Abstract
As well known, cocaine induces stimulant effects and dopamine transporter (DAT) trafficking to the plasma membrane of dopaminergic neurons. In the present study, we examined cocaine-induced hyperactivity along with cocaine-induced DAT trafficking and the recovery rate of the dopaminergic system in female rats in comparison with male rats, demonstrating interesting gender differences. Female rats are initially more sensitive to cocaine than male rats in terms of both the DAT trafficking and hyperactivity induced by cocaine. Particularly, intraperitoneal (i.p.) administration of 5 mg/kg cocaine induced significant hyperactivity and DAT trafficking in female rats but not in male rats. After repeated cocaine exposures (i.e., intraperitoneal administration of 20 mg/kg cocaine every other day from day 0 to day 32), cocaine-induced hyperactivity in female rats gradually became a clear pattern of two phases, with the first phase of the hyperactivity lasting for only a few minutes and the second phase lasting for over an hour beginning at ~30 min, which is clearly different from that of male rats. It has also been demonstrated that the striatal DAT distribution of female rats may recover faster than that of male rats after multiple cocaine exposures. Nevertheless, despite the remarkable gender differences, our recently developed long-acting cocaine hydrolase, known as CocH5-Fc(M6), can similarly and effectively block cocaine-induced DAT trafficking and hyperactivity in both male and female rats.
1. INTRODUCTION
Drug dependence (or addiction) is a debilitating neuropsychiatric disorder associated with high levels of drug intake, drug seeking, and repeated cycles of abstinence and relapse.1 Increasing studies have reported gender differences in vulnerability to substance use disorders (SUDs), including cocaine use disorder (CUD).2 Although men are more likely to be given the opportunity to try cocaine than women, both genders progress to regular use at an equal rate once exposed to cocaine.3, 4 However, women are reported to transition to the dependence faster, take more cocaine, experience more adverse consequences,5 and have more difficulty remaining abstinent.6 When the opportunity for drug use is taken into consideration, women are more likely to use cocaine at an earlier age,7 seeking higher doses,8, 9 and being more vulnerable to physical, mental, and social consequences of abuse.10, 11 Nonetheless, female subjects are still underrepresented in both preclinical and clinical research. While these gender differences in humans could be influenced by various factors, similar gender differences were observed in animal models. On the other hand, our previous study did not observe significant differences in the plasma levels of cocaine in male and female rats after the same dose of cocaine.12
As well known, although cocaine interacts with numerous proteins in the central nervous system (CNS),13 cocaine produces its stimulant effect by mainly targeting dopamine (DA) transporter (DAT),14 thus blocking DA reuptake. Cocaine intake rapidly induces DAT trafficking to the plasma membrane without significantly changing the sum of the intracellular and plasma membrane DAT,15, 16 whereas normalization of dopaminergic function is a very slow process.17 In our previous study,16 i.p. administration of cocaine at a dose of 10 mg/kg or higher induced most of DAT translocating to the plasma membrane of the dopaminergic neurons while producing significant hyperactivity in male rats. However, i.p. administration of 5 mg/kg cocaine induced no significant DAT trafficking or hyperactivity in male rats, suggesting that the minimum/threshold dose of cocaine (administered i.p.) capable of significantly inducing DAT trafficking or hyperactivity should be between 5 and 10 mg/kg for male rats.16 Further, we demonstrated that complete recovery of the striatal DAT distribution may take about 60 days after an acute cocaine exposure of one-time 20 mg/kg (i.p.) or about 90 days after repeated cocaine exposures (17 times of 20 mg/kg cocaine, i.p.).17 Due to all these features of the dopaminergic system, it has been extremely challenging to develop an effective pharmacotherapy for CUD treatment. No CUD-specific pharmacotherapy has been approved by the Food and Drug Administration (FDA) so far.
Nevertheless, through structure and mechanism-based computational design, we have successfully designed and discovered highly efficient cocaine hydrolases (CocHs)18–33 and their long-acting form denoted as CocH-Fc,34 demonstrating that these CocH-Fc proteins can efficiently convert cocaine to physiologically inactive metabolites and, thus, effectively block the physiological and reinforcing effects of cocaine.34–39 It was demonstrated that CocH-Fc also effectively blocked cocaine-induced DAT trafficking in male rats.16 In addition, a most recently reported more active CocH-Fc, denoted as CocH5-Fc(M6),36 effectively blocked cocaine-induced hyperactivity and DAT trafficking with repeated cocaine exposures by maintaining a plasma CocH5-Fc(M6) concentration ≥58.7 ± 2.9 nM in male rats.17 So, CocH5-Fc(M6) was able to protect the dopaminergic system and, thus, help the cocaine-altered DAT distribution to recover by preventing the dopaminergic system from further damage by cocaine in male rats.17
However, to the best of our knowledge, there has been no report on the recovery of the dopaminergic system in female rats after cocaine-induced DAT trafficking, and the enzyme’s effects on DAT trafficking have been explored in male rats only. Considering the aforementioned gender differences in CUD, the question remains whether CocH5-Fc(M6) can also similarly protect the dopaminergic system of female rats. In the present study, we examined cocaine-induced hyperactivity, along with cocaine-induced DAT trafficking, and the recovery rate of the dopaminergic system in female rats in comparison with male rats under the same experimental conditions, demonstrating significant gender differences. Particularly, female rats are initially more sensitive to cocaine than male rats in terms of both the DAT trafficking and hyperactivity induced by cocaine. The recovery of the dopaminergic system in female rats after repeated cocaine exposure may be faster in comparison with male rats. Nevertheless, it has been demonstrated that, despite the gender differences, the enzyme CocH5-Fc(M6) can similarly and effectively block cocaine-induced DAT trafficking and hyperactivity in both male and female rats.
