial and in patients with RRMS who were enrolled in a double-blind multicenter clinical trial designed to examine the effect of glatiramer acetate (copolymer 1; Copaxone) on relapsing disease. Patients with CPMS have sustained high levels of circulating uPAR-positive (uPAR+) monocytes. In comparison, patients with RRMS displayed variable levels of circulating uPAR+ monocytes. Mean values for uPAR in patients with RRMS were above those seen for controls but were not as high as those observed for patients with secondary progressive MS. Patients with RRMS in the clinical trial also had variable levels of monocyte uPAR. However, patients in the treatment group displayed lower levels following 2 years of treatment. In both placebo-treated and glatiramer acetate-treated patients, the percentage of circulating uPAR+ monocytes, as well as the density of uPAR expressed per cell (mean linear fluorescence intensity), increased just prior to the onset of a clinically documented exacerbation. Values fell dramatically with the development of clinical symptoms. uPAR levels in all groups correlated with both clinical activity and severity. Results indicate that monocyte activation is impatient in MS and that glatiramer acetate may have a significant effect on monocyte activation in patients with RRMS.
The pathogenesis of multiple sclerosis (MS) is a complex, multicellular process in which the infiltration of both monocytes and lymphocytes plays a key role (34, 39). Despite considerable experimental evidence and scientific opinion pointing to a role for T cells in the pathology of MS, there is little consensus on the role played by the peripheral blood monocyte. The monocyte/macrophage constitutes a large proportion of the infiltrating cells in MS lesions. Circulating monocytes from patients with MS have been shown to differ from normal monocytes in several aspects: (i) increased surface expression of interleukin-2R (52), (ii) increased oxidative burst activity (52), (iii) increased release of superoxide (15), (iv) increased phagocytic activity (31), (v) monocyte release of neopterin (17), and (vi) increased synthesis and release of a number of monokines (10, 14, 16, 23, 35, 40, 42), including members of the eicosanoid family (11, 13, 28). These results suggest that circulating monocytes from patients with MS are activated. This concept is supported by increased surface expression of costimulatory molecules (41, 51) and the expression of the monocyte activation antigen urokinase plasminogen activator receptor (uPAR) (12, 49). Of interest, however, is that the expression of uPAR and other surface markers of activation does not correlate with class II expression in these cells (4, 37). Taken together, the data suggest that circulating monocytes from patients with MS are “stimulated” and that monocytes may exist in different states of activation.
The role of uPAR in monocyte activation is not clear. However, it is known that the urokinase plasminogen activator and its receptor are important to extracellular proteolysis and to regulation of cell migration and invasiveness (7, 30). Thus, it is possible that monocytes bearing surface uPAR are primed for migration from the blood into the tissue. Whether the increased sustained pattern of monocyte uPAR expression previously reported in patients with secondary progressive MS (SPMS) is also seen in patients with relapsing-remitting MS (RRMS) is not known. We have examined uPAR expression in monocytes from patients with RRMS and SPMS as well as in 24 patients with RRMS participating in a clinical trial to determine the therapeutic efficacy of glatiramer acetate. Monocyte uPAR expression in patients with RRMS varied considerably over a wide range of values, although mean levels were significantly higher than those observed for healthy controls. Glatiramer acetate (copolymer 1 [COP-1]) significantly altered uPAR expression in RRMS patients admitted to the clinical trial after prolonged treatment. Increased uPAR expression correlated with changes in disease activity in all patient groups tested. Results also suggest that treatment was more effective in patients whose baseline uPAR levels were low at the onset of treatment.
MATERIALS AND METHODS
Patient recruitment and enrollment.
Patients with RRMS had been diagnosed with definite MS for a minimum of 2 years and had exprienced at least two documented relapses during that period of time. Patients admitted to the COP-1 study were those with RRMS who met the inclusion criteria (47, 32). Patients had experienced at least two documented elapses during the previous 2-year period but were stable for at least 30 days prior to entry. These patients had an expanded disability severity scale of 0 to 5.5 and had not received corticosteroid or other anti-inflammatory therapy for a minimum of 30 days. Patients with secondary chronic progressive MS and patients with RRMS who were not participating in the clinical trial also had not been treated with steroid or anti-inflammatory drugs for at least 3 months. Control populations consisted of (i) healthy volunteers from both the hospital personnel and community volunteers who were matched for age and sex and (ii) patients with other neurological diseases (amyotrophic lateral sclerosis, migraine headache, chronic inflammatory demyelinating polyneuropathy). All patients were examined by Wayne State University neurology faculty. All MS groups and healthy controls were studied serially.
Study design and data collection.
Patients in the clinical trial were examined before the beginning of therapy and every 3 months thereafter. Patients were examined within 5 days of an episode believed to be a possible exacerbation. At each visit two neurologists were involved: a treating neurologist and an examining neurologist. Both physicians were unaware of the treatment arm to which the patient had been assigned. Volunteers not participating in the clinical trial had been clinically evaluated within 3 months of sample donation. Samples were taken at the time of clinical examination. Samples were coded prior to study and were identified by trial number. Laboratory personnel were unaware of the patient diagnosis or of the treatment.
Monoclonal antibodies.
Mouse anti-human uPAR antibody M03F (immunoglobulin G2; 1:50) and isotype control antibody M45q (immunoglobulin G2a; 1:40) prepared during the same immunization protocols (47) were kindly provided by Robert F. Todd (University of Michigan, Ann Arbor). Secondary antibody [a fluorescein isothiocyanate-conjugated goat anti-mouse F(ab)2 fragment] was purchased from Cappel (Durham, N.C.). Monoclonal anti-human CD14 phycoerythrin-conjugated antibody was purchased from Sigma (St. Louis, Mo.).
