Abstract
Map and Eap are secreted Staphylococcus aureus proteins that interact with various extracellular matrix molecules. PCR analysis using map primers yielded positive reactions in 97.9% of S. aureus isolates but not in Staphylococcus epidermidis isolates. Cloning and sequencing of the conferring genes revealed a high degree of overall homology combined with size variability of the gene product due to various repeat numbers and early translation termination in a poly(A) region. Thus, Map and Eap may provide a potential novel tool for S. aureus identification and typing.
Staphylococcus aureus continues to be a major pathogen, and adherence of S. aureus cells to host molecules is a prerequisite for tissue colonization and overt disease. In addition to the wall-bound MSCRAMM protein superfamily (for a review, see reference 15) conferring adhesion (4), cellular invasion (2, 17), and virulence (for a review, see reference 6), other adhesive S. aureus proteins are cell secreted and in part rebind to bacterial surface structures via mechanisms not yet clearly defined. Three of these molecules, i.e., coagulase (16), Efb (the extracellular fibrinogen [Fg] binding protein) (14), and a 60-kDa protein from S. aureus strain Newman (3) subsequently termed Eap (for extracellular adherence protein) (13) have been found to bind Fg. The size and function of Eap suggest close similarity with a group of 60- to 72-kDa proteins binding to a large spectrum of host molecules, including fibronectin (Fn), vitronectin (Vn), thrombospondin, bone sialoprotein, and collagen (11). The 72-kDa protein of strain FDA 574 has been cloned and sequenced and due to its subdomain homology with the major histocompatibility complex class II β chain has been termed Map (for major histocompatibility complex class II analog protein) (10). Similarity of Eap and Map has been further suggested upon comparison of the respective N-terminal sequences and amino acid compositions (13). While these studies suggest the presence and close similarity of Eap and Map analogs in different S. aureus laboratory strains, the degree of molecular homology has not yet been corroborated and furthermore, the presence of Eap and Map analogous sequences in clinical S. aureus and Staphylococcus epidermidis isolates has not yet been determined. Therefore, this study aimed to determine the prevalence of Eap and Map analogs in various clinical and laboratory strains, to further analyze their molecular nature, and to investigate the function of recombinant gene products.
Bacterial strains.
Nine laboratory strains of S. aureus—Newman D2C (ATCC 25904), Cowan 1 (ATCC 12598), SA113 (9), 8325-4 (12), 6850 (1), ATCC 12600, DSM 20044, DSM 10231, and Wood 46 (ATCC 10832)—as well as 240 methicillin-susceptible S. aureus isolates derived from clinical specimens were tested in this study. These isolates were obtained either from blood (n = 100) or from the anterior nares (n = 140) during a German multicenter study (18). Only one isolate per patient was tested. In addition, two reference strains of S. epidermidis (ATCC 35984 and DSM 20044) as well as eight clinical S. epidermidis isolates from patients in the University Hospital of Muenster, Muenster, Germany were included. Isolates were identified at the species level as S. aureus or S. epidermidis using standard microbiological methods including commercial phenotypic testing kits (Api Staph ID 32; BioMerieux, Marcy-l'Etoile, France).
Phenotypic characterization of Map and Eap analogs.
