Abstract
Background
Cardiac injury and inflammation are common findings in COVID-19 patients. Autopsy studies have revealed cardiac microvascular endothelial damage and thrombosis in COVID-19 patients, indicative of microvascular dysfunction in which reactive oxygen species (ROS) may play a role. We explored whether the ROS producing proteins NOX2, NOX4 and NOX5 are involved in COVID-19-induced cardio-microvascular endothelial dysfunction.
Methods
Heart tissue were taken from the left (LV) and right (RV) ventricle of COVID-19 patients (n = 15) and the LV of controls (n = 14) at autopsy. The NOX2-, NOX4-, NOX5- and Nitrotyrosine (NT)-positive intramyocardial blood vessels fractions were quantitatively analyzed using immunohistochemistry.
Results
The LV NOX2+, NOX5+ and NT+ blood vessels fractions in COVID-19 patients were significantly higher than in controls. The fraction of NOX4+ blood vessels in COVID-19 patients was comparable with controls. In COVID-19 patients, the fractions of NOX2+, NOX5+ and NT+ vessels did not differ significantly between the LV and RV, and correlated positively between LV and RV in case of NOX5 (r = 0.710; p = 0.006). A negative correlation between NOX5 and NOX2 (r = −0.591; p = 0.029) and between NOX5 and disease time (r = −0.576; p = 0.034) was noted in the LV of COVID-19 patients.
Conclusion
We show the induction of NOX2 and NOX5 in the cardiac microvascular endothelium in COVID-19 patients, which may contribute to the previously observed cardio-microvascular dysfunction in COVID-19 patients. The exact roles of these NOXes in pathogenesis of COVID-19 however remain to be elucidated.
Keywords: COVID-19, Heart, NOX2, NOX5, Microvasculature
1. Introduction
Respiratory syndrome coronavirus (SARS-CoV-2)-induced coronavirus disease (COVID-19) can have a serious impact on the cardiovascular system, resulting in cardiac injury and inflammation, including viral myocarditis [[1], [2], [3]]. In addition, studies have shown cardiac microvascular involvement in COVID-19 patients. For instance, endothelial damage, intravascular thrombosis and an elevated presence of prothrombotic proteins (e.g. tissue factor (TF), factor VII and factor FXII) have been found in the cardiac microvasculature of deceased COVID19 patients [4,5]. These results indicate that cardiac microvascular endothelial dysfunction may play an important role in COVID-19-induced cardiac injury and inflammation and as such relate to poor outcome [6].
Oxidative stress, i.e. an imbalance in the production of reactive oxygen species (ROS) and antioxidant mechanisms that leads to high cellular ROS levels, is a key driver of endothelial dysfunction and is involved in proinflammatory and procoagulant activation as well as cell damage in endothelial cells [7]. Moreover, oxidative stress is believed to be an important contributor to the pathogenesis of COVID-19 [8]. Indeed, both oxidative stress-related genes such as myeloperoxidase (MPO) in alveolar and circulating leucocytes [9], as well as serum levels of the oxidative stress biomarkers malondialdehyde (MDA), total oxidant status (TOS), catalase (CAT), nitrotyrosine (NT) and super oxide dismutase (SOD) [10,11], were found to be increased in COVID-19 patients and to correlate with disease severity [12,13].
Important producers of reactive oxygen species (ROS) in the heart are the NADPH oxidases (NOXes). Under physiological conditions NOX-produced ROS can act as signaling molecules in endothelial cells that regulate vascular tone and function [14]. However, different pathophysiological conditions have been shown to lead to increased expression and over-activation of NOXes, which in turn can lead to endothelial dysfunction and cell death [15]. The NOX isozymes NOX2, NOX4 and NOX5 have been shown to be expressed in the human cardiac microvascular endothelium [14,16]. NOX2 and NOX5 are inducible superoxide-producing NOX isoforms and increased endothelial expression of NOX2 and NOX5 in the human heart has been shown under pathological conditions such as heart failure [17] and myocardial infarction [16]. In addition, increased endothelial expression and activity of NOX2 and NOX5 were found to contribute to angiotensin-II-induced cardiac inflammation, fibrosis and hypertension as well as cell death and microvascular permeability [[18], [19], [20], [21], [22], [23]]. Interestingly, homocysteine-induced TF expression in endothelial cells was found to be inhibited by the NOX inhibitor apocynin [24]. Lastly, elevated serum levels of soluble NOX2-derived peptide (sNox2-dp) were found in COVID-19 patients, indicative of increased systemic NOX2 activity, that correlated with disease severity and the occurrence of thrombotic events [25]. Conversely, NOX4 predominantly produces hydrogen peroxide (H2O2) and appears to be constitutively expressed and active in endothelial cells and was shown to be involved in angiogenesis and to promote microvascular endothelial cell survival after ischemia/reperfusion injury [26,27].
Given their important roles in microvascular endothelial (dys)function, we wondered whether NOX2, NOX4 and NOX5 are involved in COVID-19-induced cardiac microvascular dysfunction. This we have studied in deceased COVID-19 patients.
2. Materials and methods
2.1. Patients
The heart tissues used in this study were derived from 29 deceased patients. Among them, 15 patients died of clinically confirmed COVID-19. The other 14 patients served as control patients that died without any form of heart disease and were without cardiac inflammation. All these control patients died before the COVID-19 outbreak. Autopsies were performed as soon as possible after death. The time from death to autopsy was <24 h. The autopsy results of the COVID-19 patients used in this study have been previously published [28]. Transmural heart tissue samples from COVID-19 patients (left ventricles and right ventricles) and control patients (left ventricles) were collected, fixed in formalin solution and then embedded in paraffin for follow-up research.
This study was approved by the ethics committee of Amsterdam UMC (Amsterdam, the Netherlands) and followed the Declaration of Helsinki. The autopsy materials in this study were used with the consent of patients or their relatives.
