Abstract
Recent studies have shown that alveolar macrophages (AMs) not only act as phagocytes but also play a central role as potent secretory cells in various lung diseases, including pneumonia and acute respiratory distress syndrome. The behavior of AMs during disseminated candidiasis, however, is insufficiently elucidated. This study is the first to report disseminated candidiasis in AM-depleted mice and to analyze the effect of AMs on Candida-induced acute lung injury. While all AM-sufficient mice died by day 2 after infection with Candida albicans, no mortality was observed among AM-depleted mice. Unexpectedly, the CFU numbers of C. albicans isolated from the lungs of AM-depleted mice were significantly higher than those for C. albicans isolated from AM-sufficient mice. The lung wet-to-dry weight ratio was lower for AM-depleted mice than for AM-sufficient mice, although this difference was not significant. We found that bronchoalveolar lavage fluid (BALF) from AM-depleted mice in candidemia contained fewer neutrophils than BALF from AM-sufficient mice. In addition, myeloperoxidase activities in lung homogenates of AM-depleted mice were significantly lower than those in homogenates of AM-sufficient mice. A significant decrease in levels of murine macrophage inflammatory protein 2 (MIP-2), a potent chemoattractant for neutrophils, was noted in lung homogenates from AM-depleted mice compared with levels in homogenates from AM-sufficient mice. Immunohistochemical studies using anti-MIP-2 antibodies revealed that AMs were the cellular source of MIP-2 within the lung during candidemia. We observed that AM depletion decreased levels of AM-derived neutrophil chemoattractant, alleviated acute lung injury during candidemia, and prolonged the survival of mice in candidemia, even though clearance of C. albicans from the lungs was reduced.
Candida albicans infection is common in immunocompromised patients, and its importance as a blood-borne pathogen has increased as a result of widespread use of antimicrobial and chemotherapeutic agents (3). In addition to efforts to develop more effective antifungal agents, new therapeutic approaches that augment the antifungal capacity of the host's immune system are necessary. Recently studies have focused on the role of alveolar macrophages (AMs) as potent secretory cells regulating inflammatory reactions within the lung (16, 19). AMs are reported to be principal mediators in the pathogenesis of septic shock (5). Our study was undertaken to investigate the role of AMs in acute lung injury during systemic candidiasis. For this purpose, the effect of AM depletion on survival, wet-to-dry (W/D) lung weight ratios, and clearance of C. albicans was assessed.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free BALB/c mice (5- to 6-week-old males; Japan SLC Co., Kyoto, Japan) were used in all experiments. All mice were housed in the animal care facility at Kyoto Prefectural University of Medicine until the end of the experiments.
C. albicans.
C. albicans (TIMN 1623, a gift from Teikyo University, Tokyo, Japan) was maintained at −85°C in Sabouraud's broth supplemented with 5% dimethyl sulfoxide (DMSO) and transferred to Sabouraud's dextrose agar at 37°C prior to use. Yeast-phase blastospores for infusion were suspended in sterile saline, sedimented (400 × g, 10 min, 4°C), and resuspended in sterile saline to a concentration of 5 × 107 cells/ml as determined with a hemacytometer.
AM depletion.
2-Chloroadenosine (2-CA) (Sigma Chemical Co., St. Louis, Mo.) was dissolved in saline at a concentration of 1 mM. Mice were placed immobilized in a nose-only aerosol chamber and received aerosolized 2-CA for 2 h via an ultrasonic nebulizer (NE-U11B) (Omron Co., Kyoto, Japan) driven at a rate of 0.75 ml/min (14). We used the mice in experiments 24 h after 2-CA treatment.
Analysis of outcome.
Our initial experiments were designed to characterize the in vivo role of AM in the outcome of mice in candidemia. Thirty-six mice comprising three subgroups were infected intravenously with 107 C. albicans cells. The three subgroups were (i) naive mice (naive group), (ii) mice after treatment with aerosolized saline for 2 h (saline group), and (iii) AM-depleted mice after treatment with aerosolized 2-CA (AM-depleted group) (n = 12 per group). All mice were infected intravenously with C. albicans cells, and survival was observed over 5 days.
Experimental protocols.
