Abstract
Extracellular vesicles are important vectors for cell-cell communication and show potential value for diagnosis and treatment of kidney diseases. The pathologic diagnosis of kidney diseases relies on kidney biopsy, whereas collection of extracellular vesicles from urine or circulating blood may constitute a less invasive diagnostic tool. In particular, urinary extracellular vesicles released mainly from resident kidney cells might provide an alternative tool for detection of kidney injury. Because extracellular vesicles mirror many features of their parent cells, cargoes of several populations of urinary extracellular vesicles are promising biomarkers for disease processes, like diabetic kidney disease, kidney transplant, and lupus nephritis. Contrarily, extracellular vesicles derived from reparative cells, such as mesenchymal stem cells, tubular epithelial progenitor cells, and human umbilical cord blood represent promising regenerative tools for treatment of kidney diseases. Furthermore, induced pluripotent stem cells–derived and engineered extracellular vesicles are being developed for specific applications for the kidney. Nevertheless, some assumptions regarding the specificity and immunogenicity of extracellular vesicles remain to be established. This review focuses on the utility of extracellular vesicles as therapeutic and diagnostic (theranostic) tools in kidney diseases and future directions for studies.
Keywords: diagnosis, extracellular vesicles, kidney diseases
Introduction
Extracellular vesicles are nanoscale membrane vesicles released by cells that carry luminal and membrane-bound cargoes, endowing them with exceptional theranostic (therapeutic and diagnostic) properties. Consequently, since their discovery, interest in their application has been rising progressively, including in kidney applications.
Extracellular vesicles are considered to mainly include exosomes, microvesicles, and apoptotic bodies (1). Exosomes (30–150 nm) are formed primarily from multivesicular bodies, which develop from late endosomes assisted by clusters-of-differentiation 9 (CD9), CD63, endosomal sorting complex required for transport (ESCRT), and Alix proteins. Multivesicular bodies ultimately fuse with the plasma membrane and release exosomes into the extracellular space. Other than this classic ESCRT-dependent pathway, ESCRT-independent paths contribute to exosome formation and sorting of specific molecules, and they seem to involve the tetraspanin CD63 (2) and neutral sphingomyelinase-2 (3). Other ESCRT-independent paths, like amyloid precursor protein–containing extracellular vesicles, are secreted by an Alix- and Syntenin-1–dependent mechanism (4), and the Ras-related protein Rab-31 controls exosome formation via a flotillin proteins–dependent pathway (5).
Microvesicles are generally larger (100–1000 nm) and form directly from the plasma membrane through an interaction between phosphatidylserine redistribution and cytoskeletal protein contraction (6). Apoptotic bodies (500–4000 nm) form during preprogrammed cell death and are characterized by organelle and fragmented DNA content (1). Most apoptotic bodies are recognized and cleared by macrophages (7).
Extracellular Vesicle Isolation and Characterization
Extracellular vesicles are detectable in most bodily fluids and must be isolated in sufficient quantities for analysis. Of available methods, ultracentrifugation is among the most widely used and separates extracellular vesicles into a pellet that dissolves in a buffer (8). Its disadvantages include time consumption, disrupted particle integrity, and protein contamination, which might be ameliorated with one-step sucrose cushion ultracentrifugation (9). Size-exclusion chromatography can isolate size-specific relatively pure extracellular vesicles with higher yield than ultracentrifugation (10). Other technologies used to separate extracellular vesicles include tangential flow filtration (less time consuming and higher yield than ultracentrifugation), ultrafiltration (more efficient), magnetic beads conjugated with antibodies (isolate extracellular vesicles from specific cells), precipitation (low equipment demands, preserves biologic activities of extracellular vesicles, and low protein contamination), microfluidics and lab-on-a-chip technology (produce size-controlled extracellular vesicles), hydrostatic filtration dialysis (urine suitable and inexpensive), and anion exchange chromatography (rapid isolation, high yield, and low protein contamination). Recent studies have promoted ultracentrifugation and hydrostatic filtration dialysis for mRNA sequencing of urinary extracellular vesicles in diabetic kidney disease (DKD) (11) and ultrafiltration in combination with exosomal RNA columns to purify microRNAs (miRNAs) (12).
Subsequently, extracellular vesicles are identified by markers and morphology. CD9, CD63, CD81, TSG101, and Alix regulate formation and identify exosomes (13); adenosine diphosphate ribosylation factor-6 (14) and vesicle-associated membrane protein-3 (15) characterize microvesicle formation. High-throughput fluorescence correlation spectroscopy facilitates their identification (16). With electron microscopy, extracellular vesicle size can be assessed, and a nanostructure of peripheral limiting membrane can be resolved (17). Moreover, extracellular vesicles shed from cells can be directly observed with high-resolution cryogenic electron microscopy (18). Nanoparticle tracking analysis and dynamic light scattering are useful for determination of size distribution and concentration.
Extracellular vesicles are rich in proteins as well as miRNA, mRNA, long-noncoding, and other types of RNA, and they contain lipids like cholesterol, sphingomyelin, glycosphingolipid, phosphatidylserine, and ceramide. Furthermore, their surface membrane proteins (19) together with cargo act as signal vectors, making extracellular vesicles an important tool for cell-to-cell communication and, potentially, in clinical practice. This review focuses on extracellular vesicles as theranostic tools in kidney disease.
Extracellular Vesicles: Diagnostic Tools in Kidney Disease
Extracellular vesicles can be easily isolated from most body fluids, and their cargo is altered in many diseases, with urinary and circulating extracellular vesicles best characterized. Because their size may prohibit passage of circulating extracellular vesicles across the glomerular filtration barrier, urinary extracellular vesicles are traditionally considered to originate mainly from resident kidney cells (20), and their levels and characteristics are considered suitable to reflect kidney disease. Yet, circulating extracellular vesicles may also be altered in kidney disease (Figure 1A).
Figure 1.
