Abstract
BALB/c mice were intravenously injected with lipopolysaccharide (LPS) (0.05 μg/g of body weight) 7 days after being primed with zymosan. Recombinant human lactoferrin (250 μg/g of body weight), intravenously administered 1 day before the injection of LPS, significantly lessened the severity of hepatitis, as assessed by levels of serum alanine transaminase compared to those seen when casein was administered. The transient rise of serum tumor necrosis factor alpha (TNF-α) after LPS treatment was also significantly lowered by the intravenous administration of lactoferrin, suggesting that the effect of lactoferrin was due to the suppression of TNF-α production. The following results indicate that the sites of action of lactoferrin for the suppression of the development of this type of hepatitis are Kupffer cells. Gadolinium chloride, a substance known to eliminate Kupffer cells, administered 1 day before LPS, inhibited the transient rise of TNF-α and protected against the development of hepatitis. Kupffer cells isolated from mice intraperitoneally injected with recombinant human lactoferrin became refractory to LPS. The specific interaction of recombinant human lactoferrin with the Kupffer cells was shown by a binding assay, which revealed two types of binding sites on mouse Kupffer cells. Of the two dissociation constants determined in this way, the lower dissociation constant, 0.47 × 10−6 M, was within the range of the 50% effective doses for the suppression of TNF-α production. These results suggest that recombinant human lactoferrin administered to mice suppresses the production of TNF-α by Kupffer cells by directly associating with the binding sites on these cells.
Lactoferrin had long been recognized as a polypeptide capable of chelating ferrous ions, which was thought to be one of the mechanisms by which it blocks the growth of microorganisms (14). However, it has also been demonstrated that the polypeptide itself has bacteriostatic activity (27). Recently, it has been shown that lactoferrin can also function as an anti-inflammatory factor in that it regulates the production of inflammatory cytokines in a manner similar to those of other anti-inflammatory cytokines. Lactoferrin contained in secretory vesicles of neutrophils is released at sites of inflammation to suppress the production of inflammatory cytokines, while factors that promote inflammation are also released from neutrophils (4). Lactoferrin released at sites of inflammation interacts with mononuclear cells (16) and suppresses the production of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) (6). Lactoferrin is also found in most secreted fluids such as saliva, tears, cervical mucus, and amniotic fluid (5). In the organs or tissues where these fluids are secreted, lactoferrin may suppress inflammatory reactions in addition to blocking the growth of microorganisms. On the other hand, it has also been shown that lactoferrin enhances the proliferation and differentiation of colon epithelial cells (Caco-2) when added to cultures (20). Therefore, lactoferrin in milk taken by neonates and infants may suppress inflammatory reactions with a variety of causes and promote proliferation of some types of cells, such as those of the intestines. This effect is probably not limited to neonates and infants, since lactoferrin is also found in neutrophils and the secretory fluids of adults. Therefore, lactoferrin functions as an immunomodulator not only in neonates and infants but also in adults.
To assess the function of lactoferrin as an anti-inflammatory factor, we examined its efficacy in protecting against the development in mice of hepatitis induced by the administration of zymosan and lipopolysaccharide (LPS). Zymosan, a complex of yeast cell wall components, was used to prime hepatic macrophages (Kupffer cells) and to render them sensitive to a small dose of LPS (7). When a small dose of LPS is administered to zymosan-primed mice, it induces a burst of production of inflammatory cytokines, including TNF-α, eventually causing a hepatic lesion (3). Lactoferrin added properly before LPS will suppress inflammatory cytokine production and thereby prevent the development of LPS-induced hepatitis. Here we show that intravenously administered recombinant human lactoferrin protects against the development of zymosan- and LPS-induced hepatitis by interacting with Kupffer cells.
MATERIALS AND METHODS
Seven-week-old male BALB/c mice (Charles River Japan, Yokohama, Japan; n = 5) were intravenously injected with zymosan (50 μg/g of body weight; Sigma, St. Louis, Mo.). After an interval of 7 days, the mice were intravenously injected with LPS (0.05 μg/g of body weight; List Biological Laboratories Inc., Campbell, Calif.). Recombinant human lactoferrin (Agennix, Houston, Tex.) was intravenously administered 24 h before the administration of LPS. Gadolinium chloride (10 μg/g of body weight) was intraperitoneally administered in place of lactoferrin 24 h before the administration of LPS. Sixty minutes after LPS injection, blood was withdrawn to obtain serum for the measurement of serum TNF-α. Twenty-four hours after the LPS injection, blood was withdrawn to obtain serum for measurement of serum alanine transaminase (ALT) activity.