Eighteen mice from the three subgroups (n = 6) described above were studied in each of the following experiments for up to 24 h after intravenous infection with 107 C. albicans cells. Twenty-four hours after infection, all mice were sacrificed by exsanguination.
Lung water measurement.
The W/D lung weight ratio, which is an index of microvascular permeability, was determined to assess the severity of lung edema (8). Twenty-four hours after administration of C. albicans, both lungs were removed, weighed, and dried in a vacuum oven at 80°C for 24 h.
Determination of the number of CFU of C. albicans in lung tissue.
Quantitation of viable C. albicans within the lungs of infected mice was made by colony counting. Both lungs were removed aseptically and homogenized in 10 ml of sterile saline with a tissue homogenizer (Dremel, Racine, Wis.). Twenty microliters of each lung homogenate was placed on Sabouraud's dextrose agar and incubated for 24 h at 37°C, after which colonies were counted.
Bronchoalveolar lavage (BAL).
BAL was performed to determine whether AM depletion affected the migration of neutrophils or lymphocytes into lung airspaces during systemic candidiasis. Mice were anesthetized intraperitoneally with approximately 2.0 mg of pentobarbital each. The trachea was exposed and intubated with a 27-gauge needle. BAL was performed by administration of 0.5 ml of sterile saline three times, and cells in BAL fluid (BALF) were counted. Fluid recovery was routinely 90% or greater. For differential counts, BALF was centrifuged at 1,000 × g for 3 min, and the collected cells were stained with Giemsa for cytology.
Lung myeloperoxidase (MPO) assay.
Lung MPO activity was quantitated as described previously (17). Whole lungs were homogenized in 2 ml of 50 mM potassium phosphate, pH 6.0, with 5% hexadecyltrimethylammonium bromide and 5 mM EDTA. The homogenate was sonicated and centrifuged at 12,000 × g for 15 min. The supernatant was mixed 1:15 with assay buffer and read at 490 nm. MPO units were calculated as the change in absorbance over time.
Lung harvesting for cytokine analysis.
Prior to removal of the lungs, the pulmonary vasculature was perfused via the right ventricle with 1 mM phosphate-buffered saline containing 5 mM EDTA. After removal, whole lung was homogenized in 3 ml of lysis buffer containing 0.5% Triton X-100, 150 mM NaCl, 15 mM Tris, 1 mM CaCl, and 1 mM MgCl (pH 7.4), using a tissue homogenizer. The homogenate was incubated on ice for 30 min and then centrifuged at 2,500 rpm (Cytospin 2; Shandon Southern Instruments, Sewickley, Pa.) for 10 min. The supernatant was collected, passed through a 0.45-μm-pore-size filter (Gelman Science, Ann Arbor, Mich.), and then stored at −30°C until assessment of cytokine levels (11).
Cytokine enzyme-linked immunosorbent assays.
Murine macrophage inflammatory protein-2 (MIP-2) exhibits potent neutrophil chemotactic activity and is thought to be a key mediator of neutrophil recruitment (1). To determine whether the impaired recruitment of neutrophils observed in AM-depleted mice is the result of decreased levels of this C-X-C chemokine, we measured the level of MIP-2 in lung homogenate. Quantitation of MIP-2 was performed according to the directions for a radioimmunoassay kit for MIP-2 (R&D Systems, Minneapolis, Minn.).
Immunohistochemical localization of antigenic MIP-2.
To confirm the cellular source of MIP-2, immunohistochemical staining of lung sections and BALF with polyclonal anti-murine MIP-2 antisera was performed (11). Sections of lung and BAL differentials from mice 24 h after intravenous administration of C. albicans were prepared for immunohistochemical localization of MIP-2 antigen. Polyclonal anti-murine MIP-2 antisera were produced by immunization of rabbits with recombinant murine MIP-2 (R&D Systems) in multiple intradermal sites with complete Freund's adjuvant. The lungs were removed, embedded in OCT compound (Miles, Elkhart, Ind.), frozen in liquid nitrogen, cut in 5-μm sections on a cryostat, placed on slides, and fixed in acetone for 10 min. BALF was centrifuged at 1,000 × g for 3 min, and the collected cells were placed on slides. After blocking nonspecific binding sites with bovine serum albumin (Sigma Chemical Co.), we performed an immunohistochemical analysis with a 1:1,000 dilution of rabbit anti-murine MIP-2 antisera. The secondary antibody was the anti-rabbit immunoglobulin-horseradish peroxidase-linked F(ab′)2 fragment (Amersham, Little Chalfont, Buckinghamshire, United Kingdom). All slides were visualized with 3,3′-diaminobenzidine (Sigma Chemical Co.).
