Abstract
The recombinant product (rK39) of the 39-amino-acid repeats encoded by a kinesin-like protein-encoding gene of Leishmania chagasi was evaluated by enzyme-linked immunosorbent assay (ELISA) for diagnostic potential and the ability to predict the response to therapy in Indian kala-azar or visceral leishmaniasis (VL); we also compared its performance with that of crude soluble antigen (CSA). At the diagnosis of VL, the anti-rK39 antibody titer was 59-fold higher than the anti-CSA antibody titer. With successful therapy, antibody titers declined steeply at the end of treatment and during follow-up. In contrast, patients who relapsed showed increased titers of antibodies to rK39. The extremely high levels of anti-rK39 antibodies in VL cases suggest the application of rK39 for sensitive and specific serodiagnosis, and rK39 ELISA is also valuable in monitoring drug therapy and detecting relapse of the disease.
Indian kala-azar or visceral leishmaniasis (VL) is a potentially fatal disease caused by Leishmania donovani. It is endemic in eastern parts of India and often turns epidemic (22, 29). Definitive diagnosis of this disease continues to require demonstration of parasites in splenic or bone marrow aspirates through invasive procedures. Recent efforts to improve the diagnosis of Indian VL and post-kala-azar dermal leishmaniasis (PKDL) have been made by detecting anti-parasite antibodies (9, 11) via indirect hemagglutination (22, 29), indirect immunofluorescence (6), direct agglutination (26), latex agglutination (8), and enzyme-linked immunosorbent assay (ELISA) (5, 10).
Recently, a kinesin-related protein-encoding gene has been discovered in Leishmania chagasi that contains a repetitive 117-bp sequence encoding 39 amino acid residues (K39) conserved at the C-terminal end in all of the VL-causing isolates examined so far (4). The recombinant product of K39 (rK39) has proven to be a very sensitive and specific antigen in an ELISA for the serodiagnosis of VL from the endemic foci in Brazil, China, Pakistan, and Sudan (4, 18). In the present study, we evaluated the ability of titers of antibodies against rK39 to diagnose active disease and predict either a successful response to therapy or a relapse of the disease and compared its performance with that of crude soluble lysate of L. donovani promastigotes. The crude soluble antigens (CSA) used in this study were largely L. donovani whole promastigotes or their soluble lysates.
MATERIALS AND METHODS
Patients.
The Ethical Committee of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, approved this study. The first study group consisted of sera from 43 patients with parasitologically proven VL that were tested by ELISA using the rK39 antigen (kind gift of Steven G. Reed, Corixa Corporation, Seattle, Wash.), as well as by crude soluble lysate. The second study group consisted of 17 L. donovani-infected patients who were under going therapy. Titers of antibodies to rK39 and crude soluble lysate were determined for these patients at sequential time points, i.e., at diagnosis (D0), at the end of treatment (D30), and at the 6-month (6M) follow-up after therapy. The third study group comprised nine patients who relapsed after an initial successful treatment for VL and in whose splenic aspirates parasites were detected. These patients were evaluated serologically for titers of antibody against rK39 to determine the ability of this marker to predict relapse. Sixteen normal, healthy individuals living in areas where the disease is endemic and sera collected from patients with tuberculosis (n = 10), malaria (n = 4), or leprosy (n = 8) were also studied.
CSA.
CSA was prepared in accordance with a method described elsewhere (7). Briefly, antigen was prepared by six cycles of freezing (−70°C) and thawing (37°C) of a suspension of 2 × 108 parasites/ml in phosphate-buffered saline (PBS; pH 7.4). The extract was then centrifuged at 20,000 × g for 15 min. The supernatant was collected and stored in aliquots at −20°C. The protein content of the antigen preparation (CSA) was estimated by the method of Lowry et al. (15). The first and second extractions of promastigote antigen yielded 9.4 and 3.3 mg of protein per ml, respectively.
rK39 antigen (4).
