Abstract
Embryonic exposure to ethanol increases the risk for alcohol use disorder in humans and stimulates alcohol-related behaviors in different animal models. Evidence in rats and zebrafish suggests that this phenomenon induced by ethanol at low-moderate concentrations involves a stimulatory effect on neurogenesis and density of hypothalamic neurons expressing the peptides, hypocretin/orexin (Hcrt) and melanin-concentrating hormone (MCH), known to promote alcohol consumption. Building on our report in zebrafish showing that ethanol induces ectopic expression of Hcrt neurons outside the hypothalamus, we investigated here whether embryonic ethanol exposure also induces ectopic peptide neurons in rats similar to zebrafish, affects their morphological characteristics, and if these ectopic neurons are functional and have a role in the ethanol-induced disturbances in behavior. We demonstrate in rats that ethanol at a low-moderate dose, in addition to increasing Hcrt and MCH neurons in the lateral hypothalamus where they are normally concentrated, induces ectopic expression of these peptide neurons further anterior in the nucleus accumbens core and ventromedial caudate putamen where they have not been previously observed and causes morphological changes relative to normally located hypothalamic neurons. Similar to rats, embryonic ethanol exposure at a low-moderate dose in zebrafish induces ectopic Hcrt neurons anterior to the hypothalamus and alters their morphology. Notably, laser ablation of these ectopic Hcrt neurons blocks the behavioral effects induced by ethanol exposure, including increased anxiety and locomotor activity. These findings suggest that the ectopic peptide neurons are functional and contribute to the ethanol-induced behavioral disturbances related to the overconsumption of alcohol.
Keywords: Alcohol, Embryonic ethanol, Ectopic neurons
Graphical Abstract:
Embryonic ethanol exposure at low-moderate concentrations, while causing behavioral disturbances, increases density of neurons expressing hypocretin/orexin (Hcrt) or melanin-concentrating hormone (MCH) in the hypothalamus where they are normally located. Here we report in both rats and zebrafish that embryonic ethanol induces ectopic expression of Hcrt and MCH neurons with altered morphology outside the hypothalamus, and targeted ablation of these ectopic as well as normally located neurons in zebrafish blocks the ethanol-induced behavioral changes, demonstrating their function in mediating these behaviors.

1. Introduction
The risk for alcohol use disorder (AUD) and alcohol-related behaviors is increased by gestational exposure to alcohol1. This phenomenon is similarly demonstrated in different animal models and believed to involve disturbances in neuropeptide systems in the brain2,3. Recent studies in rodents have focused on the neuropeptides, hypocretin/orexin (Hcrt) and melanin-concentrating hormone (MCH), which are expressed in neurons concentrated in the lateral hypothalamus (LH)4,5 and play a role in promoting alcohol consumption and related behaviors including arousal, anxiety and motivation6–8. Embryonic ethanol at low-moderate doses in rats stimulates neurogenesis and increases the density of these Hcrt and MCH neurons in the LH while stimulating ethanol consumption and associated behaviors2,9, suggesting their function in mediating these behaviors.
Similar effects to these are also observed in studies of Hcrt neurons and ethanol-related behaviors in zebrafish3,10,11. Zebrafish have a well-conserved Hcrt system12 and are an especially useful model for investigating the neuronal changes induced by ethanol and other drugs13,14. As described in adult rats2,15, voluntary consumption of ethanol in adult zebrafish stimulates the expression of Hcrt neurons concentrated in the anterior hypothalamus (AH) while increasing locomotor and aggressive behaviors, and central injection of an Hcrt agonist itself increases the consumption of ethanol10. Further, embryonic ethanol exposure in zebrafish as in rats increases the expression, genesis and number of Hcrt neurons in the AH and voluntary ethanol consumption and other behaviors related to intake such as locomotor activity3,10,11. In both rats and zebrafish, there is some evidence that embryonic ethanol also affects the morphology of neurons, increasing their size and dendritic branching16,17. The migration of neurons in rodents and zebrafish is also affected by ethanol, which alters the timing and directionality of migration18,19 and causes neurons to fail to reach their final destination20, to be expressed in ectopic locations outside of their normal location11, and to cluster at inappropriate sites leading to the formation of heterotopias21. There is clear evidence that maternal ethanol consumption during pregnancy in humans causes heterotopias in the brains of children with fetal alcohol spectrum disorders (FASD)22. While these ethanol-induced heterotopias are believed to contribute to neurological disorders such as epilepsy23, little is known about the specific characteristics of the ectopic neurons and whether they are in fact functional and involved in mediating the behavioral disturbances induced by ethanol.
Thus, the goal of this report was to examine both rats and zebrafish and determine whether embryonic ethanol exposure at a low-moderate dose, in addition to stimulating the normally located hypothalamic peptide neurons, causes ectopic expression of these neurons and alters their morphology and whether these ectopic peptide neurons persist and are involved in ethanol-induced behavioral disturbances related to the overconsumption of alcohol.
2. Materials and Methods
2.1. Animals and housing
All animals were housed within a fully accredited AAALAC facility in accordance with protocols approved by The Rockefeller University Animal Care and Use Committee and consistent with the NIH Guide to the Care and Use of Laboratory Animals. Adult female and male Sprague-Dawley rats, obtained from Charles River Breeding Laboratories, were maintained under standard lighting conditions (22 °C, 12:12-h light–dark cycle with lights off at 7 am), with food (LabDiet Rodent Chow 5001) and filtered water available ad libitum. Rats were bred and the sex of the offspring was determined as previously described24,25. Only female offspring were examined based on our previously studies showing them to be significantly more affected than males by embryonic ethanol exposure2,26. Female newborn rats on postnatal day 9 (P9) or early-adolescent P30 offspring were sacrificed and perfused, and their brains were excised, fixed, and frozen as previously described27.
We also studied larval zebrafish (Danio rerio) between 6–12 days post-fertilization (dpf). These zebrafish included hcrt:EGFP of an AB/wild-type genetic background28 and vglut2a:DsRed29 transgenic lines of a wild-type genetic background, which were housed (12:12 hour light-dark cycle with lights on at 7 am) with recirculating water flow at a temperature between 28–29°C and a pH between 6.9–7.4 as previously described11. With MCH transgenic lines not available to us, we could only study the development of Hcrt peptide neurons in zebrafish. Since larval zebrafish do not exhibit sexual dimorphism30 and also have no sex-linked markers31, we were unable to test only female zebrafish.
2.2. Embryonic ethanol treatment
Pregnant rats as described previously2,26 received intraorally from embryonic day 10 (E10) to E15 either a 2 g/kg/d ethanol solution (30% v/v), referred to as “Ethanol”, or a control solution of maltose–dextrin, referred to as “Control”. The dosing regimen involved a 1 g/kg gavage occurring 2 hours after the start of the dark cycle and a second gavage of 1 g/kg occurring 7 hours later. There were no differences between groups in terms of the dam’s body weight (224 to 251 g), chow intake (60 to 80 kcal/d), and litter size (7 to 14), with no spontaneous abortions.
Embryonic exposure of zebrafish to ethanol was performed as described in our previous reports3,10. Briefly, at 22 hours post-fertilization (hpf), embryos were placed in a fresh solution of either 0.0% or 0.5% (vol/vol %) ethanol for 2 hours and then washed and returned to the incubator.
2.3. iDISCO brain clearing
Tissue clearing and whole mount immunolabeling of the entire brain of P9 female rat brains were performed as described32, to determine how embryonic ethanol affects the density and location of the entire populations of both Hcrt and MCH neurons. Brain samples were incubated for 7 days in primary antibodies, Rabbit anti-Orexin A (1:500, Cat. #H-003–30, Phoenix Pharmaceuticals Inc) or Rabbit anti-MCH (1:500, Cat. #H-070–47, Phoenix Pharmaceuticals Inc) and then for 7 days in a secondary antibody, Donkey anti-Rabbit Alexa Fluor 647 (1:200, Cat # ab150075, Abcam).
2.4. Immunofluorescence
Immunofluorescence (IF) was performed in both rat brain sections and whole zebrafish brains. Briefly, as described previously2, female P9 and P30 rat brains were cut at 30 μm with a cryostat, and free-floating coronal sections containing the LH and forebrain regions where peptide neurons were detected were incubated in the primary antibodies, Rabbit anti-Orexin A (1:500) with mouse anti-NeuN (1:100, Cat. #MAB377, EMD Millipore) or Rabbit anti-MCH (1:1000) with mouse anti-NeuN (1:100) for 72 hours. The sections were then incubated for 90 min in a combination of Biotin-conjugated Horse anti-Rabbit (1:100, Cat. #: BA-1100, Vector laboratories, Inc.) for Hcrt and MCH, and Cy3-conjugated Donkey anti-Mouse (1:100, Cat. #: 715–165-151, Jackson Immuno Research Labs) for NeuN. This was followed by 30 min incubation in streptavidin-HRP (1:100, Perkin Elmer) and 5 min incubation in fluorescein tyramide (1:50, Perkin Elmer) to amplify Hcrt and MCH immunolabeling. Sections were counterstained with DAPI.
For IF in zebrafish, hcrt:EGFP fish at 12 dpf were fixed overnight at 4°C in 4% PFA in PBST (1% Tween-20), and whole brains were excised for IF labeling as previously described33,34. Briefly, samples were incubated overnight in Chicken anti-GFP primary antibody (1:500, Cat # GFP-1010, Aves Labs) and Mouse anti-HuC/D antibody (1:500, Cat #A-21271, Invitrogen). This was followed by overnight incubation in Alexa fluor 488 anti-Chicken secondary antibody (1:500; Invitrogen, A32931) and Alexa fluor 647 anti-Mouse secondary antibody (1:100; Abcam, ab150111) with DAPI (1:200).