2.2. Immunohistochemistry
Glass-mounted tissue-sections (4 μm) of the heart tissue were used for immunohistochemical analysis. Deparaffinization and rehydration were respectively completed in fresh xylene and a range of concentrations of alcohol (from 100% alcohol to 70% alcohol). The sections were placed in 0.3% H2O2 for 20 min to inhibit endogenous peroxidase activity. Tris-EDTA buffer (pH 9.0; NOX2 and CD31) and Citrate buffer (pH 6.0; NOX4, NOX5 and NT) were used for antigen retrieval at sub-boiling temperature (98 °C) for 20 min. All sections were blocked with blocking solution for 10 min at room temperature (RT). The sections were then incubated with rabbit-anti-human NOX2 antibody (Dako; ab80508, 1:100 dilution for 1 h at RT), rabbit-anti-human NOX5 antibody (abcam; ab191010, 1:1000 dilution for 1 h at RT), mouse-anti-human CD31 antibody (Dako; M0823, 1:200 dilution, overnight at 4 °C), rabbit-anti-nitrotyrosine (Chemicon; AB5411, 1:500 dilution, overnight at 4 °C) or with rabbit-anti-human NOX4 antibody (Novus; NB110–58849, 1:100 dilution, overnight at 4 °C). After three washes with TBS, the slides were incubated with envision secondary antibodies (Dako, K5007, undiluted for 30 min at RT, for NOX2, NOX5 and NT staining; Avantor, VWRKDPVM110HRP, undiluted for 30 min at RT, for CD31 staining) or with biotinylated anti rabbit IgG (1:300 dilution, Dako E0431, 30 min at RT) and biotin-based amplification regent (Vector; PK-4000, undiluted, for 1 h at RT, for NOX4 staining). Staining was visualized with 3,3′-diaminobenzidine (DAB) (0.1 mg/mL, Dako) and then counterstained sections with hematoxylin. In this study, slides were included that were stained without primary antibody as a negative control and these yielded no staining (not shown).
2.3. Immunohistochemical analyses
The intramyocardial blood vessels wherein endothelial cells stained positive for NOX2, NOX4, NOX5 and NT were identified as positive blood vessels. The positive intramyocardial blood vessels were counted using light microscopy at 200× magnification. The surface area of each tissue was determined on scanned slides using a PathScan Enabler IV slide scanner (Meyer Instruments, Houston, TX, USA) and QuickPhoto Micro analysis software (Promicra Prague, Czech Republic). The numbers of positive blood vessels were divided by the tissue surface areas to obtain the immunohistochemical score. The total numbers of blood vessels were counted on serial CD31-stained tissue sections using Digital Pathology Solution (Philips) and were subsequently divided by the surface areas (cm2) of the analyzed tissues. The numbers of NOX2+, NOX5+ and NT+ blood vessels per cm2 where then divided by the total numbers of blood vessels per cm2 in the corresponding tissue sections to obtain the fraction of NOX2+, NOX5+ and NT+ blood vessels. Immunoscoring was performed by researchers (Z.J. and L.W.) who were blinded to the clinical data.
2.4. Immunofluorescence and imaging
Glass-mounted tissue-sections (4 μm) of the frozen heart tissue were used for immunofluorescent staining. All sections were blocked with blocking solution for 10 min at RT. The sections were then incubated with mouse-anti-human NOX2 antibody (abcam; ab80897, 1:200 dilution) and rabbit-anti-human CD31 antibody (abcam; ab28364, 1:50 dilution) for 2 h at RT for NOX2 and CD31 double staining, or with rabbit-anti-human NOX5 antibody (abcam; ab191010, 1:1000 dilution) and mouse-anti-human CD31 antibody (Dako; M0823, 1:200 dilution) for 2 h at RT for NOX5 and CD31 double staining. Then the slides were washed with PBS for three times and incubated with DAPI and secondary antibodies (Invitrogen, A11029, Alexa Fluor 488 conjugated goat-anti-mouse IgG, 1:500 dilution for NOX2; Jackson immunoresearch, 711–545-152, Alexa Fluor 488 conjugated Donkey-anti-rabbit IgG, 1:500 dilution for NOX5; Invitrogen, A11036, Alexa Fluor 568 conjugated goat-anti-rabbit IgG, 1:500 dilution and Alexa Fluor 568 conjugated goat-anti-mouse IgG, 1:500 dilution for CD31) for 1 h at RT in dark chamber. In this study, slides were included that were stained without primary antibody as a negative control and these yielded no staining (not shown).
The wild-field fluorescence microscope (Leica DM5000B) and camera (Leica DFC500) were used to observe and image slides at 100× magnification.
2.5. Statistics
For graph design GraphPad Prism software version 9 (San Diego, CA, USA) was used. SPSS software (version 26.0, Armonk, NY, USA) was used to statistically analyze the data. Differences in gender distribution were analyzed with a Chi-Squared test. To evaluate differences between two groups, t-test were used for normal distributed data and Mann-Whitney U test were used for non-Gaussian distributed data. A p value <0.05 was considered statistically significant. A Spearman rank correlation coefficient (r) was used to determine linear relations between two groups, whereby −0.29 < r < 0.29 was considered as a correlation absence, −0.49 < r < −0.30 or 0.30 < r < 0.49 was considered as a moderate correlation, and − 1.0 < r < −0.50 or 0.50 < r < 1.0 was considered as a strong correlation. In the text the values are given as median [Interquartile range] unless stated otherwise.
3. Results
3.1. Patient characteristics
The patients under investigation in this study have been used in a previous COVID-19 study [4]. The clinical characteristics of the included patients are presented in Table 1 . Both groups were in majority male (Control: 10 male (71.4%); COVID-19: 12 male (80.0%)). There was a significant difference in age between the two groups. The mean [SD] age of the COVID-19 patients (67.4 [9.5]) was significantly higher than controls (53.0 [14.0], p = 0.003). All COVID-19 patients were hospitalized, of whom 13 (87%) were ventilated for 6 to 53 days. The time between first COVID-19 symptoms to death ranged from 5 to 44 days. Prior to admittance, 8 COVID-19 patients were hypertensive for whom 6 received antihypertensive medication, 1 had arrhythmia, 1 had congestive heart failure and 2 had prior cardiac ischemia. Four COVID-19 patients received thrombocyte aggregation inhibitors prior to admittance.