Isolation of peripheral blood mononuclear cells.
Mononuclear cells were obtained from defibrinated venous blood by density separation following centrifugation for 30 min on Lympho-Paque (Nyegaard & Co, Oslo, Norway). The mononuclear layer was collected, washed three times, and resuspended in phosphate-buffered saline plus 2% heat-inactivated autologous serum. Mononuclear cells were counted on a hemocytometer, and 106 cells/ml were distributed to polystyrene tubes (12 by 75 mm; Becton-Dickinson Labware, Lincoln Park, N.J.).
Immunofluorescence flow cytometric analysis.
Primary antibody (200 ml) plus sodium azide (0.01%) was added to mononuclear cells in antibody excess. Cells plus antibody were incubated for 30 min at 4°C. Fluorescein isothiocynate-conjugated secondary antibody (1:100 dilution; saturation density) was applied after three washes in phosphate-buffered saline plus 2% bovine serum albumin. Anti-CD14 P phycoerythrin-conjugated antibody (1:100 dilution) was added last. After incubation at 4°C for 30 min, the cells were washed and fixed with 3% paraformaldehyde and kept in a refrigerator until the next day. The stained cells were then examined with a FACScan instrument (Becton and Dickinson, San Jose, Calif.) and analyzed by using the PC lysis II program. The monocyte population was first gated with the monocyte-specific marker CD14 (log forward-angle light scatter verses log 90° light scatter). Specific immunoreactivity for uPAR was determined by subtracting the value for the isotype control antibody M45q from the value for uPAR. Results were presented as percent positive cells and the mean channel number adjusted to a linear scale (mean linear fluorescence intensity [MLFI]) ± standard deviation (SD) (32). MLFI is an indicator of the density of antigen expression on a given cell. A minimum of 10,000 monocytes were analyzed.
RESULTS
Peripheral blood monocytes from patients with MS.
No statistically significant difference in percent total leukocytes, morphological appearance of cells, or cell viability was observed between leukocyte populations isolated from any of the patient groups and healthy controls (data not shown). The total number of monocytes, defined as CD14+ cells, was similar in all treatment groups as well as healthy controls over the study period (Table 1).
TABLE 1.
Percentage of CD14+ monocytes in patients with MS
| Group | % CD14+ monocytes (mean ± SD) at mo:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 25 | 27 | 30 | |
| Placebo | —a | 7 ± 3.4 | 9.1 ± 3.2 | 6.7 ± 4.4 | 10.6 ± 4.8 | 13.5 ± 3.23 | 7.8 ± 1.8 | 12.3 ± 6.6 | 15.5 ± 8.9 | 11.5 ± 6.6 | 9.1 ± 5 | 7.5 ± 0.7 |
| COP-1 | 9.9 ± 3.1 | 7.8 ± 4.2 | 7.6 ± 4.7 | 10.8 ± 7.5 | 13.4 ± 7.5 | 12.6 ± 5.3 | 13.5 ± 4.9 | 8.9 ± 1.6 | 16.3 ± 3.7 | 12.8 ± 2.5 | 11.9 ± 1.3 | 3.1 |
| Control | 12 | — | 9.6 | — | 11 | 11 | — | 7.1 | 8.3 | — | — | — |
—, not done.
Expression of monocyte uPAR in healthy controls and patients with RRMS and SPMS.
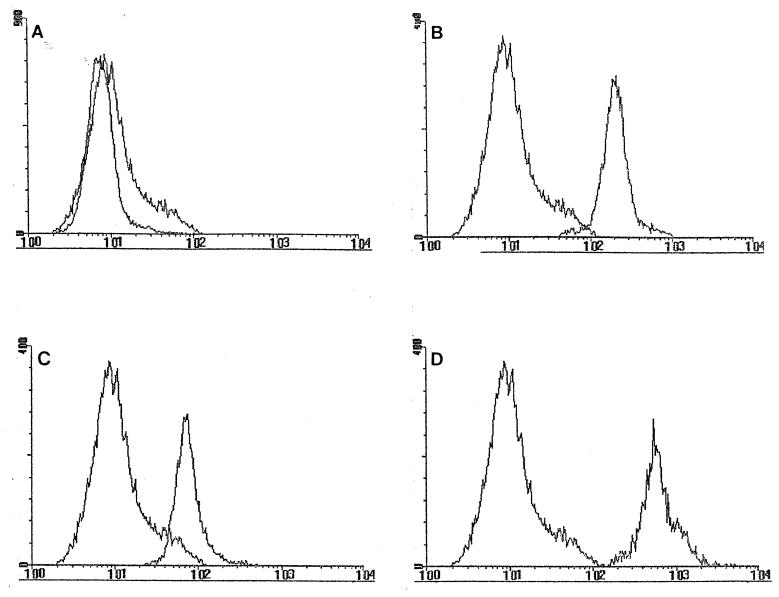
The surface expression of uPAR was examined by flow cytometry. All control volunteers displayed low percentages of circulating uPAR-positive (uPAR+) CD14+ monocytes. Those monocytes expressing uPAR did so at a low density. These cells exhibited a low MLFI, confirming previous studies by our laboratory (12, 49). When control monocytes were activated with lipopolysaccharide (LPS; 10 μg/ml) the percent CD14+ uPAR+ cells and the MLFI for uPAR+ monocytes were increased (Fig. 1A and B and Table 2). The increased percentage of uPAR+ monocytes correlated positively with increased MLFI.
FIG. 1.