A genomic library was constructed with sheared DNA of L. chagasi (MHOM/BR/82/BA-2, C1) in Lambda ZAP11 (Stratagene) and screened with preadsorbed serum (21) of an L. donovani patient. rK39 was purified from a 25 to 40% ammonium sulfate fraction of bacterial lysate by preparative isoelectric focusing with a Roto cell for isoelectric focusing and 10% 3/10 ampholytes (pH range, 3.5 to 9.5) in the presence of 8 M urea and 10 mM dithiothreitol. Peak fractions were concentrated by ammonium sulfate precipitation and dialyzed against 25 mM Tris-HCl (pH 8)–150 mM NaCl. Protein concentrations were determined by using the Pierce bicinchoninic acid assay, and purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining (13).
ELISA.
Ninety-six-well microtiter plates were coated with 25 ng of rK39 or 5 μg of CSA protein (crude soluble fraction of promastogotes isolated from an Indian patient with VL [HOM/IN/96/70]) per well overnight at 4°C. Plates were then aspirated, blocked with PBS containing 1 or 5% (wt/vol) bovine serum albumin (BSA) for 2 h at room temperature, and then washed six times with PBS containing 0.1% Tween 20 (PBS-T). Sera were serially diluted in PBS containing 0.1% BSA (1% for CSA), 0.1% Tween 20 was added to the wells, and the sera were incubated for 30 min at room temperature for rK39 or for 1 h at 37°C for CSA. The wells were then washed six times with PBS-T and incubated for 30 min with protein A-horseradish peroxidase (1/2,000 dilution; Bangalore Genei) in PBS containing 0.1% BSA and 0.1% BSA and 1.1% Tween 20 and 1 h at 37°C for CSA (goat anti-human immunoglobulin G conjugated with horseradish peroxidase, 1/5,000 dilution). Plates were then washed six times in PBS-T and incubated with tetramethylbenzidine or ortho-phenylenediamine substrate for a further 15 and 30 min, respectively. The reaction was stopped by the addition of 1 N sulfuric acid, and the optical density at 450 or 492 nm was determined. The cutoff points for rK39 and crude soluble lysate were determined from the serum dilution with optical densities of 0.102 and 0.346, respectively, calculated as the mean of the negative controls plus 3 standard deviations. Each serum sample was assayed in triplicate, and negative and positive serum samples were used as standards.
Statistical analysis.
The statistical significance of the results of the study was determined by Student's t test, and a P value of 0.05 was considered significant.
RESULTS
At the diagnosis of VL, the anti-rK39 antibody, titer was 59 times as high as the anti-CSA titer. The mean titers of antibodies against rK39 and CSA were 4.9 × 105 and 8.2 × 103, and the titers ranged from 1.6 × 104 to 1.0 × 106 and from 5.1 × 102 to 6.6 × 104, respectively.
In the second group, antibody titers were estimated in the serial serum samples to determine whether serum antibody titers against rK39 declined during successful drug treatment and compared antibody with titers against CSA. All of the patients in this study, including those who relapsed, had a negative splenic aspirate at 30 days of treatment. These patients were monitored clinically for at least 6 months before a definite cure was declared; all of the patients were cured at 6 months. Anti-rK39 and CSA antibody levels of 17 paired pre- and posttreatment and 6-month follow-up sera of VL patients were evaluated. There was a 2.7-fold decline in the titers of antibodies against both CSA and rK39 posttreatment, but at 6 months follow-up, a further decline of 5.36-fold (14 times the pretreatment level) in the anti-rK39 antibody titer was observed. However, the anti-CSA antibody titer showed a 0.85-fold increase compared to the posttreatment titer. Sixteen (94%) of 17 and 8 (47%) of 17 patients showed a decrease in anti-rK39 and anti-CSA antibody titers posttreatment and at follow-up (Table 1).
TABLE 1.
Titers of antibodies against CSA and rK39 antigen in VL patients before treatment, posttreatment, and at 6-month follow-up
| Time | Mean antibody titer ± SEM
|
|
|---|---|---|
| CSA | rK39 | |
| D0 | 9,970 ± 3,764 | 265,035 ± 6,043 |
| D30 | 3,674 ± 1,863b | 98,785 ± 39,621b |
| 6M | 4,276 ± 2,039cd | 18,432 ± 7,906cd |
Seventeen samples were tested at each time point.
