Abstract
We assessed the diagnostic performance of the Biofire® Filmarray® Pneumonia Plus panel (FA-PP) compared to standard culture in Intensive Care Unit patients with suspected ventilator-associated lower respiratory tract infection in the COVID-19 era. We determined whether its implementation in routine diagnostic algorithms would be cost-beneficial from a hospital perspective.
Of 163 specimens, 96 (59%) returned negative results with FA-PP and conventional culture, and 29 specimens (17.8%) were positive with both diagnostic methods and yielded concordant qualitative bacterial identification/isolation. Thirty-nine specimens (23.9%) gave discordant results (positive via FA-PP and negative via culture). Real-life adjustments of empirical antimicrobial therapy (EAT) after FA-PP results resulted in additional costs beyond EAT alone of 1868.7 €. Adequate EAT adjustments upon FA-PP results would have resulted in a saving of 6675.8 €. In conclusion, the data presented supports the potential utility of FA-PP for early EAT adjustment in patients with ventilator-associated lower respiratory tract infection.
Keywords: Biofire® Filmarray® Pneumonia Plus, Lower respiratory tract infection, Intensive Care Unit, COVID-19
1. Introduction
Ventilator-associated lower respiratory tract bacterial infection (VA-LRTBI), including tracheobronchitis (VAT) and pneumonia (VAP), occurs frequently in intensive care unit (ICU) patients, resulting in high morbidity and mortality and dramatically raising healthcare-associated costs [1], [2], [3]. Suspicion of VA-LRTBI prompts initiation of broad-spectrum empirical antimicrobial therapy (EAT) [4,5]. The inadequacy of EAT, rather common in clinical settings with a high prevalence of multidrug-resistant bacteria (MDRB), or failure to de-escalate to narrower-spectrum therapies in a timely manner promote increased ICU morbidity and mortality and MDRB selection and spread [6]. Rapid turnaround times of microbiological results, particularly regarding the antimicrobial susceptibility of the putative causative agent, are therefore imperative for appropriate therapeutic management of VA-LRTBI [4,5]. Standard semiquantitative culture-based and antimicrobial susceptibility testing procedures performed on endotracheal aspirates or bronchoscopic specimens are lengthy, returning results approximately 48 to 72 hours after specimen reception. The use of molecular diagnostic approaches that allow rapid bacterial identification and documentation of the presence of genotypic resistance traits in the causative bacteria may provide clinically actionable results within 2 hours. One such approach is the BioFire® FilmArray® Pneumonia/Pneumonia plus Panel (FA-PP) (BioFire Diagnostics, LLC, Salt Lake City, UT), a multiplex PCR panel that allows detection of 15 bacteria commonly involved in VA-LRTBI, providing semiquantitative estimates of bacterial load and 7 genetic markers of antibiotic resistance (mecA/C and MREJ, blaCTX-M,blaKPC, blaVIM, blaOXA-48-like, blaIMP, blaNDM) This test has been extensively evaluated and proven to increase the diagnostic yield in LRTBI compared with standard culture-based methods [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. FA-PP shows great promise in improving the therapeutic management of VA-LRTBI, however, its positioning in laboratory diagnostic algorithms remains to be defined [22].
Patients with COVID-19 admitted to the ICU are at high risk of developing secondary bacterial infections [23]; here, in addition to assessing the diagnostic performance of FA-PP in ICU patients with suspicion of VA-LRTBI in the COVID-19 era compared to standard procedures, we also investigated whether its implementation in routine diagnostic algorithms would help reduce the direct costs related to diagnostic testing and antimicrobial therapy in patients with suspected VA-LRTBI from a hospital perspective.
2. Material and methods
2.1. Patients
In this single-center, retrospective and observational study, we included consecutive mechanically-ventilated (MV) patients, admitted to the ICU between March 2020 and April 2021, who underwent routine testing by FA-PP (Table 1 ). As per local protocol, FA-PP was requested upon suspicion of VA-LRTBI (2 or more of the following indicators: fever, hypoxemia, compatible imaging, increase in LRT secretions and acute-phase reactants), in line with American and European consensus guidelines [4,5]. Conventional semiquantitative cultures were performed in parallel for all patients. Selected data regarding demographics, clinical condition and outcomes, and antibiotic use was gathered from electronic medical records by the attending physicians at the ICU (NC and MLB). A database was built and analyzed by JF, MAC, EG, IT and EA. No antibiotic stewardship programs had been implemented at the ICU at the time of initiation of the current study; although consensus protocols for antimicrobial therapy were in place within the study period, antimicrobials were ultimately prescribed at the physician's discretion. An informed consent waiver was obtained from the local institutional review board (Ethical Committee of Hospital Clínico Universitario INCLIVA) for data collection from the laboratory and medical records.
Table 1.