Expression of uPAR by peripheral blood monocytes. (A) The fluorescence-activated cell sorter histograms indicate that healthy controls express very low levels of uPAR. (B) Treatment with LPS significantly increased both the percentage of uPAR+ monocytes and the MLFI. (C) RRMS patients express variable levels of uPAR, although the expressed uPAR at higher level than the controls did. (D) SPMS patients express uniformly high levels of uPAR compared to the levels expressed by healthy controls.
TABLE 2.
Density of uPAR expression on CD14+ monocytes from patients with SPMS, and RRMS and control volunteers
| Group (no. of subjects) | % uPAR+ cells | Mean ± SD MLFI for uPAR+ cellsb |
|---|---|---|
| SPMS (10) | 93 ± 12c | 710 ± 60c |
| RRMS (20) | 45 ± 20c | 421 ± 142c |
| Controls (15) | 18 ± 2 | 110 ± 14 |
| Neurological control (5) | 16 ± 3 | 128 ± 16 |
| Controls treated with LPS (4) | 68 ± 11c | 594 ± 72c |
Percent positively stained cells were determined by flow cytometry. CD14+ cells were gated, and their numbers were calculated following subtraction of cells counted with nonspecific isotype control antibody.
The MLFI was determined by flow cytometry.
Significantly different (P < 0.01) from values for healthy controls or untreated monocytes.
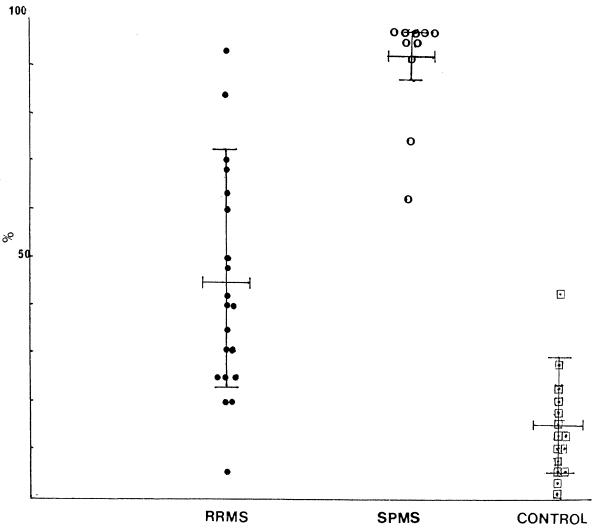
Patients with RRMS expressed variable levels of uPAR, expressed as both a percentage and MLFI (Table 2 and Fig. 1C). However, mean values were significantly above those for healthy controls and neurological controls (Table 2). The range of values for patients with RRMS is shown in Fig. 2. Monocytes from patients with SPMS had increased levels of expression of uPAR compared with that for monocytes from healthy controls (Table 2 and Fig. 1D), again supporting previous data. Unlike the range of values for patients with RRMS, the range of values for patients with SPMS was at a significantly higher level.
FIG. 2.
Range of percent uPAR+ monocytes in patients with RRMS (not participating in the trial) (●), patients with SPMS (○), and healthy controls (□). Values are expressed as means ± SDs calculated over the study period. Patients were not currently experiencing an exacerbation.
Longitudinal variation in monocyte uPAR expression.
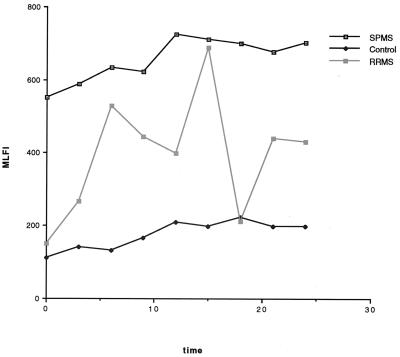
Longitudinal changes in the percent circulating CD14+ uPAR+ monocytes and in the density of uPAR expression are shown for representative patients in Fig. 3. Little fluctuation was observed in values for monocytes from healthy control volunteers. The percentage of uPAR+ monocytes as well as the MLFI (Fig. 3) for monocytes from patients with SPMS remained high during the study period, confirming previous results. Both the values for percent uPAR+ monocytes and the density of uPAR expression for patients with RRMS fluctuated significantly over the study period.
FIG. 3.
Longitudinal changes in monocyte uPAR expression. Fluctuations in MLFI for representative volunteers from the control, RRMS, and SPMS groups are graphed. Healthy control volunteers expressed low levels of uPAR+ monocytes. Values for patients with SPMS were consistently high. Patients with RRMS exhibited variable levels of uPAR.
Expression of monocyte uPAR in patients with RRMS participating in the glatiramer acetate clinical trial.
The expression of uPAR on the monocyte surface in both placebo and glatiramer acetate-treated patients was examined by dual-label flow cytometry. Patients with RRMS treated with either placebo or glatiramer acetate had significantly (P < 0.05) greater percentages of uPAR+ monocytes as well as a greater density (MLFI) of uPAR expression than healthy controls (Table 3). When studied longitudinally, the monocytes of placebo-treated patients expressed uPAR at variable percentages, and the density of uPAR expression was also variable. This pattern was similar to that obtained for patients with RRMS not participating in the clinical trial. In patients treated with glatiramer acetate, we observed a trend toward decreased longitudinal fluctuation in circulating CD14+ uPAR+ monocytes. The uPAR density (MLFI) decreased, but it was not until patients had been treated for nearly 2 years (Table 4) that these values reached statistical significance. As a group, mean values were statistically significantly lower in glatiramer acetate-treated patients after 2 years of treatment. It was also noted that some treated patients exhibited highly variable fluctuations in uPAR expression, while others displayed relatively low levels of fluctuation throughout the treatment protocol. We questioned whether this represented a subset of patients with RRMS.
