Abstract
Advances in cancer therapy have significantly improved overall patient survival; however, AKI remains a common complication in patients with cancer, occurring in anywhere from 11% to 22% of patients, depending on patient-related or cancer-specific factors. Critically ill patients with cancer as well as patients with certain malignancies (e.g., leukemias, lymphomas, multiple myeloma, and renal cell carcinoma) are at highest risk of developing AKI. AKI may be a consequence of the underlying malignancy itself or from the wide array of therapies used to treat it. Cancer-associated AKI can affect virtually every compartment of the nephron and can present as subclinical AKI or as overt acute tubular injury, tubulointerstitial nephritis, or thrombotic microangiopathy, among others. AKI can have major repercussions for patients with cancer, potentially jeopardizing further eligibility for therapy and leading to greater morbidity and mortality. This review highlights the epidemiology of AKI in critically ill patients with cancer, risk factors for AKI, and common pathologies associated with certain cancer therapies, as well as the management of AKI in different clinical scenarios. It highlights gaps in our knowledge of AKI in patients with cancer, including the lack of validated biomarkers, as well as evidence-based therapies to prevent AKI and its deleterious consequences.
Keywords: critical care nephrology and acute kidney injury series, onconephrology, cancer, AKI, drug nephrotoxicity, acute kidney injury
Introduction
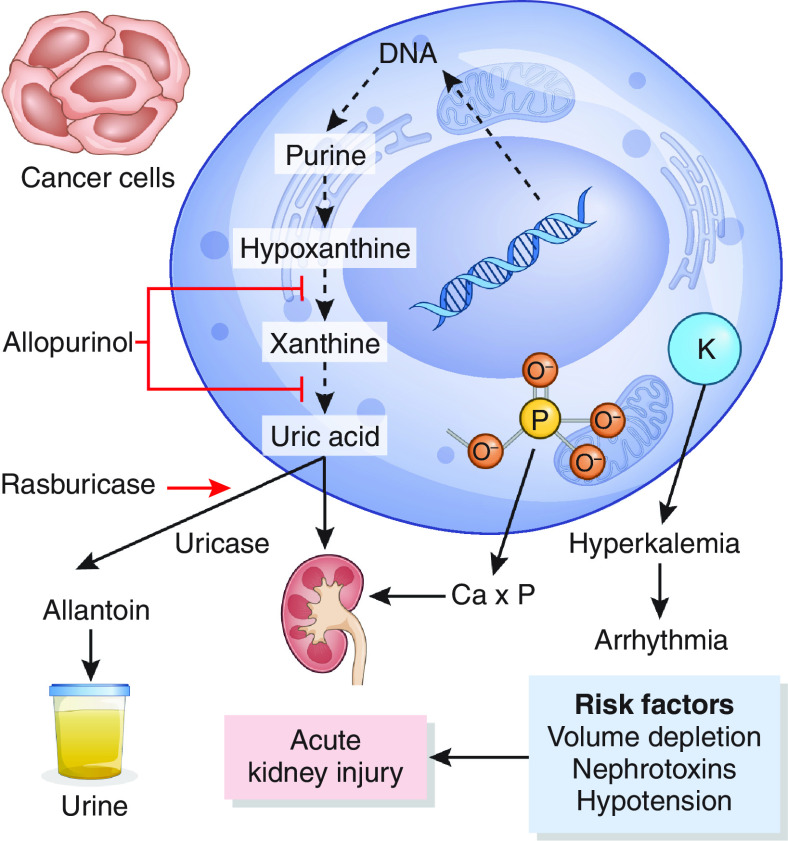
The field of hematology/oncology has made great strides toward treating cancer; however, AKI remains a common complication, occurring in 9% of patients initiating systemic therapy (1). Malignancies associated with the highest 5-year risk of AKI include multiple myeloma (26%), bladder cancer (19%), and leukemia (15%) (1). Other studies have found a high risk of AKI among patients with urogenital cancers and liver cancer (2). Additional high-risk populations include critically ill patients with cancer admitted to the intensive care unit (ICU) as well as those who have undergone hematopoietic stem cell transplant (HSCT) (3,4). The spectrum of AKI is broad (Figure 1) and is driven by cancer-related, patient-specific, and treatment-related factors. Noncancer-related risk factors for AKI include advanced age, CKD, diabetes, and concomitant administration of diuretics and renin-angiotensin receptor blockers (1), antibiotics, or intravenous contrast (5). AKI can have devastating consequences, including higher costs of hospitalization (6), lower rates of cancer remission (7), and even higher mortality (8). Early recognition and prevention are therefore critical to minimize the consequences of AKI.
Figure 1.
The spectrum of AKI is broad in patients with cancer. Ca+, hypercalcemia; HSCT, hematopoietic stem cell transplant; ICU, intensive care unit; NSAID, nonsteroidal anti-inflammatory agent; RAASi, renin-angiotensin-aldosterone system inhibitor; TLS, tumor lysis syndrome; TMA, thrombotic microangiopathy. Adapted from ref. 137, with permission.
Cancer-Associated AKI
Monoclonal Gammopathies and AKI
AKI commonly occurs in the setting of monoclonal gammopathies and results from the abnormal deposition or activity of a paraprotein in the kidney. Multiple myeloma is the most common malignant monoclonal gammopathy and is associated with AKI in up to half of cases (9). Patients with multiple myeloma and kidney impairment have higher mortality compared with those without kidney involvement (10). Cast nephropathy is a myeloma-defining event and occurs when an excess of free light chains binds with uromodulin glycoproteins in the ascending loop of Henle, forming obstructing casts and initiating an inflammatory cascade (11). The diagnosis of cast nephropathy relies on the measurement of serum free light chains, with quantitative measurement of κ- and λ-free light chains, as well as serum and urine protein electrophoresis. The treatment of cast nephropathy has seen a paradigm shift over the past decade with the advent of clone-directed therapy, which causes apoptosis of malignant plasma cells and inhibits NF-KB pathways that are implicated in interstitial inflammation and subsequent fibrosis (12). Extracorporeal therapy has been used to enhance the removal of free light chains, but its use remains controversial. Multiple small studies have shown no mortality benefit for therapeutic plasma exchange (13–15). Two trials examined the utility of high-cutoff hemodialysis for light chain removal; the European Trial of Free Light Chain Removal by Extended Hemodialysis in Cast Nephropathy found no difference in major clinical outcomes among patients who did and did not receive high-cutoff hemodialysis, whereas the Multiple Myeloma and Renal Failure due to Myeloma Cast Nephropathy trial demonstrated a possible reduction in dialysis dependence at 12 months among patients who received high-cutoff hemodialysis (16,17). High-cutoff hemodialysis is not recommended on the basis of available data, and its availability is limited in the United States. Plasmapheresis has also been studied among patients with a new diagnosis of multiple myeloma and AKI, but the largest trial to date did not show a difference in the composite outcome of death, dialysis dependence, or eGFR<30 ml/min per m2 at 6 months between the placebo and the plasmapheresis arm (15).
Until recently, only malignant monoclonal gammopathies were implicated as pathogenic and therefore warranting treatment; however, it is now recognized that some B cell or plasma cell clonal proliferative disorders may not meet hematologic criteria for a malignant monoclonal gammopathy yet can cause AKI and progressive kidney disease (e.g., monoclonal gammopathies of renal significance). These lesions are often classified on the basis of histopathology (Figure 2) and include a wide spectrum of disorders, including amyloidosis, cryoglobulinemia, monoclonal Ig deposition disease, and proximal tubular disorders like Fanconi syndrome. Data suggest that patients with monoclonal gammopathies of renal significance are at high risk for progression to kidney failure if not treated with clone-directed therapy (e.g., bortezomib) (18). Kidney biopsy should be pursued in patients with unexplained AKI, an M spike on serum protein electrophoresis, and/or an abnormal ratio of serum free light chains, as clone-directed therapy may improve kidney and hematologic outcomes (19).
Figure 2.
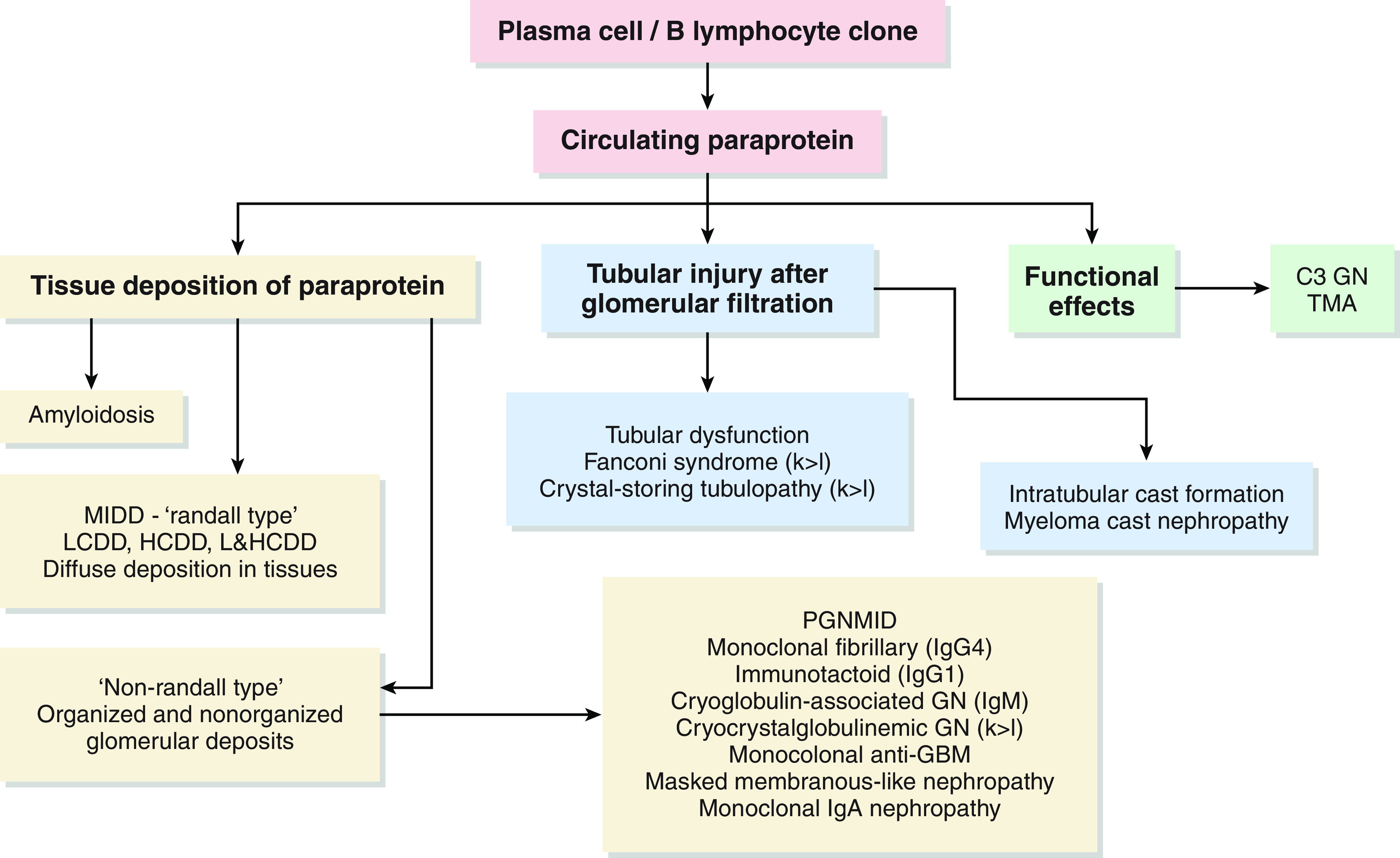
Causes of AKI in monoclonal gammopathies are classified based on histopathology. C3 GN, complement 3–related GN; GBM, glomerular basement membrane; HCDD, heavy chain deposition disease; k>l, κ-light chain>λ-light chain; LCDD, light chain deposition disease; L&HCDD, light and heavy chain deposition disease; MIDD, monoclonal immunoglobulin deposition disease; PGNMID: proliferative GN with monoclonal Ig deposits. Adapted from Bijol and Rennke (Kidney Week 2021 presentation), with permission.
