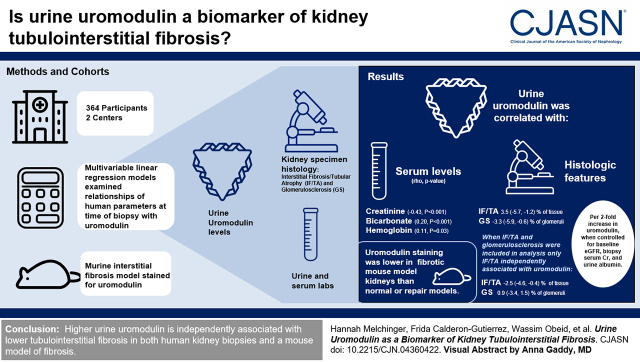
Visual Abstract
Keywords: biomarker, kidney biopsy, interstitial fibrosis, glomerulosclerosis, cross-sectional analysis, uromodulin
Abstract
Background and objectives
Uromodulin, produced exclusively in the kidney’s thick ascending limb, is a biomarker of kidney tubular health. However, the relationship between urine uromodulin and histologic changes in the kidney tubulointerstitium has not been characterized. In this study, we test the association of urine uromodulin with kidney histologic findings in humans and mice.
Design, setting, participants, & measurements
We investigated the independent association of urine uromodulin measured at the time of kidney biopsy with histologic features in 364 participants at two academic medical centers from 2015 to 2018 using multivariable linear regression models. This relationship was further examined by comparison of uromodulin staining in murine models of kidney fibrosis and repair.
Results
We found urine uromodulin to be correlated with serum creatinine (rho=−0.43; P<0.001), bicarbonate (0.20; P<0.001), and hemoglobin (0.11; P=0.03) at the time of biopsy but not with urine albumin (−0.07; P=0.34). Multivariable models controlling for prebiopsy GFR, serum creatinine at biopsy, and urine albumin showed higher uromodulin to be associated with lower severity of interstitial fibrosis/tubular atrophy and glomerulosclerosis (interstitial fibrosis/tubular atrophy: −3.5% [95% confidence intervals, −5.7% to −1.2%] and glomerulosclerosis: −3.3% [95% confidence intervals, −5.9% to −0.6%] per two-fold difference in uromodulin). However, when both interstitial fibrosis/tubular atrophy and glomerulosclerosis were included in multivariable analysis, only interstitial fibrosis/tubular atrophy was independently associated with uromodulin (interstitial fibrosis/tubular atrophy: −2.5% [95% confidence intervals, −4.6% to −0.4%] and glomerulosclerosis: −0.9% [95% confidence intervals, −3.4% to 1.5%] per two-fold difference in uromodulin). In mouse kidneys, uromodulin staining was found to be lower in the fibrotic model than in normal or repaired models.
Conclusions
Higher urine uromodulin is independently associated with lower tubulointerstitial fibrosis in both human kidney biopsies and a mouse model of fibrosis.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2022_08_10_CJN04360422.mp3.
Introduction
In clinical practice, kidney diseases are diagnosed and monitored using markers of glomerular function, such as eGFR using serum creatinine, or glomerular damage, such as urine albumin or protein. It is well recognized that the prognosis of kidney disease correlates not only with glomerular function but also with tubular health. As a result, many biomarkers of tubular health and disease are being evaluated to provide a comprehensive assessment of kidney health and disease (1).
Uromodulin, also known as Tamm–Horsfall protein, is a recognized biomarker of kidney tubular function. Uromodulin is produced exclusively in segments of the kidney tubule, including the thick ascending limb of the loop of Henle and the early distal convoluted tubule, and it is the most abundant glycoprotein found in typical urine (2). Uromodulin plays a role in the regulation of transport processes in the early distal convoluted tubule (3); the control of BP, water balance, and urine concentration (4); and protection against kidney stone formation and urinary tract infections. Genome-wide association studies have identified variants in the UMOD gene region as some of the strongest predictors of kidney function later in life (5,6). Epidemiologic studies have also shown that higher urine uromodulin is associated with lower risk of kidney disease (7).
We hypothesized that higher urine uromodulin levels indicate better kidney tubular health. Therefore, we predicted that histologic analysis of patients with higher urine uromodulin would reveal less severe chronic tubular damage, specifically decreased interstitial fibrosis and tubular atrophy (IF/TA). In this study, we test this hypothesis by examining the association of urine uromodulin with histologic findings from a cohort of participants who had urine samples collected at the time of their kidney biopsy. We then confirmed the association of uromodulin with kidney fibrosis using experimental animal models.
Materials and Methods
Recruitment and Setting of Patients Undergoing Biopsy
This is a substudy of the previously described Yale biopsy cohort (8,9). We enrolled patients undergoing a kidney biopsy at two Yale-affiliated sites, Yale New Haven Hospital and Saint Raphael’s Hospital, between January 2015 and June 2018. Both hospitals are in New Haven, Connecticut. We enrolled participants using consecutive sampling, excluding patients undergoing biopsies for evaluation of either transplanted kidneys or kidney malignancies. For this substudy, we excluded participants who either failed to provide a urine sample for analysis or did not undergo a biopsy after enrollment. This study was approved by the Yale Human Investigation Committee under approval number 11110009286. All participants provided written informed consent.
Data Sources
Exposure: Uromodulin Level.
Urine samples were collected a median (interquartile range [IQR]) of 1.6 (IQR, 1.1–2.4) hours before biopsy and centrifuged, with supernatant stored at −80°C for a median (IQR) of 1.1 (IQR, 0.7–1.4) years before analysis. Urine supernatant samples were thawed using a single controlled thaw for analysis. We used a uromodulin assay from Mesoscale Discovery with a mean (SD) coefficient of variation of 3% (±2.5) as previously described (7,10). The personnel performing biomarker assays were blinded to clinical data, including histologic features. In the primary analysis, we indexed uromodulin biomarkers by urine creatinine to account for differences in urine concentration. In a sensitivity analysis, we used “raw” or unindexed uromodulin values. We performed urine dipstick analysis with the Clintek status analyzer (Siemens Healthcare Diagnostics Inc.) and urine sediment microscopy using a Laxco LMCYBF (Fisher Scientific).