Table 1.
Clinical Characteristics of Patients in this study.
| Characteristic | Control (n = 14) | COVID-19 (n = 15) |
|---|---|---|
| Age, Mean (SD) | 53.0(14.0) | 67.4(9.5)⁎⁎ |
| Gender (n, %) | ||
| Female | 4 (29%) | 3 (20%) |
| Male | 10 (71%) | 12 (80%) |
| COVID-19 Disease time, mean days (range) (first symptoms to death) | N.A. | 21 (5–44) |
| Ventilated, yes/no (%) | 0/14 (0%) | 13/2 (87% / 13%) |
| Ventilation time, mean days (range) | N.A. | 17 (6–53) |
| Comorbidities | ||
| Hypertension | n.k. | 8 (53%) |
| Arrhythmia | 0 (0%) | 1 (7%) |
| Pulmonary disease | 2 (14%) | 1 (7%) |
| Cardiac ischemia | 0 (0%) | 2 (13%) |
| CHF | 0 (0%) | 1 (7%) |
| Diabetes | 0 (0%) | 1 (7%) |
| Active Malignancy | 4 (29%) | 2 (13%) |
| Neurologic | 2 (14%) | 3 (20%) |
| Medication before admittance | ||
| Antihypertensive | n.k. | 6 (40%) |
| Analgesics | n.k. | 1 (7%) |
| Oral antidiabetics | n.k. | 2 (13%) |
| Thrombocyte aggregation inhibitors | n.k. | 4 (27%) |
| Medication during admittance | ||
| Antibiotics | n.k. | 14 (93%) |
| Chloroquine | n.k. | 8 (53%) |
| Anti-viral drugs | n.k. | 6 (40%) |
| Steroids | n.k. | 5 (33%) |
| Anti-fungal | n.k. | 6 (40%) |
| Cardiac symptoms during admittance | ||
| Arrhythmia | 0 (0%) | 10 (67%) |
| CHF | 0 (0%) | 1 (7%) |
| Post-mortem diagnosed myocarditis | ||
| lymphocytic | 0 (0%) | 7 (47%) |
| DCI | 0 (0%) | 8 (53%) |
| Thrombotic events | ||
| Deep vein thrombosis | 0 (0%) | 5 (33%) |
| pulmonary embolism | 1 (7%) | 4 (27%) |
| Intravascular thrombi | 0 (0%) | 7 (47%) |
| Supra infections | ||
| Aspergillus | 0 (0%) | 5 (33%) |
| Fungal | 0 (0%) | 1 (7%) |
| Bacterial | 0 (0%) | 1 (7%) |
| Cause of death | ||
| COVID-19 pulmonary disease | 0 (0%) | 9 (60%) |
| Multiple organ failure | 0 (0%) | 4 (27%) |
| Pulmonary embolism | 0 (0%) | 1 (7%) |
| Brain infarction (Stroke) | 2 (14%) | 1 (7%) |
| Rupture aneurysm brain | 2 (14%) | 0 (0%) |
| Rupture aneurysm aorta | 1 (7%) | 0 (0%) |
| Pneumonia and sepsis | 4 (29%) | 0 (0%) |
| Pneumonia | 2 (14%) | 0 (0%) |
| Epileptic insult | 1 (7%) | 0 (0%) |
| Car accident | 1 (7%) | 0 (0%) |
| Aortic dissection | 1 (7%) | 0 (0%) |
COVID-19 indicates patients with SARS-CoV-2 infection. Control indicates patients died not related with any form of heart disease and did not have inflammation of the heart. Controls died at least 1 year prior to the COVID-19 outbreak.
CHF indicates congestive heart failure. N.A. indicates not applicable; n.k. indicates not known (i.e. the information was either not known or not retrievable); CHF indicates congestive heart failure; DCI indicates diffuse cardiac inflammation.
Indicates p < 0.01 compared with control group.
A majority of COVID-19 patients (n = 10 (67%)) developed cardiac arrhythmia during hospitalization. Furthermore, thrombotic events were prevalent in the COVID-19 group with deep-vein thrombosis and pulmonary embolism being observed in 5 (33%) and 4 (27%) patients respectively, indicative of systemic hypercoagulability. A majority of COVID-19 patients had leukocytosis during admittance (n = 13 (87%)). All COVID-19 patients showed signs of cardiac inflammation, e.g. increases in CD45+ lymphocytes, CD3+ T lymphocytes, CD68+ macrophages and myeloperoxidase (MPO) + neutrophils in the myocardium, that was accompanied by injury in dispersed small cardiomyocyte clusters or individual cells [4,28]. In 7 COVID-19 patients the cardiac inflammation was consistent with lymphocytic myocarditis (LM), with an infiltrate that was mainly composed of clusters of adherent T lymphocytes, and a lesser extent of macrophages [29]. The other 8 COVID-19 patients presented with a dispersed mixed infiltration of lymphocytes, macrophages and neutrophils that we refer to as diffuse cardiac inflammation (DCI) [4].