2. MATERIALS AND METHODS
2.1. Animals
All female and male Sprague–Dawley rats used in this study were ordered from Envigo (Indianapolis, IN). The rats were 9–11 weeks old when the experiments began, which is consistent with the age of the male rats used in our previous studies.17 Animals were housed initially as two rats (with the same gender) per cage. All rats were kept at room temperature of ~22°C, allowing ad libitum access to food and water. Animal housing areas were maintained on a 12-hour light/12-hour dark cycle, with lights on at 8:00 AM. On all the testing days, experiments were performed in light between 9:00 AM and 6:00 PM. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (NIH). The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky.
2.2. Drugs and materials
CocH5-Fc(M6) is an Fc fusion protein with CocH5 (which is the A199S/F227A/P285A/S287G/A328W/Y332G mutant of human butyrylcholinesterase) fused with a mutant (A1V/M38Y/S40T/T42E/D142E/L144M) of Fc region of human IgG1, and it was developed in our previous studies.18, 34, 36 Cocaine was provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD). Anti-DAT antibody (mouse monoclonal antibody, catalog #MA524796), and HRP-conjugated goat anti-mouse (catalog #G21040) polyclonal antibody, protease and phosphatase inhibitor cocktail (100-X), Sulfo-NHS-SS-biotin (sulfosuccinimidyl-2-[biotinamido]ethyl-1,3-dithiopropionate), and monomeric avidin agarose were purchased from Thermo Fisher Scientific (Waltham, MA). Antibodies for rat Na+/K+ ATPase (mouse monoclonal antibody, catalog #sc-48345), β-actin (Horseradish peroxidase (HRP)-conjugated mouse polyclonal antibody, catalog #sc-47778), and protein phosphatase 2A (PP2A, mouse monoclonal antibody, catalog #sc-13601) were all ordered from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals and surgery tools were purchased from either VWR international (Radnor, PA), Thermo Fisher Scientific (Waltham, MA), or Sigma-Aldrich (St. Louis, MO).
2.3. DAT cellular distribution assays
Biotinylation and Western blotting assays were performed using our previously described method,16, 36 with the loaded plasma/intracellular protein isolated from an equal amount of no more than 1.5 μg/well of protein from each sample’s synaptosomes lysate.
2.4. Locomotor activity testing in female rats
Rats were placed in high-density, non-porous plastic chambers (50-cm L × 50-cm W × 38-cm H) in a quiet room and allowed to acclimate before testing. On the test day, following one-hour acclimation, rats were administered either a dose of 20 mg/kg cocaine or saline (i.p.). To examine whether CocH5-Fc(M6) at a blood concentration of ≥60 nM can fully block the physiological effects of 20 mg/kg cocaine (i.p.) in female rats as observed previously in male rats,17 we first gave a group of female rats a dose of ~0.6 mg/kg CocH5-Fc(M6) (i.v.), then monitored their plasma CocH5-Fc(M6) concentrations. When the desired plasma CocH5-Fc(M6) level was reached, the female rats were allowed to have one-hour acclimation in the test chambers and then given a dose of 20 mg/kg cocaine (i.p.). After cocaine injection, rats were immediately returned to the test chambers. The distances traveled every 5 minutes were tracked and recorded for two hours after cocaine administration using the ANY-maze video tracking system (San Diego Instruments, San Diego, CA) in our animal lab. Upon completion of the tests, animals were returned to their home cages.
2.5. Determination of plasma enzyme concentrations
To measure plasma enzyme concentrations, blood samples (around 75 μL) were collected from saphenous veins using heparin-treated capillary tubes at different time-points after enzyme administration. Collected blood samples were centrifuged for 5 minutes at a speed of 8,000 g, and separated plasma was kept at 4°C before use for analysis. A sensitive radiometric assay as described previously35 was performed to measure the enzyme concentration in plasma. Briefly, 10 μL of plasma sample was mixed with 140 μL of 0.1 M phosphate buffer (pH = 7.4). 50 μL of 400 μM [3H](−)-cocaine was added to the mixture to initiate enzymatic reaction. The reaction was performed at room temperature and was stopped by adding 200 μL of 0.1 M HCl. The radioactive product benzoic acid was extracted by toluene for scintillation counting. The enzyme concentration ([E]) was calculated based on equation [E] = Vmax/kcat, where Vmax is the maximum rate the enzymatic reaction can achieve, kcat is the turnover number of the enzyme. Enzyme kinetic parameters were obtained from our previous studies.18, 36
2.6. Determination of cocaine levels in brain
To determine the concentrations of cocaine in the brain, rat brain samples (n = 4 for each group) were quickly removed at 10 min after cocaine administration. Surface blood was blotted away, and brain samples were weighed and immediately frozen in 1% trichloroacetic acid (TCA) containing 250 μM paraoxon on dry ice. The brain samples were stored at −80 °C until use. For analysis, thawed brain samples were homogenized in cold 1% TCA containing 250 μM paraoxon (5 mL of homogenizing solution per gram of brain sample) using a homogenizer motor with a 7 × 95 mm saw-tooth generator probe (VWR International LLC, Radnor, PA). The homogenate was then centrifuged at 17,000 g at 4 °C for 20 minutes. 200 μL of supernatant was collected and mixed with 50 μL of 50 ng/mL internal standards. The mixture was then extracted by 750 μL of hexane. The resulting inorganic phase was collected and mixed with 750 μL of 1% TCA. The mixture was then subjected to a one-step solid-phase extraction (SPE) using mixed-mode cation-exchange solid phase extraction cartridges (Oasis MCX 1 cc Vac Cartridge, 10 mg) from Waters (Milford, MA). The loaded sample was washed twice with 1 mL methanol, followed by elution using 1 mL methanol/7.5% ammonium hydroxide (95:5, v/v).
