Abstract
Paired serum-oral fluid samples from 127 hepatitis C virus (HCV)-positive and 31 HCV-negative patients were tested for the presence of anti-HCV using the Ortho HCV 3.0 ELISA. Using the immunoglobulin G (IgG)-specific detection antibody provided with the HCV 3.0 ELISA we attained 100% sensitivity and specificity with serum samples; however, sensitivity in oral fluid samples was only 81%. By modifying the HCV 3.0 ELISA to utilize a secondary antibody cocktail that recognizes not only IgG but IgA and IgM as well, we attained 100% specificity and sensitivity with oral fluid samples.
The use of oral fluid in diagnostic tests provides many advantages over traditional serum-based analyses. Oral fluid collection is rapid and noninvasive and eliminates the risks of needle exposure. Furthermore, oral fluid can be collected by nonmedical personnel, thus relieving health care professionals of the time-consuming and economic burden of obtaining serum samples. Indeed, oral fluid-based assays may prove to be the preferred method of testing for infants and young children and in developing nations, as well as for patient groups where blood collection is difficult, such as intravenous drug users, who constitute a significant portion of total hepatitis C virus (HCV) cases.
Assays developed to utilize oral fluid instead of serum have shown promise in the detection of virus-specific antibodies in patients infected with human immunodeficiency virus (9), HBV (3), HAV (14), and rubella (12) and following immunization with HAV (8), rotavirus (17), and poliovirus (18). Recently, attempts to detect HCV-specific antibodies using oral fluid with modified serum-based enzyme-linked immunosorbent assays (ELISA) have also shown promise (4, 5, 13, 15, 16). Using a modified protocol to test oral fluid in the Ortho HCV 3.0 ELISA, McIntyre et al. (10) achieved 72% sensitivity and 98% specificity from a group of 18 HCV-seropositive and 49 HCV-seronegative donors. In the same study, 100% sensitivity and specificity were achieved using a modified protocol with the MONOLISA HCV assay (Sanofi Diagnostics Pasteur). It is unclear what factors led to the differences in sensitivity between the kits, however, and these results indicate that individual HCV assays must be optimized for use with oral fluid samples, as minor differences in design may affect the outcome of the test significantly.
Oral fluid consists of a mixture of salivary gland secretions and gingival crevicular fluid, the former being enriched with immunoglobulin A (IgA) and the latter being a mixture of predominately IgG and IgM (11, 13). While the relative proportions of the individual classes of immunoglobulins are thought to be similar in serum and oral fluid, the overall concentration of immunoglobulins in oral fluid is likely 800- to 1,000-fold less than that in serum (11). Indeed, this dramatic reduction in the concentration of antibodies in oral fluid may be responsible for the decreased detection sensitivity of anti-HCV antibodies in oral fluid; serum-based immunoassays modified to test for HCV in oral fluid utilize tracer antibodies that recognize only antibodies of the IgG class while other classes of antibodies remain undetected (5, 10, 13). With the relatively low levels of antibodies present in oral fluid overall, it is likely that many of the false negatives obtained using modified serum-based assays to test oral fluid are the result of HCV-positive patients possessing levels of anti-HCV IgG in their oral fluid that are so low as to be undetectable by immunoassays recognizing only IgG class antibodies.
In the present study, we hypothesized that the detection of multiple classes of anti-HCV in oral fluid could increase the detection sensitivity of the Ortho HCV 3.0 ELISA to levels comparable with those attained using serum samples. Patients for this study were preselected from 11 participating clinical sites and shown to be either HCV positive or negative based on a clinical diagnosis according to the Centers for Disease Control and Prevention testing algorithm (1). The status of serum samples was further confirmed by repeat in-house testing using the Ortho HCV 3.0 ELISA following the manufacturer's instructions. Oral fluid samples were collected using a Salivette kit (Sarstedt Research), whereby a polyester-coated cotton plug is placed in the mouth of the patient until saturation and is then centrifuged in a carrier tube for 5 min to extract the oral fluid. The Salivette system was chosen for its ease of use and because it does not use a sample buffer to dilute the specimens as does the Omni-Sal system (Saliva Diagnostic Systems). Paired samples were shipped overnight at 4°C and processed immediately upon arrival. Samples were then stored at −80°C until testing.