D0 versus D30, P < 0.001.
D0 versus 6M, P < 0.001.
D30 versus 6M, P < 0.05.
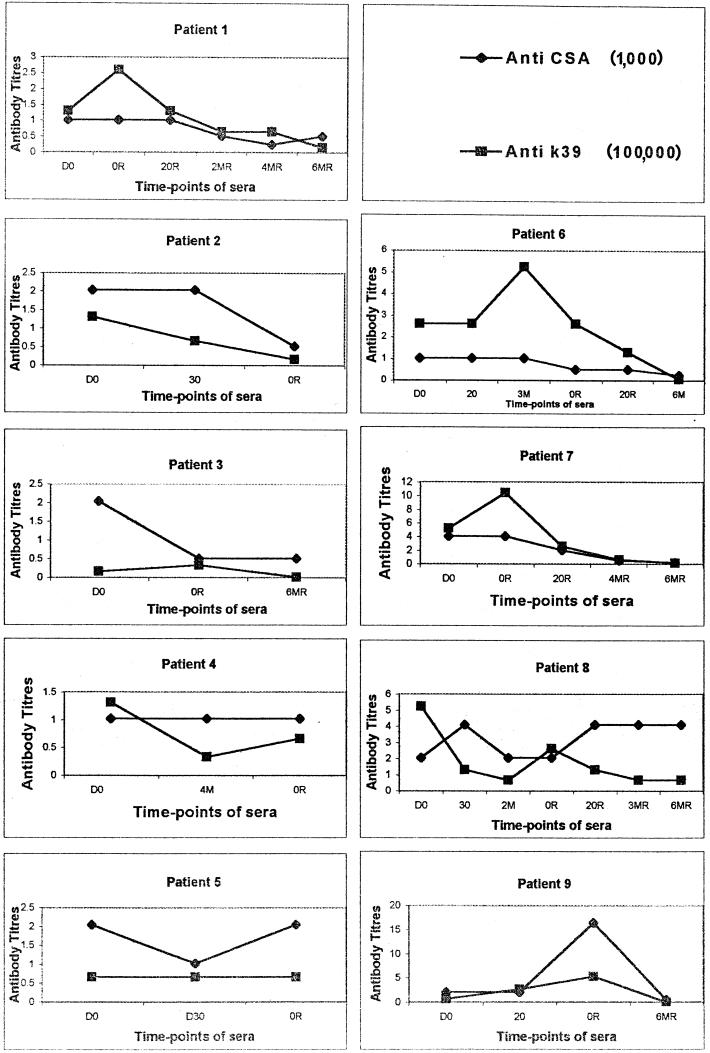
The kinetics of anti-rK39 and CSA antibody titers in patients who demonstrated a clinical relapse after an initial cure revealed interesting results. Patients 5 and 6, on relapse, showed titers similar to those observed at the baseline. However, in patients 1, 3, 7, and 9, the titers observed on relapse were higher than the baseline values, whereas in patients 2, 4, and 8, the anti-rK39 antibody titers on relapse were lower than the baseline titers. At relapse, however, such clear trends in the deviation of titers were not seen when CSA was used (Fig. 1).
FIG. 1.
Titers of anti-CSA and anti-rK39 antibodies of VL patients (n = 9) at D0 (at diagnosis), 20 (day 20 of treatment), 3M (3 months posttreatment), 4M (4 months posttreatment), 0R (at relapse), 20R (day 20 of treatment after relapse), 3MR (3 months posttreatment after relapse), 4MR (4 months posttreatment after relapse), and 6MR (6 months posttreatment after relapse). The anti-CSA and anti-rK39 antibody titers were individually determined by ELISA as described in Materials and Methods.
