Abstract
RATIONALE:
We studied an aldehyde-labeling reagent, N-{2-[(4-aminophenoxy)methyl]benzyl}-N,N-diethylethanaminium bromide (CAX-A), containing an aniline functional group, for the detection of aldehydes with high specificity.
METHODS:
Six standard aldehydes were labeled by CAX-A and analyzed by LC-ESI-Orbitrap-MS. The aldehydes (40 nmol each) were derivatized with CAX-A in the presence of sodium cyanoborohydride at room temperature overnight. The labeling reaction was applied to two urine samples for the detection of putative aldehydes.
RESULTS:
All six standard CAX-aldehyde derivatives were detected as precursor ions by dilution to 830 fmol/injection (S/N 587 to 1573). A total of 2,184 MS1 features were detected overall in urines and blanks, of which 14 were putative aldehydes found only in the urines.
CONCLUSIONS:
CAX-A can provide three levels of specificity for aldehyde detection. First is the known labeling specificity of the aniline functional group for aldehydes, which we confirmed here by observing a significant peak only from the aldehyde (S/N = 3388) when a mixture of an aldehyde, a ketone (no peak), and quinone (S/N = 2.3) was tested. Second is the ease of formation of an analyte-characteristic first product ion (via anchimeric-assisted loss of triethylamine as a neutral) in MS2 from a CAX-labeled analyte. Third is the formation of a characteristic second product ion via loss of CO in MS3. CAX-A enables specific, convenient detection of putative aldehydes in urine.
Keywords: Mass tags, derivatization, aldehydes, urine
INTRODUCTION
Three derivatizing agents have been reported for the specific detection of aldehydes by LCMS, to the best of our knowledge. Eggink and colleagues developed 4-[2-(trimethylammonio)ethoxy]benzaminium halide (4-APC), which has an aniline functional group1. This reagent was used to derivatize standard aldehydes (C6-C10) in buffer in the 0–500 nM range. The limits of detection (LOD) were reported as being in the 3–33 nM range. LODs were defined as the concentration corresponding to the S/N ratio of 3. To evaluate specificity, pentanal and pentanone were tested and the latter was said to be “poorly derivatized” after 20 h. El Maghrabey and coworkers developed 9,10-phenanthrequinone (9,10-PQ), to derivatize aldehydes (C3-C10) spiked into human serum2. Calibration curves with six concentrations (not specified) were plotted. LODs, defined as the concentration giving a S/N of 3, were in the 0.004 – 0.1 nM range. Ketones and carboxylic acids were said to be not detected. Cao et al. developed a method abbreviated as “NCAPQ” or (N-[1-chloroalkyl]-pyridinium quaternization), where pyridine and thionyl chloride were reacted with standard fatty aldehydes3. Calibration curves were generated for 13 mixed fatty aldehydes (C6-C18) at six concentrations. Detection limits of <0.3 pg/mL were reported. Fatty-ketones, -alcohols and -acids were not detected. Chevelleau et al. used an untargeted approach to derivatize various classes of aldehydes in rat fecal matter using the brominated derivatizing reagent 1-[(ammoniooxy)methyl]-2-bromobenzene chloride (BBHA)4, although this reagent was previously shown to detect ketones in relatively low yields5. Other reagents that enhance the detection of carbonyls in general including aldehydes, ketones, or carboxylic acids have been reported6–17.
Here we present a new aldehyde labeling reagent, N-{2-[(4-aminophenoxy)methyl]benzyl}-N,N-diethylethanaminium bromide, or CAX-A. Similar to the reagent described above1, CAX-A possesses an aniline functional group. It also contains a quaternary amine as a cationic xylyl (CAX) group, to enable anchimeric-assisted formation of an analyte-characteristic first product ion (from the loss of triethylamine as a neutral) in MS218. Further, it boosts specificity by forming a second product ion (loss of CO) in MS3. Urine has been previously tested for aldehydes; an extensive review has been provided by Dator and colleagues.17 Our labeling reaction has been applied to two urine samples spiked with two standard aldehydes (benzaldehyde-d6, and 2-trifluoromethoxybenzaldehyde), leading to the nontargeted detection of 14 putative aldehydes as reported here.
EXPERIMENTAL
Chemicals and Materials
N-Boc-4-hydroxyaniline, α,α’-dibromo-o-xylene, benzaldehyde, sodium bicarbonate, 2-chloroacetaldehyde, benzaldehyde-d6, 2-cyclohexylbenzaldehyde, sodium cyanoborohydride, triethylamine (TEA), hydrochloric acid, dichloromethane (DCM), tert-butyl-(4-hydroxyphenyl)carbamate, and ethanol (EtOH) were from Sigma Aldrich (St. Louis, MO, USA). Diethyl ether, trans-cinnamaldehyde and heptaldehyde were from Alfa Aesar (Ward Hill, MA, USA). Ethyl acetate (EtOAc), hexane, LCMS-grade methanol (MeOH) and acetonitrile (ACN) were from Fisher Chemicals (Fairlawn, NJ, USA). Tetrahydrofuran (THF), formic acid, 99%, and 2-trifluoromethoxybenzaldehyde were from Acros Organics (Fairlawn, NJ, USA). OASIS® HLB extraction cartridges (1 cc, 30 mg) were from Waters Corporation, Ireland. Clear Eppendorf tubes (0.5 mL) were purchased from Eppendorf North America Inc, USA, and plastic Luer lock syringe caps were from Gejoy, Amazon. Snap-cap microcentrifuge tubes (1.5 mL), and the 15 mL centrifuge tubes were from Fisherbrand (Fisher Scientific, USA). HPLC vials and vial caps were from Thermo Scientific, Germany.
