Abstract
Inflammatory foci induced by murine cytomegalovirus infection in normocholesterolemic mice were present temporarily in the aortic wall, but some of these foci developed into advanced lesions that persisted late after infection. The early foci induced by virus infection were significantly exacerbated following a single inoculation with Chlamydia pneumoniae.
Infection by certain pathogens in early childhood, the chronic presence of the initiating agents in the arteries, and the contribution of additional risk factors may cause an inflammatory process that progresses to full atherosclerosis in adults (16). Chlamydia pneumoniae and human cytomegalovirus (HCMV) are the major microbial agents involved in the pathogenesis of atherosclerosis (4, 6, 8, 12).
Murine CMV (MCMV), a frequent model for the human infection, induced inflammatory foci in the aortas of normocholesterolemic mice (1). Inoculation of C. pneumoniae induced inflammatory lesions or atherosclerosis in the rabbit aorta (9, 11). Inflammatory changes arose in the aortas in normocholesterolemic C57/BL mice after repeated intranasal C. pneumoniae inoculations but not after a single inoculation. (2). The combined effect of C. pneumoniae and MCMV on inflammatory focus development in the normocholesterolemic mouse aorta has not been addressed. In the present study, we examined the duration of the inflammatory foci and the expression of MCMV immediate-early (IE) transcripts in the mouse aorta, the frequency of aortic lesion development and the sizes of such lesions, and the effects of infections with MCMV and C. pneumoniae, in a temporal sequence consistent with that in human infections, on the development of aortic pathological changes in mice without genetic disorders in lipid metabolism.
In a previous study (1), inflammatory foci were detected in the aortic wall in normocholesterolemic MCMV-infected BALB/c mice on days 25 to 36 after infection. Irradiation at the time of infection increased the extents of viral antigen expression and inflammatory lesions. In the present study, 6- to 8-week-old BALB/c male mice (Charles River) were gamma irradiated (450 rads) and infected intraperitoneally with 4 × 105 PFU of salivary gland-adapted MCMV (strain Smith; American Type Culture Collection [ATCC]), inoculated intranasally with 2 × 104 to 4 × 104 inclusing-forming units of C. pneumoniae (TWAR; ATCC), or infected with both pathogens. Mice received regular chow throughout the study and were sacrificed at different times after infection. For histopathological analysis and in situ hybridization, aortic sections were processed (1, 15).
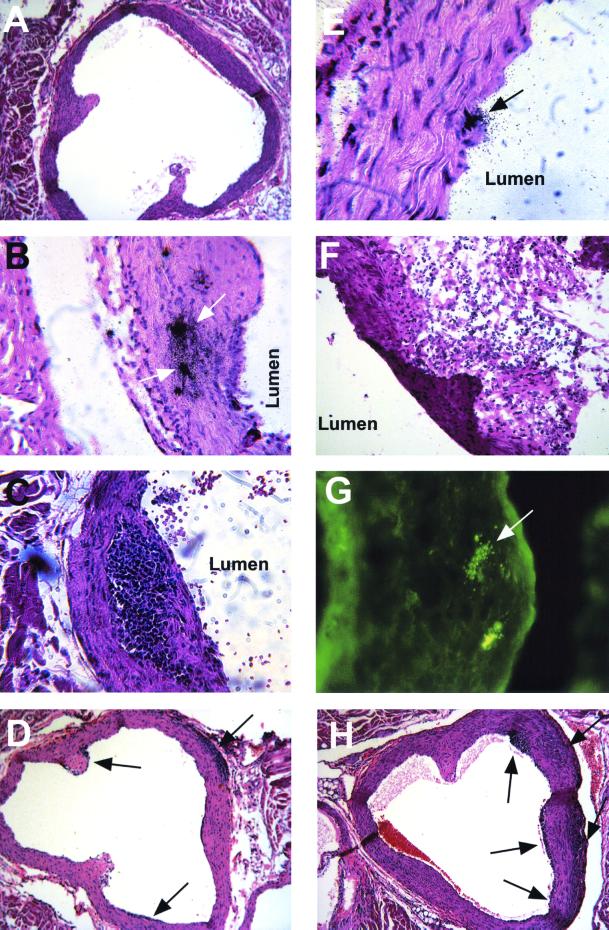
No aortic changes were observed in uninfected mice or in mice infected with tissue culture-adapted MCMV or with heat-inactivated salivary gland-adapted MCMV and sacrificed at any time after infection (Fig. 1A) (1). By week 6, inflammatory foci were seen in the aortas of 8 of 17 salivary gland-adapted MCMV-infected mice, and in situ hybridization revealed MCMV-IE-1 mRNA expression in the aortic walls in these animals (Fig. 1B). However, pathological aortic changes occurred in only 1 of 11 and 1 of 6 mice by 11 and 18 weeks after infection, respectively. Aortic sections from these two mice demonstrated intimal thickening (data not shown) or subendothelial lesions characterized by mononuclear inflammatory cell accumulations (Fig. 1C). Separately, 30 mice were infected with MCMV and sacrificed 28 to 30 weeks after infection; aortas from 4 of the mice exhibited chronic inflammatory foci, as exemplified in Fig. 1C.