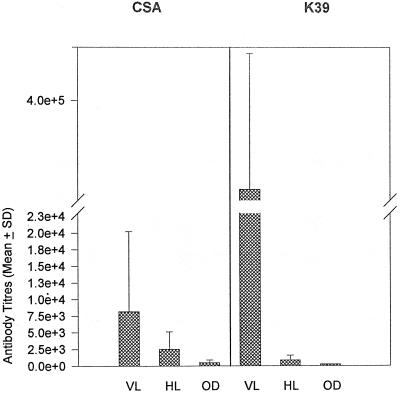
In 16 healthy controls drawn from areas where the disease is endemic, the mean titers of antibodies against rK39 and CSA were 2,470 ± 506 and 6,511 ± 5,671, respectively. All of these controls had anti-rK39 antibody titers of less than 2,048, except one control who was positive at a 1:131,072 dilution, and titers of antibody against CSA ranged upto an 8,192-fold dilution. Similarly, we took sera from 22 patients with other diseases, like leprosy, tuberculosis, and malaria, and in all of these sera the anti-rK39 titers were ≤256, whereas the anti-CSA antibody titers varied between 256 and 2,048 (Fig. 2).
FIG. 2.
Titers of anti-CSA and anti-rK39 antibodies in patients with symptomatic Indian VL, healthy controls (HL), and those with other infectious diseases (OD), like tuberculosis, leprosy, and malaria. The anti-CSA and anti-rK39 antibody titers were individually determined by ELISA as described in Materials and Methods. Results are reported as mean antibody titers ± the standard deviation.
DISCUSSION
All patients with active VL showed very high titers of antibodies against the rK39 antigen, with the lowest titer seen at a 1:16,000 serum dilution. The anti-rK39 antibody titers were 74-fold higher than those observed in the sera of the controls tested. In most of these controls, the anti-rK39 antibody titers were <2,048, except in one whose serum was positive at a 1:131,072 dilution. That individual showed no overt symptoms of active VL at the time of blood collection. This may be due to a subclinical VL infection, as no clinical or laboratory markers have been described to identify the disease before it manifests itself clinically. Subclinical VL with few or no symptoms and positive antileishmanial serology or documented seroconversion (1, 2), in which organ aspirate direct smears are often negative for parasites (12, 16), is common in several areas where the disease is endemic (1, 2, 12, 16) and may occur in India too (19, 23). At least some false-positive responses might be expected among patients with subclinical infections, especially those who do not eventually self-cure (2). However, the frequency of subclinical infection in India has not been clearly established. We believe that the presence of such a high titer in only 1 the 22 controls tested is best explained by the high specificity of the anti-rK39 antibody for active VL (2, 4, 18, 25).
With the CSA ELISA, considerable overlap in antibody titers existed between the controls and patients with active VL, and use of CSA for diagnosis, especially in an area where the disease is endemic, can pose difficulties. rK39, however, can be safely used with confidence because an overlap in titers is rare, as rK39 is highly specific. Other diseases, like tuberculosis, leprosy, and malaria, that are prevalent in areas where VL is endemic and are known to cross-react with leishmanial antigen pose diagnostic difficulties when the parasitic antigen is used (25, 27). Nonspecific cross-reactivity might have contributed to the higher anti-CSA antibody titers seen in the healthy controls tested. With rK39, no such cross-reactivity was seen and antibody titers in sera from patients with these disorders were ≤256 and there was no overlap, in contrast to the findings observed when CSA was used.
The kinetics of anti-rK39 and -CSA antibody titers in patients who relapsed after an initial cure revealed interesting results. In these patients, the titers of antibodies against both rK39 and CSA fell immediately after treatment to 37% of the baseline values. However, an antibody rise to levels higher than the initial pretreatment levels was seen in these patients when they presented again with active disease. Once a second course of treatment was successfully instituted, the titers fell along predictable lines (Fig. 2). This rise in titers indicated a relapse and could be used as a marker for an incomplete cure or a relapse. Under these conditions the rK39 and CSA antigens performed equally.
At the 6-month follow-up, a sharp decline of 14-fold was seen with rK39, and this could be taken as a characteristic of a definitive cure, in contrast to a relapse. With CSA, however, the fall was only 2.3-fold at 6 months. Anti-CSA antibody titers at 6 months remained high in several patients, making it impossible to use them as a marker for a cure.