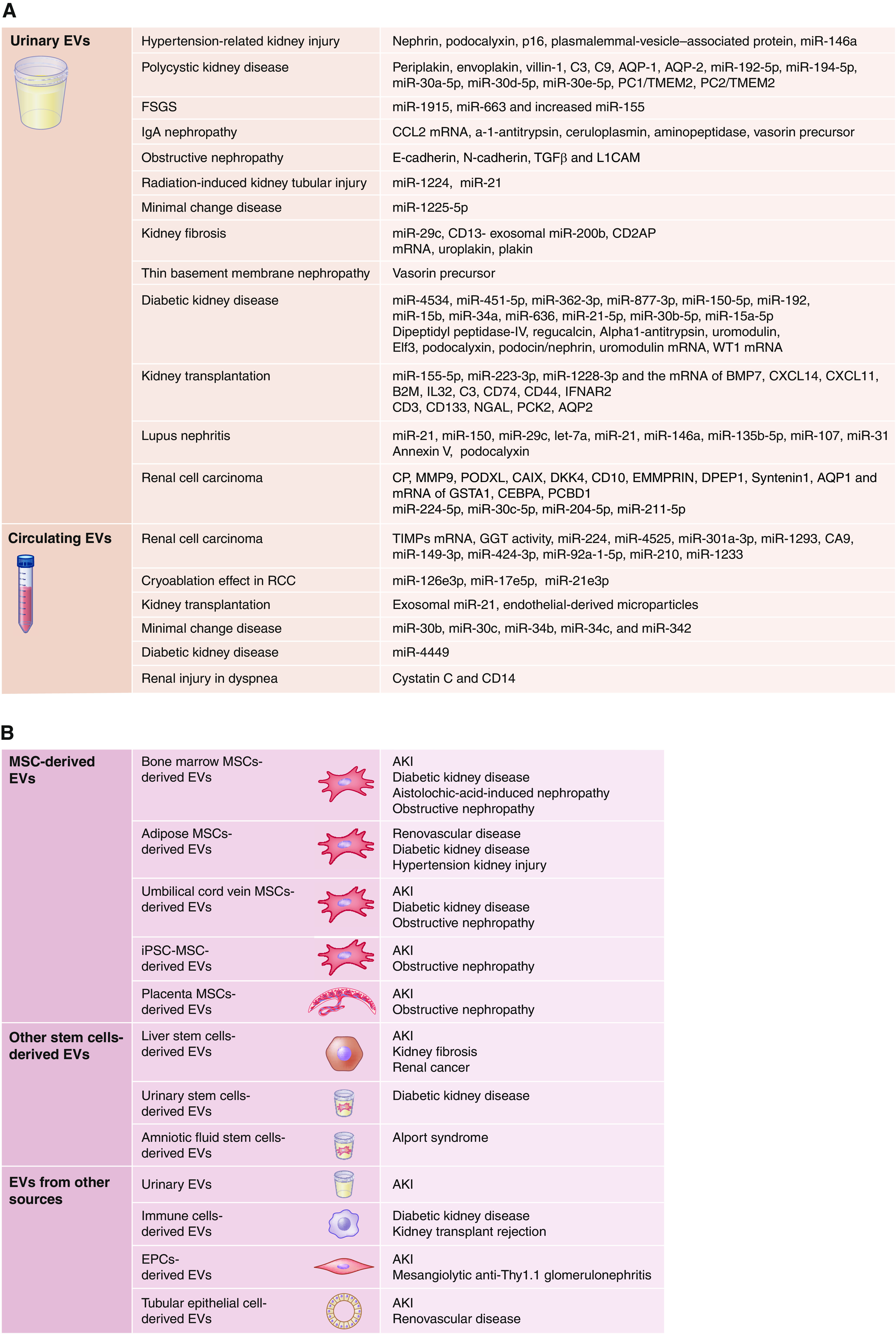
Extracellular vesicles (EVs) are ubiquitous diagnostic and therapeutic tools in kidney diseases. (A) EV-based biomarkers of kidney diseases include urinary and circulating EVs. Urinary EVs have been widely applied to detect kidney diseases or injury, including diabetic kidney disease (DKD), kidney transplantation, lupus nephritis, renal cell carcinoma (RCC), hypertension, polycystic kidney disease, FSGS, IgA nephropathy, obstructive nephropathy, radiation-induced kidney injury, minimal change kidney disease, kidney fibrosis, and basement membrane nephropathy. Circulating EVs have been studied in fewer diseases (including RCC, kidney transplantation, minimal change kidney disease, DKD, and renal injury in dyspnea). (B) EVs harvested from different tissues can serve as therapeutic tools in kidney diseases. Proposed sources for EVs include tissue mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), induced pluripotent stem cells (iPSCs), tubular epithelial cells, urine, and human placenta. B2M, beta-2 microglobulin; BMP, bone morphogenetic protein; CAIX, carbonic anhydrase IX; CD, cluster of differentiation; CEBPA, CCAAT/enhancer-binding protein alpha; CP, ceruloplasmin; CXCL, C-X-C motif chemokine ligand; DDK, dickkopf-related protein; DPEP, dipeptidase; EMMRIN, extracellular matrix metalloproteinase inducer; GGT, γ-glutamyltransferase; GSTA, glutathione S-transferase A; IFNAR, interferon-α/β receptor; IgA, immunoglobulin A; IL, interleukin; L1CAM, L1 cell adhesion molecule; miR, microRNA; MMP, matrix metalloproteinase; NGAL, neutrophil gelatinase-associated lipocalin; PC, polycystin; PCBD, pterin-4-alpha-carbinolamine dehydratase; PCK, phosphoenolpyruvate carboxykinase; PODXL, podocalyxin-like protein; RVD, renovascular disease; TIMPs, tissue inhibitors of metalloproteinases; TGF-β, transforming growth factor β; TMEM, transmembrane protein.
Urinary Extracellular Vesicles
Studies have demonstrated the rich heterogeneity of urinary extracellular vesicles and their suitability for detection of kidney disease (21). For example, proteomics highlighted their resemblance to several kidney proteins and utility in tracking kidney pathology (22); the most promising extracellular vesicle biomarkers are listed in Supplemental Table 1. Typical changes have been described in specific kidney diseases.
Diabetic Kidney Disease.
Several urinary extracellular vesicle miRNAs and proteins are altered in DKD (Supplemental Table 1) and linked to its onset. Urinary microvesicle dipeptidyl peptidase IV level correlates with the urinary albumin-creatinine-ratio in participants with DKD (23), and regucalcin is decreased (24). In particular, podocyte markers, like podocalyxin+, or a high podocin-nephrin ratio in extracellular vesicles suggest glomerular injury in DKD (25). Additional studies are needed to identify the best markers to detect and reflect DKD severity and how they are affected by glucosuria or protein complexes (26).
Kidney Transplant.
In patients with allograft rejection, the numbers of CD3+ urinary extracellular vesicles are elevated (27), and their mRNA and miRNA are useful for diagnosis (Supplemental Table 1). Interestingly, CD133+ urinary extracellular vesicles continuously released during homeostatic turnover of the nephron may reflect graft function (28). Moreover, neutrophil gelatinase–associated lipocalin in urinary extracellular vesicles may predict graft recovery (29), as does extracellular vesicle phosphoenolpyruvate carboxykinase (30). Furthermore, acute disturbance of water homeostasis in kidneys post-transplant might be related to decreased urinary extracellular vesicles expression of aquaporin-2 (31). The miRNA bkv-miR-B1–5p secreted by the BK virus that may cause nephropathy in kidney transplant recipients was detected in the blood and urine of patients (32). Therefore, urinary extracellular vesicles in patients after kidney transplant may reflect graft status, function, and complications.
Lupus Nephritis.
Glomerular podocytes have been implicated in glomerular injury in patients with lupus nephritis. Importantly, urinary podocyte microparticles (annexin-V and podocalyxin+) track with disease activity (33), and several miRNAs were also proposed as biomarkers of lupus nephritis (Supplemental Table 1). Yet, their selectivity for the pathogenesis of lupus nephritis must be defined before therapeutic targets are selected.
Renal Cell Carcinoma.
Distinct protein content was identified in urinary extracellular vesicles of patients with renal cell carcinoma (RCC) (34), which might serve as biomarker reservoirs (Supplemental Table 1). Specifically, patients with clear-cell RCC have altered urinary extracellular vesicle RNA and miRNAs, and in murine RCC, miR‐204‐5p and miR‐211‐5p are elevated (35). However, it remains unclear whether these changes reflect the pathogenesis of the disease or are secondary to the phenotype.
Hypertension-Related Kidney Injury.
Patients with hypertension release increased numbers of urinary extracellular vesicles bearing markers reflecting kidney injury, including nephrin+ and podocalyxin+ (podocyte injury) (36), urate-transporter-1+/p16+ (proximal tubular senescence) (37), and plasmalemmal vesicle–associated protein+ (microvascular injury) urinary extracellular vesicles (38). Furthermore, the exosome miR-146a level inversely associates with albuminuria and early kidney injury (39). Therefore, urinary extracellular vesicles might pinpoint injuries to specific kidney compartments. The ability to detect kidney injury preceding albuminuria would be invaluable for early treatment.
Urinary Extracellular Vesicles in Other Kidney Diseases.
Urinary extracellular vesicles have been applied to elucidate other kidney diseases as well. For example, elevated levels of C-C motif chemokine ligand-2 mRNA (40), a-1-antitrypsin, and ceruloplasmin, as well as reduced aminopeptidase and vasorin precursor, were observed in IgA nephropathy, whereas in thin basement membrane nephropathy, vasorin precursor increased (41). Polycystin-transmembrane protein-2 ratio in urinary extracellular vesicles is a good marker in polycystic kidney disease (42), and preeclampsia is associated with an elevated urinary podocin+-nephrin+ extracellular vesicles ratio (43). Additionally, decreases in the urinary extracellular vesicle CD133 indicate glomerular damage (44), and decreases in vacuolar adenosine triphosphatase B1 identify distal tubular acidosis (45). We found increased levels of podocyte-derived urinary extracellular vesicles in patients with metabolic syndrome–related kidney disease (46). Urinary extracellular vesicles miR-26a (47) and WT1 mRNA (48) may also reflect podocyte injury. Notably, palmitic acid can promote extracellular vesicle production by renal tubular epithelial cells in vitro (49), suggesting that high levels of urinary extracellular vesicles derived from tubular epithelial cells may be a marker of lipotoxicity. Therefore, characterization of urinary extracellular vesicle populations may aid in localizing kidney injury to specific cell types.
