Abstract
Cancer is one of the major medical challenges, with an unacceptably high death toll worldwide. In Morocco, medicinal plants continue to play a pivotal therapeutic role despite the development of modern sanitation systems. In the current study, an ethnobotanical survey was carried out at the Moroccan national institute of oncology, Rabat, and we aimed at (1) establishing an exhaustive inventory of indigenous knowledge of Moroccan medicinal plants used to manage cancer and (2) confirming the reported ethnopharmacological uses through bibliometric review. An ethnobotanical survey was conducted with 291 cancer patients at the Moroccan National Institute of Oncology, Rabat, during a period of 4 months, from February to May 2019, through semistructured interviews. Ethnobotanical indices, including informant consensus factor (FIC), use report (UR), relative frequency citation (RFC), botanical family use value (FUV), fidelity level (FL), and index of agreement on remedies (IAR), were employed in data analyses. The survey revealed that 39 medicinal plants belonging to 27 botanical families and 38 genera were used to treat cancer. The most used ethnospecies were Aristolochia longa with the highest RFC value (0.096), followed by Nigella sativa, Ephedra alata, Euphorbia resinifera, and Lavandula dentata, éwith RFC values of 0.072, 0.054, 0.044, and 0.044, respectively. In regard to the plant families, Lamiaceae contributed the highest number of plants with five species (FUV = 0.034), followed by Asteraceae (4 species; FUV = 0.020), and Fabaceae (4 species; FUV = 0.020). The leaves are the most popular plant part used by the studied population against cancer; otherwise, decoction was the most commonly used method for remedy preparation and the highest FIC was noticed for uterine cancer treatment (0.86). Considering these findings, further investigations into the recorded plant species should be performed to assess phytochemical constituents and pharmaceutical benefits in order to identify their active compounds for any drug formulations.
1. Introduction
Cancer is one of the leading causes of death in humans [1, 2]. According to the World Health Organization (WHO) and the International Agency for Research on Cancer (IARC), there were approximately 9.6 million deaths and 18.1 million new cancer cases worldwide in 2018 [3]. The estimated number of new cancer cases and deaths worldwide by 2040 is around 27.5 million and 16.3 million, respectively [4]. In Morocco in 2020, there were 59,370 new cases and 35,265 cancer deaths for both sexes and all ages. Breast cancer was the most common type of cancer in Morocco (19.8%), followed by lung (12.4%), colorectal (7.7%), prostate (7.5%), non-Hodgkin's lymphoma (4.1%), and other cancers (48.5%) [5].
To date, chemotherapy, surgery, and radiotherapy remain the most frequently employed options for cancer treatment, but their toxicity and side effects restrict their usage [6, 7]. Chemotherapy and/or radiotherapy may lead to some serious complications, such as fatigue, chronic pain, oral mucositis, anorexia, gastrointestinal toxicity, hepatotoxicity, nephrotoxicity, insomnia, edema, depression/anxiety, or constipation [6, 7]. These effects are difficult to manage and can significantly affect the quality of a patient's life [2].
Furthermore, due to poverty and the high cost of biological analysis and treatment, access to this type of treatment remains limited in rural Morocco and other developing countries [6]. As a result, cancer has become one of the medical challenges that has resulted in an unacceptably high death toll in these areas [8, 9]. Thus, there is a need to revert to homegrown solutions, such as exploring the flora of our own country, Morocco, which is part of the North African botanical block and is home to approximately 4,200 taxa, 32% (1350 taxa) of which are endemic [9].
In practice, 55% to 90% of the general population frequently reports using medicinal plants to treat Moroccan cancer patients [2]. Several ethnobotanical studies conducted in Morocco have also revealed significant traditional uses of medicinal plants for cancer management. Aboufaras et al. recently conducted a survey with 530 cancer patients at Beni Mellal's Regional Oncology Center. They reported that Aristolochia longa L., Euphorbia resinifera Berg, and Nigella sativa L. are recommended for the treatment of a variety of cancers, including breast, cervix, lung, colorectal, oral, stomach, and bladder cancers [2].
Furthermore, many plant-derived anticancer agents, such as vinca alkaloids (vinblastine, vincristine, and vindesine), epipodophyllotoxins (etoposide and teniposide), taxanes (paclitaxel and docetaxel), and camptothecin derivatives (camptothecin and irinotecan), have been used successfully in clinical studies [10–13].
As a result, it is critical to identify natural agents present in medicinal plants that may have the anticarcinogenic potential [1, 14–18]. Because ethnobotanical and ethnopharmacological surveys, including those conducted with patient interviews, are effective methods for documenting and identifying medicinal plants used in traditional pharmacopeia systems, the current study was carried out at the Moroccan National Institute of Oncology (Sidi Mohamed Ben Abdellah) to record medicinal plant species used in the management of various types of cancer and to identify the potential bioactive phytochemicals in the claimed plants as well as their potential mechanisms of action via an in-depth literature revision.
2. Materials and Methods
2.1. Study Area
Morocco's Rabat-Sale-Kenitra region is in the northwest (34° 02′ 00″ N, 6° 50′ 00″ W) (Figure 1). It has a population of about 4,580,866 people and an area of 18,194 km2 (2.56% of the national territory). The Tangier-Tetouan-Al-Hoceima region borders it on the north, the Fez-Meknes region on the east, the Beni Mellal-Khenifra and Casablanca-Settat regions on the south, and the Atlantic Ocean on the west [19].
Figure 1.
Geographical location of the study area.
The study area has three prefectures: Rabat, Sale, and Skhirate-Temara, and it has four provinces: Kenitra, Sidi Slimane, Khemisset, and Sidi Kacem. The Rabat-Sale-Kenitra region concentrates the majority of the Kingdom's demographic, economic, administrative, and cultural flows. This development is mainly due to the administrative weight of Rabat, the capital of Morocco. Its exceptional geographical position, its human resources, and its natural potential make it a platform of development and a crossroad for investment and partnership [19].
The Rabat-Sale-Kenitra region has a remarkable natural and environmental heritage with numerous sites playing a major role in the conservation of biodiversity and ecosystems. This region contains over 408 vascular plant species and subspecies from 261 genera and 62 botanical families, accounting for approximately 10% of the Moroccan vascular flora [20, 21]. Remarkably, this region contains many natural reservoirs, such as the Mamora forest, which is one of the world's most important ecological reserves of Quercus suber [16].
2.2. Ethnobotanical Fieldwork
In order to collect and document indigenous knowledge of Moroccan medicinal plants used to manage cancer, a series of ethnobotanical surveys were carried out at the Moroccan national institute of oncology, Rabat (Sidi Mohamed Ben Abdellah). The target population was 1216 cancer patients who consulted consecutively during the study period. The size of the sample was estimated using the following formula [2]:
| (1) |
where n: Sample size; N: population size = 1216, z: z-score = 1.96; e: error margin = 5%; and p: standard deviation = 0.5.
The minimum sample size estimated was 291 participants, with a confidence level of 99%. Indeed, among 1216 (N) patients, 291 (n) informants with different types of cancer were selected randomly. No special selection criteria were used in the choice of the participants.
The data were collected during a period of 4 months, from February to May 2019, through semistructured interviews using the Moroccan Arabic dialect, and the time spent in each interview varied from 20 to 30 min. This survey was designed first to establish the sociodemographic features of cancer patients (name, age, gender, education level, ethnicity, place of residence, and income of participants) and the traditional remedies used in cancer treatment. In fact, the questionnaire included different ethnobotanical and ethnomedicinal information such as vernacular and scientific names; plant families; plant parts used; preparation methods; administration modes; posology; side effects; and therapeutic combinations.
2.3. Species Identification, Systematization, and Preservation
The species taxonomic identification was undertaken based on standard Moroccan floras, including the practice flora of Morocco [22], vascular flora of Morocco, inventory and chorology [23], traditional Moroccan pharmacopeia [24], and Moroccan medicinal plants [25]. The botanical names of inventoried species were checked at the Phytovigilance Department of Moroccan Poison Control and the Pharmacovigilance Center, Rabat, from online botanical databases, namely: The Plant List (https://www.theplantlist.org/), African Plant Database (https://www.ville-ge.ch/musinfo/bd/cjb/africa/recherche.php) and the International Plant Names Index (https://www.ipni.org).
The listed plant species with their correct nomenclature were arranged alphabetically by family names based on the Angiosperm Phylogeny Group-IV (APG-IV) classification 2016 and vernacular names. Moreover, an EPPO code for each plant species was provided from an online database, the European and Mediterranean Plant Protection Organization (https://www.eppo.int). An EPPO code is an encoded identifier of species names, both scientific and vernacular. Finally, all the preserved specimens in the form of dried plants, parts of plants or pressed plant samples have been deposited in the herbarium of the Department of Biology, Faculty of Sciences and Technologies, University of Sidi Mohamed Ben Abdellah Fez, with defined ID numbers (BLMUP).
2.4. Quantitative Data Analysis
To describe the collected ethnopharmacological information, data were first sorted in the Microsoft Excel database and arranged in use reports (UR, a citation of one plant used by one respondent). Then, the one-way analysis of variance (ANOVA) was used to analyze the socio-demographic data of the patients in order to determine whether there were any significant variations between the means as well as correlations between the variables (Independent Samples T-Test, p values ≤0.05 were considered statistically significant). Ethnobotanical quantitative indices were also used to discuss our findings and to clearly indicate their novelty value compared with other ethnobotanical studies, which included the informant consensus factor (FIC), the use report (UR), the relative frequency citation (RFC), the botanical family use value (FUV), the fidelity level (FL), and the index of agreement on remedies (IAR). Moreover, principal component analysis (PCA) was performed based on the ethnobotanical indices using XLSTAT statistics version 2016 software.
2.4.1. Informant Consensus Factor (FIC)
The FIC shows the homogeneity of the exchange of traditional knowledge between informants concerning the use of taxa to treat various disease categories. FIC was calculated using the following formula [26]:
| (2) |
where Nur refers to the number of usage reports for each ailment (type of cancer) and Nt is the number of species used for the same ailment. A high value (close to 1) of FIC indicated that the reported plant is primarily recognized as an anticancer agent by the informants.
With (0 < FIC < 1).
2.4.2. Fidelity Level (FL)
The fidelity level (FL) indicates the effective effects of a given plant species against a particular ailment. FL was calculated according to Sreekeesoon & Mahomoodally [27].
| (3) |
where Ip is the number of informants who used a specific species for a particular type of cancer, and Lu is the total number of respondents who indicated all uses of the given species for the treatment of any cancer.
2.4.3. Relative Frequency Citation (RFC)
The relative citation frequency (RFC) was calculated by dividing the citation frequency (FC) by the population size (N) [28].
| (4) |
This index demonstrates the local relative importance of each species.
2.4.4. Family Importance Value (FIV)
The family importance value (FIV) shows the significance of botanical families. It was established to evaluate the biological taxonomic value of species and was calculated as follows [27]:
| (5) |
where RFC = the use value of the species belonging to the family; Ns = the number of species in the family.
2.4.5. Index of Agreement on Remedies (IAR)
The index of agreement on remedies (IAR) was designed to measure the value of species for which there is consensus on more than one traditional use. Indeed, a species with a high number of citations in several cancers may rank higher than plants with more citations in any single type of cancer. This index was calculated according to the following formula [29]:
| (6) |
With Nr, the total number of use citations for a specific plant across all use categories, and Na, the number of mentioned categories.
