Visual Abstract
Keywords: transgender, gender affirming hormone therapy, kidney function, systematic review, meta-analysis, hormones
Abstract
Background and objectives
Gender-affirming hormone therapy modifies body composition and lean muscle mass in transgender persons. We sought to characterize the change in serum creatinine, other kidney function biomarkers, and GFR in transgender persons initiating masculinizing and feminizing gender-affirming hormone therapy.
Design, setting, participants, & measurements
We searched PubMed, EMBASE, the Cochrane Library, and ClinicalTrials.gov from inception to September 16, 2020 for randomized controlled trials, observational studies, and case series that evaluated the change in serum creatinine, other kidney function biomarkers, and GFR before and after the initiation of gender-affirming hormone therapy in adult transgender persons. Two reviewers independently screened and abstracted data, and disagreements were resolved by a third reviewer. A random effects meta-analysis was performed to determine the change in outcomes over follow-up of 3, 6, and 12 months.
Results
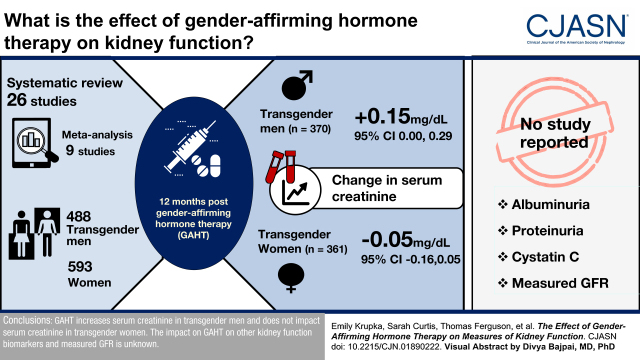
Of the 4758 eligible studies, 26 met the inclusion criteria, including nine studies that recruited 488 transgender men and 593 women in which data were meta-analyzed. There was heterogeneity in study design, populations, gender-affirming hormone therapy routes, and dosing. At 12 months after initiating gender-affirming hormone therapy, serum creatinine increased by 0.15 mg/dl (95% confidence interval, 0.00 to 0.29) in 370 transgender men and decreased by −0.05 mg/dl (95% confidence interval, −0.16 to 0.05) in 361 transgender women. No study reported the effect of gender-affirming hormone therapy on albuminuria, proteinuria, cystatin C, or measured GFR.
Conclusions
Gender-affirming hormone therapy increases serum creatinine in transgender men and does not affect serum creatinine in transgender women. The effect on gender-affirming hormone therapy on other kidney function biomarkers and measured GFR is unknown.
Clinical Trial registry name and registration number:
Change in Kidney Function Biomarkers in Transgender Persons on Gender Affirmation Hormone Therapy–A Systematic Review and Meta-Analysis, CRD42020214248
Introduction
It is estimated that 0.3%–0.6% of the adult population identifies as transgender, which amounts to approximately 150,000 transgender persons in Canada, 1 million in the United States, and 25 million worldwide (1–3). Gender-affirming hormone therapy (GAHT) shifts an individual’s sex hormone milieu to biochemically reflect their affirmed gender identity (4,5). For transgender men, GAHT typically includes injectable or transdermal testosterone therapy, and for transgender women, GAHT typically includes exogenous oral, sublingual, transdermal, or injectable estradiol and antiandrogen therapy (cyproterone acetate, GnRH agonists, mineralocorticoid receptor antagonists, 5α-reductase inhibitors, and bicalutamide) with or without progesterone. Nonbinary and gender-diverse persons may also be treated with either masculinizing or feminizing GAHT. GAHT is known to alter body composition, including lean muscle mass and body fat, in transgender persons (6,7).
Creatinine is a biomarker derived from skeletal muscle used to estimate GFR and is influenced by non-GFR determinants (i.e., age, sex/gender, and race) (8). The degree to which GAHT affects serum creatinine and other kidney function biomarkers (cystatin C, albuminuria, and proteinuria) given its effect on body composition is unclear (9). Similarly, whether GAHT affects GFR independent of changes in kidney function biomarkers related to changes in body composition is unknown. These issues are important because both sex/gender and kidney function biomarkers are incorporated into estimating GFR, which is a cornerstone in CKD detection, drug dosing, prognosis, and management.
We completed a systematic review and meta-analysis to characterize the change in serum creatinine, other kidney function biomarkers, and GFR in adult transgender persons initiating masculinizing and feminizing GAHT in order to inform the care of this patient population (10).
Materials and Methods
Data Sources and Searches
To identify studies, the population, intervention, comparison, outcomes, and timing criteria for the search strategy were used, and the protocol was registered at PROSPERO (11). In collaboration with a medical librarian (N.A.), original research articles were identified from the following databases: PubMed, EMBASE, the Cochrane Library, and ClinicalTrials.gov. Our search of these databases ranged from the date of their establishment until September 16, 2020. The search strategy was tailored to each database and used a combination of key terms, including “transgender,” “trans,” “gender,” “sex,” “creatinine,” “cystatin c,” “glomerular filtration rate,” “gender-affirmation,” “hormone therapy,” “estrogen,” “testosterone,” “anti-androgen,” “spironolactone,” “cyproterone acetate,” and “gonadotropin-releasing hormone agonist.” The search strategy is shown in Supplemental Table 1. The gray literature was not assessed.
Target studies included those that enrolled adult (aged 18+) transgender persons (men, women, nonbinary, or gender diverse) who had received treatment with GAHT of any type and had serum creatinine, creatinine clearance, BUN, cystatin C, eGFR, measured GFR, albuminuria, or proteinuria (spot or 24-hour values) reported at baseline (prior to the initiation of GAHT) and after 3, 6, and 12 months or longer on GAHT. We also included studies that compared outcomes between transgender persons either pre- or post-GAHT and matched cisgender controls cross-sectionally. Types of studies included randomized controlled trials and prospective, retrospective, or cross-sectional observational cohort studies, including case series but not case reports. There was no limit on study size, duration, or setting. Articles in languages other than English, French, Spanish, and German were excluded. All citations were uploaded in Covidence (12).
Study Selection
Two individuals (S.C. and E.K.) independently reviewed titles and abstracts of the studies identified by the search strategy, and those that did not match the inclusion criteria were excluded. For the remaining studies, full texts were retrieved and reviewed for inclusion. A third reviewer (D.C.) reviewed conflicts, and final decisions were made by consensus. References of included studies were also reviewed to identify relevant studies not captured by the search strategy.