2.5. Zebrafish brain registration
Live transgenic 6 dpf hcrt:EGFP zebrafish were crossed with vglut2a:DsRed zebrafish that exhibit pan-neuronal expression and permit alignment and registration of brain images to an annotated brain atlas, to identify the anatomical location and size of each Hcrt neuron, as we have previously reported11. We used Advanced Normalization Tools (ANTs) software35 on the Rockefeller University’s High-Performance Computing cluster to perform registrations and align brains using parameters as described36. The registered brains for Ethanol and Control zebrafish were averaged and then aligned to the Zebrafish Brain Browser36 to evaluate the anatomical location of each Hcrt neuron. We were unable to use DAPI staining within this experiment due to the use of live zebrafish. Overlays of n = 4 registered images were created for each group, and each image was analyzed individually.
2.6. Microscopy and image analysis
iDISCO samples of P9 rat brains were imaged on a light-sheet microscope (Ultramicroscope II, LaVision Biotec), using a 4x objective lens with a z step size of 2.5 μm. Whole-brain images were then analyzed using Imaris 9.8.0 software (Bitplane). In addition to examining the LH where peptide neurons are most concentrated4,5, experimenters blind to testing conditions, with no a priori evidence guiding them, reviewed manually each whole-brain image searching for Hcrt and MCH expression in regions outside the hypothalamus and detected ectopic peptide neurons only in the nucleus accumbens core (NAcC) and ventromedial caudate putamen (vmCP). These two forebrain areas together with the LH were then cropped with the ‘Surface’ function in Imaris software, using dimensions derived from a rat brain atlas37. The ‘Spots’ function in Imaris was used for counts of Hcrt and MCH neurons in the LH, NAcC and vmCP, which were then divided by the volumes of these areas to determine neuron density. The IF samples of P9 rat brain sections containing the LH, NAcC and vmCP were then imaged with a 40x objective lens on a LSM 780 inverted laser scanning confocal microscope (Zeiss), with a z step of 1.0 μm. These IF images were then analyzed in Imaris software to quantify neuron density and size, and processes seen emanating from the soma of each peptide neuron were quantified manually. It is possible that some of the smaller neuronal processes may not have been detected, due to an insufficient imaging resolution or having been removed by the brain sectioning procedure. Based on previous anatomical studies4,5, Hcrt and MCH neurons localized in the LH were defined as normally located neurons, while neurons outside of their normal location detected in the NAcC and vmCP were defined as ectopic.
Live 6 dpf zebrafish brains were imaged with a 25x objective lens on a LSM 780, with a z step of 1.0 μm. These 6 dpf brain images were individually analyzed in Imaris software using the ‘Spots’ and ‘Surface’ functions, to quantify total Hcrt neuron number and Hcrt neuron size. Based on previous anatomical studies12, Hcrt neurons localized in the AH were defined as normally located neurons, while Hcrt neurons detected in the preoptic area (POA) anterior to the AH were defined as ectopic. IF samples of 12 dpf zebrafish brains were imaged with a 40x objective lens on a LSM 780 with a z step of 1.0 μm. Images were then analyzed in Imaris software using the ‘Spots’ function for number of Hcrt neurons and ‘Surface’ function for Hcrt size, and processes emanating from the soma of each neuron were quantified manually.
2.7. Laser ablation
Laser ablation was performed as previously described38 with minor modifications. Control and Ethanol zebrafish at 8 dpf were anesthetized and mounted in 1.5% LMP agarose containing 15 mM Tricaine on a microscope slide. For ablation of ectopic POA Hcrt neurons, we targeted the 3–4 neurons located on both left and right sides at least 20 μm away from the most anterior AH Hcrt neurons, and for normally located AH Hcrt neuron ablation, we targeted 3–4 Hcrt neurons that were in the most anterior region of the AH. We ablated only 3–4 neurons in each zebrafish to avoid heat damage to surrounding tissue and cells caused by repeated laser applications39, yielding a survival rate of approximately 90%. The laser ablations were performed with a bleaching function using a Zeiss LSM 880 microscope with a 40x objective. This was accomplished by scanning 100 iterations within each region of interest, which was manually drawn around the soma of each targeted Hcrt neuron with a 800-nm beam delivered from a Coherent laser. Laser power was attenuated with neutral gray filters to avoid excessive tissue damage, and confocal z stack images were acquired both before and after laser ablation. After ablation, larvae were carefully removed from agarose and placed in embryo water to recover overnight (~20 hours) and were then used for behavioral testing the following day at 9 dpf.
2.8. Behavioral testing
Following the 0.0% or 0.5% ethanol treatment and ablation, zebrafish at 9 dpf underwent testing within a Noldus (Wageningen, Netherlands) DanioVision behavior chamber to measure both anxiety-like and locomotor behaviors40. Behavior was analyzed for the following groups: “Control” (no mounting or ablation) and “Ethanol” (no mounting or ablation) to control for mounting and ablation, “Control Mounted” (no ablation) and “Ethanol Mounted” (no ablation) to control for mounting, “Control AH Ablation” and “Ethanol AH Ablation” to test the effects of ablation of normally located AH neurons, and “Ethanol POA Ablation” to test the effects of ectopic Hcrt ablation in the POA. Zebrafish were individually transferred from their home tanks into a well of a standard 12-well culture plate containing fresh embryo media, and this plate was immediately placed into the behavior chamber. Zebrafish first underwent a 10-minute free swimming test, to assess locomotor activity by measuring distance traveled and also thigmotaxis by measuring percentage of time spent in the perimeter of the arena to examine anxiety-like behavior41. Zebrafish were then returned to the incubator for 1 hour of recovery and put into a new 12-well plate for a 10-minute light-dark preference test to assess light preference as measured by the percentage of time spent in the light half of the environment which indicates anxiety in larval zebrafish42. Zebrafish activity was tracked by Noldus Ethovision XT 16 software, followed by manual correction of swim tracks if necessary.
2.9. Statistical analyses
All iDISCO and IF data from P9 rats evaluating Hcrt and MCH neuron density, size, and number of processes were analyzed using unpaired t-tests for measures obtained from the LH, NAcC and vmCP. All IF data obtained from P30 rats were analyzed using a one-way ANOVA, followed by Dunnett’s post-hoc multiple comparisons test. Zebrafish live-imaging, IF and behavioral data were analyzed using one-way ANOVA, followed by Dunnet’s or Sidak’s post-hoc test. Normality of each group was assessed using Shapiro–Wilk and Kolmogorov–Smirnov tests. All tests were two-tailed, and significance was determined at p < 0.05. All data were analyzed using Prism (version 8, GraphPad, San Diego, CA) and are presented as mean ± SEM in the figures and table.
3. Results
3.1. Embryonic ethanol exposure increases density and alters morphology of peptide neurons in LH of newborn rats.
Using iDISCO tissue clearing to examine the entire population of peptide neurons throughout the brain and IF to characterize their morphology, we first tested in P9 rats whether embryonic ethanol exposure at a low-moderate concentration affects the density and morphology of Hcrt and MCH neurons in the hypothalamus, specifically the LH where they are most concentrated4,5, followed by an analysis of areas outside the hypothalamus to determine if ethanol induces ectopic peptide neurons as shown in zebrafish11. With iDISCO, we found that ethanol increased the density throughout the whole LH of neurons expressing Hcrt (Figure 1A; Ethanol LH = 4.38 × 10−4 ± 0.55 vs Control LH = 2.02 × 10−4 ± 0.55; t (6) = 3.023, p = 0.023) or MCH (Figure 1B; Ethanol LH = 3.91 × 10−4 ± 0.24 vs Control LH = 2.54 × 10−4 ± 0.48; t (6) = 2.553, p = 0.043). We then confirmed these results in the LH using IF, as illustrated in the photomicrographs of Hcrt (Figures 1C) and MCH (Figure 1D) neurons. In addition to increasing the density of LH Hcrt neurons (Figure 1E; t (4) = 3.794, p = 0.019), Ethanol compared to Control increased their size (Figure 1F; t (296) = 4.29, p = 0.0001) and number of processes emanating from the soma (Figure 1G; t (263) = 3.71, p = 0.0003), as shown in the single-cell enlargements within each photomicrograph (Figures 1C, D). Similarly, it increased the density (Figure 1H; t (4) = 4.941, p = 0.007)) and size (Figure 1I; t (502) = 8.075, p = 0.0001) of MCH neurons in the LH, although not their number of processes (Figure 1J; t (401) = 1.953, p = 0.051).
Figure 1:

Embryonic ethanol exposure (2 g/kg/day) from embryonic day 10 (E10) to E15 increases the density and alters the morphology of Hcrt and MCH neurons in lateral hypothalamus (LH) of female postnatal day 9 (P9) rats. (A, B) Representative photomicrographs (4x) of entire population of Hcrt (green) and MCH (red) neurons in LH, stained using iDISCO clearing, illustrate the increase in peptide neuron density in Ethanol compared to Control rats. Scale bar, 50 μm. (C, D) Photomicrographs (10x) of Hcrt (green) and MCH (red) neurons in LH, stained on brain sections using IF, similarly illustrate the increase in neuron density in Ethanol compared to Control rats, with enlargements (upper left corners) of individual Hcrt and MCH neurons from small white dotted boxes showing the ethanol-induced changes in neuron size and number of processes (indicated by white arrowheads). Scale bar, 30 μm. (E, F, G). Bar graphs (mean ± SEM, n = 3–4/group) indicate that Ethanol compared to Control increases the density and size of Hcrt neurons in LH and number of processes per Hcrt neuron stained using IF. (H, I, J) Bar graphs (mean ± SEM, n = 3–4/group) show that Ethanol compared to Control rats increases the density and size of MCH neurons in LH stained using IF, but not the number of processes per MCH neuron. *p < 0.05, **p < 0.01, ****p < 0.001, ****p < 0.0001. Abbreviations: Hcrt: Hypocretin/Orexin; IF: Immunofluorescence; LH: lateral hypothalamus; MCH: Melanin-concentrating hormone; P9: Postnatal day 9.