TABLE 3.
Baseline monocyte uPAR expression in patients participating in the COP-1 clinical trial
| Treatment (no. of subjects) | % Monocytesa | MLFIa |
|---|---|---|
| COP-1 (12) | 24 ± 21 | 250 ± 140b |
| Placebo (12) | 22 ± 19 | 241 ± 154b |
| Healthy controls (12) | 12 ± 10 | 126 ± 33 |
Values are means ± SDs.
Statistically significant (P < 0.05) compared to control values.
TABLE 4.
Serial study of monocyte uPAR expression in patients participating in glatiramer acetate treatment study
| Mo | MLFI
|
% uPAR+ cells
|
||
|---|---|---|---|---|
| Treated group | Placebo group | Treated group | Placebo group | |
| 12 | 365 ± 76a (10)b | 365 ± 76 (10) | 54 ± 13 (10) | 44 ± 11 (10) |
| 18 | 288 ± 46 (5) | 230 ± 39 (4) | 39 ± 13 (5) | 30 ± 8 (6) |
| 21 | 166 ± 51 (9) | 452 ± 32 (9) | 63 ± 13 (8) | 52 ± 13 (9) |
| 24 | 216 ± 22 (9) | 311 ± 61 (9) | 77 ± 11 (6) | 18 ± 7 (9) |
| 27 | 213 ± 14 (9) | 333 ± 38 (9) | 24 ± 12 (10) | 73 ± 9 (10) |
| 30 | NDc | ND | 23 ± 6 (10) | 62 ± 10 (10) |
Values are means ± SDs.
Values in parentheses are numbers of subjects.
ND, not determined.
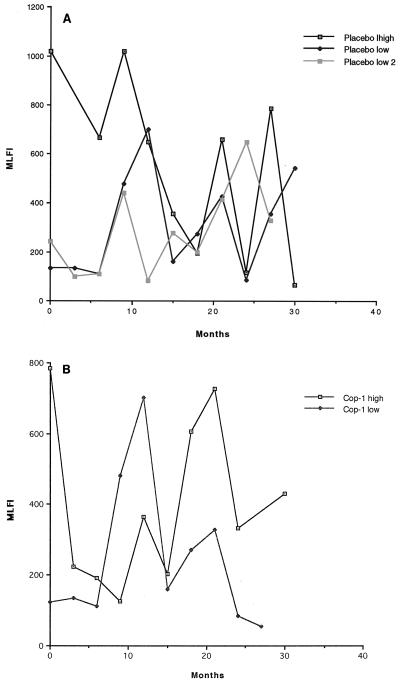
To study this, values were reanalyzed by grouping patients according to whether they exhibited high or low baseline uPAR+ values at the start of the trial. Patients were grouped as follows: placebo-treated patients with high baseline (MLFIs, ≥300) of uPAR+ monocytes (group A), placebo-treated patients with low baseline MLFI levels (<300) of uPAR+ monocytes (group B), glatiramer acetate-treated patients with high baseline MLFI levels of uPAR+ monocytes (group C), and glatiramer acetate-treated patients with low baseline MLFI levels of uPAR+ monocytes (group D) (Table 5). In Fig. 4 we graphed longitudinal values for representative patients from each group. Group A (placebo with high baseline levels) exhibited highly variable patterns of uPAR expression characterized by peaks and valleys, as did patients in group B (placebo with low starting baseline levels) (Fig. 4A). Glatiramer acetate-treated patients (Fig. 4B) who began the trial with high baseline levels of uPAR+ monocytes also exhibited highly variable levels of uPAR expression similar to that in placebo-treated patients (Fig. 4A), although after prolonged treatment some patients appeared to express lower levels. These patients were those who began the trial with low baseline levels of uPAR+ monocytes. These results suggest that glatiramer acetate may have had a greater effect on uPAR expression in patients in this subset. One possible explanation is that uPAR expression, which is an indication of monocyte activation, changes with disease severity and/or activity and that patients in group D may have started the trial with less active disease.
TABLE 5.
Baseline expression of uPAR in treatment subgroups
| Group (no. of subjects)a | % | MLFI |
|---|---|---|
| Placebo, low (9) | 24 ± 21b | 115 ± 14 |
| Placebo, high (3) | 77 ± 16 | 590 ± 380 |
| COP-1, low (6) | 28 ± 23 | 113 ± 15 |
| COP-1, high (6) | 78 ± 31c | 582 ± 209c |
Patient groups were subdivided into those who began the study with baseline levels which were at control levels (MLFI, <300 [low]) and high levels (MLFI, ≥300 [high]).
Values are means ± SDs.
Statistically significantly different from the group with low baseline levels (P < 0.001).
FIG. 4.
Longitudinal changes in patients exhibiting high and low levels of monocyte uPAR expression. Representative placebo-treated patients (A) grouped by high baseline levels of uPAR and low baseline levels of uPAR are compared to glatiramer acetate-treated patients (B) grouped according to high baseline levels and low baseline levels. Glatiramer acetate-treated patients who started the trial with low baseline uPAR values exhibited less overall fluctuation in uPAR expression and lower mean levels than treated patients with high levels at the start of the trial and than placebo patients.
Correlation with clinical activity.