Main patient baseline characteristics on admission to the Intensive Care Unit (ICU).
| Parameter | Patients |
|---|---|
| Median age, years (IQR) | 62 (21–80) |
| Number of days of ICU stay (IQR) | 19 (9–66) |
| Male sex, no. (%) | 75 (68.8) |
| SARS-CoV-2 pneumonia no. (%) | 75 (68.8) |
| Clinical conditions prompting FA-PP request | |
| Increased volume of LRT secretions; no. (%) | 23 (21) |
| Increased volume of LRT secretions and compatible imaging of LRT infection; no. (%) | 30 (27.5) |
| Increased volume of LRT secretions and hypoxemia; no. (%) | 12 (11.0) |
| Fever and increased volume of LRT secretions; no. (%) | 8 (7.3) |
| Increased volume of LRT secretions and increase in acute phase reactants; no. (%) | 4 (3.6) |
| Fever, increased volume of LRT secretions and hypoxemia; no. (%) | 16 (14.6) |
| Increased volume of LRT secretions, compatible imaging of LRT infection and increase in acute phase reactants; no. (%) | 10 (10.9) |
| Increased volume of LRT secretions, compatible imaging of LRT infection, hypoxemia and increase in acute phase reactants; no. (%) | 3 (2.7) |
| Fever, increased volume of LRT secretions and hypoxemia; no. (%) | 1 (0.9) |
| Fever, increased volume of LRT secretions, and increase in acute phase reactants; no. (%) | 1 (0.9) |
| Fever, increased volume of LRT secretions, compatible imaging of LRT infection, hypoxemia and increase in acute phase reactants; no. (%) | 2 (1.8) |
| Comorbidities, no. (%) | |
| Obesity (body mass index ≥30 kg/m2) | 16 (14.7) |
| Hypertension | 51 (46.8) |
| Diabetes mellitus | 26 (23.8) |
| Immunosuppression | 10 (9.2) |
| Transplantation | 1 (0.9) |
| Chronic renal disease | 3 (2.7) |
| Chronic respiratory disease | 17 (15.6) |
| Outcomes in ICU | |
| Invasive mechanical ventilation | 109 (100) |
| ECMO | 5 (4.5) |
| ICU mortality | 55 (50.5) |
| Empirical antibiotic treatment, no. (%) | 99 (91) |
ECMO = extracorporeal membrane oxygenation; FA-PP = FilmArray Pneumonia Plus Panel; IQR = interquartile range; LRT = lower respiratory tract.
2.2. Microbiological testing
Tracheal aspirates (TAs) were processed in parallel with conventional culture-based procedures and FA-PP. The former included Gram staining and semiquantitative cultures (reported as CFU/mL) on selective/differential media, including colistin-nalidixic blood agar, MacConkey agar and chocolate agar, all purchased from Becton, Dickinson and Company (NJ). The plates were inoculated with 1 µL calibrated loops and then incubated at 35 °C in CO2-enriched air and examined for growth at 24 and 48 hours. Isolated colonies were identified using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) using the Bruker Microflex LT instrument, MBT Compass software ver. 4.1 and Compass Library DB-8468 (Bruker Daltonics, MA). Antimicrobial susceptibility testing (AST) was performed by microdilution (PM33 panels, PM MDR MicroScan; Beckman Coulter) or disc diffusion in Mueller Hinton Fastidious Agar (Becton Dickinson, Sparks, MD) and results evaluated following European Committee on Antimicrobial Susceptibility Testing (EUCAST) interpretative criteria (https://www.eucast.org/clinical_breakpoints/). Bacterial growth (CFU/mL) was always reported quantitatively. Negative cultures were those from which bacteria were not recovered or reported as “normal oropharyngeal flora.” The FA-PP plus is an automated multiplex PCR test for the rapid detection of 27 bacteria and viruses and 7 genetic markers of antibiotic resistance, with a hands-on time of around 5 minutes and total analysis time of about an hour. Bacterial detections were categorized as negative if ≤ 103.5 copies/mL were detected, while positive results were reported in a semiquantitative manner and specified as 104, 105, 106 or ≥ 107 copies/mL. No further testing was performed to resolve discrepancies between comparison methods; in this respect, for analysis purposes, we assumed both methods had 100% specificity. Although initially intended to be carried out 24/7, FA-PP was ultimately performed upon physician's request and following the manufacturer's instructions; the results were reported to the clinician in real-time.
2.3. Antimicrobial therapy
As per local protocol, based partly upon recent guidelines [24] and according to hospital and Health Department epidemiology, a combination of 2 antibiotics displaying antipseudomonal activity including a beta-lactam/beta-lactamase inhibitor (such as Ceftolozane/Tazobactam, Ceftazidime/Avibactam, Meropenem or Piperacillin/Tazobactam) plus amikacin, ciprofloxacin or inhaled/intravenous colistin, and an another covering Methicillin-resistant Staphylococcus aureus (MRSA) (mainly Linezolid) was used as EAT in patients with suspicion of VA-LRTBI. The following EAT adjustments based upon FA-PP results were deemed suitable as they presumably provided therapeutic coverage for the bacteria involved (either confirmed or not by AST): (1) antibiotic de-escalation, consisting of discontinuation of MRSA coverage upon negative result for Staphylococcus aureus; withdrawal of 1 antipseudomonal agent and coverage of MRSA upon detection of Enterobacterales, Haemophilus influenzae or Moraxella catarrhalis; discontinuation of 1 antipseudomonal agent upon detection of MRSA or other Gram-positive bacteria; spectrum reduction of antipseudomonal agent by switching to ertapenem upon detection of ESBL producing-Enterobacterales or, more usually, if negative for genetic markers of antibiotic resistance in gram-negative microorganisms. (2) Antibiotic escalation achieving potential coverage (or actual coverage when antimicrobial susceptibility data were available) of the bacteria detected.