3.2. Embryonic ethanol exposure induces ectopic Hcrt and MCH neurons with altered morphology in newborn rats.
In the same whole-brain images collected from iDISCO rat samples as above, we next identified distinct populations of ectopic peptide-expressing neurons in two important areas, the NAcC and nearby vmCP, of ethanol-exposed rats. Analysis of the NAcC (Figure 2A) revealed Hcrt neurons in Ethanol rats that were not detected in Control rats and were less dense than Hcrt neurons in the LH of Ethanol rats (Figure 2B; Ethanol NAcC = 0.021 × 10−4 ± 0.007 vs Ethanol LH = 4.38 × 10−4 ± 0.55; t (6) = 7.504, p = 0.0003). These ectopic Hcrt neurons in the NAcC were confirmed in IF brain sections (Figure 2C), where they were found to co-label NeuN (Figure 2D, Boxes 1–2), indicating they are mature neurons. These ethanol-induced Hcrt neurons in the NAcC compared to those in the LH of Ethanol rats, in addition to being less dense (Figure 2E; t (5) = 5.995, p = 0.002), were smaller in size (Figure 2F; t (210) = 4.815, p = 0.0001) and had fewer processes emanating from the soma (Figure 2G; t (204) = 7.643, p = 0.0001), as shown in the single-cell enlargements (Figure 2D, Box 2). Further analysis of iDISCO images similarly revealed ectopic MCH neurons in the same NAcC area of the Ethanol but not Control rats that were less dense than the MCH neurons in the LH of Ethanol rats (Figure 2H; Ethanol NAcC = 0.021× 10−4 ± 0.004 vs Ethanol LH = 3.91 × 10−4 ± 0.24; t (5) = 12.89, p = 0.0001). These findings were confirmed using IF (Figure 2I), which showed these ectopic MCH neurons to co-label NeuN (Figure 2J, Boxes 3–4), to be less dense than the LH neurons (Figure 2K; t (5) = 7.175, p = 0.0008), smaller in size (Figure 2L; t (335) = 2.132, p = 0.033), and to have fewer processes (Figure 2M; t (313) = 3.572, p = 0.0004), as shown in the single-cell enlargements (Figure 2J, Box 4).
Figure 2:

Embryonic ethanol exposure (2 g/kg/day) from embryonic day 10 (E10) to E15 induces ectopic Hcrt and MCH neurons with altered morphology in nucleus accumbens core (NAcC) of female postnatal day 9 (P9) rats. (A) Coronal brain section showing the NAcC at the anterior-posterior level of bregma A5.9 mm38. (B) Representative photomicrographs (4x) illustrate ectopic Hcrt neurons (green) in NAcC of Ethanol rats, stained using iDISCO clearing and not detected in Control rats. Scale bar, 15 μm. (C) Photomicrographs (10x) illustrate ectopic Hcrt neurons (green) in NAcC of Ethanol rats, stained in brain sections using IF and not detected in Control rats. Scale bar, 30 μm. (D) Photomicrographs (40x) of brain sections stained using IF illustrate ectopic Hcrt (green) neurons in the NAcC of Ethanol rats, which when enlarged in white dotted Box 1 and further enlarged in Box 2 show co-labeling with NeuN (white) and illustrate their size and neuronal process (identified by white arrowhead). Scale bars, 80 μm (low magnification) and 10 μm (high magnification). (E, F, G) Bar graphs (mean ± SEM, n = 3–4/group) show ectopic Hcrt neurons in NAcC of Ethanol rats, which are less dense than LH neurons in the same Ethanol rats and also smaller in size and have fewer processes per neuron as shown using IF. (H) Representative photomicrographs (4x) illustrate ectopic MCH neurons (red) in NAcC of Ethanol rats, stained using iDISCO clearing and not detected in Control rats. Scale bar, 15 μm. (I) Photomicrographs (10x) illustrate ectopic MCH neurons (red) in NAcC of Ethanol rats, stained in brain sections using IF and not detected in Control rats. Scale bar, 30 μm. (J) Photomicrographs (40x) of brain sections stained using IF illustrate ectopic MCH (red) neurons in the NAcC of Ethanol rats, which when enlarged in white dotted Box 3 and further enlarged in Box 4 show co-labeling with NeuN (white) and illustrate their size and neuronal process (identified by white arrowhead). Scale bars, 80 μm (low magnification) and 10 μm (high magnification). (K, L, M) Bar graphs (mean ± SEM, n = 3–4/group) show ethanol-induced ectopic MCH neurons in NAcC of Ethanol rats, which as with Hcrt are less dense than LH neurons in Ethanol rats and are smaller in size and have fewer processes per neuron as shown using IF. *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001. Abbreviations: Hcrt: Hypocretin/Orexin; IF: Immunofluorescence; MCH: Melanin-concentrating hormone; NAcC: Nucleus Accumbens Core; P9: Postnatal day 9.
Similar results were obtained for ectopic peptide neurons detected in the nearby vmCP (Figure 3A). Analysis of Hcrt in the iDISCO images of the vmCP revealed neurons in the Ethanol but not Control rats that were less dense than in the LH (Figure 3B; Ethanol vmCP = 0.016 × 10−4 ± 0.002 vs Ethanol LH = 4.38 × 10−4 ± 0.55; t (6) = 7.618, p = 0.0003), and these neurons were confirmed in IF brain sections (Figure 3C) and found to co-label NeuN (Figure 3D, Boxes 1–2). Compared to Hcrt neurons in the LH of Ethanol rats, these ectopic Hcrt neurons in the vmCP, in addition to being less dense (Figure 3E; t (4) = 3.974, p = 0.016), were smaller in size (Figure 3F; t (206) = 3.956, p = 0.0001) and had fewer processes from the soma (Figure 3G; t (224) = 8.568, p = 0.0001), as shown in the single-cell enlargements (Figure 3D, Box 2). The iDISCO images similarly revealed ectopic MCH neurons in the vmCP of Ethanol but not Control rats that were also less dense than in the LH (Figure 3H; Ethanol vmCP = 0.012 × 10−4 ± 0.001 vs Ethanol LH = 3.91 × 10−4 ± 0.24; t (5) = 13.28, p = 0.0001). These results were confirmed using IF (Figure 3I) with the MCH neurons co-labeling NeuN (Figure 3J, Boxes 3–4), and while less dense than LH neurons (Figure 3K, t (4) = 4.593, p = 0.010), they were smaller in size (Figure 3L, t (352) = 4.030, p = 0.0001) and had fewer processes (Figure 3M; t (328) = 3.70, p = 0.0003), as shown in the single-cell enlargements (Figure 3J, Box 4).
Figure 3:

Embryonic ethanol exposure (2 g/kg/day) from embryonic day 10 (E10) to E15 induces ectopic Hcrt and MCH neurons with altered morphology in the ventromedial caudate putamen (vmCP) of female postnatal day 9 (P9) rats. (A) Coronal brain section showing the vmCP at the anterior-posterior level of bregma A5.9 mm in rat brain atlas38. (B) Representative photomicrographs (4x) illustrate ectopic Hcrt neurons (green) in vmCP of Ethanol rats, stained using iDISCO clearing and not detected in Control rats. Scale bar, 15 μm. (C) Photomicrographs (10x) illustrate ectopic Hcrt neurons (green) in vmCP of Ethanol rats, stained in brain sections using IF and not detected in Control rats. Scale bar, 30 μm. (D) Photomicrographs (40x) of brain sections stained using IF illustrate ectopic Hcrt (green) neurons in the vmCP of Ethanol rats, which when enlarged in white dotted Box 1 and further enlarged in Box 2 show co-labeling with NeuN (white), and illustrate their size and neuronal process (identified by white arrowhead). Scale bars, 80 μm (low magnification) and 10 μm (high magnification). (E, F, G) Bar graphs (mean ± SEM, n = 3–4/group) show ectopic Hcrt neurons in vmCP of Ethanol rats, which are less dense than Ethanol LH neurons and are smaller in size and have fewer processes per neuron shown using IF. (H) Representative photomicrographs (4x) illustrate ectopic MCH neurons (red) in vmCP of Ethanol rats, stained using iDISCO clearing and not detected in Control rats. Scale bar, 15 μm. (I) (C) Photomicrographs (10x) illustrate ectopic MCH neurons (red) in vmCP of Ethanol rats, stained in brain sections using IF and not detected in Control rats. Scale bar, 30 μm. (J) Photomicrographs (40x) of brain sections stained using IF illustrate ectopic MCH (red) neurons in the vmCP of Ethanol rats, which when enlarged in white dotted Box 3 and further enlarged in Box 4 show co-labeling with NeuN (white) and illustrate their size and neuronal process (identified by white arrowhead). Scale bars, 80 μm (low magnification) and 10 μm (high magnification). (K, L, M) Bar graphs (mean ± SEM, n = 3–4/group) show ectopic MCH neurons in vmCP of Ethanol rats, which similar to Hcrt neurons are less dense than Ethanol LH neurons and are smaller in size and have fewer processes per neuron shown using IF. *p < 0.05, **p < 0.01, ****p < 0.0001. Abbreviations: Hcrt: Hypocretin/Orexin; IF: Immunofluorescence; MCH: Melanin-concentrating hormone; V: Ventricle; P9: Postnatal day 9; vmCP: ventromedial caudate putamen.