3.2. NOX2, NOX5 and NT are increased in the cardiac microvasculature of COVID-19 patients
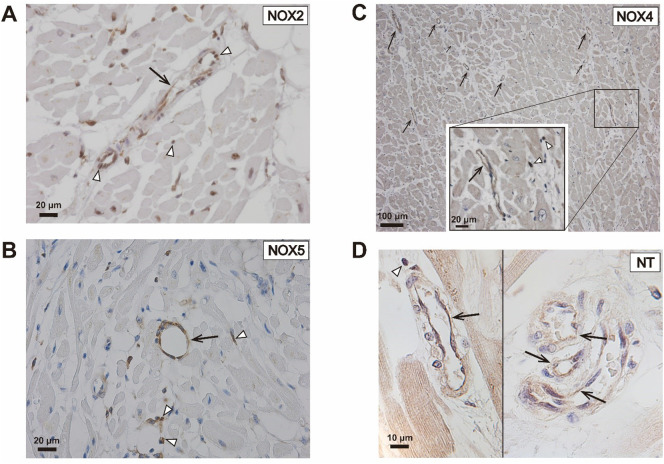
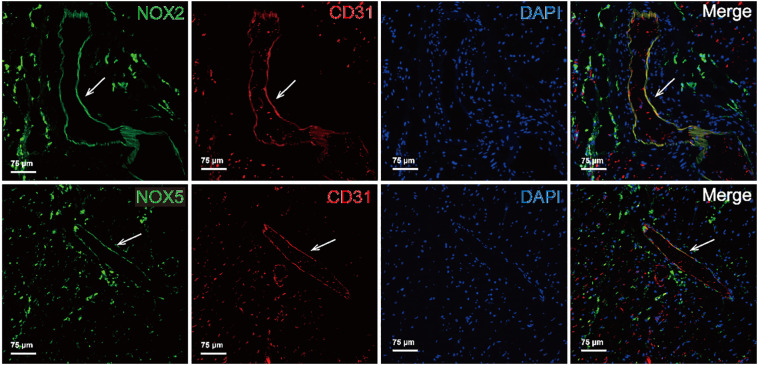
NOX2 (Fig. 1A), NOX4 (Fig. 1C) and NOX5 (Fig. 1B) were present in the cardiac microvascular endothelium. It has to be noticed that NOX2, NOX4 and NOX5 were also present in infiltrating inflammatory cells, most notably in neutrophils and macrophages (Fig. 1). NOX4 was found in the endothelium of almost all intramyocardial blood vessels in all patients, without significant differences between controls and COVID-19 patients (data not shown). Immunofluorescent staining showed that NOX2 and NOX5 co-localized with the endothelial cell marker CD31, confirming the presence of these NOXes in the cardiac microvascular endothelium of patients with COVID-19 (Fig. 2 ).
Fig. 1.
Immunohistochemical staining of NOXes and NT in the cardiac microvasculature of COVID-19 patients.
Shown are examples of immunohistochemical staining for (A) NOX2, (B) NOX5, (C) NOX4 and (D) NT in the cardiac microvasculature in the LV of COVID-19 patients. The black arrows indicate blood vessels with NOX- and NT-positive endothelial cells, while the white arrowheads indicate NOX- and NT-positive extravasated leucocytes.
Fig. 2.
Immunofluorescent staining of NOXes and CD31 in the cardiac microvasculature of COVID-19 patients.
Shown are examples of immunofluorescent double staining for NOX2 + CD31 (the top row) and NOX5 + CD31 (the bottom row) in the cardiac microvasculature in the LV of COVID-19 patients. The white arrows indicate blood vessels with NOXes-positive and CD31-positive endothelium. NOXes were stained as green as shown in the first column. CD31 was stained as red as shown in the second column, the nucleic acid was stained as blue in the third column. The images shown in the fourth column are the merge of NOXes, CD31and nucleic acids. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
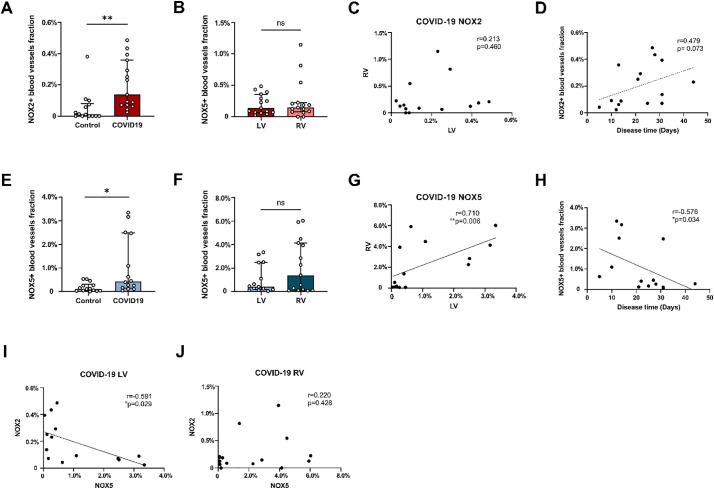
There was no significant difference in the total number of blood vessels per cm2 in the LV between controls and COVID19 patients (data not shown). In the left ventricle (LV) of COVID-19 patients the fraction of NOX2+ (0.14% [0.07%–0.36%]) and NOX5+ (0.43% [0.15%–2.48%]) intramyocardial blood vessels were significantly increased compared with the LV in controls (NOX2+: 0.012% [0–0.081%], p < 0.01; NOX5+: 0.12% [0.037%–0.32%], p < 0.05). Notably, the average fraction of NOX5+ intramyocardial blood vessels was approximately 3-fold higher than that of NOX2+ intramyocardial blood vessels in COVID-19 patients (Fig. 3A and E). In COVID-19 patients, the fractions of NOX2+ and NOX5+ blood vessels did not differ significantly between the left and right ventricle (RV) (Fig. 3B and F). We did not find a correlation between the fractions of NOX2+ blood vessels in the LV and RV (Fig. 3C), whereas a strong positive correlation was found between the fractions of NOX5+ vessels in the LV and RV (r = 0.710; p = 0.006) (Fig. 3G).
Fig. 3.
Quantification of the presence of NOX2 and NOX5 in the cardiac microvasculature of COVID-19 patients.
