Abstract
Microvascular angina (MVA), historically called cardiac syndrome X, refers to angina with nonobstructive coronary artery disease. This female-predominant cardiovascular disorder adds considerable health-related costs due to repeated diagnostic angiography and frequent hospital admissions. Despite the high prevalence of this diagnosis in patients undergoing coronary angiography, it is still a therapeutic challenge for cardiologists. Unlike obstructive coronary artery disease, with multiple evidence-based therapies and management guidelines, little is known regarding the management of MVA. During the last decade, many therapeutic interventions have been suggested for the treatment of MVA. However, there is a lack of summarization tab and update of current knowledge about pharmacologic management of MVA, mostly due to unclear pathophysiology. In this article, we have reviewed the underlying mechanisms of MVA and the outcomes of various medications in patients with this disease. Contrary to vasospastic angina in which normal angiogram is observed as well, nitrates are not effective in the treatment of MVA. Beta-blockers and calcium channel blockers have the strongest evidence of improving the symptoms. Moreover, the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, statins, estrogen, and novel antianginal drugs has had promising outcomes. Investigations are still ongoing for vitamin D, omega-3, incretins, and n-acetyl cysteine, which have resulted in beneficial initial outcomes. We believe that the employment of the available results and results of the future large-scale trials into cardiac care guidelines would help reduce the global cost of cardiac care tremendously.
1. Introduction
Microvascular angina (MVA) was first described by Kemp in 1973 as an angina-like chest pain without any evidence of coronary obstruction in angiogram [1]. Although there has been controversy in the definition of MVA, recently in 2018, The Coronary Vasomotion Disorders International Study Group published standardized criteria for diagnosis of MVA as presence of symptoms of myocardial ischemia accompanied with evidence of ischemia on electrocardiogram (ECG) or on cardiac imaging along with the absence of obstructive coronary artery disease (CAD) and evidence of impaired coronary microcirculation [2]. Advancements in diagnostic imaging have caused a rise in the prevalence of MVA in recent years, with 50% of about 400,000 suspected CAD patients reported having normal angiography with no established CAD or nonobstructive CAD in a study across the United States [3, 4]. In the recent CE-MARC2 study, 68% of patients with angina and nonobstructive coronary angiogram had abnormal microvascular function [5]. Although, the true prevalence of MVA is not known and is difficult to establish but above data does suggest a high prevalence and high burden on health care. MVA is a female-predominant disorder with women comprising about 70% of patients MVA were diagnosed in 43.1% of women and only 14% of the men with typical angina undergoing coronary angiography [6]. In a study conducted in the United States, Hispanics contributed 21%, and non-White races accounted for 38% of MVA patients [7].
Although earlier MVA was considered a benign condition, the Women's Ischemia Syndrome Evaluation (WISE) study reported the 5-year risk of adverse cardiovascular events 3 to 6 times higher in women with MVA compared to normal asymptomatic women [8]. Moreover, MVA has adverse effects on patients' quality of life due to recurrent chest pain and further imposes increased lifetime cost of healthcare mostly due to frequent hospitalizations and diagnostic procedures [9, 10].
In the last decade, despite advances in our understanding of MVA, it still remains a therapeutic challenge for physicians. In this review, we primarily focus on the pharmacologic management of MVA.
2. Pathophysiology
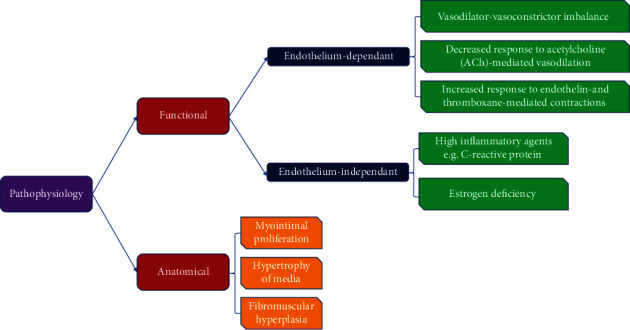
Various anatomic and functional pathophysiologic mechanisms involving coronary microcirculation are proposed to lead to MVA. Of note, MVA must not be confused with vasospastic angina which classically is a disorder of major epicardial coronary arteries.