Data Extraction
We created a data extraction form to capture the following information: study characteristics (first author, year of publication, country, setting, study design, number of participants, and duration), participant characteristics (age, sex/gender, race, comorbidities, GAHT including type, dose, and frequency during study), gender confirmation surgery, follow-up duration, and outcomes pre- and post-GAHT (serum creatinine; cystatin C; urine protein-creatinine ratio; urine albumin-creatinine ratio; 24-hour urine for creatinine and albuminuria/proteinuria; eGFR by the Modification of Diet in Renal Disease [13] equation, the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] [8] equation, or other equations; measured GFR using any methodology; and eGFR bias [i.e., the difference between eGFR and measured GFR using cisgender and transgender in eGFR equations]). We contacted all corresponding authors of included studies for missing or additional outcomes in case they were not reported in the original manuscript. Creatinine in micromoles per liter was converted to milligrams per deciliter using a factor of 0.0113. If a study used multiple follow-up time points, values at baseline and those closest to 3 months, 6 months, 9 months, and 1 year were recorded. If a study reported outcomes over a time period (e.g., 3–6 and 6–18 months), only the earliest time point was used (i.e., 3 and 6 months, respectively). We did not analyze outcomes beyond 1 year given their limited reporting and variability in the timing of assessments. If the variance of an outcome of interest was not reported, it was derived from the interquartile range or standard error of the mean (SEM). If neither of these were reported, it was assumed to be equal to that of earlier time points if available or was the overall mean of the study. Two reviewers (S.C. and E.K.) independently extracted data; inconsistencies were corrected and resolved by consensus and in consultation with a third reviewer (D.C.). The National Institutes of Health Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group was utilized to determine the quality of included studies in the meta-analysis only. This tool is composed of 12 questions used to assess the risk of types of bias, such as selection, reporting, or observer bias. The methodologic quality of included studies was assessed by two authors (S.C. and E.K.) separately. Each study was defined as poor, fair, or good.
Data Synthesis and Analyses
A random effects meta-analysis for longitudinal effect estimates with 95% confidence intervals (95% CIs) of GAHT on change in outcomes at 3, 6, and 12 months was performed using estimated within-study and between-study correlations using PROC MIXED with autoregressive within-study and between-study covariance matrices (14). Modeling were performed separately for transgender men on GAHT and transgender women on GAHT. All analyses were done in SAS version 9.4 (Cary, NC). In order to estimate heterogeneity as measured by τ and I2, we also performed a random effects meta-analysis at 3, 6, and 12 months using the DerSimonian and Laird method in RevMan, acknowledging that this meta-analytic approach is less credible than the primary meta-analytic approach described above due to the presence of repeated correlated measures. We did not perform any sensitivity analyses.
Results
Search Results and Study Characteristics
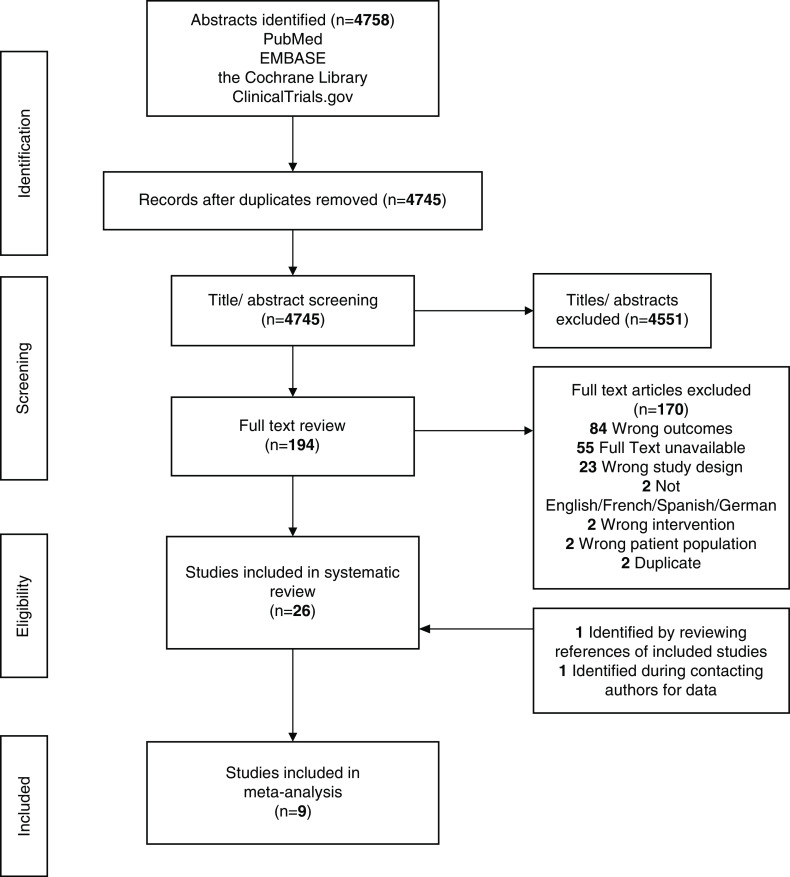
From 4758 eligible studies, 26 met the inclusion criteria. The studies are summarized in Table 1 (18,19,24–47), Supplemental Table 2 (transgender men on GAHT), and Supplemental Table 3 (transgender women on GAHT). Included studies were published between 1998 and 2019 and included three randomized controlled trials, 12 prospective studies, five retrospective studies, and six cross-sectional studies. Studies were mostly from the United States and Europe, with heterogeneity across designs, populations, GAHT route, dose, and follow-up duration. No study included nonbinary or gender-diverse individuals on GAHT. Participants were typically younger without any evidence of CKD. Twenty-three studies reported serum creatinine. BUN was reported infrequently (seven studies), and no study reported cystatin C. eGFR and creatinine clearance (Cockcroft Gault) were reported in two and three studies, respectively; 24-hour urine creatinine was reported in two studies, and creatinine clearance calculated by a 24-hour urine collection was reported in one study. Urinalysis and urine creatinine were reported in one study each. Albuminuria, proteinuria, measured GFR, and eGFR performance were not reported in any study.
Table 1.