Unlike protein and nucleic acid, lipid metabolites in extracellular vesicles as biomarkers for kidney diseases have been studied in less detail. However, a differential lipid composition in urinary exosomes was found in RCC compared with healthy individuals (50). In addition, the presence of phosphatidylserine and other lipid species identified prostate cancer in vivo (51) and in vitro (52). Clearly, lipid metabolites in extracellular vesicles can be potential markers for kidney diseases, and additional research is needed in this area of extracellular vesicles biology.
Changes in urinary extracellular vesicles have also been described in animal models of drug-induced nephrotoxicity (53), ischemia-reperfusion injury (54), AKI (55), and polyarteritis nodosa nephritis (56). Yet, species differences must be considered in application of data obtained from animal models.
Given that extracellular vesicles in the urine originate mainly from kidney cells, it might be feasible to identify their parent cells. Podocyte-derived extracellular vesicles are nephrin, podocalyxin, calyxin, or podocin positive (37,46,57). Extracellular vesicles of proximal tubular cell origin can be identified by megalin, aquaporin-1, osteoprotegerin, and urate transporter-1 (37,57,58), whereas CD144, CD31, or plasmalemmal vesicle–associated protein markers suggest endothelial cell origin (38,57). In addition, uromodulin is a marker for the ascending limb of Henle loop and prominin-2 for distal tubules (37). Therefore, specific markers may assist in localizing cellular alterations to specific nephron segments.
Circulating Extracellular Vesicles
Circulating extracellular vesicles have broader origins than urinary extracellular vesicles and possibly account for a fraction of kidney-derived extracellular vesicles. In particular, they have been linked to diagnosis or severity of RCC (Supplemental Table 2). Furthermore, after kidney transplant, increased circulating extracellular vesicle miR-21 levels distinguish the degree of interstitial fibrosis more accurately than serum creatinine and proteinuria (59). In patients with minimal change nephrotic syndrome, several mRNAs are elevated (Supplemental Table 2), and concentrations of miR-4449 in circulating extracellular vesicles are higher in patients with diabetes with versus without DKD (60). Therefore, the content of circulating extracellular vesicles may reflect CKD subtypes and complications. Nevertheless, the systemic distribution and specificity of these markers for kidney conditions warrant careful verification.
Notably, the perception that circulating extracellular vesicles do not contribute to urinary extracellular vesicles warrants reconsideration. Although usually only small circulating particles (<10 nm) undergo glomerular filtration, larger nanoparticles (300–400 nm) may undergo transcytosis across the peritubular capillary endothelium and accumulate in proximal tubular epithelial cells (61), and they might thus be released into the tubular lumen and appear in the urine. Possibly, a reverse route might be used by kidney-derived extracellular vesicles to reach the systemic circulation. Therefore, the precise source and ultimate destiny of urinary and circulating extracellular vesicles need to be fate mapped. Moreover, circulating extracellular vesicles may more easily cross an injured glomerular filtration barrier, so that increased levels of urinary extracellular vesicles originating from the circulation may indicate glomerular injury.
Extracellular Vesicles as Therapeutic Tools in Kidney Disease
Beyond serving as biomarkers, the extracellular vesicle cargos of proteins, nucleic acids, and lipids may mediate tissue repair, at least when derived from curative cells. Compared with their parent cells, extracellular vesicles are more stable, are traditionally considered to be less immunogenic, and enable longer storage. Because of their small size, they penetrate tissues better, and intra-arterial injections of extracellular vesicles may pose a lower risk of embolization than cell injection. Therefore, since their first therapeutic application in murine AKI (62), extracellular vesicles have been applied widely, including small clinical studies (63). Meta-analyses have shown that extracellular vesicles improve kidney function in experimental AKI and CKD models (64,65). However, their effects depend on their cells of origin.
Mesenchymal Stem Cell–/Stromal Cell–Derived Extracellular Vesicles
Mesenchymal stem/stromal cells used in regenerative medicine can be isolated from bone marrow, adipose tissue, and umbilical cord vein blood and reside in solid organs, such as the liver, kidney, heart, and pancreas. Extracellular vesicles replicate the beneficial therapeutic function of their parent cells but with a lower risk of rejection (66), and they have therefore been widely studied in kidney diseases (Figure 1B).
Bone Marrow Mesenchymal Stem Cell–Derived Extracellular Vesicles.
Bone marrow mesenchymal stem cell–derived extracellular vesicles are beneficial in AKI, possibly by their miRNA load (67), but they are not all equally effective. Extracellular vesicles with medium flotation density enriched with exosomal markers were the most effective for tubular epithelial cell protection (68). Similarly, kidney function improved with exosomal-enriched but not with microvesicle-enriched secretome of bone marrow mesenchymal stem cells (69).
In animal models of diabetic nephropathy, these extracellular vesicles blunted albuminuria and relieved inflammation and fibrosis (70). They protected kidney function in some respects better than their parent mesenchymal stem cells (71), attenuated kidney fibrosis in unilateral ureteral obstruction (72), and ameliorated kidney injury in nephropathy (73). However, in rats with kidney transplant rejection, bone marrow mesenchymal stem cell extracellular vesicles did not prolong graft and recipient survival (74). Clearly, their utility might be dictated by the underlying disease. In addition, given the invasiveness of bone marrow collection, an allogeneic source might be warranted for clinical applications.
Adipose Mesenchymal Stem Cell–Derived Extracellular Vesicles.
In our studies, adipose mesenchymal stem cell–derived extracellular vesicles improved intrarenal microvascular density in pigs with renovascular disease, possibly by attenuating kidney inflammation and fibrosis (75–77). Like bone marrow mesenchymal stem cell extracellular vesicles, adipose mesenchymal stem cell extracellular vesicles ameliorated diabetic nephropathy. In db/db mice, adipose mesenchymal stem cell extracellular vesicles decreased podocyte apoptosis and enhanced autophagy, possibly by regulating miR-486/Smad1/mammalian target of rapamycin signaling (78).
However, the adipose tissue niche dictates the renoprotective function of these extracellular vesicles, as obesity or metabolic syndrome changes their cargoes and attenuates their therapeutic potency (79,80). Consequently, in pigs with renovascular disease, lean but not metabolic syndrome extracellular vesicles restored kidney function (81). Therefore, the ambient microenvironment of donor mesenchymal stem cells and extracellular vesicles should be taken into account when planning autologous transplantation (Figure 2). Future developments may need to improve mesenchymal stem cell function before autologous delivery.
Figure 2.