A quantitative LC-MS/MS protocol described in our previous study40 was used to quantify cocaine and its metabolites in the brain samples (with calibration curves determined by using the homogenized brain samples). Briefly, extracted samples were evaporated and re-suspended in 0.1% formic acid before loading to a Shimadzu HPLC system (Shimadzu, Kyoto, Japan), consisting of a DGU-20A/3R degasser, LC-20 AD binary pumps, CBM-20A controller, and SIL-20A/HT autosampler. Mobile phase A consisted of 0.1% formic acid and mobile phase B consisted of 0.1% formic acid: acetonitrile (10: 90, v/v). Samples were loaded to an Atlantis T3 (100 Å, 3 μm, 2.1 mm × 150 mm I.D) column (Waters, Milford, MA) and were eluted by gradient. AB SCIEX tripleTOF™ 5600 (AB SCIEX, Redwood City, CA) was applied in positive ion and high sensitivity mode for analysis.
2.7. Statistics
Data are presented as mean ± SEM, and n represents the number of independent experiments for each group. Data from immunoblotting experiments were analyzed using one-way ANOVA or t-test for each of the fractions (biotinylated and non-biotinylated). The accepted level of statistical significance was p < 0.05.
3. RESULTS
3.1. Cocaine-induced hyperactivity in female rats by acute cocaine administration
In our previously reported study,16 we determined the hyperactivity in male rats induced by a single i.p. dose of cocaine at various dose levels (5, 10, 20, 40, and 60 mg/kg), demonstrating that a cocaine dose of 10 mg/kg or higher (i.p.) significantly induced hyperactivity in male rats, whereas 5 mg/kg cocaine (i.p.) did not significantly induce hyperactivity in male rats.
In Figure 1, we compared 5 or 20 mg/kg cocaine (i.p.)-induced hyperactivity in both male and female rats – Figure 1A and 1C for male rats,16 and Figure 1B and 1D for female rats – determined by using the same experimental methods and conditions. Depicted in Figure 1E are the total traveled distances during the first two hours after the cocaine administration to different groups of rats. The female rats were significantly more sensitive to cocaine exposure for a given dose of cocaine (5 or 20 mg/kg, i.p.) than the male rats, as seen in Figure 1E. Particularly, 5 mg/kg cocaine (i.p.) did induce significant hyperactivity in female rats, but not in male rats (Figure 1E).17
Figure 1.

Cocaine-induced hyperactivity in male and female rats. For each group of rats (n = 8), the animals were first allowed to acclimate to the test chambers for 60 min before i.p. administration of cocaine or saline. The cocaine dose used was 5 mg/kg for panels A and B or 20 mg/kg for panels C and D. All data for male rats in panels A and C came from our previously reported study.17 The data in panels B and D were obtained in the present study. The locomotor activity data are plotted as the mean ± SEM meters traveled in a 5-min bin during the locomotor activity test for seven hours. Panel E shows the total traveled distances for the first 2 hours after cocaine or saline administration for all groups in (A) to (D). Statistical significance (t-test): * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.2. DAT trafficking induced by acute cocaine administration in female rats
In our previous studies,16, 17 we demonstrated that in normal male rat brain, striatal DAT mainly existed in the intracellular fraction, with only a very small fraction (~5.7%) existing in the plasma membrane in rats. The small fraction of ~5.7% is close to the synaptosomal surface faction (~7%) of DAT in rats reported by Johnson et al.,41 but is less than the previously reported surface DAT fraction in cultured PC12-hDAT cells (~25–40%). Cocaine at an i.p. dose of 10 mg/kg or higher, induced translocating of most DAT into the plasma membrane (with only <5% remaining in the intracellular fraction) at 24 hours after cocaine administration in rats that were naïve to cocaine.
In this study, we first determined the striatal DAT distribution on the plasma membrane and intracellular fractions in female rats after a single dose of cocaine at two different dose levels (5 or 20 mg/kg cocaine, i.p.) that were found important for male rats.17 Likewise, rat brain samples were collected at 24 h after i.p. administration of cocaine or saline.
Figure 2A shows western blots of the striatal DAT distribution in both the plasma membrane fraction (biotinylated) and intracellular fraction (non-biotinylated) of the samples collected at 24 hours after saline or 20 mg/kg cocaine exposure (i.p.). The data for all the male groups have been reported previously,16 and the data for female groups in Figure 2 are reported here for the first time. The corresponding numeric data are summarized in Figure 2B. Na+/K+ ATPase α and PP2A are presented as controls for the plasma membrane fraction and intracellular fraction, respectively. β-actin was used as a loading control. All these signals of the protein were used to normalize the DAT distribution while converting the images into numeric data.
Figure 2.