Statistical analysis.
All data, which are expressed as means ± standard errors of the means, were analyzed by one-way analysis of variance. The differences between groups were compared by Fisher's PLSD test. A P value of ≤0.05 was considered statistically significant.
RESULTS
Effects of AM depletion on survival.
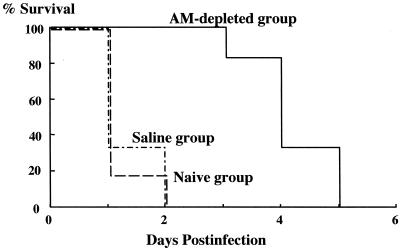
Survival in the naive and saline groups decreased substantially after infection with C. albicans. One hundred percent lethality was noted by day 2 postinfection. Delayed mortality was observed during the 5-day postinfection follow-up with the AM-depleted mice (Fig. 1).
FIG. 1.
Effect of AM depletion on survival in candidemia. All mice in the three groups were infected intravenously with 107 C. albicans cells (n = 12 per group).
Lung CFU.
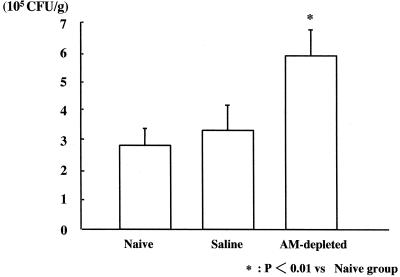
The numbers of CFU isolated from lung homogenates were significantly higher in the AM-depleted group (5.97 [± 0.89] × 105 CFU/g) than in the naive and saline groups (2.87 [± 0.56] × 105 cfu/g and 3.34 [± 0.81] × 105 CFU/g, respectively) (Fig. 2).
FIG. 2.
Effect of AM depletion on CFUs in the lung 24 h after intravenous infection with C. albicans (n = 6 per group).
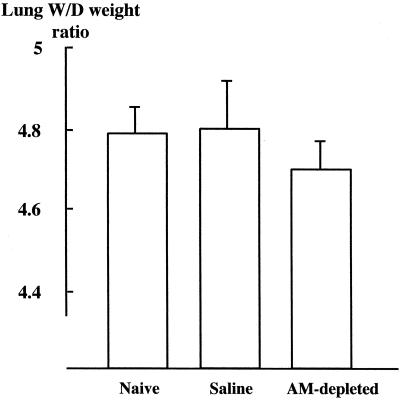
Lung W/D weight ratio.
The lung W/D weight ratio was lower in the AM-depleted group (4.70 ± 0.05) than in the naive and saline groups (4.79 ± 0.06 and 4.80 ± 0.14, respectively) (not significant) (Fig. 3).
FIG. 3.
Effect of AM depletion on lung W/D weight ratios 24 h after intravenous infection with C. albicans (n = 6 per group).
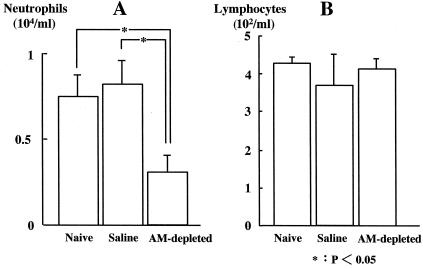
Cell counts in BALF.
Neutrophils in BALF were significantly fewer in the AM-depleted group (0.31 [± 0.11] × 104/ml) than in the naive and saline groups (0.75 [± 0.17] × 104/ml and 0.78 [± 0.19] × 104/ml, respectively) (Fig. 4A). There was no significant difference in lymphocyte counts among the three groups (Fig. 4B).
FIG. 4.