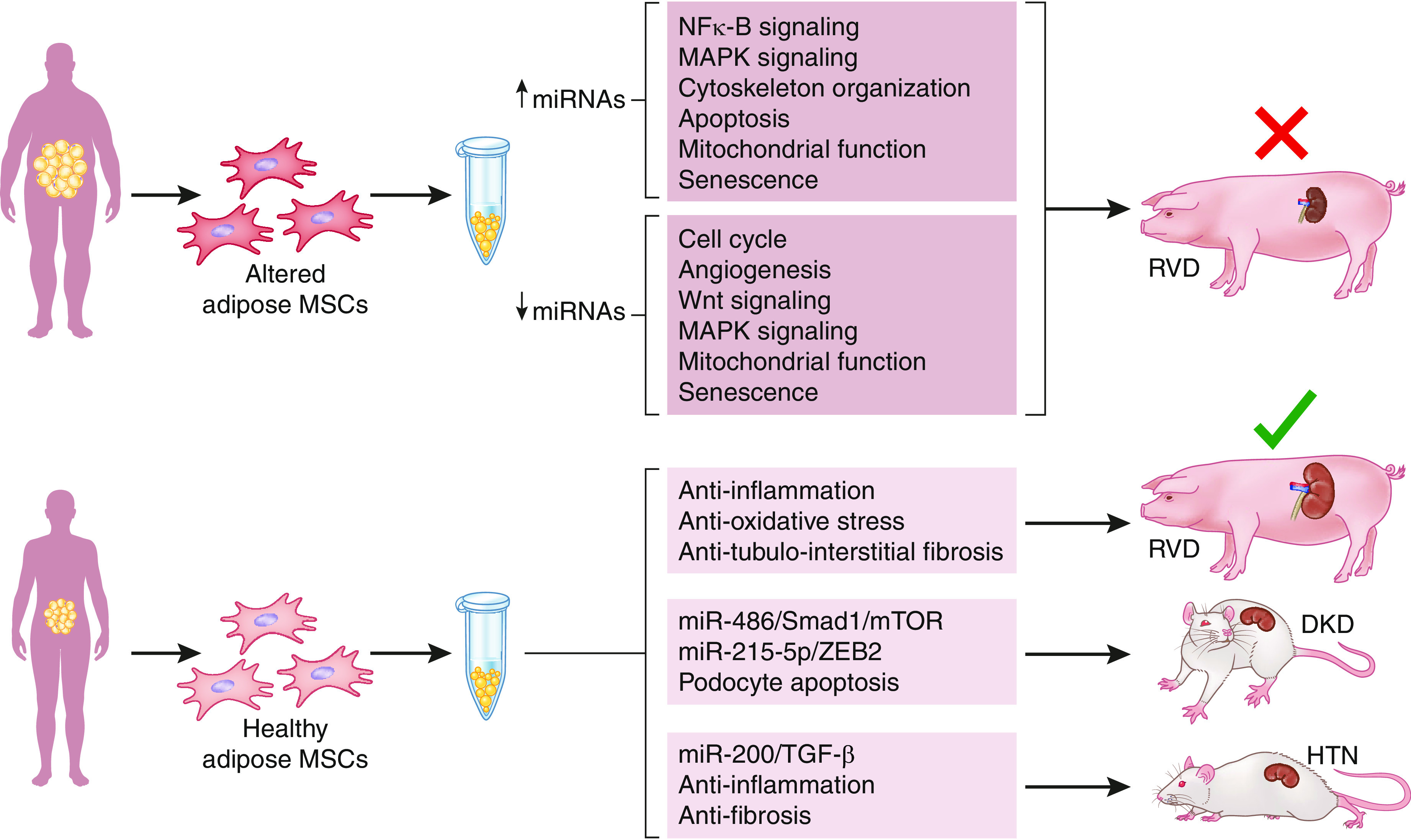
Obesity blunts the efficacy of adipose MSC-derived EVs as a therapeutic tool in kidney diseases. Lean adipose MSC-derived EVs cover curative effects, whereas obesity or metabolic syndrome changes their cargoes and blunts their ability to alleviate cell or organ injury. HTN, hypertensive nephropathy; MAPK, mitogen-activated protein kinase; miRNA, microRNA; mTOR, mammalian target of rapamycin; RVD, renovascular disease.
Umbilical Cord Vein Blood Mesenchymal Stem Cell–Derived Extracellular Vesicles.
The first clinical trial of extracellular vesicles used umbilical cord vein blood mesenchymal stem cell extracellular vesicles in 20 patients with CKD, in whom kidney function improved with no adverse effects (63). In animal models, such extracellular vesicles improved kidney function in ischemia-reperfusion injury and restarted the cell cycle in murine diabetic nephropathy (82). Notably, umbilical cord extracts, including growth factors, extracellular matrices, and exosomes, also increased the therapeutic effect of bone marrow mesenchymal stem cells in diabetic nephropathy (83).
Induced Pluripotent Stem Cell–Derived Extracellular Vesicles.
Induced pluripotent stem cells (iPSCs) are produced by reprogramming adult mesenchymal cells that can differentiate into various lineages, including mesenchymal stem cells. Importantly, although tissue-specific mesenchymal stem cells can be safely passaged to few generations, iPSCs can renew numerous times (84). Therefore, iPSC mesenchymal stem cell extracellular vesicles are receiving escalating attention in regenerative therapy. Like adipose mesenchymal stem cell–derived extracellular vesicles, iPSC mesenchymal stem cell extracellular vesicles exert antifibrotic, antioxidative stress, anti-inflammatory, and antiapoptosis functions (85,86).
Endothelial Progenitor Cell–Derived Extracellular Vesicles
Endothelial progenitor cells originate from bone marrow and bear some stem cell characteristics, but they are lineage committed. Extracellular vesicles released by these cells stimulate proliferation, differentiation, and angiogenesis, which make them a potential therapeutic tool. In rats, endothelial progenitor cell extracellular vesicles ameliorate kidney dysfunction and damage by enhancing tubular epithelial cell proliferation and reducing inflammation, possibly through their miRNA content (87). In rat GN, endothelial progenitor cell extracellular vesicles also ameliorated kidney function through immune/inflammatory regulation and antiapoptosis (88).
Tubular Epithelial Cell–Derived Extracellular Vesicles
Extracellular vesicles released by tubular epithelial cells mediate their crosstalk with other kidney resident and inflammatory cells (89,90). Notably, tubular epithelial cell extracellular vesicles have been implicated in the pathophysiology of several kidney diseases, such as diabetic nephropathy (91). Nonetheless, extracellular vesicles derived from healthy tubular epithelial cells might be effective regenerative tools. Tubular epithelial cell extracellular vesicles accelerate kidney recovery in ischemia-reperfusion injury by alleviating tissue damage, partly by activating transcription factor-3 and CD26 (92,93). We identified renoprotective effects of scattered tubular-like cell–derived extracellular vesicles. Scattered tubular-like cell extracellular vesicles improved mitochondrial function of injured tubular epithelial cells in vitro and stenotic kidney perfusion in vivo (94). However, unlike mesenchymal stem cells, tubular epithelial cells are generally not immune privileged, limiting the application of their extracellular vesicles to autologous formulations (95).
Extracellular vesicles derived from other sources that were studied in kidney disease include liver stem cells, urine, and immune cells.
Engineered Extracellular Vesicles in Kidney Disease
Clearly, extracellular vesicles show potential for kidney damage repair. However, variable stability and retention of certain extracellular vesicle populations affect their efficacy, and an unwholesome ancestry might yield suboptimal extracellular vesicles. The easiest and most widely studied method to engineer superior extracellular vesicles applies hypoxia (a form of stress), which induces the release of large numbers of extracellular vesicles with enhanced properties (96). Their efficacy has also been augmented by transfection with miRNA, specific miRNA mimics, or antagomirs (97,98). Protein overexpression of the pluripotency factor oct-4 in mesenchymal stem cell extracellular vesicles also enhanced their antiapoptotic effects in AKI (99). Intriguingly, kidney-targeting macrophage-derived microvesicles containing dexamethasone suppressed kidney inflammation and fibrosis (100), leveraging extracellular vesicles as a drug delivery vehicle. Also, extracellular vesicles harvested from parent cells engineered to release erythropoietin or neurotrophic factor improved anemia and peritubular capillary loss, respectively (101,102).
In addition to their cargo, the extracellular vesicle surface can be modified to harness their potential. Encapsulation in a collagen matrix (103) or hydrogel (104) augmented their kidney retention and repair thanks to continuous and steady release. Importantly, extracellular vesicles coated with anti-KIM1 antibodies showed better homing and restoration of kidney function and blunting of inflammation (105). Selective targeting is an exciting approach that may allow for the decrease of the dose and the minimization of off-target accumulation of extracellular vesicles. Of particular interest are strategies that leverage the known pathways of extracellular vesicles physiology (production, trafficking, release, etc.). Recently, two endogenous “scaffold” proteins of extracellular vesicles, PTGFRN and BASP1, have been identified. PTGFRN enables high-density surface display, and BASP1 promotes luminal loading of various molecules (106), providing a potential strategy for extracellular vesicle engineering. For example, antibodies against kidney cell proteins can be fused to PTGFRN and glucocorticoids loaded with BASP1 protein to specifically target kidney cells and deliver glucocorticoids to improve the efficacy and reduce side effects. Alternatively, lysosome-associated membrane protein-2, glycosylphosphatidylinositol, and C1C2 domain of lactadherin binding with phosphatidylserine can be exploited as anchors for kidney cell marker proteins. Also, because the source, size, and composition of extracellular vesicles can affect their preferential target (107), it is important to ensure which extracellular vesicles preferentially target the kidney.