Impaired vasodilation response, either via endothelium-dependent or endothelium-independent mechanisms, is frequently implied in the pathogenesis of MVA [11]. A vasodilator-vasoconstrictor imbalance depicted as a reduced nitric oxide (NO) release as well as high plasma levels of endothelin-1 may also be a contributing factor for endothelial dysfunction in MVA patients [12–15]. In a recent study on patients with MVA and patients with vasospastic angina, resistant arteries of patients with MVA showed decreased response to acetylcholine- (ACh-) mediated vasodilation and increased response to endothelin- and thromboxane-mediated contractions. The microvascular vasoactive responses in both MVA and vasospastic angina patients tended toward an augmented vasoconstriction [16]. ACh provocation test in patients with angina and no obstructive epicardial CAD demonstrated a microvascular spasm in almost half of them, bringing ischemic ECG patterns [17]. Similarly, a European study conducted on patients with angina and normal angiogram revealed a female-male odds ratio of 4.2 in patients with MVA, suggesting that the female predominance is due to the higher sensitivity of women to develop vasomotor dysfunction at lower ACh levels [18]. Moreover, endothelium-independent microvascular dysfunction in patients with angina without obstructive coronary arteries was suggested to be associated with multiple proinflammatory and coagulation factors including von Willebrand factor and Galectin-4 [19].
Endothelial dysfunction in patients with MVA seems to be multifactorial, and it is conceivable that risk factors like hypertension, hypercholesterolemia, smoking, diabetes mellitus, and insulin resistance can contribute to its development [20]. Cosín-Sales et al. demonstrated that high plasma C-reactive protein was associated with endothelial impairment, which indicates the potential role of inflammation in the pathogenesis of MVA [21]. This inflammatory response can decrease NO bioavailability and as a result microvascular dysfunction [22]. The high prevalence of MVA in menopausal women has also suggested the pathogenic effect of estrogen deficiency in the development of endothelial dysfunction [23].
In addition to functional abnormalities, some studies support the structural abnormalities of coronary microvasculature as a mechanism for MVA [24, 25]. In a histologic study conducted by Mosseri et al. on MVA patients, myointimal proliferation, hypertrophy of media, and fibromuscular hyperplasia have been reported as possible causes of MVA development [25].
A number of studies have related the signs and symptoms of MVA patients to neurologic abnormalities including cardiac adrenergic hyperactivity, cardiac neurasthenia, and low threshold of chest pain [26, 27]. Increased activity of membrane sodium-hydrogen exchanger [28], reduced number of epithelial progenitor cells [29], and psychological morbidities [30] are among other pathophysiologic mechanisms proposed for MVA. Figure 1 summarizes the pathophysiologic mechanisms behind MVA.
Figure 1.
Summary of pathophysiology behind microvascular angina.
3. Pharmacologic Management of MVA
The majority of patients with MVA do have some extent of concomitant coronary atherosclerosis as seen in studies using the intravascular ultrasound [31]. Thus, aggressive risk factor modification and guideline-based management of hypertension, hyperlipidemia, diabetes, lifestyle changes, smoking, and obesity are indicated in most patients.
The main goal of pharmacologic interventions in patients with MVA is to control symptoms and improve patients' quality of life. Nitrates, statins, calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEI), and beta-blockers are the most commonly administered drugs for MVA patients. However, at least in part due to heterogeneous mechanisms of MVA pathophysiology, the mentioned medications have modest efficacies. Hence, the management of MVA remains a significant challenge. In the following section, we will review the mechanisms and outcomes of treatment with conventional and novel drugs in MVA patients.
3.1. Beta-Blockers
Blocking beta-adrenergic receptors improves myocardial ischemia by lowering heart rate, myocardial contractility, blood pressure, and consequently myocardial oxygen consumption. Additionally, beta-blockers enhance coronary blood flow (CBF) by diastolic time prolongation [32, 33]. Beta-blockers have been shown to be effective in relieving angina in about 75% of patients with MVA. In comparison to the placebo, propranolol significantly reduced the 24-h frequency of ischemic episodes (0.7 ± 0.6 vs. 3.9 ± 1.8, respectively, p < 0.0005) [34]. Atenolol also has been shown to reduce the number of ischemic episodes (0.44 ± 0.55 vs. 4.8 ± 4, p < 0.01) and improve symptoms, diastolic function, and exercise tolerance in MVA patients [35, 36]. Beta-blockers have been shown to have more favorable effects than CCBs in angina with a normal coronary angiogram [34, 36]. In comparison to the placebo, a significant decrease in the average number of daily ischemic episodes has been recorded during propranolol therapy (0.7 ± 0.6 vs. 3.9 ± 1.8, p < 0.0005); however, no significant association was found in the verapamil group (3.4 ± 1.7 vs. 3.9 ± 1.8) [34].