Analysis of neutrophil (A) and lymphocyte (B) counts in BALF 24 h after intravenous infection with C. albicans (n = 6 per group).
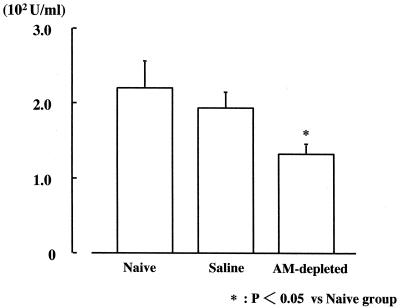
Lung MPO activity.
Lung MPO activity was significantly lower in the AM-depleted group (1.33 [±0.14] × 102 U/ml) than in the naive and saline groups (2.20 [±0.36] × 102 and 1.93 [± 0.20] × 102 U/ml, respectively) (Fig. 5).
FIG. 5.
MPO activity 24 h after intravenous infection with C. albicans (n = 6 per group).
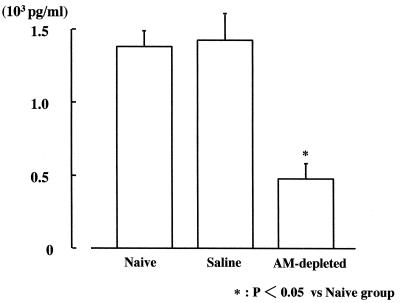
MIP-2.
The MIP-2 level in the AM-depleted group (0.49 [±0.12] × 103 pg/ml) was significantly lower than in the naive and saline groups (1.40 [±0.09] × 103 and 1.42 [±0.21] × 103 pg/ml, respectively) (Fig. 6).
FIG. 6.
Composite MIP-2 protein production in lung homogenates 24 h after intravenous infection with C. albicans.
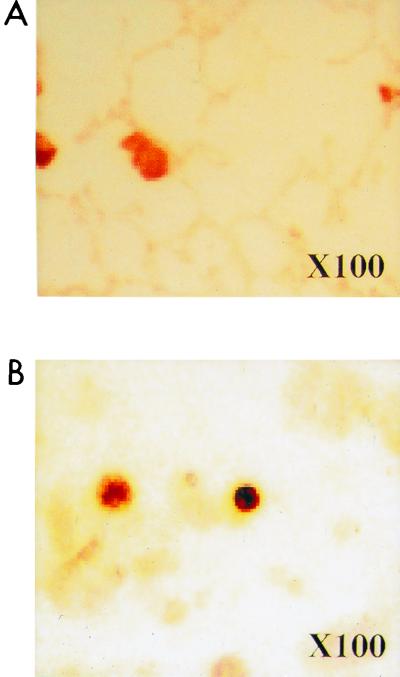
Immunohistochemical localization of antigenic MIP-2.
Cell-associated MIP-2 was present within AMs (Fig. 7A and B). Staining for MIP-2 was specific since no staining was observed in a section of lung incubated with purified immunoglobulin G from control serum. This result supports the theory that AMs are the cellular source of MIP-2 within the lung.
FIG. 7.
Immunohistochemical staining of lung sections (A) and BALF (B) for MIP-2 24 h after intravenous infection with C. albicans.
DISCUSSION
AMs are found in the alveoli and alveolar ducts of the lung and are the only macrophages that live in an aerobic environment. Recent studies have shown that AMs not only act as phagocytes but also function as potent secretory cells in various lung diseases. For example, Hashimoto et al. (13) reported that AMs play a protective role in Pseudomonas pneumonia by promoting the initial recruitment of neutrophils into infected air spaces. Some researchers have reported that AMs play a central role in the pathogenesis of acute lung injury during sepsis (18, 10). However, the behavior of AMs during disseminated candidiasis is insufficiently elucidated. Lechner et al. (15) reported that tumor necrosis factor (TNF) levels in BALF of neutropenic animals with candidemia are elevated, whereas TNF levels in serum remain low, even at death. They assumed that impaired clearance of yeast-phase C. albicans by circulating polymorphonuclear cells leads to extensive yeast mycelium transformation in the pulmonary vasculature, hyphal outgrowth from pulmonary capillaries, and increased TNF production by AMs in response to hyphal elements in the distal air spaces. Our present report is the first documentation of disseminated candidiasis in AM-depleted mice and the first analysis of the effect of AMs in Candida-induced acute lung injury.