Hypercalcemia
Malignancy is the most common cause of hypercalcemia among patients admitted to the ICU. Mechanisms of hypercalcemia include secretion of parathyroid hormone–related protein (e.g., by solid tumors, T cell leukemias, and lymphomas), local osteolysis with release of cytokines, and, less commonly, ectopic parathyroid hormone secretion and tumor production of 1,25-hydroxyvitamin D. Kidney manifestations of hypercalcemia include arterial vasoconstriction leading to AKI, distal renal tubular acidosis, and nephrogenic diabetes insipidus. Management of severe hypercalcemia involves immediate, aggressive volume expansion with isotonic saline, loop diuretics in patients who develop signs or symptoms of volume overload, and calcitonin. However, these therapies each have transient effects, and patients need more definitive therapy in the form of either bisphosphonates or denosumab. Zoledronic acid is preferred in the setting of hypercalcemia of malignancy, but pamidronate or denosumab can be considered in patients with severe kidney dysfunction (20). In patients with calcium levels over 18 mg/dl, oliguric AKI, or severe neurologic symptoms, hemodialysis may be indicated.
Other Etiologies of Cancer-Associated AKI
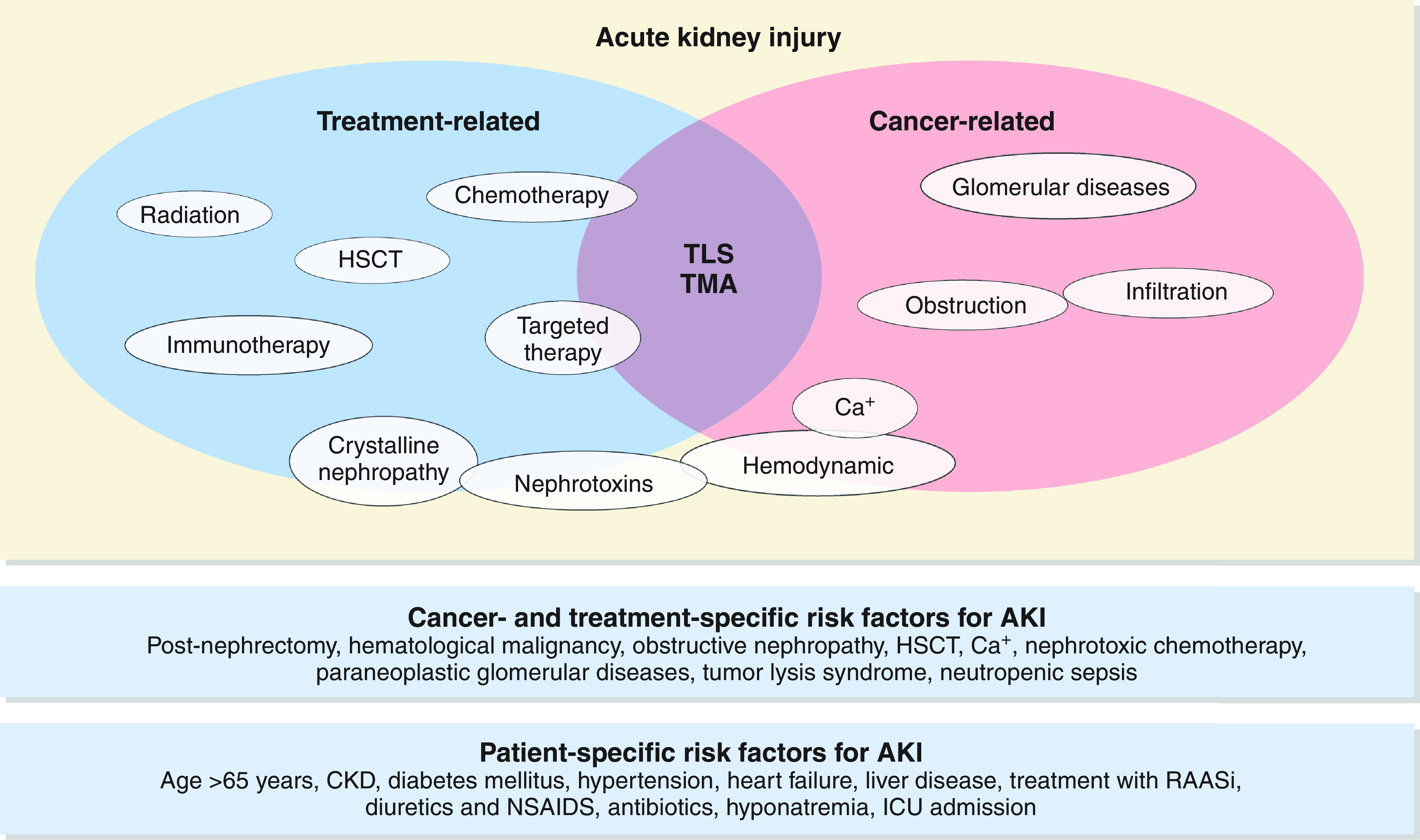
Direct parenchymal involvement is an uncommon cause of AKI. Tumor cell infiltration of the interstitium may result in compression of the tubules and damage to the kidney vasculature, thereby leading to AKI. Leukemic or lymphomatous infiltration of the interstitium manifests as unilateral or bilateral enlargement of the kidneys; however, this is rarely the primary driver of severe AKI in patients with hematologic malignancies and is often an incidental finding (21,22). Autopsy series suggest that the prevalence of kidney infiltration in hematologic malignancies ranges from 33% in the setting of acute myeloid leukemia to 63% in chronic lymphoblastic leukemia (23). Patients with acute myeloid leukemia and certain lymphocytic leukemias may also develop leukostasis, which can rarely cause AKI due to the formation of intravascular leukocyte thrombi and fibrin strands in the kidney vasculature (24,25). Aggressive forms of B cell lymphomas can lead to intraglomerular infiltration by tumor cells, which can manifest as nephrotic-range proteinuria, hematuria, kidney enlargement, and even severe AKI. Obstruction (both intrinsic obstruction and extrinsic compression) is a common cause of AKI, particularly in patients with genitourinary cancers. The pathophysiology and management of obstruction in patients with cancer is outlined in Figure 3.
Figure 3.
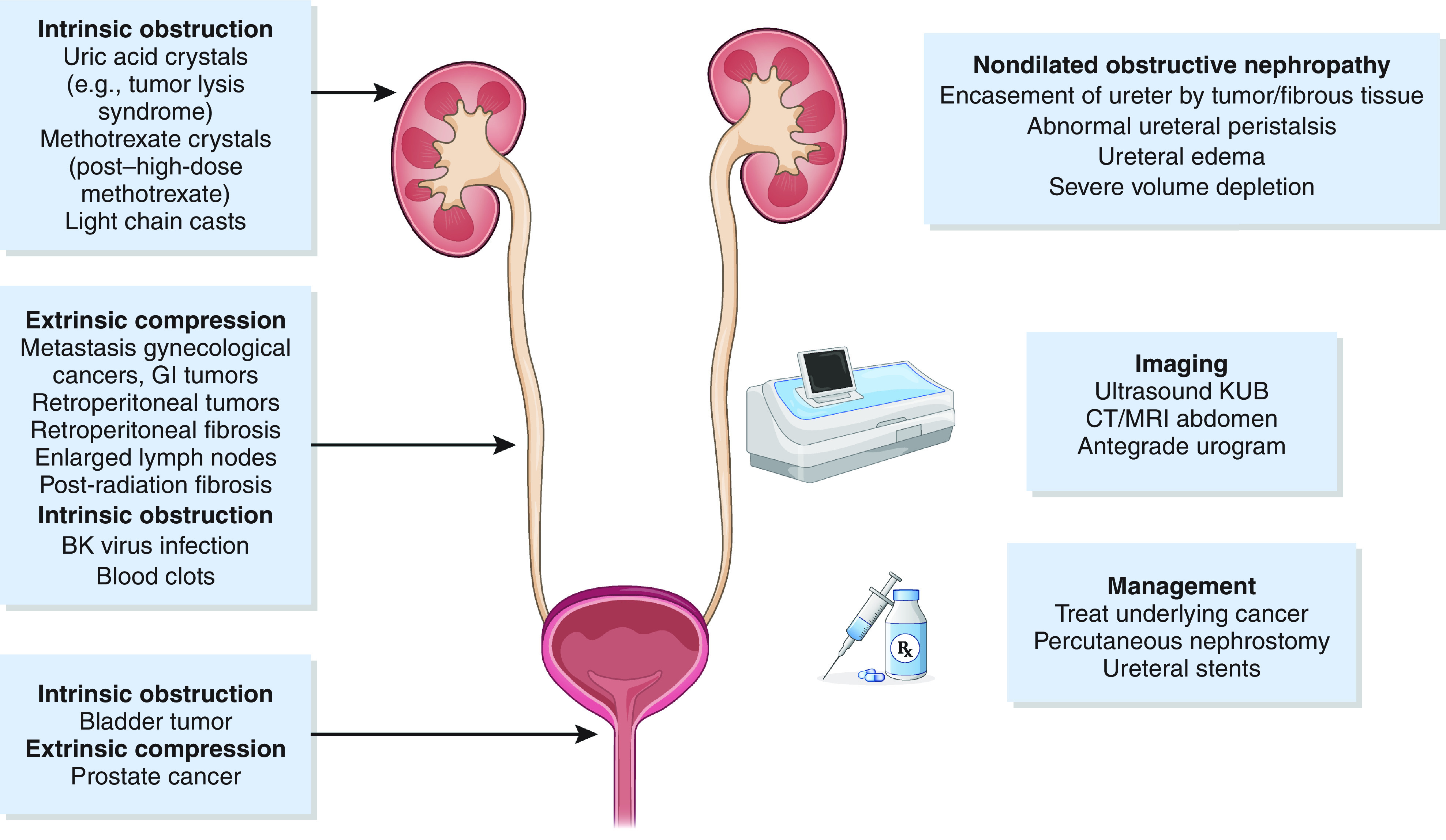
Obstructive nephropathy may be extrinsic or intrinsic to the genitourinary system. CT, computed tomography; GI, gastrointestinal; KUB, kidney, ureter, bladder; MRI, magnetic resonance imaging.
Cancer Treatments and AKI
Conventional Chemotherapy
Conventional chemotherapies continue to be the cornerstone of treatment for many malignancies; however, several agents are associated with AKI, affecting nearly every segment of the nephron and manifesting as tubular injury, tubulointerstitial nephritis, glomerular disease, and thrombotic microangiopathy (TMA) (Table 1).
Table 1.