Unlike earlier generations of beta-blockers, third-generation drugs such as nebivolol and carvedilol have more endothelium-dependent vasodilatory effects. Substantial increases in the serum levels of endothelial function markers including plasma NO, L-arginine, and L-arginine/asymmetric dimethylarginine ratio have been reported in the administration of nebivolol compared to metoprolol [37]. In addition, a study by Togni et al. revealed that intracoronary nebivolol increased the coronary flow reserve (CFR) [38]. In a randomized placebo-controlled trial on MVA patients, Kaski et al. have shown that two-thirds of patients who had received carvedilol did not have angina at peak exercise (p < 0.01), in one-third ST segment shift was less than 1 mm during exercise tolerance test (p < 0.05), and these proportions were significantly higher in carvedilol group than placebo group [39]. To explain the mechanisms of these significant effects of third-generation beta-blockers on endothelium, several theories have been proposed including adenosine triphosphate (ATP) efflux which stimulates P2Y-purinoceptor-mediated NO release [40], overexpression of inducible NO synthase by beta3-adrenergic receptor activation [41], and decrease in reactive oxygen species (ROS) production in endothelial cells [42, 43]. Overall, it seems that beta-blockers can be the first line of treatment for MVA patients [34] and, if required, they could be used in combination with CCBs.
3.2. Calcium Channel Blockers
CCBs reduce myocardial oxygen demand through their negative inotropic (nondihydropyridine) and vasodilatory effects and subsequent decrease in heart afterload [44]. Molecular-based studies have indicated that CCBs are able to protect endothelium against free radical injuries [45, 46], and amlodipine as a dihydropyridine CCB has been shown to increase nitrate production [47]. In addition, effect of CCBs in reducing microvascular tone and relieving spasm [48] raises CCBs as a potential treatment for patients with MVA.
A meta-analysis compared the effects of four CCBs in vasospastic angina patients which suggested that benidipine outperformed amlodipine, nifedipine, and diltiazem in terms of attack suppression [49]. Different clinical trials have shown discordant results about the efficacy of CCBs in patients with MVA. Cannon et al. compared the efficacy of nifedipine and verapamil versus placebo in patients with angina pectoris along with angiographically normal coronary arteries and reported fewer episodes of angina, longer exercise duration, and fewer nitroglycerin consumption in CCB users [50]. While many studies support CCBs' effects on controlling angina in MVA patients [50–52], there are some studies showing conflicting results. Amlodipine showed no benefit in reducing chest pain episodes in a trial by Lanza et al. [36]. Although intravenous administration of diltiazem failed to increase CFR in patients with MVA [53], sustained-release capsules of diltiazem have been reported to significantly improve chest pain, treadmill exercise test, and CBF [52]. It has been suggested that long-acting L-type CCB is more favorable than short-acting ones for the coronary microcirculation [54]. Moreover, combination therapy of diltiazem with statins (fluvastatin) has been shown to improve coronary flow and prolong time to 1 mm ST segment depression in patients with MVA, to a higher extent than CCB or statins alone [55].
3.3. Statins
Statins beyond their lipid-lowering effect by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase have noticeable effects on improving endothelial function through increasing NO bioavailability, antioxidant properties, and suppressing inflammatory responses [56].
A large number of clinical trials have demonstrated statins' effect on improving endothelial dysfunction [57–60]. A systematic review in 2011 also showed significant improvement in both peripheral and coronary endothelial function after statin therapy, highlighting its potential use in MVA [61]. Moreover, there are several studies that have evaluated the effect of statins on MVA patients. In a randomized placebo-controlled trial on 40 known cases of MVA, simvastatin 20 mg/day increased the relative brachial artery flow-mediated dilation by 52% [62]. In another randomized controlled trial in patients with MVA, 3 months prescription of pravastatin (40mg/day) significantly improved the exercise-induced ischemia and flow-mediated dilatation [63]. A combination of statins and CCBs has been tested on 68 MVA patients divided into three groups including fluvastatin (40 mg/day), diltiazem (90 mg/day), and a combination of fluvastatin (40 mg/day) and diltiazem (90 mg/day). At the end of 90 days, improvement in CFR (23.2%, 12.4%, and 29.1% respectively) was higher in patients who received combination therapy [55].
Taking all these lines of evidence together, statins should be regarded as a major drug class in the management of patients with MVA, particularly in combination with CCBs.
3.4. ACEI/ARBs
Angiotensin II is a vasoconstrictor agent which increases superoxide production by its effect on nicotinamide adenine dinucleotide phosphate -NADPH- and nicotinamide adenine dinucleotide –NADH [64, 65]. ACEIs or angiotensin receptor blockers (ARBs) through their effect on superoxide dismutase activation, thereby decreasing ROS, improve microvascular dysfunction [66, 67]. In addition, these drugs stimulate NO production by lowering the bradykinin degradation [68].