2.5. Ethical Statement and Consent to Participate
This study has been carried out with the permission of the committee for ethical research of the Moroccan Poison Control and Pharmacovigilance Center and the Moroccan National Institute of Oncology, under the number 310/2019. The data was collected with respect to confidentiality, anonymity, and consent. All patients were informed about the objective of this investigation. Oral and signed consent was obtained from the study participants.
2.6. Literature-Based Validation of the Data
Based on previous ethnobotanical investigations of Moroccan anticancer flora, we performed a bibliometric survey using different scientific search engines, including Google Scholar, Web of Science, Scopus, ScienceDirect, SpringerLink, Medline, SciFinder, and PubMed. The inventoried plants in this study were compared with other ethnobotanical surveys of plants used for cancer management in Morocco and other neighboring countries (Algeria, Tunisia, and Libya). This comparison allowed us to assess the originality and uniqueness of traditional uses sustained by the studied population and to report new plant citations and new medicinal practices against cancer. Furthermore, the data on the in vitro and in vivo anticancer properties of these plants, as well as the mechanisms underlying their activities, were summarized. Finally, the major chemical compounds demonstrating anticancer properties were also reviewed in this study, and ChemDraw Ultra 16.0 software was used to draw their chemical structures. The IUPAC names of the reported compounds were checked from PubChem databases (pubchem.ncbi.nlm.nih.gov).
3. Results and Discussion
3.1. Sociodemographic Data
According to the age distribution (Table 1, Figure 2), 55% of respondents were between the ages of 40 and 60, followed by those between the ages of 20 and 40 (29%), and only a few informants were older than 60 years old (16%). According to our findings, people over the age of 40 were the most likely to use medicinal plants to treat various types of cancer. As a result, the difference in age and indigenous knowledge was statistically significant (P=0.001). These findings could be explained by the fact that older people have more knowledge about the use of medicinal plants and their benefits as a result of practical knowledge passed down from older generations [16, 30]. Other ethnobotanical surveys carried out on a national and international scale showed similar results [16, 30–32].
Table 1.
Informant sociodemographic profile.
| Sociodemographic characteristic | Variables | Numbers of informants (%) | Number of used ethnospecies (%) | p value |
|---|---|---|---|---|
| Age | 20–40 | 85(29%) | 9(53.84%) | 0.001 ∗ |
| 40–60 | 159(55%) | 21(53.84%) | ||
| More than 60 | 47(16%) | 33(84.61%) | ||
|
| ||||
| Gender | Male | 128(44%) | 26(66.66%) | 0.140 |
| Female | 163(54%) | 37(94.87%) | ||
|
| ||||
| Education level | No | 105(36%) | 29(74.35%) | 0.360 |
| Primary | 67(23%) | 32(82.05%) | ||
| Secondary | 81(28%) | 17(43.58%) | ||
| University | 38(13%) | 8(20.51%) | ||
|
| ||||
| Location | Rural | 176(60%) | 37(94.87%) | 0.140 |
| Urban | 125(40%) | 30(76.92%) | ||
|
| ||||
| Socio-economic situation | High | 14(5%) | 18(46.15%) | 0.424 |
| Intermediate | 148(51%) | 27(29.23%) | ||
| Low | 129(44%) | 34(87.17%) | ||
Values with ∗ indicate the significant correlation between the number of reported species with the age variable ( ∗p < 0.05).
Figure 2.
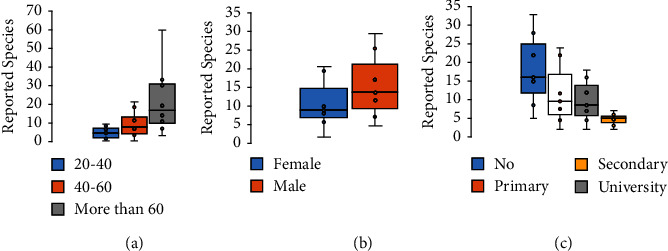
Number of reported ethnospecies variations according to (a) age; (b) gender; and (c) education level.
Among the 292 patients interviewed, 54% were women and 44% were men (Table 1, Figure 2). This demonstrates that women are more familiar with traditional medicine than men because women typically care for their families using medicinal plants as herbal remedies. These findings are consistent with those of other studies conducted in Messina, Morocco [33], Moulay Yacoub, Northeast Morocco [34], and Kinmen, Taiwan [31].
Our survey showed that most of the informants were illiterate (36%), 28% had a secondary level, 23% had a primary school education, and only 13% had a university level. These findings show that informants with lower levels of education have more frequent contact with medicinal plants, whereas those with higher levels of education have less exposure to medicinal plants and less relevant knowledge of traditional medicine. Several studies have found that illiterate people are more interested in medicinal plants than people with higher educational levels [2, 33–35].
In terms of habitat, findings revealed that 60% of informants lived in rural areas, while 40% lived in urban areas. People living in rural areas are closely associated with nature because of poverty and lack of access to health facilities. These findings are consistent with those reported in previous ethnobotanical surveys [36, 37].
This study showed that 51% of informants were in an intermediate socioeconomic situation, 44% were in a low socioeconomic situation, and only 5% were in a high socioeconomic situation. These findings are consistent with those of Benkhaira et al. [30]. This could be explained by the high cost of modern medical treatments, which creates a barrier to access and drives people to seek traditional treatments [38].
3.2. Diversity of Medicinal Plants Used to Treat Cancer
In the present ethnopharmacological survey, 39 medicinal plants belonging to 27 botanical families and 38 genera were used to treat cancer. Ethnobotanical knowledge of the inventoried plants is mentioned in Table 2, which includes the data on botanical families, vernacular and scientific names, parts used, methods of preparation, traditional dosage, route of administration, and ethnobotanical indices. The most used ethnospecies were Aristolochia longa L. with the highest RFC value (0.096), followed by Nigella sativa L., Ephedra alata Decne, Euphorbia resinifera Berg, Lavandula dentata L., and Berberis hispanica Boiss. & Reut., with RFC values of 0.072, 0.054, 0.044, 0.044, and 0.041, respectively (Table 2, Figure 3). The highest RFC values indicated that these species had an increasing traditional interest in the local area for treating cancer. Accordingly, further investigations into these species should be performed to assess phytochemical constituents and pharmaceutical benefits in order to identify their active compounds for any drug formulations [45].
Table 2.
Herbal remedies used by local people for the treatment of cancer.
| Family | Scientific name (EPPO code) | Voucher | Vernacular name Arabe (English) | Used part | Method of preparation/Route of administration | Common traditional dosages | FL (%) | FC | UR | RFC | FUV | IAR | Toxicity (lethal dose (LD50)) | Revised literature |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amaryllidaceae | Allium cepa L. (ALLCE 23211) | BLMUP306 | Bassela (onion) | Bulbs | Inf, Dec/In | Glass | 60% brain; 40% leukemia | 5 | 2 | 0.017 | 0.012 | 0.75 | LD50 = 3000 mg/kg b.w. (body weight) | [35] |
| Allium sativum L. (ALLSA 23233) | BLMUP307 | Toma (garlic) | Bulbs | Dec, Mac/In | 3 Units | 50% oral; 50% pancreatic | 2 | 2 | 0.006 | ND | LD50 = 5000 mg/kg b.w | [16] | ||
|
| ||||||||||||||
| Apiaceae | Ammodaucus leucotrichus coss. (AMKLE 23511) | BLMUP308 | Kamoun essoufi (wooly cumin) | Fruits | Pow/In | Spoon | 75% oral; 25% prostate | 4 | 2 | 0.013 | 0.009 | 0.66 | LD50 = 520 mg/kg b.w | [35] |
| Pimpinella anisum L. (PIMAN40505) | BLMUP309 | Habat hlawa (anise) | Seeds | Dec, Pow/In | Spoon, pinch | 100% gastro-intestinal | 2 | 1 | 0.006 | 1 | LD50 = 25 ± 2 g/kg b.w | [39] | ||
|
| ||||||||||||||
| Apocynaceae | Nerium oleander L. (NEROL 38433) | BLMUP310 | Dafla (oleander) | Leaves, flowers | Dec/Ex | Handful | 100% skin cancer | 1 | 1 | 0.003 | 0.003 | ND | LD50 = 57 mg/Kg b.w | [16] |
|
| ||||||||||||||
| Aristolochiaceae | Aristolochia longa L. (ARPLO 24116) | BLMUP311 | Berztem (pipevine) | Roots, whole plant | Dec, Pow/In, Ex | Handful spoon, pinch | 35% uterine cancer; 21% skin cancer | 28 | 7 | 0.096 | 0.10 | 0.77 | LD50 = 407.38 mg/kg b.w | [40] |
|
| ||||||||||||||
| Asteraceae | Artemisia herba-alba Asso (ARTHA 24166) | BLMUP312 | Chih (white mugwort) | Aerial parts, leaves | Inf, Dec/In, Ex | Coffee spoon | 36% pancreatic | 11 | 5 | 0.037 | 0.014 | 0.66 | LD50 = 2600 mg/kg b.w | [30] |
| Chamaemelum nobile (L.) All. (ANTNO 23736) | BLMUP313 | Babonj (chamomile) | Flowers | Inf/In | Spoon | 100% gastro-intestinal | 2 | 1 | 0.006 | 1 | LD50 = 5000 mg/kg b.w | [41] | ||