Synthesis
tert-Butyl-{4-[2-(bromomethyl)benzyloxy]phenyl}carbamate
tert-Butyl-(4-hydroxyphenyl)carbamate (1.2 g, 5.73 mmol) and sodium bicarbonate (1.16 g, 13.76 mmol) were added to 60 mL anhydrous THF in a 250 mL round bottom (rb) flask. The mixture was refluxed under nitrogen for 1 hour. A solution of α,α’-dibromo-o-xylene (9.08 g, 34.4 mmol) in 40 mL THF was added drop-wise over a period of 30 min. The contents were then refluxed for 24 h, cooled to rt, and filtered to remove inorganics (using filter paper in Buchner funnel). The filtrate was dried in a rotary evaporator, and the residue was chromatographed on a 400 mesh flash silica gel column (EtOAc/hexane,1 to 30%) to yield product 1 (1.14 g, 51%).
N-{2-[(4-((tert-butoxycarbonyl)amino)phenoxy)methyl]benzyl}-N,N-diethylethanaminium bromide
Compound 1 (1.1 g, 2.8 mmol), triethylamine (1.7 g, 16.82 mmol) and 60 mL of anhydrous DCM were added to a 250 mL rb flask, followed by stirring at rt under nitrogen for 16 h. The solvent was removed in a rotary evaporator and the residue was triturated with cold, anhydrous ether to give a white solid which was filtered and dried under high vacuum to furnish product 2 (1.29 g, 93%).
N-{2-[(4-aminophenoxy)methyl]benzyl}-N,N-diethylethanaminium bromide (CAX-A)
Compound 2 (1.2 g, 2.44 mmol) was added to 40 mL of EtOH in a 100 mL rb flask, followed by 5 ml of HCl, and stirred at rt for 4 h under nitrogen. After vacuum drying, the residue was triturated with ice-cold anhydrous ether to yield CAX-A (690 mg, 72%). The final product was an amorphous, off-white solid, which was stored at −20º C.
Stock solutions
Stock solutions (20 mM) of 6 aldehyde standards were prepared in LCMS-grade ACN: 2-chloroacetaldehyde (1.6 mg/ml), benzaldehyde-d6 (2.2 mg/ml), heptaldehyde (2.3 mg/ml), trans-cinnamaldehyde (2.6 mg/ml), 2-cyclohexylbenzaldehyde (3.8 mg/ml) and 2-trifluoromethoxybenzaldehyde (3.8 mg/ml). CAX-A (3.27 mg/ml, 8.3 mM) and NaCNBH3 (31.5 mg/ml, 502 mM) were separately prepared in LCMS-grade MeOH and stored at −20ºC.
Preparation of CAX-aldehyde standards and blanks (Reaction Mixture I)
From 20 mM stock solutions, 2-chloroacetaldehyde (2 μL, 40 nmol, 6.1 μg), benzaldehyde-d6 (2 μL, 40 nmol, 4.5 μg), heptaldehyde (2 μL, 40 nmol, 4.6 μg), trans-cinnamaldehyde (2 μL, 40 nmol, 5.3 μg), 2-cyclohexylbenzaldehyde (2 μL, 40 nmol, 7.5 μg) and 2-trifluoromethoxybenzaldehyde (2 μL, 40 nmol, 7.6 μg) were mixed with CAX-A (10 μL, 83 nmol, 33 μg) and NaCNBH3 (10 μL, 5 μmol, 315 μg) in a clear, 0.5 mL Eppendorf tube in triplicate. The reaction mixture (32 μL) was vortexed for ~10–20 sec and incubated overnight (~17–24 h) in the dark at RT, giving Reaction Mixture I for the samples. For the blanks, 12 μL ACN replaced the aldehydes. This gave Reaction Mixture I for the blanks.