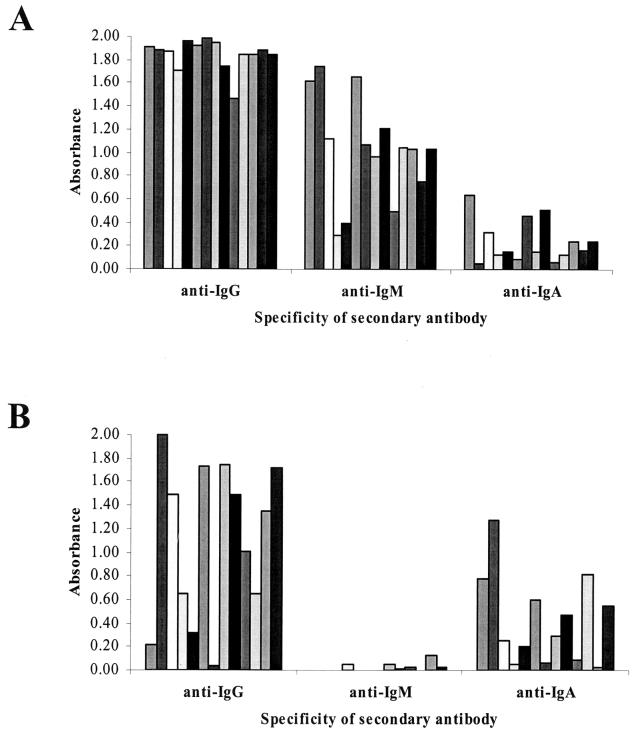
To determine if specific classes of antibodies were preferentially enriched in serum or oral fluid samples, we examined the composition of anti-HCV present in both fluids. Fourteen paired HCV-positive oral fluid-serum samples (with sufficient volumes of oral fluid for multiple ELISA) were chosen for ELISA analysis and examined using secondary enzyme-conjugated antibodies (Jackson Immunoresearch) that recognize only IgG, IgM, or IgA, respectively, to identify the different classes of anti-HCV detectable in oral fluid (Fig. 1). Modification of the HCV 3.0 ELISA was necessary to achieve optimal detection sensitivity and specificity; compared to the manufacturer's instructions for use with serum, the oral fluid sample volume was increased from 10 to 100 μl per well and the sample incubation time was increased from 1 h at 37°C to overnight at 4°C. Furthermore, a more sensitive two-part TMB substrate kit (Pierce) was used for all testing in place of the o-phenylenediamine tablets supplied with the HCV 3.0 kit. Analysis of the optical densities (OD) generated by these 14 samples showed that anti-HCV of the IgG and IgM class was most abundant in serum samples (mean OD = 1.85 and 1.03, respectively), with little IgA-class anti-HCV present (OD = 0.24) (Fig. 1A). These samples were not treated for rheumatoid factor, however, and thus it is possible that elevated levels of anti-IgM reactivity in serum samples may be attributable to the presence of this interfering substance (7). In contrast, while IgG (OD = 1.10) remained the major class of anti-HCV detectable in oral fluid samples by the HCV 3.0 assay, a higher level of anti-HCV IgA (OD = 0.42) was also detectable, while nearly no anti-HCV IgM was present (OD = 0.02) (Fig. 1B). Statistically, the mean OD of anti-HCV of the IgG and IgM class is significantly reduced in oral fluid compared to serum (P < 0.01), while the OD of IgA-class anti-HCV is not significantly different (P > 0.01). Interestingly, in a number of oral fluid samples possessing low anti-HCV IgG levels, a significant amount of anti-HCV IgA is detectable (Fig. 1B), which might contribute to a higher overall OD and thus render a positive result. Indeed, the ability to detect anti-HCV of the IgA class may also increase the likelihood of detection early during the course of infection, as IgA is known to be present during the earliest stages of the immune response (6).