| Dittrichia viscosa (L.) Greuter (INUVI 34981) | BLMUP314 | Magraman (false yellowhead) | Aerial parts | Dec/In | ND | 100% brain cancer | 1 | 1 | 0.003 | ND | LD50 = 1720.25 mg/kg/b.w. | [41] | ||
| Saussurea costus (Falc.) Lipsch. (SAULA 44090) | BLMUP315 | Quist AL-Hindi (Indian costus) | Whole plant | Pow/In | Pinch | 75% uterine cancer | 4 | 2 | 0.013 | 0.66 | LD50 > 1000 mg/kg b.w | [16] | ||
|
| ||||||||||||||
| Berberidaceae | Berberis hispanica Boiss. & Reut. (BEBHI 25054) | BLMUP316 | Aghris (barberry) | Roots | Pow, Dec/In, Ex | Glass | 33% uterine; 33% skin | 12 | 4 | 0.041 | 0.041 | 0.72 | LD50 = 1016.16 mg/kg b.w | [40] |
|
| ||||||||||||||
| Brassicaceae | Lepidium sativum L. (LEPSA 35947) | BLMUP317 | Hab-errachad (garden cress) | Seeds, leaves | Pow, Mac/In | Pinch | 100% prostate | 1 | 1 | 0.003 | 0.003 | ND | LD50 = 639 mg/kg b.w | [41] |
|
| ||||||||||||||
| Cactaceae | Opuntia ficus-indica (L.) Mill. (OPUFI 39060) | BLMUP318 | Hindia (barbary fig) | Fruits, whole plants | Dec, Inf/In, Ex | Glass | 66% gastro-intestinal | 5 | 2 | 0.017 | 0.017 | 0.75 | LD50 = 2.72 ml/kg body | [21] |
| Capparaceae | Capparis spinosa L. (CPPSP 28130) | BLMUP319 | Kabar (flinders rose) | Leaves, fruits | Pow/Ex | Spoon | 62% gastro-intestinal; 25% pancreatic | 8 | 3 | 0.027 | 0.027 | 0.71 | LD50 = 5140 mg/kg b.w | [42] |
|
| ||||||||||||||
| Caryophyllaceae | Corrigiola telephiifolia Pourr. (CGLTE 26812) | BLMUP320 | Serghina (corrigiola) | Whole plant, leaves | Dec/In | Coffee spoon | 83% uterine; 17% kidney | 6 | 2 | 0.020 | 0.020 | 0.8 | LD50 = 14.000 mg/kg b.w | [42] |
|
| ||||||||||||||
| Chenopodiaceae | Chenopodium ambrosioides L. (CHEAM 26847) | BLMUP321 | Mkhinza (sweet pigweed) | Leaves | Dec/In, Ex | Spoon | 40% uterine; 20% skin; 10% prostate; 10% pancreatic | 10 | 6 | 0.034 | 0.034 | 0.44 | LD50 = 460 mg/kg b.w | [43] |
|
| ||||||||||||||
| Cupressaceae | Juniperus phoenicea L. (IUPPH 35388) | BLMUP322 | Aaraar (phoenician juniper) | Aerial parts | Pow, Dec/In, Ex | Spoon | 100% kidney | 2 | 1 | 0.006 | 0.006 | 1 | LD50 = 3432 mg/kg b.w | [16] |
|
| ||||||||||||||
| Ephedraceae | Ephedra alata Decne (EPEAL 86676) | BLMUP323 | Andla (ephedra) | Leaves | Inf, Pow/In, Ex | Spoon | ND | 16 | 5 | 0.054 | 0.054 | 0.73 | LD50 = 500 mg/kg b.w | [42] |
|
| ||||||||||||||
| Euphorbiaceae | Euphorbia resinifera O. Berg (EPHRN 31428) | BLMUP324 | Daghmous (spurge) | Leaves | Pow, Dec/In, Ex | Pinch | 38% skin; 38% uterine; 15% gastro-intestinal; | 13 | 4 | 0.044 | 0.044 | 0.75 | LD50 > 2.5 mg/kg b.w | [42] |
| Fabaceae | Ceratonia siliqua L. (CEQSI 26680) | BLMUP325 | SAnnâ hram (carob) | Leaves | Dec/In | Glass | 60% skin; 20% oral; 20% gastro-intestinal | 5 | 3 | 0.017 | 0.011 | 0.5 | LD50 > 5000 mg/kg b.w | [44] |
| Glycyrrhiza glabra L. (GYCGL 33535) | BLMUP326 | Araq-sűs (liquorice) | Roots, stems | Pow, Dec/In | Spoon | 66% leukemia; 33% skin | 3 | 2 | 0.010 | 0.5 | DL50 > 1000 mg/kg b.w | [21] | ||
| Retama monosperma (L.) Boiss. (RTARE98145) | BLMUP327 | R'tum (bridal broom) | Aerial parts | Dec/In | Pinch | 100% oral cancer | 1 | 1 | 0.003 | ND | LD50 = 1995 mg/kg b.w | [41] | ||
| Trigonella foenum-graecum L. (TRKFG47367) | BLMUP328 | Helba (fenugreek) | Seeds | Pow/In | Pinch | 75% oral; 25% gastro-intestinal | 4 | 2 | 0,013 | 0.66 | LD50 = 7000 mg/kg b.w | [39] | ||
|
| ||||||||||||||
| Iridaceae | Crocus sativus L. (CVOSA28993) | BLMUP329 | Safran (saffron) | Roots | Pow/In, Ex | Spoon | 100% leukemia | 3 | 1 | 0.010 | 0.010 | 2 | LD50 = 1.48 ml/kg b.w | [39] |
|
| ||||||||||||||
| Lamiaceae | Lavandula dentata L. (LAVDE35748) | BLMUP330 | Khzama (fringed lavender) | Leaves | Dec/In | Spoon | ND | 13 | 7 | 0.044 | 0.024 | 0.5 | LD50 = 2000 mg/kg b.w | [23] |
| Marrubium vulgare L. (MAQVU 37199) | BLMUP331 | Marwita (white horehound) | Leaves, Aerial parts | Dec/In, Ex | Glass | 33% uterine; 22% oral | 9 | 6 | 0.030 | 0.37 | LD50 > 2000 mg/kg b.w | [39] | ||
| Origanum compactum Benth. (ORICO93447) | BLMUP332 | Zaatar (oregano) | Aerial parts | Dec, Inf/In | Handful | 37% gastro-intestinal; 37% oral | 8 | 4 | 0.027 | 0.57 | LD50 > 5000 mg/kg b.w | [16] | ||
| Rosmarinus officinalis L. (RMSOF 43318) | BLMUP333 | Azir (rosemary) | Aerial parts | Dec, Inf/In | Glass | 33% oral; 33% pancreatic; 33% prostate | 3 | 3 | 0.010 | ND | LD50 = 561 mg/kg b.w | [35] | ||
| Salvia officinalis L. (SALOF44001) | BLMUP334 | Salmiya (sage) | Leaves | Inf, Dec/In | Glass | 75% pancreatic; 25% oral | 4 | 2 | 0.013 | 0.66 | LD50 = 1287.3 mg/Kg b.w | [35] | ||
|
| ||||||||||||||
| Linaceae | Linum usitatissimum L. (LIUUT 36286) | BLMUP335 | Zeri-it-lktan (flax seed) | Seeds | Pow/In, Ex | Coffee spoon | 28% oral; 28% uterine; 14% leukemia | 7 | 3 | 0.024 | 0.024 | 0.66 | LD50 = 3.76 mg/Kg b.w | [23] |
|
| ||||||||||||||
| Lauraceae | Cinnamomum camphora (L.) J. Presl (CINCA 27139) | BLMUP336 | Kafour (camphor) | Whole plant | Fum/Ex | Spoon | 30% brain; 20% skin | 5 | 2 | 0.017 | 0.017 | 0.75 | LD50 = 5100 mg/kg b.w | [23] |
|
| ||||||||||||||
| Moraceae | Ficus carica L. (FIUCA 32494) | BLMUP337 | Karmous (fig) | Fruits | Pow/In | Spoon | 75% uterine; 12% skin; 12% pancreatic; | 6 | 4 | 0.020 | 0.020 | 0.4 | LD50 > 6000 mg/kg b.w | [21] |
|
| ||||||||||||||
| Myrtaceae | Myrtus communis L. (MYVCO 38308) | BLMUP338 | Rihan (true myrtle) | Leaves | Inf, Fum/In, Ex | Handful | 33% pancreatic; 33% skin | 8 | 3 | 0.027 | 0.027 | 0.71 | LD50 = 473 mg/kg b.w | [42] |
|
| ||||||||||||||
| Oleaceae | Olea europaea L. (OLVEU38940) | BLMUP339 | Zitoun (olive) | Leaves | Dec, Inf/In, Ex | Glass | 30% pancreatic; 20% kidney | 10 | 5 | 0.034 | 0.034 | 0.55 | LD50 = 3475 mg/kg b.w | [35] |
|
| ||||||||||||||
| Renonculaceae | Nigella sativa L. (NIGSA 38473) | BLMUP34 | Sanouj (black cumin) | Seeds | Pow, Dec/In, Ex | Coffee spoon | 24% skin; 14% gastro-intestinal | 21 | 6 | 0.072 | 0.072 | 0.75 | LD50 = 1,853 mg/kg b.w | [40] |
|
| ||||||||||||||
| Rosaceae | Rosa × damascene Herrm. (ROSDM 43433) | BLMUP341 | Ward-lbeldi (damask rose) | Flowers | Dec, Fum/Ex | Handful | 66% skin; 33% gastro-intestinal | 3 | 3 | 0.010 | 0.010 | ND | LD50 > 2500 mg/kg b.w | [16] |
|
| ||||||||||||||
| Sapotaceae | Argania spinosa (L.) Skeels (ARJSI 24031) | BLMUP342 | Argane (argan) | Fruits | Pow, Cat/In, Ex | Glass | 100% pancreatic | 1 | 1 | 0.003 | 0.003 | ND | LD50 = 1300 mg/kg b.w | [44] |
|
| ||||||||||||||
| Urticaceae | Urtica dioica L. (URTDI 47803) | BLMUP343 | Harriga (common nettle) | Aerial parts | Dec, Cat/In, Ex | Handful | 64% uterine; 36% skin | 11 | 2 | 0.037 | 0.037 | 0.9 | LD50 = 3625 mg/kg b.w | [44] |
|
| ||||||||||||||
| Zingiberaceae | Zingiber officinale Roscoe (ZINOF 48967) | BLMUP344 | Skinjbir (ginger) | Rhizomes | Pow/In | Glass | 50% kidney; 50% gastro-intestinal | 2 | 2 | 0.006 | 0.006 | ND | LD50 = 4525.5 mg/kg b.w | [16] |
Inf, infusion; Dec, decoction; Pow, powder; Mac, maceration; Fum, fumigation; Cat, cataplasm; In, internal uses, Ex, external uses.
Figure 3.

Main ethnospecies used against cancer by the informants. (a) Olea europaea, (b) Nerium oleander, (c) Chenopodium ambrosioides, (d) Crocus sativus, (e) Nigella sativa, and (f) Aristolochia longa.
Principal component analysis (PCA) showed two top principal components (eigenvalue ≥1), accounting for 84.7% of the total variation of parameters studied. To facilitate the selection of species based on the ethnobotanical parameters studied, a biplot was created using two first principal components (F1 and F2). F1 explained 63.35% of the total variation and was strongly influenced by the FC, RFC, UR, and FL. While F2 accounted for 21.35% of the total variation and was significantly influenced by the IAR index, A. longa and N. sativa were associated positively with RFC, FC, and UR, while C. sativus was associated positively with the IAR index (Figure 4). These results indicated the frequent use of A. longa and N. sativa to treat cancer by the studied population, demonstrating the relative medicinal importance of these two species in the present local area. The common use of A. longa and N. sativa was already confirmed in Moroccan traditional pharmacopeia, which showed the medicinal values of these two species against cancer as well as related diseases. To that end, efforts must be made to prioritize these species for preservation and protection, as their traditional uses may place the local people under threat due to over-exploitation.
Figure 4.
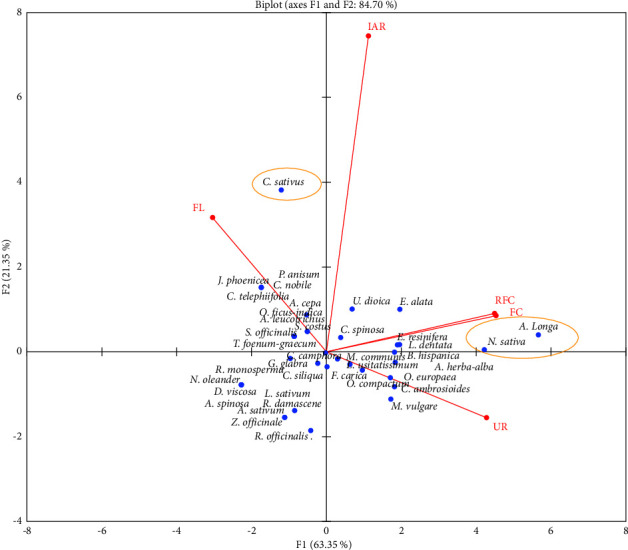
Biplot of principal components analysis for an ethnobotanical index of the reported species.