Characteristics of included studies (pre/post and cross-sectional)
| Study | Author and Year | Country | Study Design | Population | Previous Gender-Affirming Hormone Therapy | Gender Confirmation Surgery | Follow-Up | Outcomes | Meta-Analysis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Bunck et al. (25) 2006 | The Netherlands | Randomized controlled trial, AI versus placebo | 30 TM | 18–24 mo | Yes | 12 wk | Creatinine, urinary creatinine | No |
| 2 | Fernandez et al. (26) 2016 | United States | Retrospective | 19 TM, 33 TW | N/A | No | 3–6, 6–18 mo | Creatinine | Yes |
| 3 | Giltay et al. (27) 1998 | The Netherlands | Prospective | 17 TM, 17 TW | N/A | No | 4 mo | Creatinine, 24-h urine creatinine | Yes |
| 4 | Giltay et al. (28) 2008 | The Netherlands | Prospective | 14 TM, 15 TW | N/A | No | 4, 12 mo | Creatinine, 24-h urine creatinine | No (duplicate data) |
| 5 | Humble et al. (29) 2019 | United States | Retrospective | 150 TM, 152 TW | N/A | 24% TM, 19% TW | 6, 12 mo | BUN, creatinine, eGFR | Yes |
| 6 | Hiransuthikul et al. (30) 2019 | Thailand | Prospective | 20 TW | N/A | No | 15 wk | Creatinine clearance | No |
| 7 | Jain et al. (31) 2019 | United States | Retrospective | 92 TW | N/A | No | 3 mo, annually, mean 3.4±1.7 yr | BUN, creatinine | No |
| 8 | Kurahashi et al. (32) 2013 | Japan | Prospective | 160 TM | N/A | No | 3, 6, 12 mo | Urinalysis, creatinine | No |
| 9 | Meriggiola et al. (33) 2008 | Italy | Randomized, open-label, uncontrolled safety study | 15 TM | 71.4±30.5 mo | Yes | 6, 18, 30, 42, 54 wk | BUN, creatinine | No |
| 10 | Nicholls et al. (34) 1973 | United Kingdom | Prospective | 22 TM | N/A | No | 2 wk to 10 mo (mean 10 wk) | 24-h urine for creatinine clearance | No |
| 11 | Shieh et al. (35) 2019 | United States | Prospective | 8 CisM, 8 TW | 1–27 yr | No | 7 d | Creatinine, eGFR, creatinine clearance | No |
| 12 | SoRelle et al. (36) 2019 | United States | Retrospective | Baseline: 62 TM, 87 TW; post-GAHT: 89 TM, 133 TW | ≥6 mo | Table 1 | N/A | BUN, creatinine | Yes |
| 13 | van Kesteren et al. (37) 1998 | The Netherlands | Prospective | 19 TM, 20 TW | N/A; surgery 20.3±4.1 after GAHT | Yes | 12 mo, 25.2±10.4 mo after surgery | Creatinine | Yes |
| 14 | van Velzen et al. (17) 2019 | ENIGI, The Netherlands, Belgium, Norway, Italy | Prospective | 188 TM, 242 TW | N/A | No | 12 mo | Creatinine | No (duplicate data) |
| 15 | Vita et al. (38) 2018 | Italy | Retrospective | 11 TM, 21 TW | N/A | No | Mean 30 (25.9) mo | Creatinine | Yes |
| 16 | Vlot et al. (18) 2019 | ENIGI, The Netherlands, Belgium, Norway, Italy | Prospective | 132 TM, 121 TW | N/A | No | q3,12 mo | Creatinine | No (duplicate data) |
| 17 | Wierckx et al. (19) 2014 | ENIGI, Norway, Belgium | Prospective | 53 TM, 53 TW | N/A | No | q3 mo,12 mo | Creatinine | No (duplicate data) |
| 18 | Yahyaoui et al. (39) 2008 | Spain | Prospective | 47 TM, 22 TW | N/A | No | 1, 2 yr | Creatinine | Yes |
| 19 | Angus et al. (40) 2019 | Australia | Cross-sectional | 80 TW | ≥6 mo, median 1.5 yr (0.9–2.6) | No | N/A | BUN, creatinine | No |
| 20 | Becerra Fernández et al. (41) 1999 | Spain | Cross-sectional | 26 TM, 31 TW | Minimum 6 mo, maximum 10 yr | No | N/A | BUN, creatinine | No |
| 21 | Cottrell et al. (42) 2018 | United States | Cross-sectional | 4 TW, 4 CisM, 4 CisW | 1–9 yr | No | N/A | Creatinine clearance | No |
| 22 | Roberts et al. (43) 2014 | United States | Cross-sectional | 55 TW, 20 CisM, 20 CisW | ≥6 mo to ≤10 yr follow-up | No | Median 4 yr | BUN, creatinine | No |
| 23 | Van Caenegem et al. (44) 2012 | Belgium | Cross-sectional | 50 TM GAHT, 16 TM no GAHT, 50 CisW, 16 CisW | 9.9 yr (range, 3.2–27.5) | Yes | N/A | Creatinine | No |
| 24 | Wierckx et al. (45) 2012 | Belgium | Cross-sectional | 50 TM, 50 TW | TM=10 yr, TW=9.2 yr | Yes | N/A | Creatinine | No |
| 25 | Giltay et al. (47) 2003 | The Netherlands | Open-label, randomized trial | 40 TW | N/A | No | 2, 4 mo | Creatinine | Yes |
| 26 | Scharff et al. (46) 2019 | ENIGI, The Netherlands, Belgium, Norway | Prospective | 278 TM, 248 TW | N/A | No | 3, 12 mo | Creatinine | Yes |
AI, aromatase inhibitor; TM, transgender man; TW, transgender woman; N/A, not applicable; CisM, cisgender man; GAHT, gender-affirming hormone therapy; ENIGI, European Network for the Investigation of Gender Incongruence; q3, every; CisW, cisgender woman.