Nephrotoxicity associated with conventional chemotherapy
| Chemotherapeutic Agent | Nephrotoxic Effect |
|---|---|
| Platins (cisplatin, carboplatin, oxaliplatin) | ATI, proximal tubulopathy, TMA, salt wasting, hypomagnesemia |
| Methotrexate | ATI, crystalline nephropathy |
| Gemcitabine | TMA |
| Pemetrexed | Proximal tubulopathy, ATI, nephrogenic diabetes insipidus |
| Ifosfamide | Proximal tubulopathy, ATI, hemorrhagic cystitis |
| Cyclophosphamide | Hemorrhagic cystitis, hyponatremia |
| Nitrosureas (carmustine, lomustine, streptozocin) | Chronic interstitial nephritis, uric acid nephrolithiasis and diabetes insipidus (streptozocin) |
| Mitomycin C | TMA |
| Melphalan | SIADH |
| Trabectidin | Rhabdomyolysis |
ATI, acute tubular injury; TMA, thrombotic microangiopathy; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Of the platinum-based therapies, cisplatin results in the highest incidence of AKI, occurring in 32% of adults receiving a single dose (26). Cisplatin-associated AKI is dose dependent and frequently presents as nonoliguric AKI (27). Risk factors for cisplatin-associated AKI include female sex, older age, smoking, and hypoalbuminemia (28). Proposed mechanisms include proximal tubular injury from oxidative stress and inflammation (28–30), apoptosis (31,32), mitochondrial injury (33), and DNA damage (34) (Figure 4). Therapies like intravenous fluids, mannitol, and magnesium have been proposed for the prevention of cisplatin-associated AKI; however, data from well-designed randomized controlled trials are lacking (35). Cisplatin is also associated with magnesium wasting, which may potentiate and exacerbate AKI (36). Amiloride (37) and sodium-glucose cotransporter-2 inhibitors (38) warrant further study as potential therapies for refractory hypomagnesemia.
Figure 4.
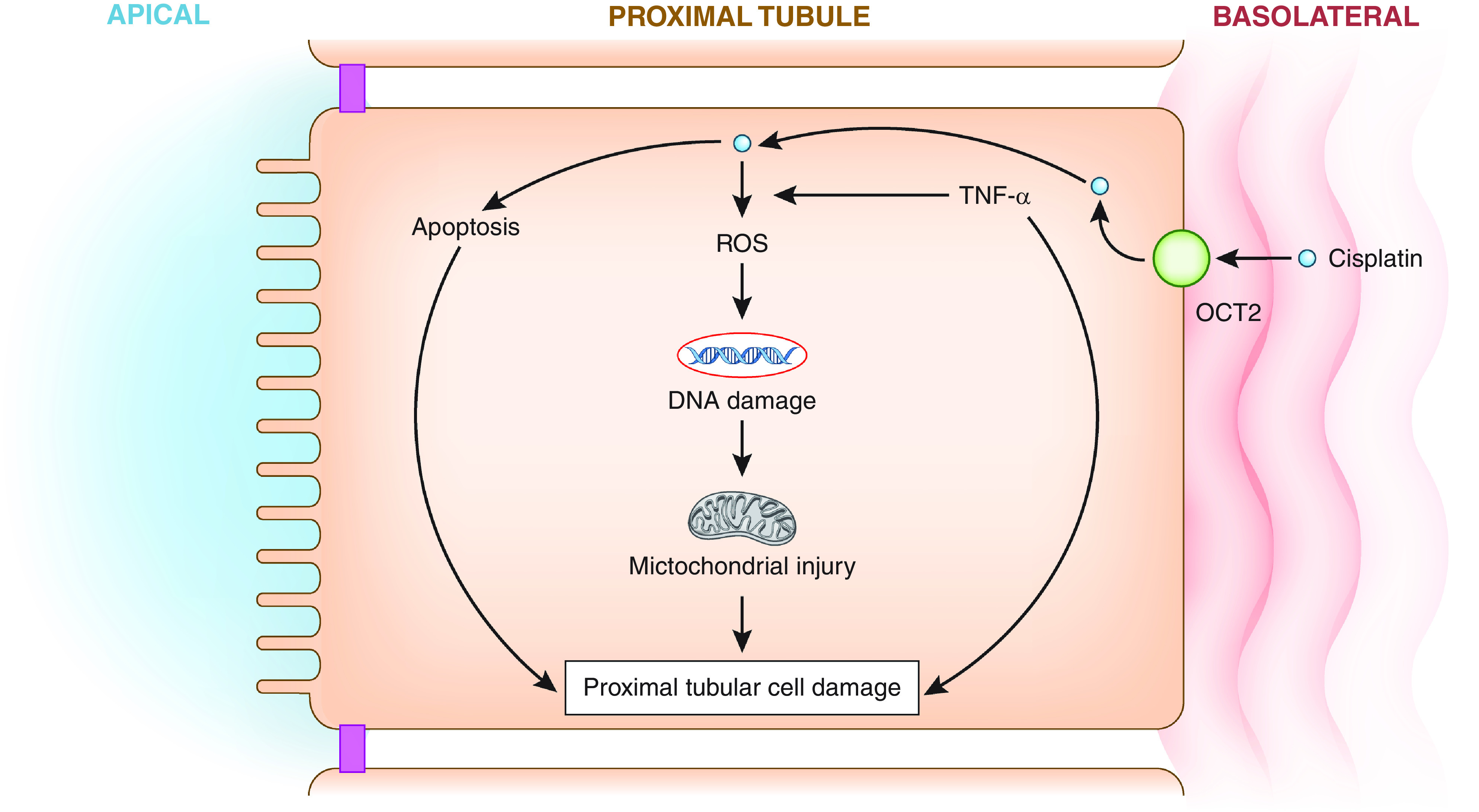
Cisplatin-AKI is mediated by apoptosis, inflammation, DNA damage, and mitochondrial injury. Reactive oxygen species (ROS) are markers of oxidative stress and lead to DNA damage and mitochondrial injury. TNF-α is a proinflammatory cytokine. OCT, organic cation transporter.
Methotrexate is a folate derivative and antimetabolite that can cause nephrotoxicity through the accumulation of intratubular crystals, which precipitate at an acidic pH (39). Patients with a history of nephrotoxicity in the setting of high-dose methotrexate and those with preexisting CKD are at highest risk for developing methotrexate-associated AKI (40). The mainstay for prevention of MTX-associated AKI is aggressive hydration and urinary alkalinization, along with discontinuation of drugs that can impair methotrexate excretion (e.g., nonsteroidal anti-inflammatory drugs and penicillin derivatives). Leucovorin is an active metabolite of folic acid and allows purine/pyrimidine synthesis to occur in the presence of methotrexate, rescuing cells from toxicity, including methotrexate-associated AKI. Glucarpidase, a recombinant bacterial enzyme that cleaves methotrexate to inactive metabolites, is highly effective at rapidly reducing methotrexate concentrations; however, its use is limited by lack of availability and cost (41). Use of fixed dose glucarpidase should be considered for patients with 48-hour methotrexate levels >5 mmol/L and serum creatinine increases >50% from baseline (42). Dialysis has limited utility in methotrexate-associated AKI due to a rebound in plasma levels after dialysis is discontinued, although successive sessions of high-flux hemodialysis may help reduce methotrexate concentrations (43,44).
Gemcitabine is an antimetabolite used for solid organ tumors, including carcinomas of the pancreas, lung, and breast. Although relatively rare, TMA is the primary kidney lesion associated with gemcitabine. Patients typically present with hypertension along with anemia, thrombocytopenia, increased lactate dehydrogenase levels, and low haptoglobin levels (45,46). Treatment is largely supportive; however, eculizumab has recently demonstrated some success in achieving hematologic remission in a small series of patients with severe AKI and TMA from gemcitabine (47,48). Determining which patients with chemotherapy-associated TMA should receive eculizumab remains a challenge, particularly considering its exorbitant cost. Those with diagnosed mutations in the complement inhibitory pathway (e.g., mutations in complement factor H) may stand to benefit the most, but this warrants further study.
Pemetrexed is an antifolate agent that blocks key enzymes involved in purine and pyrimidine syntheses, thereby reducing tumor cell growth and inducing apoptosis (49). Pemetrexed is used alone or in combination with other potentially nephrotoxic agents, such as carboplatin or cisplatin. The incidence of AKI from pemetrexed is widely variable but may be higher in patients treated with combination therapy that also includes pemetrexed, as well as in patients receiving higher cumulative doses over time (50–52). Kidney biopsies among patients presenting with AKI from pemetrexed typically show tubulointerstitial injury and tubular atrophy (53). Prevention of AKI includes minimizing concomitant nephrotoxic medications use, administration of folic acid and vitamin B12, and adequate hydration. In some cases, the drug may need to be discontinued altogether. Although pemetrexed-induced AKI may be reversible upon discontinuation, some patients may develop progressive CKD (53).
Both cyclophosphamide and ifosfamide are associated with hemorrhagic cystitis. However, ifosfamide is considered more nephrotoxic than cyclophosphamide. Biopsies of patients with AKI from ifosfamide demonstrate acute tubular necrosis and mitochondrial toxicity on electron microscopy (54). Clinically, patients with ifosfamide nephrotoxicity may present with proximal tubular dysfunction (e.g., tubular proteinuria; decreased sodium, glucose, and phosphate reabsorption; and frank glycosuria and phosphaturia). Most studies of AKI following treatment with ifosfamide have included pediatric patients (55,56), although studies in adults suggest that up to 18% develop moderate kidney dysfunction (57). Risk factors include preexisting CKD, higher cumulative dose, and concomitant use of other potentially nephrotoxic medications (54,56,58). Guidelines for dose reduction vary considerably. There are no specific preventative therapies for ifosfamide-induced AKI. Prevention of hemorrhagic cystitis from both cyclophosphamide and ifosfamide includes aggressive hydration and use of mesna, a chemoprotective agent.
Immunotherapy
Immune checkpoint inhibitors (ICPis) have revolutionized the treatment of a wide range of malignancies. ICPis block cytotoxic T lymphocyte–associated protein 4 or programmed cell death protein 1/programmed death ligand 1, thereby increasing cytotoxic T cell activity and proliferation. Although the increased T cell activation facilitates the antitumor response of ICPis, it also can lead to immune-related adverse events, including AKI. The estimated incidence of AKI directly attributed to the immune checkpoint inhibitor (ICPi-AKI) is 2%–5%, although definitions of ICPi-AKI vary across studies (59–64).
The predominant lesion found on biopsy among patients with ICPi-AKI is acute tubulointerstitial nephritis (61,64–67), although other lesions have been described (68,69). PPIs may predispose to acute tubulointerstitial nephritis through loss of tolerance from activation of drug-specific T cells and, therefore, should be discontinued in any patient with suspected ICPi-AKI. A history of prior or concomitant extrarenal immune-related adverse events, such as rash, thyroiditis, or colitis, should raise suspicion for ICPi-AKI as well (67,70).
There are no clinical features that reliably distinguish ICPi-AKI from other potential etiologies. Although ICPi-AKI occurs at a median of 14–16 weeks (60,67), the latency period between ICPi initiation and ICPi-AKI is highly variable, with some patients developing AKI within a few days and others developing AKI >1 year after initiation. Patients may present with sterile pyuria and subnephrotic-range proteinuria, but neither one is sensitive nor specific for ICPi-AKI (60,67).
In patients with suspected ICPi-AKI, there is considerable debate about whether patients should undergo biopsy versus empirical treatment with glucocorticoids. Guidelines published by the National Comprehensive Cancer Network recommend biopsy in patients with more than three-fold rise in serum creatinine (71). Recently, the Society for Immunotherapy Cancer acknowledged that, given the lack of specific clinical features for ICPi-AKI, kidney biopsy should be strongly considered when feasible, particularly when a plausible alternative etiology for AKI exists or urine studies are suggestive of glomerular disease (72). Patients with ICPi-AKI generally have favorable outcomes, with approximately two thirds of patients with ICPi-AKI achieving kidney recovery (67). Data suggest that early treatment with glucocorticoids is associated with a higher odds of kidney recovery (67). Data on dose and duration of glucocorticoid therapy are limited, although a single-center study showed that patients who are tapered rapidly over a median of 20 versus 38 days had equivalent outcomes (73). Although infliximab and mycophenolate mofetil have been trialed in cases of ICPi-AKI refractory to corticosteroids (67,73), larger studies are needed to determine their utility in ICPi-AKI.