Extracellular vesicle uptake is a low-yield process (108), and modification with a virus-derived fusogenic protein can significantly improve their cargo delivery efficiency (109). In addition, clathrin-mediated endocytosis increases extracellular vesicle uptake, whereas caveolin-dependent endocytosis is a negative regulator (110). Theoretically, given that LDL is taken up by cells through clathrin-mediated endocytosis, LDL coating may increase extracellular vesicle uptake through the same route and, in turn, magnify cargo delivery. Following uptake, some contents of extracellular vesicles are degraded by the lysosome, whereas some escape degradation by an unclear mechanism (111). Enabling extracellular vesicles to escape postuptake degradation can increase the efficiency of cargo delivery. Increasing extracellular vesicle circulation time can also improve the efficiency of cargo delivery and might be achievable by upregulating CD47 (112).
Challenges and Future Directions in the Study of Extracellular Vesicles
Despite comprehensive studies, extracellular vesicle application as a therapeutic tool has mostly been confined to animal models, with few small clinical trials published, possibly because their production cost, sensitivity, and specificity have yet to be validated against benchmarks, like biopsies or imaging modalities, in large clinical studies. Standardization of extracellular vesicle production and characterization is paramount to establish irrefutable confidence in their ability to deliver consistent regenerative effects (113). Improving methods of extracellular vesicle, isolation, and cargo detection is critical because they remain relatively expensive and complex. Randomized controlled clinical trials are needed to verify their efficacy and safety in kidney diseases and compare extracellular vesicles harvested from different sources. The administration route and dose of extracellular vesicles also affect their distribution. Intra-arterial or intrarenal injection has been the main route of extracellular vesicle delivery to decrease lung and liver uptake, but specific targeting may permit systemic delivery in kidney diseases. A dose of 100 μg/kg (protein per body weight) is effective for umbilical cord mesenchymal stem cell–derived extracellular vesicles to ameliorate CKD in patients (63). Similarly, delivery of 1011 particles of mesenchymal stem cell–derived extracellular vesicles reduces pig ischemic kidney damage (114), and the amount obtained from 106 to 107 mesenchymal stem cells per kilogram of body weight reduce graft-versus-host disease in patients (115). However, their dose-effect relationships should be determined in uniform units, and the source, scalability, and regimen of extracellular vesicles should be optimized. Moreover, although large-scale cell culture systems (e.g., stirred tank bioreactors) can be used for mass production of extracellular vesicles, stem cells cannot expand indefinitely, confounding their clinical value as sources for extracellular vesicles. iPSCs characterized by continuous renewal might be an ideal source for extracellular vesicles, but supporting animal and clinical studies are needed. Engineered retrofitting may further improve extracellular vesicle retention and decrease off-target effects, decreasing their dose and cost (116). Such new promising directions to enhance efficacy and stability may paint a brighter future for engineered extracellular vesicles. Confidence in the diagnostic validity of their cargo would be enhanced by linking it mechanistically to pathophysiologic processes in specific diseases and by reproducing findings at independent laboratories. This would also require strict standardization of harvesting, characterization, and delivery regimens.
Furthermore, although extracellular vesicles are conventionally assumed to provoke little immune response (66) relative to their parent cells, this notion has not been rigorously tested. In particular, extracellular vesicles derived from nonimmune-privileged cells, like tubular epithelial cells, might well maintain their immunogenicity (117), warranting caution during in vivo applications.
In conclusion, extracellular vesicles bear an exciting promise and might evolve to constitute efficacious and versatile off-the-shelf biologic products. Additional studies are needed to standardize and increase the production; validate efficacy; and optimize targeted homing, local retention, and therapeutic cargo of these novel particles. Hopefully, such studies would establish the feasibility of these particles as a useful theranostic tool in kidney disease.
Disclosures
W. Huang is supported by China scholarship council and reports research funding from the National Natural Science Foundation of China and honoraria from Dongzhimen Hospital. A. Lerman reports consultancy agreements with AstraZeneca, Itamar Medical, Phillips, and Shahal Telecommunication and serves as an advisor to CureSpec. L.O. Lerman reports consultancy agreements with AstraZeneca, Butterfly Biosciences, and CureSpec; research funding from AstraZeneca; honoraria from AstraZeneca, Butterfly Biosciences, and CureSpec; patents or royalties from Cohbar and Stealth Biopharmaceuticals; serving in an advisory or leadership role with AstraZeneca and CureSpec; and other interests or relationships with the American Heart Association and the National Institutes of Health. The remaining author has nothing to disclose.
Funding
This study was supported by National Institutes of Health grants DK120292, DK122734, and AG062104.
Supplementary Material
Acknowledgments
Randall J. Fritz (Mayo Clinic) substantively edited the manuscript. The scientific publications staff at Mayo Clinic provided proofreading, administrative, and clerical support. We also thank the China Scholarship Council for support.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
L.O. Lerman conceptualized the study; W. Huang was responsible for formal analysis; W. Huang was responsible for methodology; L.O. Lerman was responsible for funding acquisition; L.O. Lerman provided supervision; W. Huang wrote the original draft; and W. Huang, A. Lerman, L.O. Lerman, and X.-Y. Zhu reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16751221/-/DCSupplemental.
Supplemental Table 1. Promising biomarkers of kidney disease in urinary EVs.
Supplemental Table 2. Circulating EVs as biomarkers in kidney disease.