In regard to the plant families, Lamiaceae contributed the highest number of plants with five species (FUV = 0.034), followed by Asteraceae (4 species; FUV = 0.020), Fabaceae (4 species; FUV = 0.020) and Apiaceae (2 species; FUV = 0.13) as shown in Figure 5. This is consistent with our previous studies carried out in different areas in Morocco, including the Rabat-Sale-Kenitra, Fez, and Taounat regions [16, 30, 35], where we found the dominance of the Lamiaceae, Asteraceae, Fabaceae, and Apiaceae families. Moreover, the predominance of these plant families has also been proven in numerous ethnobotanical surveys conducted in other Moroccan regions [38, 45–47], as well as in other neighboring countries like Algeria [48, 49] and Tunisia [50]. Furthermore, the Lamiaceae and Asteraceae families have been specifically reported in traditional medicine against cancer. Besides that, these two botanical families dominate Moroccan flora and are found throughout the country [43].
Figure 5.
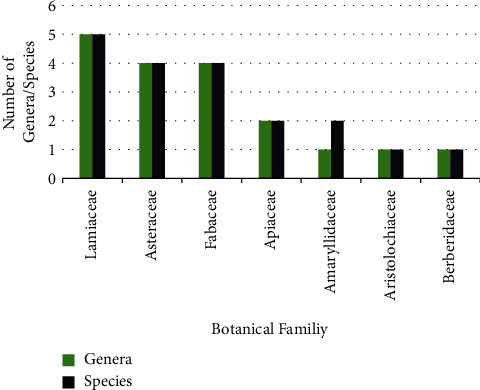
Number of ethnospecies in each family used to treat cancer.
On the other hand, the Lamiaceae and Asteraceae families have been linked to a variety of pharmacological effects, including antibacterial, antifungal, anti-inflammatory, antidiabetic, antiviral, antioxidant, and insecticidal properties [51–54]. These biological properties are particularly related to their valuable chemical constituents, which have shown promising therapeutic potential [18, 55].
3.3. Parts of the Medicinal Plants Used
Different parts of plants are used as medicine to create traditional cancer treatments, including leaves, seeds, stems, fruits, flowers, rhizomes, and roots. The studied population prefers the leaves against cancer (33%), followed by aerial parts (14%), seeds (13%), whole plants (11%), roots (7%), and fruits (6%) (Figure 6). Similarly, ethnobotanical research surveys conducted in Morocco revealed that leaves were a major dominant plant part used in the preparation of traditional remedies [21, 39, 42, 44, 45]. Similar findings were reported in other international ethnobotanical studies in Ghana [56], Lebanon [57], and Turkey [58].
Figure 6.
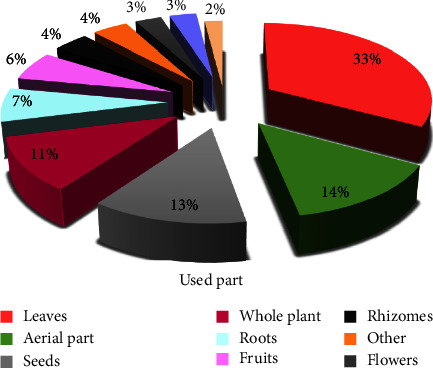
Frequency of different plant parts used.
The common traditional use of one part over another varies depending on its active fraction. The availability, ease of harvesting, and simple uses in herbal medicine remedies may explain why leaves were preferred. Furthermore, the leaves are thought to be photosynthesis and secondary metabolite storage sites, which are involved in a variety of health-promoting effects. They contain more phenolic compounds, alkaloids, heterosides, and essential oils. However, when compared to other plant organs such as fruits, roots, and stems, exploiting and harvesting this plant part is a relatively sustainable practice; harvesting the roots may result in the annihilation and disappearance of the plants.
3.4. Methods of Remedy Preparations
According to the current ethnobotanical study, several methods are used for remedy preparation. Figure 7 shows that decoction is the most used method (38%) for herbal preparation against cancer at various stages of development, followed by infusion (27%), and powder (23%). Other methods, such as cataplasm (5%), maceration (5%), and fumigation (2%), are rarely used by the studied population. These findings may be supported by the fact that decoction allows for better extraction of the most active ingredients and reduces or eliminates the toxicity of certain polyherbal prescriptions [38]. The predominance of decoction in our findings is consistent with previous research in the Rabat-Sale-Kenitra, Taounat, and Fez regions [16, 30, 35]. Other ethnobotanical studies in Morocco [38, 42, 44, 45] corroborate these findings.
Figure 7.
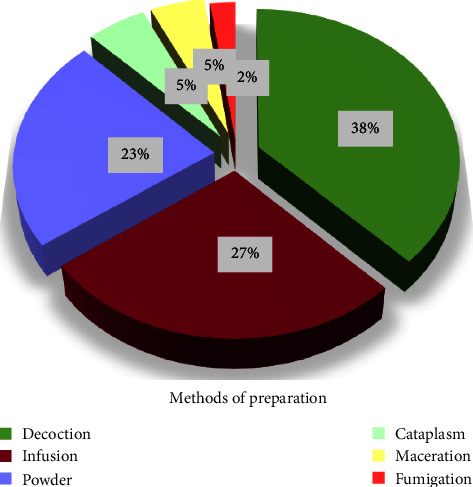
Frequency of different preparation methods.
In terms of administration, the findings revealed that most herbal remedies are taken orally. Other modes of administration, such as inhalation and massage, were also used in some traditional recipes. These findings have also been reported in national and international traditional pharmacopeias, indicating that the oral route is the most common and acceptable mode for patients to receive plants [39, 48, 49, 58].
3.5. Cancer Categories and Their FIC Values
Plants identified in this survey were used to treat nine types of cancer, which can be classified as brain, gastrointestinal, kidney, leukemia, oral, pancreatic, prostate, skin, and uterine cancer.
In this survey, plants identified were used to treat nine categories of cancer, which can be grouped into brain, gastrointestinal, kidney, leukemia, oral, pancreatic, prostate, skin, and uterine cancer. The informant consensus factor (FIC) values of the claimed plants in this survey ranged between 0.36 and 0.83 (Table 3), indicating a lack of consensus on patients suffering from a specific type of cancer in comparison to traditional knowledge, as well as a lack of knowledge exchange among people in different areas [59]. This disparity in knowledge among the cancer patients studied may be explained by their belief that all types of cancer are the same disease. In contrast, certain cancer types, such as uterine and skin cancer, showed interesting agreement among patients regarding the use of plant species (FIC values of 0.83 and 0.6, respectively). These highest FIC values demonstrated reasonable reliability in agreement with the use of plants against cancer.
Table 3.
Cancer categories and their FIC values.
| Cancer type | Nur | Nt | FIC | Plants species (FC) |
|---|---|---|---|---|
| Brain cancer | 9 | 5 | 0.5 | Dittrichia viscosa (L.) Greuter (1), Allium cepa L. (3), Artemisia herba-alba Asso. (1), Cinnamomum camphora (L.) J. Presl, (3), Origanum compactum Benth. (1) |
|
| ||||
| Gastro-intestinal cancer | 34 | 16 | 0.54 | Aristolochia longa L. (3), Zingiber officinale Roscoe (1), Ficus carica L. (1), Pimpinella anisum L. (2), Chamaemelum nobile (L.) All. (2), Capparis spinosa L. (5), Saussurea costus (Falc.) Lipsch. (1), Opuntia ficus-indica (L.) Mill. (4), Chenopodium ambrosioides L. (1), Trigonella foenum-graecum L. (1), Rosa × damascene Herrm, (1), Nigella sativa L. (3), Marrubium vulgare L. (1), Origanum compactum Benth. (3), Ceratonia siliqua L. (1), Euphorbia resinifera O. Berg. (2) |
|
| ||||
| Kidney cancer | 14 | 8 | 0.46 | Aristolochia longa L. (1), Ficus carica L (1), Corrigiola telephiifolia Pourr. (1), Juniperus phoenicea L. (2), Zingiber officinale Roscoe (1), Artemisia herba-alba Asso (2), Berberis hispanica Boiss. & Reut. (3), Olea europaea L. (2) |
|
| ||||
| Leukemia | 12 | 7 | 0.45 | Linum usitatissimum L. (1), Allium cepa L. (2), Crocus sativus L. (3), Glycyrrhiza glabra L. (2), Nigella sativa L. (1), Euphorbia resinifera O. Berg, (1), Aristolochia longa L. (2) |
|
| ||||
| Oral cancer | 27 | 14 | 0.5 | Olea europaea L. (4), Linum usitatissimum L. (2), Salvia officinalis L. (1), Allium sativum L. (1), Ammodaucus leucotrichus Coss. (3), Ceratonia siliqua L. (1), Origanum compactum Benth. (3), Retama monosperma (L.) Boiss. (1), Marrubium vulgare L. (2), Trigonella foenum-graecum L. (3), Artemisia herba-alba Asso, (1), Chenopodium ambrosioides L. (1) Rosmarinus officinalis L. (1) Nigella sativa L. (2) |
|
| ||||
| Pancreatic cancer | 24 | 14 | 0.43 | Argania spinosa (L.) Skeels (1), Aristolochia longa L. (1), Capparis spinosa L. (2), Chenopodium ambrosioides L. (1), Rosmarinus officinalis L. (1), Nigella sativa L. (1), Ficus carica L. (2), Salvia officinalis L. (3), Allium sativum L. (1), Artemisia herba-alba Asso. (4), Myrtus communis L. (1), Olea europaea L. (3), Marrubium vulgare L. (1), Origanum compactum Benth. (2) |
|
| ||||
| Prostate cancer | 12 | 8 | 0.36 | Aristolochia longa L. (5), Rosmarinus officinalis L. (1), Lepidium sativum L. (1), Ammodaucus leucotrichus Coss. (1), Berberis hispanica Boiss. & Reut. (1), Chenopodium ambrosioides L. (1), Olea europaea L. (1), Marrubium vulgare L. (1) |
|
| ||||
| Skin cancer | 44 | 18 | 0.6 | Aristolochia longa L. (6), Glycyrrhiza glabra L. (1), Nigella sativa L. (5), Olea europaea L. (1), Myrtus communis L. (1), Urtica dioica L. (4), Chenopodium ambrosioides L. (2), Nerium oleander L. (1), Ficus carica L. (2), Marrubium vulgare L. (1), Euphorbia resinifera O. Berg, (5), Cinnamomum camphora (L.) J. Presl, (2), Rosa × damascene Herrm. (1), Artemisia herba-alba Asso. (2), Opuntia ficus-indica (L.) Mill. (2), Berberis hispanica Boiss. & Reut. (4), Capparis spinosa L. (1), Ceratonia siliqua L. (3) |
|
| ||||
| Uterine cancer | 60 | 11 | 0.83 | Aristolochia longa L. (10), Nigella sativa L. (11), Myrtus communis L. (6), Urtica dioica L. (7), Euphorbia resinifera O. Berg, (5), Linum usitatissimum L. (2) , Saussurea costus (Falc.) Lipsch. (3), Corrigiola telephiifolia Pourr. (5), Chenopodium ambrosioides L. (4), Marrubium vulgare L. (3), Berberis hispanica Boiss. & Reut. (4) |
Nur, number of use report; Nt, number of plant species used by category.