FIG. 1.
Characterization of multiple classes of anti-HCV present in serum and oral fluid. Paired serum-oral fluid samples were screened by HCV 3.0 ELISA using enzyme-conjugated antibodies specific for human IgG, IgM, or IgA, respectively. (A) In serum, high levels of anti-HCV IgG- and IgM-class antibodies are detectable, while relatively little anti-HCV IgA is present. (B) In oral fluid, the majority of antibodies detectable are of the IgG or IgA class, with little or no anti-HCV IgM present.
We then examined whether the detection of multiple classes of anti-HCV antibodies, instead of IgG alone, could increase the sensitivity of the Ortho HCV 3.0 ELISA in a modified oral fluid-based format. Paired oral fluid-serum samples from 127 known HCV-seropositive and 31 HCV-seronegative donors were screened using the HCV 3.0 assay according to the manufacturer's instructions, using the monoclonal anti-human IgG-peroxidase detection antibody. Using serum samples, we achieved 100% sensitivity and specificity with the HCV 3.0 assay (Table 1). Because there is no accepted cutoff value for oral fluid in the HCV 3.0 kit, sensitivity and specificity were determined by receiver-operator curve analysis at the 95% confidence interval as well as by determining a cutoff 3.5 standard deviations above the mean of the 31 HCV-negative samples. Using the modified incubation protocol mentioned previously, along with the anti-IgG conjugate antibody of the HCV 3.0 kit, detection sensitivity was reduced to 81% (103 of 127 samples), while specificity remained 100%.
TABLE 1.
Sensitivity and specificity of HCV 3.0 assay using paired serum and oral fluid samples with different enzyme-conjugated secondary antibodies
| Conjugate | Result with oral fluid | No. of samples with result with serum
|
|
|---|---|---|---|
| Positive | Negative | ||
| Monoclonal anti-human IgGa | Positive | 103 | 0 |
| Negative | 24 | 31 | |
| Goat anti-human IgG-IgM-IgAb | Positive | 127 | 0 |
| Negative | 0 | 31 | |
Sensitivity, 81%; specificity, 100%.
Sensitivity, 100%; specificity, 100%.
Oral fluid samples were then rescreened using a 1:16,000 dilution of peroxidase-labeled goat anti-human IgG-IgM-IgA antibody cocktail (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) in phosphate-buffered saline–1% bovine serum albumin–10% goat serum instead of the monoclonal anti-human IgG provided with the HCV 3.0 kit. This antibody dilution proved to have the greatest signal/noise ratio in titration studies and was used in all studies in which the antibody cocktail was included. Using this modified protocol, anti-HCV was detected in patient oral fluid samples with 100% sensitivity and specificity by receiver-operator curve analysis or using the calculated 3.5-standard deviation cutoff (cutoff = 0.026) (Table 1). All oral fluid samples from HCV-positive individuals that were initially scored as HCV negative by the Ortho HCV 3.0 anti-IgG conjugate were subsequently scored as HCV positive when the anti-IgG-IgM-IgA cocktail was used (Table 2).
TABLE 2.