FIG. 1.
Aortic inflammatory changes in the mouse aorta following infection with MCMV, C. pneumoniae, or both pathogens. (A) No inflammation in the aortic wall of an uninfected mouse (magnification, ×90). (B) MCMV-IE-1 mRNA expression (arrows) in the aortic wall 6 weeks after MCMV infection, as detected by in situ hybridization using as a probe an EcoRI fragment encoding MCMV IE gene 1, obtained from vector pcDNAI/Amp (pcDNA-pp89) (from D. H. Spector, University of California, La Jolla) and labeled with 35S by nick translation (magnification, ×180). (C) Subendothelial inflammatory lesion in the aortic wall 18 weeks after MCMV infection (magnification, ×180; hematoxylin-eosin [HE] stain). (D) Inflammatory foci (arrows) in the aorta 7 weeks after MCMV infection, an example of aortic lesions for which size data are shown in Table 1 (magnification, ×90). (E) C. pneumoniae ompA signals (arrow) in the aorta 7 days after infection, as detected by in situ hybridization using a probe generated by PCR amplification of the gene and labeled with 35S by nick translation and HE staining, but no inflammatory changes (magnification, ×360). (F) Massive periaortic inflammation on day 14 after three C. pneumoniae inoculations (4 weeks apart) (frozen section; magnification, ×180; HE stain). (G) C. pneumoniae-specific antigens (arrow) in the aortic wall at the area of the aortic cusp of the same mouse as that shown in panel F (magnification, ×180; immunofluorescence assay of frozen section using pooled sera from C. pneumoniae-immunized mice). (H) Inflammatory foci (arrows) in the aorta of a doubly infected mouse 7 weeks after MCMV infection (3 weeks after C. pneumoniae infection), an example of aortic lesions for which size data are shown in Table 1 (magnification, ×90).
To determine whether C. pneumoniae alone induces inflammatory changes in the aorta, mice were inoculated with a McCoy cell preparation (ATCC) or C. pneumoniae one to three times. Bacterial replication was confirmed by culturing lung homogenates from all infected mice on days 3, 7, and 11 (10). In situ hybridization disclosed C. pneumoniae ompA DNA sequences on days 7 and 14 after infection in a few heart muscle cells from all mice (data not shown) and in aortic endothelial cells of one of three mice at each time point (Fig. 1E). No inflammatory foci were seen in the aortic walls in mice inoculated with an uninfected McCoy cell preparation or C. pneumoniae once or twice (4-week interval) and sacrificed 14 to 50 days after inoculation. However, massive periaortic inflammation was concentrated around the aortic cusps in four of seven mice inoculated three times with C. pneumoniae and sacrificed 14 days after the last inoculation, suggesting an immunopathological mechanism for the development of lesions (Fig. 1F), and C. pneumoniae antigens were detected in aortic walls by an immunofluorescence assay (Fig. 1G). Irradiation at the time of infection did not change the pathology of the aorta after single or repeated inoculations with C. pneumoniae.
The effect of superinfection with a single C. pneumoniae dose on inflammatory changes in the aortas in mice previously infected with MCMV was tested with mice left uninfected or inoculated with MCMV and superinfected or not superinfected 4 weeks later with C. pneumoniae (Table 1). At 7 weeks after MCMV infection, more mice (seven of eight) among those infected with MCMV followed by C. pneumoniae than among those infected with MCMV alone (four of nine) showed positive histopathological aortic changes. The mean area of inflammation in the aortas in doubly infected mice was 2.8-fold (P < 0.01) that in mice inoculated with MCMV only (Fig. 1D and H). Separately, mice were infected with MCMV or C. pneumoniae alone or with MCMV and then with C. pneumoniae 4 weeks later or were left uninfected. Hearts were obtained later than in earlier experiments (11 weeks after initial infection) and processed for histopathological analysis. Similar to the previous results for the prevalence of aortic lesions, 9 of 17 doubly infected mice exhibited inflammatory foci as compared with only 1 of 11 MCMV-infected mice in the same period.