3.6. Scientific Evidence and Mechanisms of Action of Anticancer Medicinal Plants
In this study, the 39 identified plants against cancer have been pharmacologically validated through in vitro and in vivo studies [16, 60–65]. In Table 4, we listed the mechanisms of anticancer properties of the inventoried plants used traditionally against cancer by the studied population.
Table 4.
Anticancer properties of the medicinal plants cited in the study area.
| Plant species | Used parts | Used extracts | Experimental model | Key results | References |
|---|---|---|---|---|---|
| Allium cepa L. | Bulbes | Ethanol, methanol, aqueous extracts | HepG2 human liver cancer cell lines HeLa cervical cancer cell line | Exhibited significant antigenotoxic activity in HepG2 cells decreased the levels of intracellular ROS at 1–100 μg/mL concentrations. Showed anticancer effect on HeLa cells (IC50 = 4.8 μM) | [66, 67] |
|
| |||||
| Allium sativum L. | Bulbes | Ethanol | Breast (MDAMB231), prostate (PC3), colon (HCT-15), hepatic cancer (Hep3B) cell lines | IC50 (MDAMB231) = 5.748 mg/mL IC50(PC3) = 3.333 mg/mL IC50(HCT-15) = 3.746 mg/mL IC50(Hep3B) = 3.301 mg/mL | [65] |
|
| |||||
| Ammodaucus leucotrichus coss. | Aerial parts | Essential oil | HCT116 colon cancer cell line HePG2 (human hepatocellular carcinoma cell line | IC50 = 72.6 μg/mL and 110.6 μg/mL, respectively | [64] |
|
| |||||
| Pimpinella anisum L. | Seeds | Alcoholic extracts and essential oil | Gastric cancer cell line | IC50 = 30 µg/mL | [68] |
|
| |||||
| Nerium oleander L. | Flowers | Essential oil | Ehrlich ascites carcinoma cells (EAC) | Inhibited significantly the growth of EAC cells | [60] |
|
| |||||
| Aristolochia longa L. | Roots | Aqueous extract | Burkitt's lymphoma BL41 cells | Induced cell death in a dose-dependent manner triggered cell apoptosis, associated with a loss of mitochondrial membrane integrity and the upregulation of caspases-9 and -3 followed by PARP cleavage | [69] |
|
| |||||
| Artemisia herba-alba Asso | Aerial parts | Essential oil | P815 murin mastocytoma and BSR kidney adenocarcinoma of hamster cell lines | Exhibited significant cytotoxic effect | [70] |
|
| |||||
| Chamaemelum nobile (L.) All. | Aerial part | Aqueous extract | MCF7 | IC50 = 168.40 μg/mL | [71] |
|
| |||||
| Dittrichia viscosa (L.) Greuter | Aerial part | Ethanol extract | Human breast cancer (estrogen receptor-negative; MDA-MB-231) and prostate cancer (PC3) cell lines | Significantly suppressed cell proliferation and induced cell apoptosis in all the studied cell lines | [72] |
|
| |||||
| Saussurea costus (Falc.) Lipsch. | Roots | Hexane extract | Human colon (HCT116) cells | IC50 of 20.07 ± 7.25 μg/mL | [62] |
|
| |||||
| Berberis hispanica Boiss. & Reut | Roots | Methanol extracts | Breast MDA-MB-231 and MCF-7 cell lines | Exhibited significant antiproliferative potential with IC50 values of 6.55 ± 0.58 and 17.95 ± 0.58 µg/mL respectively | [63] |
|
| |||||
| Lepidium sativum L. | Leaves | Aqueous extract | Cellosaurus CAL-27 cell lines | Induced noticeable damage to DNA, and up-regulated the levels of mitochondrial reactive oxygen species (ROS), leading to apoptosis (up to 30% and 60%) | [73] |
|
| |||||
| Opuntia ficus-indica (L.) Mill. | Fruit | Ethanol extract | PC3 and MCF7 | IC50 values of 5775.7 and 6311.3 μg/ml, respectively | [74] |
|
| |||||
| Capparis spinosa L. | Leaves | Ethanolic extract | Ehrlich ascites carcinoma in Swiss albino mice | Induced apoptosis through the activation of caspase 3 and BCL2 proteins | [75] |
|
| |||||
| Corrigiola telephiifolia Pourr | Roots | Dichloromethane extract | Murine colon adenocarcinoma (CT-26) cells | IC50 = 80 ± 4.56 | [61] |
|
| |||||
| Chenopodium ambrosioides L. | Leaves | EO | Liver cancer SMMC-7721 cells | Inhibited cell proliferation, and cell cycle division at Go/G1 phase. Caused caspase-dependent apoptosis | [76] |
|
| |||||
| Juniperus phoenicea L. | Aerial parts | Chloroform fraction | MCF-7 cancer cell | Suppressed cell proliferation, induced cell cycle arrest at the G1 phase, and cell apoptosis | [77] |
|
| |||||
| Ephedra alata Decne | Aerial parts | Methanol extract | 4T1 breast cancer cells | Inhibited cell growth and migration in a dose and time-dependent manner and triggered apoptosis, reduced ROS production, and induced caspases activation | [78] |
|
| |||||
| Euphorbia resinifera O. Berg | Aerial parts | Hexane extract | Rhabdomyosarcoma cancerous, kidney adenocarcinoma of hamster and monkey kidney cancerous cell lines | IC50 values between 50.7 ± 4,89 and 266.43 ± 10.20 µg/mL | [79] |
|
| |||||
| Ceratonia siliqua L. | Seeds | Ethanol extract | Human glioblastoma cancer cells | Decreased cell viability in a concentration-dependent manner | [80] |
|
| |||||
| Glycyrrhiza glabra L. | Roots | Methanol extract | Carcinoma (Caco-2) and prostate carcinoma (PC-3) cell lines | IC50 values ranged between 40 and 40.6 μg/ml | [81] |
|
| |||||
| Retama monosperma (L.) Boiss. | Leaves | n-Hexane extract | Jurkat cells | Promoted cell cycle arrest and triggered extrinsic pathway-dependent apoptosis | [82] |
|
| |||||
| Trigonella foenum-graecum L. | Seeds | Ethanol extract | HepG2 cells | Showed antiangiogenic activity, inducing cell cycle arrest at the G2/M phase after 12 and 48 h of treatment and remarkable arrest at the G1/S phase after 24 h of treatment | [83] |
|
| |||||
| Crocus sativus L. | Flowers | Ethanol extract | Breast cancer cells MCF-7 | Caspase activation and increased expression of Bax protein lead to cell apoptosis | [84] |
|
| |||||
| Lavandula dentata L. | Aerial parts | Dichloromethane extract | Erlish cell line | Showed interesting antitumor activity at a concentration of 300 µl/ml | [85] |
|
| |||||
| Marrubium vulgare L. | Aerial parts | Methanol extract | Jurkat cells | IC50 > 50 μg/mL | [86] |
|
| |||||
| Origanum compactum Benth. | Aerial parts | Ethyl acetate extract | MCF-7 cells | IC50 = 279.51 ± 16 µg/mL | [87] |
|
| |||||
| Rosmarinus officinalis L. | Aerial parts | EO | Rhabdomyosarcoma cells | IC50 = 113.42 ± 4.6 µg/mL | [88] |
|
| |||||
| Salvia officinalis L. | Aerial parts | EO | MCF7, LNCaP, and HeLa cells | Reduced cell viability after a 48-hour incubation at concentrations of 100 and 200 μg/mL | [89] |
|
| |||||
| Linum usitatissimum L. | Seeds | Methanol extract and ethyl acetate fraction | HepG2 and MCF7 cells | IC50 = 60 ± 0.24 μg/ml (HepG2) IC50 = 29.4 ± 0.12 μg/ml (MCF7) IC50 = 94.7 ± 0.21 μg/ml IC50 = 227 ± 0.48 μg/ml, respectively | [90] |
|
| |||||
| Cinnamomum camphora (L.) J. Presl | ND | ND | Cervical carcinoma HeLa cells | Pinoresinol inhibited significantly cell growth and proliferation | [91] |
|
| |||||
| Ficus carica L. | Latex | ND | MDA-MB-231 cells | Exhibited promising antimetastatic effects through ERK2, CREB, and AKT2 signaling pathways. Showed genotoxic and cytotoxic effects in MDA-MB-231 cells | [92] |
|
| |||||
| Argania spinosa (L.) Skeels | Fruits | Methanol n-butanol extracts | HTC hepatoma cell lines | Significant antiproliferative effect inhibition of ERK1/2 activation was also associated with decreased DNA synthesis | [93] |
|
| |||||
| Urtica dioica L. | Roots | Methanolic extract | Balb/c mouse model of benign prostatic hyperplasia | Oral treatment at a dose of 5 mg for 28 days reduced hyperplasia with 51.4% growth inhibition | [94] |
|
| |||||
| Zingiber officinale Roscoe | Roots | EO | Swiss albino mice | Significantly reduced the volume of solid tumor development by 54.4% At a concentration of 500 mg/kg body weight | [95] |
EO, essential oil.
These medicinal plants have shown promising anticancer properties targeting multiple checkpoints, including angiogenesis, inflammation inhibition, cell cycle arrest, metastasis, induction of cell apoptosis, and autophagy, thanks to their valuable chemical constituents (Figure 8). A. longa is the most frequently used medicinal plant by informants to treat cancer. Indeed, aqueous extracts of A. longa have been shown to induce cell death in a dose-dependent manner, resulting in cell apoptosis associated with a loss of mitochondrial membrane integrity and the up-regulation of caspases-9 and -3 followed by PARP cleavage [48]. Furthermore, C. sativus ethanol extracts demonstrated promising anticancer properties in MCF-7 breast cancer cells [84]. These extracts induced caspase-dependent apoptosis by increasing the expression of the Bax protein. Furthermore, n-hexane extracts from Retama monosperma leaves induced cell cycle arrest and extrinsic pathway-dependent apoptosis in Jurkat cells [82]. Trigonella foenum-graecum seeds inhibited angiogenesis in HepG2 cells, inducing cell cycle arrest at the G2/M phase after 12 and 48 hours of treatment, and a remarkable arrest at the G1/S phase after 24 hours [83]. Ephedra alata is another species with anticancer potential, significantly inhibiting cell growth and migration in 4T1 breast cancer cells in a dose and time-dependent manner [78].
Figure 8.
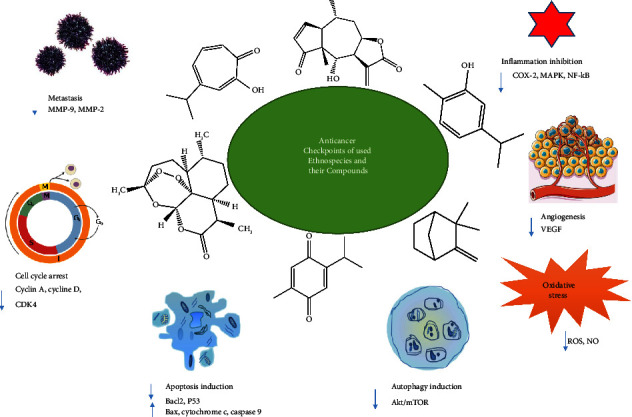
Anticancer molecular targets of reported medicinal plants and their bioactive molecules.