Shown are the fractions of NOX2+ (A) and NOX5+ (E) blood vessels in the left ventricle (LV) in control and COVID-19 patients and the fractions of NOX2+ (B) and NOX5+ (F) blood vessels in the LV and right ventricle (RV), as well as the correlation analysis of the NOXes between LV and RV in COVID-19 patients (C and G). The correlation analyses between NOX2 (D) or NOX5 (H) expression in the LV of COVID-19 patients and disease time are also shown. In addition, the correlation analyses between NOX2 and NOX5 in the LV(I) and RV(J) of COVID-19 patients are shown. The bars in the graphs represent median [IQR]. Each point represents the score of an individual patient.
*P < 0.05, **P < 0.01 and ns indicates not significant.
We next analyzed a putative correlation between the fractions of NOX2+ and NOX5+ blood vessels. No correlation was found between NOX2 and NOX5 in the LV of control patients (data not shown). In COVID-19 patients, a negative correlation was found between NOX2 and NOX5 in the LV (r = −0.591; p = 0.029) (Fig. 3I), but not in the RV (Fig. 3J). In addition, both in controls and COVID-19 patients the fractions of NOX2+ and NOX5+ blood vessels did not correlate with age (data not shown). Moreover, a moderate positive correlation, albeit not significant (r = 0.479; p = 0.073) was found between the fractions of NOX2+ blood vessels and disease time in the LV (Fig. 3D), but not in the RV (data not shown), while a strong negative correlation was found between the fractions of NOX5+ blood vessels in the LV and disease time (r = −0.576; p = 0.034) (Fig. 3H), but not in the RV (data not shown). Whereas no significant correlation between NOX5 and NOX2 was found in the LV of COVID-19 patients when disease time was used as a control variable (r = −0.464; p = 0.110).
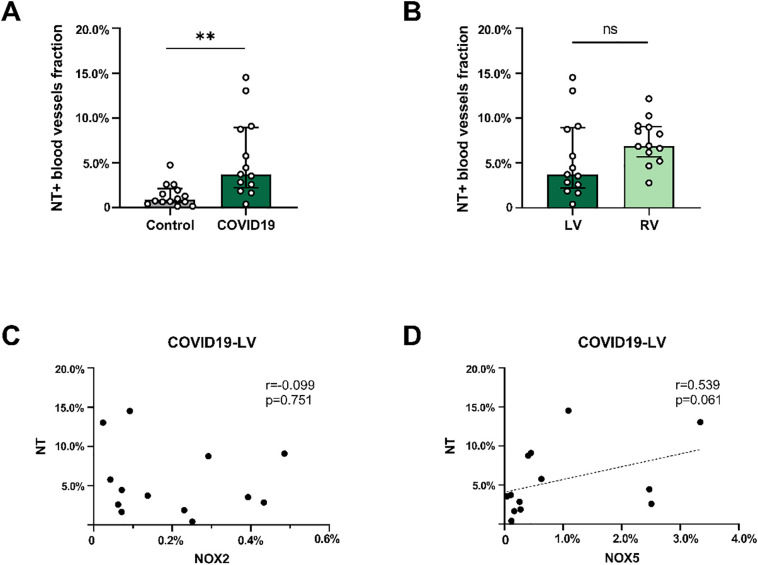
In addition, the fraction of NT+ (3.73% [2.24%–8.92%]) blood vessels in the LV of COVID-19 patients was significantly increased compared with the LV in controls (0.87% [0.61%–2.12%]) (Fig. 4A). The fractions of NT+ blood vessels did not differ significantly between the left and right ventricle in COVID-19 patients (Fig. 4B). No significant correlation between NT and NOX2 was found in COVID-19 patients (Fig. 4C), whereas a strong positive correlation between NT and NOX5 was found in the LV, albeit not significant (r = 0.539; p = 0.061)(Fig. 4D).
Fig. 4.
Quantification of the presence of NT in the cardiac microvasculature of COVID-19 patients.
Shown are the fractions of NT+ (A) blood vessels in the left ventricle (LV) in control and COVID-19 patients and the fractions of NT+ (B) blood vessels in the LV and right ventricle (RV) in COVID-19 patients. The correlation analyses between NOX2 (C) or NOX5 (D) expression in the LV of COVID-19 patients and NT are also shown. The bars in the graphs represent median [IQR]. Each point represents the score of an individual patient.
**P < 0.01 and ns indicates not significant.
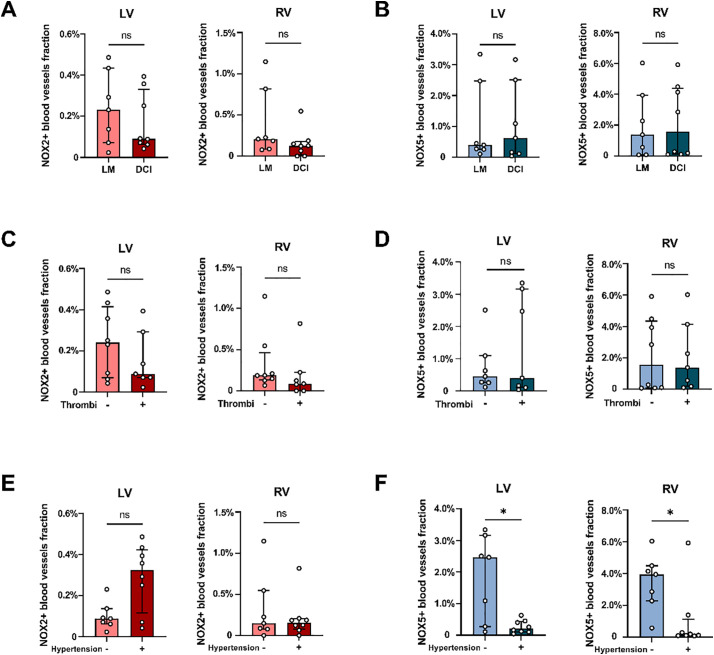
Among the 15 patients in the COVID-19 group, 7 patients were diagnosed with LM and 8 patients with DCI. The fractions of NOX2+ and NOX5+ blood vessels however did not differ significantly between the LM patients and DCI patients, neither in the LV nor in the RV (Fig. 5A and B). Furthermore, in seven out of 15 COVID-19 patients (47%), intravascular thrombi were found in the heart. However, no differences were found in the fractions of NOX2+ and NOX5+ vessels between COVID-19 patients with and without intravascular thrombi, neither in the LV nor in the RV (Fig. 5C and D). Prior to admittance 8 COVID-19 patients were hypertensive. The average fraction of NOX2+ blood vessels in LV of hypertensive patients appeared higher than in patients without hypertension, albeit not significant (p = 0.054), while they were similar in the RV (Fig. 5E). Surprisingly however, the fraction of NOX5+ blood vessels in patients with hypertension was significantly lower than in patients without hypertension, both in LV (hypertension-: 2.47% [0.27%–3.17%], hypertension+: 0.21% [0.10%–0.44%], p < 0.05) and RV (hypertension-: 3.93% [2.27%-4.48], hypertension+: 0.15% [0.087%–1.11%], p < 0.05) (Fig. 5F).