TABLE 1.
Exacerbation of MCMV-induced inflammatory foci by C. pneumoniaea
| Infection of mice
|
No. of mice with pathological aortic changes/no. tested | Size of areas with inflammatory foci (mean ± SD μm2) | |
|---|---|---|---|
| MCMV | C. pneumoniae | ||
| − | − | 0/6 | |
| − | + | 0/10 | |
| + | − | 4/9 | 1.04 × 105 ± 9 × 104b |
| + | + | 7/8 | 2.92 × 105 ± 1.6 × 105b |
Mice were irradiated and infected with MCMV and then with C. pneumoniae 4 weeks later. At 7 weeks after MCMV infection, mice were sacrificed and aortic sections were processed for histopathological analysis. The size of areas with inflammatory foci in 28 sections from seven doubly infected mice was compared with that in 16 sections from four MCMV-infected mice with pathological aortic changes. Images of each aortic section were captured with a high-resolution digital video camera (Hamamatsu model C4742-95-10NR) attached to a microscope and were stored in digital format in a computer using imaging software (Qued Imaging Inc., Pittsburgh, Pa.). Areas with inflammatory foci were measured with image analysis software (Image J, version 1.07; National Institute of Health; downloaded from http://rsb.info.nih.gov and expressed in square micrometers. Student's two-tailed t test was used to compare the sizes of inflammatory foci in the positive sections.
Values were significantly different from each other (P < 0.01).
These results are consistent with the C. pneumoniae predilection for causing atheromatous lesions and the acceleration of the atherosclerotic process in apoE-deficient or hypercholesterolemic mice (5, 7, 14). The increased pathological aortic changes in mice infected with MCMV followed by C. pneumoniae might reflect a higher susceptibility to C. pneumoniae infection in MCMV-induced aortic lesions than in the normal aortic wall. Alternatively, C. pneumoniae infection might lead to local MCMV reactivation in the aortic wall by some intracellular effect of the bacterial infection or induce the production of proinflammatory cytokines that exacerbate existing MCMV-induced lesions. Interestingly, a trans-activating effect of C. pneumoniae infection on the HCMV IE promoter in transfected tissue culture cells has been reported (17). In our experiments, McCoy cells simultaneously infected in vitro with MCMV (0.1 PFU) and C. pneumoniae (0.1 inclusion-forming unit) showed a 3.10- to 4.78-fold-higher yield of MCMV than cultures infected with MCMV alone 4 and 5 days after infection (2.25 × 103 PFU versus 7.25 × 102 PFU on day 4 and 2.75 × 103 PFU versus 5.75 × 102 PFU on day 5, respectively), consistent with the above-mentioned effect on the HCMV IE promoter (17). Nevertheless, further studies are needed to clarify the mechanism(s) whereby C. pneumoniae superinfection exacerbates MCMV-induced aortic lesions in mice.
Although our experiments do not address the specificity of C. pneumoniae superinfection in enhancing pathological aortic changes and although exacerbation of MCMV-induced pathological aortic changes by other superinfections remains possible, C. pneumoniae but not C. trachomatis enhances lesion development in mice prone to atherogenicity (3).
apoE knockout mice infected with either MCMV or C. pneumoniae demonstrated increased lesion size, but infection with both pathogens did not increase lesion size to a greater extent (5). Normocholesterolemic mice without background aortic lesions provide a different study design for evaluation of the effects of infections with one versus two pathogens on lesion size. In these mice, a single infection with C. pneumoniae only exacerbated the lesions initiated by earlier MCMV infection and itself induced no inflammatory lesions. Thus, unlike in apoE-deficient mice, there are no background lesions to complicate lesion size evaluation and mask any enhancing effect of infection.
Animal models of atherosclerosis highlight its multifactorial origin, including elevated serum lipid levels and autoimmune mechanisms (13, 18). The combined effect of MCMV and C. pneumoniae in normocholesterolemic mice indicates involvement of further interacting mechanisms in the development of chronic arterial diseases.
Acknowledgments
This work was supported by grants ETT T-10 592/96, ETT 05-263/96, MKM FKFP 2025/97, OTKA F 030404, and OTKA T 034954 and by the W. W. Smith Charitable Trust.