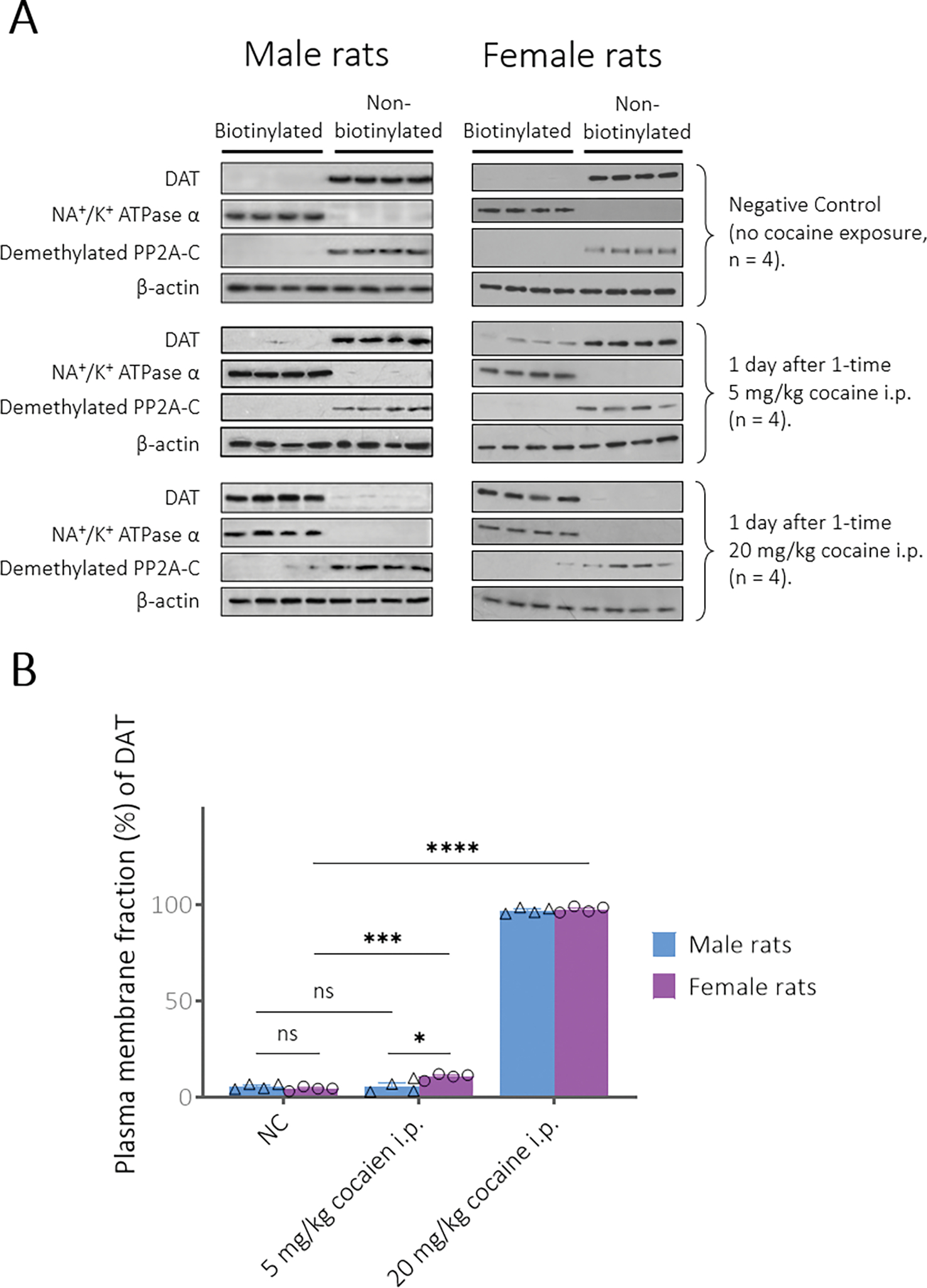
(A) Striatal DAT immunoreactivity after acute exposure to cocaine (n = 4 rats per group). For the immunoblotting results of striatal DAT distribution in the biotinylated membrane fraction and non-biotinylated intracellular fraction, Na+/K+ ATPase and PP2A serve as biotinylation controls, while β-actin serves as a loading control. (B) Normalized DAT expression values are represented as the mean ± SEM for DAT density following different time periods after cocaine exposure expressed as a percentage of total DAT (biotinylated plus non-biotinylated). Statistical analysis (t-test): * p < 0.05, *** p < 0.001, and **** p < 0.0001.
In comparison with the male control data (~5.7%), cocaine-naïve female rats showed a similar plasma membrane DAT level (4.5 ± 1.0%). 20 mg/kg cocaine (i.p.) raised the plasma membrane DAT to a similarly high level in both male rats (96.9 ± 1.5%) and female rats (97.5 ± 1.5%). However, unlike in male rats (~5.8%), i.p. administration of 5 mg/kg cocaine significantly increased the plasma membrane fraction of DAT to 10.7 ± 1.6% in female rats. The observed gender difference in the DAT trafficking data is consistent with the aforementioned gender difference in the cocaine-induced hyperactivity data.
3.3. Recovery of the dopaminergic system in female rats after cocaine exposure for multiple times
To check recovery process of the DAT distribution after cocaine exposure multiple times in female rats, we prepared additional four groups of rats (n = 4 per group) that were given an i.p. dose of 20 mg/kg cocaine every other day from day 0 (first dose) to day 32 (final dose) for a total of 17 doses. Brain samples (n = 4 per group) were collected at 24 hours, 30 days, 60 days, or 90 days after the final dose of cocaine.
To further explore the physiological differences between male and female rats under the impact of the repeated i.p. administration of 20 mg/kg cocaine for 17 times within 32 days, we also recorded the locomotor activity of female rats (n = 16) during the first two hours after each cocaine administration. The data for the male group17 is also included in Figure 3 for comparison with the female group. Figure 3A to 3Q show the locomotor activity of male and female groups of rats after each injection of 20 mg/kg cocaine (i.p.), and Figure 3R summarizes all these data by comparing the accumulated traveled distances recorded each time after cocaine injection in all locomotor activity tests for the two groups. As seen in Figure 3R, the female rats showed the highest hyperactivity (in terms of the total distance traveled) on day 0, whereas the male rats showed their highest hyperactivity on day 4. As a result, the largest difference between the female and male groups in terms of the total distance traveled occurred on day 0, the total distance (~493 m) for the female rats is about two times of that (~245 m) for the male rats.
Figure 3.

Hyperactivity in both female rats (this study) and male rats (our previous report17) induced by each dose of 20 mg/kg cocaine (i.p.) given every other day from days 0 to 32 (panels A to Q). The locomotor activity data are plotted as the mean ± SEM meters traveled in a 5-min bin during the locomotor activity test for 3 hours (including 2 hours after the cocaine administration). Panel R summarizes the accumulated traveled distances (meters) within the recorded first 2 hours after cocaine administration. Outcomes of statistical analysis (t-test) on the statistical analysis of the accumulated traveled distances in panel R between male and female groups: **** p < 0.0001 on day 0; * p < 0.05 on day 2 and day 4; ns (p > 0.05) from days 6 to 32.