Preparation of urine samples (Reaction Mixture II)
Urine samples were labeled with CAX-A in a Waters OASIS® HLB cartridge. Two urine samples (1 male, 1 female, IRB-approved) were obtained for preliminary analysis; 1 mL of each was centrifuged for 10 min at 10,000 RPM, of which 200 μL was taken for derivatization. After the cartridge was conditioned with 1 mL of MeOH:ACN 50:50, v/v, 200 μL of urine spiked with two internal standards (benzaldehyde-d6, 5 μL, 98 pmol, and CF3-benzaldehyde, 5 μL, 69 pmol) was applied followed by CAX-A (10 μL, 83 nmol) and excess NaCNBH3 (10 μL, 5 μmol). The exit port of the cartridge was capped off with a plastic Leur lock syringe cap, and the top of the cartridge was sealed with parafilm. The secured cartridge was gently swirled for 10 seconds and incubated overnight at RT. The next day, the cartridge was eluted with 800 μL of 50:50 ACN:MeOH, v/v. This eluent was dried in a Thermo Savant SPD111V SpeedVac®, and reconstituted in 60 μL initial mobile phase (94% A, 6% B, 0.1% formic acid), giving Reaction Mixture II.
Instrumentation
An LC-LTQ Orbitrap XL (Thermo Scientific, Tewksbury, MA, USA) equipped with an ESI source, Dionex Ultimate® 3000 LC system and XCalibur® 2.0.7 data package was employed. Samples were resolved on a Thermo AQUASIL C-18 column (250 × 1 mm, 5 μm) using a binary gradient in which mobile phase A was 0.1% formic acid in water, and mobile phase B was 0.1% formic acid in ACN. The flow rate was 40 μL/min and the column oven was maintained at 31ºC. The gradient was 6% B from 0–4 min, ramped up to 95% B from 4–17 min, and held at 95% B from 17–48 min.
LC-LTQ Orbitrap XL analysis of CAX-aldehyde standards
For injection of 830 fmol each of CAX-aldehyde standards, a 2 μL aliquot of Reaction Mixture I (separate for standards and blanks) was diluted into 998 μL of ACN (1:500 dilution) and then 10 μL of this dilution was added to 40 μL of initial mobile phase (94% A, 6% B, 0.1% formic acid), giving overall a 1:2500 dilution, of which 5 μL was injected.
The instrument was set to run 5 scan events: one full scan event (FTMS, mass range 300–1000 m/z) followed by 2 data-dependent MS2, and 2 data-dependent MS3 scans of the two most abundant precursor ions in the ion trap. The maximum ion time in the FTMS mode was 80 ms with 2 microscans, with automatic gain control of 5xE6 and mass resolution of 30,000 (FWHM at m/z 400). The maximum ion time for the ion trap in the MS2 mode was 100 ms, with 2 microscans, using a 30% normalized collision energy and a 2 Da isolation width.
LC-LTQ-Orbitrap XL analysis of urine samples
From Reaction Mixture II, 2 μL was injected into an LTQ-Orbitrap XL. The instrument was set to run 3 scan events: one full scan event (FTMS, m/z 300–1000) followed by 2 data-dependent MS2 scans of the two most abundant precursor ions in the ion trap. An inclusion and an exclusion list were created in addition to the data-dependent MS2 scan modes. The inclusion list contained putative aldehydes that displayed a 101.12 Da neutral loss in MS2; this was determined based on preliminary testing. All other parameters were kept the same as above. LC features (m/z, RT, intensity) were extracted with MZmine software version 2.53, and the corresponding m/z values (corrected for the mass of CAX-A = −297.2325 Da) were searched for putative aldehydes on Human Metabolome database (HMDB) with a mass tolerance of 10 ppm.
RESULTS AND DISCUSSION
CAX-A synthesis
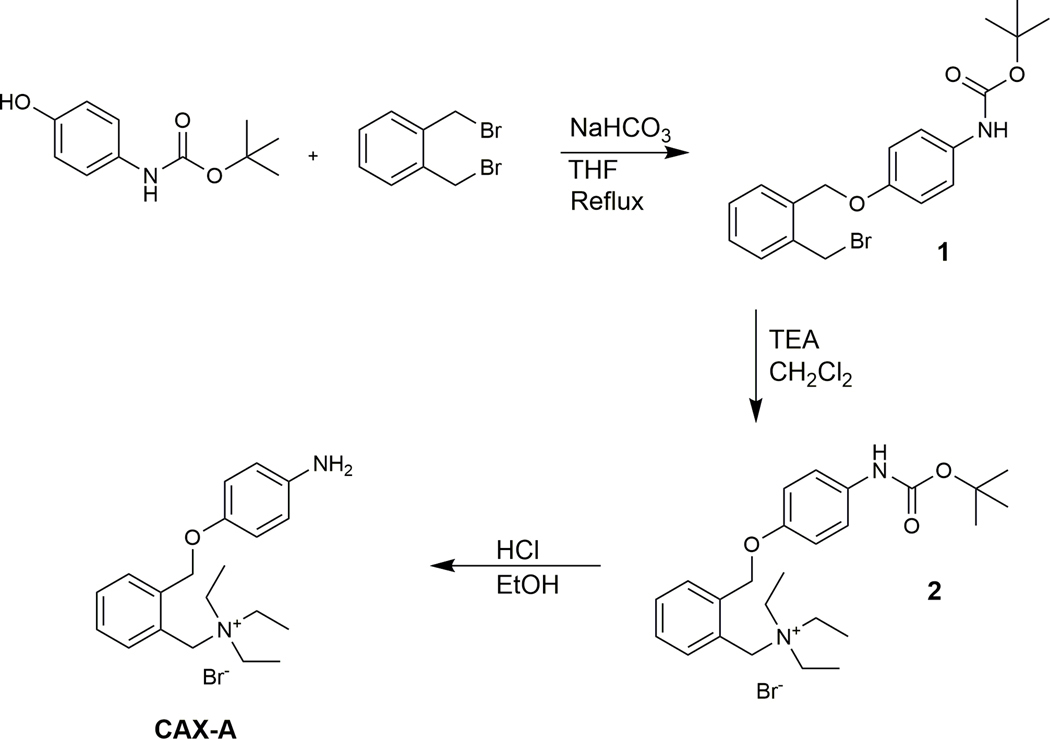
In this study, we present a new labeling reagent, CAX-A, with the goal of improving the detection of aldehydes by MS. CAX-A was synthesized in three steps as described in Figure 1.