Outcome: Histologic Data.
We collected data on histologic features through review of clinical histology reports, noting both histologic diagnosis and the severity of histologic features. This included data on IF/TA, glomerulosclerosis, infiltrate, eosinophils, arterionephrosclerosis, tubulitis, tubular injury, and crescents (Supplemental Table 1). We recorded IF/TA as a percentage of the biopsy affected on the basis of clinical histology reports. Wherever possible, we also converted qualitative and quantitative terms into ordinal categories.
Other Data Sources.
We collected clinical history; laboratory results, including serum creatinine and urine protein-creatinine ratio; medications; demographic data; and imaging findings, including kidney size, through review of participants’ electronic health records within the Epic electronic health record (Epic Inc., Verona, WI) and through the Yale Joint Data Analytics Team’s data repository as previously described (11). We performed urine albumin and creatinine measurements from stored urine samples using a Randox RX Daytona machine. We calculated eGFR from serum creatinine values using the 2021 race-free creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation (12). We defined baseline creatinine as the median creatinine values in the 30-day to 6-month period before the kidney biopsy. We defined AKI as an increase in creatinine values by 50% within 7 days or 0.3 mg/dl within 48 hours from their lowest level. We defined acute kidney disease as an increase in creatinine values by 50%, a decrease in eGFR by >35%, or a new decrease in eGFR to <60 ml/min within 3 months (13).
Animal Surgery and Immunohistochemistry for Mouse Kidney Sections
We included C57BL/6 (Envigo) wild-type mice (9–11 weeks) in this work. To establish the unilateral ischemia/reperfusion injury (“fibrotic”) model, we induced warm kidney ischemia using a nontraumatic microaneurysm clip (FST Micro Clamps) on the left kidney pedicle for 27 minutes, leaving the right kidney intact. To establish the unilateral ischemia/reperfusion injury with contralateral nephrectomy (“repaired”) model, we surgically removed the right kidney at the time of left kidney ischemia, as previously described (14,15). We euthanized injured mice (n=8 per model) on day 30 postsurgery. We also included age-matched uninjured (“normal”) wild-type mice as experimental controls (n=7). We have previously shown that mice with kidney fibrosis have higher collagen deposition (29%±5% of the whole kidney) than those with repair (17%±6%) or uninjured normal control kidneys (3%±0.8% collagen deposition) (7). For immunohistochemistry (IHC) analysis, we fixed the kidneys in 10% neutral buffered formalin and embedded them in paraffin. We detected uromodulin expression by IHC using the primary antibody against UMOD (#sc-20631; Santa Cruz Biotechnology) as previously described (15). We used citrate buffer antigen retrieval and omitted primary antibodies as negative controls. Finally, we used the IHC Profiler in ImageJ (National Institutes of Health) to quantify the total area of UMOD-positive staining in the entire kidney cross-section. All animal protocols were approved by the Yale University Animal Care and Use Committee.
Statistical Analyses
We presented characteristics of participants at biopsy as median (IQR) or number (proportion) by uromodulin tertiles. We tested differences between groups using chi-squared or nonparametric trend tests. We tested the correlation of urine uromodulin with markers of kidney health and histologic features obtained on the biopsy using Spearman correlation tests. We tested the association of various histologic features with log2 and tertiles of the uromodulin level using linear regression analysis. Model 1 investigated the univariable association of histologic features with uromodulin. Model 2 controlled for baseline eGFR, serum creatinine at biopsy, and urine albumin-creatinine ratio. Model 3 was only analyzed for histologic features significantly associated with uromodulin in model 2 and additionally controlled for IF/TA and glomerulosclerosis. In a supplementary analysis, we further adjusted our analyses for histologic diagnosis. We report β-coefficients of linear regression models (and 95% confidence intervals [95% CIs]), which represent unit differences in outcome (IF/TA expressed as a percentage of kidney tissue or glomerulosclerosis expressed as a percentage of glomeruli sampled) per unit (either tertile or two-fold) difference in urine uromodulin level. Degree of missingness is noted in Supplemental Table 2. Our primary analysis was a complete case analysis such that only participants with nonmissing data on histologic features were included in the analysis. In a sensitivity analysis, we performed multiple imputations to account for missing data. Human data were analyzed using STATA statistical software (version 14.2). Animal data are expressed as mean±SD. We performed ordinary one-way ANOVA followed by the Tukey multiple comparison test for subgroup comparison using Prism 8 (GraphPad Software). We considered a two-sided P-value of <0.05 as statistically significant.
Results
Participant Characteristics
Of the 392 participants enrolled in the Yale biopsy cohort between 2015 and 2018, we included 364 in the analysis, excluding those who did not provide a urine sample (n=22) or who did not undergo a biopsy after enrollment (n=6) (Supplemental Figure 1). Patients with higher urine uromodulin were less likely to be Black and less likely to have diabetes or CKD (Table 1). We did not find uromodulin levels to be associated with baseline factors such as age, sex, body mass index, or diagnoses (such as hypertension or cirrhosis). Most patients underwent a biopsy for an indication of AKI (n=147) or acute kidney disease (n=137).
Table 1.