ALT activity was measured using a colorimetric diagnostic kit (Wako Pure Chemical Industries Ltd., Osaka, Japan). Serum TNF-α was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Amersham Pharmacia, Tokyo, Japan).
Mice were intraperitoneally administered recombinant human lactoferrin 18 h prior to the isolation of Kupffer cells. Kupffer cells were isolated from the mice by a collagenase perfusion method and cultured as described below. The cells from the four mice administered identical protein samples were combined and spread on 24-well culture plates at a density of 105 cells/well. Various doses of LPS were added 4 h after the start of culture. The culture supernatants were collected 4 h later and assayed for TNF-α by an ELISA method. The results were normalized to the values for mice given 0 ng of LPS/ml.
Mouse Kupffer cells were isolated from the collagenase-perfused livers of the mice by the conventional method using differential centrifugation (10). Isolated Kupffer cells were cultured in RPMI 1640 supplemented with 5% fetal calf serum in 24-well culture plates at a density of 105 cells/well. One hour after the start of culture, recombinant human lactoferrin was added to the cultures at various doses. Eighteen hours later, the cells were washed with Hanks' balanced salt solution and then medium without serum was added to the cultures. LPS (1 ng/ml) was added to the cultures to induce the cells to produce TNF-α. The levels of TNF-α in the culture supernatants were estimated using an ELISA kit.
RAW 264 cells were cultured in RPMI 1640 medium supplemented with 10% serum in 24-well culture plates at a density of 105 cells/well. The efficacy of suppression of TNF-α production by a recombinant human lactoferrin was assessed by a protocol similar to that described above for the Kupffer cells.
The binding of recombinant human lactoferrin to the mouse Kupffer cells was assessed using 125I-labeled lactoferrin. Recombinant human lactoferrin was labeled using Iodo beads (Pierce, Rockford, Ill.). Recombinant human lactoferrin (100 μg) and 125I (1 mCi) were dissolved in 0.5 ml of phosphate-buffered saline (PBS). To the solution was then added two Iodo beads to start the reaction. The reaction was terminated after incubation at room temperature for 20 min by removing the beads. Unreacted 125I was separated from the lactoferrin using a prepacked gel filtration column, PD10 (Amersham Pharmacia), which had been equilibrated with 0.1% PBS. The specific radioactivity of the 125I-labeled lactoferrin was 107 cpm/μg of protein.
125I-labeled lactoferrin (1.5 × 105 cpm/μg of protein) in 0.1% bovine serum albumin was added to 0.4 ml of RAW 264 culture (1 × 105 cells/well). The cells were incubated at 4°C for 1 h and then washed with cold PBS. The nonspecific binding of lactoferrin was assessed by estimating the binding of 125I-labeled lactoferrin in a solution with an excess amount of lactoferrin (100 mg). The radioactivity, bound to the cells, was measured using cell lysates obtained by treatment with 0.5 M KOH.
Results are expressed as means ± standard deviations. Fisher's PSLD test (parametric) and Mann-Whitney's U test (nonparametric) were performed to compare means (ALT and TNF-α) in different groups, and P values less than 0.05 were considered to be statistically significant.
RESULTS
The doses of zymosan and LPS capable of promoting hepatitis were tested prior to the experiments to analyze the protective effect of lactoferrin. Doses of zymosan ranging from 5 to 250 μg/g of body weight and of LPS ranging from 0.005 to 0.5 μg/g of body weight were tested. Among these doses, the combination of 50 μg of zymosan per g of body weight and 0.05 μg/g of LPS per g of body weight was found to be appropriate to induce experimental hepatitis in BALB/c mice (data not shown). At the sites of the lesions caused by zymosan and LPS, deformation of hepatocytes and accumulation of nonparenchymal cells were observed. The morphology of the hepatic sinusoidal cells indicated that these cells were mainly macrophages. In the areas surrounding the lesions, neutrophils infiltrating the sinusoidal endothelial cell layer were observed. These results indicated that the foci of hepatic inflammation involving the death of hepatocytes and the accumulation of inflammatory cells were caused by these doses of zymosan and LPS.
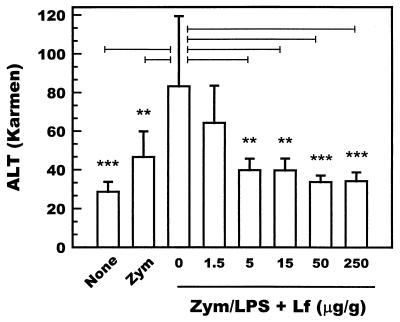
Using these doses of zymosan and LPS, the protective effect of lactoferrin was tested at doses ranging from 1.5 to 250 μg/g of body weight (n = 5). Recombinant human lactoferrin administered intravenously protected against the development of hepatitis at doses higher than 5 μg/g of body weight as assessed by the levels of serum ALT (Fig. 1). These results indicate that intravenously administered recombinant human lactoferrin suppresses the development of hepatitis.