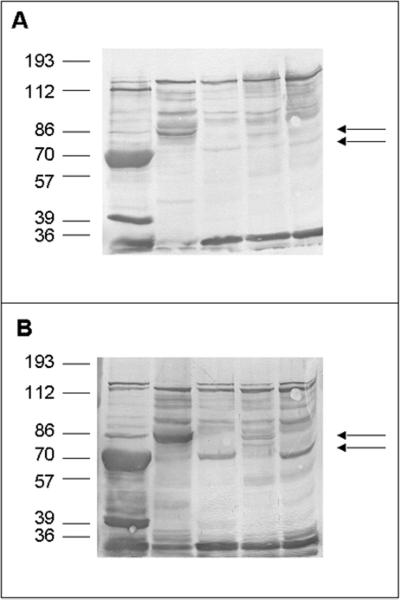
Bacterial cell surface proteins were selectively extracted by heating bacteria in 2% sodium dodecyl sulfate (SDS) in 100 mM Tris-HCl, pH 6.8, without significant release of intracellular contents or cells lysis (8). Briefly, bacteria were grown overnight, pelleted, resuspended in 2% SDS (Sigma-Aldrich, Deisenhofen, Germany) in 125 mM Tris-HCl (pH 7.0), heated at 95°C (3 min), and then centrifuged (10,000 × g, 5 min). The supernatant was dialyzed against distilled water to remove SDS and stored at −20°C. Human Fn (Chemicon, Temecula, Calif.), human Fg (Calbiochem, San Diego, Calif.), and Vn (purified according to the method presented in reference 19) were labeled with biotin (Boehringer, Mannheim, Germany) according to the instructions of the supplier. For Western ligand analysis, the cell surface extracts of S. aureus strains Newman, Cowan 1, Wood 46, 8325-4, and SA113 separated by SDS-polyacrylamide gel electrophoresis (PAGE) were blotted electrophoretically onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), membranes were blocked with 3% bovine serum albumin, and proteins were probed with biotinylated Vn and Fn. A protein running between markers of 70 and 86 kDa was found in extracts of strain Wood 46, while in extracts of strains Newman, 8325-4, Cowan, and SA113, a protein close to the marker of 70 kDa was detected after incubation with Fn and Vn (Fig. 1). The 70-kDa protein of strain Newman (presumably corresponding to previously published Eap) was detected in remarkably large amounts and confirmed to be a Map analog based upon sequence comparison of the 18-amino-acid N-terminal sequence (AAKPL DKSSS SLHHG YSK; 89% identity to Map from S. aureus FDA 574 [EMBL accession no. U20503], 95.6% identity to a partially [121 amino acids] sequenced 70-kDa outer membrane protein precursor from S. aureus ATCC 25923 [EMBL accession no. X13404], and 74% identity to a 70-kDa outer surface binding protein [p70] from S. aureus Wood 46 [EMBL accession no. Y10419]).
FIG. 1.
Western ligand blot analysis with biotinylated Fn and Vn of S. aureus cell surface proteins. Cell surface proteins were extracted by boiling whole cells in 100 mM Tris-HCl, pH 6.8, containing 2% SDS. After separation by SDS-PAGE, proteins were blotted onto a nitrocellulose membrane and probed with biotin-labeled Fn (A) and Vn (B), and bands were visualized in a color reaction using avidin alkaline phosphatase. From left to right, lanes contain Newman, Wood 46, 8325-4, Cowan 1, and SA113. Upper arrow in each panel, Eap of Wood 46; lower arrow in each panel, Eap of the other strains tested.
Incidence and polymorphism of map analogs in clinical staphylococcal isolates.
For isolation of genomic DNA, staphylococci from 0.5 ml of an overnight brain heart infusion culture were pelleted by centrifugation at 5,000 × g for 20 min, resuspended in 180 μl of TE buffer (20 mM Tris chloride, 2 mM EDTA [pH 8.0]) with 7.5 μl of recombinant lysostaphin (5 mg/ml; Sigma-Aldrich) by vortex mixing, and incubated at 37°C for 30 min. DNA was subsequently extracted with a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. Nucleic acid samples were eluted with distilled water and adjusted to a final concentration of 1 μg/ml according to A260 values. The PCR amplification was performed in a thermal cycler with a hot bonnet (iCycler; Bio-Rad, Munich, Germany). The PCR mixture consisted of 1 μg of DNA; 10 mM Tris-HCl, pH 8.3; 10 mM KCl; 3 mM MgCl2; a 1 μM concentration of the primer; a 200 μM concentration (each) of dATP, dCTP, dGTP, and dTTP; 5 U of Taq DNA polymerase (QBIOgene, Heidelberg, Germany); and double-distilled water added to a final volume of 50 μl. A total of 30 PCR cycles were run under the following conditions: DNA denaturation at 95°C for 1 min (5 min for the first cycle), primer annealing at 50°C for 1 min, and DNA extension at 72°C for 2 min. After the final cycle, the reaction was terminated by keeping it at 72°C for 10 min. Ten microliters of the product was analyzed on 1% ethidium bromide-stained agarose gel.