To examine the in vivo role of AMs in candidiasis, we used a previously described technique (14) to deplete mice of AMs. Treatment with 1 mM aerosolized 2-CA for 2 h reduced the number of AMs in BALF to less than or equal to 30% of control values, and the number remained low for at least 72 h after 2-CA administration. Studies have shown that intratracheal administration of drugs may induce neutrophil chemotaxis to the lung (4, 6). In contrast to methods used previously, administration of aerosolized 2-CA did not affect leukocyte or lymphocyte counts in BALF, and no mucosal edema or cellular infiltration in lung tissue was observed microscopically. Moreover, 2-CA did not increase permeability of the lung vasculature and did not affect the viability of other phagocytes.
While all AM-sufficient mice died by day 2 after infection with C. albicans, no mortality was observed among AM-depleted mice until day 3. This result shows a beneficial effect of depletion of AMs at the onset of candidiasis. The striking increase in survival of AM-depleted infected mice prompted us to examine whether AM depletion affected clearance of C. albicans from the lungs. But, unexpectedly, the CFU numbers for C. albicans isolated from lungs of AM-depleted mice were significantly higher than for AM-sufficient mice. This finding indicates that increases in levels of C. albicans may not be an essential factor in the outcome of the early phase of candidemia. The lung W/D weight ratio was lower in AM-depleted mice than in AM-sufficient mice; however, this difference was not significant. We hypothesized that AM depletion may reduce lung edema and may prolong survival of mice in candidemia. Researchers have shown that neutrophils play an essential role in the initial clearance of C. albicans during candidemia (2, 20). To determine whether the decreased clearance of C. albicans observed in AM-depleted mice was a result of impaired recruitment of neutrophils, we analyzed cell counts in BALF 24 h after intravenous infection with C. albicans. In our model, we found that BALF from AM-depleted mice with candidemia contained fewer neutrophils than fluid from AM-sufficient mice and that MPO activity in lung homogenates of AM-depleted mice was significantly lower than that of AM-sufficient mice. Therefore, we supposed that impaired recruitment of neutrophils into the lungs of AM-depleted mice may permit the number of yeasts to increase but may mitigate inflammation in lung tissue.
MIP-2 is a potent chemoattractant for neutrophils in rodents and has been reported to play a significant role in recruiting neutrophils during endotoxin-induced lung injury (12). We hypothesized that in Candida-induced lung injury, the impaired recruitment of neutrophils into the lungs of AM-depleted mice was the result of decreased MIP-2 levels in the lung. The levels of MIP-2 in lung homogenates from AM-depleted mice were significantly lower than those in lungs from AM-sufficient mice. Immunohistochemical studies with an anti-MIP-2 antibody revealed that AMs are the cellular sources of MIP-2 within the lung during candidemia.
We did not detect any other neutrophil chemoattractants and did not examine whether pretreatment with anti-MIP-2 antibodies can prevent Candida-induced lung damage and death in this model. However, in a murine Klebsiella pneumonia model, Greenberger et al. (11) showed that neutralization of MIP-2 by intraperitoneal administration of anti-MIP-2 antibodies attenuates neutrophil recruitment and bacterial clearance in lung tissue. We assume that pretreatment with anti-MIP-2 antibodies prevents neutrophil-induced lung damage and death during the early stage of lung damage but would not significantly improve survival because of worsened candidemia in the late stage. An effective combination of antifungal agents may improve late-stage survival.
How injected C. albicans stimulates AMs remains unclear. Castro et al. (7) reported that C. albicans induces release of inflammatory mediators, such as arachidonic acid, interleukin-1 beta, and TNF-alpha. In addition, Filler et al. (9) showed that C. albicans stimulates endothelial cells to produce several cytokines and leukocyte adhesion molecules. We show here that reduction of stimulated AMs decreases AM-derived neutrophil chemoattractant and alleviates acute lung injury during candidemia. If the mechanism of stimulation of AMs by C. albicans is elucidated, more effective prevention and treatment of Candida-induced lung injury may be possible. In summary, our results indicate that AM depletion and decreases in AM-derived chemoattractant for neutrophils mitigate Candida-induced lung injury and prolong survival of mice in candidemia.