Figure 1.
Reaction scheme for the synthesis of CAX-A. CAX-A, N-{2-[(4-aminophenoxy)methyl]benzyl}-N,N-diethylethanaminium bromide
Labeling reaction
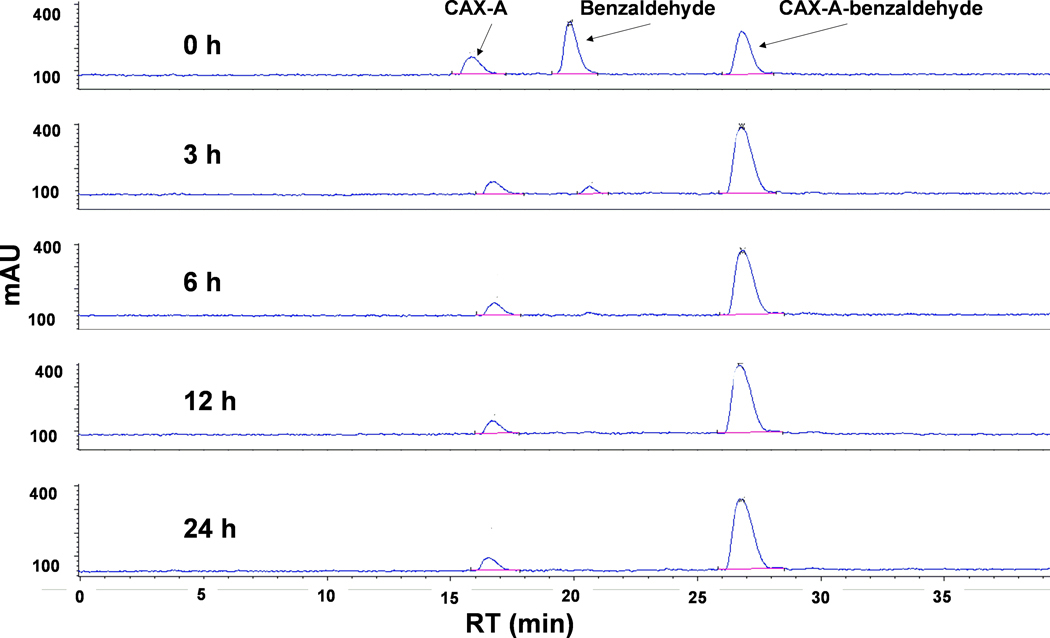
In Figure 2 is shown the CAX-labeling reaction that we applied to standard aldehydes. While reductive labeling of aldehydes with amines is known19–22, our labeling conditions are somewhat new. Aldehydes (40 nmol each) were derivatized with 83 nmol of CAX-A and excess NaCNBH3 in 1:1 ACN:MeOH, v/v, in the dark at room temperature overnight. A reaction time of overnight was selected after observing, based on HPLC analysis, that the peak for unreacted benzaldehyde was reduced to 24% after the first hour, to 12% after 3 h, and to nearly zero after 6 hours (Figure 3).
Figure 2.
Labeling of an aldehyde with CAX-A in the presence of sodium cyanoborohydride. CAX-A, N-{2-[(4-aminophenoxy)methyl]benzyl}-N,N-diethylethanaminium bromide
Figure 3.
Labeling reaction of benzaldehyde with CAX-A, monitored by HPLC at 0, 3, 6, 12, and 24 h. Retention time values of CAX-A, benzaldehyde, and CAX-A-benzaldehyde derivatives are 16.7, 20.6, and 26.8 min, respectively. CAX-A, N-{2-[(4-aminophenoxy)methyl]benzyl}-N,N-diethylethanaminium bromide
Aldehydes tested
The six standard aldehydes we chose to study are listed in Table 1, along with their exact masses and the exact masses of the corresponding CAX-derivatives. As seen, we chose aldehydes with a diversity of properties: saturated and containing an electronegative group (3), perdeuterated aryl as a candidate internal standard (4), saturated aliphatic (5), α,β-unsaturated (6), sterically hindered aromatic (7), and trifluoromethoxy aryl (8).