Baseline characteristics by uromodulin tertile
| Characteristic | Tertile 1, n=122 | Tertile 2, n=121 | Tertile 3, n=121 |
|---|---|---|---|
| Demographics | |||
| Age, yr | 58 (49–66) | 59 (46–67) | 57 (43–68) |
| Women, n (%) | 52 (43) | 51 (42) | 59 (49) |
| Black race, n (%) | 42 (35) | 23 (19) | 26 (22) |
| Diabetes, n (%) | 51 (43) | 46 (38) | 31 (26) |
| Hypertension, n (%) | 93 (76) | 91 (75) | 82 (68) |
| Cirrhosis, n (%) | 8 (7) | 9 (8) | 10 (8) |
| CKD, n (%) | 87 (80) | 84 (72) | 56 (50) |
| Body mass index, kg/m2 | 30 (25–33) | 29 (25–35) | 28 (24–33) |
| Medication use, n (%) | |||
| PPI use | 44 (36) | 44 (37) | 46 (38) |
| NSAID use | 20 (17) | 17 (14) | 29 (24) |
| Antibiotic use | 42 (35) | 53 (45) | 67 (56) |
| Prebiopsy laboratory features | |||
| Creatinine, mg/dla | 1.8 (1.3–2.6) | 1.4 (1.1–2.0) | 1.2 (0.8–1.6) |
| eGFR, ml/mina | 35 (24–58) | 50 (30–68) | 63 (42–99) |
| Protein-creatinine ratio, mg/mg | 2.1 (0.7–4.4) | 1.7 (0.7–4.6) | 1.5 (0.5–4.6) |
| Albumin-creatinine ratio, mg/mg | 1.0 (0.1–2.2) | 0.7 (0.1–2.7) | 0.7 (0.1–1.9) |
| Features at biopsy | |||
| AKI or AKD, n (%) | 100 (82) | 95 (79) | 78 (64) |
| Dialysis, n (%) | 6 (5) | 4 (3) | 7 (6) |
| Creatinine, mg/dl | 4.0 (2.3–6.3) | 2.5 (1.7–3.6) | 1.9 (1.2–3.0) |
| BUN, mg/dl | 49 (32–72) | 36 (27–52) | 32 (21–57) |
| Bicarbonate, serum, mEq/L | 20 (17–23) | 22 (19–25) | 23 (20–25) |
| Hemoglobin, g/dl | 10.4 (8.7–11.9) | 10.5 (8.9–11.9) | 10.8 (9.2–12.9) |
| Kidney size, cm | 11.2 (10.2–12.3) | 11.4 (10.8–12.2) | 11.6 (10.7–12.7) |
Data are interquartile ranges or proportion. Tertile 1, 0.14–0.81 μg/g; tertile 2, 0.82–1.65 μg/g; tertile 3, 1.67–12.24 μg/g. Chi-squared and nonparametric trend tests. PPI, proton pump inhibitor; NSAID, nonsteroidal anti-inflammatory drug; AKD, acute kidney disease.
Prebiospy creatinine and eGFR values were obtained 1–6 months prior to kidney biopsy.
Correlation of Uromodulin with Biomarkers of Glomerular and Tubular Function, Injury, and Inflammation
Serum creatinine and eGFR measured before and at the time of biopsy were strongly correlated with urine uromodulin. Patients with higher uromodulin tended to have lower serum creatinine and higher eGFR, and they were more likely to have AKI or acute kidney disease (Table 1, Supplemental Table 3). However, neither baseline protein-creatinine ratio nor baseline albumin-creatinine ratio were correlated with urine uromodulin. Urine uromodulin was higher in those with greater kidney size, an indirect marker of kidney mass, and in those with higher levels of serum bicarbonate and hemoglobin, indirect markers of kidney tubular function. Among novel kidney biomarkers, we noted that urine uromodulin was negatively correlated with markers of kidney inflammation, such as urine IL-6, TNFα, chitinase 3 like 1 (YKL-40), and neutrophil gelatinase–associated lipocalin (Supplemental Table 4).
Histologic Features and Uromodulin in Humans
Uromodulin levels were lower with increasing severity of IF/TA, glomerulosclerosis, interstitial infiltrate, interstitial eosinophils, and arterionephrosclerosis (Figure 1). However, in multivariable analysis controlling for baseline (prebiopsy) eGFR, baseline urine albumin-creatinine ratio, and serum creatinine at biopsy, only IF/TA and glomerulosclerosis were significantly associated with urine uromodulin (Supplemental Table 5). We noted correlation coefficients of −0.31 (P<0.001) and −0.21 (P<0.001) for urine uromodulin with IF/TA and glomerulosclerosis, respectively (Figure 2). IF/TA was significantly associated with urine uromodulin when we further controlled for glomerulosclerosis (IF/TA per two-fold difference in uromodulin, −2.5%; 95% CI, −4.6% to −0.4% of kidney tissue) but not vice versa (glomerulosclerosis per two-fold difference in uromodulin, 0.9%; 95% CI, −3.4% to 1.5% of glomeruli sampled) (Table 2, model 3). Further controlling for the histologic diagnosis showed similar results (Supplemental Table 6). We noted similar results when analyzing uromodulin levels as tertiles (Table 2) and when uromodulin levels were analyzed without indexing to urine creatinine (Supplemental Table 7). An analysis using multiple imputations to account for missing data showed similar results (Supplemental Table 8).
Figure 1.