2.4. Statistical analysis
Positive and negative percent agreements between comparison methods (PPA and NPA, respectively), both qualitative (positive vs. negative result) and quantitative (according to the number of copies/mL of bacterial DNA returned by the FA-PP assay), were reported throughout the study. Receiver operating characteristic (ROC) analysis was conducted to evaluate the performance of the FA-PP assay according to the quantitative results returned by the standard cultures. Logistic regression models providing Odds ratios (ORs) and 95% confidence intervals (CIs) were built to assess the association between DNA copies/mL detected by FA-PP and the probability of obtaining a positive culture. Correlations between variables were assessed by the Pearson test. Two-sided exact P values were reported and a P-value <0.05 was considered statistically significant. The analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL). Graphical design was performed using GraphPad Software Inc v6.0 (CA).
2.5. Cost-benefit analysis
The cost-benefit analysis in the current study focused on the economic impact (increased spending or saving) in Euros linked to changes in EAT made according to the results obtained from the FA-PP assay.
The direct cost of FA-PP (155 €/test), which included purchase charges, equipment maintenance and proficiency testing, was obtained from our Hospital's Financial Department. Technical and regular staff costs were 12.9 €/h and 22.9 €/h according to the Financial Department of the Conselleria de Sanitat Universal (Valencian Community Government). The time required (by technical staff) to carry out the FA-PP was estimated at 5 minutes, with an equivalent time invested in validation and communication of the results by the staff in charge. The total cost of EAT modifications following receipt of FA-PP data was calculated by adding or subtracting the price of antibiotics added or discontinued from EAT, adjusted to their defined daily dose (DDD), and assuming that FA-PP provides results 48 hours earlier than conventional cultures. The purchase price of the antibiotics was supplied by the Hospital's Pharmacy Unit. For patients tested more than once via FA-PP, data for cost-benefit analyses were derived from the first test.
3. Results
A total of 815 patients were admitted to the ICU within the study period, of which 280 underwent MV; of these, 109 patients (median age, 62 years; range, 21–80; 75 male) were screened using the FA-PP assay upon physician's request (Supplementary Fig. 1). Seventy-five patients (68.8%) had COVID-19.
3.1. Performance comparison of standard culture procedures and FA-PP
A total of 163 TA from 109 patients were processed in parallel for standard culture and FA-PP. In detail, a single specimen was collected from 78 patients, a median of 10 days after ICU admission (range, 1–31 days). Eighteen patients had a second TA sample obtained at a median of 22 days after ICU admission (range, 10–40), 9 had a third specimen (median, 28 days; range, 20–37) and 6 a had fourth (median, 39 days; range, 32–52). Most specimens (145/163) were collected from patients who had been started on EAT within the preceding 48 hours. Detailed data on bacterial detection by FA-PP (any genome copy value/mL) or recovery of bacteria included in the FA-PP panel by culture (any CFU/mL count) are shown in Supplementary Tables 1 and 2, respectively. Of the 163 specimens, 96 (59%) returned negative results with both FA-PP and conventional culture (only considering bacteria included in the FA-PP panel); among these 96, Haemophilus parainfluenzae, not included in the FA-PP panel, was cultured in 1 specimen. A total of 29 specimens (17.8%) were positive with both diagnostic methods and yielded concordant qualitative bacterial identification/isolation (n = 29 targets) (Table 2 ); of note, Stenotrophomonas maltophilia, not included in the FA-PP panel, was recovered from 1 specimen.
Table 2.
Concordant bacterial detection by the FilmArray Pneumonia plus panel and isolation by conventional cultures from tracheal aspirates from mechanically-ventilated patients.