FIG. 1.
Levels of ALT activity in the sera of zymosan- and LPS-treated mice (n = 5). Mice were intravenously administered zymosan (50 μg/g of body weight), followed by an intravenous administration of LPS (0.05 μg/g of body weight) after an interval of 7 days. None, recombinant human lactoferrin (1.5 to 250 μg/g of body weight) was intravenously administered 24 h before the administration of LPS. Serum samples were prepared from the blood collected 24 h after the administration of LPS. Bars represent standard deviations. Bars marked with ∗∗ and ∗∗∗ indicate results that are significantly different from those for the 0-μg/ml lactoferrin group at a P of <0.01 and <0.001, respectively. Zym, zymosan alone; Zym/LPS, zymosan and LPS; Lf, lactoferrin.
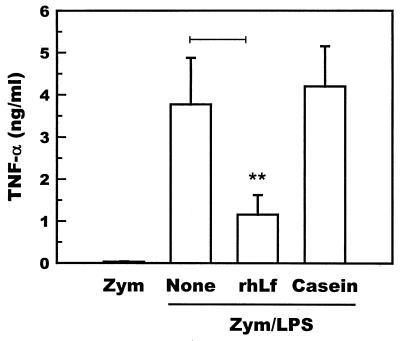
To determine the involvement of TNF-α in the development of hepatitis, the levels of transient TNF-α were measured. The levels of TNF-α in the sera collected 60 min after the administration of LPS were significantly increased compared to those in the sera of mice that were not challenged with LPS (n = 5). The increase in serum TNF-α was significantly suppressed when the mice were administered recombinant human lactoferrin 1 day before the administration of LPS (Fig. 2), indicating that lactoferrin suppresses the transient increase in TNF-α caused by LPS in zymosan-primed mice.
FIG. 2.
Levels of TNF-α in the sera of zymosan-primed mice (n = 5) after an LPS challenge. None, recombinant human lactoferrin (0.25 mg/g of body weight) or bovine casein (0.25 mg/g of body weight) was intravenously administered 24 h before LPS. The sera were prepared from the blood samples collected 60 min after LPS. Bars represent standard deviations. The bar marked with ∗∗ indicates results that are significantly different from those of the None group at a P of <0.01. Zym; zymosan alone; Zym/LPS, zymosan and LPS; rhLf, recombinant human lactoferrin.
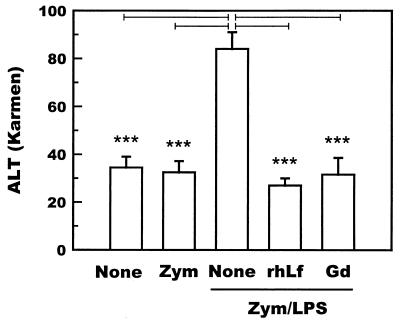
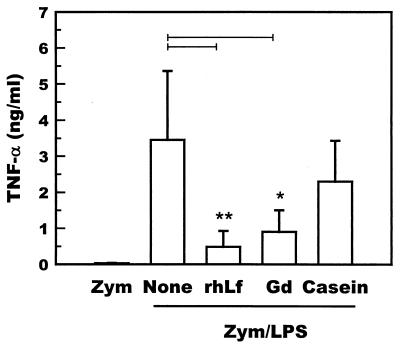
Gadolinium chloride was administered to zymosan-primed mice (n = 5) to examine the involvement of hepatic macrophages in the development of hepatitis. Gadolinium chloride administered to zymosan-primed mice 1 day before the administration of LPS significantly suppressed the LPS-induced increase in the serum ALT level (Fig. 3). The levels of TNF-α 60 min after LPS injection were also significantly reduced by the administration of gadolinium chloride (Fig. 4). These results indicate that hepatic macrophages are responsible for the development of hepatic lesions in the zymosan-LPS hepatitis model.
FIG. 3.