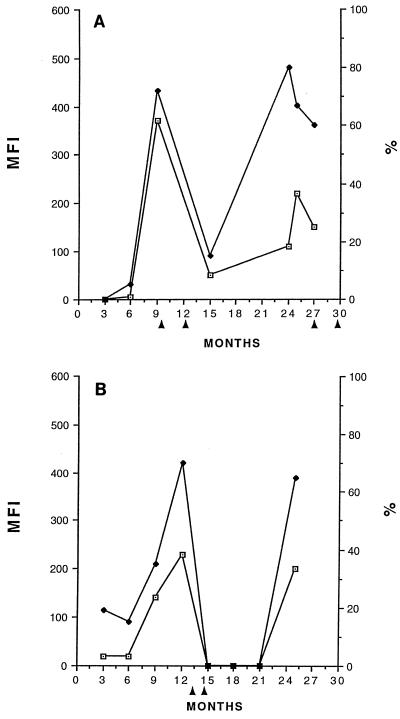
Patients participating in the clinical trial were instructed to report an exacerbation of their disease. Samples were obtained during and after a clinically documented exacerbation. Monocytes isolated from patients prior to a clinically documented exacerbation exhibited high uPAR values (Fig. 5). The values decreased after the onset of new symptoms. This pattern was observed in both the placebo and the treated groups. However, treatment with glatiramer acetate significantly lowered the rate of exacerbation in patients with low levels of uPAR expression and lowered the preexacerbation rises in the MLFI and percentage of circulating uPAR+ monocytes (Fig. 5B) in this subset. The exacerbation rates for patients with low baseline levels of uPAR+ monocytes were 1.166% ± 0.3% (n = 9) and 1.66% ± 0.6% (n = 9) for glatiramer acetate- and placebo-treated patients, respectively. The difference between these groups was statistically significant (P < 0.01 by Student's t test). The exacerbation rates for patients with high baseline levels of uPAR+ monocytes were 1.25% ± 0.4% (n = 6) and 1.5% ± 0.5% (n = 5) for glatiramer acetate- and placebo-treated patients, respectively.
FIG. 5.
Correlation of uPAR expression with clinical activity. MLFI (MFI; ⊡) and percent (⧫) uPAR+ monocytes increased prior to a clinically documented exacerbation in placebo-treated (A) and glatiramer acetate (COP-1)-treated (B) patients. Arrows point to the onset of exacerbation. Data for representative patients were chosen for presentation.
Correlation with EDSS.
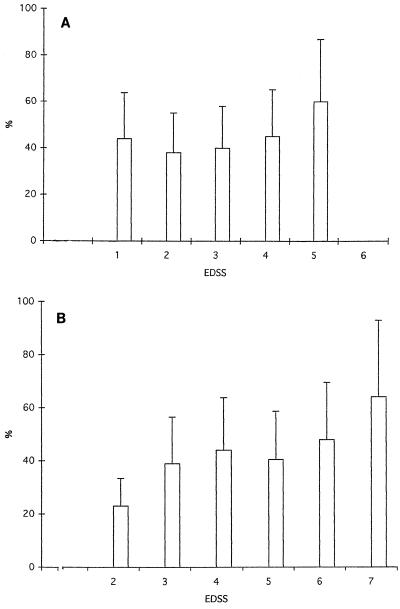
Disability ratings for glatiramer acetate- and placebo-treated patients are shown in Table 6. Scores were compared to MLFI and to percent uPAR+ monocytes for both the placebo and the treated groups longitudinally over time. The percentage of uPAR+ monocytes increased with an increase in the Kurtzke Expanded Disability Status Score (EDSS) (Fig. 6). The higher the disability rating, the higher the percentage of uPAR+ monocytes. The MLFI, however, did not correlate with EDSS (data not shown). However, increased numbers of patients need to be studied in order to confirm these results.
TABLE 6.
Mean disability ratings for placebo and COP-1-treated groups
| Group (n) | Mean ± SD rating at mo:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 25 | 27 | 30 | |
| Placebo (12) | 2.1 ± 1.2 | 1.8 ± 1.1 | 2.4 ± 1.4 | 2.2 ± 1.4 | 2.5 ± 1.4 | 2.4 ± 1.8 | 1.3 ± 1.2 | 2.3 ± 1.4 | 3.4 ± 1.8 | 3.5 ± 2.1 | 2.7 ± 2 | 2.2 |
| COP-1 (12) | 3.2 ± 1.1 | 3.8 ± 1.9 | 3.8 ± 2.2 | 4 ± 1.8 | 3 ± 1.8 | 3.7 ± 1.8 | 2.5 ± 1 | 3.7 ± 2.4 | 3.5 ± 1.6 | 4.5 ± 2.4 | 4.8 ± 2.4 | 3 |
FIG. 6.
Correlation of percent uPAR+ monocytes with EDSS. Expression of uPAR on peripheral blood monocytes was found to correlate with EDSS in patients treated with placebo (A) and glatiramer acetate (B). The correlations for MLFI did not reach statistical significance.
DISCUSSION
Monocyte surface expression of uPAR is thought to reflect a state of monocyte activation (7, 12, 20, 49). uPAR is also expressed by T-effector cells (47) and by many other cells such as endothelial cells, microglial cells (48), and metastatic tumor cells (for a review, see reference 47) thought to be primed to migrate. uPAR is a principal initiator of the plasminogen-plasmin cascade and contributes indirectly, through plasmin, to the activation of gelatinase. Gelatinase is a highly active matrix-degrading metalloproteinase. Both enzymes are important to cell migration (7), disruption of the blood-brain barrier (7, 10), and myelin degradation (10, 27, 43) and may be important in experimental allergic encephalomyelitis and MS (10, 18, 25). uPAR expression is modulated by endotoxin (LPS) and cytokines such as tumor necrosis factor alpha and interferon gamma (24). Both cytokines have been implicated in the pathogenesis of MS (9, 33).