3.3. Ectopic Hcrt and MCH neurons in the NAcC and vmCP persist and are still evident in early-adolescent rats.
To determine if the ethanol-induced ectopic Hcrt and MCH neurons with altered morphology are long lasting, we next examined using IF the LH, NAcC and vmCP of brains obtained from early-adolescent P30 female rats. We found that Ethanol compared to Control increased the density of Hcrt neurons (Figure 4A; F (3,10) = 3.69, p = 0.008) in the LH where they are normally located and also the size of these neurons (Figure 4B; F (3,86) = 1.007, p = 0.001) and number of processes from their soma (Figure 4C; F (3,86) = 0.473, p = 0.036). It also induced ectopic Hcrt neurons in both the NAcC and vmCP that were not evident in Control brains, as illustrated in the photomicrographs (Figure 4D). Compared to LH neurons in Ethanol rats, these ectopic Hcrt neurons in the NAcC while less dense (Figure 4A; p = 0.0001) were smaller in size (Figure 4B; p = 0.003) and had fewer processes (Figure 4C; p = 0.004), and those in the nearby vmCP were also less dense (Figure 4A; p = 0.0005) and smaller in size (Figure 4B; p = 0.002) and had fewer processes (Figure 4C; p = 0.0001), as shown in the single-cell enlargements within each photomicrograph (Figure 4D). Our analysis of MCH neurons in the LH of early-adolescent rats revealed similar results as in newborn rats, showing that Ethanol compared to Control increased their density (Figure 4E; F (3,17) = 1.151, p = 0.0001) and size (Figure 4F; F (3,137) = 1.658, p = 0.032) but not their number of processes (Figure 4G; F (3,198) = 2.279, p = 0.838), and it induced ectopic MCH neurons in the NAcC and vmCP that were not evident in Control brains, as shown in the photomicrographs (Figure 4H). Once again, compared to LH neurons in ethanol rats, these ectopic MCH neurons in the NAcC were reduced in density (Figure 4E; p = 0.0001), size (Figure 4F; p = 0.0001), and processes (Figure 4G; p = 0.0001), as were those in the vmCP which were less dense (Figure 4E; p = 0.0001) and smaller in size (Figure 4F; p = 0.024) and had fewer processes (Figure 4G; p = 0.001), as shown in the single-cell enlargements within each photomicrograph (Figure 4H).
Figure 4:

The ectopic Hcrt and MCH neurons seen in newborn rats after ethanol exposure (2 g/kg/day) from embryonic day 10 (E10) to E15 are long lasting, similarly evident with altered morphology in nucleus accumbens core (NAcC) and ventromedial caudate putamen (vmCP) of early-adolescent female rats on postnatal day 30 (P30). (A, B, C) As shown using IF in newborn rats, bar graphs (mean ± SEM, n = 3–4/group) show that Ethanol compared to Control increases in early-adolescent rats the density, size and processes of Hcrt neurons in LH, and compared to these LH neurons, the ectopic Hcrt neurons in NAc and vmCP of Ethanol rats not detected in Control rats are reduced in density, are smaller in size, and have fewer processes. (D) Photomicrographs (40x) of Hcrt neurons (green) stained in brain sections using IF illustrate the increase in density in LH of Ethanol compared to Control rats and the ectopic neurons in the NAcC and vmCP detected in Ethanol but not Control rats. Individual Hcrt neurons in white dotted boxes are further enlarged (upper left corners) to illustrate the ethanol-induced changes in neuron size and number of processes (indicated by white arrowheads). Scale bar, 20 μm. (E, F, G) As shown using IF in newborn rats, bar graphs (mean ± SEM, n = 4–5/group) show that Ethanol compared to Control increases in early-adolescent rats the density, size and number of processes of MCH neurons in LH, and compared to these LH neurons, the ectopic MCH neurons in NAc and vmCP of Ethanol rats not detected in Control rats are reduced in density, are smaller in size, and have fewer processes. (H) (D) Photomicrographs (40x) of MCH neurons (red) stained in brain sections using IF illustrate the increase in density in LH of Ethanol compared to Control rats and the ectopic neurons in the NAcC and vmCP detected in Ethanol but not Control rats. Individual MCH neurons in white dotted boxes are further enlarged (upper left corners) to illustrate the ethanol-induced changes in neuron size and number of processes (indicated by white arrowheads). Scale bar, 20 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Abbreviations: Hcrt: Hypocretin/Orexin; IF: Immunofluorescence; LH: lateral hypothalamus; MCH: Melanin-concentrating hormone; NAcC: Nucleus Accumbens Core; P30: Postnatal day 30; vmCP: ventromedial Caudate Putamen.
3.4. Embryonic ethanol exposure similarly induces ectopic Hcrt neurons with altered morphology in zebrafish.
We next tested in zebrafish if a low-moderate concentration of ethanol (0.5% v/v) produces, similar to the rat, ectopically located Hcrt neurons with altered morphology. Using live-imaging, we registered whole-brain 6 dpf images to the Zebrafish Brain Browser36 to evaluate the precise anatomical location of each Hcrt neuron. In the AH where they are normally concentrated, Ethanol compared to Control increased the density of Hcrt neurons (Figure 5A; F (2,11) = 1.139, p = 0.002) and also the size of these neurons (Figure 5B; F (2,11) = 1.098, p = 0.0001). As previously reported11, Ethanol also induced ectopic Hcrt neurons not evident in Control zebrafish, causing 15–20% of the total population of Hcrt neurons to be located outside their normal location in the AH, further anterior within the POA up to 50 uM beyond the border of the AH (Figure 5C, D, Boxes 1–2). As in the rat, these ectopically located Hcrt neurons, in addition to being less dense (Figure 5A; p = 0.0001) than those in the AH of Ethanol zebrafish, were also significantly smaller in size (Figure 5B; p = 0.0001).
Figure 5:
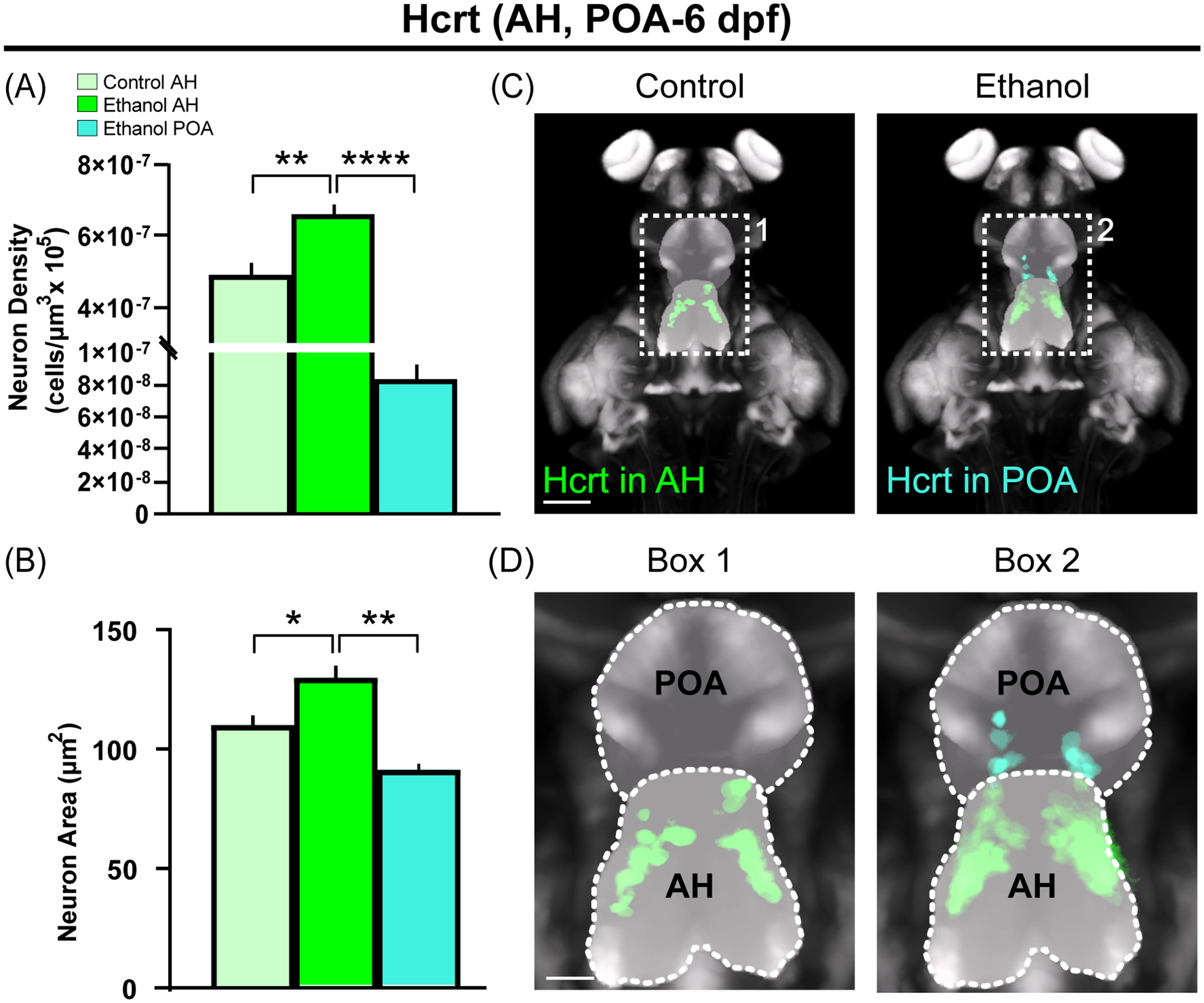
Embryonic ethanol exposure (0.5% v/v) early in development (22–24 hpf), while increasing the density of Hcrt neurons in the anterior hypothalamus (AH) where they are normally located, induces in zebrafish at 6 dpf ectopic Hcrt neurons with altered morphology in the preoptic area (POA) located anterior to the AH. (A, B) Bar graphs (mean ± SEM, n = 4/group) show that Ethanol compared to Control increases in zebrafish the density and size of Hcrt neurons in AH, and compared to these Ethanol AH neurons, the ectopic Hcrt neurons in the POA of Ethanol zebrafish are lower in density and smaller in size. (C) Photomicrographs (25x) show an overlay of n = 4 images for each condition of zebrafish brains at 6 dpf obtained using live-imaging, with pan-neuronal vglut2a:DsRed expression (grey) shown together with Hcrt neurons in AH (green) of Ethanol compared to Control zebrafish and also ectopic Hcrt neurons revealed in POA (cyan) of Ethanol zebrafish. (D) Enlargements of Box 1 and Box 2 show the outline of the AH and POA as derived from the Zebrafish Brain Browser37. Scale bars: 100 μm (low magnification); 20 μm (high magnification). *p < 0.05, **p < 0.01, ****p < 0.0001. Abbreviations: AH: anterior hypothalamus; dpf: days post fertilization; Hcrt: Hypocretin/Orexin; POA: Preoptic area.