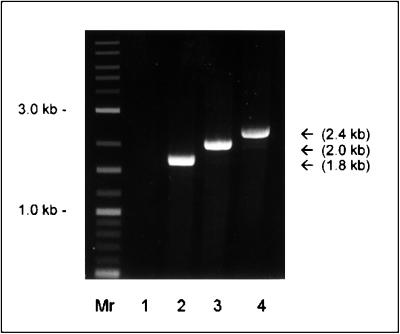
Using primer P2 (Table 1), which was designed according to the published map sequence (10), and primer P3, which was designed according to the eap-N sequence in strain Newman (as described below), the presence of map analogues was demonstrated in 235 of 240 (97.9%) S. aureus isolates. Based upon PCR product sizes, the map-positive clinical strains could be attributed to two groups—group I (1.8-kbp product), consisting of blood isolates (14 of 100 [14.0%]) and nasal isolates (24 of 140 [17.9%]), and group II (2-kbp product), consisting of blood isolates (85 of 100 [85.0%]) and nasal isolates (112 of 140 [80.0%]). An additional group III product (2.4 kbp) was only found in the laboratory strain Wood 46 but not in any clinical isolate (Fig. 2). Five clinical S. aureus isolates, i.e., 1 of 100 (1.0%) blood isolates and 4 of 140 (2.9%) nasal isolates, were identified as map negative. Analysis of the origin of the clinical isolates tested (blood isolates versus nasal isolates) did not reveal significant differences with respect to the attribution to the different groups (chi-square test, P ≥ 0.59). In addition, two reference strains of S. epidermidis (ATCC 35984 and DSM 20044) and 10 clinical S. epidermidis isolates were also tested, yet map was not detected in any of these 12 S. epidermidis isolates. Three S. aureus strains (clinical isolate 7 and strains Newman, and Wood 46) representing each group were selected for further characterization, and map analogs were amplified using primers P1 and P2 for clinical isolate 7 and P5 and P2 for the Newman and Wood isolates.
TABLE 1.
Primer sequences used in this study
| Primer | Sequence | Comments |
|---|---|---|
| P1 | 5′CTC GGA TCC ATG AAA TTT AAG TCA TTG ATT ACA ACA ACA TTA GCA TTA GG 3′ | Nucleotides 71–111 of map; BamHI site underlined |
| P2 | 5′ CTC GGT ACC TTA AAA TTT AAT TTC AAT GTC TAC TTT TTT AAT GTC 3′ | Nucleotides 2139–2105 of map; KpnI site underlined |
| P3 | 5′CTC GGA TCC GCA GCT AAG CCA TTA GAT AAA TCA TCA AGT TCG TTA CAC C3′ | Nucleotides 91–130 of eap-N; BamHI site underlined |
| P4 | 5′CTC GGA TCC GCA GCT AAG CCA TTA GAT AAA TCA TCA AGT TTA TTA CAT C3′ | Nucleotides 91–130 of eap-W; BamHI site underlined |
| P5 | 5′CTC GGA TCC AAA ATA GAG AAA GTC TGG CTA TAA TTA AGT TGC 3′ | Nucleotides 3–35 of map; BamHI site underlined |
FIG. 2.
Agarose (1%) gel stained with ethidium bromide showing the PCR product amplified with the eap and map primers P2 and P3 from genomic DNA of S. aureus as described in Methods and Materials. Lanes: Mr, DNA molecular size marker (combined 100-bp and 1-kb DNA ladder [New England Biolabs]); 1, S. epidermidis DSM 20044; 2, S. aureus clinical isolate 7 (1.8 kb); 3, S. aureus Newman (2.0 kb); 4, S. aureus Wood 46 corresponding to group III (2.4 kb).
The PCR products were ligated in pCR 2.1 vector (Invitrogen, Carlsbad, Calif.), and the ligation mixture was transformed in Escherichia coli INVαF′ (Invitrogen). According to the designation of the protein in strain Newman referred to as Eap (13), the plasmid containing the map-analogous sequence from S. aureus Newman D2C was referred to as pEap-N, and the plasmids with sequences from clinical isolate 7 and strain Wood 46 were designated pEap-7 and pEap-W, respectively.
Sequencing of eap-N, eap-7, and eap-W.