Suppression of the levels of ALT activity in the sera of zymosan- and LPS-treated mice (n = 5) by intraperitoneal administration of gadolinium chloride. Mice were intravenously administered zymosan (50 μg/g of body weight), followed by intravenous administration of LPS (0.05 μg/g of body weight) after an interval of 7 days. None, recombinant human lactoferrin (0.25 mg/g of body weight) or gadolinium chloride (10 μg/g of body weight) was intraperitoneally administered 24 h before LPS. Serum samples were prepared from the blood collected 24 h after the administration of LPS. Bars represent standard deviations. Bars marked with ∗∗∗ indicate results that are significantly different from those of the None group at a P of <0.001. Zym, zymosan alone; Zym/LPS, zymosan and LPS; Gd, gadolinium chloride; rhLf, recombinant human lactoferrin.
FIG. 4.
Suppression of the levels of TNF-α in the sera of zymosan- and LPS-treated mice (n = 5) by intraperitoneal administration of gadolinium chloride. Mice were intravenously administered zymosan (50 μg/g of body weight), followed by intravenous administration of LPS (0.05 μg/g of body weight) after an interval of 7 days. None, recombinant human lactoferrin (0.25 mg/g of body weight) or gadolinium chloride (10 μg/g of body weight) was intraperitoneally administered 24 h before LPS. Serum samples were prepared from the blood collected 60 min after LPS. Bars represent standard deviations. Bars marked with ∗ and ∗∗ indicate results that are significantly different from those for the None group at a P of <0.05 and <0.01, respectively. Zym, zymosan alone; Zym/LPS, zymosan and LPS; Gd, gadolinium chloride; rhLf, recombinant human lactoferrin.
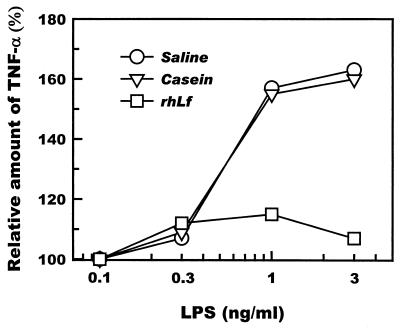
To investigate the effects of recombinant human lactoferrin administered to mice on Kupffer cells, mice were intraperitoneally administered recombinant human lactoferrin (250 μg/g of body weight) 18 h prior to the isolation of Kupffer cells. Kupffer cells isolated from these mice showed a significantly diminished ability to respond to LPS as assessed by their production of TNF-α (Fig. 5). No such effect was seen when Kupffer cells isolated from mice intraperitoneally administered saline or casein were challenged with LPS.
FIG. 5.
Suppression of TNF-α production by Kupffer cells isolated from mice that were intraperitoneally administered recombinant human lactoferrin (rhLf) (250 μg/g of body weight) 18 h prior to cell isolation. Kupffer cells were challenged with various doses of LPS 4 h after the start of culture. The culture supernatants were collected and assayed for TNF-α by an ELISA method. Saline and casein were similarly administered as controls. The results from the pooled cells of four animals were normalized to the values for mice given 0 ng of LPS per ml.
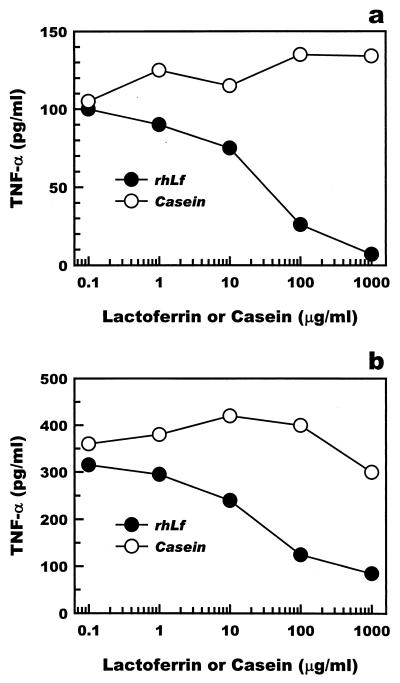
Recombinant human lactoferrin was added to primary cultures of Kupffer cells or to cultures of the mouse monocyte cell line RAW 264 to assess the efficacy of lactoferrin in the suppression of LPS-induced TNF-α production by macrophages in vitro. Recombinant human lactoferrin was added to cultured macrophages 24 h prior to LPS administration. TNF-α production by either Kupffer cells or RAW 264 cells was suppressed by recombinant human lactoferrin at doses higher than 10 μg/ml (Fig. 6).
FIG. 6.
Suppression of TNF-α production by mouse Kupffer cells and by mouse RAW 264 monocytes. Various amounts of recombinant human lactoferrin (rhLf) were added to the cultures 18 h prior to activation by LPS (1 ng/ml). The culture supernatants were collected to measure the secreted level of TNF-α. The cells were washed with medium before LPS was administered. (a) Mouse Kupffer cells; (b) RAW 264 cells.