This effect was mediated by the induction of apoptosis and caspase activation, as well as the up-regulation of reactive oxygen species (ROS) production [78]. Other Asteraceae plant species, such as Artemisia herba-alba, Chamaemelum nobile, Saussurea costus, and Dittrichia viscosa, demonstrated significant anticancer properties against various cell lines, with IC50 values ranging from 20.07 to 168.40 g/mL [62, 70–72]. Artemisinin, a major naturally occurring compound found in A. herba-alba, is thought to be an effective anticancer agent. Artemisinin's anticancer activity has been linked to several molecular mechanisms, including apoptosis induction, cell cycle arrest, metastasis inhibition, and increased oxidative stress via ROS and NO production [96, 97].
Plants in the Lamiaceae family, on the other hand, are distinguished by the presence of specific structures involved in the production and secretion of volatile compounds as secondary metabolites, such as thymol, carvacrol, carvone, carveol, and hinokitiol (Figure 9). Several plants, including Lavandula dentata, Marrubium vulgare, Origanum compactum, Rosmarinus officinalis, and Salvia officinalis, contain these compounds, which are responsible for their anticancer properties. Essential oils extracted from the aerial parts of S. officinalis reduced cell viability in breast MCF7 cancer, human LNCaP prostate adenocarcinoma, and HeLa cell lines after a 48-hour incubation at concentrations of 100 and 200 g/mL [89]. Furthermore, O. compactum essential oils have high cytotoxicity against MCF-7 cells, with an IC50 of 113.42 ± 4.6 g/mL [87].
Figure 9.
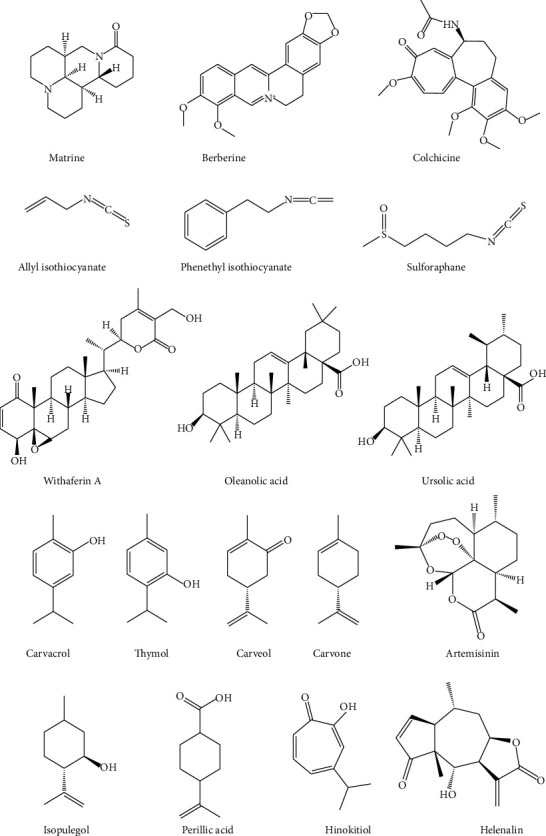
Some phytochemical compounds with potent anticancer properties.
Carvacrol and thymol, remarkably, have been assigned anticancer potential against various cancer cell lines, with varying modes of action [98–100]. Indeed, thymol has been shown to inhibit the G0/G1 phase transition of the cell lines cellosaurus P-815 and MCF-7 [100]. In human promyelocytic leukemia (HL-60) cells, thymol induces both caspase-dependent and caspase-independent apoptosis [98]. Carvacrol has also been identified as an effective anticancer agent, inducing cell apoptosis in HepG2 cell lines via caspase activation and the mitogen-activated protein kinase (MAPK) pathway [101]. Furthermore, in vivo studies showed that carvacrol has the potential to prevent liver cancer in male Wistar albino rats induced by diethylnitrosamine [99].
4. Conclusions
This ethnobotanical survey revealed that the locals have extensive indigenous knowledge of the use of medicinal plants, particularly for cancer treatment. These valuable traditional practices reflect the rich and varied floristic patrimony of the studied area. More efforts, however, must be made to prioritize certain medicinal plants for preservation and protection, as their preferred traditional uses may endanger them due to over-exploitation. Furthermore, based on the claimed results and suggested discussions, some identified medicinal plants may have a promising role in the treatment of various types of cancer at various stages of development, including prostate, gastrointestinal, uterine, leukemia, brain, skin, oral, kidney, and pancreatic cancer. However, this medicinal value requires further clarification and should be used with caution. Further research into these plant species is needed to assess phytochemical constituents and pharmaceutical benefits in order to identify active compounds for any drug formulations. Furthermore, data on the toxicity of medicinal plants is essential for ensuring the safety of their uses as well as standardizing their accurate posology and therapeutic doses.
Acknowledgments
All the authors kindly acknowledge the committee of research of the Moroccan national institute of oncology and the cancer patients for their contribution to realizing this study. This research was supported by Conseil Départemental d'Eure et Loir, and Conseil Régional Centre-Val de Loire.
Contributor Information
Naoufal El Hachlafi, Email: naoufal.elhachlafi@usmba.ac.ma.
Mohamed Addi, Email: m.addi@ump.ac.ma.
Christophe Hano, Email: hano@univ-orleans.fr.
Data Availability
All the data supporting the findings of this study are included in this article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Khan H., Saeedi M., Nabavi S. M., Mubarak M. S., Bishayee A. Glycosides from medicinal plants as potential anticancer agents: emerging trends towards future drugs. Current Medicinal Chemistry . 2019;26(13):2389–2406. doi: 10.2174/0929867325666180403145137. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed A., Karima S., Nadia O. The use of medicinal plants against cancer: an ethnobotanical study in the Beni mellal-khenifra region in Morocco. European Journal of Integrative Medicine . 2022;52 doi: 10.1016/j.eujim.2022.102137.102137 [DOI] [Google Scholar]
- 3.Mattiuzzi C., Lippi G. Current cancer epidemiology. Journal of Epidemiology and Global Health . 2019;9(4):p. 217. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center M., Siegel R., Jemal A. Global cancer facts & figures. American Cancer Society . 2011;3:1–52. [Google Scholar]
- 5.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 6.Alami Merrouni I., Elachouri M. Anticancer medicinal plants used by Moroccan people: ethnobotanical, preclinical, phytochemical and clinical evidence. Journal of Ethnopharmacology . 2021;266 doi: 10.1016/j.jep.2020.113435.113435 [DOI] [PubMed] [Google Scholar]
- 7.Vibala B. V., Praseetha P. K., Vijayakumar S. Evaluating new strategies for anticancer molecules from ethnic medicinal plants through in silico and biological approach-A review. Gene Reports . 2020;18 doi: 10.1016/j.genrep.2019.100553.100553 [DOI] [Google Scholar]
- 8.Khan M. I., Bouyahya A., Hachlafi N. E. L., et al. Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: a review on recent investigations. Environmental Science and Pollution Research . 2022;29(17):24411–24444. doi: 10.1007/s11356-021-17795-7. [DOI] [PubMed] [Google Scholar]
- 9.Omara T., Kiprop A. K., Ramkat R. C., et al. Medicinal plants used in traditional management of cancer in Uganda: a review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evidence-Based Complementary and Alternative Medicine . 2020;2020:26. doi: 10.1155/2020/3529081.3529081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashraf M. A. Phytochemicals as potential anticancer drugs: time to ponder nature’s bounty. BioMed Research International . 2020;2020 doi: 10.1155/2020/8602879.8602879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai A. G., Qazi G. N., Ganju R. K., et al. Medicinal plants and cancer chemoprevention. Current Drug Metabolism . 2008;9(7):581–591. doi: 10.2174/138920008785821657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mechchate H., El Allam A., El Omari N., et al. Vegetables and their bioactive compounds as anti-aging drugs. Molecules . 2022;27(7):p. 2316. doi: 10.3390/molecules27072316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouyahya A., El Hachlafi N., Aanniz T., et al. Natural bioactive compounds targeting histone deacetylases in human cancers: recent updates. Molecules . 2022;27(8):p. 2568. doi: 10.3390/molecules27082568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balahbib A., El Omari N., Hachlafi N. E. L., et al. Health beneficial and pharmacological properties of p-cymene. Food and Chemical Toxicology . 2021;153 doi: 10.1016/j.fct.2021.112259.112259 [DOI] [PubMed] [Google Scholar]
- 15.Bouyahya A., El Omari N., Hakkur M., et al. Sources, health benefits, and biological properties of zeaxanthin. Trends in Food Science & Technology . 2021;118:519–538. doi: 10.1016/j.tifs.2021.10.017. [DOI] [Google Scholar]
- 16.El Hachlafi N., Chebat A., Soulaymani Bencheikh R., Fikri-Benbrahim K. Ethnopharmacological study of medicinal plants used for chronic diseases treatment in Rabat-Sale-Kenitra region (Morocco) Ethnobotany Research and Applications . 2020;20:1–23. doi: 10.32859/era.20.2.1-23. [DOI] [Google Scholar]
- 17.El Hachlafi N., Lakhdar F., Khouchlaa A., et al. Health benefits and pharmacological properties of hinokitiol. Processes . 2021;9:p. 1680. doi: 10.3390/pr9091680. [DOI] [Google Scholar]
- 18.Chamkhi I., Charfi S., El Hachlafi N., et al. Genetic diversity, antimicrobial, nutritional, and phytochemical properties of chenopodium album: a comprehensive review. Food Research International . 2022;154 doi: 10.1016/j.foodres.2022.110979.110979 [DOI] [PubMed] [Google Scholar]
- 19.High Commission for Planning. Note de présentation des premiers résultats du Recensement Général de la Population et de l’Habitat 2014 . High Commission for Planning; 2015. [Google Scholar]
- 20.Benabid A. Flore et écosystèmes du Maroc: evaluation et préservation de la biodiversité. 2000.
- 21.Bouayyadi L., El Hafian M., Zidane L. Étude floristique et ethnobotanique de la flore médicinale dans la région du Gharb, Maroc. Journal of Applied Biosciences . 2015;93(1):8770–8788. doi: 10.4314/jab.v93i1.10. [DOI] [Google Scholar]
- 22.Fennane M., Tattou M. I., Mathez J. Flore Pratique du Maroc: Manuel de Détermination des Plantes Vasculaires. Pteridophyta, Gymnospermae, Angiospermae (Lauraceae-Neuradaceae) Institut Scientifique; 1999. [Google Scholar]
- 23.Fennane M., Tattou M. I. Flore vasculaire du Maroc: Inventaire et chorologie . Department de Botanique et d’Ecologie Vegetale; 2005. Pteridophyta, gymnospermae, angiospermae. [Google Scholar]
- 24.Bellakhdar J. The traditional Moroccan pharmacopoeia: ancient Arabic medicine and popular knowledge. 1997.
- 25.Hmamouchi M. Les plantes médicinales et aromatiques marocaines. 2001.