| Bacteria | No. of tracheal aspirates |
|---|---|
| Monomicrobial | |
| Acinetobacter baumannii | 1 |
| Escherichia coli | 1 |
| Haemophilus influenzae | 1 |
| Klebsiella aerogenes | 5 |
| Klebsiella oxytoca | 1 |
| Klebsiella pneumoniae | 3 |
| Pseudomonas aeruginosa | 4 |
| Serratia marcescens | 1 |
| Staphylococcus aureus | 9 |
| Polymicrobial | |
| Escherichia coli + Klebsiella aerogenes | 1 |
| Klebsiella pneumoniae + Klebsiella aerogenes | 2 |
Results provided by the 2 diagnostic methods were discordant in the remaining 39 specimens (23.9%) (Table 3 ), of which 28 returned positive results with FA-PP and negative results with culture. Regarding the discordant specimens, a total of 65 bacterial targets were detected by FA-PP, either individually (monobacterial) or in combination (polybacterial), of which only 11 could be recovered by culture. Bacteria missed by culturing were Gram-positive cocci (GPC) (n = 17), Enterobacterales (n = 16), Haemophilus influenzae/Moraxella catarrhalis (n = 11), Pseudomonas aeruginosa (n = 9) and Acinetobacter baumannii (n = 1). In one of these discordant specimens, Klebsiella oxytoca and Klebsiella pneumoniae group were identified by FA-PP, whereas Klebsiella pneumoniae and Citrobacter freundii were recovered by culture.
Table 3.
Discordant bacterial detection by the FilmArray Pneumonia plus panel and isolation by conventional cultures from tracheal aspirates from mechanically-ventilated patients.
| Detection by the FilmArray Pneumonia plus Panel | Recovery from bacterial cultures | no. of tracheal aspirates |
|---|---|---|
| Escherichia coli | None | 1 |
| Haemophilus influenzae | 1 | |
| Klebsiella aerogenes | 2 | |
| Klebsiella pneumoniae | 3 | |
| Pseudomonas aeruginosa | 6 | |
| Staphylococcus aureus | 4 | |
| Streptococcus pneumoniae | 1 | |
| Haemophilus influenzae + Staphylococcus aureus | 2 | |
| Klebsiella aerogenes+ Pseudomonas aeruginosa | 1 | |
| Klebsiella pneumoniae+ Haemophilus influenzae | 1 | |
| Pseudomonas aeruginosa + Staphylococcus aureus | 2 | |
| Streptococcus pneumoniae+ Serratia marcescens | 1 | |
| Staphylococcus aureus + Proteus mirabilis + Klebsiella pneumoniae | 1 | |
| Haemophilus influenzae+ Klebsiella aerogenes+ Acinetobacter baumannii + Staphylococcus aureus | 1 | |
| Escherichia coli | Stenotrophomonas maltophilia | 1 |
| Escherichia coli + Haemophilus influenzae | Escherichia coli | 1 |
| Pseudomonas aeruginosa + Staphylococcus aureus | Pseudomonas aeruginosa | 1 |
| Haemophilus influenzae + Klebsiella aerogenes | Klebsiella aerogenes | 1 |
| Haemophilus influenzae + Staphylococcus aureus + Streptococcus agalactiae | Staphylococcus aureus | 1 |
| Haemophilus influenzae + Streptococcus pneumoniae | Streptococcus pneumoniae | 1 |
| Klebsiella pneumoniae + Klebsiella oxytoca | Klebsiella pneumoniae + Citrobacter freundii | 1 |
| Klebsiella pneumoniae + Proteus mirabilis | Klebsiella pneumoniae | 1 |
| Klebsiella aerogenes + Staphylococcus aureus + Moraxella catarrhalis | Klebsiella aerogenes + Staphylococcus aureus | 1 |
| Moraxella catarrhalis +Streptococcus pneumoniae | Moraxella catarrhalis | 1 |
| Staphylococcus aureus + Haemophilus influenzae + Streptococcus pneumoniae | Streptococcus pneumoniae | 1 |
| None | Haemophilus parainfluenze | 1 |
The qualitative PPA value between FA-PP and standard culture when considering all bacterial targets was 44.2% (Supplementary Table 3). PPA was above 50% for most Enterobacterales and lower for nonfermenting Gram-negative bacilli (NF-GNB), Gram-positive bacterial targets and other GNB (Haemophilus influenzae). In turn, no specimen testing negative via FA-PP returned positive results via culture (for bacterial species included in the molecular panel). The overall qualitative NPA was 99.8%.