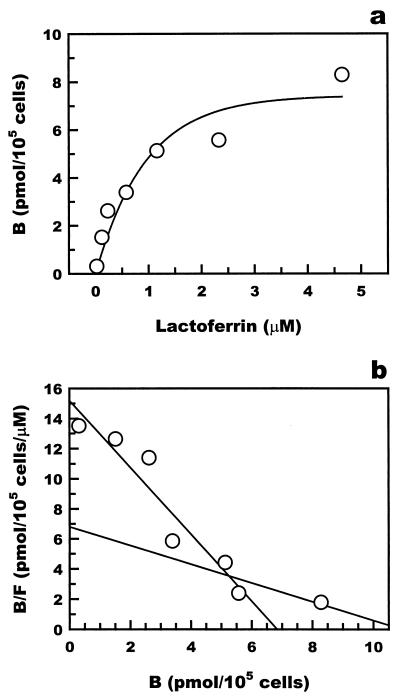
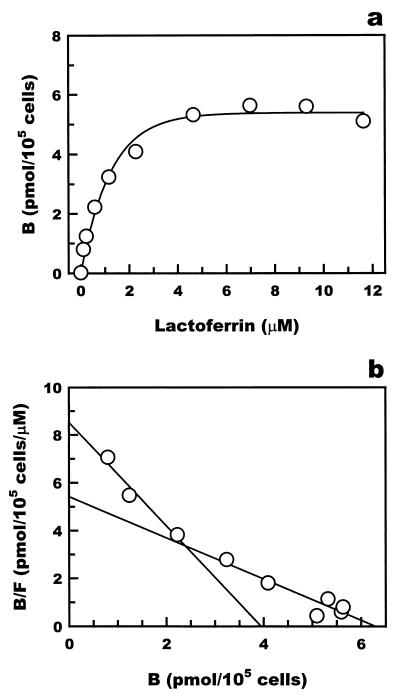
The interaction of lactoferrin with macrophages was examined by a binding assay using 125I-labeled recombinant human lactoferrin. The profiles of binding of 125I-lactoferrin to mouse Kupffer cells and RAW 264 cells are shown in Fig. 7 and 8, respectively. The Scatchard plot of the results indicated that 125I-lactoferrin bound to both Kupffer cells and RAW 264 cells with the dissociation constants 0.47 × 10−6 and 1.82 × 10−6 M for Kupffer cells and 0.46 × 10−6 and 1.48 × 10−6 M for RAW 264 cells. The lower dissociation constants, 0.47 × 10−6 M for Kupffer cells and 0.46 × 10−6 M for RAW 264 cells, were comparable to the 50% effective dose of lactoferrin for the suppression of TNF-α production, 20 μg/ml, in both types of cells.
FIG. 7.
Binding of 125I-labeled recombinant human lactoferrin to mouse Kupffer cells. (a) 125I-labeled recombinant human lactoferrin specifically bound to mouse Kupffer cells. (b) Scatchard analysis of the binding of recombinant human lactoferrin. Scatchard analysis showed two Kd values. B, lactoferrin bound; F, lactoferrin free.
FIG. 8.
Binding of 125I-labeled recombinant human lactoferrin to the mouse monocyte cell line RAW 264. (a) 125I-labeled recombinant human lactoferrin specifically bound to RAW 264 cells. (b) Scatchard analysis of the binding of recombinant human lactoferrin. Scatchard analysis showed two Kd values. B, lactoferrin bound; F, lactoferrin free.
DISCUSSION
The results of this study indicated that lactoferrin prevents the development of hepatic lesions caused by LPS. The action of lactoferrin was probably due to the suppression of the production of TNF-α by Kupffer cells, as supported by the observation that the elimination of macrophages by the administration of gadolinium chloride prevented the formation of hepatic lesions by LPS. Based on these results, we speculated that lactoferrin protects against the development of hepatitis by suppressing TNF-α production by Kupffer cells. The relationship between the mechanism of the development of hepatitis caused by LPS in zymosan-primed mice and the mechanism of the action of lactoferrin to suppress this type of hepatitis is discussed below.
Neutrophils recruited to infectious sites will secrete various substances, each of which has a role as a promoter or a suppressor of infection (4). Lactoferrin is one of the factors secreted from neutrophils and is supposed to function as a suppressor of inflammation in that it interacts with macrophages and suppresses the production of inflammatory cytokines by these cells (6). In fact, human milk lactoferrin suppresses the production of IL-6 by human mononuclear cells (THP-1) (15), or of TNF-α and IL-1 by mononuclear cells in human mixed-lymphocyte cultures (6), when added before the administration of the pathogenic promoter of cytokine production, LPS. Lactoferrin secreted at inflammatory sites might buffer inflammatory reactions and localize inflammation to a limited area.