Plasmid DNA was prepared using the Qiagen plasmid Mini kit and used as a template for sequencing on an ABI 310 genetic analyzer. Sequencing was started with the M13 reverse and the T7 standard primers of the vector upstream and downstream of insert DNA, respectively, and continued by primer walking, with subsequent primers being synthesized according to obtained sequence information. Nucleotide and deduced protein sequences were analyzed with the program of the European Molecular Biology Laboratory (EMBL) outstation European Bioinformatic Institute (EBI) (Cambridge, United Kingdom, http://www.ebi.ac.uk/). We have determined the complete nucleotide sequences encoding Eap from strain Newman 2DC (eap-N), from clinical isolate 7 (eap-7), and from strain Wood 46 (eap-W). In these sequences, the putative translation start codon ATG is preceded by a putative ribosomal binding sequence characteristic of gram-positive bacteria. The first 30 amino acids have features typical of a bacterial signal peptide. Sequence data are summarized in Table 2. The deduced amino acids sequence of Eap-W and the deposited sequence of mature peptide of p70 are almost identical (99%), confirming the sequencing results of strain Wood 46 from two different laboratories. The deposited sequence of p70 is lacking a signal peptide sequence, and the eap-W sequence now provides the complete sequence information on Eap of Wood 46. The deposited nucleotide sequence of strain Newman (Kreikemeyer et al., EMBL accession no. AJ223806 [for brevity, in this report, it is designated map-N]) is lacking a part of the 5′ sequence, yet on the protein level this sequence is 98% identical to the complete Eap-N sequence presented here.
TABLE 2.
Comparisons of the eap genes and the Eap proteins of S. aureus strains used in this studya
| Strain | Gene designation | ORF size (bp) | %GC content | Full-length polypeptide
|
Mature peptide
|
Alignment scoreb (%) | ||
|---|---|---|---|---|---|---|---|---|
| Molecular mass (kDa) | No. of aa | Molecular mass (kDa) | No. of aa | |||||
| Newman | eap-N | 1,751 | 27.8 | 65.4 | 583 | 62.3 | 533 | 81 |
| Isolate 7 | eap-7 | 1,755 | 29.6 | 65.2 | 584 | 62.2 | 554 | 96 |
| Wood 46 | eap-W | 2,061 | 28 | 77.1 | 687 | 74.0 | 657 | 75 |
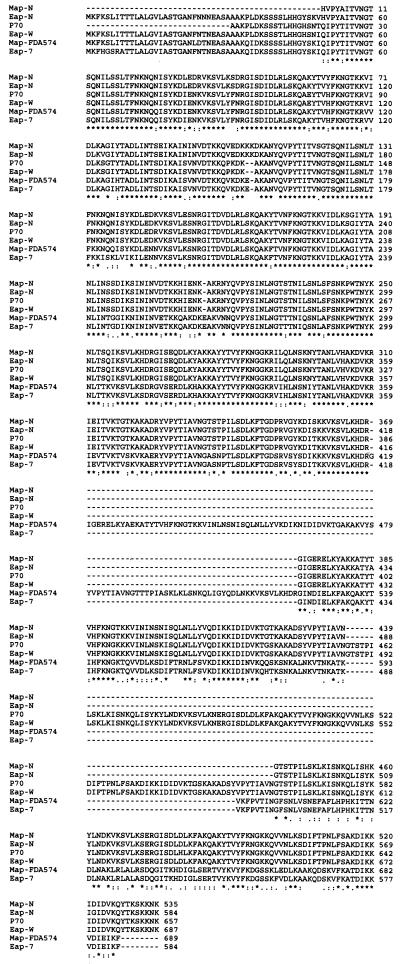
Multiple alignments of deduced amino acid sequences of Eap-N, Eap-W, and Eap-7 as well as deposited sequences of the p70 protein of strain Wood 46, Map of strain FDA574, and Map-N are shown in Fig. 3. An alignment score with Map-FDA574 between 74 and 96% demonstrates the high similarity of Eap and Map analogs. Sequence comparison of Eap-N and Map-N and Map-FDA574 confirms the high homology of Eap and Map as already suggested by amino-terminal sequence analysis of Eap (13). Of note, the PCR products of genomic DNAs of S. aureus Newman D2C and Wood 46 with primers P1 and P2 were 2,056 and 2,364 bp, respectively. A stop codon preceded by nine adenine bases (starting at nucleotide 1740 in Newman D2C and nucleotide 2049 in Wood 46) resulted in mature peptides of 65.5 and 74 kDa for Newman D2C and Wood 46, respectively. This finding suggests an insertional point mutation of an additional adenine base in the poly(A) region which causes the premature termination of translation. If this region consisted of eight adenine bases, or if translation continued in a second open reading frame, mature peptides of 77 and 85.55 kDa for Newman D2C and Wood 46 would be expected, respectively. Analysis of the published sequences of Map-N and p70 suggests that events similar to the one observed in Eap-N and Eap-W have occurred in these strains. The multiple-sequence alignment presented in Fig. 4 suggests that in the absence of an insertional point mutation in the poly(A) region, all Eap and Map proteins have almost identical amino-terminal and carboxy-terminal sequences with high homology to Map of FDA574.