One population that warrants special consideration is patients with a history of kidney transplant, due to the high risk of rejection and concerns surrounding efficacy of ICPis in patients on maintenance immunosuppression. In a multicenter study of 69 patients treated with ICPis, 29 (42%) developed acute rejection, 19 of whom lost their allograft (74). Median time to rejection was earlier (e.g., 24 days: interquartile range, 20–56). Both increasing immunosuppression and use of mammalian target of rapamycin inhibitors were associated with a lower risk of rejection. Several trials are underway that will specifically examine the role of different immunosuppressants in patients with kidney transplant receiving ICPis (NCT03816332 and NCT04339062).
Cellular Therapies
Chimeric antigen receptor T (CAR-T) cell therapy has emerged as a breakthrough therapy for hematologic malignancies, like acute lymphoblastic leukemia, recurrent B cell lymphoma, and mantle cell lymphoma, with newer generation chimeric antigen receptors now being developed for solid tumors and multiple myeloma. CAR-T cells are manufactured by genetically engineering a patient’s T cells to target specific tumor antigens. These cells are expanded ex vivo into hundreds of millions of cells and reinfused in the patient, leading to the production of inflammatory cytokines. Cytokine release syndrome (CRS) is common after CAR-T infusion, manifesting as fever, tachycardia, and multiorgan dysfunction. AKI occurs due to cytokine-mediated capillary leak and intravascular volume depletion, with decreased kidney perfusion. Risk factors for AKI include a history of prior autologous or allogeneic stem cell transplantation, requirement for ICU-level care, and higher grades of CRS (75). Real-world studies suggest that AKI occurs in 19%–30% of patients, usually in the context of CRS (76,77); however, the incidence is lower (5%) in patients receiving a certain type of chimeric antigen receptor, tisagenlecleucel, perhaps due to its attenuated inflammatory profile (78). Most cases of AKI present as prerenal azotemia reversible with hemodynamic support, although some patients can progress to acute tubular necrosis and the need for KRT (77). Tocilizumab, an IL-6 inhibitor, and dexamethasone may reduce the risk of CRS and therefore AKI related to CAR-T therapy (76,79).
Targeted Therapies
The introduction of novel molecularly targeted therapies in the last two decades has significantly improved patient survival compared with standard conventional chemotherapies for certain type of cancers (80). However, the toxicity profiles associated with these agents are qualitatively different from those seen with traditional cytotoxic chemotherapy and comprise a wide spectrum of pathologies (Table 2). As these targeted agents become more common in oncologic practice, it is vital that their various toxicities are recognized and investigated.
Table 2.
AKI from targeted therapies
| Drug Class | Example | Indications | Mechanism of Toxicity | Adverse Event |
|---|---|---|---|---|
| VEGF inhibitors (122,123) | Microvascular rarefaction, decrease NO, endothelial injury, podocyte injury | Hypertension, proteinuria, TMA, MCD/FSGS, ATIN | ||
| VEGF antibody | Bevacizumab | CRC, relapsing GBM | ||
| Ranibizumab | AMD, DR | |||
| Recombinant fusion protein | Aflibercept | AMD, DR, macular edema | ||
| mAb against VEGFR2 | Ramucirumab | Gastric Ca, NSCLC, CRC | ||
| Multitargeted TKI | Sunitinib | RCC, GIST, pancreatic NET | ||
| Sorafenib | RCC, HCC, thyroid Ca | |||
| Axitinib | Pancreatic Ca, RCC, CML | |||
| Pazopanib | RCC, soft tissue sarcoma | |||
| Vandetanib | Medullary thyroid Ca | |||
| Cediranib | Relapsed ovarian Ca | |||
| Lenvatinib | Advanced RCC, HCC | |||
| Motesanib | NSCLC | |||
| Regorafenib | CRC, GIST | |||
| BCR-ABL TKI (124) | Imatinib, dasatinib ponatinib | CML | Endothelial, tubular, podocyte injury | ATI (imatinib), TLS, TMA, nephrotic syndrome |
| Human EGFRi (125) | Trastuzumab, pertuzumab | Breast cancer | Cardiotoxicity | CRS, HTN |
| FGFRi (126) | Derazatinib, dovitinib, lucatanib | Breast, gastric, biliary, urothelial, and SCLC | Endothelial cell injury, decreased NO, capillary rarefaction | TMA, proteinuria |
| BRAF inhibitors (127) | Vemurafenib, dabrafenib | Metastatic melanoma | Mediated by ERK activation | ATI, ATIN |
| Bcl-2 inhibitors (128) | Venetoclax | CLL, SLL, AML | Tubular injury | TLS, AKI |
| CDK4/6 inhibitors (121) | Palbociclib, abemaciclib, ribociclib | HR+, HER2− breast Ca | Inhibit metformin uptake by OCT2; MATE1 and MATE2 transporters | Pseudo-AKI, ATI |
| Immunomodulators (129) | Lenalidomide, pomalidomide, thalidomide | Multiple myeloma | Endothelial cell injury | AKI, ATIN, Fanconi syndrome, MCD, crystal nephropathy |
| mTOR inhibitors (130) | Temsirolimus, everolimus | VEGF inhibition, reduced cubilin and megalin | ATI, podocytopathies | |
| ALK inhibitors (131) | Crizotinib, ceritinib alectinib, brigatinib lorlatinib | Small cell lung cancer | Interact with proximal tubule transporter channels, renal artery myocyte vacuolization | Pseudo-AKI, prerenal AKI, ATI, kidney cysts |
| PARP inhibitors (132) | Olaparib, nirapanib talazoparib | BRCA-mutated breast cancer, relapsed epithelial ovarian Ca | Interact with PCT transporter channels | Pseudo-AKI |
| Proteasome inhibitors (133) | Bortezomib, ixazomib, carfilzomib | Multiple myeloma, MCL | Direct microvascular toxicity; cytokines leading to autoantibody formation | TMA, HTN |
| Bruton tyrosine kinase inhibitor (134) | Ibrutinib | CLL/SLL, MCL, WM, marginal zone lymphoma | Endothelial and tubular injury | Hypertension, TLS ATI |
| XPO 1 inhibitor (135) | Selinexor | Multiple myeloma | Nausea and vomiting | Prerenal AKI, hyponatremia |
| CD22-directed cytotoxin (136) | Moxetumomab pasudotox | Hairy cell leukemia | Capillary leak syndrome, nausea/vomiting | Prerenal AKI, HUS |
VEGF, vascular endothelial growth factor; NO, nitric oxide; TMA, thrombotic microangiopathy; MCD, minimal change disease; ATIN, acute tubulointerstitial nephritis; CRC, colorectal cancer; GBM, glioblastoma multiforme; AMD, age-related macular degeneration; DR, diabetic retinopathy; VEGFR2, vascular endothelial growth factor receptor 2; Ca, carcinoma; NSCLC, nonsmall cell lung cancer; TKI, tyrosine kinase inhibitor; RCC, renal cell cancer; GIST, gastrointestinal stromal tumor; NET, neuroendocrine tumor; HCC, hepatocellular carcinoma; CML, chronic myeloid leukemia; BCR-ABL, breakpoint cluster region-tyrosine protein kinase ABL-1 gene; ATI, acute tubular injury; TLS, tumor lysis syndrome; EGFRi, epidermal growth factor receptor inhibitor; CRS, cytokine release syndrome; HTN, hypertension; FGFRi, fibroblast growth factor receptor inhibitor; SCLC, small cell lung cancer; BRAF, proto-oncogene B-raf; ERK, extracellular signal–regulated kinase; Bcl-2, B cell lymphoma-2 gene; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; AML, acute myeloid leukemia; CDK4/6, cyclin-dependent kinase 4/6; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; OCT2, organic cation transporter 2; MATE, multidrug and toxin extrusion; mTOR, mammalian target of rapamycin; ALK, anaplastic lymphoma kinase; PARP, poly-ADP-ribose polymerase; BRCA, breast cancer gene; PCT, proximal convoluted tubule; MCL, mantel cell lymphoma; WM, Waldenstrom macroglobulinemia; XPO 1, exportin-1; CD22, cluster differentiation-22; HUS, hemolytic uremic syndrome.
AKI after Hematopoietic Stem Cell Transplant
HSCT is a curative treatment for many benign and malignant hematologic conditions; however, patients undergoing HSCT are at high risk for AKI due to both patient-specific factors (pretransplant diabetes mellitus, hypertension, CKD, sepsis, mechanical ventilation, and ICU admission) and characteristics of HSCT (myeloablative versus nonmyeloablative) itself. The incidence of AKI ranges from 12% to 50% in myeloablative autologous HSCT, from 19% to 66% in myeloablative allogenic HSCT, and from 29% to 54% in nonmyeloablative allogenic SCT (81–83). The risk of needing KRT is highest in myeloablative allogenic SCT (approximately 17%) (84). AKI occurring within 30 days of engraftment is associated with high mortality; among patients post-HSCT who are treated with KRT, the incidence of death may approach 55%–100% (85,86). Figure 5 outlines the pathophysiology of AKI in the setting of HSCT. Therapy should be directed at the underlying etiology (e.g., treatment of sepsis with antibiotics, dose reduction of calcineurin inhibitors and consideration for eculizumab in the setting of TMA [87], and use of defibrotide in hepatic sinusoidal obstruction syndrome [88]).
Figure 5.