REFERENCES
- 1.Appelberg R. Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell-mediated neutrophil recruitment. J Leukoc Biol. 1992;52:303–306. doi: 10.1002/jlb.52.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck-Sagué C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 4.Berg J T, Lee S T, Thepen T, Lee C Y, Tsan M F. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J Appl Physiol. 1993;74:2812–2819. doi: 10.1152/jappl.1993.74.6.2812. [DOI] [PubMed] [Google Scholar]
- 5.Brigham K L, Meyrick B. Endotoxin and lung injury. Am J Respir Dis. 1986;133:913–927. [PubMed] [Google Scholar]
- 6.Broug-Holub E, Toews G B, Van Iwaarden J F, Strieter R M, Kunkel S L, Paine R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro M, Bjoraker J A, Rohrbach M S, Limper A H. Candida albicans induces the release of inflammatory mediators from human peripheral blood monocytes. Inflammation. 1996;20:107–122. doi: 10.1007/BF01487749. [DOI] [PubMed] [Google Scholar]
- 8.Collins J A, Braitberg A, Butcher H R., Jr Changes in lung and body weight and lung water content in rats treated for hemorrhage with various fluids. Surgery. 1973;73:401–411. [PubMed] [Google Scholar]
- 9.Filler S G, Pfunder A S, Spellberg B J, Spellberg J P, Edwards J E., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goya T, Abe M, Shimura H, Torisu M. Characteristics of alveolar macrophages in experimental septic lung. J Leukoc Biol. 1992;52:236–243. doi: 10.1002/jlb.52.2.236. [DOI] [PubMed] [Google Scholar]
- 11.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Laichalk L L, McGillicuddy D C, Standiford T J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Feng L, Yoshimura T, Redick J, Fu S M, Rose C E., Jr Intra-alveolar macrophage-inflammatory peptide 2 induces rapid neutrophil localization in the lung. Am J Respir Cell Mol Biol. 1996;15:656–663. doi: 10.1165/ajrcmb.15.5.8918372. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto S, Pittet J-F, Hong K, Folkesson H, Bagby G, Kobzik L, Frevert C, Watanabe K, Tsurufuji S, Wiener-Kronish J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol. 1996;270:819–828. doi: 10.1152/ajplung.1996.270.5.L819. [DOI] [PubMed] [Google Scholar]
- 14.Kubota Y, Iwasaki Y, Harada H, Yokomura I, Ueda M, Hashimoto S, Nakagawa M. Depletion of alveolar macrophages by treatment with 2-chloroadenosine aerosol. Clin Diagn Lab Immunol. 1999;6:452–456. doi: 10.1128/cdli.6.4.452-456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lechner A J, Tredway T L, Brink D S, Klein C A, Matuschak G M. Differential systemic and intrapulmonary TNF-alpha production in Candida sepsis during immunosuppression. Am J Physiol. 1992;263:526–535. doi: 10.1152/ajplung.1992.263.5.L526. [DOI] [PubMed] [Google Scholar]
- 16.Nathan C F. Secretory products of macrophages. J Clin Investig. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remick D G, Stieter R M, Eskandari M K. Role of tumor necrosis factor-alpha in lipopolysaccharide-induced pathologic alterations. Am J Pathol. 1990;136:49–60. [PMC free article] [PubMed] [Google Scholar]
- 18.Rinaldo J E, Henson J E, Dauber J H, Henson P M. Role of alveolar macrophages in endotoxin-induced neutrophilic alveolitis in rats. Tissue Cell. 1985;17:461–472. doi: 10.1016/0040-8166(85)90025-4. [DOI] [PubMed] [Google Scholar]
- 19.Sibille Y, Reynolds H Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 20.Tansho S, Abe S, Tansho T, Yamaguchi H. Effective inhibition of Candida albicans growth by the combination of murine peritoneal neutrophils and activated macrophages. Microbiol Immunol. 1999;43:235–240. doi: 10.1111/j.1348-0421.1999.tb02398.x. [DOI] [PubMed] [Google Scholar]