Table 1.
Aldehydes selected for CAX-labeling
| Aldehyde | Number | Exact Mass (Da) | Exact Mass of CAX-Aldehyde (Da) |
|---|---|---|---|
| 2-chloroacetaldehyde | 3 | 77.9872 | 375.2198 |
| benzaldehyde-D6 | 4 | 112.0795 | 409.3121 |
| heptaldehyde | 5 | 114.1045 | 411.3370 |
| trans-cinnamaldehyde | 6 | 132.0575 | 429.2900 |
| 2-cyclohexylbenzaldehyde | 7 | 188.1201 | 485.3526 |
| 2-trifluoromethoxybenzaldehyde | 8 | 190.0242 | 487.2567 |
Detection of CAX-aldehydes by LC-MS in the MS1 mode
LC-LTQ-Orbitrap-MS:
Standard aldehydes (40 nmol each) were labeled by CAX-A in triplicate. Corresponding blanks (aldehydes missing) were also tested in triplicate. Aliquots (2 μL) of these reaction mixtures (40 nmol) and blanks were diluted and injected in triplicate at 830 fmol/injection. The S/N values for CAX-3 to 8 were 978, 1435, 1573, 587, 1243, and 1307 respectively (Figure 4A). Retention times were 21.52, 21.37, 21.21, 21.45, 26.12, and 24.02 min, respectively for CAX-3 to 8, which is roughly the same order as their masses.
Figure 4.
LC-ESI-Orbitrap-MS of six CAX-aldehyde standards injected at 830 fmol per injection (65–157 pg per injection). A, Extracted ion chromatograms of six CAX aldehydes in the MS1 mode showing m/z, RT, and normalized intensities (NL, intensity normalized to the base peak in the selected time range, 0–48 min). Retention times for CAX aldehydes 3–8 are 21.52, 21.37, 21.21, 21.45, 26.12, and 24.02 min, respectively. B, Mass spectra of six CAX aldehydes in the MS2 mode featuring neutral loss of triethylamine, 101.12 Da. RT, retention time
Detection of CAX-aldehydes by LC-MS in the MS2 mode
LC-LTQ-Orbitrap-MS2:
In the linear ion trap (ITMS), under CID conditions, all CAX-aldehydes experienced a neutral loss of triethylamine, 101.12 Da, forming an intense first product ion (Figure 4B). In Figure 5, a representative FT-MS2 spectrum for CAX-4 shows additional fragment ions at m/z 280.1955, 225.1122, 212.1044 and 162.1269 (discussed below). At 830 fmol/injection, the normalized intensity or NL values (normalized to the same largest peak in the m/z range 300–1000, NL is part of the instrument software) for the first product ions of CAX-3 to 8 were 2.40E4, 2.74E5, 2.11E5, 1.13E3, 1.75E4, and 1.69E4 respectively (Figure 4B).
Figure 5.
FT-MS2 spectrum of CAX-4 (m/z 409.3121 Da), showing the first product ion at m/z 308.1903 (further peak identification is shown in Figure 6)
Detection of CAX-aldehydes by LC-MS in the MS3 mode
LC-LTQ-Orbitrap-MS3:
All CAX-aldehydes, when detected by MS3, gave a peak corresponding to loss of CO (27.99 Da) from the first product ion. We present data from CAX-4 as an example. In Figure 5 is shown the formation of the first product ion at m/z 308.1903 from the parent ion at m/z 409.3121 (FT-MS2 data). Then, in Figure 6, a second product ion at m/z 280.1954 (FT-MS3 data) is seen from additional loss of CO (27.9947 Da), ruling out C2H4 (28.0313 Da) as a fragment loss. Also shown in this figure are tentative assignments for two other ions at m/z 225.1122, and 212.1043. The former ion might form from m/z 308.1903 via loss of the benzylic-d6 moiety, while the latter via a four-center transfer of a hydrogen to the phenyl-d5 group. A scheme rationalizing the loss of CO from 308.1916 is shown in Figure 7.
Figure 6.
FT-MS3 spectrum of first product ion at m/z 308.1903, showing a characteristic loss of CO (27.99 Da), and assigned fragments at m/z 212.1043 and 225.1122
Figure 7.