References
- 1.Akers JC, Gonda D, Kim R, Carter BS, Chen CC: Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 113: 1–11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G: Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126: 5553–5565, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M: Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Cone AS, Hurwitz SN, Lee GS, Yuan X, Zhou Y, Li Y, Meckes DG Jr.: Alix and Syntenin-1 direct amyloid precursor protein trafficking into extracellular vesicles. BMC Mol Cell Biol 21: 58, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei D, Zhan W, Gao Y, Huang L, Gong R, Wang W, Zhang R, Wu Y, Gao S, Kang T: RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res 31: 157–177, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C: Microvesicles: Mediators of extracellular communication during cancer progression. J Cell Sci 123: 1603–1611, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erwig LP, Henson PM: Clearance of apoptotic cells by phagocytes. Cell Death Differ 15: 243–250, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Gardiner C, Di Vizio D, Sahoo S, Théry C, Witwer KW, Wauben M, Hill AF: Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J Extracell Vesicles 5: 32945, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK, Nayak B, Mohanty S: An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res Ther 9: 180, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monguió-Tortajada M, Gálvez-Montón C, Bayes-Genis A, Roura S, Borràs FE: Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell Mol Life Sci 76: 2369–2382, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreiro K, Dwivedi OP, Leparc G, Rolser M, Delic D, Forsblom C, Groop PH, Groop L, Huber TB, Puhka M, Holthofer H: Comparison of urinary extracellular vesicle isolation methods for transcriptomic biomarker research in diabetic kidney disease. J Extracell Vesicles 10: e12038, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Channavajjhala SK, Rossato M, Morandini F, Castagna A, Pizzolo F, Bazzoni F, Olivieri O: Optimizing the purification and analysis of miRNAs from urinary exosomes. Clin Chem Lab Med 52: 345–354, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Toh WS, Lai RC, Zhang B, Lim SK: MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans 46: 843–853, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Souza-Schorey C, van Donselaar E, Hsu VW, Yang C, Stahl PD, Peters PJ: ARF6 targets recycling vesicles to the plasma membrane: Insights from an ultrastructural investigation. J Cell Biol 140: 603–616, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clancy JW, Sedgwick A, Rosse C, Muralidharan-Chari V, Raposo G, Method M, Chavrier P, D’Souza-Schorey C: Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat Commun 6: 6919, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu X, Song Y, Masud A, Nuti K, DeRouchey JE, Richards CI: High-throughput fluorescence correlation spectroscopy enables analysis of surface components of cell-derived vesicles. Anal Bioanal Chem 412: 2589–2597, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capomaccio S, Cappelli K, Bazzucchi C, Coletti M, Gialletti R, Moriconi F, Passamonti F, Pepe M, Petrini S, Mecocci S, Silvestrelli M, Pascucci L: Equine adipose-derived mesenchymal stromal cells release extracellular vesicles enclosing different subsets of small RNAs. Stem Cells Int 2019: 4957806, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koifman N, Biran I, Aharon A, Brenner B, Talmon Y: A direct-imaging cryo-EM study of shedding extracellular vesicles from leukemic monocytes. J Struct Biol 198: 177–185, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Wu P, Zhang B, Ocansey DKW, Xu W, Qian H: Extracellular vesicles: A bright star of nanomedicine. Biomaterials 269: 120467, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z: Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311: F844–F851, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun IO, Lerman LO: Urinary extracellular vesicles as biomarkers of kidney disease: From diagnostics to therapeutics. Diagnostics (Basel) 10: 311, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, Poulsen SB, Murali SK, Grimm PR, Su XT, Delpire E, Welling PA, Ellison DH, Fenton RA: Large-scale proteomic assessment of urinary extracellular vesicles highlights their reliability in reflecting protein changes in the kidney. J Am Soc Nephrol 32: 2195–2209, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun AL, Deng JT, Guan GJ, Chen SH, Liu YT, Cheng J, Li ZW, Zhuang XH, Sun FD, Deng HP: Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diab Vasc Dis Res 9: 301–308, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Zubiri I, Posada-Ayala M, Benito-Martin A, Maroto AS, Martin-Lorenzo M, Cannata-Ortiz P, de la Cuesta F, Gonzalez-Calero L, Barderas MG, Fernandez-Fernandez B, Ortiz A, Vivanco F, Alvarez-Llamas G: Kidney tissue proteomics reveals regucalcin downregulation in response to diabetic nephropathy with reflection in urinary exosomes. Transl Res 166: 474–484.e4, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR: Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol 25: 1401–1407, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wachalska M, Koppers-Lalic D, van Eijndhoven M, Pegtel M, Geldof AA, Lipinska AD, van Moorselaar RJ, Bijnsdorp IV: Protein complexes in urine interfere with extracellular vesicle biomarker studies. J Circ Biomark 5: 4, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Lin HY, Assaker JP, Jeong S, Huang CH, Kurdi T, Lee K, Fraser K, Min C, Eskandari S, Routray S, Tannous B, Abdi R, Riella L, Chandraker A, Castro CM, Weissleder R, Lee H, Azzi JR: Integrated kidney exosome analysis for the detection of kidney transplant rejection. ACS Nano 11: 11041–11046, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimuccio V, Ranghino A, Praticò Barbato L, Fop F, Biancone L, Camussi G, Bussolati B: Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS One 9: e104490, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez S, Suazo C, Boltansky A, Ursu M, Carvajal D, Innocenti G, Vukusich A, Hurtado M, Villanueva S, Carreño JE, Rogelio A, Irarrazabal CE: Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant Proc 45: 3719–3723, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Braun F, Rinschen M, Buchner D, Bohl K, Plagmann I, Bachurski D, Richard Späth M, Antczak P, Göbel H, Klein C, Lackmann JW, Kretz O, Puelles VG, Wahba R, Hallek M, Schermer B, Benzing T, Huber TB, Beyer A, Stippel D, Kurschat CE, Müller RU: The proteomic landscape of small urinary extracellular vesicles during kidney transplantation. J Extracell Vesicles 10: e12026, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshikawa-Hori S, Yokota-Ikeda N, Sonoda H, Ikeda M: Urinary extracellular vesicular release of aquaporins in patients with renal transplantation. BMC Nephrol 20: 216, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MH, Lee YH, Seo JW, Moon H, Kim JS, Kim YG, Jeong KH, Moon JY, Lee TW, Ihm CG, Kim CD, Park JB, Chung BH, Kim YH, Lee SH: Urinary exosomal viral microRNA as a marker of BK virus nephropathy in kidney transplant recipients. PLoS One 12: e0190068, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Hu ZB, Chen PP, Lu CC, Zhang JX, Li XQ, Yuan BY, Huang SJ, Ma KL: Urinary podocyte microparticles are associated with disease activity and renal injury in systemic lupus erythematosus. BMC Nephrol 20: 303, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raimondo F, Morosi L, Corbetta S, Chinello C, Brambilla P, Della Mina P, Villa A, Albo G, Battaglia C, Bosari S, Magni F, Pitto M: Differential protein profiling of renal cell carcinoma urinary exosomes. Mol Biosyst 9: 1220–1233, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Kurahashi R, Kadomatsu T, Baba M, Hara C, Itoh H, Miyata K, Endo M, Morinaga J, Terada K, Araki K, Eto M, Schmidt LS, Kamba T, Linehan WM, Oike Y: MicroRNA-204-5p: A novel candidate urinary biomarker of Xp11.2 translocation renal cell carcinoma. Cancer Sci 110: 1897–1908, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon SH, Woollard JR, Saad A, Garovic VD, Zand L, Jordan KL, Textor SC, Lerman LO: Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol Dial Transplant 32: 800–807, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santelli A, Sun IO, Eirin A, Abumoawad AM, Woollard JR, Lerman A, Textor SC, Puranik AS, Lerman LO: Senescent kidney cells in hypertensive patients release urinary extracellular vesicles. J Am Heart Assoc 8: e012584, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun IO, Santelli A, Abumoawad A, Eirin A, Ferguson CM, Woollard JR, Lerman A, Textor SC, Puranik AS, Lerman LO: Loss of renal peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension 72: 1180–1188, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Hernandez J, Olivares D, Forner MJ, Ortega A, Solaz E, Martinez F, Chaves FJ, Redon J, Cortes R: Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J Transl Med 16: 228, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Y, Lv LL, Wu WJ, Li ZL, Chen J, Ni HF, Zhou LT, Tang TT, Wang FM, Wang B, Chen PS, Crowley SD, Liu BC: Urinary exosomes and exosomal CCL2 mRNA as biomarkers of active histologic injury in IgA nephropathy. Am J Pathol 188: 2542–2552, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Moon PG, Lee JE, You S, Kim TK, Cho JH, Kim IS, Kwon TH, Kim CD, Park SH, Hwang D, Kim YL, Baek MC: Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 11: 2459–2475, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Hogan MC, Bakeberg JL, Gainullin VG, Irazabal MV, Harmon AJ, Lieske JC, Charlesworth MC, Johnson KL, Madden BJ, Zenka RM, McCormick DJ, Sundsbak JL, Heyer CM, Torres VE, Harris PC, Ward CJ: Identification of biomarkers for PKD1 using urinary exosomes. J Am Soc Nephrol 26: 1661–1670, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilani SI, Anderson UD, Jayachandran M, Weissgerber TL, Zand L, White WM, Milic N, Suarez MLG, Vallapureddy RR, Nääv Å, Erlandsson L, Lieske JC, Grande JP, Nath KA, Hansson SR, Garovic VD: Urinary extracellular vesicles of podocyte origin and renal injury in preeclampsia. J Am Soc Nephrol 28: 3363–3372, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimuccio V, Peruzzi L, Brizzi MF, Cocchi E, Fop F, Boido A, Gili M, Gallo S, Biancone L, Camussi G, Bussolati B: Acute and chronic glomerular damage is associated with reduced CD133 expression in urinary extracellular vesicles. Am J Physiol Renal Physiol 318: F486–F495, 2020 [DOI] [PubMed] [Google Scholar]