Using IF with excised brains of zebrafish at 12 dpf, we next found that the effect of embryonic ethanol exposure on ectopic neurons persists into this older age. We observed effects of ethanol at 12 dpf very similar to those in the younger zebrafish and in the rat. Ethanol compared to Control increased in the AH the density (Figure 6A; F (2,11) = 1.607, p = 0.001) and size (Figure 6B; F (2,11) = 1.311, p = 0.0001) of Hcrt neurons and the number of processes emanating from the soma of these AH neurons (Figure 6C; F (2,279) = 8.292, p = 0.035), and it also caused some of the Hcrt neurons to be ectopically located outside the AH, within the POA up to 50 uM anterior to the AH, which compared to Hcrt neurons normally located in the AH of Ethanol zebrafish were less dense (Figure 6A; p = 0.0001) and smaller in size (Figure 6B; p = 0.0001), and the number of their processes per neuron were apparently but not significantly reduced (Figure 6C; p = 0.535). The increase in Hcrt density within the AH induced by Ethanol compared to Control and the presence of ectopically located POA neurons detected in the Ethanol but not Control group are illustrated in the photomicrographs (Figure 6D), with the effects of ethanol on their size and processes shown in single-cell enlargements of neurons located in the AH and POA (upper left and right corners). Essentially all of the Hcrt neurons in the AH and POA were found to co-label the neuronal differentiation marker HuC (Figure 6E) as shown in the single-cell enlargements (upper left and right corners), indicating that they are mature neurons.
Figure 6:
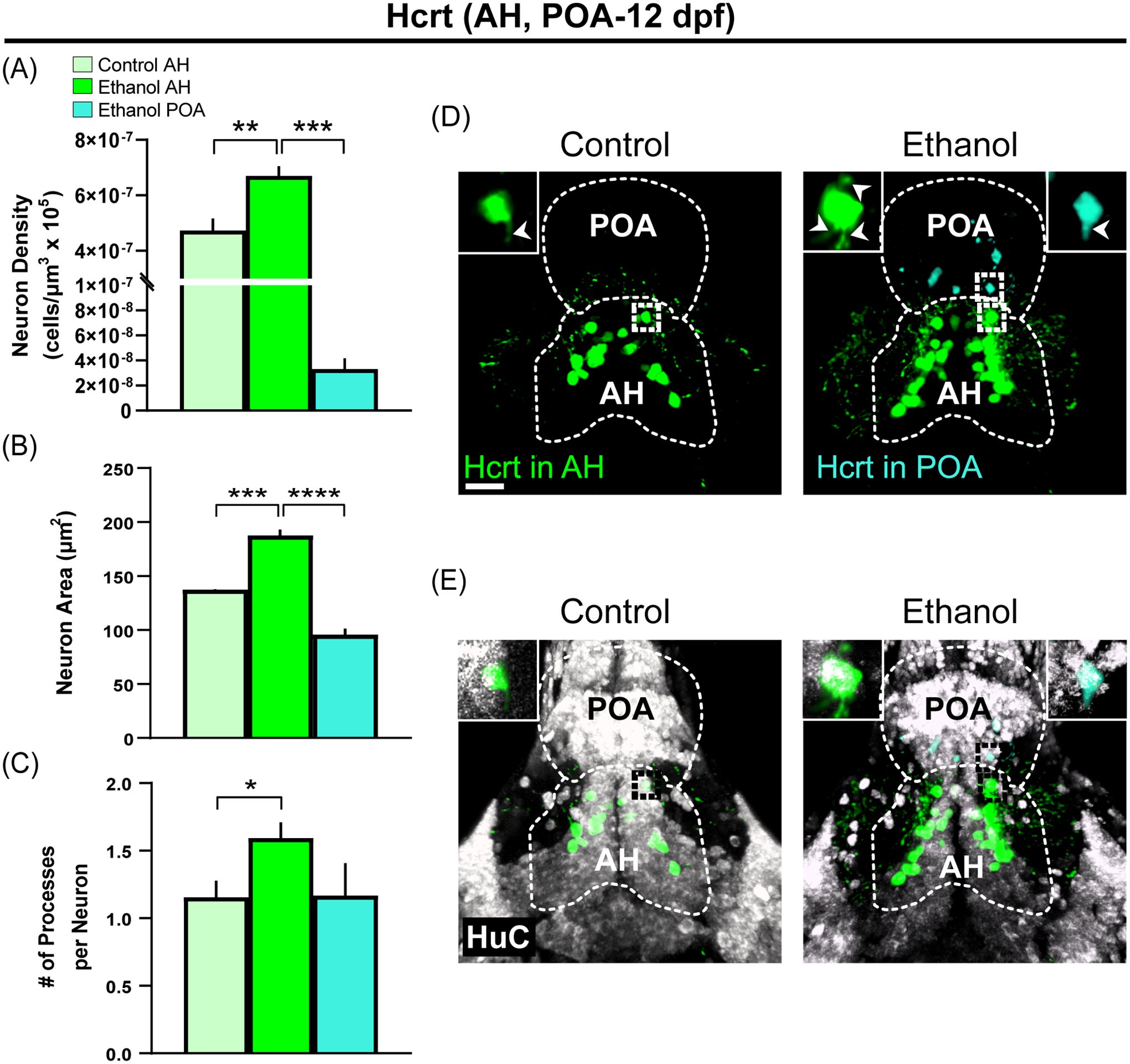
Ectopic Hcrt neurons, induced with altered morphology in Hcrt-EGFP zebrafish by embryonic ethanol exposure (0.5% v/v) in the preoptic area (POA) anterior to AH where they are normally concentrated, are similarly observed and found to persist into an older age at 12 dpf. (A, B, C) Bar graphs (mean ± SEM, n = 4–5/group) show that Ethanol compared to Control increases in 12 dpf zebrafish the density, size and number of processes per Hcrt neurons in AH, and compared to these Ethanol AH neurons, the ectopic Hcrt neurons in Ethanol POA are less dense and smaller in size and show a tendency toward reduced processes not statistically significant. (D) Photomicrographs (25x) of the 12 dpf zebrafish brain using IF show the ethanol-induced increase compared to control in density of Hcrt neurons (green) normally located in the AH and the ectopic Hcrt neurons (cyan) observed in the POA only after ethanol. Individual Hcrt neurons in white dotted boxes are further enlarged for the AH (upper left corners) and POA (upper right corner) to illustrate the ethanol-induced changes in neuron size and number of processes (indicated by white arrowheads). Scale bar, 20 μm. (E) Photomicrographs (25x) of the 12 dpf zebrafish brain using IF show staining of the neuronal marker HuC (white) and its double labeling with Hcrt neurons located in the AH (green) and POA (cyan). Individual neurons in black dotted boxes are further enlarged for the AH (upper left corners) and POA (upper right corner) to illustrate double labeling with HuC. Scale bar, 20 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Abbreviations: AH: anterior hypothalamus; dpf: days post fertilization; Hcrt: Hypocretin/Orexin; POA: Preoptic area.
3.5. Targeted ablation of ectopic peptide neurons in zebrafish blocks ethanol-induced behaviors.
To determine if these ectopic Hcrt neurons in the POA have specific behavioral functions along with the normally located neurons in the AH, we examined in larval zebrafish the effect of embryonic ethanol exposure on different behaviors known to be related to the consumption of ethanol and used targeted laser ablation of these neurons to determine their involvement in mediating these ethanol-induced behaviors. Showing that Ethanol compared to Control increased anxiety-like behaviors consistent with prior reports3 as revealed by increased thigmotaxis (Figure 7A; F (6,78) = 1.480, p = 0.048) and light preference (Figure 7B; F (6,77) = 0.787, p = 0.007), we found the ethanol-induced increase in these behaviors to be blocked by laser ablation of the ectopic Hcrt neurons in the POA of the Ethanol POA Ablation group (Figure 7A, B, E) for both thigmotaxis (p = 0.014) and light preference (p = 0.0002) and also by ablation of the same number of Hcrt neurons in the AH of the Ethanol AH Ablation group (Figure 7A, B, E) for both thigmotaxis (p = 0.040) and light preference (p = 0.029). Ethanol compared to Control, in addition to inducing ectopic Hcrt neurons in the POA (Figure 7D), also increased locomotor activity (Figure 7C; F (6,79) = 1.455, p = 0.037) as previously reported43, and notably, this ethanol-induced increase in locomotor behavior was totally blocked only by laser ablation of the ectopic POA Hcrt neurons in the Ethanol POA ablation group (Figure 7C, E; p = 0.0143) while unaffected by ablation of the normally located neurons in the AH of the Ethanol AH ablation group (Figure 7C, E; p = 0.999). Thus, in contrast to the anxiety-like behaviors which appear to involve both the AH and POA neurons, this result suggests that the ethanol-induced increase in locomotor activity is mediated specifically by the ectopic Hcrt neurons in the POA. These conclusions are further supported by our findings in additional zebrafish control groups, which show no changes in the behaviors induced by agarose mounting itself in the Control Mounted or Ethanol Mounted groups or by the ablation process itself in the Control AH Ablation group (Table 1).