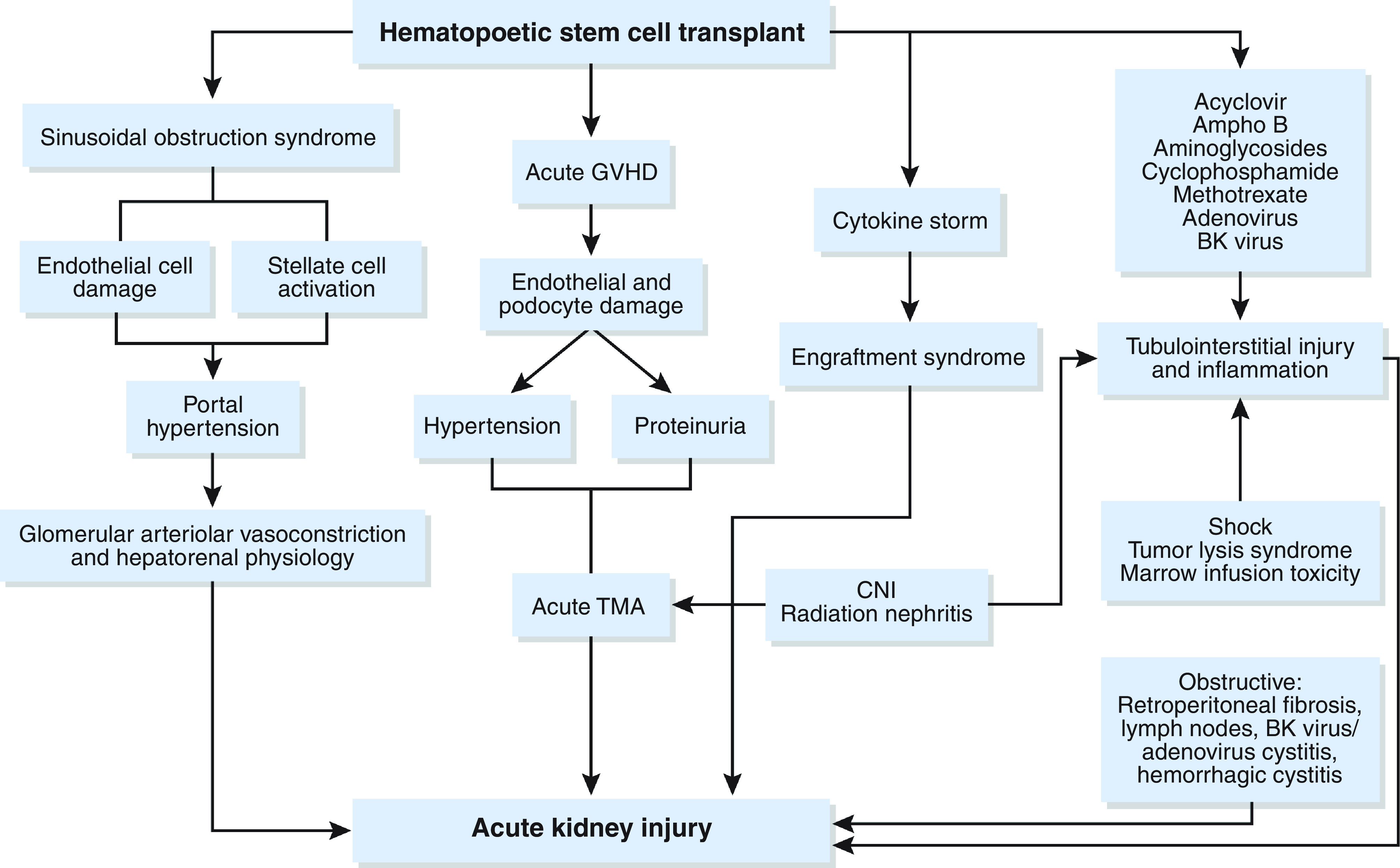
The pathophysiology of AKI from HSCT is variable. AmphoB, amphotericin B; CNI, calcineurin inhibitor; GVHD, graft-versus-host disease. Adapted from ref. 122, with permission.
AKI Related to Both Cancer and Cancer-Directed Therapies
Hemodynamic-Associated AKI
Patients with cancer are susceptible to a plethora of hemodynamic insults. Nausea, vomiting, diarrhea, and anorexia occur in up to 70% of these patients, often in the setting of conventional chemotherapy with and without radiation, thereby predisposing to volume depletion and prerenal azotemia (89). Newer therapies, like ICPis, can also predispose to immune-mediated colitis and significant gastrointestinal losses, thereby leading to volume depletion (90). Even in the absence of gastrointestinal losses, hemodynamic-mediated insults can occur. Hypercalcemia, which complicates up to 30% of all malignancies (91), can cause vasoconstriction of the afferent renal artery and degradation of aquaporins in the distal convoluted tubule, thereby predisposing to prerenal azotemia (elaborated on further in the section on Hypercalcemia) (91). Contrast-associated AKI can similarly cause kidney vasoconstriction and intraglomerular hemodynamic changes, although this is not well studied in patients with cancer (5). Other forms of hemodynamic-mediated AKI include cardiorenal syndrome from anthracyclines and the human epidermal growth factor receptor 2 modulator, trastuzumab (92), as well as hepatorenal syndrome (e.g., sinusoidal obstruction syndrome post-HSCT) (93). Capillary leak is very common in patients in the ICU both with and without cancer, and it can occur due to cytokine release and increased endothelial permeability from sepsis (94) or in the postoperative setting (95). A careful medical history, physical examination, and inspection of the urine sediment can help differentiate hemodynamic causes from other etiologies of AKI and also assist with appropriate fluid management.
Thrombotic Microangiopathy
TMA is a disorder characterized by microvascular thrombosis, thrombocytopenia, microangiopathic hemolytic anemia, and end organ damage (96). TMA in patients with cancer can occur both due to the underlying malignancy as well as from the therapies used to treat it. TMA has been reported in patients with mucin-producing adenocarcinomas, in particular gastric and breast adenocarcinoma (97). The pathogenesis of cancer-associated TMA is not well understood; however, there is no evidence to support the role of ADAMTS-13 deficiency (98). One plausible mechanism for microangiopathic hemolytic anemia is red blood cell fragmentation due to direct contact with tumor emboli, as autopsies of patients with TMA demonstrate intraluminal fibrin thrombi in the blood vessels (99,100). TMA can also occur in the setting of therapies used to treat cancer. Agents commonly implicated include conventional chemotherapies (e.g., gemcitabine, mitomycin C, and cisplatin) as well as targeted therapies (including antivascular endothelial growth factor inhibitors like bevacizumab, tyrosine kinase inhibitors, and selective proteasome inhibitors [e.g., carfilzomib] used in the treatment of myeloma) (101). TMA in the setting of conventional chemotherapy is often dose dependent, irreversible, and associated with a high risk of progressive CKD and mortality (102). In contrast, TMA in the setting of antivascular endothelial growth factor agents is usually not dose related, is often reversible, and less reliably presents with characteristic hematologic findings. Furthermore, prognosis appears to improve with discontinuation of therapy (102). TMA can also occur after HSCT in anywhere from 2% to 39% of patients and is associated with high morbidity and mortality (103). This wide variation seen is due to a lack of fixed diagnostic criteria as well as inclusion of both adult and pediatric patients in the studies.
In general, management of TMA in patients with cancer is largely supportive and has not been shown to respond to plasma exchange or infusion (104). There are case reports and series describing successful treatment of chemotherapy-associated TMA and HSCT-TMA with eculizumab; however, larger studies are needed to verify these findings (48,87,105,106).
Tumor Lysis Syndrome
Tumor lysis syndrome is a constellation of metabolic derangements, namely hyperkalemia, hyperphosphatemia, and hyperuricemia, that results from the rapid degradation of tumor cells. Tumor lysis syndrome is considered an oncologic emergency due to the risk of life-threatening arrhythmias and respiratory failure as well as AKI. It can occur spontaneously but most often develops during treatment of hematologic malignancies. Tumor lysis syndrome can also occur following use of targeted therapies, like venetoclax, bortezomib, and rituximab, as well as BRAF/MEK inhibitors (107–111). Tumor cell breakdown results in the release of purines, which are converted to intermediate products (e.g., hypoxanthine and xanthine) and then metabolized to uric acid (Figure 6). AKI occurs secondary to calcium phosphate and uric acid deposition, although uric acid can also perpetuate AKI through crystalline-independent pathways, including inflammation and kidney vasoconstriction, as well as decreased kidney perfusion (112–114). Tumor lysis syndrome is classified using the Cairo-Bishop criteria, which define laboratory tumor lysis syndrome as a 25% decrease in serum calcium and/or a 25% increase in uric acid, potassium, or phosphate levels from baseline (115). These abnormalities must occur within 3 days preceding or 7 days following the initiation of chemotherapy. Clinical tumor lysis syndrome is defined as laboratory tumor lysis syndrome in addition to clinical manifestations, such as arrhythmia, seizures, AKI, or sudden death.
Figure 6.
Pathophysiology of AKI in the setting of TLS and therapeutic targets. Allopurinol inhibits xanthine oxidase, an enzyme that converts hypoxanthine to xanthine and xanthine to uric acid. Rasburicase is a recombinant urate oxidase that converts uric acid into a more soluble metabolite, allantoin. Ca×P, calcium phosphate product; K, potassium.
Prevention involves aggressive volume repletion for all patients at risk for tumor lysis syndrome to maintain high urinary flow rates and promote uric acid, potassium, and phosphate excretion. Urinary alkalinization is no longer recommended due to the risk of calcium-phosphate precipitation. Allopurinol and febuxostat are xanthine oxidase inhibitors that are recommended for prophylaxis in patients with low to intermediate risk of tumor lysis syndrome. Importantly, allopurinol has no effect on preexisting uric acid levels. Furthermore, the manufacturer’s label suggests that the dose of allopurinol should be reduced in patients with a creatinine clearance below 20 ml/min (116), and therefore, caution should be used with higher doses in the setting of severe oliguric AKI. High-risk patients may benefit from rasburicase, a recombinant urate oxidase that converts uric acid to its more soluble form, allantoin. Rasburicase has been shown to rapidly reduce uric acid levels in both pediatric and adult patients at high risk for tumor lysis syndrome (117,118), but it is contraindicated in the setting of glucose-6-phosphate dehydrogenase deficiency due to the risk of severe hemolysis. Some patients may require early initiation of KRT to increase clearance of potassium, phosphate, and uric acid.
Future Directions
In the era of precision medicine, there is considerable interest in identifying biomarkers of AKI, and this extends to the field of onconephrology as well. Markers of AKI, like serum creatinine, rise only after significant injury has occurred and have decreased utility in patients with reduced muscle mass. Although several novel markers for early diagnosis of AKI show promise in preclinical studies, few have been sufficiently evaluated in patients with cancer, and most are markers of tubular injury studied in the context of cisplatin nephrotoxicity. More recent studies have explored whether certain blood and urine biomarkers are associated with nephrotoxicity from immunotherapy. For example, one single-center, retrospective study identified higher levels of C-reactive protein and urinary retinol binding protein in patients with ICPi-AKI and, specifically, in those with acute tubulointerstitial nephritis as the dominant lesion on kidney biopsy (119).
Biomarkers may also help differentiate “pseudo-AKI” from true AKI. For instance, both poly-ADP-ribose polymerase inhibitors and cyclin-dependent kinase 4/6 have been associated with pseudo-AKI (120,121) (Table 2), with elevations in serum creatinine without a true decline in GFR. Measurement of cystatin C levels and kidney iothalamate clearance may help differentiate pseudo-AKI from true AKI, but additional biomarkers with anatomic specificity are urgently needed. The paucity of data on biomarkers in onconephrology is due in part to the lack of a “gold standard,” where patients are often treated empirically for their AKI, irrespective of the cause. Furthermore, our understanding of the mechanisms underlying AKI in patients with cancer is limited, highlighting the need for larger translational studies with longitudinal biospecimen collection.
AKI has major repercussions for patients with cancer and can lead to ineligibility for further therapy, prolonged hospitalizations, and higher mortality. As patients with cancer are living longer, they must also grapple with the sequelae of recurrent AKI events, including CKD. A more thorough understanding of both patient- and cancer-specific predisposing risk factors is needed to help risk-stratify patients and optimize their cancer care. Furthermore, large-scale genetic and biomarker-based studies may provide insight into potential therapeutic targets. Given the complexities of managing AKI in the patient with cancer, a multidisciplinary team of hematologists, oncologists, and nephrologists is needed to provide quality care to this vulnerable subset of patients.
Disclosures
K.D. Jhaveri reports employment with Northwell Health; is a founder and copresident of the American Society of Onco-Nephrology; reports consultancy agreements with Astex Pharmaceuticals, ChemoCentryx, Chinook, GlaxoSmithKline, Natera, and Travere Therapeutics; reports honoraria from the American Society of Nephrology, the International Society of Nephrology, and UpToDate.com; reports serving on the editorial boards of American Journal of Kidney Diseases, CJASN, Clinical Kidney Journal, Journal of Onconephrology, Kidney International, and Nephrology Dialysis Transplantation; reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation; and reports other interests/relationships as the President of American Nephrologist of Indian Origin. P. Gudsoorkar reports serving as an editorial board member for Advances in Chronic Kidney Disease; serving as a scientific advisor or member of the medical advisory board of the National Kidney Foundation–Northern Kentucky & Southern Ohio; and serving as a member and fellow of the National Kidney Foundation. S. Gupta reports research funding from BTG International and GE HealthCare and is a founder and copresident of the American Society of Onco-Nephrology.