If lactoferrin is present at the site of infection before infection by pathogens can be established, macrophages may recognize pathogens but may not produce inflammatory cytokines. This speculation was supported by the experiment reported by Machnicki et al. (13) in which lactoferrin was intravenously administered to mice before the administration of LPS and the levels of TNF-α in peripheral blood were significantly decreased. For lactoferrin to suppress LPS-induced TNF-α production, it had to be injected at least 8 h before the administration of LPS. Administration of lactoferrin prior to LPS probably induced some reactions that suppressed the production of inflammatory cytokines. The period of time before the administration of LPS that is required for lactoferrin to show its effect might be due to these reactions. Once the interaction of lactoferrin with macrophages has been established, inflammatory reactions that should follow the recognition of pathogens by macrophages may not proceed, resulting in the suppression of inflammation.
Zymosan, a complex of yeast cell wall components, has been reported to activate macrophages (7). Interaction with zymosan will activate macrophages to produce inflammatory cytokines (3). These primed macrophages will react to small doses of LPS to promote a burst of production of TNF-α (26). Therefore, the primary target of LPS seems to be primed macrophages. However, pathogens have been suggested to induce an increase in the Th1-type T-cell population, which may be responsible for LPS-induced inflammation. Heat-inactivated Propionibacterium acnes administered to mice accumulated in the liver to a higher degree than in other organs (24) and increased the Th1-cell population in the liver (28). This increase in Th1-cell population was dependent on the mouse strain. In C57BL/6 mice, P. acnes induced an increase in the Th1-cell population, while in BALB/c mice, no such increase was observed (28). It has been suggested that the production of gamma interferon, which is necessary for the differentiation of T cells to the Th1 type, is not induced (25, 28). In the experiments described here, we used the BALB/c strain to minimize the participation of Th1 cells in the formation of liver lesions. Thus, macrophages are likely to be major effector cells in the action of lactoferrin in our experiments.
Gadolinium chloride has been used to decrease the number of Kupffer cells in the liver (11). The mechanism of action of gadolinium chloride is not known, but it has been suggested to eliminate larger Kupffer cell populations in the liver while depressing the activity of smaller Kupffer cells (1, 8, 11, 17, 22, 23). Without Kupffer cells, the liver will not produce TNF-α in response to LPS. Consequently, the liver will not be damaged by TNF-α produced by Kupffer cells. As described in this paper, gadolinium chloride protected against damage to the liver by a small dose of LPS. This result indicated that Kupffer cells are involved in the development of hepatitis and supported the speculation that macrophages are major sites of the action of lactoferrin.
This idea was supported by the observation that recombinant human lactoferrin suppressed the production of TNF-α by Kupffer cells when they were exposed to LPS. Our in vitro and ex vivo experiments employing Kupffer cells indicated that lactoferrin suppressed TNF-α production by Kupffer cells via a direct interaction with these cells. In the in vitro assay, Kupffer cells were cultured in media supplemented with recombinant human lactoferrin for 18 h prior to the administration of LPS. Kupffer cells were extensively washed to remove residual lactoferrin before LPS was added. Therefore, it was unlikely that LPS was trapped by lactoferrin in culture media. Rather, it was likely that lactoferrin directly interacted with Kupffer cells and rendered them refractory to LPS. In the ex vivo assay, mice was administered recombinant human lactoferrin 18 h prior to the isolation of Kupffer cells. Kupffer cells were transferred to cultures and then activated with LPS. Since the production of TNF-α by these Kupffer cells was suppressed, intraperitoneally administered recombinant-human lactoferrin probably exerted its effect by interacting with Kupffer cells. Lactoferrin intravenously administered to rats most specifically accumulated in Kupffer cells (21). Although not all the molecules that accumulated in Kupffer cells functioned to suppress TNF-α production, some should have functionally affected Kupffer cells to lower the rate of TNF-α production.
Our analysis of the binding of 125I-labeled recombinant human lactoferrin with mouse Kupffer cells indicated two dissociation constants, 0.47 × 10−6 and 1.82 × 10−6 M, which were comparable to those reported previously for RAW 264 cells and those obtained by our analysis. The low dissociation constant (higher affinity) corresponded to the level of the 50% effective dose of recombinant human lactoferrin for the suppression of TNF-α production, suggesting that recombinant human lactoferrin suppressed Kupffer cells by binding to the high-affinity binding sites. These results suggested that some of the lactoferrin molecules associated with Kupffer cells may function to suppress the production of TNF-α in these cells.