Scheme (as two steps a and b) for rationalizing the loss of CO (27.99 Da) from the first product ion at m/z 308.19 from CAX-4, yielding m/z 280.1954 (see Figure 6)
Specificity for labeling aldehydes
Our method can enhance the specificity for detecting aldehydes in three ways. First is the specificity of the functional group aniline1 of CAX-A for coupling to aldehydes. We evaluated this by reacting a mixture of aldehyde 4, para-benzoquinone, and 4-phenyl-2-butanone with CAX-A at the 10 μg level. A peak for CAX-4 was detected at a NL value of 1.11E6 (S/N = 3388), while CAX derivatives of the ketone and quinone were not detected (Figure 8). A peak at 20.5 min (m/z 445.3168, NL = 1.84E4, S/N = 49), which was 10.1 ppm off the expected mass (m/z = 445.3213) of the ketone derivative was rejected based on high mass accuracy displayed by the aldehyde derivative (1.5 ppm) in Figure 8A. While aniline can give mono-β and di-β addition onto benzoquinone under strong conditions,23 no evidence of such products were observed from the mild conditions employed here, other than a peak at m/z 419.2923, S/N of 2.3 (1467x lower than the S/N achieved by the CAX-aldehyde, CAX-4). Second, specificity is enhanced by detection of a characteristic first product ion in high yield, a designed property of the CAX group.18 Third is the opportunity to further confirm a CAX-aldehyde by means of an MS3 experiment, based on observing a characteristic loss of CO.
Figure 8.
Extracted ion chromatograms using LC-ESI-Orbitrap-MS after an aldehyde (4), a ketone (4-phenyl-2-butanone), and a quinone (para-benzoquinone) were subjected to CAX labeling. A, The NL value for CAX-4 is 1.16E6. B, No CAX-labeled ketone was detected. The candidate peak at 20.5 min in B was rejected based on its mass (off by 10.1 ppm)
Analysis of urine samples
Two urine samples were derivatized in Waters OASIS® HLB cartridges and analyzed by LC-LTQ-Orbitrap-MS and LC-LTQ-MS2. An HMDB database search of 2,184 MS1 features from blanks and urines yielded 113 putative aldehyde hits, out of which 42 were non-isomeric aldehydes and 14 were unique to urine samples (Table 2). As detailed in Table 2, 7 putative aldehydes were detected in both urine, 4 only in the male urine, and 3 only in the female urine. Out of the 14 putative aldehydes that were specific to urine, data-dependent MS2 scans (101.12 Da neutral loss) were detected for 9. Figure 9A shows a representative total ion chromatogram (TIC) of one urine sample, and Figure 9B shows the extracted ion chromatogram (XIC) of one putative aldehyde detected in both urines. The NL value of the putative aldehyde peak at m/z 387.2637 (18.4 min, potentially glyceraldehyde, error 3 ppm) is 3.86E5. Also shown in Figure 9B, inset, is the ion at m/z 286.16, which arises from the loss of 101.12 Da (triethylamine) from the CAX-aldehyde in the MS2 mode. The postulated ion at m/z 268.18 might be due to the loss of -OH group in the form of water (17.98 Da). Sample variability was accounted for by the addition of internal standards. The CVs for the relative intensities of the internal standards (from triplicate samples) ranged from 2.3 to 19.7% (shown for one urine in Figure S1).
Table 2:
HMDB putative aldehyde hits (14) detected in two urine samples. a. CAX-labeled aldehyde mass, b. hits found in male urine and c. hits found in female urine.
| Observed Mass (m/z, Da)*a | RT (min) | (Accurate Mass, Da) | Error (PPM) | HMDB ID | Name b,c |
|---|---|---|---|---|---|
| 90.0316 (387.2636) | 18.4 | 90.03169 | 3 | HMDB0001051 | Glyceraldehyde b,c |
| 110.047 (407.2788) | 23.8 | 110.0480 | 9 | HMDB0003905 | Imidazole-4-acetaldehyde c |
| 122.0366 (419.2686) | 19.8 | 122.0367 | 2 | HMDB0011718 HMDB0186744 HMDB0034170 |
4-Hydroxybenzaldehyde 3-hydroxybenzaldehyde Hydroxybenzaldehyde (2-Furanyl)-2-propenalb |
| 130.0636 (427.2965) | 22.5 | 130.0629 | 8 | HMDB0012882 HMDB0163705 |
Adipate semialdehyde 3-(hydroxymethyl)-4-oxopentanal b,c |
| 134.0574 (431.2894) | 18.2 | 134.0579 | 7 | HMDB0167973 | 3,4,5-trihydroxypentanal b,c |
| 159.0682 (456.3002) | 21.1 | 159.0684 | 3 | HMDB0001190 | Indoleacetaldehyde b |
| 160.0540 (457.2860) | 19.9 | 160.0524 | 10 | HMDB0036073 HMDB0041009 |