Fig. 5.
The presence of NOX2 and NOX5 in the cardiac microvasculature of COVID-19 patients related to cardiac inflammation, cardiac thrombosis and hypertension.
Shown are the fractions of NOX2+ (A) and NOX5+ (B) blood vessels in the left ventricle (LV) and right ventricle (RV) of COVID-19 patients with lymphocytic myocarditis (LM, n = 7) and with diffuse cardiac inflammation (DCI, n = 8). In addition, the fractions of NOX2+ (C) and NOX5+ (D) blood vessels in the LV and RV of COVID-19 patients with (+; n = 7) or without (−; n = 8) intravascular thrombi are also shown. Furthermore, the fractions of NOX2+ (E) and NOX5+ (F) blood vessels in the LV and RV of COVID-19 patients with (+; n = 8) or without (−; n = 7) hypertension are shown. *P < 0.05 and ns indicates not significant.
4. Discussion
In this study, we analyzed the cardiac microvascular endothelial levels of NOX2, NOX4, NOX5 and NT in COVID-19 patients. We found that the microvascular endothelial NOX2, NOX5 and NT levels in the hearts of COVID-19 patients were significantly higher than those in controls, while NOX4 levels did not differ between these groups. These results indicate a role for oxidative stress in COVID-19-induced cardiac microvascular endothelial dysfunction related to NOX2 and NOX5.
Several studies have shown that endothelial dysfunction is an important manifestation of COVID-19 [30], also in the heart. We have previously found increased levels of the advanced glycation end-product N(ε)-Carboxymethyllysine (CML) in the cardiac microvascular endothelium of these COVID-19 cases, which is a biomarker of endothelial inflammation and cell damage [4]. This coincided with a pro-thrombotic microvascular activation, as indicated by the increased expression of procoagulant tissue factor (TF) and the decrease of anticoagulant Dipeptidyl Peptidase 4 (DPP4), as well as the occurrence of cardiac microvascular thrombosis [4,28]. Both increased CML accumulation and TF expression and loss of DPP4 in endothelial cells have been shown to relate to NOX2 activity [24,31]. In addition, thrombin, a known inducer of TF expression in endothelial cells, was also shown to induce NOX5 expression and activity in human microvascular endothelial cells, [32] while depletion of NOX5 via siRNA reduced ROS levels and expression of ICAM-1 and VCAM-1 in human aortic endothelial cells [33]. Moreover, thrombin generation was found to be enhanced in COVID-19 patients [34]. These results thus support a role for NOX2- and NOX5-derived ROS in COVID-19-induced endothelial thrombogenicity in the heart.
In addition, increased NOX2- and NOX5-related ROS production may negatively affect the bioavailability of nitric oxide (NO) in endothelial cells through inactivation of endothelial NO synthase (eNOS) [19,35] or increased consumption of NO through high superoxide levels, thereby negatively affecting the antioxidant properties and vasomotor function of NO. Indeed, the increased levels of NOX2 and NOX5 coincided with elevated levels of NT in our study, indicative of oxidative stress in the cardiac microvascular endothelium in COVID-19 patients. In line herewith, blood levels of NO were recently shown to be significantly reduced in conjunction with increased NT levels in COVID-19 patients [36]. indicative for reduced bioavailability of NO and increased oxidative stress, which supports this hypothesis.
Multiple factors may contribute to the upregulation of cardiac microvascular endothelial NOX2 and NOX5. These include the high levels of circulating pro-inflammatory cytokines that often accompany COVID-19 as well as the increased cardiac inflammation and local ischemia, induced by the microvascular thrombosis, we found in these COVID-19 patients [4,28]. Indeed, we previously found increased microvascular endothelial NOX5 expression in patients after acute myocardial infarction [16], suggesting a possible role for ischemia in the induction of endothelial NOX5. In addition, the vasoactive agent angiotensin II (AngII) was found to induce NOX5 expression in endothelial cells [20], as well as NOX2 [37]. However, although plasma AngII levels were reported to be upregulated in patients with especially severe/critical COVID-19 [38], other studies have reported no differences in plasma AngII levels between COVID-19 patients and healthy controls [39,40].
Interestingly, we found a borderline significant (p = 0.054) increase in the fraction of NOX2+ and a significant decrease in NOX5+ blood vessels in hypertensive COVID-19 patients compared to patients without hypertension. Both NOX2 and NOX5 have been found to play an important role in the pathogenesis of hypertension [41]. In various hypertension models NOX2 was found to be upregulated [37,42], which is in line with our results. However, higher NOX5 levels were reported in circulating endothelial microparticles in hypertensive compared to normotensive humans and mice expressing human NOX5 in endothelial cells developed hypertension [19]. Our finding of lower NOX5 levels are thus inconsistent herewith. Of note, 6 out of 8 hypertensive COVID-19 patients received antihypertensive medication and it was shown that antihypertensive Ca2+ channel blockers reduce the intracellular Ca2+ concentration [20,32], and attenuate AngII-induced endothelial NOX5 expression [20]. However, whether the use of antihypertensive drugs underlies the observed lower endothelial NOX5 in these hypertensive COVID-19 patients remains to be elucidated.