The Effect of Gender-Affirming Hormone Therapy on Serum Creatinine, Other Markers of Kidney Function, and Glomerular Filtration Rate
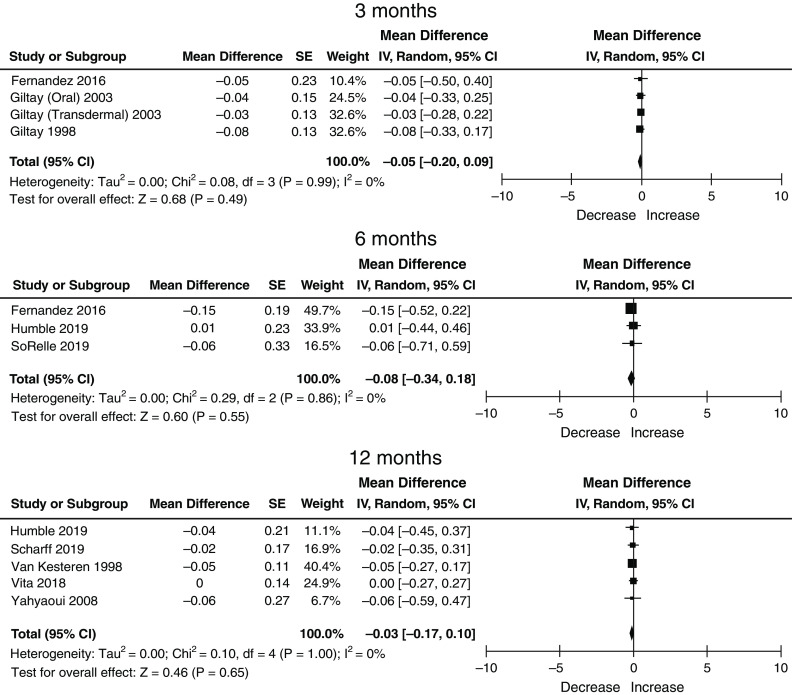
There were nine studies with data available for meta-analysis without duplicate data. The quality of included studies is shown in Supplemental Figure 1. Seven studies were deemed to be of fair quality, and two studies were deemed to be of poor quality. No study was deemed to be of good quality. No study reported a sample size calculation, and no study was blinded; also, there were issues regarding the generalizability of cohorts (as most were single center with younger, healthier populations); the lack of reporting or use of standardized, calibrated creatinine assays (Supplemental Table 4); and loss to follow-up. There were a total of 488 transgender men, of which 33 (two studies), 92 (three studies), and 370 (five studies) contributed data to the 3-, 6-, and 12-month post-GAHT time points. In transgender men, the mean baseline creatinine (SD) prior to the initiation of GAHT was 0.77 mg/dl (0.49). Serum creatinine increased after initiating GAHT by 0.14 mg/dl (95% CI, −0.07 to 0.35) at 3 months, 0.13 mg/dl (95% CI, −0.14 to 0.41) at 6 months, and 0.15 mg/dl (95% CI, 0.00 to 0.29) at 12 months (Table 2).
Table 2.
The change in serum creatinine over time in transgender men treated with gender-affirming hormone therapy
| Time, mo | Change in Serum Creatinine, mg/dl | SEM | P Value | 95% Confidence Interval |
|---|---|---|---|---|
| 3 | 0.14 | 0.11 | 0.19 | −0.07 to 0.35 |
| 6 | 0.13 | 0.14 | 0.34 | −0.14 to 0.41 |
| 12 | 0.15 | 0.07 | 0.05 | 0.00 to 0.29 |
Random effects using estimated within-study and between-study correlations using PROC MIXED with autoregressive within-study and between-study covariance matrices (14).
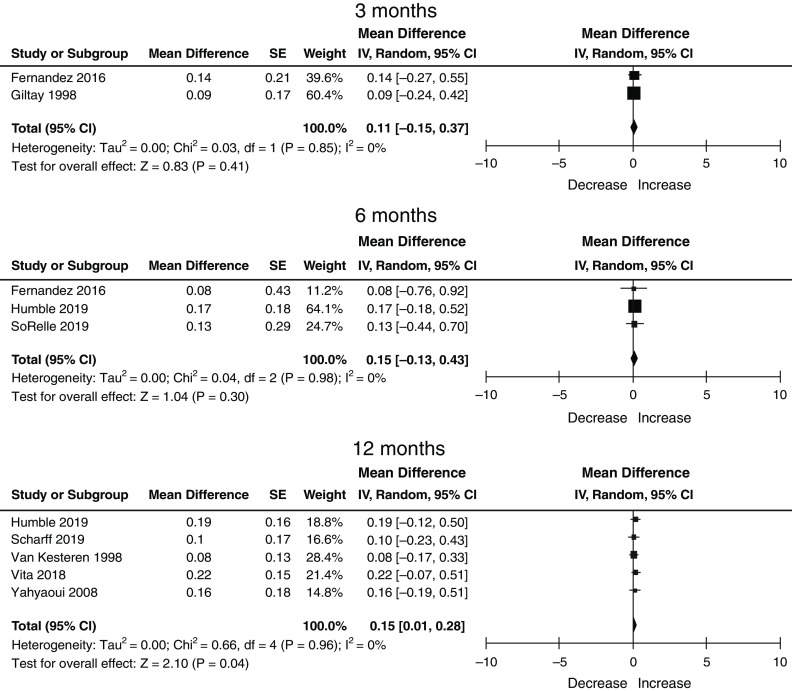
There were a total of 529 transgender women, of which 77 (four studies), 167 (three studies), and 361 (five studies) contributed data to the 3-, 6-, and 12-month post-GAHT time points. In transgender women, the mean baseline serum creatinine (SD) prior to the initiation of GAHT was 0.93 (0.58). Serum creatinine decreased after initiating GAHT by 0.00 mg/dl (95% CI, −0.12 to 0.13) at 3 months, −0.05 mg/dl (95% CI, −0.24 to 0.13) at 6 months, and −0.05 (95% CI, −0.16 to 0.05) at 12 months (Table 3). Significant heterogeneity was not detected in estimates for either transgender men or women at any time point (Figures 1–3). We did not attempt to perform a meta-regression or multivariable regression to identify study-level or patient-level factors independently associated with changes in serum creatinine (i.e., age, race, GAHT type/route/dosing, achieved hormone levels, and baseline biomarker values) due to the number of studies and the lack of individual patient-level data.
Table 3.
The change in serum creatinine over time in transgender women treated with gender-affirming hormone therapy
| Time, mo | Change in Serum Creatinine, mg/dl | SEM | P Value | 95% Confidence Interval |
|---|---|---|---|---|
| 3 | 0.00 | 0.06 | 0.94 | −0.12 to 0.13 |
| 6 | −0.05 | 0.10 | 0.57 | −0.24 to 0.13 |
| 12 | −0.05 | 0.05 | 0.29 | −0.16 to 0.05 |
Random effects using estimated within-study and between-study correlations using PROC MIXED with autoregressive within-study and between-study covariance matrices (14).
Figure 1.
PRISMA flow diagram. PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Figure 3.
Changes in serum creatinine in transgender women on GAHT at 3, 6, 12 months: nine studies, n=593, random effects, DerSimonian and Laird method. 95% CI, 95% confidence interval; IV, weighted mean difference SE, standard error.