7-Methyl-2-benzofurancarboxaldehyde Viburtinal b,c |
| 164.0469 (461.2789) | 22.7 | 164.0473 | 2 | HMDB0141768 HMDB0141767 HMDB0134031 HMDB0134029 |
3-(4-hydroxyphenyl)oxirane-2-carbaldehyde 3-(2-hydroxyphenyl)oxirane-2-carbaldehyde (2Z)-3-(3,4-dihydroxyphenyl)prop-2-enal 3-(2,5-dihydroxyphenyl)prop-2-enal (2E)-3-(3,4-dihydroxyphenyl)prop-2-enal 3-(2,3-dihydroxyphenyl)prop-2-enalb |
| 190.0477 (487.2797) | 21.2 | 190.0486 | 3 | HMDB0037815 | 5-(Methylthio)-2-[(methylthio)methyl]-2- pentenal b |
| 194.0779 (491.3099) | 17.7 | 194.0765 | 8 | HMDB0151284 | 2-benzyl-3-(methylsulfanyl)propanal b,c |
| 210.0730 (507.3050) | 17.5 | 210.0714 | 7 | HMDB0151295 HMDB0151298 HMDB0151294 HMDB0151292 HMDB0151293 |
2-[(4-hydroxyphenyl)methyl]-3-(methylsulfanyl)propanal 2-benzyl-2-hydroxy-3-(methylsulfanyl)propanal 2-benzyl-3-methanesulfinylpropanal 2-[(3-hydroxyphenyl)methyl]-3-(methylsulfanyl)propanal 2-[(2-hydroxyphenyl)methyl]-3-(methylsulfanyl)propanal 3-hydroxy-2-[(methylsulfanyl)methyl]-3-phenylpropanal b,c |
| 294.0867 (591.3187) | 21.6 | 294.0892 | 8 | HMDB0131333 | 3-[2-(2H-1,3-benzodioxol-5-yl)-1-benzofuran-5-yl]propanal c |
| 309.1361 (606.3681) | 19.9 | 309.1364 | 2 | HMDB0165792 | 2-[3,4-dimethoxy-10- (methylamino)phenanthren-1-yl]acetaldehyde b,c |
| 312.0969(609.3289) | 22.1 | 312.0997 | 9 | HMDB0149082 | 3-[2-(3,4-dihydroxyphenyl)-7-methoxy-1-benzofuran-5-yl]propanal c |
Figure 9.
A, TIC of a urine sample; B, XIC at m/z 387.2637 (potentially CAX glyceraldehyde, RT 18.4 min, NL = 3.86E5); inset shows loss of 101.12 Da in MS2 mode. RT, retention time; TIC, total ion chromatogram; XIC, extracted ion chromatogram
Conclusions:
An advantageous method is presented for detection of aldehydes. Derivatization is conducted at RT in the presence of sodium cyanoborohydride which avoids the formation of isomeric products (a limitation for hydrazide and oxime functional groups for aldehyde labeling). High sensitivity is achieved by means of a CAX mass tag. Specificity can be enhanced in three ways as described above. Fourteen putative aldehydes were detected in two urine samples that did not appear in the blanks.
Supplementary Material
Acknowledgements:
This work was funded by the Environmental Cancer Research Program, and the National Institute of Environmental Sciences, Grant/Award Number: P42ES017198
Funding Information:
Environmental Cancer Research Program and, the National Institute of Environmental Sciences, Grant/Award Number: P42ES017198
REFERENCES
- 1.Eggink M, Wijtmans M, Ekkebus R, et al. Development of a Selective ESI-MS Derivatization Reagent: Synthesis and Optimization for the Analysis of Aldehydes in Biological Mixtures. Anal Chem. 2008;80(23):9042–9051. doi: 10.1021/ac801429w [DOI] [PubMed] [Google Scholar]
- 2.El-Maghrabey M, Kishikawa N, Kuroda N. 9,10-Phenanthrenequinone as a mass-tagging reagent for ultra-sensitive liquid chromatography–tandem mass spectrometry assay of aliphatic aldehydes in human serum. Journal of Chromatography A. 2016;1462:80–89. doi: 10.1016/j.chroma.2016.07.082 [DOI] [PubMed] [Google Scholar]
- 3.Cao Y, Guan Q, Sun T, Qi W, Guo Y. Charged tag founded in N-(1-chloroalkyl)pyridinium quaternization for quantification of fatty aldehydes. Analytica Chimica Acta. 2016;937:80–86. doi: 10.1016/j.aca.2016.05.032 [DOI] [PubMed] [Google Scholar]
- 4.Chevolleau S, Noguer-Meireles MH, Mervant L, et al. Towards Aldehydomics: Untargeted Trapping and Analysis of Reactive Diet-Related Carbonyl Compounds Formed in the Intestinal Lumen. Antioxidants (Basel). 2021;10(8):1261. doi: 10.3390/antiox10081261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jouanin I, Chevolleau S, Canlet C, et al. Facile Oxime Ether Synthesis: Free Carbonyl Compound Derivatization by a Brominated O-Benzylhydroxylamine. Synthetic Communications. 2015;45(13):1585–1591. doi: 10.1080/00397911.2015.1035791 [DOI] [Google Scholar]
- 6.Eggink M, Wijtmans M, Kretschmer A, et al. Targeted LC–MS derivatization for aldehydes and carboxylic acids with a new derivatization agent 4-APEBA. Anal Bioanal Chem. 2010;397(2):665–675. doi: 10.1007/s00216-010-3575-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel D, Meinema AC, Permentier H, Hopfgartner G, Bischoff R. Integrated Quantification and Identification of Aldehydes and Ketones in Biological Samples. Anal Chem. 2014;86(10):5089–5100. doi: 10.1021/ac500810r [DOI] [PubMed] [Google Scholar]