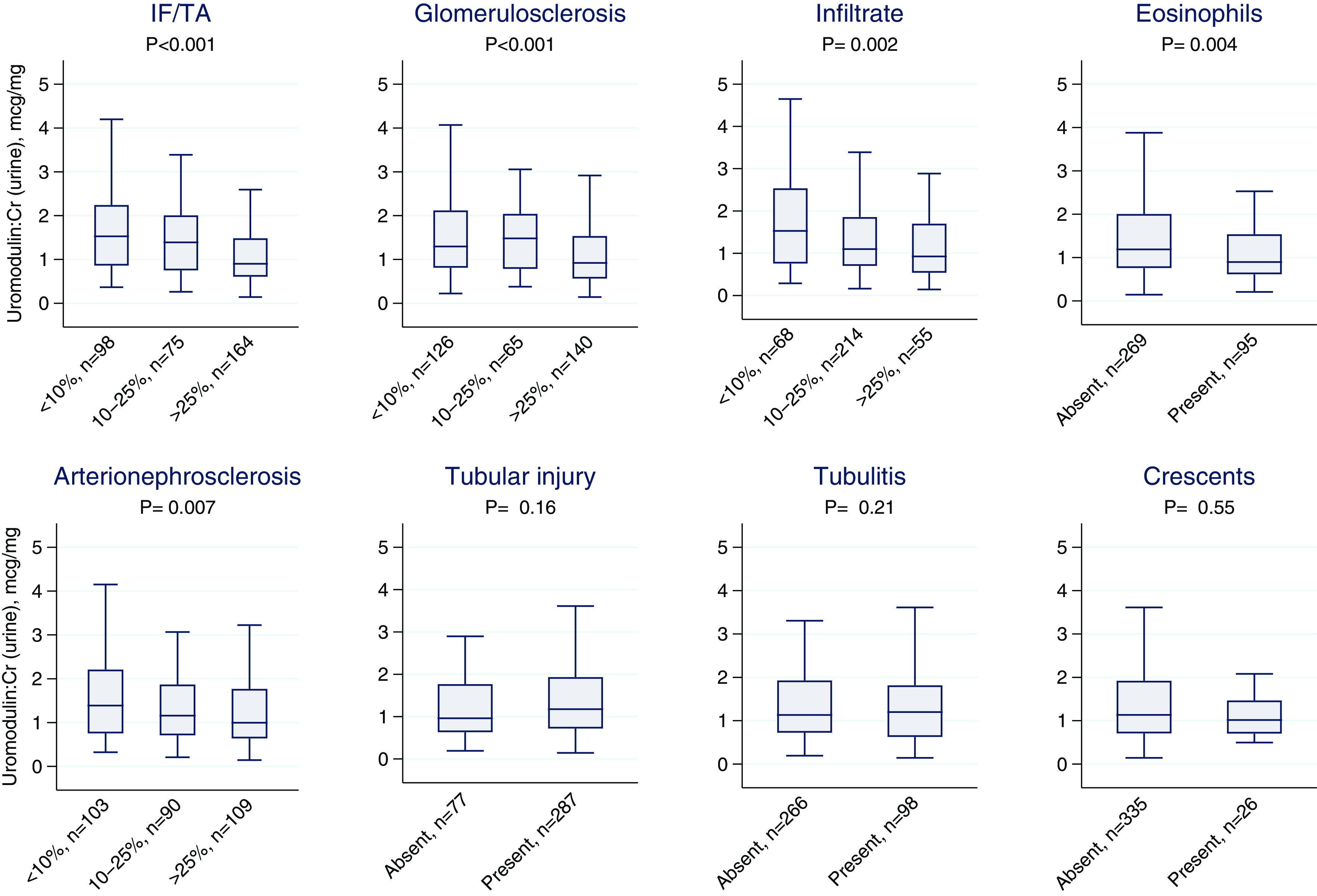
Association of urine uromodulin level and kidney histology. Nonparametric trend test. IF/TA, interstitial fibrosis and tubular atrophy.
Figure 2.
Correlation of interstitial fibrosis and urine uromodulin. Nonparametric (Spearman) correlation coefficient. Lines and shaded areas are the quadratic fit prediction and its 95% confidence interval, respectively.
Table 2.
Association of urine uromodulin with tubulointerstitial and glomerular fibrosis
| Histologic Feature | Difference in Histologic Feature per Unit Difference in Uromodulin | ||
|---|---|---|---|
| Model 1 (95% Confidence Intervals) | Model 2 (95% Confidence Intervals) | Model 3 (95% Confidence Intervals) | |
| Interstitial fibrosis, % of kidney tissue a | |||
| Per two-fold difference | −7.2 (−9.4 to −5.1)c | −3.5 (−5.7 to −1.2)c | −2.5 (−4.6 to −0.4)c |
| Tertile 1, 0.14–0.81 μg/g | Ref. | Ref. | Ref. |
| Tertile 2, 0.82–1.65 μg/g | −3.1 (−9.1 to 2.9) | 0.7 (−5.1 to 6.3) | 1.2 (−4.0 to 6.4) |
| Tertile 3, 1.67–12.24 μg/g | −16.5 (−22.6 to −10.6)c | −6.9 (−12.9 to −0.9)c | −6.8 (−12.3 to −1.3)c |
| Glomerulosclerosis, % of glomeruli b | |||
| Per two-fold difference | −6.4 (−8.8 to −4.0)c | −3.3 (−5.9 to −0.6)c | −0.9 (−3.4 to 1.5) |
| Tertile 1, 0.14–0.81 μg/g | Ref. | Ref. | Ref. |
| Tertile 2, 0.82–1.65 μg/g | −6.4 (−13.3 to 0.6)c | −2.3 (−9.1 to 4.5) | −1.7 (−7.8 to 4.5) |
| Tertile 3, 1.67–12.24 μg/g | −12.1 (−19.1 to −5.2)c | −2.6 (−10.0 to 4.7) | 2.3 (−4.3 to 9.0) |
Linear regression analysis with outcome as the histologic feature (interstitial fibrosis or glomerulosclerosis) and exposure as the uromodulin level (log2 or tertile). Values represent unit differences in outcome (percentage of total biopsy tissue for interstitial fibrosis/tubular atrophy or percentage of glomeruli that have global sclerosis for glomerulosclerosis) for each unit (two-fold or tertile) difference of urine uromodulin level. Model 1 is univariable. Model 2 controls for baseline eGFR, baseline urine albumin-creatinine ratio, and serum creatinine at biopsy. Model 3 additionally controls for glomerulosclerosis and interstitial fibrosis. Ref., reference.
Model 3 additionally controlled for glomerulosclerosis.
Model 3 additionally controlled for interstitial fibrosis.
Significant associations.