Since the findings indicate a decline in anti-rK39 antibody titers at the end of therapy with a steep decline at 6 months, it may become possible to distinguish between those who achieve a permanent cure as opposed to those who are either refractory or relapse after an initial apparent cure. There are no predictors of a successful response to therapy, except parasitological methods, which have limited applications.
PKDL is an important sequel seen in 10 to 20% of kala-azar patients (20). The majority of patients with PKDL have a history of kala-azar with or without treatment (17), and PKDL appears to be a result of kala-azar (24). A close relationship between VL and PKDL is suggested by several studies. There are antigenic similarities between the strains of L. donovani causing VL and PKDL, suggesting that the parasite causing the original visceral infection was also responsible for PKDL (3). Isozyme characterization of isolates from Indian cases of PKDL has been shown to be indistinguishable from those of Indian kala-azar (zymodeme LON-41) (14). Recently, a study by Salotra et al. (20) revealed that the humoral immune response of PKDL patients is quite distinct from that of kala-azar patients.
Thus, the rK39 antigen has great utility in the unambiguous diagnosis of VL and is much superior to crude parasite antigen. Several rapid immunochromatographic formats are under evaluation (28), and if the high degree of sensitivity and specificity demonstrated in our experiments could be transferred to rapid testing formats, invasive and risky procedures like spleen or bone marrow aspiration could be eliminated for the majority of patients presenting for the first time with VL. The sharp decline in titers correlated well with a definite cure and can be applied in clinics, whereas in relapsing patients a rise after an initial fall was a good indicator. Although it is difficult, if semiquantitative strips could be manufactured, an attempt could be made to predict cure and relapse.
ACKNOWLEDGMENTS
We thank S. G. Reed, Corixa Corporation, Seattle, Wash., for providing rK39 antigen.
The senior research fellowship awarded to R. Kumar by the University Grants Commission, New Delhi, India, is thankfully acknowledged.
REFERENCES
- 1.Badaro R, Jones T C, Carvalho E M, Sampiao D, Reed S G, Barral A, Teixeira R, Johnson W D., Jr New perspectives on a sublinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 2.Badaro R, Benson D, Eulalio M C, Friere M, Cunha S, Netto E M, Pedral-Sampiao D, Madureira C, Burns J M, Hoghton R L, David J R, Reed S G. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 1996;173:758–761. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 3.Bray R S, Ashford R W, Mukherjee A M, Sengupta P C. Studies on the immunology and serology of leishmaniasis. IX. Serological investigation of the parasites of Indian kala-azar and Indian post-kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1973;67:125–129. doi: 10.1016/0035-9203(73)90330-1. [DOI] [PubMed] [Google Scholar]
- 4.Burns J M, Shreffler W G, Benson D R, Ghalib H W, Badaro R, Reed S G. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhry A, Guru P Y, Saxena R P, Tandon A, Saxena K C. Enzyme-linked immunosorbent assay in the diagnosis of kala-azar in Bhadohi (Varanasi), India. Trans R Soc Trop Med Hyg. 1990;84:363–366. doi: 10.1016/0035-9203(90)90319-a. [DOI] [PubMed] [Google Scholar]
- 6.Choudhry A, Puri A, Guru P Y, Saxena R P, Saxena K C. An indirect fluorescent antibody (IFA) test for the serodiagnosis of kala-azar. J Commun Dis. 1992;24:32–36. [PubMed] [Google Scholar]
- 7.Cillari E, Milano S, Dieli M, Maltese E, DiRosa S, Mansueto S, Salerno A, Liew F Y. Reduction in the number of UCHL-1+ cells and IL-2 production in the peripheral blood of patients with visceral leishmaniasis. J Immunol. 1991;146:1026–1030. [PubMed] [Google Scholar]