- 26.Heinrich M., Ankli A., Frei B., Weimann C., Sticher O. Medicinal plants in Mexico: healers’ consensus and cultural importance. Social Science & Medicine . 1998;47(11):1859–1871. doi: 10.1016/s0277-9536(98)00181-6. [DOI] [PubMed] [Google Scholar]
- 27.Sreekeesoon D. P., Mahomoodally M. F. Ethnopharmacological analysis of medicinal plants and animals used in the treatment and management of pain in Mauritius. Journal of Ethnopharmacology . 2014;157:181–200. doi: 10.1016/j.jep.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Tardío J., Pardo-de-Santayana M. Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain) 1. Economic Botany . 2008;62:24–39. doi: 10.1007/s12231-007-9004-5. [DOI] [Google Scholar]
- 29.Doyle B. J., Asiala C. M., Fernández D. M. Relative importance and knowledge distribution of medicinal plants in a Kichwa community in the Ecuadorian Amazon. Ethnobiology Letters . 2017;8:1–14. doi: 10.14237/ebl.8.1.2017.777. [DOI] [Google Scholar]
- 30.Benkhaira N., Ech-chibani N., Fikri-Benbrahim K. Ethnobotanical survey on the medicinal usage of two common medicinal plants in Taounate region: artemisia herba-alba Asso and Ormenis mixta (L.) Dumort. Ethnobotany Research and Applications . 2021;22:1–19. doi: 10.32859/era.22.48.1-19. [DOI] [Google Scholar]
- 31.Iqbal M. S., Ahmad K. S., Ali M. A., et al. An ethnobotanical study of wetland flora of head maralla Punjab Pakistan. PLoS One . 2021;16(10) doi: 10.1371/journal.pone.0258167.e0258167 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Lee C., Kim S.-Y., Eum S., et al. Ethnobotanical study on medicinal plants used by local van kieu ethnic people of bac huong hoa nature reserve, Vietnam. Journal of Ethnopharmacology . 2019;231:283–294. doi: 10.1016/j.jep.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Ghanimi R., Ouhammou A., Ahouach A., Cherkaoui M. Ethnobotanical study on wild edible plants traditionally used by Messiwa people, Morocco. Journal of Ethnobiology and Ethnomedicine . 2022;18:16–12. doi: 10.1186/s13002-022-00500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Khomsi M., Dandani Y., Chaachouay N., Hmouni D. Ethnobotanical study of plants used for medicinal, cosmetic, and food purposes in the region of Moulay Yacoub, Northeast of Morocco. Journal of Pharmacy & Pharmacognosy Research . 2022;10(1):13–29. doi: 10.56499/jppres21.1084_10.1.13. [DOI] [Google Scholar]
- 35.Benkhaira N., Koraichi S. I., Fikri-Benbrahim K. Ethnobotanical survey on plants used by traditional healers to fight against COVID-19 in Fez city, Northern Morocco. Ethnobotany Research and Applications . 2021;21:1–18. doi: 10.32859/era.21.27.1-18. [DOI] [Google Scholar]
- 36.Kharchoufa L., Bouhrim M., Bencheikh N., et al. Potential toxicity of medicinal plants inventoried in Northeastern Morocco: an ethnobotanical approach. Plants . 2021;10(6):p. 1108. doi: 10.3390/plants10061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yangdon P., Araki T., Rahayu Y. Y. S., Norbu K. Ethnobotanical study of wild edible fruits in eastern Bhutan. Journal of Ethnobiology and Ethnomedicine . 2022;18:27–17. doi: 10.1186/s13002-022-00526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bencheikh N., Elbouzidi A., Kharchoufa L., et al. Inventory of medicinal plants used traditionally to manage kidney diseases in north-eastern Morocco: ethnobotanical fieldwork and pharmacological evidence. Plants . 2021;10(9):p. 1966. doi: 10.3390/plants10091966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salhi S., Fadli M., Zidane L., Douira A. Etudes floristique et ethnobotanique des plantes médicinales de la ville de Kénitra (Maroc) Lazaroa . 2010;31:133–143. doi: 10.5209/rev_laza.2010.v31.9. [DOI] [Google Scholar]
- 40.Bouyahya A., Abrini J., Et-Touys A., Bakri Y., Dakka N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. European Journal of Integrative Medicine . 2017;13:9–25. doi: 10.1016/j.eujim.2017.06.004. [DOI] [Google Scholar]
- 41.Eddouks M., Maghrani M., Lemhadri A., Ouahidi M.-L., Jouad H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet) Journal of Ethnopharmacology . 2002;82(2-3):97–103. doi: 10.1016/s0378-8741(02)00164-2. [DOI] [PubMed] [Google Scholar]
- 42.Chebat A., Skalli S., Errihani H., et al. Étude de prévalence des effets indésirables liés à l’utilisation des plantes médicinales par les patients de l’Institut National d’Oncologie, Rabat. Phytothérapie . 2014;12(1):25–32. doi: 10.1007/s10298-013-0828-4. [DOI] [Google Scholar]
- 43.El Hachlafi N., Chebat A., Fikri-Benbrahim K. Ethnopharmacology, phytochemistry, and pharmacological properties of thymus satureioides coss. Evidence-Based Complementary and Alternative Medicine . 2021;2021:23. doi: 10.1155/2021/6673838.6673838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghourri M., Zidane L., Douira A. Catalogue des plantes médicinales utilisées dans le traitement de la lithiase rénale dans la province de Tan-Tan (Maroc saharien) International Journal of Biological and Chemical Sciences . 2014;7(4):1688–1700. doi: 10.4314/ijbcs.v7i4.24. [DOI] [Google Scholar]
- 45.Chaachouay N., Benkhnigue O., Fadli M., El Ibaoui H., Zidane L. Ethnobotanical and ethnopharmacological studies of medicinal and aromatic plants used in the treatment of metabolic diseases in the Moroccan Rif. Heliyon . 2019;5(10) doi: 10.1016/j.heliyon.2019.e02191.e02191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orch H., Douira A., Zidane L. Étude ethnobotanique des plantes médicinales utilisées dans le traitement du diabète, et des maladies cardiaques dans la région d’Izarène (Nord du Maroc) Journal of Applied Biosciences . 2015;86(1):7940–7956. doi: 10.4314/jab.v86i1.3. [DOI] [Google Scholar]
- 47.Kachmar M. R., Naceiri Mrabti H., Bellahmar M., et al. Traditional knowledge of medicinal plants used in the Northeastern part of Morocco. Evidence-Based Complementary and Alternative Medicine . 2021;2021:20. doi: 10.1155/2021/6002949.6002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benarba B. Medicinal plants used by traditional healers from South-West Algeria: an ethnobotanical study. Journal of Intercultural Ethnopharmacology . 2016;5(4):p. 320. doi: 10.5455/jice.20160814115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farah R., Mahfoud H. M., Mohamed D. O. H., et al. Ethnobotanical study of some medicinal plants from Hoggar, Algeria. Journal of Medicinal Plants Research . 2015;9(30):820–827. doi: 10.5897/jmpr2015.5805. [DOI] [Google Scholar]
- 50.Karous O., Ben Haj Jilani I., Ghrabi-Gammar Z. Ethnobotanical study on plant used by semi-nomad descendants’ community in ouled dabbeb—southern Tunisia. Plants . 2021;10(4):p. 642. doi: 10.3390/plants10040642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benbrahim K., Chraibi M., Farah A., Elamin O., Iraqui H. Characterization, antioxidant, antimycobacterial, antimicrobial effcts of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol. Journal of Advanced Pharmaceutical Technology & Research . 2020;11(1):p. 25. doi: 10.4103/japtr.japtr_74_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouyahya A., Dakka N., Talbaoui A., et al. Correlation between phenological changes, chemical composition and biological activities of the essential oil from Moroccan endemic Oregano (Origanum compactum Benth) Industrial Crops and Products . 2017;108:729–737. doi: 10.1016/j.indcrop.2017.07.033. [DOI] [Google Scholar]
- 53.Benali T., Bouyahya A., Habbadi K., et al. Chemical composition and antibacterial activity of the essential oil and extracts of Cistus ladaniferus subsp. ladanifer and Mentha suaveolens against phytopathogenic bacteria and their ecofriendly management of phytopathogenic bacteria. Biocatalysis and Agricultural Biotechnology . 2020;28 doi: 10.1016/j.bcab.2020.101696.101696 [DOI] [Google Scholar]
- 54.Benkhaira N., Ibnsouda Koraichi S., Fikri-Benbrahim K. Ruta Montana (L.) L: an insight into its medicinal value, phytochemistry, biological properties, and toxicity. Journal of Herbmed Pharmacology . 2022;11(3):305–319. doi: 10.34172/jhp.2022.36. [DOI] [Google Scholar]
- 55.Benkhaira N., ibnsouda Koraichi S., Fikri-Benbrahim K. In vitro methods to study antioxidant and some biological activities of essential oils: a review. Biointerface Research in Applied Chemistry . 2021;12 [Google Scholar]
- 56.Agyare C., Spiegler V., Asase A., Scholz M., Hempel G., Hensel A. An ethnopharmacological survey of medicinal plants traditionally used for cancer treatment in the Ashanti region, Ghana. Journal of Ethnopharmacology . 2018;212:137–152. doi: 10.1016/j.jep.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Baydoun S., Chalak L., Dalleh H., Arnold N. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. Journal of Ethnopharmacology . 2015;173:139–156. doi: 10.1016/j.jep.2015.06.052. [DOI] [PubMed] [Google Scholar]
- 58.Palabaş Uzun S., Koca C. Ethnobotanical survey of medicinal plants traded in herbal markets of Kahramanmaraş. Plant Diversity . 2020;42(6):443–454. doi: 10.1016/j.pld.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Qura’n S. Ethnobotanical survey of folk toxic plants in southern part of Jordan. Toxicon . 2005;46(2):119–129. doi: 10.1016/j.toxicon.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Ali H. F. M., El-Ella F. M. A., Nasr N. F. Screening of chemical analysis, antioxidant antimicrobial and antitumor activities of essential oil of oleander (Nerium oleander) flower. International Journal of Biological Chemistry . 2010;4:190–202. doi: 10.3923/ijbc.2010.190.202. [DOI] [Google Scholar]
- 61.Doudach L., Meddah B., Fanzi M., Khatib A.-M., Lalou C., Hammani K. Cytotoxic & antioxidant activity of various extracts of Corrigiola telephiifolia Pourr. IJPPS . 2013;5:154–158. [Google Scholar]
- 62.Mohsen E., El-Far A. H., Godugu K., Elsayed F., Mousa S. A., Younis I. Y. SPME and solvent-based GC–MS metabolite profiling of Egyptian marketed Saussurea costus (Falc.) Lipsch. concerning its anticancer activity. Phytomedicine Plus . 2022;2(1) doi: 10.1016/j.phyplu.2021.100209.100209 [DOI] [Google Scholar]
- 63.El Fakir L., Bouothmany K., Alotaibi A., et al. Antioxidant and understanding the anticancer properties in human prostate and breast cancer cell lines of chemically characterized methanol extract from berberis hispanica Boiss. Applied Sciences . 2021;11(8):p. 3510. doi: 10.3390/app11083510. [DOI] [Google Scholar]