Discrepant analysis of select patient oral fluid samples possessing low levels of anti-HCV IgG
| Patient no. | Ortho HCV 3.0 ELISA result
|
||
|---|---|---|---|
| Oral fluid with:
|
Serum with anti-IgG conjugate | ||
| Anti-IgG conjugate | Anti-IgG-IgM-IgA cocktail | ||
| 103–19 | 0.014 | >3.5 | 2.42 |
| 109–03 | 0.014 | 2.24 | 2.25 |
| 103–15 | 0.149 | 2.60 | 2.07 |
| 109–01 | 0.213 | 2.77 | 2.33 |
| 103–01 | 0.293 | >3.5 | 2.34 |
| 103–37 | 0.357 | 2.29 | 1.56 |
| 108–06 | 0.378 | >3.5 | 2.40 |
Our results indicate that the use of a secondary antibody cocktail that recognizes not only IgG but IgA and IgM as well may aid in the detection of the relatively low levels of anti-HCV antibodies present within oral fluid and thus increase detection sensitivity. This increase in detection sensitivity when such an antibody cocktail is used is in good agreement with our data showing that a significant percentage of anti-HCV antibodies in oral fluid exist in the form of IgA-class antibody molecules (Fig. 1). A recent study by Van Doornum et al. (16) showed that anti-HCV could be detected with up to 88% sensitivity in oral fluid by using a modified protocol with the MONOLISA anti-HCV Plus kit. Similar to the Ortho HCV 3.0 assay, however, this kit utilizes an anti-human IgG conjugate antibody, and it is therefore incapable of detecting IgA-class anti-HCV present in oral fluid samples. Furthermore, in contrast to the HCV 3.0 assay, the MONOLISA does not incorporate proteins from the core region of the HCV genome, and sensitivity in oral fluid may be reduced by the inability to capture antibodies directed against this highly antigenic region. By detecting multiple classes of antibodies, and through the use of an ELISA with a high percentage of the total antigenic sequences of HCV coated onto the solid phase, we were able to increase our detection sensitivity to levels comparable to those obtained from serum-based analysis.
Thus, the results of this study suggest that while the detection of non-IgG-class anti-HCV is unnecessary in serum, where overall antibody concentrations are extremely high and IgG-class immunoglobulins predominate, detection of anti-HCV IgG, IgM, and IgA in oral fluid samples may prove to be of benefit in correctly diagnosing patients on the basis of samples possessing relatively low levels of anti-HCV IgG. Indeed, patient oral fluid samples with low anti-HCV IgG levels may escape detection in immunoassays that recognize only IgG class immunoglobulins. By effectively increasing the pool of antibodies detectable in oral fluid samples, it may be possible to overcome the intrinsic difficulty of detecting the extremely low levels of antibodies in oral fluid and allow the generation of novel non-blood-based immunoassays.
Acknowledgments
We thank Alan Day, Tabitha Wagner, Katherine Paweletz, and Heather Schessler for their technical assistance.
Funding for this project was kindly provided by the Schering-Plough Corporation.
REFERENCES
- 1.Alter M J, Margolis H S, et al. Recommendations for prevention and control of hepatitis C (HCV) infection and HCV-related chronic disease. Morb Mortal Wkly Rep. 1998;47(RR-19):26–28. [PubMed] [Google Scholar]
- 2.Alter M J, Purcell R H, Shih J W, Melpolder J C, Houghton M, Choo Q L, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Aryeh H, Ur I, Ben-Parath E. The relationship between antigenemia and excretion of hepatitis B surface antigen in human whole saliva and in gingival crevicular fluid. Arch Oral Biol. 1985;30:97–99. doi: 10.1016/0003-9969(85)90032-9. [DOI] [PubMed] [Google Scholar]