The microvascular endothelial levels of NOX2 and NOX5 were negatively correlated in the LV of COVID-19 patients. One possible explanation is that the increased expression and activity of one NOX isoform directly or indirectly inhibits the other. However, as far as we are aware such cross-talk between NOX isoforms has not been shown before. Disease time may also be a factor herein. The microvascular endothelial NOX5 levels namely correlated negatively with disease time, while NOX2 showed a moderate positive association with disease time, albeit not significant. These data suggest that NOX5 may be involved predominantly early, while the involvement of NOX2 may occur later in COVID-19-related cardiac pathology. We also found that the numbers of NOX2+ and NOX5+ intramyocardial blood vessels in the RV were similar to those in the LV in the COVID-19 patients. Although we were unable to verify this in RV tissue from control patients, this suggests that NOX2 and NOX5 were also increased in the microvascular endothelium of the RV. As our COVID-19 patients all had severe diffuse alveolar damage (DAD) [28], putative hemodynamic changes caused by reduced lung perfusion, induced by pulmonary microvascular damage and thrombosis, could result in an increased RV afterload and may be an additional underlying factor herein.
We found no effects of COVID-19 on the number of NOX4+ blood vessels in the heart. NOX4 was previously found to be constitutively expressed in rat cardiac microvascular endothelial cells in vitro [15], which is in line with the abundant expression we found in our patients. However, we did not verify whether the expression levels of NOX4 in the individual endothelial cells were affected, nor whether its activity or its function change by COVID-19. In contrast to NOX2 and NOX5, NOX4 activity in endothelial cells is generally considered to be beneficial. For instance, overexpression of NOX4 in endothelial cells was found to promote angiogenesis through increased eNOS activation and NO production [26]. However, genetic knockout of NOX4 was found to greatly protect from cerebral ischemia-induced oxidative stress and blood-brain-barrier leakage in mice [43], indicating a deleterious effect of endothelial NOX4. Interestingly, this damaging role for NOX4 was shown to be unique for the brain as NOX4 knockout did not affect the heart or hindlimb after experimental ischemia in mice [44].
In conclusion, in this study we show the induction of the oxidative stress-associated enzymes NOX2 and NOX5 in the cardiac microvascular endothelium in COVID-19 patients. These NOX isoforms may thus be a contributor to the previously observed microvascular dysfunction in the hearts of COVID-19 patients. However, the exact roles of these NOXes in pathogenesis of COVID-19 remain to be elucidated.
Disclosure
None.
CRediT authorship contribution statement
Zhu Jiang: Methodology, Formal analysis, Data curation, Visualization, Writing – original draft. Linghe Wu: Visualization, Formal analysis, Data curation, Writing – review & editing. Britt van der Leeden: Visualization, Writing – review & editing. Albert C. van Rossum: Resources, Methodology, Writing – review & editing. Hans W.M. Niessen: Resources, Project administration, Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. Paul A.J. Krijnen: Resources, Project administration, Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
None.
Acknowledgements
This work was supported by the China Scholarship Council (Beijing, China, grant number 202008320278 to ZJ; Beijing, China, grant number 201708260020 to LW); Heath Holland (The Hague, The Netherlands, Sector Life Sciences & Health (LSH) - Topconsortia for Knowledge and Innovation's (TKI) grant, number LSHM19106 to BvdL).
References
- 1.Faconti L., Chowienczyk P.J., Shah A.M. Cardiovascular disease, heart failure and COVID-19. J. Renin-Angiotensin-Aldosterone Syst. 2020;21 doi: 10.1177/1470320320926903. 1470320320926903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of Cardiac Injury with Mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen B.H., Shi N.N., Wu C.W., An D.A., Shi Y.X., Wesemann L.D., et al. Early cardiac involvement in patients with acute COVID-19 infection identified by multiparametric cardiovascular magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging. 2021;22:844–851. doi: 10.1093/ehjci/jeab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu L., Baylan U., van der Leeden B., Schurink B., Roos E., Schalkwijk C.G., et al. Cardiac inflammation and microvascular procoagulant changes are decreased in second wave compared to first wave deceased COVID-19 patients. Int. J. Cardiol. 2022;349:157–165. doi: 10.1016/j.ijcard.2021.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrini D., Kawakami R., Guagliumi G., Sakamoto A., Kawai K., Gianatti A., et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 6.Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC working Group for Atherosclerosis and Vascular Biology, and the ESC Council of basic cardiovascular science. Cardiovasc. Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joffre J., Hellman J. Oxidative stress and endothelial dysfunction in Sepsis and acute inflammation. Antioxid. Redox Signal. 2021;35:1291–1307. doi: 10.1089/ars.2021.0027. [DOI] [PubMed] [Google Scholar]
- 8.Chernyak B.V., Popova E.N., Prikhodko A.S., Grebenchikov O.A., Zinovkina L.A., Zinovkin R.A. COVID-19 and oxidative stress. Biochemistry (Mosc) 2020;85:1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saheb Sharif-Askari N., Saheb Sharif-Askari F., Mdkhana B., Hussain Alsayed H.A., Alsafar H., Alrais Z.F., et al. Upregulation of oxidative stress gene markers during SARS-COV-2 viral infection. Free Radic. Biol. Med. 2021;172:688–698. doi: 10.1016/j.freeradbiomed.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehri F., Rahbar A.H., Ghane E.T., Souri B., Esfahani M. The comparison of oxidative markers between COVID-19 patients and healthy subjects. Arch. Med. Res. 2021;52(8):843–849. doi: 10.1016/j.arcmed.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soto M.E., Guarner-Lans V., Diaz-Diaz E., Manzano-Pech L., Palacios-Chavarria A., Valdez-Vazquez R.R., et al. Hyperglycemia and loss of redox homeostasis in COVID-19 patients. Cells. 2022:11. doi: 10.3390/cells11060932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laforge M., Elbim C., Frere C., Hemadi M., Massaad C., Nuss P., et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedford F. 2021. Removing Fuel from Coronavirus Fire: Blocking Superoxide through NOX Inhibition. [Google Scholar]