Figure 2.
Changes in serum creatinine in transgender men on gender-affirming hormone therapy (GAHT) at 3, 6, and 12 months: eight studies, n=488, random effects, DerSimonian and Laird method. 95% CI, 95% confidence interval; IV, weighted mean difference; SE, standard error.
There were a total of 94 transgender men, of which 79 (two studies) and 15 (one study) contributed data to the 6- and 12-month post-GAHT time points for BUN. In transgender men, the mean baseline BUN (SD) prior to the initiation of GAHT was 10.94 mg/dl (0.02). BUN did not significantly change after initiating GAHT at 6 months (−0.28 mg/dl; 95% CI, −3.53 to 2.98) or 12 months (0.60 mg/dl; 95% CI, −2.61 to 3.81) (Table 4). There were a total of 198 transgender women, of which 126 (two studies) and 47 (one study) contributed data to the 6- and 12-month post-GAHT time points for BUN. In transgender women, the mean baseline BUN (SD) prior to the initiation of GAHT was 12.18 mg/dl (0.10). BUN did not significantly change after initiating GAHT at 6 months (0.21 mg/dl; 95% CI, −1.63 to 2.05) or 12 months (0.92 mg/dl; 95% CI, −1.14 to 2.98) (Table 5).
Table 4.
The change in BUN over time in transgender men treated with gender-affirming hormone therapy
| Time, mo | Change in BUN, mg/dl | SEM | P Value | 95% Confidence Interval |
|---|---|---|---|---|
| 6 | −0.28 | 1.66 | 0.87 | −3.53 to 2.98 |
| 12 | 0.60 | 1.64 | 0.72 | −2.61 to 3.81 |
Random effects using estimated within-study and between-study correlations using PROC MIXED with autoregressive within-study and between-study covariance matrices (14).
Table 5.
The change in BUN over time in transgender women treated with gender-affirming hormone therapy
| Time, mo | Change in BUN, mg/dl | SEM | P Value | 95% Confidence Interval |
|---|---|---|---|---|
| 6 | 0.21 | 0.94 | 0.83 | −1.63 to 2.05 |
| 12 | 0.92 | 1.05 | 0.38 | −1.14 to 2.98 |
Random effects using estimated within-study and between-study correlations using PROC MIXED with autoregressive within-study and between-study covariance matrices (14).
The limited number of studies for kidney function biomarkers other than serum creatinine and BUN precluded a meta-analysis for other outcomes of interest. However, in the two studies that reported 24-hour urine creatinine excretion, there were statistically significant changes in transgender men but not transgender women at 4 and 12 months of follow-up. Giltay et al. (27) reported that the urinary creatinine excretion rate (millimoles per 24 hours) did not change in 17 transgender women treated with ethinyl estradiol at 100 µg/d in combination with cyproterone acetate at 100 mg/d from baseline to 4 months (15.0 [SD 4.1] to 15.3 [SD 3.5]; P=0.62). However, it did increase from 10.3 (SD 2.8) to 12.1 (SD 3.7; P=0.008) from baseline to 4 months in 17 transgender men treated with testosterone esters at 250 mg every 2 weeks. Giltay et al. (28) also reported that the urinary creatinine excretion rate (millimoles per 24 hours) did not change in 15 transgender women treated with the same GAHT described above from baseline (14.7 [SD 4.1]) to 12 months (13.9 [SD 3.2]; P=0.19). However, it did increase from 10.8 (SD 2.7) at baseline to 14.0 (SD 3.8) at 12 months (P=0.01) in 14 transgender men treated with 250-mg intramuscular testosterone esters every 2 weeks. Lastly, in the one study that reported creatinine clearance measured by 24-hour urine, Nicholls et al. (34) showed that there was no difference (103 ml/min [SD 23] before treatment and 116 ml/min [SD 20] after treatment) in 22 transgender women (21 received stilboestrol at a mean of 20.25 mg/d, whereas one received ethinylestradiol at 0.4 mg daily) over a mean follow-up of 10 weeks (range, 2 weeks to 10 months).
Discussion
In this systematic review of 26 studies and meta-analysis of nine studies including 488 adult transgender men and 529 adult transgender women on masculinizing and feminizing GAHT, serum creatinine increased in transgender men and did not significantly change in transgender women initiating GAHT. BUN did not change after the initiation of GAHT in either transgender men or women. No study reported on the effect of GAHT on other kidney function biomarkers, including cystatin C, albuminuria, proteinuria, or measured GFR.
Previous studies addressing the effect of GAHT on kidney function were, individually, small with limited power to precisely quantify differences in biomarkers of kidney function. Our finding that an increase in serum creatinine in transgender men occurs by 12 months on GAHT and encompasses a clinically meaningful change requires awareness by health care providers, including primary care physicians, endocrinologists, and nephrologists. For example, a 25-year-old transgender man whose baseline serum creatinine is 0.77 mg/dl (eGFR=127 ml/min per 1.73 m2 by CKD-EPI) and whose serum creatinine 12 months post-GAHT is either 0.92 mg/dl (+0.15 mg/dl or the mean changes in serum creatinine as above, which corresponds to an eGFR of 118 ml/min per 1.73 m2) or 1.06 mg/dl (0.29 mg/dl or the 95% upper CI as above, which corresponds to an eGFR of 100 ml/min per 1.73 m2) represents an approximately 10%–15% decline in eGFR (8). This presumably reflects the corresponding increase in muscle mass due to the use of testosterone rather than a true change in GFR (6,7) and would not require further investigation in the absence of other signs of kidney disease. In transgender women, on the other hand, serum creatinine did not change significantly over 3, 6, and 12 months on GAHT. However, it should be acknowledged that across both transgender men and women, there is a spectrum of change in serum creatinine likely due to changes in lean muscle mass as a result of GAHT type, dose, adherence, and achieved target hormone levels as well as potentially other non-GFR determinants of serum creatinine, such as diet (21). Lastly, this interpretation assumes that changes in serum creatinine are due to changes in lean muscle mass due to GAHT and not GFR. This is currently unknown and possibly erroneous given the association of female sex/gender with adverse outcomes in CKD (22–24).