- 64.Naima B., Abdelkrim R., Ouarda B., N Salah N., A M Larbi B. Chemical composition, antimicrobial, antioxidant and anticancer activities of essential oil from Ammodaucus leucotrichus Cosson & Durieu (Apiaceae) growing in South Algeria. Bulletin of the Chemical Society of Ethiopia . 2019;33(3):541–549. doi: 10.4314/bcse.v33i3.14. [DOI] [Google Scholar]
- 65.Gore G. G., Satish S., Ganpule A., Srivastava S., Athavale M. Garlic (Allium sativum) exhibits anticancer and anticancer stem cell activity on breast, prostate, colon, hepatic and cervical cancer cell lines. International Journal of Herbal Medicine . 2021;9:93–99. [Google Scholar]
- 66.Kim J., Kim J.-S., Park E. Antioxidative and antigenotoxic effects of onion peel extracts in non-cellular and cellular systems. Food Science and Biotechnology . 2013;22(5):1–8. doi: 10.1007/s10068-013-0228-0. [DOI] [Google Scholar]
- 67.Nile A., Gansukh E., Park G.-S., Kim D.-H., Hariram Nile S. Novel insights on the multi-functional properties of flavonol glucosides from red onion (Allium cepa L) solid waste–In vitro and in silico approach. Food Chemistry . 2021;335 doi: 10.1016/j.foodchem.2020.127650.127650 [DOI] [PubMed] [Google Scholar]
- 68.Asadi H., Rahamooz-Haghighi S. Anti-proliferative effect of the extracts and essential oil of Pimpinella anisum on gastric cancer cells. Journal of Herbmed Pharmacology . 2016;5 [Google Scholar]
- 69.Benarba B., Aoues A., Vazquez A., Ambroise G., Meddah B. Aristolochia longa aqueous extract triggers the mitochondrial pathway of apoptosis in BL41 Burkitt’s lymphoma cells. International Journal of Green Pharmacy . 2012;6(1):p. 45. doi: 10.4103/0973-8258.97128. [DOI] [Google Scholar]
- 70.Tilaoui M., Ait Mouse H., Jaafari A., Zyad A. Comparative phytochemical analysis of essential oils from different biological parts of Artemisia herba alba and their cytotoxic effect on cancer cells. PLoS One . 2015;10(7) doi: 10.1371/journal.pone.0131799.e0131799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Snafi, Medical A. E. Importance of anthemis nobilis (chamaemelum nobile)-a review. Asian Journal of Pharmaceutical Science and Technology . 2016;6:89–95. [Google Scholar]
- 72.Sevgi E., Dag A., Kızılarslan-Hançer Ç., Atasoy S., Kurt B. Z., Aksakal Ö. Evaluation of cytotoxic and antioxidant potential of Dittrichia viscosa (L.) Greuter used in traditional medicine. Journal of Ethnopharmacology . 2021;276 doi: 10.1016/j.jep.2021.114211.114211 [DOI] [PubMed] [Google Scholar]
- 73.AlObaidi L. A. H. Study the anticancer effect of Lepidium sativum leaves extract on squamous cell carcinoma (CAL-27) cell lines. Journal of Natural Sciences Research . 2014;4:48–52. [Google Scholar]
- 74.Heikal A., Abd El-Sadek M. E., Salama A., Taha H. S. Comparative study between in vivo-and in vitro-derived extracts of cactus (Opuntis ficus-indica L. Mill) against prostate and mammary cancer cell lines. Heliyon . 2021;7(9) doi: 10.1016/j.heliyon.2021.e08016.e08016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Z Mohammed F., A Gurigis A., Gamal A. Assessment of antiproliferative activity of Capparis spinosa L extract against Ehrlich ascites carcinoma in Swiss albino mice. Biochemistry Letters . 2018;13(1):54–78. doi: 10.21608/blj.2018.47583. [DOI] [Google Scholar]
- 76.Kasali F. M., Tusiimire J., Kadima J. N., Agaba A. G. Ethnomedical uses, chemical constituents, and evidence-based pharmacological properties of Chenopodium ambrosioides L.: extensive overview. Future Journal of Pharmaceutical Sciences . 2021;7:153–236. doi: 10.1186/s43094-021-00306-3. [DOI] [Google Scholar]
- 77.Barnawi I. O., Nasr F. A., Noman O. M., et al. Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis. Open Chemistry . 2021;19(1):119–127. doi: 10.1515/chem-2021-0195. [DOI] [Google Scholar]
- 78.Sioud F., Dhouafi Z., Lahmar A., Elgueder D., Chekir-Ghedira L. A novel anticancer effect of Ephedra alata Decne in breast cancer cells. Nutrition and Cancer . 2022;74(9):3403–3412. doi: 10.1080/01635581.2022.2072907. [DOI] [PubMed] [Google Scholar]
- 79.Talbaoui A., El Hamdaoui L., Bouyahya A., El Moussaouiti M., Bakri Y. Chemical composition, in vitro cytotoxic, and antibacterial activities of Moroccan medicinal plants Euphorbia resinifera and Marrubium vulgare. Biointerface Research in Applied Chemistry . 2020;10:7343–7355. [Google Scholar]
- 80.Amessis-Ouchemoukh N., Ouchemoukh S., Meziant N., et al. Bioactive metabolites involved in the antioxidant, anticancer and anticalpain activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. extracts. Industrial Crops and Products . 2017;95:6–17. doi: 10.1016/j.indcrop.2016.10.007. [DOI] [Google Scholar]
- 81.Al-Snafi A. E. Glycyrrhiza glabra: A phytochemical and pharmacological review. IOSR Journal of Pharmacy . 2018;8:1–17. [Google Scholar]
- 82.Belayachi L., Aceves-Luquero C., Merghoub N., et al. Retama monosperma n-hexane extract induces cell cycle arrest and extrinsic pathway-dependent apoptosis in Jurkat cells. BMC Complementary Medicine and Therapies . 2014;14:38–12. doi: 10.1186/1472-6882-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Dabbagh B., Elhaty I. A., Al Hrout A., et al. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complementary Medicine and Therapies . 2018;18:240–312. doi: 10.1186/s12906-018-2285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lambrianidou A., Koutsougianni F., Papapostolou I., Dimas K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules . 2020;26(1):p. 86. doi: 10.3390/molecules26010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aly M. M., Al-Ghamdi M., Bafeel S. O., Khedr A. M. Antimicrobial qctivities and phytochemical analysis of the essential oil of Lavandula dentata and Plectranthus tenuiflorus, collected from Al Baha region, Saudi Arabia. Life Science Journal . 2013;10 [Google Scholar]
- 86.Belayachi L., Aceves-Luquero C., Merghoub N., et al. Screening of North African medicinal plant extracts for cytotoxic activity against tumor cell lines. European Journal of Medicinal Plants . 2013;3:310–332. doi: 10.9734/ejmp/2013/3403. [DOI] [Google Scholar]
- 87.Chaouki W., Leger D. Y., Eljastimi J., Beneytout J.-L., Hmamouchi M. Antiproliferative effect of extracts from Aristolochia baetica and Origanum compactum on human breast cancer cell line MCF-7. Pharmaceutical Biology . 2010;48(3):269–274. doi: 10.3109/13880200903096588. [DOI] [PubMed] [Google Scholar]
- 88.Bouyahya A., Dakka N., Lagrouh F., Abrini J., Bakri Y. In vitro antiproliferative and antidermatophyte activities of essential oils from three moroccan medicinal plants. Journal of Biologically Active Products from Nature . 2018;8(3):144–153. doi: 10.1080/22311866.2018.1496032. [DOI] [Google Scholar]
- 89.Privitera G., Luca T., Castorina S., Passanisi R., Ruberto G., Napoli E. Anticancer activity of Salvia officinalis essential oil and its principal constituents against hormone-dependent tumour cells. Asian Pacific Journal of Tropical Biomedicine . 2019;9(1):p. 24. doi: 10.4103/2221-1691.250266. [DOI] [Google Scholar]
- 90.Morsi E. A., Ahmed H. O., Abdel-Hady H., El-Sayed M., Shemis M. A. GC-analysis, and antioxidant, anti-inflammatory, and anticancer activities of some extracts and fractions of Linum usitatissimum. Current Bioactive Compounds . 2020;16(9):1306–1318. doi: 10.2174/1573407216666200206095954. [DOI] [Google Scholar]
- 91.Zhou H., Huang R., Su T., et al. A c-MWCNTs/AuNPs-based electrochemical cytosensor to evaluate the anticancer activity of pinoresinol from Cinnamomum camphora against HeLa cells. Bioelectrochemistry . 2022;146 doi: 10.1016/j.bioelechem.2022.108133.108133 [DOI] [PubMed] [Google Scholar]
- 92.AlGhalban F. M., Khan A. A., Khattak M. N. K. Comparative anticancer activities of Ficus carica and Ficus salicifolia latex in MDA-MB-231 cells. Saudi Journal of Biological Sciences . 2021;28(6):3225–3234. doi: 10.1016/j.sjbs.2021.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samane S., Noel J., Charrouf Z., Amarouch H., Haddad P. S. Insulin-sensitizing and anti-proliferative effects of argania spinosa seed extracts. Evidence-Based Complementary and Alternative Medicine . 2006;3:317–327. doi: 10.1093/ecam/nel015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esposito S., Bianco A., Russo R., Di Maro A., Isernia C., Pedone P. V. Therapeutic perspectives of molecules from urtica dioica extracts for cancer treatment. Molecules . 2019;24(15):p. 2753. doi: 10.3390/molecules24152753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeena K., Liju V. B., Kuttan R. Antitumor and cytotoxic activity of ginger essential oil (zingiber officinale Roscoe) International Journal of Pharmacy and Pharmaceutical Sciences . 2015;7:341–344. [Google Scholar]
- 96.Cavallo F., De Giovanni C., Nanni P., Forni G., Lollini P.-L. 2011: the immune hallmarks of cancer. Cancer Immunology, Immunotherapy . 2011;60(3):319–326. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng X. r., Liu Z. x., Liu F., et al. Holotransferrin enhances selective anticancer activity of artemisinin against human hepatocellular carcinoma cells. Journal of Huazhong University of Science and Technology [Medical Sciences] . 2013;33(6):862–865. doi: 10.1007/s11596-013-1212-x. [DOI] [PubMed] [Google Scholar]
- 98.Deb D. D., Parimala G., Saravana Devi S., Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chemico-Biological Interactions . 2011;193(1):97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 99.Jayakumar S., Madankumar A., Asokkumar S., et al. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Molecular and Cellular Biochemistry . 2012;360(1-2):51–60. doi: 10.1007/s11010-011-1043-7. [DOI] [PubMed] [Google Scholar]
- 100.Jaafari A., Tilaoui M., Mouse H. A., et al. Comparative study of the antitumor effect of natural monoterpenes: relationship to cell cycle analysis. Revista Brasileira de Farmacognosia . 2012;22(3):534–540. doi: 10.1590/s0102-695x2012005000021. [DOI] [Google Scholar]
- 101.Nakamura Y., Miyamoto M., Murakami A., Ohigashi H., Osawa T., Uchida K. A phase II detoxification enzyme inducer from lemongrass: identification of citral and involvement of electrophilic reaction in the enzyme induction. Biochemical and Biophysical Research Communications . 2003;302(3):593–600. doi: 10.1016/s0006-291x(03)00219-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting the findings of this study are included in this article.