Overall, in response to the first three doses of cocaine (days 0, 2, and 4), female rats showed significantly higher hyperactivity than male rats, then the cocaine-induced hyperactivity in terms of the total distances traveled gradually decreased for both groups from day to day, and eventually reached a similar level during the later locomotor activity sessions of the experiment. Interestingly, starting on day 2 of the experiment, the locomotor activity of the female group always had a sharp decrease after the initial 5 to 10 min and reached a second peak between 50 to 70 min, showing a two-phase hyperactivity pattern. Particularly, beginning on day 12, the first phase of the hyperactivity lasted for only a few minutes and quickly ceased, whereas the second began at ~30 min and lasted for longer than an hour (see Figure 3). There was no toxicity sign observed for all rats between the end of the first phase and the beginning of the second phase. The rats just stayed in the corners of the chambers licking their bodies such as legs and tails, while some of the rats fell asleep. The observed cocaine-induced hyperactivity pattern of female rats is remarkably different from that of male rats.
The data in Figure 4 shows a time course of striatal DAT distribution (fraction) over 90 days after the final cocaine exposure in the female rats. Interestingly, as shown in Figure 4, the plasma membrane DAT fraction was upregulated from 4.5 ± 1.0% (negative control) to 91.7 ± 0.8% one day (24 hours) after the last cocaine exposure, and then gradually recovered to 31.4 ± 2.3% (after 30 days), 9.0 ± 2.6% (60 days), and 4.8 ± 0.5% (90 days). The plasma membrane DAT fractions in female rats on all the days (1, 30, 60, and 90 days) after the 17 times of 20 mg/kg cocaine administration (i.p.) were significantly lower than the corresponding plasma membrane DAT fractions in male rats, as seen in Figure 5. We previously demonstrated in male rats17 that cocaine-induced upregulation of plasma membrane DAT fraction can last for a longer period after the repeated dosing of 20 mg/kg cocaine, compared to that with a single dose of 20 mg/kg cocaine. The average plasma membrane DAT fraction after i.p. administration of 20 mg/kg cocaine for 17 times in male rats was upregulated from ~5.7% (negative control) to 97.1 ± 0.2% on day 1 and decreased to 40.8 ± 3.1% on day 30, 15.3 ± 1.2% on day 60, and then 12.3 ± 1.3% on day 90 after the last exposure of 20 mg/kg cocaine (i.p.).17 According to the data in Figure 4, the initial recovery process during the first 30 days was relatively faster (from 91.7 ± 0.8% on day 1 to 31.4 ± 2.3% on day 30) and then slowed down afterward. Remarkably, the plasma membrane fraction (9.0 ± 2.6%) on day 60 was not significantly different from the negative control (4.5 ± 1.0%). So, the striatal DAT distribution of female rats may recover to the normal status faster than that of male rats after the repeated dosing of cocaine (for a total of 17 doses of 20 mg/kg cocaine in 32 days).
Figure 4.

(A) Time course of striatal DAT immunoreactivity in female rats after multiple times of cocaine exposure (n = 4 rats per group). All groups of female rats received a dose of 20 mg/kg cocaine (i.p.) every other day from days 0 to 32 days for a total of 17 doses. (B) Normalized DAT fraction values are represented as the mean ± SEM for DAT fraction following different periods of cocaine abstinence, expressed as the percentage of total DAT (biotinylated plus non-biotinylated). Statistical analysis (One-way ANOVA): * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Figure 5.

Gender differences in the normalized plasma membrane fraction (%, represented as the mean ± SEM) of total DAT on different days after repeated administration of 20 mg/kg cocaine for 17 times. The data for female rats came from Figure 4B, and the data for male rats came from our previous report.17 Statistical analysis (t-test)): * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
3.4. Impact of enzyme CocH5-Fc(M6) on DAT trafficking
After understanding the pattern of DAT trafficking in response to cocaine exposure and the timing for recovery of the dopaminergic system in male and female rats, we wanted to further test the potential impact of CocH5-Fc(M6) on the effects of cocaine in female rats. Particularly, we wanted to see whether the enzyme can help the cocaine-altered dopaminergic system to recover by preventing further damage from repeated cocaine exposures in female rats as it did for male rats. For this purpose, we designed a simplified version of the experiment (that we performed previously in male rats) for female rats. Specifically, in our previous study,17 we demonstrated that CocH5-Fc(M6) was able to powerfully hydrolyze cocaine in blood, effectively preventing cocaine from reaching the brain, and eliminating the cocaine-induced hyperactivity. Additionally, the locomotor activity data in Figure 3 showed that the influence of 20 mg/kg cocaine i.p. in both male and female rats tend towards very similar levels (in terms of the total distances traveled in two hours) after the first several doses of cocaine. So, the main idea for this experiment is to see whether CocH5-Fc(M6) can also provide female rats with effective protection when the plasma concentration of the enzyme becomes as low as 58.7 ± 2.9 nM (the lowest concentration of the enzyme observed during the 32 days of repeated i.p. administration of 20 mg/kg cocaine in male rats). In this study, one group of female rats (n = 4) received a dose of ~0.6 mg/kg CocH5-Fc(M6) i.v. and then their plasma enzyme concentrations were monitored afterward along with blood sample collection for analysis. At the time of the locomotor activity test, the female rats were put in the test chambers for acclimation and administrated 20 mg/kg cocaine (i.p.) when the plasma CocH5-Fc(M6) level in the female rats was decreased to 67.8 ± 11.1 nM at 90 minutes post enzyme injection on day 0 (that was very close to the lowest enzyme concentration of 58.7 ± 2.9 nM found previously in male rats17). The locomotor activity was monitored for 6 hours after the cocaine administration, and the brain samples of the female rats were collected at 24 hours after cocaine injection for DAT analysis.