FIG. 3.
Alignment of the deduced amino acid sequences of Eap-N, Eap-7, and Eap-W with published sequences of Map FDA574, p70, and Map-N. Alignment was determined with the analysis program, multiple sequence alignments 2CLUSTAL W (version 1.74), available at http://www2.ebi.ac.uk. Asterisks indicate consensus amino acids; dots indicate gaps introduced to maximize alignment. References and EBL data bank accession numbers are detailed in text.
FIG. 4.
Alignment of the deduced amino acid sequences of map FDA574, Eap-7, Eap-N, and Eap-W. Alignment was performed after deleting one A residue at nucleotide 1792 in eap-N (referred to as Eap-N_8A) and 2112 in eap-W (Eap-W_8A) with the sequence analysis program as described in Fig. 3.
Expression and functional analysis of recombinant eap
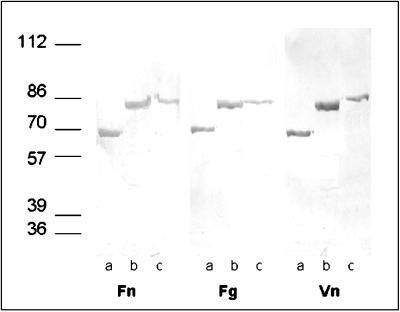
eap-N, eap-7, and eap-W (lacking the signal peptide) were amplified by PCR with upper primer P3 for eap-N and eap-7 or upper primer P4 for eap-W and lower primer P2. PCR products were ligated into the QIAexpress pQE30 vector (Qiagen). The ligation mixture was then transformed in E. coli M-15. Six-His-tagged Map fusion proteins were expressed and purified according to the protocol provided by the manufacturer (Qiagen). The expression of His-tagged recombinant Eap using vector pQE30 in E. coli M15 allows single-step purification using Ni-nitrilotriacetic acid affinity resin. SDS-PAGE of recombinant Eap (Eap-W, 74 kDa; Eap-N, 65 kDa [in some expression and purification experiments, 74-kDa proteins were observed] [Fig. 5]; Eap-7, 65 kDa) revealed proteins of similar size compared to the Eap analogs from the respective S. aureus strains extracted with 2% SDS. The recombinant proteins also showed binding to biotin-labeled Fn, Fg, and Vn in Western ligand blots (Fig. 5). Control blots without ligands but with alkaline phosphatase-conjugated avidin did not reveal a signal.
FIG. 5.
Western ligand blot analysis of recombinant proteins Eap-7 (lanes a), Eap-N (lanes b), and Eap-W (lanes c) with Fn, Fg, and Vn. Recombinant proteins were affinity purified as described in the text. After separation by SDS-PAGE, proteins were blotted onto nitrocellulose membrane and probed with biotin-labeled ligands, and spots were visualized by alkaline phosphatase reaction. The values on the left are molecular masses in kilodaltons. Note that in this experiment migration of Eap-N corresponds to a larger protein size than that of Eap-N in Fig. 1 (see also text).
Expression and localization of Eap analogs in clinical staphylococcal isolates.