Figure 7:

Ethanol exposure (0.5% v/v) during early embryonic development (22–24 hpf), while increasing Hcrt neurons in AH and POA, produces changes in anxiety-like and locomotion behaviors in zebrafish at 9 dpf that are altered by targeted laser ablation of the Hcrt neurons in POA and AH. (A,B) Bar graphs (mean ± SEM, n = 9–15/group) show that Ethanol increases anxiety-like behaviors, indicated by an increase in perecentage of time spent in the perimeter of the arena (thigmotaxis) and in the light half of the arena during the light-dark test (light preference), and these behavioral effects are blocked by laser ablation of the ectopic Hcrt neurons (3–4) in POA of the Ethanol POA Ablation group and also by ablation of normally located Hcrt neurons (3–4) in the AH of Ethanol AH Ablation group. (C) Bar graphs (mean ± SEM) show that Ethanol compared to Control also increases locomotor behavior, as indicated an increase in distance traveled, and this effect is blocked by laser ablation of ectopic POA Hcrt neurons (3–4) in Ethanol POA Ablation group but unaffected by laser ablation of the same number of Hcrt neurons in AH in Ethanol AH Ablation group. (D) Representative photomicrographs of non-ablated zebrafish brains at 8 dpf show Hcrt neurons in their normal location in AH (green) of the Ethanol and Control groups and ectopic Hcrt neurons in POA (cyan) of the Ethanol group. Scale bar, 10 μm. (E) Represetnative photomicrographs of zebrafish from Ethanol AH Ablation and Ethanol POA Ablation groups illustrate before and after ablation of Hcrt neurons in the AH (green) where they are normally located or ectopic Hcrt neurons in the POA (cyan). Before ablation, white arrowheads indicate normally located Hcrt neurons in AH of Ethanol AH Ablation group or ectopic Hcrt neurons in POA of Ethanol POA Ablation group, which were targeted for ablation, and then after ablation, arrows indicate the absence of these specific neurons. Scale bar: 10 μm. *p < 0.05, **p < 0.01, ***p < 0.001. Abbreviations: AH: anterior hypothalamus; dpf: days post fertilization; Hcrt: Hypocretin/Orexin; POA: Preoptic area.
Table 1:
Behavioral results from additional zebrafish control groups.
| Control a Mean ± SEM |
Control Mounted b Mean ± SEM |
p value | Control a Mean ± SEM |
Control AH Ablation c Mean ± SEM |
p value | Ethanol d Mean ± SEM |
Ethanol Mounted e Mean ± SEM |
p value | |
|---|---|---|---|---|---|---|---|---|---|
| Thigmotaxis (% time in outside zone) | 60.56 ± 6.12 | 62.92 ± 5.24 | 0.99 | 60.56 ± 6.12 | 51.84 ± 8.27 | 0.91 | 84.52 ± 4.7 | 74.67 ± 3.97 | 0.78 |
| Light Preference (% time in light zone) | 51.53 ± 5.79 | 62.23 ± 5.26 | 0.64 | 51.53 ± 5.79 | 56.21 ± 3.76 | 0.99 | 75.81 ± 5.20 | 67.19 ± 4.06 | 0.73 |
| Locomotion (Distance traveled in cm) | 41.91 ± 5.11 | 49.19 ± 6.27 | 0.98 | 41.91 ± 5.11 | 51.72 ± 8.49 | 0.90 | 67.41 ± 5.08 | 63.27 ± 7.00 | 0.99 |
Control: Control zebrafish with no mounting or ablation.
Control Mounted: Control zebrafish with no ablation mounted in agarose and imaged on a confocal microscope.
Control AH Ablation: Control zebrafish mounted in agarose and imaged on a confocal microscope with ablation of Hcrt neurons in AH.
. Ethanol: Ethanol-exposed zebrafish (0.5%) with no mounting or ablation.
Ethanol Mounted: Ethanol-exposed zebrafish (0.5%) with no ablation mounted in agarose and imaged on a confocal microscope.
4. Discussion
Recent studies demonstrate at different ages that embryonic ethanol exposure at low-moderate doses increases the density as well as proliferation and differentiation of Hcrt and MCH neurons in the LH where they are normally concentrated2,15. In addition to confirming here in newborn rats this ethanol-induced increase in density of these LH peptide neurons, we provide new evidence that ethanol induces ectopic Hcrt and MCH neurons outside the hypothalamus in more anterior regions. These ectopic peptide-expressing neurons are detected in the NAcC and nearby vmCP, areas where they have not previously been observed in anatomical studies of rats4,5 and are not evident here in Control rats. These populations of ectopic peptide neurons in the forebrain structures are found to co-label the neuronal differentiation marker NeuN, suggesting that they are mature neurons which are likely active and integrated into the local neural circuitry44. The existence of these mature ectopic Hcrt and MCH neurons in newborn rats suggests that ethanol is altering their anterior migration as they develop in the embryo, possibly through changes in neuroimmune factors such as chemokines known to stimulate neuronal migration and, in turn, to be increased by embryonic ethanol exposure in both rats45 and zebrafish46. This is consistent with evidence that low doses of embryonic ethanol in rats promote premature migration and increase density of cortical GABAergic interneurons19, a phenomenon that may involve the stimulation of chemokine and growth factor signaling26,47. Furthermore, these ethanol-induced ectopic Hcrt and MCH neurons in the NAcC and vmCP are still evident in early-adolescent rats indicating that these effects of ethanol are long lasting, consistent with the detection of heterotopias in the brains of adolescent children with FASD22. Given that these adjacent forebrain regions mediate behaviors related to alcohol drinking48,49 and are affected by ethanol exposure during embryonic development50,51, the abnormal and long-lasting presence of Hcrt and MCH neurons within the NAcC and vmCP likely contributes to persistent ethanol-induced behavioral disturbances described in both clinical and pre-clinical studies52,53.
Our results also demonstrate that embryonic ethanol exposure at a low-moderate dose alters the morphology of these peptide neurons in newborn and early-adolescent rats. In the LH where they are normally concentrated, ethanol increases the size of both Hcrt and MCH neurons, similar to a study of hypothalamic Hcrt neurons in mice16, and it also increases the number of processes emanating from the soma of each Hcrt neuron, consistent with evidence showing embryonic ethanol to increase the complexity and intersections of dendrites in rodents17. While the direct impact on behavior of these morphological changes in LH neurons is unknown, their increased density here is likely to produce an overall increase in neuropeptide signaling from the hypothalamus and into extra-hypothalamic areas involved in mediating ethanol-related behaviors6. Compared to these LH neurons in ethanol-exposed rats, we also find that the ectopic Hcrt and MCH neurons in the NAcC and vmCP are smaller in size and have fewer processes emanating from the soma, possibly reflecting evidence that ethanol at a moderate dose increases neuronal size in the hypothalamus16 but decreases neuronal size in extra-hypothalamic areas54. With ethanol at low-moderate doses unlikely to inhibit neuronal development or induce apoptosis observed at higher doses9, these ectopic neurons may actually be more excitable, as indicated by studies showing neurons with smaller surface areas to produce action potentials with a lower input55. Evidence in primates suggests that morphological and synaptic changes in the putamen are associated with alcohol drinking56, and it is thus possible that these ectopic peptide-expressing neurons in the NAcC and vmCP with altered morphology, perhaps together with normally located neurons in the LH, may directly contribute to ethanol-related behaviors induced by embryonic exposure. While the density, morphology and ectopic location of both Hcrt and MCH neurons were similarly affected by embryonic ethanol exposure, there is evidence that exposure during adulthood to other drugs of misuse increases Hcrt density while having no effect on MCH neurons57,58. This difference is likely due to the fact that embryonic peptide neurons when differentiating from neural progenitor cells are more susceptible than mature neurons to drug-induced disturbances. It is notable that these other substances administered at older ages, such as fentanyl57 and cocaine59, stimulate the density of Hcrt neurons similar to the effects of embryonic ethanol exposure and animals with more motivation for these substances have a greater number of Hcrt neurons. This suggests that there are common underlying mechanisms mediating these drug effects on this neuropeptide neuronal population, which in turn drives behaviors related to substance use disorders.