Kidney Fibrosis and Uromodulin in Animal Models
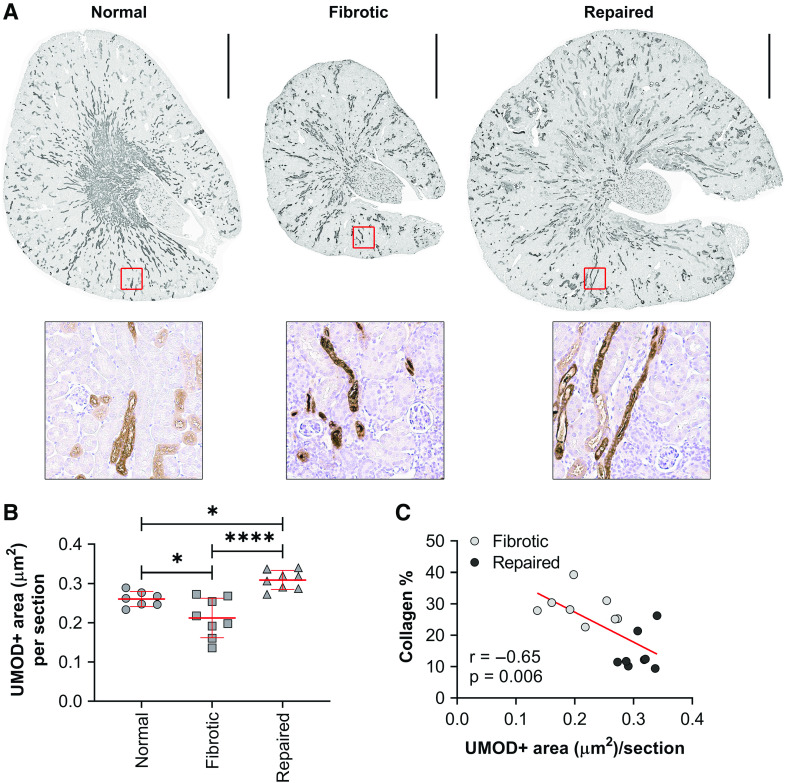
We included eight mice with kidney fibrosis after injury, eight mice with kidney repair after injury, and seven uninjured normal control kidneys. We noted that fibrotic mice had lower kidney area staining for uromodulin than repaired mice (mean difference in area staining for uromodulin, −0.10; 95% CI of the difference, −0.14 to −0.05 µm2; P<0.001) and normal control kidneys (−0.05; 95% CI of the difference, −0.00 to −0.09 µm2; P=0.04) (Figure 3, A and B, Supplemental Table 9). We also found that uromodulin negatively correlated with collagen deposition (r=−0.65) after injury (Figure 3C).
Figure 3.
Fibrotic kidneys show decreased uromodulin expression in animal models. (A) Representative images of the whole-kidney sections immunostained for UMOD from fibrotic (n=8), repaired (n=8), and normal uninjured (n=7) mouse kidneys. Wild-type mice were subjected to 27 minutes of unilateral ischemia/reperfusion injury with contralateral kidney intact (fibrotic model) or unilateral ischemia/reperfusion injury with contralateral nephrectomy (repaired model) and harvested at 30 days after injury. Normal uninjured mice were age matched to fibrotic and repaired mice. Scale bar, 1 mm. (B) Uromodulin-positive areas of the entire kidney sections were quantified. n=7–8 kidneys per group. *P=0.05; ****P<0.001. (C) Scatterplot and best fit line showing the correlation between collagen and UMOD staining.
Discussion
In this observational study, we show higher urine uromodulin levels to be associated with less severe histologic findings of IF/TA, independent of existing markers of kidney function and damage. We confirm these findings in an experimental murine model showing lower levels of uromodulin staining in kidneys with fibrosis as compared with kidneys that were successfully repaired after injury.
This association of uromodulin with IF/TA strengthens its role as a biomarker of tubular health and function. IF/TA is the final common pathway to multiple etiologies of kidney damage (16), and the degree of fibrosis is an important prognostic factor in CKD of various etiologies (17–19). However, because of the risks associated with the kidney biopsy procedure (20–22), this information is not readily available for clinical use. Therefore, a biomarker associated with interstitial fibrosis could serve an important role not only in determining prognosis but also in evaluating favorable response to therapy. Another study that compared urine uromodulin levels with kidney histologic findings noted a trend toward higher tubular atrophy scores in those with lower urine uromodulin (P=0.08) in 70 participants with kidney biopsy (23). Our larger sample size allowed us to not only to confirm this relationship but also demonstrate that this association is independent of other clinically available biomarkers such as serum creatinine or urine albumin. We also confirmed lower expression of uromodulin in murine models of fibrosed kidneys as compared with normal or repaired kidneys; these findings agree with the literature, which has shown increased uromodulin in mice recovering postinjury and impaired recovery in UMOD knockout mice post-AKI (24).
We noted urine uromodulin to be correlated with markers of tubular function and health but not with glomerular damage. For example, uromodulin correlated with serum bicarbonate and hemoglobin, markers associated with kidney tubulointerstitium, but not with urine albumin- or protein-creatinine ratio, markers of glomerular damage. Similarly, although uromodulin was independently associated with both interstitial fibrosis and glomerulosclerosis, the association between uromodulin and glomerulosclerosis was null after controlling for the degree of interstitial fibrosis. This supports the argument that uromodulin may specifically reflect tubular function and health (25).
Our study describes the potential of urine uromodulin as a marker of irreversible kidney damage, adding to the existing literature that demonstrates the kidney-specific expression of this biomarker (26) and its abundance in human urine. Large prospective observational studies have shown higher urine uromodulin levels to be associated with better long-term kidney function. Garimella et al. (25) noted that study participants with higher levels of urine uromodulin were at lower risk of eGFR decline, independent of conventional risk factors for CKD prognosis such as eGFR and urine albumin-creatinine ratio. In the Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury study composed of over 1500 hospitalized patients, Puthumana et al. (7) showed that higher urine uromodulin levels measured after hospital discharge were associated with lower decline in eGFR and lower occurrence of CKD. Other studies have described similar relationships between serum uromodulin and kidney disease (27,28).