Figure 6 shows the locomotor activity data (Figure 6A), DAT distribution (Figure 6B), and normalized percentage DAT level compared to the negative control group and 20 mg/kg cocaine (i.p.) group (Figure 6C). These data indicate that even when the plasma level of CocH5-Fc(M6) was dropped to 67.8 ± 11.1 nM, the enzyme can still prevent cocaine-induced DAT trafficking and cocaine-induced hyperactivity in female rats.
Figure 6.

(A) Effect of CoocH5-Fc(M6) on cocaine-induced hyperactivity in female rats (n = 4). Rats were first allowed to acclimate to the test chambers before i.p. administration of cocaine or saline. 20 mg/kg cocaine (i.p.) was given to rats when their plasma CocH5-Fc(M6) level was 67.8 ± 11.1 nM. The locomotor data are plotted as the mean ± SEM meters traveled in a 5-min bin during locomotor activity for seven hours. (B) Time course of striatal DAT immunoreactivity at 24 hours after the administration of 20 mg/kg cocaine (i.p.). (C) Normalized DAT expression values are mean ± SEM for DAT density of the negative control group, one-time 20 mg/kg cocaine i.p. group, and the enzyme pre-treated group, expressed as a percentage of total DAT (biotinylated plus non-biotinylated). Statistical analysis (One-way ANOVA): * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
3.5. Cocaine concentrations in female rat brain
We also determined cocaine concentrations in the whole brains of additional 6 groups of male and female rats (n = 4 per group) at 10 min after i.p. administration of 5 or 20 mg/kg cocaine (Figure 7). As we discussed previously,16, 17 we chose to collect the brain samples at the 10 min time-point because our previous study revealed that the blood cocaine concentrations reached the peaks (Cmax) at ~10 min after i.p. administration of 5 or 20 mg/kg cocaine in rats. So, we wanted to know the brain cocaine concentrations at 10 min associated with all our above data.
Figure 7.

Cocaine concentrations in the whole brains of rats at 10 min after i.p. administration of cocaine at various doses: 5 mg/kg cocaine (i.p.), 20 mg/kg cocaine (i.p.), and 20 mg/kg cocaine (i.p.) in the presence of 60.9 ± 7.6 nM CocH5-Fc(M6) in plasma for both male and female groups of rats (n = 4 per group). Statistical analysis (t-test): * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001; there was no significant difference between male and female rats in the brain cocaine level under the same dose condition.
As we demonstrated previously16 in male rats, the higher the dose of cocaine, the higher the average cocaine concentration in the brain. Similar results were obtained in the current study with both male and female rats (Figure 7). The average cocaine concentration in the whole brain tested in this study was 1,209 ± 60 ng/g associated with 5 mg/kg cocaine (i.p.), and 8,386 ± 586 ng/g associated with 20 mg/kg cocaine in male rats. The higher the dose of cocaine injected, the higher the brain cocaine concentration observed. As shown in Figure 7, the brain cocaine concentrations in female groups of rats that received 5 and 20 mg/kg cocaine (i.p.) were about 1,305 ± 161 and 8,499 ± 1,388 ng/g, respectively, which are comparable to those observed in the corresponding male groups. Further, for the CocH5-Fc(M6)-treated male and female groups of rats, at a similar plasma CocH5-Fc(M6) level of 60.9 ± 7.6 nM, 20 mg/kg cocaine was administrated (i.p.) and brain samples were collected at 10 min after the cocaine administration. The average brain cocaine concentration was only 126 ± 34 ng/g in the female rats and 150 ± 7 ng/g in the male rats, all are far lower than the corresponding group without the enzyme administration, as seen in Figure 7. This data further suggested that even at a plasma concentration of CocH5-Fc(M6) as low as 60.9 ± 7.6 nM, the enzyme can still effectively prevent cocaine (20 mg/kg, i.p.) from reaching the brain in both male and female rats.
4. DISCUSSION
Concerning cocaine-induced DAT trafficking, we previously demonstrated16, 17 that, in male rats, cocaine at an intraperitoneal (i.p.) dose of 10 mg/kg or higher induced trafficking of most DAT to the plasma membrane (with only <5% remained in the intracellular fraction) at 24 hours after cocaine injection, and the recovery of the dopaminergic system (in terms of intracellular and plasma membrane fractions of DAT) after administration of 20 mg/kg cocaine (i.p.) may take up to 60 days (after one-time 20 mg/kg cocaine, i.p.) or 90 days (after 17-times 20 mg/kg cocaine, i.p.). In the present study, we performed some of the corresponding experiments on female rats to examine the gender differences in terms of the physiological effects of cocaine. For all the experiments mentioned above, we first compared the physiological effects of cocaine after i.p. administration of a single dose (5 or 20 mg/kg) of cocaine. According to the data obtained, after i.p. administration of 5 mg/kg cocaine, whereas both male and female rats had similar cocaine concentrations in the brain, female rats showed significantly higher hyperactivity induced by the same dose of cocaine compared to the male rats treated with cocaine in the same way (Figure 1E). Correspondingly, female rats also had significantly higher membrane DAT fraction than male rats (Figure 2B). After i.p. administration of 20 mg/kg cocaine, whereas both male and female groups have most of their DAT on the membrane (~97% of total DAT) (Figure 2B), female rats have significantly higher cocaine-induced hyperactivity levels (Figure 1E).
According to our hyperactivity data, female rats are more sensitive to cocaine than male rats, which is not surprising. However, our hyperactivity data have demonstrated for the first time that after repeated exposures to cocaine, female rats have a remarkably different cocaine-induced hyperactivity pattern (showing two phases) compared to male rats. For this reason, studies from shorter locomotor activity sessions (such as 20-min locomotor activity sessions as reported in literature42) for female rats will not reflect the longer-lasting second phase of cocaine-induced hyperactivity. These remarkable gender differences may be associated with different striatal DAT densities between male and female rats. As reported previously,43, 44 female rats have a higher density of striatal DA uptake sites (DAT) and greater striatal DA synthesis and release than male rats.