Similar to the rat, our studies in zebrafish with low-moderate doses and at different ages demonstrate that embryonic ethanol exposure increases the density, as well as proliferation, differentiation and migration, of Hcrt neurons in the AH where they are normally concentrated3,10,11. We show here in zebrafish that ethanol, while increasing the density of Hcrt neurons in the AH, induces ectopic Hcrt neurons in the POA where they are not normally detected. This region exists anterior to the AH, similar to the ectopic neurons in the rat detected in brain regions anterior to their normal hypothalamic location. This agrees with our earlier study of Hcrt neurons11 and other studies in zebrafish showing ethanol to induce ectopic expression of oxytocin60, cranial neural crest cells61, and facial branchial motor neurons62. The POA in zebrafish, analogous to the mammalian paraventricular nucleus63, is known to have a role in mediating somatic behaviors and also aggressive behaviors64 shown to be increased by embryonic ethanol exposure in zebrafish3,10. As in rats, ethanol in zebrafish increases the size of Hcrt neurons in the AH where they are normally concentrated and number of their processes emanating from the soma. Also, compared to these Hcrt neurons in the AH, the ethanol-induced ectopic Hcrt neurons in the POA as in the forebrain of rats are morphologically altered, smaller in size with fewer processes, and they co-label the neuronal differentiation marker HuC, suggesting that they are mature neurons likely functionally active in mediating behavior44.
An important finding of this study is that ectopic Hcrt neurons do, in fact, have a functional role in mediating the behavioral disturbances, an increase in anxiety and locomotor behaviors, shown here and previously2,10 to be induced by embryonic ethanol exposure. Anxiety-like behaviors which are mediated by the Hcrt system7 are associated with elevations in voluntary ethanol consumption induced by ethanol exposure in zebrafish3,10, are predictive of ethanol intake in rodents65, and are positively related to alcohol consumption in humans66. Here we demonstrate in ethanol-exposed zebrafish that laser ablation of the ectopic Hcrt neurons in the POA, similar to ablation of Hcrt neurons in the AH, blocks the increase in anxiety-like behaviors, suggesting the involvement of both neuronal populations in these behaviors. Notably, we find that locomotor activity induced by ethanol, a behavior closely related to and predictive of ethanol consumption67 and mediated by Hcrt neurons in zebrafish and rats68, is blocked by laser ablation of the Hcrt neurons in the POA while unaffected by ablation of the normally located AH neurons, suggesting these ectopic POA neurons have a particularly important role. These Hcrt neurons may have a unique function through altered signaling to noradrenergic populations in the locus coeruleus, which in zebrafish are shown to receive input from Hcrt neurons and to be required for increased locomotor activity following optogenetic stimulation of Hcrt neurons69. However, with Hcrt neurons known to be genetically heterogenous and to express other neurochemicals and neuropeptides70, we must note the limitations of this ablation experiment and any conclusions regarding the role of specific neuronal systems in mediating the ethanol-induced behaviors. With the proportion of ectopic neurons relative to total number of Hcrt and MCH neurons being smaller in the rat than zebrafish and these ectopic neurons having a small number of projections and perhaps limited neural connectivity, future studies would benefit from directly testing in the rat model the behavioral function of these ectopic neurons by repeating and expanding with more detailed analyses our study described here in zebrafish. This could be done through targeted microinjections of an antisense morpholino into the NacC and vmCP followed by analysis of alcohol preference and consumption or by measuring the neuronal activity of these ectopic neurons after behavior to determine if they are participating in local neural circuitry59.
While both clinical and animal studies have shown embryonic ethanol exposure to produce ectopic neurons in the brain21,22,62, this report provides new evidence in both rats and zebrafish for ectopic neurons that express specific peptides known to promote alcohol-related behaviors. It also characterizes the morphology of these neurons, showing them to be mature neurons but different from normally located neurons, and it presents in zebrafish the first direct evidence that these ectopic neurons are functional and involved in mediating ethanol-induced disturbances in behavior. With ethanol-exposed rat offspring exhibiting similar behavioral changes as shown in zebrafish2 while expressing ectopic peptide neurons, both Hcrt and MCH, in forebrain structures having major functions in the use and misuse of alcohol and other drugs48,49, these ectopic neurons in rats identified as mature neurons are also likely to be involved in the increased alcohol consumption and related behaviors induced by ethanol. Further studies of this nature in zebrafish as well as rats should help to elucidate the precise functional role of ethanol-induced heterotopias found in the brains of children with FASD22.
Acknowledgements:
This research was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health, under Award Numbers R01AA024798 (S.F.L.), R01AA027653 (S.F.L.) and F32AA027702 (A.D.C.).
Funding information:
National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health, Grant/Award Numbers: R01AA024798 (S.F.L), R01AA027653 (S.F.L), F32AA027702 (A.D.C)
Footnotes
Conflict of interest: The authors declare no competing interests.
Data availability:
The data that support the findings of this study are available on request from the corresponding author.
References:
- 1.Gupta KK, Gupta VK, Shirasaka T. An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol Clin Exp Res. 2016;40(8):1594–1602. [DOI] [PubMed] [Google Scholar]
- 2.Chang GQ, Karatayev O, Halkina V, Edelstien J, Ramirez E, Leibowitz SF. Hypothalamic CCL2/CCR2 Chemokine System: Role in Sexually Dimorphic Effects of Maternal Ethanol Exposure on Melanin-Concentrating Hormone and Behavior in Adolescent Offspring. J Neurosci. 2018;38(42):9072–9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collier AD, Yasmin N, Khalizova N, Campbell S, Onoichenco A, Fam M, Albeg AS, Leibowitz SF. Sexually dimorphic and asymmetric effects of embryonic ethanol exposure on hypocretin/orexin neurons as related to behavioral changes in zebrafish. Sci Rep. 2021;11(1):16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull. 1985;15(6):635–649. [DOI] [PubMed] [Google Scholar]
- 5.De Lecea L, Kilduff T, Peyron C, Gao X-B, Foye P, Danielson P, Fukuhara C, Battenberg E, Gautvik V, Bartlett Fn. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences. 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morganstern I, Gulati G, Leibowitz SF. Role of melanin-concentrating hormone in drug use disorders. Brain Res. 2020;1741:146872. [DOI] [PubMed] [Google Scholar]
- 7.Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012;198:133–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James MH, Mahler SV, Moorman DE, Aston-Jones G. A decade of orexin/hypocretin and addiction: where are we now? Behav Neurosci Orexin/Hypocretin. 2016:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang GQ, Karatayev O, Liang SC, Barson JR, Leibowitz SF. Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: possible mechanism for offspring ethanol overconsumption. Neurosci. 2012;222:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterling ME, Chang GQ, Karatayev O, Chang SY, Leibowitz SF. Effects of embryonic ethanol exposure at low doses on neuronal development, voluntary ethanol consumption and related behaviors in larval and adult zebrafish: Role of hypothalamic orexigenic peptides. Behav Brain Res. 2016;304:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collier AD, Halkina V, Min SS, Roberts MY, Campbell SD, Camidge K, Leibowitz SF. Embryonic Ethanol Exposure Affects the Early Development, Migration, and Location of Hypocretin/Orexin Neurons in Zebrafish. Alcohol Clin Exp Res. 2019;43(8):1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbaz I, Levitas-Djerbi T, Appelbaum L. The hypocretin/orexin neuronal networks in zebrafish. Behav Neurosci Orexin/Hypocretin. 2017;33:75–92. [DOI] [PubMed] [Google Scholar]
- 13.Alsakran A, Kudoh T. Zebrafish as a Model for Fetal Alcohol Spectrum Disorders. Front Pharmacol. 2021;12:721924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovely CB, Fernandes Y, Eberhart JK. Fishing for Fetal Alcohol Spectrum Disorders: Zebrafish as a Model for Ethanol Teratogenesis. Zebrafish. 2016;13(5):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neurosci. 2015;310:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olateju OI, Bhagwandin A, Ihunwo AO, Manger PR. Changes in the cholinergic, catecholaminergic, orexinergic and serotonergic structures forming part of the sleep systems of adult mice exposed to intrauterine alcohol. Front Neuroanat. 2017;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Naik V, Orzabal M, Lunde-Young R, Ramadoss J. Morphological alteration in rat hippocampal neuronal dendrites following chronic binge prenatal alcohol exposure. Brain Res. 2021;1768:147587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole GJ, Zhang C, Ojiaku P, Bell V, Devkota S, Mukhopadhyay S. Effects of ethanol exposure on nervous system development in zebrafish. Int Rev Cell Mol Biol. 2012;299:255–315. [DOI] [PubMed] [Google Scholar]
- 19.Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci. 2008;28(8):1854–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumada T, Jiang Y, Cameron DB, Komuro H. How does alcohol impair neuronal migration? J Neurosci Res. 2007;85(3):465–470. [DOI] [PubMed] [Google Scholar]