Although multiple studies support the role of urine uromodulin as a biomarker of tubulointerstitial health, function, and long-term prognosis, some studies show contradicting results. For example, a variant in the UMOD region associated with a greater risk of CKD was found to associate with higher levels of uromodulin, and higher uromodulin levels were found to associate with future incidence of CKD (29). Additionally, one recent Mendelian randomization study demonstrated the independent role of uromodulin in causing kidney disease (30), while another suggested that risk factors for CKD increase with uromodulin levels (31,32). Several hypotheses have been proposed to reconcile the different findings of these genetic and observational studies. One hypothesis notes an increase in per nephron uromodulin production with kidney damage, suggesting that individuals with early kidney damage but without complete loss of tubules may have higher uromodulin levels and higher future risk of CKD (2). The translation of these genetic findings into biologic understanding of the association of uromodulin with CKD is also somewhat problematic (2). Our findings support the hypothesis that the association of higher urine uromodulin with lower decline in kidney function is likely due to uromodulin being a marker of less fibrosis.
Our findings have implications for clinical practice, research, and future trials. This study, along with the multiple studies discussed above, shows that assessment of uromodulin levels accurately captures tubular health and can therefore provide a more complete picture of kidney health in a patient with CKD. Biomarkers that correlate with histologic findings could also support clinical trials in CKD; after further validation, uromodulin can be assessed along with GFR and albuminuria to evaluate the effect of kidney-specific therapies in early-phase clinical trials.
Our study has several strengths. First, our demonstration of the independent association of a promising kidney biomarker with histologic findings of fibrosis represents an important step toward the implementation of this biomarker in clinical practice. Second, our study conducted prospective enrollment of consecutive participants with standardization of sample collection, processing, storage, and biomarker measurement, making our results reliable and reproducible. Third, although long-term −80°C storage beyond a year and repeated freeze-thaw cycles could lead to degradation of uromodulin, we measured uromodulin after a relatively short duration of storage and a single freeze-thaw cycle. Finally, the personnel measuring biomarker levels were blinded to histologic features.
Our study also had some limitations. First is the potential for selection bias; all participants had kidney disease significant enough to warrant a biopsy, limiting the generalizability of this study for those without severe kidney disease. Second, as this study did not include individuals with kidney transplants, our findings may not be generalizable to this group of patients. Third, we did not investigate biomarker expression in human tissue but instead showed kidney expression in murine models of fibrosis and repair. Fourth, we used a single spot urine collection, which may be less informative than the steady-state data captured by a timed (e.g., 24-hour) urine collection. However, we conducted all of our analyses both with and without indexing of uromodulin to urine creatinine ratios to account for intraday urine concentration differences. Moreover, our findings may be more applicable to real-world conditions, where spot urine collection is more logistically feasible than timed collections. Importantly, both spot and 24-hour urine uromodulin concentration are linearly and positively associated with eGFR (4). Fifth, although this study used surrogates, such as serum bicarbonate and kidney size, to ascertain kidney tubular function and kidney mass, there may exist better, more specific methods for these assessments. However, we believe that assessment with more accurate measures would only strengthen the association of uromodulin with tubular function. Finally, our study did not evaluate the association of uromodulin with long-term outcomes. However, this relationship has been established by numerous studies as noted above.
In conclusion, we show that urine uromodulin correlates with IF/TA on histology independent of currently available clinical tests, such as eGFR, creatinine, and albuminuria. We noted similar results in an animal model of kidney fibrosis. Although uromodulin had a similar relationship with glomerulosclerosis, this was not independent of IF/TA. These findings support the role of urine uromodulin as a biomarker of kidney tubular health.
Disclosures
F. Calderon-Gutierrez reports ownership interest in Facebook, Google, and Tesla. M. Kashgarian reports patents or royalties with Abcam and Calbiochem. M. Kuperman reports employment with Arkana Laboratories. D.G. Moledina reports ownership interest in Predict AIN, LLC; reports research funding from National Institute of Diabetes and Digestive and Kidney Diseases grants K23DK117065, R01DK12681, R01128087, UH3DK114866, and P30DK079310; reports honoraria from the British Medical Journal, the National Kidney Foundation, and Remedy Health Media; is a coinventor of the pending patent application “Methods and Systems for Diagnosis of Acute Interstitial Nephritis,” which was subject to an option for a license agreement with Renalytix AI Inc.; and serves as an editorial board member of Kidney360. C.R. Parikh reports consultancy agreements with Genfit Biopharmaceutical Company; serves as a member of the advisory board of and owns equity in Renalytix AI Inc.; reports research funding from the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases; reports an advisory or leadership role for Genfit Biopharmaceutical Company; and is a coinventor of the pending patent application “Methods and Systems for Diagnosis of Acute Interstitial Nephritis,” which was subject to an option for a license agreement with Renalytix AI Inc. F.P. Wilson is the owner of Efference, LLC, a medical communications company; reports consultancy agreements with Translational Catalyst, LLC; reports research funding from Amgen, Boehringer-Ingelheim, Vifor, and Whoop; serves on the editorial boards of American Journal of Kidney Disease and CJASN; and reports other interests or relationships as a medical columnist for Medscape. All remaining authors have nothing to disclose.
Funding
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases awards K23DK117065 (to D.G. Moledina), R01DK128087 (to D.G. Moledina), R01DK126815 (to D.G. Moledina and L.G. Cantley), UH3DK114866 (to D.G. Moledina, C.R. Parikh, and F.P. Wilson), and K01DK120783 (to L. Xu) and Yale O’Brien Center grant P30DK079310.