Funding
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases grant K23 DK125672.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
S. Gupta, P. Gudsoorkar, and K.D. Jhaveri were responsible for visualization; P. Gudsoorkar and S. Gupta wrote the original draft; and P. Gudsoorkar, S. Gupta, and K.D. Jhaveri reviewed and edited the manuscript.
References
- 1.Kitchlu A, McArthur E, Amir E, Booth CM, Sutradhar R, Majeed H, Nash DM, Silver SA, Garg AX, Chan CT, Kim SJ, Wald R: Acute kidney injury in patients receiving systemic treatment for cancer: A population-based cohort study. J Natl Cancer Inst 111: 727–736, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiansen CF, Johansen MB, Langeberg WJ, Fryzek JP, Sørensen HT: Incidence of acute kidney injury in cancer patients: A Danish population-based cohort study. Eur J Intern Med 22: 399–406, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Soares M, Salluh JI, Carvalho MS, Darmon M, Rocco JR, Spector N: Prognosis of critically ill patients with cancer and acute renal dysfunction. J Clin Oncol 24: 4003–4010, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Parikh CR, McSweeney PA, Korular D, Ecder T, Merouani A, Taylor J, Slat-Vasquez V, Shpall EJ, Jones RB, Bearman SI, Schrier RW: Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int 62: 566–573, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cosmai L, Porta C, Privitera C, Gesualdo L, Procopio G, Gori S, Laghi A: Acute kidney injury from contrast-enhanced CT procedures in patients with cancer: White paper to highlight its clinical relevance and discuss applicable preventive strategies. ESMO Open 5: e000618, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salahudeen AK, Doshi SM, Pawar T, Nowshad G, Lahoti A, Shah P: Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin J Am Soc Nephrol 8: 347–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canet E, Zafrani L, Lambert J, Thieblemont C, Galicier L, Schnell D, Raffoux E, Lengline E, Chevret S, Darmon M, Azoulay E: Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: Impact on remission and survival. PLoS One 8: e55870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang E, Park M, Park PG, Park N, Jung Y, Kang U, Kang HG, Kim DK, Oh KH, Joo KW, Kim YS, Yoon HJ, Lee H: Acute kidney injury predicts all-cause mortality in patients with cancer. Cancer Med 8: 2740–2750, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eleutherakis-Papaiakovou V, Bamias A, Gika D, Simeonidis A, Pouli A, Anagnostopoulos A, Michali E, Economopoulos T, Zervas K, Dimopoulos MA; Greek Myeloma Study Group : Renal failure in multiple myeloma: Incidence, correlations, and prognostic significance. Leuk Lymphoma 48: 337–341, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Zafar MU, Tarar Z, Ghous G, Farooq U, Lash BW: Effect of acute kidney injury on hospital-based outcomes in patients with multiple myeloma. J Clin Oncol 39[15 Suppl]: e20006, 2021 [Google Scholar]
- 11.Sanders PW: Pathogenesis and treatment of myeloma kidney. J Lab Clin Med 124: 484–488, 1994 [PubMed] [Google Scholar]
- 12.Sanders PW: Mechanisms of light chain injury along the tubular nephron. J Am Soc Nephrol 23: 1777–1781, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Zucchelli P, Pasquali S, Cagnoli L, Ferrari G: Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int 33: 1175–1180, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Johnson WJ, Kyle RA, Pineda AA, O’Brien PC, Holley KE: Treatment of renal failure associated with multiple myeloma. Plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med 150: 863–869, 1990 [PubMed] [Google Scholar]
- 15.Clark WF, Stewart AK, Rock GA, Sternbach M, Sutton DM, Barrett BJ, Heidenheim AP, Garg AX, Churchill DN; Canadian Apheresis Group : Plasma exchange when myeloma presents as acute renal failure: A randomized, controlled trial. Ann Intern Med 143: 777–784, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hutchison CA, Cockwell P, Moroz V, Bradwell AR, Fifer L, Gillmore JD, Jesky MD, Storr M, Wessels J, Winearls CG, Weisel K, Heyne N, Cook M: High cutoff versus high-flux haemodialysis for myeloma cast nephropathy in patients receiving bortezomib-based chemotherapy (EuLITE): A phase 2 randomised controlled trial. Lancet Haematol 6: e217–e228, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Bridoux F, Carron PL, Pegourie B, Alamartine E, Augeul-Meunier K, Karras A, Joly B, Peraldi MN, Arnulf B, Vigneau C, Lamy T, Wynckel A, Kolb B, Royer B, Rabot N, Benboubker L, Combe C, Jaccard A, Moulin B, Knebelmann B, Chevret S, Fermand JP; MYRE Study Group : Effect of high-cutoff hemodialysis vs conventional hemodialysis on hemodialysis independence among patients with myeloma cast nephropathy: A randomized clinical trial. JAMA 318: 2099–2110, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klomjit N, Leung N, Fervenza F, Sethi S, Zand L: Rate and predictors of finding monoclonal gammopathy of renal significance (MGRS) lesions on kidney biopsy in patients with monoclonal gammopathy. J Am Soc Nephrol 31: 2400–2411, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaador K, Peeters H, Minnema MC, Nguyen TQ, Dendooven A, Vos JMI, Croockewit AJ, van de Donk NWCJ, Jacobs JFM, Wetzels JFM, Sprangers B, Abrahams AC: Monoclonal gammopathy of renal significance (MGRS) histopathologic classification, diagnostic workup, and therapeutic options. Neth J Med 77: 243–254, 2019 [PubMed] [Google Scholar]
- 20.Goldner W: Cancer-related hypercalcemia. J Oncol Pract 12: 426–432, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Richards MA, Mootoosamy I, Reznek RH, Webb JA, Lister TA: Renal involvement in patients with non-Hodgkin’s lymphoma: Clinical and pathological features in 23 cases. Hematol Oncol 8: 105–110, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Törnroth T, Heiro M, Marcussen N, Franssila K: Lymphomas diagnosed by percutaneous kidney biopsy. Am J Kidney Dis 42: 960–971, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hardy F: Comparison of fluid resin and compression molding methods in processing dimensional changes. J Prosthet Dent 39: 375–377, 1978 [DOI] [PubMed] [Google Scholar]
- 24.McKee LC Jr., Collins RD: Intravascular leukocyte thrombi and aggregates as a cause of morbidity and mortality in leukemia. Medicine (Baltimore) 53: 463–478, 1974 [DOI] [PubMed] [Google Scholar]
- 25.Bewersdorf JP, Zeidan AM: Hyperleukocytosis and leukostasis in acute myeloid leukemia: Can a better understanding of the underlying molecular pathophysiology lead to novel treatments? Cells 9: 2310, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD: Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol 11: 1173–1179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Motwani SS, McMahon GM, Humphreys BD, Partridge AH, Waikar SS, Curhan GC: Development and validation of a risk prediction model for acute kidney injury after the first course of cisplatin. J Clin Oncol 36: 682–688, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidera Y, Kawakami H, Sakiyama T, Okamoto K, Tanaka K, Takeda M, Kaneda H, Nishina S, Tsurutani J, Fujiwara K, Nomura M, Yamazoe Y, Chiba Y, Nishida S, Tamura T, Nakagawa K: Risk factors for cisplatin-induced nephrotoxicity and potential of magnesium supplementation for renal protection. PLoS One 9: e101902, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozkok A, Edelstein CL: Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res Int 2014: 967826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang M, Dong Z: Regulation and pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol Exp Ther 327: 300–307, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Wei Q, Dong G, Franklin J, Dong Z: The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72: 53–62, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Zsengellér ZK, Ellezian L, Brown D, Horváth B, Mukhopadhyay P, Kalyanaraman B, Parikh SM, Karumanchi SA, Stillman IE, Pacher P: Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem 60: 521–529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu S, Pabla N, Tang C, He L, Dong Z: DNA damage response in cisplatin-induced nephrotoxicity. Arch Toxicol 89: 2197–2205, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lajer H, Kristensen M, Hansen HH, Nielsen S, Frøkiaer J, Ostergaard LF, Christensen S, Daugaard G, Jonassen TE: Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemother Pharmacol 56: 535–542, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Dai LJ, Raymond L, Friedman PA, Quamme GA: Mechanisms of amiloride stimulation of Mg2+ uptake in immortalized mouse distal convoluted tubule cells. Am J Physiol 272: F249–F256, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Ray EC, Boyd-Shiwarski CR, Liu P, Novacic D, Cassiman D: SGLT2 inhibitors for treatment of refractory hypomagnesemia: A case report of 3 patients. Kidney Med 2: 359–364, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garneau AP, Riopel J, Isenring P: Acute methotrexate-induced crystal nephropathy. N Engl J Med 373: 2691–2693, 2015 [DOI] [PubMed] [Google Scholar]
- 40.May J, Carson KR, Butler S, Liu W, Bartlett NL, Wagner-Johnston ND: High incidence of methotrexate associated renal toxicity in patients with lymphoma: A retrospective analysis. Leuk Lymphoma 55: 1345–1349, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widemann BC, Balis FM, Kim A, Boron M, Jayaprakash N, Shalabi A, O’Brien M, Eby M, Cole DE, Murphy RF, Fox E, Ivy P, Adamson PC: Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J Clin Oncol 28: 3979–3986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truong H, Leung N: Fixed-dose glucarpidase for toxic methotrexate levels and acute kidney injury in adult lymphoma patients: Case series. Clin Lymphoma Myeloma Leuk 21: e497–e502, 2021 [DOI] [PubMed] [Google Scholar]
- 43.Kawabata K, Makino H, Nagake Y, Tokioka H, Matsumi M, Morita Y, Ota K, Shikata K, Ota Z: A case of methotrexate-induced acute renal failure successfully treated with plasma perfusion and sequential hemodialysis. Nephron 71: 233–234, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Thierry FX, Vernier I, Dueymes JM, Roche H, Canal P, Meeus F, Pourrat JP, Conté JJ: Acute renal failure after high-dose methotrexate therapy. Role of hemodialysis and plasma exchange in methotrexate removal. Nephron 51: 416–417, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Glezerman I, Kris MG, Miller V, Seshan S, Flombaum CD: Gemcitabine nephrotoxicity and hemolytic uremic syndrome: Report of 29 cases from a single institution. Clin Nephrol 71: 130–139, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Daviet F, Rouby F, Poullin P, Moussi-Francès J, Sallée M, Burtey S, Mancini J, Duffaud F, Sabatier R, Pourroy B, Grandvuillemin A, Grange S, Frémeaux-Bacchi V, Coppo P, Micallef J, Jourde-Chiche N: Thrombotic microangiopathy associated with gemcitabine use: Presentation and outcome in a national French retrospective cohort. Br J Clin Pharmacol 85: 403–412, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grall M, Daviet F, Chiche NJ, Provot F, Presne C, Coindre JP, Pouteil-Noble C, Karras A, Guerrot D, François A, Benhamou Y, Veyradier A, Frémeaux-Bacchi V, Coppo P, Grangé S: Eculizumab in gemcitabine-induced thrombotic microangiopathy: Experience of the French thrombotic microangiopathies reference centre. BMC Nephrol 22: 267, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Efe O, Goyal L, Galway A, Zhu AX, Niles JL, Zonozi R: Treatment of gemcitabine-induced thrombotic microangiopathy followed by gemcitabine rechallenge with eculizumab. Kidney Int Rep 6: 1464–1468, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLeod HL, Cassidy J, Powrie RH, Priest DG, Zorbas MA, Synold TW, Shibata S, Spicer D, Bissett D, Pithavala YK, Collier MA, Paradiso LJ, Roberts JD: Pharmacokinetic and pharmacodynamic evaluation of the glycinamide ribonucleotide formyltransferase inhibitor AG2034. Clin Cancer Res 6: 2677–2684, 2000 [PubMed] [Google Scholar]