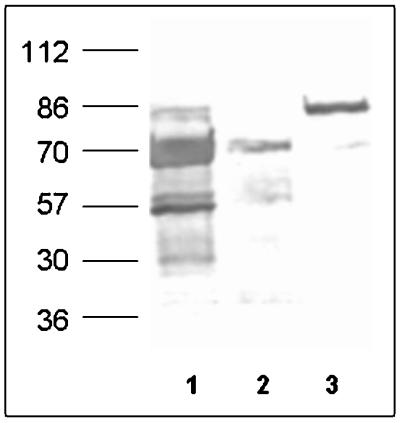
To confirm the expression of eap in isolates with a positive PCR for eap, Western immunoblots were probed with anti-Eap antibodies developed against recombinant Eap-N. Polyclonal antibodies were raised in two rabbits according to the method presented in reference 7 by injecting 50 μg of Ni-nitrilotriacetic acid-purified Eap-N and two consecutive booster injections. Naturally occurring antistaphylococcal antibodies were immunodepleted by mixing serum with 100 volumes of 2% SDS extract from a clinical isolate which does not contain eap as determined by the PCR analysis method and SDS-PAGE. SDS-PAGE-separated S. aureus surface proteins extracted with 2% SDS-containing buffer by heating at 95°C for 3 min were blotted onto a nitrocellulose membrane and probed with antiserum (dilution, 1:10,000), and blots were developed using alkaline phosphatase-conjugated goat anti-rabbit immunoglobulins (dilution 1:3,000; Dako A/S, Glostrup, Denmark). Of 20 clinical isolates tested, Eap expression was observed in 14 isolates belonging to groups I and II as determined by PCR analysis. Variation in the amount of Eap expression was observed, with maximum protein production by strain Newman (Fig. 6).
FIG. 6.
Western immunoblot analysis of SDS surface protein extracts from three S. aureus isolates with anti-Eap antibodies. Lane 1, S. aureus Newman; lane 2, S. aureus isolate 7; lane 3, S. aureus Wood 46.
For confirmation of the extracellular localization, samples of 10 of these 14 Eap-positive S. aureus isolates, including strains Newman and Wood 46 and clinical isolate 7 as well as five of six isolates not expressing Eap, were further studied using anti Eap-N antibodies to elicit agglutination reactions. Viable washed bacteria from a fresh overnight culture were suspended in phosphate-buffered saline (PBS) (Gibco) containing 0.5% bovine serum albumin (Sigma) to an optical density at 578 nm of 1. To 50 μl of bacterial suspension, crude antiserum (working dilution, 1:1,000) preadsorbed with a 2% SDS cell surface extract from a Δeap deletion mutant of strain Newman (M. Hussain, unpublished data) was added on a glass slide, and the agglutination reaction at room temperature was scored after 3 min as negative or positive. Antibodies raised against recombinant Eap-N were able to agglutinate all 10 tested isolates of S. aureus expressing Eap as determined by Western immunoblot analysis, indicating cross-reactivity of the anti-EapN antibodies with Eap proteins of other groups. In contrast, all five tested isolates in Western blots not expressing Eap did not show an agglutination reaction. Agglutination reactions performed with S. aureus isolates using preimmune serum yielded a negative reaction in all instances.
Previously, it has been demonstrated that Eap forms oligomers, rebinds to S. aureus cell surfaces via interaction putatively with a recently described neutral phosphatase (5), and mediates agglutination of strain Newman (13). We could extend these findings by demonstrating agglutination of a panel of staphylococcal isolates by recombinant Eap analogs. Soluble recombinant Eap analogs (Eap-7, Eap-N, and Eap-W; 10 μg of recombinant protein mixed with 50 μl of bacterial suspension containing 5 × 107 CFU in PBS on a glass slide at room temperature for 3 min) were all capable of agglutinating cells of all of the 10 tested isolates of S. aureus and a single strain each of S. epidermidis, Staphylococcus haemolyticus, Staphylococcus saprophyticus, Staphylococcus capitis, and Staphylococcus lugdunensis but did not agglutinate cells of Micrococcus luteus. PBS was used as a negative control. This observation indicates that the Eap-mediated agglutination reaction is representative while it may also be specific for staphylococci.