- 14.Larsen B.T., Bubolz A.H., Mendoza S.A., Pritchard K.A., Jr., Gutterman D.D. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2009;29:739–745. doi: 10.1161/ATVBAHA.108.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirker A., Zhang M., Shah A.M. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res. Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn N.E., Meischl C., Kawahara T., Musters R.J., Verhoef V.M., van der Velden J., et al. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am. J. Pathol. 2012;180:2222–2229. doi: 10.1016/j.ajpath.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Lam C.S., Brutsaert D.L. Endothelial dysfunction: a pathophysiologic factor in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2012;60:1787–1789. doi: 10.1016/j.jacc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch C.E., Chaubey S., Zeng L., Yu B., Ivetic A., Walker S.J., et al. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J. Am. Coll. Cardiol. 2014;63:2734–2741. doi: 10.1016/j.jacc.2014.02.572. [DOI] [PubMed] [Google Scholar]
- 19.Elbatreek M.H., Sadegh S., Anastasi E., Guney E., Nogales C., Kacprowski T., et al. NOX5-induced uncoupling of endothelial NO synthase is a causal mechanism and theragnostic target of an age-related hypertension endotype. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montezano A.C., Burger D., Paravicini T.M., Chignalia A.Z., Yusuf H., Almasri M., et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ. Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J.M., Fan L.M., George V.T., Brooks G. Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic. Biol. Med. 2007;43:976–986. doi: 10.1016/j.freeradbiomed.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casas A.I., Kleikers P.W., Geuss E., Langhauser F., Adler T., Busch D.H., et al. Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J. Clin. Invest. 2019;129:1772–1778. doi: 10.1172/JCI124283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu F., Szczepaniak W.S., Shiva S., Liu H., Wang Y., Wang L., et al. Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury. Am. J. Phys. Lung Cell. Mol. Phys. 2014;307:L987–L997. doi: 10.1152/ajplung.00063.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korkmaz H., Hahn N., Jansen K., Musters R., van Bezu J., van Wieringen W., et al. Homocysteine-induced inverse expression of tissue factor and DPP4 in endothelial cells is related to NADPH oxidase activity. Physiol. Intern. 2019;106:29–38. doi: 10.1556/2060.106.2019.05. [DOI] [PubMed] [Google Scholar]
- 25.Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R., et al. Nox2 activation in Covid-19. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craige S.M., Chen K., Pei Y., Li C., Huang X., Chen C., et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Hong Z., Zeng C., Yu Q., Wang H. NADPH oxidase 4 promotes cardiac microvascular angiogenesis after hypoxia/reoxygenation in vitro. Free Radic. Biol. Med. 2014;69:278–288. doi: 10.1016/j.freeradbiomed.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Schurink B., Roos E., Radonic T., Barbe E., Bouman C.S.C., de Boer H.H., et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caforio A.L., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur. Heart J. 2013;34(2636–48) doi: 10.1093/eurheartj/eht210. 48a-48d. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNicolantonio J.J., McCarty M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart. 2020:7. doi: 10.1136/openhrt-2020-001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BelAiba R.S., Djordjevic T., Petry A., Diemer K., Bonello S., Banfi B., et al. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 33.Pai W.Y., Lo W.Y., Hsu T., Peng C.T., Wang H.J. Angiotensin-(1-7) inhibits thrombin-induced endothelial phenotypic changes and reactive oxygen species production via NADPH oxidase 5 downregulation. Front. Physiol. 2017;8:994. doi: 10.3389/fphys.2017.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campello E., Bulato C., Spiezia L., Boscolo A., Poletto F., Cola M., et al. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin. Chem. Lab. Med. 2021;59:1323–1330. doi: 10.1515/cclm-2021-0108. [DOI] [PubMed] [Google Scholar]
- 35.Judkins C.P., Diep H., Broughton B.R., Mast A.E., Hooker E.U., Miller A.A., et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 36.Dominic P., Ahmad J., Bhandari R., Pardue S., Solorzano J., Jaisingh K., et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dikalov S.I., Nazarewicz R.R., Bikineyeva A., Hilenski L., Lassegue B., Griendling K.K., et al. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2014;20:281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z., Hu R., Zhang C., Ren W., Yu A., Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care. 2020;24:290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieder M., Wirth L., Pollmeier L., Jeserich M., Goller I., Baldus N., et al. Serum ACE2, angiotensin II, and aldosterone levels are unchanged in patients with COVID-19. Am. J. Hypertens. 2021;34:278–281. doi: 10.1093/ajh/hpaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henry B.M., Benoit S., Lippi G., Benoit J. Letter to the editor - circulating plasma levels of angiotensin II and aldosterone in patients with coronavirus disease 2019 (COVID-19): a preliminary report. Prog. Cardiovasc. Dis. 2020;63:702–703. doi: 10.1016/j.pcad.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedeek M., Hebert R.L., Kennedy C.R., Burns K.D., Touyz R.M. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr. Opin. Nephrol. Hypertens. 2009;18:122–127. doi: 10.1097/MNH.0b013e32832923c3. [DOI] [PubMed] [Google Scholar]
- 42.Murdoch C.E., Alom-Ruiz S.P., Wang M., Zhang M., Walker S., Yu B., et al. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res. Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinschnitz C., Grund H., Wingler K., Armitage M.E., Jones E., Mittal M., et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casas A.I., Geuss E., Kleikers P.W.M., Mencl S., Herrmann A.M., Buendia I., et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. U. S. A. 2017;114:12315–12320. doi: 10.1073/pnas.1705034114. [DOI] [PMC free article] [PubMed] [Google Scholar]