- 45.Pathare G, Dhayat NA, Mohebbi N, Wagner CA, Bobulescu IA, Moe OW, Fuster DG: Changes in V-ATPase subunits of human urinary exosomes reflect the renal response to acute acid/alkali loading and the defects in distal renal tubular acidosis. Kidney Int 93: 871–880, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang LH, Zhu XY, Eirin A, Nargesi AA, Woollard JR, Santelli A, Sun IO, Textor SC, Lerman LO: Early podocyte injury and elevated levels of urinary podocyte-derived extracellular vesicles in swine with metabolic syndrome: Role of podocyte mitochondria. Am J Physiol Renal Physiol 317: F12–F22, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ichii O, Otsuka-Kanazawa S, Horino T, Kimura J, Nakamura T, Matsumoto M, Toi M, Kon Y: Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS One 9: e110383, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H, Kajiyama H, Tsuji T, Hu X, Leelahavanichkul A, Vento S, Frank R, Kopp JB, Trachtman H, Star RA, Yuen PS: Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol 305: F553–F559, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cobbs A, Chen X, Zhang Y, George J, Huang MB, Bond V, Thompson W, Zhao X: Saturated fatty acid stimulates production of extracellular vesicles by renal tubular epithelial cells. Mol Cell Biochem 458: 113–124, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Boccio P, Raimondo F, Pieragostino D, Morosi L, Cozzi G, Sacchetta P, Magni F, Pitto M, Urbani A: A hyphenated microLC-Q-TOF-MS platform for exosomal lipidomics investigations: Application to RCC urinary exosomes. Electrophoresis 33: 689–696, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, Sandvig K, Llorente A: Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer 70: 122–132, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Brzozowski JS, Jankowski H, Bond DR, McCague SB, Munro BR, Predebon MJ, Scarlett CJ, Skelding KA, Weidenhofer J: Lipidomic profiling of extracellular vesicles derived from prostate and prostate cancer cell lines. Lipids Health Dis 17: 211, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito M, Horie S, Yasuhara H, Kashimura A, Sugiyama E, Saijo T, Mizuno H, Kitajima H, Todoroki K: Metabolomic profiling of urine-derived extracellular vesicles from rat model of drug-induced acute kidney injury. Biochem Biophys Res Commun 546: 103–110, 2021 [DOI] [PubMed] [Google Scholar]
- 54.Sonoda H, Lee BR, Park KH, Nihalani D, Yoon JH, Ikeda M, Kwon SH: miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci Rep 9: 4692, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bulacio RP, Nosetto EC, Brandoni A, Torres AM: Novel finding of caveolin-2 in apical membranes of proximal tubule and first detection of caveolin-2 in urine: A promising biomarker of renal disease. J Cell Biochem 120: 4966–4974, 2019 [DOI] [PubMed] [Google Scholar]
- 56.Fujitaka K, Murakami T, Takeuchi M, Kakimoto T, Mochida H, Arakawa K: mRNAs in urinary nano-extracellular vesicles as potential biomarkers for non-invasive kidney biopsy. Biomed Rep 14: 11, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai FH, Wu WY, Zhou XJ, Yu XJ, Lv JC, Wang SX, Liu G, Yang L: Diagnostic roles of urinary kidney microvesicles in diabetic nephropathy. Ann Transl Med 8: 1431, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benito-Martin A, Ucero AC, Zubiri I, Posada-Ayala M, Fernandez-Fernandez B, Cannata-Ortiz P, Sanchez-Nino MD, Ruiz-Ortega M, Egido J, Alvarez-Llamas G, Ortiz A: Osteoprotegerin in exosome-like vesicles from human cultured tubular cells and urine. PLoS One 8: e72387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saejong S, Townamchai N, Somparn P, Tangtanatakul P, Ondee T, Hirankarn N, Leelahavanichkul A: MicroRNA-21 in plasma exosome, but not from whole plasma, as a biomarker for the severe interstitial fibrosis and tubular atrophy (IF/TA) in post-renal transplantation [published online ahead of print June 21, 2020]. Asian Pac J Allergy Immunol 10.12932/AP-101019-0656 [DOI] [PubMed] [Google Scholar]
- 60.Kim H, Bae YU, Jeon JS, Noh H, Park HK, Byun DW, Han DC, Ryu S, Kwon SH: The circulating exosomal microRNAs related to albuminuria in patients with diabetic nephropathy. J Transl Med 17: 236, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams RM, Shah J, Ng BD, Minton DR, Gudas LJ, Park CY, Heller DA: Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett 15: 2358–2364, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G: Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20: 1053–1067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H: Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res 20: 21, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nowak N, Yamanouchi M, Satake E: The nephroprotective properties of extracellular vesicles in experimental models of chronic kidney disease: A systematic review. Stem Cell Rev Rep 18: 902–932, 2022. 10.1007/s12015-021-10189-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C, Wang J, Hu J, Fu B, Mao Z, Zhang H, Cai G, Chen X, Sun X: Extracellular vesicles for acute kidney injury in preclinical rodent models: A meta-analysis. Stem Cell Res Ther 11: 11, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle K, Chalmers J, Schmittgen TD, Phelps MA: Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles 6: 1324730, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang C, Zhu G, He W, Yin H, Lin F, Gou X, Li X: BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. FASEB J 33: 5440–5456, 2019 [DOI] [PubMed] [Google Scholar]
- 68.Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, Quesenberry P, Camussi G: Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev Rep 13: 226–243, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R, Neri F, Kholia S, Giunti S, Wen S, Quesenberry P, Camussi G: Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A 23: 1262–1273, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M: Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep 6: 34842, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He J, Wang Y, Lu X, Zhu B, Pei X, Wu J, Zhao W: Micro vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton) 20: 591–600, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Shi Z, Wang Q, Zhang Y, Jiang D: Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem Cell Res Ther 11: 253, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kholia S, Herrera Sanchez MB, Cedrino M, Papadimitriou E, Tapparo M, Deregibus MC, Bruno S, Antico F, Brizzi MF, Quesenberry PJ, Camussi G: Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front Cell Dev Biol 8: 188, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramirez-Bajo MJ, Rovira J, Lazo-Rodriguez M, Banon-Maneus E, Tubita V, Moya-Rull D, Hierro-Garcia N, Ventura-Aguiar P, Oppenheimer F, Campistol JM, Diekmann F: Impact of mesenchymal stromal cells and their extracellular vesicles in a rat model of kidney rejection. Front Cell Dev Biol 8: 10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song T, Eirin A, Zhu X, Zhao Y, Krier JD, Tang H, Jordan KL, Woollard JR, Taner T, Lerman A, Lerman LO: Mesenchymal stem cell-derived extracellular vesicles induce regulatory T cells to ameliorate chronic kidney injury. Hypertension 75: 1223–1232, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ, Lerman LO: Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant 27: 1080–1095, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO: Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 92: 114–124, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, Huang H: Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther 10: 95, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eirin A, Meng Y, Zhu XY, Li Y, Saadiq IM, Jordan KL, Tang H, Lerman A, van Wijnen AJ, Lerman LO: The micro-RNA cargo of extracellular vesicles released by human adipose tissue-derived mesenchymal stem cells is modified by obesity. Front Cell Dev Biol 9: 660851, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Meng