We thank Katalin Hegedus, Aniko Salaki, and Zsuzsa Rosztoczy for excellent technical assistance; Anthony Lucente and Xinyu Zhao for help in computer imaging; and Elsa Aglow and Klara Pinter for histotechnology work. We also thank the editorial department of The Wistar Institute for preparing the manuscript.
REFERENCES
- 1.Berencsi K, Endrész V, Klurfeld D, Kari L, Kritchevsky D, Gönczöl E. Early atherosclerotic plaques in the aorta following cytomegalovirus infection of mice. Cell Adhesion Commun. 1998;5:39–47. doi: 10.3109/15419069809005597. [DOI] [PubMed] [Google Scholar]
- 2.Blessing E, Lin T M, Campbell L A, Rosenfeld M E, Lloyd D, Kuo C C. Chlamydia pneumoniae induces inflammatory changes in the heart and aorta of normocholesterolemic C57BL/6J mice. Infect Immun. 2000;68:4765–4768. doi: 10.1128/iai.68.8.4765-4768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blessing E, Nagano S, Campbell L A, Rosenfeld M E, Kuo C-C. Effect of Chlamydia trachomatis infection on atherosclerosis in apolipoprotein E-deficient mice. Infect Immun. 2000;68:7195–7197. doi: 10.1128/iai.68.12.7195-7197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burian K, Kis Z, Virok D, Endresz V, Prohaszka Z, Duba J, Berencsi K, Boda K, Horvath L, Romics L, Fust G, Gönczöl E. Independent and joint effects of antibodies to human heat-shock protein 60 and Chlamydia pneumoniae infection in the development of coronary atherosclerosis. Circulation. 2001;103:1503–1508. doi: 10.1161/01.cir.103.11.1503. [DOI] [PubMed] [Google Scholar]
- 5.Burnett M S, Gaydos C A, Madico G E, Glad S M, Paigen B, Quinn T C, Epstein S E. Atherosclerosis in apoE knockout mice infected with multiple pathogens. J Infect Dis. 2001;183:226–231. doi: 10.1086/317938. [DOI] [PubMed] [Google Scholar]
- 6.Campbell L A, Kuo C-C, Grayston J T. Chlamydia pneumoniae and cardiovascular disease. Emerg Infect Dis. 1998;4:571–579. doi: 10.3201/eid0404.980407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell L A, Moazed T C, Kuo C C, Grayston K T. Preclinical models for Chlamydia pneumoniae and cardiovascular disease: hypercholesterolemic mice. Clin Microbiol Infect. 1998;4:S23–S32. [PubMed] [Google Scholar]
- 8.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 9.Fong I W, Chiu B, Viira E, Jang D, Mahony J B. De novo induction of atherosclerosis by Chlamydia pneumoniae in a rabbit model. Infect Immun. 1999;67:6048–6055. doi: 10.1128/iai.67.11.6048-6055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng Y, Berencsi K, Gyulai Z, Valyi-Nagy T, Gönczöl E, Trinchieri G. Roles of interleukin-12 and gamma interferon in murine Chlamydia pneumoniae infection. Infect Immun. 2000;68:2245–2253. doi: 10.1128/iai.68.4.2245-2253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laitinen K, Laurila A, Pyhala L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis. An assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. doi: 10.1161/01.cir.96.11.4095. [DOI] [PubMed] [Google Scholar]
- 13.Lichtmann A H, Cybulsky M, Luscinskas F W. Immunology of atherosclerosis: the promise of mouse models. Am J Pathol. 1996;149:351–357. [PMC free article] [PubMed] [Google Scholar]
- 14.Moazed T C, Campbell L A, Rosenfeld M E, Grayston J T, Kuo C C. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- 15.Paigen B, Morrow A, Holmes P A, Mitchell D, Williams R A. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 16.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 17.Wanishsawad C, Zhou Y F, Epstein S E. Chlamydia pneumoniae-induced transactivation of the major immediate early promoter of cytomegalovirus: potential synergy of infectious agents in the pathogenesis of atherosclerosis. J Infect Dis. 2000;181:787–790. doi: 10.1086/315235. [DOI] [PubMed] [Google Scholar]
- 18.Wick G, Schett G, Amberger A, et al. Is atherosclerosis an immunologically mediated disease? Immunol Today. 1995;16:27–33. doi: 10.1016/0167-5699(95)80067-0. [DOI] [PubMed] [Google Scholar]