This study has important clinical, research, and health policy implications. From a clinical perspective, physicians should consider measuring GFR when considering important treatment decisions in patients undergoing GAHT given its potential effect on serum creatinine, especially in transgender men. These decisions could include referral to nephrology, initiation or discontinuation of disease-modifying therapy (e.g., renin-angiotensin-aldosterone system inhibitors, sodium-glucose cotransport 2 inhibitors, and mineralocorticoid receptor antagonists), dosing for medications with narrow therapeutic indices, and/or referral for kidney transplantation and dialysis initiation. From a research perspective, measured GFR studies and studies on other established kidney biomarkers (cystatin C, β-trace protein, and β2-microglobulin) are needed in transgender populations treated with and without GAHT. From a health policy perspective, education efforts on the metabolic changes that occur with GAHT are needed for all health care providers (i.e., primary care providers, endocrinologists, surgeons, and subspecialists) who provide care to transgender patients who may not be aware of the specialized literature on kidney biomarkers.
To our knowledge, this is the first systematic review and meta-analysis to examine the effect of GAHT on kidney function biomarkers and GFR in any transgender population. It used a liberal search strategy with limited exclusion criteria to capture all relevant studies and meta-analyzed outcomes over a series of clinically relevant time points after the initiation of GAHT. Limitations include the low certainty of evidence given the inclusion of pre/post studies mostly of fair but not good quality due to a lack of blinding; concerns regarding generalizability and loss to follow-up; the lack of inclusion of pediatrics, adolescent, and nonbinary/gender-diverse populations; its inclusion of mostly studies from the United States and Europe; and a lack of information regarding creatinine assay standardization and calibration across studies. Studies also focused mostly on creatinine and did not include other kidney function biomarkers such as cystatin C, albuminuria/proteinuria, eGFR, and measured GFR, so the effect on GAHT on these outcomes is uncertain as well as the influence of GAHT type, route of administration, and dose (we were unable to perform any sensitivity analyses due to the inability to properly categorize exposures, and further subgroup analyses would be underpowered). Included studies were predominantly in younger, healthier populations without CKD, and therefore, how GAHT affects kidney function biomarkers in those with established CKD is unknown (15,16). Studies in transgender women who used mineralocorticoid receptor antagonists as antiandrogen therapy universally did not account for its known antihypertensive effects, which decrease GFR and subsequently influence serum levels of kidney biomarkers and proteinuria. Lastly, we are missing some patients from the multicenter ENIGI (17–19) cohort as we conservatively only included one study (46) with the most comprehensive data as suggested by the study’s authors to remove the possibility of any duplicate data.
In summary, our systematic review and meta-analysis show that GAHT increases serum creatinine to a clinically significant degree in transgender men but does not significantly affect serum creatinine in transgender women; however, patients with CKD were not represented. Awareness of the serum creatinine trajectory post-GAHT is important to frame the need for diagnostic testing for AKI or CKD and nephrology consultation. Additional research is needed to determine the effect of GAHT on cystatin C, albuminuria, proteinuria, and measured GFR in addition to how to accurately and precisely estimate GFR in transgender populations treated with and without GAHT (9). Specifically, high-quality, adequately powered prospective observational studies with repeated measurements are needed in diverse populations (adults, pediatrics, and the elderly) evaluating different types of GAHT (routes and dosing) in both transgender men and women across the spectrum of health and CKD.
Disclosures
S.B. Ahmed reports serving as an advisory board member of the Canadian Institutes of Health Research Institute of Gender and Health (volunteer position), as a Canadian Medical Association Journal Governance Council Member (elected position), and as the Education Chair of the Organization for the Study of Sex Differences (volunteer position). D. Collister reports employment with Alberta Health Services; consultancy agreements with Akebia; research funding from Boehringer Ingelheim/Research Manitoba, the Canadian Institutes of Health Research, and the Kidney Foundation of Canada; and serving in an advisory or leadership role for the Canadian Nephrology Trials Network (Executive Committee and Scientific Operations Committee). S. Curtis and R. Whitlock report employment with the Chronic Disease Innovation Centre. T. Ferguson reports employment with Chronic Disease Innovation Centre; consultancy agreements with Clinpredict, Quanta Dialysis Technologies, and Strategic Health Resources (Tricida Inc., Protagonist Therapeutics); ownership interest in Klinrisk Inc.; and honoraria from Baxter Canada. A.C. Millar reports consultancy agreements with Novo Nordisk Canada, honoraria from Dexcom Canada, and speakers bureau for Dexcom Canada. N. Tangri reports consultancy agreements with Marizyme, Mesentech Inc., PulseData Inc., Renibus, and Tricida Inc.; ownership interest in Clinpredict Ltd., Klinrisk, Marizyme, Mesentech Inc., PulseData Inc., Quanta, Renibus, and Tricida Inc.; research funding from AstraZeneca Inc., Bayer, BI-Lilly, Janssen, Otsuka, and Tricida Inc.; honoraria from AstraZeneca Inc., Bayer, BI-Lilly, Janssen, Otsuka Pharmaceuticals, and Pfizer; patents or royalties from Klinrisk and Marizyme; serving in an advisory or leadership role for Clinpredict, Klinrisk, and Tricida Inc.; other interests or relationships with Clinpredict and the National Kidney Foundation; and is the founder of Klinrisk. M. Walsh reports employment with the Ontario Renal Network; research funding from the British Heart Foundation, the Canadian Institutes of Health Research, the Health Research Council, the National Health and Medical Research Council, the National Institutes of Health Research, and Vifor (no salary support received through any research funding); serving in an advisory or leadership role for Bayer (steering committee, payment to institution) and Otsuka (national leader, payment to institution); and other interests or relationships with Novo Nordisk (event adjudication, payment to institution). All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
D. Collister is supported by a Kidney Foundation of Canada Kidney Research Scientist Core Education and National Training (KRESCENT) new investigator award.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Advancing Kidney Health Equity: Influences of Gender-Affirming Hormone Therapy on Kidney Function,” on pages 1281–1283.
Author Contributions
D. Collister conceptualized the study; D. Collister, S. Curtis, and E. Krupka were responsible for data curation; D. Collister was responsible for investigation; D. Collister, T. Ferguson, and R. Whitlock were responsible for formal analysis; D. Collister was responsible for methodology; D. Collister was responsible for validation; D. Collister was responsible for visualization; D. Collister provided supervision; D. Collister wrote the original draft; and S.B. Ahmed, N. Askin, D. Collister, S. Curtis, M. Dahl, T. Ferguson, R. Fung, E. Krupka, A.C. Millar, N. Tangri, M. Walsh, and R. Whitlock reviewed and edited the manuscript.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01890222/-/DCSupplemental.