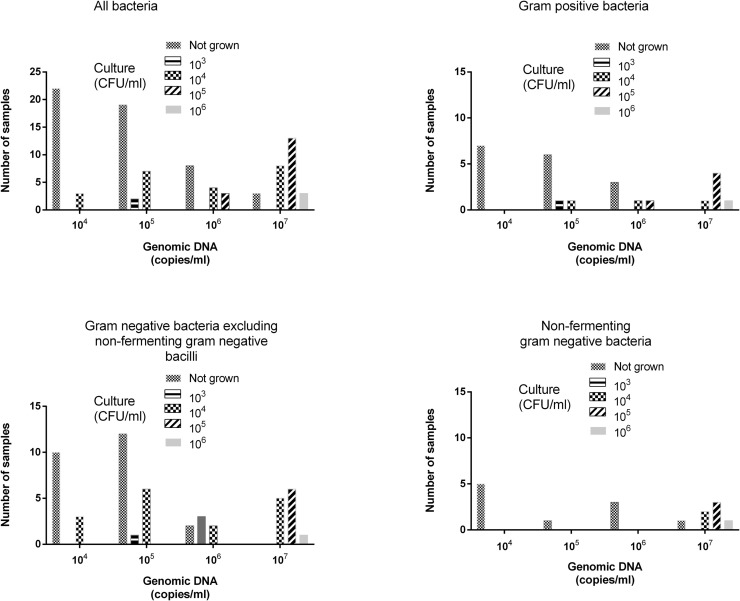
As shown in Fig. 1 , semiquantitative values reported by FA-PP were higher than those yielded by culture (median 107 copies/mL; range, 104–107 vs median, 104 CFU/mL; range, 103–107), although moderately correlated (rho = 0.65; P = 0.001), resulting in a PPA (within the same log10 value) that decreased in parallel with bacterial load (88%, 74%, 57%, and 45% for 107, 106, 105, and 104 genome copies/CFU mL, respectively) (Supplementary Table 4). A value greater than 4.5 log10 copies/mL predicted a positive culture with a sensitivity and specificity of 89% and 79.5%, respectively, as determined by ROC analysis; in turn, a value of ≥ 5 log10 copies/mL was significant (P < 0.001) with a positive culture result in logistic regression models (OR, 30.9; 95% CI, 11.24–85.16). Likewise, an increase of 1 log10 in the amount of genomic DNA quantified by FA-PP was associated with a positive culture result (OR, 2.69; 95% CI, 1.89–3.83; P = <0.001).
Fig. 1.
Quantitative result agreement between the FilmArray Pneumonia Plus panel (FA-PP) (in copies/mL) and standard of care culture (in CFU/mL) for all bacterial targets detected with FA-PP.
Overall, concordant quantitative results were more likely when Gram-negative bacteria (excluding NF-GNB) rather than Gram-positive and NF-GNB were involved. Specimens returning negative results with culture and positive results with FA-PP had bacterial DNA burdens ranging between 104 and 107 copies/mL, irrespective of the bacteria involved. Bacterial species recovered in clinically significant counts (≥105 CFU/mL) yielded 106 (n = 3) or 107 (n = 16) copies/mL; a wide range of bacterial genomic copies/mL (104–107) were quantified in specimens in which bacterial counts were 104 CFU/mL.
Regarding the identification of genotypic markers of antibiotic resistance, FA-PP allowed the detection of the mecA gene in 5 TA, from which MRSA could be recovered by culture in only 1, and the VIM-1 gene in 1 TA, from which a VIM-1-harboring Pseudomonas aeruginosa isolate was cultured.
Of interest, Entero/Rhinovirus were detected in 4 specimens.
3.2. Adjustment of EAT upon FA-PP results
Among the 99 patients undergoing EAT at the time of first FA-PP screening, 44 tested positive for 1 or more bacterial targets and 55 returned negative results (Table 4 ). For those testing positive with FA-PP, antibiotic prescription involving either de-escalation, escalation, or continuation following receipt of the FA-PP results was deemed suitable in 27 of 44; regarding those testing negative with FA-PP, suitable decisions were made in 22 of 55. Overall, most of the interventions categorized as inadequate were due to failure to discontinue MRSA antibiotic coverage (36/50). Of these 36 cases, in 29 the antibiotic not withdrawn was linezolid and on 7 occasions ceftaroline. In order of frequency, the other antibiotics not withdrawn against the FA-PP result were fluoroquinolones (10.5%), amikacin (5.3%), ceftazidime-avibactam (5.3%), or other antibiotics in smaller proportions. Importantly, modifications in antibiotic therapy based upon FA-PP results were implemented upon the attending physician's criteria and were not related to semiquantitative bacterial DNA burden estimated by assay.
Table 4.
Antibiotic prescription upon receipt of the FilmArray Pneumonia plus panel results in mechanically-ventilated patients undergoing empirical antimicrobial therapy.
| Antibiotic prescription upon receipt of FA-PP results | No. of patients | Suitable/unsuitable |
|---|---|---|
| In patients testing positive by the FA-PP | ||
| Continuation | 35 | 21/14 |
| Escalation | 5 | 3/2 |
| De-escalation | 4 | 3/1 |
| In patients testing negative by the FA-PP | ||
| Continuation | 42 | 16/26 |
| De-escalation | 9 | 6/3 |
| Escalation | 4 | 0/4 |
| FA-PP, FilmArray Pneumonia Plus panel |
3.3. Costs incurred due to adjustment of EAT upon FA-PP results
When selectively focusing on patients undergoing EAT (n = 99) at the time of FA-PP testing, the total cost incurred by FA-PP (99 tests) during the study period was 15,640 €; this figure included purchase (15,345 €) and personnel-associated costs (296 €). Real-life adjustment (de-escalation or escalation) of antimicrobial therapy following FA-PP results resulted in an added cost beyond EAT of 1868.7 €. Interestingly, suitable EAT adjustments upon FA-PP results, as defined in the methods section, would have resulted in a saving of 6675.8 €.