In the DAT recovery experiment, after the repeated i.p. administration of 20 mg/kg cocaine for 17 times, the membrane DAT fraction in the female rats was 91.7 ± 0.8% at 24 hours after last cocaine exposure, 31.4 ± 2.3% on day 30, 9.0 ± 2.6% on day 60, and 4.8 ± 0.5% on day 90 (Figure 4B). In comparison, the membrane DAT fraction in the male rats still had 40.8 ± 3.1% on day 30, 15.3 ± 1.2% on day 60, and 12.3 ± 1.3% on day 90 (Figure 2B), showing significant gender difference (Figure 5). These results suggest that the striatal DAT distribution of female rats may recover faster than that of male rats after multiple cocaine exposures (i.e., i.p. administration of 20 mg/kg cocaine for 17 times). Previous review45 and research on different drugs46 suggested that DAT recovery in female is no harder than in male. Besides, it was reported that in human, depressed patients with initially higher levels of DAT would have larger decreases in DAT binding,47, 48 which suggests that females with higher DAT density would have greater loss of DAT availability in depression than that in male. However, in terms of an animal model relative to cocaine-induced DAT trafficking and long-time cocaine abstinence, this is the first time to report these significant gender differences here. Further, to confirm the effectiveness of CocH5-Fc(M6) treatment for cocaine dependence by protecting cocaine from altering the normal distribution of striatal DAT in female rats, we also tested the CocH5-Fc(M6) treatment in female rats for comparison with male rats. The enhanced sensitivity to cocaine in female rats makes this experiment more challenging. However, our results suggest that, even at a similar plasma concentration of CocH5-Fc(M6) which is as low as ~61 nM, both gender groups have acquired complete protection against cocaine exposure (20 mg/kg cocaine, i.p.) due to the high catalytic efficiency of CocH5-Fc(M6) for cocaine hydrolysis.36 Hence, it is reasonable to conclude that CocH5-Fc(M6) maintained at a plasma level of ≥61 nM can effectively protect the dopaminergic system from the physiological effects of cocaine in both male and female rats. Overall, the findings in this study implies that administration of a long-acting cocaine-metabolizing enzyme like CocH5-Fc(M6) could serve as a potentially promising strategy to block the physiological effects of cocaine and, thus, help the cocaine-affected dopaminergic system to recover in both males and females with cocaine user disorder.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH grants U01 DA051079, R01 DA056646, U18 DA052319, UH2/UH3 DA041115, R01 DA035552, R01 DA032910, and R01 DA013930).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Reid AG, Lingford-Hughes AR, Cancela LM, Kalivas PW. Substance abuse disorders. Handb Clin Neurol 2012, 106:419–431. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology 2005, 30:1006–1018. [DOI] [PubMed] [Google Scholar]
- 3.Kasperski SJ, Vincent KB, Caldeira KM, Garnier-Dykstra LM, O’Grady KE, Arria AM. College students’ use of cocaine: results from a longitudinal study. Addict Behav 2011, 36:408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. Addiction 1999, 94:1413–1419. [DOI] [PubMed] [Google Scholar]
- 5.Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 2016, 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res 2006, 8:627–638. [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend 2002, 68:65–85. [DOI] [PubMed] [Google Scholar]
- 8.Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am 1999, 22:241–252. [DOI] [PubMed] [Google Scholar]
- 9.Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol 1999, 60:252–260. [DOI] [PubMed] [Google Scholar]
- 10.Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am 2010, 33:339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis 2003, 22:61–74. [DOI] [PubMed] [Google Scholar]
- 12.Zheng X, Zhou Z, Zhang T, Jin Z, Chen X, Deng J, Zhan C-G, Zheng F. Effectiveness of a Cocaine Hydrolase for Cocaine Toxicity Treatment in Male and Female Rats. AAPS J. 2017, 20:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heard K, Palmer R, Zahniser NR. Mechanisms of acute cocaine toxicity. Open Pharmacol J 2008, 2:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Zhan CG. How dopamine transporter interacts with dopamine: insights from molecular modeling and simulation. Biophys J 2007, 93:3627–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little KY, Zhang L, Desmond T, Frey KA, Dalack GW, Cassin BJ. Striatal dopaminergic abnormalities in human cocaine users. Am J Psychiatry 1999, 156:238–245. [DOI] [PubMed] [Google Scholar]
- 16.Deng J, Kim K, Zheng X, Shang L, Zhan C-G, Zheng F. Cocaine hydrolase blocks cocaine-induced dopamine transporter trafficking to the plasma membrane. Addict. Biol. 2022, 27:e13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng J, Zhang T, Zheng X, Shang L, Zhan C-G, Zheng F. Recovery of dopaminergic system after cocaine exposure and impact of a long-acting cocaine hydrolase. Addict. Biol 2022:11 May 2022. https://doi.org/2010.1111/adb.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng F, Xue L, Hou S, Liu J, Zhan M, Yang W, Zhan C-G. A highly efficient cocaine-detoxifying enzyme obtained by computational design. Nature Commun. 2014, 5:3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D, Tong M, Tai HH, Woods JH, Zhan C-G. Most efficient cocaine hydrolase designed by virtual screening of transition states. J. Am. Chem. Soc. 2008, 130:12148–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue L, Ko MC, Tong M, Yang W, Hou S, Fang L, Liu J, Zheng F, Woods JH, Tai HH, et al. Design, preparation, and characterization of high-activity mutants of human butyrylcholinesterase specific for detoxification of cocaine. Mol. Pharmacol. 