We report that patients with RRMS express higher levels of the monocyte activation antigen uPAR than healthy controls. These values fluctuated over a wide range when measured in a longitudinal fashion. Such variations are in contrast to the consistently high nonfluctuating levels of uPAR expression seen in monocytes from patients with SPMS (12) or to the consistently low levels of expression seen in healthy individuals (12). Changes in uPAR expression and, thus, stimulation of monocyte activity may be important in the clinical course of the disease.
Values for both MLFI and percent uPAR+ cells increased prior to the onset of a clinically documented exacerbation and rapidly decreased with the development of new clinical symptoms. During an exacerbation, uPAR levels were low and comparable to those observed in healthy controls. The dramatic increase in monocyte uPAR expression preceding the beginning of an exacerbation might suggest heightened peripheral stimulation of monocytes. That expression falls during development of new symptoms may suggest that activated monocytes migrate into the central nervous system.
Increased levels of uPAR were seen in patients with SPMS and RRMS with higher disability scores. The latter group, in general, showed less significant benefit after treatment with glatiramer acetate (1, 5, 6, 45, 50). When analysis was carried out with patients grouped by high or low starting baseline levels of uPAR, glatiramer acetate appeared to have a significant suppressive effect on monocyte expression of uPAR in patients with less active disease.
Taken together, our results suggest that the therapeutic efficacy of glatiramer acetate is likely to be greater if treatment is started very early in the disease process. Studies with much larger numbers of patients are needed to support this concept.
The mechanism responsible for glatiramer acetate-mediated suppression of monocyte uPAR expression is unclear. It is not known whether the effect on uPAR expression is a direct effect on monocytes themselves or whether it is an indirect effect mediated by other leukocyte subsets. It is thought that glatiramer acetate may activate T-suppressor cells (8) or inhibit T-cell responses to myelin basic protein (44). Evidence for the latter mechanism is equivocal (44). The suppressive effect of glatiramer acetate may be related to a cross-reactivity with myelin basic protein (44), competitively inhibiting the interaction of this molecule with antigen-presenting cells (8, 36, 44). While glatiramer acetate does not interfere with antigen processing, it appears to alter antigen presentation and T-cell activation (36). Li and colleagues (26) reported that glatiramer acetate blocked cytokine-mediated major histocompatibility complex class II antigen expression in a human monocytic cell line, suggesting that it blocks monocyte activation. The levels of production of tumor necrosis factor alpha and cathepsin D were also decreased. The level of interleukin-1 release was, however, increased in the same cells, making the data equivocal. Evidence by other investigators showing that glatiramer acetate may shift T-cell activation from the T1 to the T2 cytokine profile (2, 3, 26, 29, 46) indirectly suggests that the drug may alter monocyte release of polarizing cytokines. Thus, it is possible that glatiramer acetate may alter monocyte/macrophage function. Taken together, the results support the supposition that monocyte function is important to the pathophysiology of MS.
REFERENCES
- 1.Aharoni R, Teitelbaum D, Arnon R. T-suppressor hybridomas and interleukin-2 dependent lines induced by MBP or by spinal cord homogenate down-regulate experimental allergic encephalomyelitis. Curr J Immunol. 1993;23:17–25. doi: 10.1002/eji.1830230105. [DOI] [PubMed] [Google Scholar]
- 2.Aharoni R, Teitelbaum D, Sela M, Arnon R. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aharoni R, Teitelbaum D, Sela M, Arnon R. Bystander suppression of experimental autoimmune encephalomyelitis by T cell lines and clones of the Th2 type induced by copolymer 1. J Neuroimmunol. 1998;91:135–146. doi: 10.1016/s0165-5728(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong M A, Crocard A D, Hawkins D A, Gamble L A, Shah A, Bell A L. Class II major histocompatibility complex antigen expression on unstimulated and gamma-interferon stimulated monocytes from patients with multiple sclerosis, rheumatoid arthritis and normal controls. Autoimmunity. 1991;131:261–268. doi: 10.3109/08916939109007652. [DOI] [PubMed] [Google Scholar]
- 5.Bornstein M, Miller A, Slagle S, Weitzman M, Crystal H, Drexler E, Keilson M, Merriam A, Wassertheil-Smoller S, Spada V, Weiss W, Arnon R, Jacobsohn I, Teitelbaum D, Sela M. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med. 1987;317:408–414. doi: 10.1056/NEJM198708133170703. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein M, Miller A, Slagle S, Weitzman M, Crystal H, Drexler E, Keilson M, Merriam A, Wassertheil-Smoller S, Spada V, Weiss W, Arnon R, Jacobsohn I, Teitelbaum D, Sela M. A placebo-controlled, double blind, randomized, two-center, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurology. 1991;41:533–539. doi: 10.1212/wnl.41.4.533. [DOI] [PubMed] [Google Scholar]