It has been reported that LPS induces massive hepatic lesions by damaging endothelial cells in the hepatic sinusoid and thus subsequently inducing fibrin deposition at the sites of damage (18). The damage to sinusoidal endothelial cells is thought to be caused by activated Kupffer cells (2). Activated Kupffer cells seem to specifically attack sinusoidal endothelial cells without damaging hepatic parenchymal cells (19). One of the factors involved in the induction of damage to sinusoidal endothelial cells may be TNF-α produced by activated Kupffer cells. Other factors such as Fas might be involved in the cascade of reactions starting from the damage of sinusoidal endothelial cells and leading to hepatic lesions. However, such factors have been speculated to function in the induction of hepatic parenchymal cell death (apoptosis) (19), which takes place well after sinusoidal endothelial cell damage. Therefore, if production of inflammatory cytokines such as TNF-α by activated macrophages is inhibited, the later steps in the cascade that lead to hepatic lesions should be suppressed.
In this study, we demonstrated the protective effect of lactoferrin intravenously administered to mice against LPS-induced hepatitis. Lactoferrin in milk is naturally taken orally, so its efficacy when taken orally should also be tested. Orally administered lactoferrin has been reported to have various effects: (i) suppression of inflammation in rat footpads caused by carrageenan (30); (ii) protection against endotoxin lethal shock in germfree piglets (12); and (iii) inhibition of bacterial translocation in bovine-milk-fed mice (29). In the study on carrageenan-induced footpad inflammation, production of TNF-α and IL-6 by cultured splenocytes derived from carrageenan-treated mice was reported to be suppressed when mice were fed lactoferrin, suggesting that the effect of this polypeptide is due to the suppression of the production of inflammatory cytokines. Lactoferrin taken orally will encounter the environment of the intestinal tract, the major functions of which are nutrient absorption and immune responses. Orally administered lactoferrin might have to be absorbed before it can function or might interact with immune-responsive cells that reside in intestinal lymph nodes or Peyer's patches. Taking these points into consideration, we are currently examining the efficacy of orally administered lactoferrin for protection against the development of hepatitis in the zymosan-LPS model.
REFERENCES
- 1.Ahmad N, Gardner C R, Yurkow E J, Laskin D L. Inhibition of macrophages with gadolinium chloride alters intercellular adhesion molecule-1 expression in the liver during acute endotoxemia in rats. Hepatology. 1999;29:728–736. doi: 10.1002/hep.510290324. [DOI] [PubMed] [Google Scholar]
- 2.Arai M, Mochida S, Ohno A, Ogata I, Fujiwara K. Sinusoidal endothelial cell damage by activated hepatic macrophages in rat liver necrosis. Gastroenterology. 1993;104:1466–1471. doi: 10.1016/0016-5085(93)90357-i. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Cerami A. Tumor necrosis, chachexia, shock and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 4.Brock J. Lactoferrin: a multifunctional immunoregulatory protein. Immunol Today. 1995;16:417–419. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland M G, Bakos M A, Hilton S M, Goldblum R H. Amniotic fluid: the first feeding of mucosal immune factors. Adv Exp Med Biol. 1991;310:41–49. doi: 10.1007/978-1-4615-3838-7_4. [DOI] [PubMed] [Google Scholar]
- 6.Crouch S P M, Slater K J, Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992;80:235–240. [PubMed] [Google Scholar]
- 7.Czop J K, Fearon D T, Austen K F. Opsonin-independent phagocytosis of activators of the alternative complement pathway by human monocytes. J Immunol. 1978;120:1132–1138. [PubMed] [Google Scholar]
- 8.Hardonk M J, Dijkjis F W J, Hulstaert C E, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992;52:296–302. doi: 10.1002/jlb.52.3.296. [DOI] [PubMed] [Google Scholar]
- 9.Hardonk M J, Dijkjuis F W J, Hulstaert C E, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992;52:189–195. doi: 10.1002/jlb.52.3.296. [DOI] [PubMed] [Google Scholar]
- 10.Ikejima K, Enomoto N, Iimuro Y, Ikejima A, Fang D, Xu J, Forman D T, Brenner D A, Thurman R G. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am J Physiol. 1998;274:G669–G676. doi: 10.1152/ajpgi.1998.274.4.G669. [DOI] [PubMed] [Google Scholar]