- 50.Chauvet S, Courbebaisse M, Ronco P, Plaisier E: Pemetrexed-induced acute kidney injury leading to chronic kidney disease. Clin Nephrol 82: 402–406, 2014 [DOI] [PubMed] [Google Scholar]
- 51.de Rouw N, Boosman RJ, van de Bruinhorst H, Biesma B, van den Heuvel MM, Burger DM, Hilbrands LB, Ter Heine R, Derijks HJ: Cumulative pemetrexed dose increases the risk of nephrotoxicity. Lung Cancer 146: 30–35, 2020 [DOI] [PubMed] [Google Scholar]
- 52.Dumoulin DW, Visser S, Cornelissen R, van Gelder T, Vansteenkiste J, von der Thusen J, Aerts JGJV: Renal toxicity from pemetrexed and pembrolizumab in the era of combination therapy in patients with metastatic nonsquamous cell NSCLC. J Thorac Oncol 15: 1472–1483, 2020 [DOI] [PubMed] [Google Scholar]
- 53.Glezerman IG, Pietanza MC, Miller V, Seshan SV: Kidney tubular toxicity of maintenance pemetrexed therapy. Am J Kidney Dis 58: 817–820, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Ensergueix G, Pallet N, Joly D, Levi C, Chauvet S, Trivin C, Augusto JF, Boudet R, Aboudagga H, Touchard G, Nochy D, Essig M, Thervet E, Lazareth H, Karras A: Ifosfamide nephrotoxicity in adult patients. Clin Kidney J 13: 660–665, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skinner R, Pearson AD, Price L, Coulthard MG, Craft AW: Nephrotoxicity after ifosfamide. Arch Dis Child 65: 732–738, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skinner R, Cotterill SJ, Stevens MC; United Kingdom Children’s Cancer Study Group : Risk factors for nephrotoxicity after ifosfamide treatment in children: A UKCCSG Late Effects Group study. Br J Cancer 82: 1636–1645, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farry JK, Flombaum CD, Latcha S: Long term renal toxicity of ifosfamide in adult patients--5 year data. Eur J Cancer 48: 1326–1331, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Skinner R, Pearson AD, English MW, Price L, Wyllie RA, Coulthard MG, Craft AW: Risk factors for ifosfamide nephrotoxicity in children. Lancet 348: 578–580, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM: Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta-analysis. Nephrol Dial Transplant 34: 108–117, 2019 [DOI] [PubMed] [Google Scholar]
- 60.Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Herrmann SM, Manohar S, Shirali AC, Kitchlu A, Shirazian S, Assal A, Vijayan A, Renaghan AD, Ortiz-Melo DI, Rangarajan S, Malik AB, Hogan JJ, Dinh AR, Shin DS, Marrone KA, Mithani Z, Johnson DB, Hosseini A, Uprety D, Sharma S, Gupta S, Reynolds KL, Sise ME, Leaf DE: Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. J Am Soc Nephrol 31: 435–446, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, Cortazar FB, Leaf DE, Mooradian MJ, Villani AC, Sullivan RJ, Reynolds K, Sise ME: The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol 14: 1692–1700, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meraz-Muñoz A, Amir E, Ng P, Avila-Casado C, Ragobar C, Chan C, Kim J, Wald R, Kitchlu A: Acute kidney injury associated with immune checkpoint inhibitor therapy: Incidence, risk factors and outcomes. J Immunother Cancer 8: e000467, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.García-Carro C, Bolufer M, Bury R, Catañeda Z, Muñoz E, Felip E, Lorente D, Carreras MJ, Gabaldon A, Agraz I, Serón D, Soler MJ: Acute kidney injury as a risk factor for mortality in oncological patients receiving check-point inhibitors. Nephrol Dial Transplant 25: 887–894, 2022. 10.1093/ndt/gfab034 [DOI] [PubMed] [Google Scholar]
- 64.Stein C, Burtey S, Mancini J, Pelletier M, Sallée M, Brunet P, Berbis P, Grob JJ, Honoré S, Gaudy C, Jourde-Chiche N: Acute kidney injury in patients treated with anti-programmed death receptor-1 for advanced melanoma: A real-life study in a single-centre cohort. Nephrol Dial Transplant 36: 1664–1674, 2021 [DOI] [PubMed] [Google Scholar]
- 65.Shirali AC, Perazella MA, Gettinger S: Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287–291, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta S, Short SAP, Sise ME, Prosek JM, Madhavan SM, Soler MJ, Ostermann M, Herrmann SM, Abudayyeh A, Anand S, Glezerman I, Motwani SS, Murakami N, Wanchoo R, Ortiz-Melo DI, Rashidi A, Sprangers B, Aggarwal V, Malik AB, Loew S, Carlos CA, Chang WT, Beckerman P, Mithani Z, Shah CV, Renaghan AD, Seigneux S, Campedel L, Kitchlu A, Shin DS, Rangarajan S, Deshpande P, Coppock G, Eijgelsheim M, Seethapathy H, Lee MD, Strohbehn IA, Owen DH, Husain M, Garcia-Carro C, Bermejo S, Lumlertgul N, Seylanova N, Flanders L, Isik B, Mamlouk O, Lin JS, Garcia P, Kaghazchi A, Khanin Y, Kansal SK, Wauters E, Chandra S, Schmidt-Ott KM, Hsu RK, Tio MC, Sarvode Mothi S, Singh H, Schrag D, Jhaveri KD, Reynolds KL, Cortazar FB, Leaf DE; ICPi-AKI Consortium Investigators : Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer 9: e003467, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A: Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J Immunother Cancer 7: 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitchlu A, Jhaveri KD, Wadhwani S, Deshpande P, Harel Z, Kishibe T, Henriksen K, Wanchoo R: A systematic review of immune checkpoint inhibitor-associated glomerular disease. Kidney Int Rep 6: 66–77, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta S, Cortazar FB, Riella LV, Leaf DE: Immune checkpoint inhibitor nephrotoxicity: Update 2020. Kidney360 1: 130–140, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36: 1714–1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E, Johnson DB, Lacouture ME, Masters GA, Naidoo J, Nanni M, Perales MA, Puzanov I, Santomasso BD, Shanbhag SP, Sharma R, Skondra D, Sosman JA, Turner M, Ernstoff MS: Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 9: e002435, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee MD, Seethapathy H, Strohbehn IA, Zhao SH, Boland GM, Fadden R, Sullivan R, Reynolds KL, Sise ME: Rapid corticosteroid taper versus standard of care for immune checkpoint inhibitor induced nephritis: A single-center retrospective cohort study. J Immunother Cancer 9: e002292, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, Abdelrahim M, Khairallah P, Shirazian S, Kukla A, Owoyemi IO, Alhamad T, Husami S, Menon M, Santeusanio A, Blosser CD, Zuniga SC, Soler MJ, Moreso F, Mithani Z, Ortiz-Melo D, Jaimes EA, Gutgarts V, Lum E, Danovitch GM, Cardarelli F, Drews RE, Bassil C, Swank JL, Westphal S, Mannon RB, Shirai K, Kitchlu A, Ong S, Machado SM, Mothi SS, Ott PA, Rahma O, Hodi FS, Sise ME, Gupta S, Leaf DE, Devoe CE, Wanchoo R, Nair VV, Schmults CD, Hanna GJ, Sprangers B, Riella LV, Jhaveri KD; Immune Checkpoint Inhibitors in Solid Organ Transplant Consortium : A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int 100: 196–205, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanduri SR, Cheungpasitporn W, Thongprayoon C, Petnak T, Lin Y, Kovvuru K, Manohar S, Kashani K, Herrmann SM: Systematic review of risk factors and incidence of acute kidney injury among patients treated with CAR-T cell therapies. Kidney Int Rep 6: 1416–1422, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gutgarts V, Jain T, Zheng J, Maloy MA, Ruiz JD, Pennisi M, Jaimes EA, Perales MA, Sathick J: Acute kidney injury after CAR-T cell therapy: Low incidence and rapid recovery. Biol Blood Marrow Transplant 26: 1071–1076, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S, Seethapathy H, Strohbehn IA, Frigault MJ, O’Donnell EK, Jacobson CA, Motwani SS, Parikh SM, Curhan GC, Reynolds KL, Leaf DE, Sise ME: Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis 76: 63–71, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee MD, Strohbehn IA, Seethapathy HS, Rusibamayila N, Casey KS, Gupta S, Leaf DE, Frigault MJ, Sise ME: Acute kidney injury after the CAR-T therapy tisagenlecleucel. Am J Kidney Dis 77: 990–992, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caimi PF, Pacheco Sanchez G, Sharma A, Otegbeye F, Ahmed N, Rojas P, Patel S, Kleinsorge Block S, Schiavone J, Zamborsky K, Boughan K, Hillian A, Reese-Koc J, Maschan M, Dropulic B, Sekaly RP, de Lima M: Prophylactic tocilizumab prior to anti-CD19 CAR-T cell therapy for non-Hodgkin lymphoma. Front Immunol 12: 745320, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd: Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol 21: 508–518, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caliskan Y, Besisik SK, Sargin D, Ecder T: Early renal injury after myeloablative allogeneic and autologous hematopoietic cell transplantation. Bone Marrow Transplant 38: 141–147, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Abramson MH, Gutgarts V, Zheng J, Maloy MA, Ruiz JD, Scordo M, Jaimes EA, Sathick IJ: Acute kidney injury in the modern era of allogeneic hematopoietic stem cell transplantation. Clin J Am Soc Nephrol 16: 1318–1327, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopes JA, Jorge S, Neves M: Acute kidney injury in HCT: An update. Bone Marrow Transplant 51: 755–762, 2016 [DOI] [PubMed] [Google Scholar]
- 84.Hahn T, Rondeau C, Shaukat A, Jupudy V, Miller A, Alam AR, Baer MR, Bambach B, Bernstein Z, Chanan-Khan AA, Czuczman MS, Slack J, Wetzler M, Mookerjee BK, Silva J, McCarthy PL Jr.: Acute renal failure requiring dialysis after allogeneic blood and marrow transplantation identifies very poor prognosis patients. Bone Marrow Transplant 32: 405–410, 2003 [DOI] [PubMed] [Google Scholar]
- 85.Yang WC, Chen YT, Chang WW, Chang CH, Fan PC, Lee SY, Kao KC, Tian YC, Hung CC, Fang JT, Yang CW, Chen YC, Wang PN: Outcome predictors of allogeneic hematopoietic stem cell transplant. Am J Med Sci 345: 33–38, 2013 [DOI] [PubMed] [Google Scholar]
- 86.Parikh CR, Schrier RW, Storer B, Diaconescu R, Sorror ML, Maris MB, Maloney DG, McSweeney P, Storb R, Sandmaier BM: Comparison of ARF after myeloablative and nonmyeloablative hematopoietic cell transplantation. Am J Kidney Dis 45: 502–509, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Rudoni J, Jan A, Hosing C, Aung F, Yeh J: Eculizumab for transplant-associated thrombotic microangiopathy in adult allogeneic stem cell transplant recipients. Eur J Haematol 101: 389–398, 2018 [DOI] [PubMed] [Google Scholar]