Supplemental Table 1. Search strategy.
Supplemental Table 2. Studies of transgender men on GAHT and creatinine at baseline and follow-up (n=18 studies).
Supplemental Table 3. Studies of transgender women on GAHT and creatinine at baseline and follow-up (n=23 studies).
Supplemental Table 4. Characteristics of creatinine assays of studies included in the meta-analysis.
Supplemental Figure 1. Quality assessment.
References
- 1.Safer JD, Tangpricha V: Care of transgender persons. N Engl J Med 381: 2451–2460, 2019. 10.1056/NEJMcp1903650 [DOI] [PubMed] [Google Scholar]
- 2.Reisner SL, Poteat T, Keatley J, Cabral M, Mothopeng T, Dunham E, Holland CE, Max R, Baral SD: Global health burden and needs of transgender populations: A review. Lancet 388: 412–436, 2016. 10.1016/s0140-6736(16)00684-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collin L, Reisner SL, Tangpricha V, Goodman M: Prevalence of transgender depends on the “case” definition: A systematic review. J Sex Med 13: 613–626, 2016. 10.1016/j.jsxm.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG: Endocrine treatment of gender-dysphoric/gender-incongruent persons: An Endocrine Society Clinical practice guideline. J Clin Endocrinol Metab 102: 3869–3903, 2017. 10.1210/jc.2017-01658 [DOI] [PubMed] [Google Scholar]
- 5.World Professional Association for Transgender Health (WPATH) : Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, 7th Version, 2012. Available at: https://www.wpath.org/publications/soc. Accessed June 1, 2022
- 6.Spanos C, Bretherton I, Zajac JD, Cheung AS: Effects of gender-affirming hormone therapy on insulin resistance and body composition in transgender individuals: A systematic review. World J Diabetes 11: 66–77, 2020. 10.4239/wjd.v11.i3.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klaver M, Dekker MJHJ, de Mutsert R, Twisk JWR, den Heijer M: Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: A meta-analysis. Andrologia 49: e12660, 2017. 10.1111/and.12660 [DOI] [PubMed] [Google Scholar]
- 8.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collister D, Saad N, Christie E, Ahmed S: Providing care for transgender persons with kidney disease: A narrative review. Can J Kidney Health Dis 8: 2054358120985379, 2021. 10.1177/2054358120985379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohottige D, Lunn MR: Ensuring gender-affirming care in nephrology: Improving care for transgender and gender-expansive individuals. Clin J Am Soc Nephrol 15: 1195–1197, 2020. 10.2215/cjn.14471119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collister D, Curtis S, Krupka E: Change in Kidney Function Biomarkers in Transgender Persons on Gender Affirming Hormone Therapy–A Systematic Review and Meta-analysis. PROSPERO, 2020. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=214248. Accessed June 1, 2022
- 12.Covidence : Better systematic review management. Available at: https://www.covidence.org. Accessed June 1, 2022
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Ishak KJ, Platt RW, Joseph L, Hanley JA, Caro JJ: Meta-analysis of longitudinal studies. Clin Trials 4: 525–539, 2007. 10.1177/1740774507083567 [DOI] [PubMed] [Google Scholar]
- 15.Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robinson B, Roblin D, Silverberg MJ, Safer J, Slovis J, Tangpricha V, Goodman M: Cross-sex hormones and acute cardiovascular events in transgender persons: A cohort study. Ann Intern Med 169: 205–213, 2018. 10.7326/m17-2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connelly PJ, Marie Freel E, Perry C, Ewan J, Touyz RM, Currie G, Delles C: Gender-affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension 74: 1266–1274, 2019. 10.1161/hypertensionaha.119.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Velzen DM, Paldino A, Klaver M, Nota NM, Defreyne J, Hovingh GK, Thijs A, Simsek S, T’Sjoen G, den Heijer M: Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. J Clin Endocrinol Metab 104: 1937–1947, 2019. 10.1210/jc.2018-02138 [DOI] [PubMed] [Google Scholar]
- 18.Vlot MC, Wiepjes CM, de Jongh RT, T’Sjoen G, Heijboer AC, den Heijer M: Gender-affirming hormone treatment decreases bone turnover in transwomen and older transmen. J Bone Miner Res 34: 1862–1872, 2019. 10.1002/jbmr.3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, Kaufman JM, T’Sjoen G: Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: Results from the European network for the investigation of gender incongruence [published correction appears in J Sex Med 13: 732, 2016]. J Sex Med 11: 1999–2011, 2014. 10.1111/jsm.12571 [DOI] [PubMed] [Google Scholar]
- 20.Waikar SS, Rebholz CM, Zheng Z, Hurwitz S, Hsu CY, Feldman HI, Xie D, Liu KD, Mifflin TE, Eckfeldt JH, Kimmel PL, Vasan RS, Bonventre JV, Inker LA, Coresh J; Chronic Kidney Disease Biomarkers Consortium Investigators : Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis 72: 538–546, 2018. 10.1053/j.ajkd.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitsch D: Is there a difference in metabolic burden between men and women? Nephrol Dial Transplant 29: 1110–1112, 2014. 10.1093/ndt/gft518 [DOI] [PubMed] [Google Scholar]
- 22.Jafar TH, Schmid CH, Stark PC, Toto R, Remuzzi G, Ruggenenti P, Marcantoni C, Becker G, Shahinfar S, De Jong PE, De Zeeuw D, Kamper AL, Strangaard S, Levey AS: The rate of progression of renal disease may not be slower in women compared with men: A patient-level meta-analysis. Nephrol Dial Transplant 18: 2047–2053, 2003. 10.1093/ndt/gfg317 [DOI] [PubMed] [Google Scholar]
- 23.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR; Chronic Kidney Disease Prognosis Consortium : Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: A meta-analysis. BMJ 346: f324, 2013. 10.1136/bmj.f324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed SB, Ramesh S: Sex hormones in women with kidney disease. Nephrol Dial Transplant 31: 1787–1795, 2016. 10.1093/ndt/gfw084 [DOI] [PubMed] [Google Scholar]