- 21.Chevassus-au-Louis N, Represa A. The right neuron at the wrong place: biology of heterotopic neurons in cortical neuronal migration disorders, with special reference to associated pathologies. Cell Molec Life Sci. 1999;55(10):1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wozniak JR, Riley EP, Charness ME. Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 2019;18(8):760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christodoulou JA, Walker LM, Del Tufo SN, Katzir T, Gabrieli JD, Whitfield-Gabrieli S, Chang BS. Abnormal structural and functional brain connectivity in gray matter heterotopia. Epilepsia. 2012;53(6):1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M, Oyarzabal EA, Yang R, Murphy SJ, Hurn PD. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. J Neurosci Meth. 2008;171(2):214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohmiller JJ, Swing SP, Hanson MM. Reproduction and breeding. In: The laboratory rat. Elsevier; 2020:157–179. [Google Scholar]
- 26.Chang GQ, Yasmin N, Collier AD, Karatayev O, Khalizova N, Onoichenco A, Fam M, Albeg AS, Campbell S, Leibowitz SF. Fibroblast growth factor 2: Role in prenatal alcohol-induced stimulation of hypothalamic peptide neurons. Prog Neuropsychopharmacol Biol Psychiatry. 2022;116:110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang GQ, Karatayev O, Boorgu D, Leibowitz SF. CCL2/CCR2 Chemokine System in Embryonic Hypothalamus: Involvement in Sexually Dimorphic Stimulatory Effects of Prenatal Ethanol Exposure on Peptide-Expressing Neurons. Neurosci. 2020;424:155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appelbaum L, Wang GX, Maro GS, Mori R, Tovin A, Marin W, Yokogawa T, Kawakami K, Smith SJ, Gothilf Y, Mignot E, Mourrain P. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc Nat Acad Sci. 2009;106(51):21942–21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satou C, Kimura Y, Higashijima S. Generation of multiple classes of V0 neurons in zebrafish spinal cord: progenitor heterogeneity and temporal control of neuronal diversity. J Neurosci. 2012;32(5):1771–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos D, Luzio A, Coimbra AM. Zebrafish sex differentiation and gonad development: A review on the impact of environmental factors. Aquat Tox. 2017;191:141–163. [DOI] [PubMed] [Google Scholar]
- 31.Siegfried KR, Nüsslein-Volhard C. Germ line control of female sex determination in zebrafish. Dev Bio. 2008;324(2):277–287. [DOI] [PubMed] [Google Scholar]
- 32.Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159(4):896–910. [DOI] [PubMed] [Google Scholar]
- 33.Jacobo A, Dasgupta A, Erzberger A, Siletti K, Hudspeth AJ. Notch-Mediated Determination of Hair-Bundle Polarity in Mechanosensory Hair Cells of the Zebrafish Lateral Line. Curr Biol. 2019;29(21):3579–3587.e3577. [DOI] [PubMed] [Google Scholar]
- 34.Chang G-Q, Karatayev O, Kavya BDSS, Leibowitz SF. CCL2/CCR2 Chemokine System in Embryonic Hypothalamus: Involvement in Sexually Dimorphic Stimulatory Effects of Prenatal Ethanol Exposure on Peptide-Expressing Neurons. Neurosci. 2020;424:155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marquart GD, Tabor KM, Brown M, Strykowski JL, Varshney GK, LaFave MC, Mueller T, Burgess SM, Higashijima S, Burgess HA. A 3D Searchable Database of Transgenic Zebrafish Gal4 and Cre Lines for Functional Neuroanatomy Studies. Front Neural Circuits. 2015;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherwood NM, Timiras PS. Stereotaxic atlas of the developing rat brain. Oakland: University of California Press; 1970. [Google Scholar]
- 38.Muto A, Kawakami K. Ablation of a Neuronal Population Using a Two-photon Laser and Its Assessment Using Calcium Imaging and Behavioral Recording in Zebrafish Larvae. J Vis Exp. 2018(136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volpe BA, Fotino TH, Steiner AB. Confocal Microscope-Based Laser Ablation and Regeneration Assay in Zebrafish Interneuromast Cells. J Vis Exp. 2020(159). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merola C, Lucon‐Xiccato T, Bertolucci C, Perugini M. Behavioural effects of early‐life exposure to parabens in zebrafish larvae. J App Tox. 2021;41(11):1852–1862. [DOI] [PubMed] [Google Scholar]
- 41.Schnörr S, Steenbergen P, Richardson M, Champagne D. Measuring thigmotaxis in larval zebrafish. Behav Brain Res. 2012;228(2):367–374. [DOI] [PubMed] [Google Scholar]
- 42.Steenbergen PJ, Richardson MK, Champagne DL. The light–dark preference test for larval zebrafish. In: Zebrafish Protocols for Neurobehavioral Research. Springer; 2012:21–35. [Google Scholar]
- 43.Sterling ME, Karatayev O, Chang GQ, Algava DB, Leibowitz SF. Model of voluntary ethanol intake in zebrafish: effect on behavior and hypothalamic orexigenic peptides. Behav Brain Res. 2015;278:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gusel’nikova VV, Korzhevskiy DE. NeuN As a Neuronal Nuclear Antigen and Neuron Differentiation Marker. Acta Naturae. 2015;7(2):42–47. [PMC free article] [PubMed] [Google Scholar]
- 45.Chang GQ, Collier AD, Karatayev O, Gulati G, Boorgu D, Leibowitz SF. Moderate Prenatal Ethanol Exposure Stimulates CXCL12/CXCR4 Chemokine System in Radial Glia Progenitor Cells in Hypothalamic Neuroepithelium and Peptide Neurons in Lateral Hypothalamus of the Embryo and Postnatal Offspring. Alcohol Clin Exp Res. 2020;44(4):866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collier AD, Khalizova N, Chang GQ, Min S, Campbell S, Gulati G, Leibowitz SF. Involvement of Cxcl12a/Cxcr4b Chemokine System in Mediating the Stimulatory Effect of Embryonic Ethanol Exposure on Neuronal Density in Zebrafish Hypothalamus. Alcohol Clin Exp Res. 2020;44(12):2519–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terasaki LS, Schwarz JM. Effects of moderate prenatal alcohol exposure during early gestation in rats on inflammation across the maternal-fetal-immune interface and later-life immune function in the offspring. J Neuro Pharmacol. 2016;11(4):680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barson JR, Carr AJ, Soun JE, Sobhani NC, Leibowitz SF, Hoebel BG. Opioids in the nucleus accumbens stimulate ethanol intake. Physiol Behav. 2009;98(4):453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, Buck KJ. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res. 2011;35(10):1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fryer SL, Mattson SN, Jernigan TL, Archibald SL, Jones KL, Riley EP. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2012;36(11):1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice JP, Suggs LE, Lusk AV, Parker MO, Candelaria-Cook FT, Akers KG, Savage DD, Hamilton DA. Effects of exposure to moderate levels of ethanol during prenatal brain development on dendritic length, branching, and spine density in the nucleus accumbens and dorsal striatum of adult rats. Alcohol. 2012;46(6):577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lees B, Mewton L, Jacobus J, Valadez EA, Stapinski LA, Teesson M, Tapert SF, Squeglia LM. Association of Prenatal Alcohol Exposure With Psychological, Behavioral, and Neurodevelopmental Outcomes in Children From the Adolescent Brain Cognitive Development Study. Am J Psychiatry. 2020:appiajp202020010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobeh Rev. 2007;31(2):181–191. [DOI] [PubMed] [Google Scholar]
- 54.Zhou FC, Sari Y, Li TK, Goodlett C, Azmitia EC. Deviations in brain early serotonergic development as a result of fetal alcohol exposure. Neurotox Res. 2002;4(4):337–342. [DOI] [PubMed] [Google Scholar]
- 55.Cuntz H, Bird AD, Mittag M, Beining M, Schneider M, Mediavilla L, Hoffmann FZ, Deller T, Jedlicka P. A general principle of dendritic constancy: A neuron’s size- and shape-invariant excitability. Neuron. 2021;109(22):3647–3662.e3647. [DOI] [PubMed] [Google Scholar]
- 56.Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropyschopharm. 2011;36(12):2513–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fragale JE, James MH, Aston-Jones G. Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. Addiction biology. 2021;26(3):e12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biological psychiatry. 2019;85(11):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pantazis CB, James MH, Bentzley BS, Aston-Jones G. The number of lateral hypothalamus orexin/hypocretin neurons contributes to individual differences in cocaine demand. Addiction biology. 2020;25(4):e12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coffey CM, Solleveld PA, Fang J, Roberts AK, Hong SK, Dawid IB, Laverriere CE, Glasgow E. Novel oxytocin gene expression in the hindbrain is induced by alcohol exposure: transgenic zebrafish enable visualization of sensitive neurons. PloS one. 2013;8(1):e53991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boric K, Orio P, Viéville T, Whitlock K. Quantitative analysis of cell migration using optical flow. PloS one. 2013;8(7):e69574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buckley DM, Sidik A, Kar RD, Eberhart JK. Differentially sensitive neuronal subpopulations in the central nervous system and the formation of hindbrain heterotopias in ethanol-exposed zebrafish. Birth Def Res. 2019;12(111):700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herget U, Ryu S. Coexpression analysis of nine neuropeptides in the neurosecretory preoptic area of larval zebrafish. Front Neuroanat. 2015;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larson ET, O’Malley DM, Melloni RH. Aggression and vasotocin are associated with dominant–subordinate relationships in zebrafish. Behav Brain Res. 2006;167(1):94–102. [DOI] [PubMed] [Google Scholar]
- 65.Hayton SJ, Mahoney MK, Olmstead MC. Behavioral traits predicting alcohol drinking in outbred rats: an investigation of anxiety, novelty seeking, and cognitive flexibility. Alcohol Clin Exp Res. 2012;36(4):594–603. [DOI] [PubMed] [Google Scholar]
- 66.McCaul ME, Hutton HE, Stephens MA, Xu X, Wand GS. Anxiety, Anxiety Sensitivity, and Perceived Stress as Predictors of Recent Drinking, Alcohol Craving, and Social Stress Response in Heavy Drinkers. Alcohol Clin Exp Res. 2017;41(4):836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karatayev O, Barson JR, Carr AJ, Baylan J, Chen YW, Leibowitz SF. Predictors of ethanol consumption in adult Sprague-Dawley rats: relation to hypothalamic peptides that stimulate ethanol intake. Alcohol. 2010;44(4):323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heydendael W, Sengupta A, Beck S, Bhatnagar S. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol Behav. 2014;130:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh C, Oikonomou G, Prober DA. Norepinephrine is required to promote wakefulness and for hypocretin-induced arousal in zebrafish. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sagi D, de Lecea L, Appelbaum L. Heterogeneity of Hypocretin/Orexin Neurons. Front Neurol Neurosci. 2021;45:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