2011, 79:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng F, Zhan C-G. Are pharmacokinetic approaches feasible for treatment of cocaine addiction and overdose? Future Med. Chem. 2012, 4:125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng F, Zhan C-G. Enzyme-therapy approaches for the treatment of drug overdose and addiction. Future Med. Chem. 2011, 3:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng F, Zhan C-G. Recent progress in protein drug design and discovery with a focus on novel approaches to the development of anti-cocaine medications. Future Med. Chem 2009, 1:515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou S, Zhan M, Zheng X, Zhan C-G, Zheng F. Kinetic characterization of human butyrylcholinesterase mutants for the hydrolysis of cocaethylene. Biochem. J. 2014, 460:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan M, Hou S, Zhan C-G, Zheng F. Kinetic characterization of high-activity mutants of human butyrylcholinesterase for the cocaine metabolite norcocaine. Biochem. J. 2014, 457:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan C-G, Zheng F, Landry DW. Fundamental reaction mechanism for cocaine hydrolysis in human butyrylcholinesterase. J. Am. Chem. Soc. 2003, 125:2462–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y, Gao D, Yang W, Cho H, Yang G, Tai H-H, Zhan C-G. Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc. Natl. Acad. Sci. USA 2005, 102:16656–16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W, Xue L, Fang L, Chen X, Zhan C-G. Characterization of a high-activity mutant of human butyrylcholinesterase against (−)-cocaine. Chem. Biol. Interact. 2010, 187:148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng F, Yang W, Xue L, Hou S, Liu J, Zhan C-G. Design of high-activity mutants of human butyrylcholinesterase against (−)-cocaine: structural and energetic factors affecting the catalytic efficiency. Biochemistry 2010, 49:9113–9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng F, Zhan C-G. Actions of Butyrylcholinesterase against Cocaine. The Neuroscience of Cocaine: Mechanisms and Treatment, edited by Preedy Victor R., Academic Press; (an Imprint of Elsevier), 2017; p 663–672 2017. [Google Scholar]
- 31.Zheng F, Zhan C-G. Cocaine Hydrolases Designed from Butyrylcholinesterase. In: Montoya ID, ed. Biologics to Treat Substance Use Disorders: Vaccines, Monoclonal Antibodies, and Enzymes. Heidelberg, Germany: Springer; 2016, 187–225. [Google Scholar]
- 32.Zheng X, Chen X, Zhang T, Zhan M, Zhan C-G, Zheng F. Catalytic activities of cocaine hydrolases against the most toxic cocaine metabolite norcocaethylene. Org. Biomol. Chem. 2020, 18:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Zheng X, Zhan M, Zhou Z-Y, Zhan C-G, Zheng F. Metabolic enzymes of cocaine metabolite benzoylecgonine. ACS Chem. Biol 2016, 11:2186–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng F, Chen X, Kim K, Zhang T, Huang H, Zhou S, Zhang J, Jin Z, Zhan C-G. Structure-Based Design and Discovery of a Long-Acting Cocaine Hydrolase Mutant with Improved Binding Affinity to Neonatal Fc Receptor for Treatment of Cocaine Abuse. AAPS J. 2020, 22:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Xue L, Hou S, Jin Z, Zhang T, Zheng F, Zhan C-G. Long-acting cocaine hydrolase for addiction therapy. Proc. Natl. Acad. Sci. USA 2016, 113:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng F, Jin Z, Deng J, Chen X, Zheng X, Wang GJ, Kim K, Shang L, Zhou Z, Zhan C-G. Development of a highly efficient long-acting cocaine hydrolase entity to accelerate cocaine metabolism. Bioconjugate Chem. 2022: 10.1021/acs.bioconjchem.1022c00210. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Deng J, Zheng X, Zhang J, Zhou Z, Wei H, Zhan C-G, Zheng F. Development of a long-acting Fc-fused cocaine hydrolase with improved yield of protein expression. Chem. Biol. Interact. 2019, 306:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T, Wei H, Deng J, Zheng F, Zhan C-G. Clinical potential of a rationally engineered enzyme for treatment of cocaine dependence: Long-lasting blocking of the psychostimulant, discriminative stimulus, and reinforcing effects of cocaine. Neuropharmacology 2020, 176:108251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Cui W, Deng J, Hou S, Zhang J, Ding X, Zheng X, Wei H, Zhou Z, Kim K, et al. Development of Fc-fused Cocaine Hydrolase for Cocaine Addiction: Catalytic and Pharmacokinetic Properties. AAPS J 2018, 20:53. doi: 10.1208/s12248-12018-10214-12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Zheng X, Ding K, Zhou Z, Zhan C-G, Zheng F. A quantitative LC-MS/MS method for simultaneous determination of cocaine and its metabolites in whole blood. J. Pharm. Biomed. Anal. 2017, 134:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology 2005, 49:750–758. [DOI] [PubMed] [Google Scholar]
- 42.Wiley JL, Evans RL, Grainger DB, Nicholson KL. Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug. Pharmacol. Reports 2011, 63:1085–1092. [DOI] [PubMed] [Google Scholar]
- 43.Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology 1993, 58:16–22. [DOI] [PubMed] [Google Scholar]
- 44.Rivest R, Falardeau P, Di Paolo T. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res 1995, 692:269–272. [DOI] [PubMed] [Google Scholar]
- 45.Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res 2017, 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siciliano CA, Mauterer MI, Fordahl SC, Jones SR. Modulation of striatal dopamine dynamics by cocaine self-administration and amphetamine treatment in female rats. Eur J Neurosci 2019, 50:2740–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Argyelan M, Szabo Z, Kanyo B, Tanacs A, Kovacs Z, Janka Z, Pavics L. Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord 2005, 89:115–123. [DOI] [PubMed] [Google Scholar]
- 48.Hsiao MC, Lin KJ, Liu CY, Schatz DB. The interaction between dopamine transporter function, gender differences, and possible laterality in depression. Psychiatry Res 2013, 211:72–77. [DOI] [PubMed] [Google Scholar]