- 4.Cameron S O, Wilson K S, Good T, McMenamin J, McCarron B, Pithie A, Fox R. Detection of antibodies against hepatitis C virus in saliva: a marker of viral replication. J Viral Hepatitis. 1999;6:141–144. doi: 10.1046/j.1365-2893.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 5.Elsana S, Sikuler E, Yaari A, Shemer-Avni Y, Abu-Shakra M, Buskila D, Katzman P, Naggan L, Margalith M. HCV antibodies in saliva and urine. J Med Virol. 1998;55:24–27. [PubMed] [Google Scholar]
- 6.Freihorst J, Ogra P L. Mucosal immunity and viral infections. Ann Med. 2001;33:172–177. doi: 10.3109/07853890109002074. [DOI] [PubMed] [Google Scholar]
- 7.Genser B, Truschnig-Wilders M, Stunzner D, Landini M P, Halwachs-Baumann G. Evaluation of five commercial enzyme immunoassays for the detection of human cytomegalovirus-specific IgM antibodies in the absence of a commercially available gold standard. Clin Chem Lab Med. 2001;39:62–70. doi: 10.1515/CCLM.2001.014. [DOI] [PubMed] [Google Scholar]
- 8.Laufer D S, Hurni W, Watson B, Miller W, Ryan J, Nalin D, Brown L. Saliva and serum as diagnostic media for antibody to hepatitis A virus in adults and individuals who have received an inactivated hepatitis A vaccine. Clin Infect Dis. 1995;20:868–871. doi: 10.1093/clinids/20.4.868. [DOI] [PubMed] [Google Scholar]
- 9.Major C J, Read S E, Coates R A, Francis A, McLaughlin B J, Millson M, Shepherd F, Fanning M. Comparison of saliva and blood for human immunodeficiency virus prevalence testing. J Infect Dis. 1991;163:699–702. doi: 10.1093/infdis/163.4.699. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre P G, Laszlo J, Appleyard K, Ogden G R. Modified enzyme immunoassay to detect hepatitis C virus antibodies in oral fluid. Eur J Clin Microbiol Infect Dis. 1996;15:882–884. doi: 10.1007/BF01691223. [DOI] [PubMed] [Google Scholar]
- 11.Parry J V, Perry K R, Mortimer P P. Sensitive tests for viral antibodies in saliva; an alternative to tests on serum. Lancet. 1987;ii:72–75. doi: 10.1016/s0140-6736(87)92737-1. [DOI] [PubMed] [Google Scholar]
- 12.Saleh L H. The use of saliva for the detection of IgG and antibodies against rubella virus: comparison of indirect ELISA and antibody capture immunoassay. J Egypt Public Health Assoc. 1991;66:123–134. [PubMed] [Google Scholar]
- 13.Sherman K E, Creager R L, O'Brien S J, Sargent S, Piacentini S, Thieme T. The use of oral fluid for hepatitis C antibody screening. Am J Gastroenterol. 1994;89:2025–2027. [PubMed] [Google Scholar]
- 14.Stuart J M, Majeed F A, Cartwright K A, Room R, Parry J V, Perry K R, Begg N T. Salivary antibody testing in a school outbreak of hepatitis A. Epidemiol Infect. 1992;109:161–166. [PMC free article] [PubMed] [Google Scholar]
- 15.Thieme T, Yoshihara P, Piacentini S, Beller M. Clinical evaluation of oral samples for diagnosis of viral hepatitis. J Clin Microbiol. 1992;30:1076–1079. doi: 10.1128/jcm.30.5.1076-1079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Doornum G, Lodder A, Buimer M, Van Ameijden E, Bruisten S. Evaluation of hepatitis C antibody testing in saliva specimens collected by two different systems in comparison with HCV antibody and HCV RNA in serum. J Med Virol. 2001;64:13–20. doi: 10.1002/jmv.1011. [DOI] [PubMed] [Google Scholar]
- 17.Ward R L, Pax K A, Sherwood J R, Young E C, Schiff G M, Bernstein D I. Salivary antibody titres in adults challenged with a human rotavirus. J Med Virol. 1991;36:222–225. doi: 10.1002/jmv.1890360313. [DOI] [PubMed] [Google Scholar]
- 18.Zaman S, Carlsson B, Jalif F, Mellander L, Van Wezel A L, Bottiger M, Hanson L A. Comparison of serum and salivary antibodies in children vaccinated with oral live or parenteral inactivated poliovirus vaccines of different antigen concentrations. Acta Paediatr Scand. 1991;80:1166–1173. doi: 10.1111/j.1651-2227.1991.tb11805.x. [DOI] [PubMed] [Google Scholar]