Supplementary Material
Acknowledgments
These findings were presented at the National Kidney Foundation Spring Clinical Meeting in 2021.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
F. Calderon-Gutierrez, H. Melchinger, and D.G. Moledina conceptualized the study; F. Calderon-Gutierrez, M. Kashgarian, M. Kuperman, R. Luciano, G. Moeckel, and M.M. Shaw were responsible for data curation; D.G. Moledina, W. Obeid, and L. Xu were responsible for formal analysis; D.G. Moledina and F.P. Wilson were responsible for methodology; D.G. Moledina and M.M. Shaw were responsible for project administration; D.G. Moledina, C.R. Parikh, and F.P. Wilson were responsible for resources; D.G. Moledina and L. Xu were responsible for validation; D.G. Moledina and C.R. Parikh were responsible for funding acquisition; D.G. Moledina provided supervision; F. Calderon-Gutierrez, H. Melchinger, and D.G. Moledina wrote the original draft; and F. Calderon-Gutierrez, M. Kashgarian, M. Kuperman, R. Luciano, H. Melchinger, G. Moeckel, D.G. Moledina, W. Obeid, C.R. Parikh, M.M. Shaw, F.P. Wilson, and L. Xu reviewed and edited the manuscript.
Data Sharing Statement
Datasets have been deposited in Dryad (https://doi.org/10.5061/dryad.83bk3j9tq).
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04360422/-/DCSupplemental.
Supplemental Figure 1. STARD flow diagram.
Supplemental Table 1. Classification of histologic features.
Supplemental Table 2. Degree of missingness of key covariates.
Supplemental Table 3. Correlation of histologic features with urine uromodulin level.
Supplemental Table 4. Correlation of urine uromodulin level with other kidney biomarkers.
Supplemental Table 5. Univariable and multivariable associations of uromodulin level with histologic features.
Supplemental Table 6. Association of urine uromodulin with tubulointerstitial and glomerular fibrosis after controlling for histologic diagnosis.
Supplemental Table 7. Association of uromodulin with tubulointerstitial and glomerular fibrosis without indexing to urine creatinine.
Supplemental Table 8. Association of uromodulin with tubulointerstitial and glomerular fibrosis with multiple imputations to account for missing data.
Supplemental Table 9. Comparison of uromodulin staining on various mouse kidney biopsy models.
References
- 1.Thornley C, Dawnay A, Cattell WR: Human Tamm-Horsfall glycoprotein: Urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond) 68: 529–535, 1985. 10.1042/cs0680529 [DOI] [PubMed] [Google Scholar]
- 2.El-Achkar TM, Wu XR: Uromodulin in kidney injury: An instigator, bystander, or protector? Am J Kidney Dis 59: 452–461, 2012. 10.1053/j.ajkd.2011.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devuyst O, Bochud M: Uromodulin, kidney function, cardiovascular disease, and mortality. Kidney Int 88: 944–946, 2015. 10.1038/ki.2015.267 [DOI] [PubMed] [Google Scholar]
- 4.Devuyst O, Olinger E, Rampoldi L: Uromodulin: From physiology to rare and complex kidney disorders. Nat Rev Nephrol 13: 525–544, 2017. 10.1038/nrneph.2017.101 [DOI] [PubMed] [Google Scholar]
- 5.Gudbjartsson DF, Holm H, Indridason OS, Thorleifsson G, Edvardsson V, Sulem P, de Vegt F, d’Ancona FC, den Heijer M, Wetzels JF, Franzson L, Rafnar T, Kristjansson K, Bjornsdottir US, Eyjolfsson GI, Kiemeney LA, Kong A, Palsson R, Thorsteinsdottir U, Stefansson K: Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases [published correction appears in PLoS Genet 2010 10.1371/annotation/8e7ba8d6-a174-4a3a-93b4-510d5ca7ed1e]. PLoS Genet 6: e1001039, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009. 10.1038/ng.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puthumana J, Thiessen-Philbrook H, Xu L, Coca SG, Garg AX, Himmelfarb J, Bhatraju PK, Ikizler TA, Siew ED, Ware LB, Liu KD, Go AS, Kaufman JS, Kimmel PL, Chinchilli VM, Cantley LG, Parikh CR: Biomarkers of inflammation and repair in kidney disease progression. J Clin Invest 131: e139927, 2021. 10.1172/JCI139927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moledina DG, Wilson FP, Pober JS, Perazella MA, Singh N, Luciano RL, Obeid W, Lin H, Kuperman M, Moeckel GW, Kashgarian M, Cantley LG, Parikh CR: Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight 4: e127456, 2019. 10.1172/jci.insight.127456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moledina DG, Wilson FP, Kukova L, Obeid W, Luciano R, Kuperman M, Moeckel GW, Kashgarian M, Perazella MA, Cantley LG, Parikh CR: Urine interleukin-9 and tumor necrosis factor-α for prognosis of human acute interstitial nephritis. Nephrol Dial Transplant 36: 1851–1858, 2021. 10.1093/ndt/gfaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh CR, Liu C, Mor MK, Palevsky PM, Kaufman JS, Thiessen Philbrook H, Weisbord SD: Kidney biomarkers of injury and repair as predictors of contrast-associated AKI: A substudy of the PRESERVE trial. Am J Kidney Dis 75: 187–194, 2020. 10.1053/j.ajkd.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moledina DG, Eadon MT, Calderon F, Yamamoto Y, Shaw M, Perazella MA, Simonov M, Luciano R, Schwantes-An TH, Moeckel G, Kashgarian M, Kuperman M, Obeid W, Cantley LG, Parikh CR, Wilson FP: Development and external validation of a diagnostic model for biopsy-proven acute interstitial nephritis using electronic health record data [published online ahead of print December 4, 2021]. Nephrol Dial Transplant 10.1093/ndt/gfab346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, Grams ME, Greene T, Grubb A, Gudnason V, Gutiérrez OM, Kalil R, Karger AB, Mauer M, Navis G, Nelson RG, Poggio ED, Rodby R, Rossing P, Rule AD, Selvin E, Seegmiller JC, Shlipak MG, Torres VE, Yang W, Ballew SH, Couture SJ, Powe NR, Levey AS; Chronic Kidney Disease Epidemiology Collaboration : New creatinine- and cystatin c-based equations to estimate GFR without race. N Engl J Med 385: 1737–1749, 2021. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) Workgroup: KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 14.Xu L, Sharkey D, Cantley LG: Tubular GM-CSF promotes late MCP-1/CCR2-mediated fibrosis and inflammation after ischemia/reperfusion injury. J Am Soc Nephrol 30: 1825–1840, 2019. 10.1681/ASN.2019010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery TA, Xu L, Mason S, Chinnadurai A, Lee CG, Elias JA, Cantley LG: Breast regression protein-39/chitinase 3-like 1 promotes renal fibrosis after kidney injury via activation of myofibroblasts. J Am Soc Nephrol 28: 3218–3226, 2017. 10.1681/ASN.2017010110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockey DC, Bell PD, Hill JA: Fibrosis—A common pathway to organ injury and failure. N Engl J Med 372: 1138–1149, 2015. 10.1056/NEJMra1300575 [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Li H, Liu Y, You J, Qu Z, Yuan S, Peng Y, Liu F, Liu H: Tubular atrophy/interstitial fibrosis scores of Oxford classification combinded with proteinuria level at biopsy provides earlier risk prediction in IgA nephropathy. Sci Rep 7: 1100, 2017. 10.1038/s41598-017-01223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricaurte Archila L, Denic A, Mullan AF, Narasimhan R, Bogojevic M, Thompson RH, Leibovich BC, Sangaralingham SJ, Smith ML, Alexander MP, Rule AD: A higher foci density of interstitial fibrosis and tubular atrophy predicts progressive CKD after a radical nephrectomy for tumor. J Am Soc Nephrol 32: 2623–2633, 2021. 10.1681/ASN.2021020267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson PC, Kashgarian M, Moeckel G: Interstitial inflammation and interstitial fibrosis and tubular atrophy predict renal survival in lupus nephritis. Clin Kidney J 11: 207–218, 2018. 10.1093/ckj/sfx093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moledina DG, Luciano RL, Kukova L, Chan L, Saha A, Nadkarni G, Alfano S, Wilson FP, Perazella MA, Parikh CR: Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol 13: 1633–1640, 2018. 10.2215/CJN.04910418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korbet SM, Gashti CN, Evans JK, Whittier WL: Risk of percutaneous renal biopsy of native kidneys in the evaluation of acute kidney injury. Clin Kidney J 11: 610–615, 2018. 10.1093/ckj/sfy048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poggio ED, McClelland RL, Blank KN, Hansen S, Bansal S, Bomback AS, Canetta PA, Khairallah P, Kiryluk K, Lecker SH, McMahon GM, Palevsky PM, Parikh S, Rosas SE, Tuttle K, Vazquez MA, Vijayan A, Rovin BH; Kidney Precision Medicine Project : Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol 15: 1595–1602, 2020. 10.2215/cjn.04710420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prajczer S, Heidenreich U, Pfaller W, Kotanko P, Lhotta K, Jennings P: Evidence for a role of uromodulin in chronic kidney disease progression. Nephrol Dial Transplant 25: 1896–1903, 2010. 10.1093/ndt/gfp748 [DOI] [PubMed] [Google Scholar]
- 24.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR: Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013. 10.1152/ajprenal.00543.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garimella PS, Biggs ML, Katz R, Ix JH, Bennett MR, Devarajan P, Kestenbaum BR, Siscovick DS, Jensen MK, Shlipak MG, Chaves PH, Sarnak MJ: Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int 88: 1126–1134, 2015. 10.1038/ki.2015.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaeffer C, Devuyst O, Rampoldi L: Uromodulin: Roles in health and disease. Annu Rev Physiol 83: 477–501, 2021. 10.1146/annurev-physiol-031620-092817 [DOI] [PubMed] [Google Scholar]
- 27.Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, Block M, Kaden J, Schlumberger W: Serum uromodulin—A marker of kidney function and renal parenchymal integrity. Nephrol Dial Transplant 33: 284–295, 2018. 10.1093/ndt/gfw422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Then C, Then HL, Lechner A, Thorand B, Meisinger C, Heier M, Peters A, Koenig W, Rathmann W, Scherberich J, Seissler J: Serum uromodulin and decline of kidney function in older participants of the population-based KORA F4/FF4 study. Clin Kidney J 14: 205–211, 2020. 10.1093/ckj/sfaa032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köttgen A, Hwang S-J, Larson MG, Van Eyk JE, Fu Q, Benjamin EJ, Dehghan A, Glazer NL, Kao WH, Harris TB, Gudnason V, Shlipak MG, Yang Q, Coresh J, Levy D, Fox CS: Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol 21: 337–344, 2010. 10.1681/ASN.2009070725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponte B, Sadler MC, Olinger E, Vollenweider P, Bochud M, Padmanabhan S, Hayward C, Kutalik Z, Devuyst O: Mendelian randomization to assess causality between uromodulin, blood pressure and chronic kidney disease. Kidney Int 100: 1282–1291, 2021. 10.1016/j.kint.2021.08.032 [DOI] [PubMed] [Google Scholar]
- 31.Micanovic R, Khan S, Janosevic D, Lee ME, Hato T, Srour EF, Winfree S, Ghosh J, Tong Y, Rice SE, Dagher PC, Wu XR, El-Achkar TM: Tamm-Horsfall protein regulates mononuclear phagocytes in the kidney. J Am Soc Nephrol 29: 841–856, 2018. 10.1681/ASN.2017040409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micanovic R, LaFavers K, Garimella PS, Wu XR, El-Achkar TM: Uromodulin (Tamm-Horsfall protein): Guardian of urinary and systemic homeostasis. Nephrol Dial Transplant 35: 33–43, 2020. 10.1093/ndt/gfy394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.