- 11.Laskin D L. Xenobiotic-induced inflammation and injury in the liver. Compre Toxicol. 1997;9:151–158. [Google Scholar]
- 12.Lee W J, Farmer J L, Hilty M, Kim Y B. The protective effects of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect Immun. 1998;66:1421–1426. doi: 10.1128/iai.66.4.1421-1426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machnicki M, Zimecki M, Zagulski T. Lactoferrin regulates the release of tumor necrosis factor alpha and interleukin 6 in vivo. Int J Exp Pathol. 1993;74:433–439. [PMC free article] [PubMed] [Google Scholar]
- 14.Masson P L, Heremans J F, Prignot J, Wauters G. Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax. 1966;21:538–544. doi: 10.1136/thx.21.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattsby-Baltzer I, Roseanu A, Motas C, Elverfors J, Engberg I, Hanson L A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr Res. 1996;40:257–262. doi: 10.1203/00006450-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Miyazawa K, Mantel C, Lu L, Morrison D C, Broxmeyer H E. Lactoferrin-lipopolysaccharide (LPS): effects on lactoferrin binding to monocyte/macrophage-differentiated HL-60 cells. J Immunol. 1991;145:723–729. [PubMed] [Google Scholar]
- 17.Mizgard J P, Molina R M, Stearns R C, Brain J D, Warner A E. Gadolinium induces macrophages apoptosis. J Leukoc Biol. 1996;59:189–195. [PubMed] [Google Scholar]
- 18.Mochida S, Ohno A, Arai M, Tamatani T, Miyasaka M, Fujiwara K. Role of adhesion molecules in the development of massive hepatic necrosis in rats. Hepatology. 1996;23:320–328. doi: 10.1053/jhep.1996.v23.pm0008591859. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 20.Oguchi S, Walker W A, Sanderson I R. Iron saturation alters the effect of lactoferrin on the proliferation and differentiation of human enterocytes (Caco-2 cells) Biol Neonate. 1995;67:330–339. doi: 10.1159/000244182. [DOI] [PubMed] [Google Scholar]
- 21.Peen E, Johansson A, Engquist M, Skogh T. Hepatic and extrahepatic clearance of circulating human lactoferrin: an experimental study in rat. Eur J Haematol. 1998;61:151–159. doi: 10.1111/j.1600-0609.1998.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 22.Roland C R, Naziruddin B, Mohanakumar T, Flye M W. Gadolinium chloride inhibits Kupffer cell nitric oxide synthase induction. J Leukoc Biol. 1996;60:487–492. doi: 10.1002/jlb.60.4.487. [DOI] [PubMed] [Google Scholar]
- 23.Ruttinger D, Vollmar B, Wanner G A, Messmer K. In vivo assessment of hepatic alterations following gadolinium chloride-induced Kupffer cell blockade. J Hepatol. 1996;25:960–967. doi: 10.1016/s0168-8278(96)80302-3. [DOI] [PubMed] [Google Scholar]
- 24.Sadler T E, Cramp W A, Castro J E. Radiolabelling of Corynebacterium parvum and its distribution in mice. Br J Cancer. 1977;35:357–368. doi: 10.1038/bjc.1977.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Z, Wakil A E A, Rockey D. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci USA. 1997;94:10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibayama Y, Asaka S, Nakata K. –1994. Role of activated macrophages in augmentation of endotoxin hepatotoxicity. Exp Toxicol Pathol. 1993;45:497–502. doi: 10.1016/S0940-2993(11)80512-9. [DOI] [PubMed] [Google Scholar]
- 27.Stuart J S, Norell S, Harrington J P. Kinetic effect of human lactoferrin on the growth of Escherichia coli 0111. Int J Biochem. 1984;16:1043–1047. doi: 10.1016/0020-711x(84)90085-5. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y, Takahashi A, Watanabe K, Takayama K, Yahata T, Habu S, Nishimura T. A pivotal role of IL-12 in Th1-dependent mouse liver injury. Int Immunol. 1995;8:569–576. doi: 10.1093/intimm/8.4.569. [DOI] [PubMed] [Google Scholar]
- 29.Teraguchi S, Shin K, Ogata T, Kingaku M, Kaino A, Miyauchi H, Fukuwatari Y, Shimamura S. Orally administered bovine lactoferrin inhibits bacterial translocation in mice fed bovine milk. Appl Environ Microbiol. 1995;61:4131–4134. doi: 10.1128/aem.61.11.4131-4134.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimecki M, Miedzybrodzki R, Szymaniec A. Oral treatment of rats with bovine lactoferrin inhibits carageenan-induced inflammation: correlation with decreased cytokine production. Arch Immunol Ther Exp. 1988;46:361–365. [PubMed] [Google Scholar]