In conclusion, the present study reveals that Eap analogs are highly prevalent in various clinical and laboratory isolates of S. aureus, while they were not demonstrable in S. epidermidis isolates. Sequencing results suggest that Eap and Map sequences are highly conserved. Size differences of various eap genes are a result of the number of repeats; in addition, the size of the translated product is also determined by an insertional point mutation or ribosomal slippage at the site of a poly(A) region located at the 3′ end of the respective repeat region. Our data describe Eap as a potential novel tool for S. aureus identification and typing. Furthermore, they provide insight in the molecular architecture and function of Eap and thus contribute to our enhanced understanding of its biologic role.
Nucleotide sequence accession numbers.
The complete nucleotide sequences encoding Eap from strain Newman 2DC (eap-N), from clinical isolate 7 (eap-7), and from strain Wood 46 (eap-W) are deposited in the EMBL database under the following accession numbers: eap-N, AJ290973; eap-7, AJ243790; eap-W, AJ245439.
ACKNOWLEDGMENTS
This work has been supported by the Deutsche Forschungsgemeinschaft, Collaborative Research Center 492, Project B9.
Technical support by M. Schulte for PCR analysis and helpful discussions with J. I. Flock and R. A. Proctor are gratefully acknowledged.
REFERENCES
- 1.Balwit J M, van Langevelde P, Vann J M, Proctor R A. Gentamicin-resistant, menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis. 1994;170:1033–1036. doi: 10.1093/infdis/170.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodén M K, Flock J-I. Evidence for three different fibrinogen-binding proteins with unique properties from Staphylococcus aureus strain Newman. Microb Pathog. 1992;12:289–298. doi: 10.1016/0882-4010(92)90047-r. [DOI] [PubMed] [Google Scholar]
- 4.Flock J I. Extracellular-matrix-binding proteins as targets for the prevention of Staphylococcus aureus infections. Mol Med Today. 1999;5:532–537. doi: 10.1016/s1357-4310(99)01597-x. [DOI] [PubMed] [Google Scholar]
- 5.Flock M, Flock J I. Rebinding of extracellular adherence protein Eap to Staphylococcus aureus can occur through a surface-bound neutral phosphatase. J Bacteriol. 2001;183:3999–4003. doi: 10.1128/JB.183.13.3999-4003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster T J, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 7.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 53–138. [Google Scholar]
- 8.Hussain M, Peters G, Chhatwal G S, Herrmann M. A lithium chloride-extracted, broad-spectrum-adhesive 42-kilodalton protein of Staphylococcus epidermidis is ornithine carbamoyltransferase. Infect Immun. 1999;67:6688–6690. doi: 10.1128/iai.67.12.6688-6690.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iordanescu S, Surdeanu M. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J Gen Microbiol. 1976;96:277–281. doi: 10.1099/00221287-96-2-277. [DOI] [PubMed] [Google Scholar]
- 10.Jönsson K, McDevitt D, McGavin M H, Patti J M, Höök M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J Biol Chem. 1995;270:21457–21460. doi: 10.1074/jbc.270.37.21457. [DOI] [PubMed] [Google Scholar]
- 11.McGavin M H, Krajewska Pietrasik D, Rydén C, Höök M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 13.Palma M, Haggar A, Flock J I. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J Bacteriol. 1999;181:2840–2845. doi: 10.1128/jb.181.9.2840-2845.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palma M, Wade D, Flock M, Flock J I. Multiple binding sites in the interaction between an extracellular fibrinogen-binding protein from Staphylococcus aureus and fibrinogen. J Biol Chem. 1998;273:13177–13181. doi: 10.1074/jbc.273.21.13177. [DOI] [PubMed] [Google Scholar]
- 15.Patti J M, Höök M. Microbial adhesins recognizing extracellular matrix macromolecules. Curr Opin Cell Biol. 1994;6:752–758. doi: 10.1016/0955-0674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 16.Phonimdaeng P, O'Reilly M, Nowlan P, Bramley A J, Foster T J. The coagulase of Staphylococcus aureus 8325-4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol Microbiol. 1990;4:393–404. doi: 10.1111/j.1365-2958.1990.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 17.Sinha B, Francois P, Que Y A, Hussain M, Heilmann C, Moreillon P, Lew D, Krause K H, Peters G, Herrmann M. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect Immun. 2000;68:6871–6878. doi: 10.1128/iai.68.12.6871-6878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 19.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]