- 7.Brasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 1993;15:105–111. doi: 10.1002/bies.950150206. [DOI] [PubMed] [Google Scholar]
- 8.Burns J, Littlefield K. Failure of copolymer 1 to inhibit the human T-cell response to myelin basic protein. Neurology. 1991;41:1317–1319. doi: 10.1212/wnl.41.8.1317. [DOI] [PubMed] [Google Scholar]
- 9.Cannela B, Raine C S. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 10.Cuzner M L, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- 11.Dore-Duffy P, Donaldson J O, Koff T, Longo M, Perry W. Prostaglandin release in multiple sclerosis: correlation with disease activity. Neurology. 1986;36:1587–1590. doi: 10.1212/wnl.36.12.1587. [DOI] [PubMed] [Google Scholar]
- 12.Dore-Duffy P, Donovan C, Todd R F. Expression of monocyte activation antigen Mo3 on the surface of peripheral blood monocytes from patients with multiple sclerosis. Neurology. 1992;42:1609–1624. doi: 10.1212/wnl.42.8.1609. [DOI] [PubMed] [Google Scholar]
- 13.Dore-Duffy P, Ho S Y, Donovan C. Cerebrospinal fluid eicosanoid levels: exogenous PGE2 and LTC4 synthesis by antigen-presenting cells that migrate to the central nervous system. Neurology. 1991;41:322–324. doi: 10.1212/wnl.41.2_part_1.322. [DOI] [PubMed] [Google Scholar]
- 14.Ebadi M, Bashir R M, Heidrick M L, Hamada F M, Refaey N E, Hamed A, Helal G, Baxi M D, Cerutis D R, Lassi N K. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int. 1997;30:347–374. doi: 10.1016/s0197-0186(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 15.Fisher M, Upchurch K S, Levine P H. Monocyte and polymorphonuclear leukocyte toxic oxygen metabolite production in multiple sclerosis. Inflammation. 1988;12:123–131. doi: 10.1007/BF00916395. [DOI] [PubMed] [Google Scholar]
- 16.Fiten P, Vandenbroeck K, Dubois B, Van Coillie E, Nelissen I, Van Damme J, Ligers A, Hillert J, Andersson M, Olsson T, Opdenakker G. Microsatellite polymorphisms in the gene promoter of monocyte chemotactic protein-3 and analysis of the association between monocyte chemotactic protein-3 alleles and multiple sclerosis development. J Neuroimmunol. 1999;95:195–201. doi: 10.1016/s0165-5728(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 17.Fredrikson S, Link H, Cronroth P. CSF neopterin as a marker of disease activity in multiple sclerosis. Acta Neurol Scand. 1987;75:352–355. doi: 10.1111/j.1600-0404.1987.tb05458.x. [DOI] [PubMed] [Google Scholar]
- 18.Gijbels K, Masure S, Carton H, Opemaker C N. Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol. 1992;41:29–34. doi: 10.1016/0165-5728(92)90192-n. [DOI] [PubMed] [Google Scholar]
- 19.Hamman J P, Nix W, Dierich M P, Hopf H C. The spontaneous burst activity of peripheral blood monocytes in patients with acute polyradiculoneuritis, lymphocytic meningoencephalitis and multiple sclerosis. A comparison. J Neurol Sci. 1986;72:287–297. doi: 10.1016/0022-510x(86)90016-x. [DOI] [PubMed] [Google Scholar]
- 20.Hancock W W, Zola H, Atkins R C. Antigenic heterogeneity of human mononuclear phagocytes: immunohistological analysis using monoclonal antibodies. Blood. 1983;62:1271–1279. [PubMed] [Google Scholar]
- 21.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Schiffer R B. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 22.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Schiffer R B, Wollmer T B, Weiner L P, Wolinsky J S. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1998;50:701–708. doi: 10.1212/wnl.50.3.701. [DOI] [PubMed] [Google Scholar]
- 23.Kerschensteiner M, Gallmeier E, Behrens L, Leal V V, Misgeld T, Klinkert W E, Kolbeck R, Hoppe E, Oropexa-Wekerle R L, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchheimer J C, Nong Y H, Remold H G. IFNγ tumor necrosis factor-α and urokinase regulate the expression of urokinase receptors on human monocytes. J Immunol. 1998;141:4229–4234. [PubMed] [Google Scholar]
- 25.Koh C S, Paterson P Y. Suppression of clinical signs of cell-transferred experimental allergic encephalomyelitis and altered cerebrovascular permeability in Lewis rats treated with a plasminogen activator inhibitor. Cell Immunol. 1987;107:52–63. doi: 10.1016/0008-8749(87)90265-6. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Milo R, Panitch H, Swoveland P, Bever C T., Jr Glatiramer acetate blocks the activation of THP-1 cells by interferon-gamma. Eur J Pharmacol. 1998;342:303–310. doi: 10.1016/s0014-2999(97)01509-4. [DOI] [PubMed] [Google Scholar]
- 27.Matrisian L M. The matrix-degrading metalloproteases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 28.Merril J E, Gerner R H, Myers L W, Ellison E W. Regulation of natural killer cell cytotoxicity by prostaglandin E in the peripheral blood and cerebrospinal fluid in patients with multiple sclerosis and other neurological diseases. Part 1. Association between amount of prostaglandin produced, natural killer cell, and endogenous interferon. J Neuroimmunol. 1983;4:223–237. doi: 10.1016/0165-5728(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 29.Miller A, Shapiro S, Gershtein R, Kinarty A, Rawashdeh H, Honigman S, Lahat N. Treatment of multiple sclerosis with copolymer 1 (Copaxone): implicating mechanisms of Th1 to Th2/Th3 immune-deviation. J Neuroimmunol. 1998;92:113–121. doi: 10.1016/s0165-5728(98)00191-x. [DOI] [PubMed] [Google Scholar]