- 25.Bunck MC, Toorians AW, Lips P, Gooren LJ: The effects of the aromatase inhibitor anastrozole on bone metabolism and cardiovascular risk indices in ovariectomized, androgen-treated female-to-male transsexuals. Eur J Endocrinol 154: 569–575, 2006. 10.1530/eje.1.02126 [DOI] [PubMed] [Google Scholar]
- 26.Fernandez JD, Tannock LR: Metabolic effects of hormone therapy in transgender patients. Endocr Pract 22: 383–388, 2016. 10.4158/ep15950.or [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD: Effects of sex steroids on plasma total homocysteine levels: A study in transsexual males and females. J Clin Endocrinol Metab 83: 550–553, 1998. 10.1210/jcem.83.2.4574 [DOI] [PubMed] [Google Scholar]
- 28.Giltay EJ, Bunck MC, Gooren LJ, Zitman FG, Diamant M, Teerlink T: Effects of sex steroids on the neurotransmitter-specific aromatic amino acids phenylalanine, tyrosine, and tryptophan in transsexual subjects. Neuroendocrinology 88: 103–110, 2008. 10.1159/000135710 [DOI] [PubMed] [Google Scholar]
- 29.Humble RM, Imborek KL, Nisly N, Greene DN, Krasowski MD: Common hormone therapies used to care for transgender patients influence laboratory results. J Appl Lab Med 3: 799–814, 2019. 10.1373/jalm.2018.027078 [DOI] [PubMed] [Google Scholar]
- 30.Hiransuthikul A, Janamnuaysook R, Himmad K, Kerr SJ, Thammajaruk N, Pankam T, Phanjaroen K, Mills S, Vannakit R, Phanuphak P, Phanuphak N; iFACT Study Team : Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: The iFACT study. J Int AIDS Soc 22: e25338, 2019. 10.1002/jia2.25338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain J, Kwan D, Forcier M: Medroxyprogesterone acetate in gender-affirming therapy for transwomen: Results from a retrospective study. J Clin Endocrinol Metab 104: 5148–5156, 2019. 10.1210/jc.2018-02253 [DOI] [PubMed] [Google Scholar]
- 32.Kurahashi H, Watanabe M, Sugimoto M, Ariyoshi Y, Mahmood S, Araki M, Ishii K, Nasu Y, Nagai A, Kumon H: Testosterone replacement elevates the serum uric acid levels in patients with female to male gender identity disorder. Endocr J 60: 1321–1327, 2013. 10.1507/endocrj.ej13-0203 [DOI] [PubMed] [Google Scholar]
- 33.Meriggiola MC, Armillotta F, Costantino A, Altieri P, Saad F, Kalhorn T, Perrone AM, Ghi T, Pelusi C, Pelusi G: Effects of testosterone undecanoate administered alone or in combination with letrozole or dutasteride in female to male transsexuals. J Sex Med 5: 2442–2453, 2008. 10.1111/j.1743-6109.2008.00909.x [DOI] [PubMed] [Google Scholar]
- 34.Nicholls A, Snaith ML, Scott JT: Effect of oestrogen therapy on plasma and urinary levels of uric acid. BMJ 1: 449–451, 1973. 10.1136/bmj.1.5851.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shieh E, Marzinke MA, Fuchs EJ, Hamlin A, Bakshi R, Aung W, Breakey J, Poteat T, Brown T, Bumpus NN, Hendrix CW: Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J Int AIDS Soc 22: e25405, 2019. 10.1002/jia2.25405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SoRelle JA, Jiao R, Gao E, Veazey J, Frame I, Quinn AM, Day P, Pagels P, Gimpel N, Patel K: Impact of hormone therapy on laboratory values in transgender patients. Clin Chem 65: 170–179, 2019. 10.1373/clinchem.2018.292730 [DOI] [PubMed] [Google Scholar]
- 37.van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J: Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol (Oxf) 48: 347–354, 1998. 10.1046/j.1365-2265.1998.00396.x [DOI] [PubMed] [Google Scholar]
- 38.Vita R, Settineri S, Liotta M, Benvenga S, Trimarchi F: Changes in hormonal and metabolic parameters in transgender subjects on cross-sex hormone therapy: A cohort study. Maturitas 107: 92–96, 2018. 10.1016/j.maturitas.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 39.Yahyaoui R, Esteva I, Haro-Mora JJ, Almaraz MC, Morcillo S, Rojo-Martínez G, Martínez J, Gómez-Zumaquero JM, González I, Hernando V, Soriguer F: Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab 93: 2230–2233, 2008. 10.1210/jc.2007-2467 [DOI] [PubMed] [Google Scholar]
- 40.Angus L, Leemaqz S, Ooi O, Cundill P, Silberstein N, Locke P, Zajac JD, Cheung AS: Cyproterone acetate or spironolactone in lowering testosterone concentrations for transgender individuals receiving oestradiol therapy. Endocr Connect 8: 935–940, 2019. 10.1530/EC-19-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becerra Fernández A, de Luis Román DA, Piédrola Maroto G: [Morbidity in transsexual patients with cross-gender hormone self-treatment]. Med Clin (Barc) 113: 484–487, 1999 [PubMed] [Google Scholar]
- 42.Cottrell ML, Prince HM, Maffuid K, Poliseno A, White N, Sykes C, Nelson JA, Peery A, Dellon E, Hightow-Weidman L, Adams JL, Gay C, Kashuba AD: Altered TDF/FTC pharmacology in a transgender female cohort: Implications for PrEP. J Int AIDS Soc 21[Suppl 6], 2018 [Google Scholar]
- 43.Roberts TK, Kraft CS, French D, Ji W, Wu AH, Tangpricha V, Fantz CR: Interpreting laboratory results in transgender patients on hormone therapy. Am J Med 127: 159–162, 2014. 10.1016/j.amjmed.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 44.Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van de Peer F, Toye K, Kaufman JM, T’Sjoen G: Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J Clin Endocrinol Metab 97: 2503–2511, 2012. 10.1210/jc.2012-1187 [DOI] [PubMed] [Google Scholar]
- 45.Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, T’Sjoen G: Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 9: 2641–2651, 2012. 10.1111/j.1743-6109.2012.02876.x [DOI] [PubMed] [Google Scholar]
- 46.Scharff M, Wiepjes CM, Klaver M, Schreiner T, T’Sjoen G, den Heijer M: Change in grip strength in trans people and its association with lean body mass and bone density. Endocr Connect 8: 1020–1028, 2019. 10.1530/ec-19-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giltay EJ, Verhoef P, Gooren LJ, Geleijnse JM, Schouten EG, Stehouwer CD: Oral and transdermal estrogens both lower plasma total homocysteine in male-to-female transsexuals. Atherosclerosis 168: 139–146, 2003. 10.1016/s0021-9150(03)00090-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.