- 8.Ochs S de M, Fasciotti M, Netto ADP. Analysis of 31 Hydrazones of Carbonyl Compounds by RRLC-UV and RRLC-MS(/MS): A Comparison of Methods. Journal of Spectroscopy. 2015;2015:e890836. doi: 10.1155/2015/890836 [DOI] [Google Scholar]
- 9.Dator R, Carra’ A, Maertens L, Guidolin V, Villalta PW, Balbo S. A High Resolution/Accurate Mass (HRAM) Data-Dependent MS3 Neutral Loss Screening, Classification, and Relative Quantitation Methodology for Carbonyl Compounds in Saliva. J Am Soc Mass Spectrom. 2017;28(4):608–618. doi: 10.1007/s13361-016-1521-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng P, Higashi RM, Lane AN, et al. Quantitative profiling of carbonyl metabolites directly in crude biological extracts using chemoselective tagging and nanoESI-FTMS. Analyst. 2017;143(1):311–322. doi: 10.1039/C7AN01256J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo N, Peng CY, Zhu QF, Yuan BF, Feng YQ. Profiling of carbonyl compounds in serum by stable isotope labeling - Double precursor ion scan - Mass spectrometry analysis. Analytica Chimica Acta. 2017;967:42–51. doi: 10.1016/j.aca.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Zhao S, Dawe M, Guo K, Li L. Development of High-Performance Chemical Isotope Labeling LC–MS for Profiling the Carbonyl Submetabolome. Anal Chem. 2017;89(12):6758–6765. doi: 10.1021/acs.analchem.7b01098 [DOI] [PubMed] [Google Scholar]
- 13.Zheng SJ, Wang YL, Liu P, et al. Stable isotope labeling-solid phase extraction-mass spectrometry analysis for profiling of thiols and aldehydes in beer. Food Chem. 2017;237:399–407. doi: 10.1016/j.foodchem.2017.05.090 [DOI] [PubMed] [Google Scholar]
- 14.Martinez MP, Kannan K. Simultaneous Analysis of Seven Biomarkers of Oxidative Damage to Lipids, Proteins, and DNA in Urine. Environ Sci Technol. 2018;52(11):6647–6655. doi: 10.1021/acs.est.8b00883 [DOI] [PubMed] [Google Scholar]
- 15.Rousová J, Chintapalli MR, Lindahl A, Casey J, Kubátová A. Simultaneous determination of trace concentrations of aldehydes and carboxylic acids in particulate matter. J Chromatogr A. 2018;1544:49–61. doi: 10.1016/j.chroma.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 16.Meng X, Pang H, Sun F, et al. Simultaneous 3-Nitrophenylhydrazine Derivatization Strategy of Carbonyl, Carboxyl and Phosphoryl Submetabolome for LC-MS/MS-Based Targeted Metabolomics with Improved Sensitivity and Coverage. Anal Chem. Published online July 16, 2021. doi: 10.1021/acs.analchem.1c00767 [DOI] [PubMed] [Google Scholar]
- 17.Dator RP, Solivio MJ, Villalta PW, Balbo S. Bioanalytical and mass spectrometric methods for aldehyde profiling in biological fluids. Toxics. 2019; 7(2). doi: 10.3390/toxics702003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P, Zhang Q, Yao Y, Giese RW. Cationic Xylene Tag for Increasing Sensitivity in Mass Spectrometry. J Am Soc Mass Spectrom. 2015;26(10):1713–1721. doi: 10.1007/s13361-015-1200-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures 1. J Org Chem. 1996;61(11):3849–3862. doi: 10.1021/jo960057x [DOI] [PubMed] [Google Scholar]
- 20.Bogolubsky AV, Moroz YS, Mykhailiuk PK, et al. A One-Pot Parallel Reductive Amination of Aldehydes with Heteroaromatic Amines. ACS Comb Sci. 2014;16(8):375–380. doi: 10.1021/co5000568 [DOI] [PubMed] [Google Scholar]
- 21.McGonagle F I, MacMillan D S, Murray J F. Sneddon H, Jamieson C, B. Watson AJ. Development of a solvent selection guide for aldehyde -based direct reductive amination processes. Green Chemistry. 2013;15(5):1159–1165. doi: 10.1039/C3GC40359A [DOI] [Google Scholar]
- 22.Borch RF, Bernstein MD, Durst HD. Cyanohydridoborate anion as a selective reducing agent. J Am Chem Soc. 1971;93(12):2897–2904. doi: 10.1021/ja00741a013 [DOI] [Google Scholar]
- 23.. Stejskal J, Bober P, Trchová M, Horský J, Pilař J, Walterová Z. The oxidation of aniline with p-benzoquinone and its impact on the preparation of the conducting polymer, polyaniline. Synthetic Metals. 2014;192:66–73. doi: 10.1016/j.synthmet.2014.03.01 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.