4. Discussion
Standard culture-based diagnostic methods for VA-LRTBI are currently too slow and insensitive to guide early antimicrobial therapy decisions, which may have a major impact on ICU patients’ clinical outcomes. Molecular syndromic panels have the potential to overcome these limitations. The current study was aimed primarily at assessing the performance of FA-PP compared to standard culture for the detection of on-panel bacterial targets from a qualitative and semiquantitative standpoint, in a relatively homogeneous population including MV patients (almost 70% admitted for severe COVID-19) with suspicion of VA-LRTBI, and most (99/109) undergoing EAT (less than 48 h) at the time of first testing. In contrast to most previous studies, which included a variety of LRT specimens for microbiological studies, TA was uniformly used as the matrix for analyses herein. We also investigated the real-life impact of the receipt of FA-PP results on antimicrobial therapy prescription in our setting, gauging the potential cost-benefit of the interventions. A large number of studies have evaluated the diagnostic performance of FA-PP compared to standard culture in patients with suspected LRTBI [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]; nonetheless, cross-study comparison is not straightforward due to differences in potentially impactful parameters, such as patient's baseline clinical condition, hospitalization ward (ICU vs. non-ICU), LRTBI features (i.e., associated or not with MV), timing of FA-PP testing after hospital admission, specimens used (endotracheal aspirates vs bronchoscopic specimens), and most importantly, whether or not EAT was in place at the time of testing. Despite these dissimilarities, all studies consistently showed the superior sensitivity of FA-PP for detection of all on-panel bacterial targets [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. In line with this, we found that, compared with culture, FA-PP increased the number of specimens reported as positive by approximately 70% and the total number of bacterial targets detected by around 120%, irrespective of their nature. This provides further proof that FA-PP increases the bacterial detection yield compared with standard culture. These figures were higher than those previously published by Buchan et al., (94.8% and 63.3%) [16], and Lee et al., (a 70.3% increase in total bacterial targets detected by the PN panel) [21], probably due to the extensive use of EAT among patients in the current study. In our experience, the PPA between qualitative results returned by FA-PP and standard culture was 44% overall, and higher for Enterobacterales compared with NF-GNB, other GNB and GPC. This figure varies widely across the aforementioned studies, likely reflecting differences in bacterial targets present in the specimens and EAT usage at time of testing [16]. Similarly, in line with previously published data [8,13,[15], [16], [17],21], use of FA-PP increased the detection of antimicrobial-resistant bacteria.
From a quantitative standpoint, although a moderate correlation between genomic copies/mL and CFU/mL was found overall, FA-PP bin values were substantially higher (1–3 log10) than CFU counts, as previously shown [7,15,16,20,21]. In our experience, PPA decreased in parallel with bacterial load, and concordant results were more likely for Enterobacterales than GPC and NF-GNB. Importantly, bacterial species recovered in clinically significant counts (≥105 CFU/mL) usually yielded 107 copies/mL. Similarly, other studies showed that concordance between quantitative results was poorest when the bacterial culture count was low [16,21].
Since molecular methods cannot discriminate between viable and non-viable bacteria, actionability criteria regarding antimicrobial therapy prescription upon receipt of FA-PP results remain undefined. For patients under EAT, targeted de-escalation upon a negative FA-PP test (i.e., discontinuation of linezolid when Gram-positive bacterial targets are not detected) seems a reasonable intervention. Nevertheless, interpretation of FA-PP quantitative results remains challenging as studies establishing bacterial DNA burden thresholds for clinical significance are lacking. Further prospective randomized studies are thus warranted to determine whether instauration of antimicrobial therapy or early adaptation of EAT based upon qualitative (any genome copy count vs negative) or quantitative FA-PP results (104–107 copies/mL) are safe and effective. In this regard, Kolenda and colleagues [9] suggested that results with ≥106 copies/mL could be used for early adaptation of antimicrobial therapy. In our setting, however, bacterial DNA burdens ≥106 copies/mL were not an uncommon finding in specimens testing negative by culture or returning CFU counts <105/mL (n = 23), irrespective of the bacterial genus/species detected; thus, our data do not support the use of that CFU threshold, at least in patients undergoing EAT; further studies are warranted to solve this issue.
Physicians at our ICU made practical decisions to adjust or continue EAT based upon qualitative FA-PP results following individual non-consensus criteria (considering acute clinical deterioration in critically ill patients and the lack of prospectively validated actionable results), which added 1868.7 € to EAT costs. Among the 99 patients undergoing EAT at the time of first FA-PP screening, interventions were deemed suitable in 49 patients and categorized as unsuitable in the remaining 50 patients, the latter mostly involving failure to discontinue MRSA antibiotic coverage. Therefore, antibiotic usage could have been improved upon receipt of FA-PP results in around half the patients, resulting in overall savings of 6675.8 €. The potential for early antibiotic adjustment according to FA-PP results has been highlighted in several studies [12,16,21] and is estimated to equal a total of >18,000 hours of antibiotic sparing, presented as an average of 3.8 days/antibiotic in 1 publication [12]. Moreover, Guillotin et al., [25] evaluated the potential impact of FA-PP on antimicrobial therapy guidance for ICU patients with VAP, with number of days on broad-spectrum antimicrobial therapy as the primary endpoint; the incremental cost-effectiveness ratio was 1121 € to avoid 1 day of nonoptimized antimicrobial therapy. Nevertheless, the use of these syndromic panels in the absence of an antimicrobial stewardship program could result in an incremental cost related to the more expensive diagnostic approach
A major limitation of our study was that assessment of the real impact of adjusting or continuing EAT after FA-PP results on relevant indicators, such as length of ICU stay, MV-free days, mortality, time to clinical or microbiological cure, or ICU readmission rate was not performed, mainly due to the relatively small sample size of the study.