Y, Zhu X, Saadiq IM, Jordan KL, Eirin A, Lerman LO: Metabolic syndrome increases senescence-associated micro-RNAs in extracellular vesicles derived from swine and human mesenchymal stem/stromal cells. Cell Commun Signal 18: 124, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eirin A, Ferguson CM, Zhu XY, Saadiq IM, Tang H, Lerman A, Lerman LO: Extracellular vesicles released by adipose tissue-derived mesenchymal stromal/stem cells from obese pigs fail to repair the injured kidney. Stem Cell Res (Amst) 47: 101877, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong L, Liao G, Wang X, Li L, Zhang J, Chen Y, Liu J, Liu S, Wei L, Zhang W, Lu Y: Mesenchymal stem cells-microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19. Exp Biol Med (Maywood) 243: 1233–1242, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Saijo Y, Tsuchida H, Ishioka S, Nishikawa A, Saito T, Fujimiya M: Umbilical cord extracts improve diabetic abnormalities in bone marrow-derived mesenchymal stem cells and increase their therapeutic effects on diabetic nephropathy. Sci Rep 7: 8484, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karch SB, Fineschi V, Francia P, Scopetti M, Padovano M, Manetti F, Santurro A, Frati P, Volpe M: Role of induced pluripotent stem cells in diagnostic cardiology. World J Stem Cells 13: 331–341, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuan X, Li D, Chen X, Han C, Xu L, Huang T, Dong Z, Zhang M: Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis 8: 3200, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu L, Wu Y, Wang P, Shi M, Wang J, Ma H, Sun D: PSC-MSC-derived exosomes protect against kidney fibrosis in vivo and in vitro through the SIRT6/β-catenin signaling pathway. Int J Stem Cells 14: 310–319, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bitzer M, Ben-Dov IZ, Thum T: Microparticles and microRNAs of endothelial progenitor cells ameliorate acute kidney injury. Kidney Int 82: 375–377, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cantaluppi V, Medica D, Mannari C, Stiaccini G, Figliolini F, Dellepiane S, Quercia AD, Migliori M, Panichi V, Giovannini L, Bruno S, Tetta C, Biancone L, Camussi G: Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol Dial Transplant 30: 410–422, 2015 [DOI] [PubMed] [Google Scholar]
- 89.Lv LL, Feng Y, Wu M, Wang B, Li ZL, Zhong X, Wu WJ, Chen J, Ni HF, Tang TT, Tang RN, Lan HY, Liu BC: Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ 27: 210–226, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gildea JJ, Seaton JE, Victor KG, Reyes CM, Bigler Wang D, Pettigrew AC, Courtner CE, Shah N, Tran HT, Van Sciver RE, Carlson JM, Felder RA: Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin Biochem 47: 89–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang WJ, Xu CT, Du CL, Dong JH, Xu SB, Hu BF, Feng R, Zang DD, Meng XM, Huang C, Li J, Ma TT: Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics 12: 324–339, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dominguez JM 2nd, Dominguez JH, Xie D, Kelly KJ: Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. PLoS One 13: e0202550, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen HH, Lai PF, Lan YF, Cheng CF, Zhong WB, Lin YF, Chen TW, Lin H: Exosomal ATF3 RNA attenuates pro-inflammatory gene MCP-1 transcription in renal ischemia-reperfusion. J Cell Physiol 229: 1202–1211, 2014 [DOI] [PubMed] [Google Scholar]
- 94.Zou X, Kwon SH, Jiang K, Ferguson CM, Puranik AS, Zhu X, Lerman LO: Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci Rep 8: 1263, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harari-Steinberg O, Omer D, Gnatek Y, Pleniceanu O, Goldberg S, Cohen-Zontag O, Pri-Chen S, Kanter I, Ben Haim N, Becker E, Ankawa R, Fuchs Y, Kalisky T, Dotan Z, Dekel B: Ex vivo expanded 3D human kidney spheres engraft long term and repair chronic renal injury in mice. Cell Rep 30: 852–869.e4, 2020 [DOI] [PubMed] [Google Scholar]
- 96.Isik B, Thaler R, Goksu BB, Conley SM, Al-Khafaji H, Mohan A, Afarideh M, Abumoawad AM, Zhu XY, Krier JD, Saadiq IM, Tang H, Eirin A, Hickson LJ, van Wijnen AJ, Textor SC, Lerman LO, Herrmann SM: Hypoxic preconditioning induces epigenetic changes and modifies swine mesenchymal stem cell angiogenesis and senescence in experimental atherosclerotic renal artery stenosis. Stem Cell Res Ther 12: 240, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang A, Wang H, Wang B, Yuan Y, Klein JD, Wang XH: Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease. FASEB J 33: 13590–13601, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin J, Qian F, Zheng D, He W, Gong J, He Q: Mesenchymal stem cells attenuate renal fibrosis via exosomes-mediated delivery of microRNA Let-7i-5p antagomir. Int J Nanomedicine 16: 3565–3578, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang ZY, Hou YP, Zou XY, Xing XY, Ju GQ, Zhong L, Sun J: Oct-4 enhanced the therapeutic effects of mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. Kidney Blood Press Res 45: 95–108, 2020 [DOI] [PubMed] [Google Scholar]
- 100.Tang TT, Lv LL, Wang B, Cao JY, Feng Y, Li ZL, Wu M, Wang FM, Wen Y, Zhou LT, Ni HF, Chen PS, Gu N, Crowley SD, Liu BC: Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: An efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics 9: 4740–4755, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi HY, Kim TY, Lee M, Kim SH, Jhee JH, Lee YK, Kim HJ, Park HC: Kidney mesenchymal stem cell-derived extracellular vesicles engineered to express erythropoietin improve renal anemia in mice with chronic kidney disease. Stem Cell Rev Rep 18: 980–992, 2022. 10.1007/s12015-021-10141-x [DOI] [PubMed] [Google Scholar]
- 102.Chen L, Wang Y, Li S, Zuo B, Zhang X, Wang F, Sun D: Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics 10: 9425–9442, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Y, Cui J, Wang H, Hezam K, Zhao X, Huang H, Chen S, Han Z, Han ZC, Guo Z, Li Z: Enhanced therapeutic effects of MSC-derived extracellular vesicles with an injectable collagen matrix for experimental acute kidney injury treatment. Stem Cell Res Ther 11: 161, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang C, Shang Y, Chen X, Midgley AC, Wang Z, Zhu D, Wu J, Chen P, Wu L, Wang X, Zhang K, Wang H, Kong D, Yang Z, Li Z, Chen X: Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano 14: 12133–12147, 2020 [DOI] [PubMed] [Google Scholar]
- 105.Chen XJ, Jiang K, Ferguson CM, Tang H, Zhu X, Lerman A, Lerman LO: Augmented efficacy of exogenous extracellular vesicles targeted to injured kidneys. Signal Transduct Target Ther 5: 199, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dooley K, McConnell RE, Xu K, Lewis ND, Haupt S, Youniss MR, Martin S, Sia CL, McCoy C, Moniz RJ, Burenkova O, Sanchez-Salazar J, Jang SC, Choi B, Harrison RA, Houde D, Burzyn D, Leng C, Kirwin K, Ross NL, Finn JD, Gaidukov L, Economides KD, Estes S, Thornton JE, Kulman JD, Sathyanarayanan S, Williams DE: A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol Ther 29: 1729–1743, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P: Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp Mol Med 51: 1–12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G: Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun 12: 1864, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Somiya M, Kuroda S: Real-time luminescence assay for cytoplasmic cargo delivery of extracellular vesicles. Anal Chem 93: 5612–5620, 2021 [DOI] [PubMed] [Google Scholar]
- 110.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, Xiao ZD: Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 289: 22258–22267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Izquierdo-Useros N, Naranjo-Gómez M, Archer J, Hatch SC, Erkizia I, Blanco J, Borràs FE, Puertas MC, Connor JH, Fernández-Figueras MT, Moore L, Clotet B, Gummuluru S, Martinez-Picado J: Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113: 2732–2741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R: Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546: 498–503, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens- Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr., Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales- Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr., Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK: Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Y, Zhu X, Zhang L, Ferguson CM, Song T, Jiang K, Conley SM, Krier JD, Tang H, Saadiq I, Jordan KL, Lerman A, Lerman LO: Mesenchymal stem/stromal cells and their extracellular vesicle progeny decrease injury in poststenotic swine kidney through different mechanisms. Stem Cells Dev 29: 1190–1200, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B: MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28: 970–973, 2014 [DOI] [PubMed] [Google Scholar]
- 116.Qin B, Zhang Q, Chen D, Yu HY, Luo AX, Suo LP, Cai Y, Cai DY, Luo J, Huang JF, Xiong K: Extracellular vesicles derived from mesenchymal stem cells: A platform that can be engineered. Histol Histopathol 36: 615–632, 2021 [DOI] [PubMed] [Google Scholar]
- 117.Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, Morelli AE: Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest 126: 2805–2820, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