- 8.Cummins A J, Moody A H, Lalloo K, Chiodini P L. Rapid latex agglutination test for extraluminal amoebiasis. J Clin Pathol. 1994;47:647–648. doi: 10.1136/jcp.47.7.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghose A C, Haldar J P, Pal S C, Mishra B P, Mishra K K. Serological investigations on Indian kala-azar. Clin Exp Immunol. 1980;40:318–325. [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Srivastava J K, Ray S, Chandra R, Srivastava V K, Katiyar J C. Evaluation of enzyme-linked immunosorbent assay in the diagnosis of kala-azar in Malda district (West Bengal) Indian J Med Res. 1993;97:242–246. [PubMed] [Google Scholar]
- 11.Haldar J P, Ghose S, Saha K C, Ghose A C. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun. 1983;42:702–707. doi: 10.1128/iai.42.2.702-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho M, Siongok T K, Lyerly W, Smith D. Prevalence and disease spectrum in a new focus of visceral leishmaniasis in Kenya. Trans Med Hyg. 1982;76:741–746. doi: 10.1016/0035-9203(82)90095-5. [DOI] [PubMed] [Google Scholar]
- 13.Laemmeli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–885. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.LeBlancq S M, Peters W. Leishmania in the Old world. 4. The distribution of L. donovani sensu lato zymodemes. Trans R Soc Trop Med Hyg. 1986;80:367–377. doi: 10.1016/0035-9203(86)90320-2. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;139:265–275. [PubMed] [Google Scholar]
- 16.Marty P, Lelievre A, Quaranta J F, Rahal A, Toussaint M, Le-Fichoux Y. Use of leishmania skin test and Western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France) Trans R Soc Trop Med Hyg. 1994;88:658–659. doi: 10.1016/0035-9203(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 17.Napier E L, Das Gupta D R. A clinical study of post-kala-azar dermal leishmaniasis. Indian J Med Res. 1930;65:249–259. [PMC free article] [PubMed] [Google Scholar]
- 18.Qu J Q, Zhong L, Masoom-Yasinzai M, Abdur-Rab M, Aksu H S, Reed S G, Chang K P, Gilman-Sachs A. Serodiagnosis of Asian leishmaniasis with recombinant antigen from the repetitive domain of a leishmania kinesin. Trans R Soc Trop Med Hyg. 1994;88:543–545. doi: 10.1016/0035-9203(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 19.Sacks D L, Lal S L, Shrivastava S N, Blackwell J, Neva F A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987;138:908–913. [PubMed] [Google Scholar]
- 20.Salotra P, Raina A, Ramesh V. Western blot analysis of humoral immune response to Leishmania donovani antigens in patients with post-kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1999;93:98–101. doi: 10.1016/s0035-9203(99)90197-9. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Sanyal R K. Leishmaniasis in the Indian subcontinent. In: Chang K P, Bray R S, editors. Leishmaniasis. Amsterdam, The Netherlands: Elsevier Science Publishers; 1985. pp. 443–467. [Google Scholar]
- 23.Saran R, Gupta A K, Sharma M C. Evidence of Leishmania donovani infection in household members residing with visceral leishmaniasis patients. J Commun Dis. 1992;24:242–244. [PubMed] [Google Scholar]
- 24.Shortt H E, D'Silva H A H, Swaminath C S. Note on dermal leishmanoid. Indian J Med Res. 1928;16:239–240. [Google Scholar]
- 25.Singh S, Sacks A G, Chang K P, Reed S G. Diagnostic and prognostic value of K39 recombinant antigen in Indian leishmaniasis. J Parasitol. 1995;81:1000–1003. [PubMed] [Google Scholar]
- 26.Singla N, Singh G S, Sundar S, Vinayak V K. Evaluation of the direct agglutination test as an immunodiagnostic tool for kala-azar in India. Trans R Soc Trop Med Hyg. 1993;87:276–278. doi: 10.1016/0035-9203(93)90125-a. [DOI] [PubMed] [Google Scholar]
- 27.Smrkovski L L, Larson L L. Antigenic cross reactivity between Mycobacterium bovis (BCG) and Leishmania donovani. Infect Immun. 1997;18:561–562. doi: 10.1128/iai.18.2.561-562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundar S, Reed S G, Singh V P, Kumar P C, Murray H W. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet. 1998;351:563–565. doi: 10.1016/S0140-6736(97)04350-X. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Leishmaniasis. WHO Tech Rep Ser. 1984;701:99–108. [PubMed] [Google Scholar]