In this context, elucidation of whether the FA-PP assay outperforms the conventional culture-based approach for VA-LRTBI management would require prospective randomized controlled trials. Nevertheless, appropriate decisions on antimicrobial therapy prescription made upon results of the molecular assays may be considered as a proxy for better clinical outcomes, which would translate into cost savings from a hospital perspective [6]. Furthermore, no additional tests were carried out to confirm the true nature of bacterial targets documented by the FA-PP assay in culture-negative specimens; thus, the possibility of FA-PP assays returning false positive results could not be ruled out. Our study was also limited by its single-center nature, the use of endotracheal aspirates instead of bronchoscopic specimens, the low number of bacterial targets harboring genotypic determinants of antimicrobial resistance and the impossibility of comparing the 2 diagnostic approaches in the absence of ongoing antimicrobial therapy. That the study period (March 2020 and April 2021) overlapped with a critical time in the ICU due to COVID-19 could also be considered a limitation, as these healthcare pressures may have deterred attending physicians from making decisions on antimicrobial therapy adjustments according to FA-PP results. The novelty (and strength) of the current study stems from its purpose to evaluate the FA-PP assay from a combined diagnostic and cost-benefit approach in a rather homogeneous population.
5. Conclusions
In conclusion, the data presented herein further demonstrate that the FA-PP panel outperforms the standard of care culture-based diagnostic approach in terms of sensitivity in ICU patients with suspicion of bacterial VA-LRTBI, and support the potential for early adjustment of EAT based on its results. In addition, our study underscores the need to establish consensus criteria for antimicrobial stewardship according to FA-PP results.
Informed consent
Not applicable (as discussed with the institutional medical ethics committee).
Ethics approval
The current study was approved by the Research Ethics Committee of Hospital Clínico Universitario INCLIVA.
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors have no relevant competing interests to disclose in relation to this work.
Acknowledgments
Acknowledgments
Ignacio Torres (Río Hortega Contract; CM20/00090), Estela Giménez (Juan Rodés Contract, JR18/00053) and Eliseo Albert (Juan Rodés Contract; JR20/00011) hold contracts funded by the Carlos III Health Institute (co-financed by the European Regional Development Fund, ERDF/FEDER).
Funding
The current work received no public or private funds.
Authors’ contributions
David Navarro: Conceptualization, Methodology, Josep Ferrer, Eliseo Albert, María Ángeles Clari, Ignacio Torres.: Data curation, Writing- Original draft preparation. María Luisa Blasco, Nieves Carbonell: Supervision. Josep Ferrer, Eliseo Albert, Estela Giménez: Software, Statistics analysis and Validation. David Navarro: Writing- Reviewing and Editing,
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.diagmicrobio.2022.115847.
Appendix. Supplementary materials
References
- 1.Hunter JD. Ventilator associated pneumonia. BMJ. 2012;344:e3325. doi: 10.1136/bmj.e3325. [DOI] [PubMed] [Google Scholar]
- 2.Luo W, Xing R, Wang C. The effect of ventilator-associated pneumonia on the prognosis of intensive care unit patients within 90 days and 180 days. BMC Infect Dis. 2021;21:684. doi: 10.1186/s12879-021-06383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, de Souza BD, Cianferoni S. Diagnosis, management and prevention of ventilator-associated pneumonia: an update. Drugs. 2010;70:1927–1944. doi: 10.2165/11538080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50 doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 6.Bassetti M, Vena A, Battaglini D, Pelosi P, Giacobbe DR. The role of new antimicrobials for Gram-negative infections in daily clinical practice. Curr Opin Infect Dis. 2020;33:495–500. doi: 10.1097/QCO.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 7.Ginocchio CC, Garcia-Mondragon C, Mauerhofer B, Rindlisbacher C. Multinational evaluation of the BioFire FilmArray Pneumonia plus Panel as compared to standard of care testing. Eur J Clin Microbiol Infect Dis. 2021;40:1609–1622. doi: 10.1007/s10096-021-04195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitton B, Rule R, Said M. Laboratory evaluation of the BioFire FilmArray Pneumonia plus panel compared to conventional methods for the identification of bacteria in lower respiratory tract specimens: a prospective cross-sectional study from South Africa. Diagn Microbiol Infect Dis. 2021;99 doi: 10.1016/j.diagmicrobio.2020.115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolenda C, Ranc AG, Boisset S, Caspar Y, Carricajo A, Souche A, et al. Assessment of respiratory bacterial coinfections among severe acute respiratory syndrome coronavirus 2-positive patients hospitalized in intensive care units using conventional