- 88.Richardson P, Aggarwal S, Topaloglu O, Villa KF, Corbacioglu S: Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). Bone Marrow Transplant 54: 1951–1962, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Reilly M, Mellotte G, Ryan B, O’Connor A: Gastrointestinal side effects of cancer treatments. Ther Adv Chronic Dis 11: 2040622320970354, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Som A, Mandaliya R, Alsaadi D, Farshidpour M, Charabaty A, Malhotra N, Mattar MC: Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J Clin Cases 7: 405–418, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stewart AF: Clinical practice. Hypercalcemia associated with cancer. N Engl J Med 352: 373–379, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Fadol AP: Management of chemotherapy-induced left ventricular dysfunction and heart failure in patients with cancer while undergoing cancer treatment: The MD Anderson Practice. Front Cardiovasc Med 5: 24, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, Guinan E, Vogelsang G, Krishnan A, Giralt S, Revta C, Carreau NA, Iacobelli M, Carreras E, Ruutu T, Barbui T, Antin JH, Niederwieser D: Hepatic veno-occlusive disease following stem cell transplantation: Incidence, clinical course, and outcome. Biol Blood Marrow Transplant 16: 157–168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee WL, Slutsky AS: Sepsis and endothelial permeability. N Engl J Med 363: 689–691, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Tassani P, Schad H, Winkler C, Bernhard A, Ettner U, Braun SL, Eising GP, Kochs E, Lange R, Richter JA: Capillary leak syndrome after cardiopulmonary bypass in elective, uncomplicated coronary artery bypass grafting operations: Does it exist? J Thorac Cardiovasc Surg 123: 735–741, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Pisoni R, Ruggenenti P, Remuzzi G: Drug-induced thrombotic microangiopathy: Incidence, prevention and management. Drug Saf 24: 491–501, 2001 [DOI] [PubMed] [Google Scholar]
- 97.Lesesne JB, Rothschild N, Erickson B, Korec S, Sisk R, Keller J, Arbus M, Woolley PV, Chiazze L, Schein PS: Cancer- associated hemolytic-uremic syndrome: Analysis of 85 cases from a national registry. J Clin Oncol 7: 781–789, 1989 [DOI] [PubMed] [Google Scholar]
- 98.Sadler JE, Moake JL, Miyata T, George JN: Recent advances in thrombotic thrombocytopenic purpura. Hematology (Am Soc Hematol Educ Program) 2004: 407–423, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Brain MC, Azzopardi JG, Baker LR, Pineo GF, Roberts PD, Dacie JV: Microangiopathic haemolytic anaemia and mucin-forming adenocarcinoma. Br J Haematol 18: 183–193, 1970 [DOI] [PubMed] [Google Scholar]
- 100.Antman KH, Skarin AT, Mayer RJ, Hargreaves HK, Canellos GP: Microangiopathic hemolytic anemia and cancer: A review. Medicine (Baltimore) 58: 377–384, 1979 [DOI] [PubMed] [Google Scholar]
- 101.Reiniger G, Menke G, Boertz A, Kraus F, Rudolph W: [Interval therapy in effective treatment of angina pectoris using nitroglycerin patch systems. A controlled study with determination of nitroglycerin plasma levels]. Herz 12: 68–73, 1987 [PubMed] [Google Scholar]
- 102.Izzedine H, Perazella MA: Thrombotic microangiopathy, cancer, and cancer drugs. Am J Kidney Dis 66: 857–868, 2015 [DOI] [PubMed] [Google Scholar]
- 103.Hingorani S, Pao E, Stevenson P, Schoch G, Laskin BL, Gooley T, McDonald GB: Changes in glomerular filtration rate and impact on long-term survival among adults after hematopoietic cell transplantation: A prospective cohort study. Clin J Am Soc Nephrol 13: 866–873, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Regierer AC, Kuehnhardt D, Schulz CO, Flath B, Jehn CF, Scholz CW, Possinger K, Eucker J: Breast cancer-associated thrombotic microangiopathy. Breast Care (Basel) 6: 441–445, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vakiti A, Singh D, Pilla R, Alhaj-Moustafa M, Fitzpatrick KW: Bevacizumab-induced atypical hemolytic uremic syndrome and treatment with eculizumab. J Oncol Pharm Pract 25: 1011–1015, 2019 [DOI] [PubMed] [Google Scholar]
- 106.Gosain R, Gill A, Fuqua J, Volz LH, Kessans Knable MR, Bycroft R, Seger S, Gosain R, Rios JA, Chao JH: Gemcitabine and carfilzomib induced thrombotic microangiopathy: Eculizumab as a life-saving treatment. Clin Case Rep 5: 1926–1930, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sezer O, Vesole DH, Singhal S, Richardson P, Stadtmauer E, Jakob C, Boral AL, Esseltine DL, Mehta J: Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma 7: 233–235, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Yang H, Rosove MH, Figlin RA: Tumor lysis syndrome occurring after the administration of rituximab in lymphoproliferative disorders: High-grade non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Am J Hematol 62: 247–250, 1999 [DOI] [PubMed] [Google Scholar]
- 109.Bouclet F, Calleja A, Dilhuydy MS, Véronèse L, Pereira B, Amorim S, Cymbalista F, Herbaux C, de Guibert S, Roos-Weil D, Hivert B, Aurran T, Dupuis J, Blouet A, Tchernonog E, Laribi K, Dmytruck N, Morel P, Michallet AS, Dartigeas C, Tournilhac O, Nguyen-Khac F, Delmer A, Feugier P, Ysebaert L, Guièze R: Real-world outcomes following venetoclax therapy in patients with chronic lymphocytic leukemia or Richter syndrome: A FILO study of the French compassionate use cohort. Ann Hematol 100: 987–993, 2021 [DOI] [PubMed] [Google Scholar]
- 110.Tachibana K, Ohe S, Tanaka M, Maniwa T, Isei T: Tumor lysis syndrome induced by BRAF/MEK double blockade in a patient with metastatic melanoma: A first case report. J Dermatol 48: e324–e326, 2021 [DOI] [PubMed] [Google Scholar]
- 111.Wanchoo R, Bernabe Ramirez C, Barrientos J, Jhaveri KD: Renal involvement in chronic lymphocytic leukemia. Clin Kidney J 11: 670–680, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shimada M, Johnson RJ, May WS Jr., Lingegowda V, Sood P, Nakagawa T, Van QC, Dass B, Ejaz AA: A novel role for uric acid in acute kidney injury associated with tumour lysis syndrome. Nephrol Dial Transplant 24: 2960–2964, 2009 [DOI] [PubMed] [Google Scholar]
- 113.Kang DH, Park SK, Lee IK, Johnson RJ: Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 16: 3553–3562, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Han HJ, Lim MJ, Lee YJ, Lee JH, Yang IS, Taub M: Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am J Physiol Renal Physiol 292: F373–F381, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Cairo MS, Bishop M: Tumour lysis syndrome: New therapeutic strategies and classification. Br J Haematol 127: 3–11, 2004 [DOI] [PubMed] [Google Scholar]
- 116.US National Library of Medicine : Label: Allopurinol tablet, 2021. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=682dd8b8-fc6e-47c5-95b7-82d7ad96b750. Accessed February 2, 2022
- 117.Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, Tou C, Harvey E, Morris E, Cairo MS: A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood 97: 2998–3003, 2001 [DOI] [PubMed] [Google Scholar]
- 118.Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M, Matous J, Luger S, Dey BR, Schiller GJ, Pham D, Abboud CN, Krishnamurthy M, Brown A Jr., Laadem A, Seiter K: Control of plasma uric acid in adults at risk for tumor Lysis syndrome: Efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone--results of a multicenter phase III study. J Clin Oncol 28: 4207–4213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Isik B, Alexander MP, Manohar S, Vaughan L, Kottschade L, Markovic S, Lieske J, Kukla A, Leung N, Herrmann SM: Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int Rep 6: 1022–1031, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zibetti Dal Molin G, Westin SN, Msaouel P, Gomes LM, Dickens A, Coleman RL: Discrepancy in calculated and measured glomerular filtration rates in patients treated with PARP inhibitors. Int J Gynecol Cancer 30: 89–93, 2020 [DOI] [PubMed] [Google Scholar]
- 121.Chappell JC, Turner PK, Pak YA, Bacon J, Chiang AY, Royalty J, Hall SD, Kulanthaivel P, Bonventre JV: Abemaciclib inhibits renal tubular secretion without changing glomerular filtration rate. Clin Pharmacol Ther 105: 1187–1195, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J: Vascular and metabolic implications of novel targeted cancer therapies: Focus on kinase inhibitors. J Am Coll Cardiol 66: 1160–1178, 2015 [DOI] [PubMed] [Google Scholar]
- 123.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stanchina M, McKinnell Z, Park JH, Stein EM, Cai SF, Taylor J: BCR-ABL tyrosine kinase inhibitor (TKI)-induced nephropathy: An under-recognized phenomenon. Leuk Res Rep 14: 100211, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Russo G, Cioffi G, Di Lenarda A, Tuccia F, Bovelli D, Di Tano G, Alunni G, Gori S, Faggiano P, Tarantini L: Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Intern Emerg Med 7: 439–446, 2012 [DOI] [PubMed] [Google Scholar]
- 126.Harari PM: Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer 11: 689–708, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Jhaveri KD, Sakhiya V, Fishbane S: Nephrotoxicity of the BRAF inhibitors vemurafenib and dabrafenib. JAMA Oncol 1: 1133–1134, 2015 [DOI] [PubMed] [Google Scholar]
- 128.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF: Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374: 311–322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wanchoo R, Abudayyeh A, Doshi M, Edeani A, Glezerman IG, Monga D, Rosner M, Jhaveri KD: Renal toxicities of novel agents used for treatment of multiple myeloma. Clin J Am Soc Nephrol 12: 176–189, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaplan B, Qazi Y, Wellen JR: Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando) 28: 126–133, 2014 [DOI] [PubMed] [Google Scholar]
- 131.Bonilla M, Jhaveri KD, Izzedine H: Anaplastic lymphoma kinase inhibitors and their effect on the kidney. Clinical Kidney Journal 2022. 10.1093/ckj/sfac062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deshpande PP, Perazella MA, Jhaveri KD: PARP inhibitors and the kidney. J Onco-Nephrology 5: 42–47, 2021 [Google Scholar]
- 133.Yui JC, Van Keer J, Weiss BM, Waxman AJ, Palmer MB, D’Agati VD, Kastritis E, Dimopoulos MA, Vij R, Bansal D, Dingli D, Nasr SH, Leung N: Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol 91: E348–E352, 2016 [DOI] [PubMed] [Google Scholar]
- 134.Manohar S, Bansal A, Wanchoo R, Sakhiya V, Lucia S, Jhaveri KD: Ibrutinib induced acute tubular injury: A case series and review of the literature. Am J Hematol 94: E223–E225, 2019 [DOI] [PubMed] [Google Scholar]
- 135.Kala J, Mamlouk O, Jhaveri KD: Selinexor-associated hyponatremia: Single-center, real-world data. Kidney Int 98: 789–791, 2020 [DOI] [PubMed] [Google Scholar]
- 136.Nobre CF, Newman MJ, DeLisa A, Newman P: Moxetumomab pasudotox-tdfk for relapsed/refractory hairy cell leukemia: A review of clinical considerations. Cancer Chemother Pharmacol 84: 255–263, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meraz-Munoz A, Langote A, Jhaveri KD, Izzedine H, Gudsoorkar P: Acute kidney injury in the patient with cancer. Diagnostics (Basel) 11: 611, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]