- 30.Moller L B. Structure and function of the urokinase receptor. Blood Coagul Fibrinol. 1993;4:293–303. [PubMed] [Google Scholar]
- 31.Moller-Larsen A, Haahr S, Hollsberg P, Hansen H J. The phagocytic activity of monocytes and polymorphonuclear leukocytes against viral antigens as measured by chemoluminescence in patients with MS. J Clin Lab Immunol. 1989;29:53–58. [PubMed] [Google Scholar]
- 32.Muirhead K A, Schmitt T C, Muirhead A R. Determination of linear fluorescence intensity from flow cytometric data accumulated with logarithmic amplifiers. Cytometry. 1983;3:251–263. doi: 10.1002/cyto.990030404. [DOI] [PubMed] [Google Scholar]
- 33.Norton W T, Brosnan C F, Cammer W, Goldmintz E A. Mechanism and suppression of inflammatory demyelination. Review Acta Neurobiol Exp. 1990;40:225–235. [PubMed] [Google Scholar]
- 34.Paterson P Y, Swanborg R N. Demyelinating diseases of the central and peripheral nervous systems. Immunol Dis. 1986;11:1877–1915. [Google Scholar]
- 35.Porrini A M, Reder A T. IFN-gamma, IFN-beta, and PGE1 affect monokine secretion: relevance to monocyte activation in multiple sclerosis. Cell Immunol. 1994;157:428–438. doi: 10.1006/cimm.1994.1239. [DOI] [PubMed] [Google Scholar]
- 36.Racke M K, Martin R, McFarland H, Fritz R. Induced inhibition of antigen-specific T cell activation: interference with antigen presentation. J Neuroimmunol. 1992;37:75–84. doi: 10.1016/0165-5728(92)90157-g. [DOI] [PubMed] [Google Scholar]
- 37.Ransohoff R M, Tuohy V K, Barna B P, Rudick R A. Monocytes in active multiple sclerosis: intact regulation of HLA-DR density in vitro despite decreased HLA-DR density in vivo. J Neuroimmunol. 1992;37:169–176. doi: 10.1016/0165-5728(92)90001-2. [DOI] [PubMed] [Google Scholar]
- 38.Reder A T, Senc K, Dyskosh P V, Porrini A M. Monocyte activation in multiple sclerosis. Multiple Sclerosis. 1998;4:182. doi: 10.1177/135245859800400314. [DOI] [PubMed] [Google Scholar]
- 39.Rudick R A, Ransohoff R M. Cytokine secretion by multiple sclerosis monocytes: relationship to disease activity. Ann Neurol. 1992;49:265–270. doi: 10.1001/archneur.1992.00530270079022. [DOI] [PubMed] [Google Scholar]
- 40.Santiago E, Perex-Mediavilla L A, Lopez-Moratalla N. The role of nitric oxide in the pathogenesis of multiple sclerosis. J Physiol Biochem. 1998;54:229–237. [PubMed] [Google Scholar]
- 41.Svenningsson A, Dotevall L, Stemme S, Andersen O. Increased expression of B7-1 costimulatory molecule on cerebrospinal fluid cells of patients with multiple sclerosis and infectious central nervous system disease. J Neuroimmunol. 1997;75:59–68. doi: 10.1016/s0165-5728(96)00234-2. [DOI] [PubMed] [Google Scholar]
- 42.Tang L, Benjaponpitak S, DeKruyff R H, Umetsu D T. Reduced prevalence of allergic disease in patients with multiple sclerosis is associated with enhanced IL-12 production. J Allergy Clin Immunol. 1998;102:428–435. doi: 10.1016/s0091-6749(98)70131-9. [DOI] [PubMed] [Google Scholar]
- 43.Tani M, Ransohoff M. Do chemokines mediate inflammatory cell invasion of the central nervous system parenchyma? Brain Pathol. 1994;4:135–143. doi: 10.1111/j.1750-3639.1994.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 44.Teitelbaum D, Aharoni R, Sela M, Arnon R. Cross-reactions and specificities of monoclonal antibodies against myelin basic protein and against the synthetic copolymer 1. Proc Natl Acad Sci USA. 1991;88:9582–9632. doi: 10.1073/pnas.88.21.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teitelbaum D, Milo R, Arnon R, Sela M. Synthetic copolymer 1 inhibits human T-cell lines specific for myelin basic protein. Proc Natl Acad Sci USA. 1992;89:137–141. doi: 10.1073/pnas.89.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teitelbaum D, Arnon R, Sela M. Immunomodulation of experimental autoimmune encephalomyelitis by oral administration of copolymer 1. Proc Natl Acad Sci USA. 1999;96:3842–3847. doi: 10.1073/pnas.96.7.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Todd F E, Nadler L M, Schossman S F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981;126:1435–1442. [PubMed] [Google Scholar]
- 48.Washington R A, Becher B, Balabanov R, Antel J, Dore-Duffy P. Expression of the activation marker urokinase plasminogen-activator receptor in cultured human central nervous system microglia. J Neurosci Res. 1996;45:392–399. doi: 10.1002/(SICI)1097-4547(19960815)45:4<392::AID-JNR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Washington R, Dore-Duffy P. Role of cytoskeletal elements in expression of monocyte urokinase plasminogen activator receptor: the activation-associated antigen Mo3. Clin Diagn Lab Immunol. 1994;1:714–721. doi: 10.1128/cdli.1.6.714-721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner L H. Cop-1 therapy for multiple sclerosis. N Engl J Med. 1987;317:442–444. doi: 10.1056/NEJM198708133170708. [DOI] [PubMed] [Google Scholar]
- 51.Windhagen A, Maniak S, Gebert A, Ferger I, Heidenreich F. Costimulatory molecules B7-1 and B7-2 on CSF cells in multiple sclerosis and optic neuritis. J Neuroimmunol. 1999;96:112–120. doi: 10.1016/s0165-5728(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 52.Zaffaroni M, Ghezzi A, Callea L, Zibetti A. Interleukin-2 receptor expression on blood monocytes from patients with multiple sclerosis. Ital J Neurol Sci. 1992;13:657–660. doi: 10.1007/BF02334969. [DOI] [PubMed] [Google Scholar]