culture and BioFire, FilmArray Pneumonia Panel plus assay. Open Forum Infect Dis. 2020;7:ofaa484. doi: 10.1093/ofid/ofaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serigstad S, Markussen D, Grewal HMS, Ebbesen M, Kommedal Ø, Heggelund L, et al. Rapid syndromic PCR testing in patients with respiratory tract infections reduces time to results and improves microbial yield. Sci Rep. 2022;12:326. doi: 10.1038/s41598-021-03741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriazopoulou E, Karageorgos A, Liaskou-Antoniou L, Koufargyris P, Safarika A, Damoraki G, et al. BioFire FilmArray Pneumonia Panel for severe lower respiratory tract infections: subgroup analysis of a randomized clinical trial. Infect Dis Ther. 2021;10:1437–1449. doi: 10.1007/s40121-021-00459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maataoui N, Chemali L, Patrier J, Tran Dinh A, Le Fèvre L, Lortat-Jacob B, et al. Impact of rapid multiplex PCR on management of antibiotic therapy in COVID-19-positive patients hospitalized in intensive care unit. Eur J Clin Microbiol Infect Dis. 2021;40:2227–2234. doi: 10.1007/s10096-021-04213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy CN, Fowler R, Balada-Llasat JM, Carroll A, Stone H, Akerele O, et al. Multicenter evaluation of the BioFire FilmArray Pneumonia/Pneumonia Plus Panel for detection and quantification of agents of lower respiratory tract infection. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00128-20. e00128-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edin A, Eilers H, Allard A. Evaluation of the Biofire Filmarray Pneumonia panel plus for lower respiratory tract infections. Infect Dis (Lond) 2020;52:479–488. doi: 10.1080/23744235.2020.1755053. [DOI] [PubMed] [Google Scholar]
- 15.Yoo IY, Huh K, Shim HJ, Yun SA, Chung YN, Kang OK, et al. Evaluation of the BioFire FilmArray Pneumonia Panel for rapid detection of respiratory bacterial pathogens and antibiotic resistance genes in sputum and endotracheal aspirate specimens. Int J Infect Dis. 2020;95:326–331. doi: 10.1016/j.ijid.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Buchan BW, Windham S, Balada-Llasat JM, Leber A, Harrington A, Relich R, et al. Practical comparison of the BioFire FilmArray Pneumonia Panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00135-20. e00135-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crémet L, Gaborit B, Bouras M, Drumel T, Guillotin F, Poulain C, et al. Evaluation of the FilmArray® Pneumonia Plus Panel for rapid diagnosis of hospital-acquired pneumonia in intensive care unit patients. Front Microbiol. 2020;11:2080. doi: 10.3389/fmicb.2020.02080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouzid D, Casalino E, Mullaert J, Laurent O, Duval X, Lescure FX, et al. Added value of rapid respiratory syndromic testing at point of care versus central laboratory testing: a controlled clinical trial. J Antimicrob Chemother. 2021;76(Supplement_3):iii20–iii27. doi: 10.1093/jac/dkab241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timbrook TT, Hueth KD, Ginocchio CC. Identification of bacterial co-detections in COVID-19 critically Ill patients by BioFire® FilmArray® pneumonia panel: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. 2021;101 doi: 10.1016/j.diagmicrobio.2021.115476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Ruan SY, Pan SC, Lee TF, Chien JY, Hsueh PR. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J Microbiol Immunol Infect. 2019;52:920–928. doi: 10.1016/j.jmii.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Posteraro B, Cortazzo V, Liotti FM, Menchinelli G, Ippoliti C, De Angelis G, et al. Diagnosis and treatment of bacterial pneumonia in critically ill patients with COVID-19 using a multiplex PCR assay: a large Italian hospital's five-month experience. Microbiol Spectr. 2021;9 doi: 10.1128/Spectrum.00695-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novy E, Goury A, Thivilier C, Guillard T, Alauzet C. Algorithm for rational use of Film Array Pneumonia Panel in bacterial coinfections of critically ill ventilated COVID-19 patients. Diagn Microbiol Infect Dis. 2021;101 doi: 10.1016/j.diagmicrobio.2021.115507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grasselli G, Cattaneo E, Florio G. Secondary infections in critically ill patients with COVID-19. Crit Care. 2021;25:317. doi: 10.1186/s13054-021-03672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaragoza R, Vidal-Cortés P, Aguilar G, Borges M, Diaz E, Ferrer R, et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit Care. 2020;24:383. doi: 10.1186/s13054-020-03091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillotin F, Poulain C, Gaborit B, Bouras M, Cinotti R, Lakhal K, et al. Potential impact of rapid multiplex PCR on antimicrobial therapy guidance for ventilated hospital-acquired pneumonia in critically ill patients, a prospective observational clinical and economic study. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.804611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.