In several clinical trials, beneficial effects of an ACEI on forearm blood flow, CBF, and flow-mediated dilation have been demonstrated both in patients with CAD and in those with normal epicardial coronary arteries in the angiography [68–71]. In a double-blinded, randomized, and placebo-controlled based study, Chen et al. have found that the long-term treatment with enalapril, an ACEI, improves exercise performance and CFR in patients with MVA [71]. In the WISE trial, 16-week treatment with quinapril significantly improved CFR and angina symptoms [72]. In addition, cilazapril showed to be effective in increasing CFR in hypertensive patients in a study [73]. This effect was also observed in diabetic patients in whom coronary flow velocity increased after treatment with temocapril [74]. Although a few studies support ARBs' positive effect on microvascular dysfunction, some pieces of evidence showed no significant improvement in MVA patients [67, 75]. A randomized double-blind controlled trial examined the effect of 3-week irbesartan (150 mg daily) treatment in MVA patients which resulted in a trivial reduction in ST segment depression episodes and no significant change in treadmill exercise test [75]. It should be noted that adding eplerenone as a mineralocorticoid inhibitor to the ACEI or ARB could not reduce angina episodes and CFR in the women population [76].
To evaluate the priority of ACEI, beta-blockers, CCBs, and diuretics in MVA treatment, Higashi et al. conducted a clinical trial. In this study, the forearm blood flow response to reactive hyperemia, an index of endothelium-dependent vasodilation, was substantially higher in patients treated with ACEI than in individuals treated with either CCBs, beta-blockers, diuretic agents, or nothing [70].
Accordingly, it seems that ACEIs are highly potent medications in patients with angina and normal epicardial coronary artery angiogram, particularly in the presence of arterial hypertension. Thus overall, the evidence favors the role of ACEI in the management of MVA.
3.5. Antiplatelet Agents
In a study using intravascular ultrasound, it was found that there is a high prevalence of atherosclerosis in patients with chest pain and without obstructive CAD [31]. This suggests the beneficial use of thromboxane A2 (TXA2) inhibitors in MVA. These include low-dose aspirin and P2Y12 platelet inhibitors which can prevent vasoconstriction, platelet aggregation, and vascular injury. However, its combination with ACEI and statins tends to be more effective and is being trialed in Women's Ischemia Treatment Reduces Events in Nonobstructive CAD ((WARRIOR)-NCT03417388) [77].
4. Nitric Oxide Modulators
4.1. Nitrates
Direct relaxant effect of nitrates on vascular smooth muscles results from activation of the guanylyl cyclase signaling pathway by nitrate-released NO. Vasodilation induced by nitrates lowers cardiac preload and afterload and consequently reduces myocardial oxygen consumption [78, 79]. In addition, the vasodilatory effect of nitrates on coronary arteries increases myocardial blood supply, although the effect has been shown to be limited in the coronary microvasculature [80]. Hence, nitrates compose the main group for the medical management of patients with obstructive CAD. However, many studies have indicated the limited efficacy of nitrates in relieving angina in MVA patients [81, 82]. In addition, MVA patients did not experience proper symptom relief after administrating the sublingual nitroglycerin [2]. In a study conducted by Kaski et al., only 42% of patients with angina and normal epicardial coronary angiograms responded to the sublingual nitrate [83]. Russo et al. found out that the prescription of short-acting nitrates results in no improvement in exercise stress tests in MVA patients [82]. Even recent research has indicated that nitrates not only may not improve ischemia but also may worsen the ischemia through increasing endothelial dysfunction [84–86]. In a comparative study of atenolol, amlodipine, and isosorbide-mononitrate, the frequency of chest pain episodes during a 4-week follow-up of MVA patients was evaluated. Although atenolol significantly reduced the anginal episodes compared to placebo, nitrates and amlodipine demonstrated no significant difference [36]. Altogether, despite a high tendency to prescribe nitrates in the management of MVA, it seems that they have no significant benefit and cannot be recommended for the treatment of MVA [87].
4.2. L-Arginine
Asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) are NO synthase inhibitors that have been shown to be higher in MVA patients who develop adverse events [68, 88, 89]. L-arginine as a precursor of NO induces coronary vasodilation and counteracts ADMA. It is suggested that alterations in arginine/NO metabolic profile in addition to a rise in oxidative stress are observed in MVA patients in a similar trend to CAD cases [90]. Clinical trials have shown heterogeneous results about the efficacy of L-arginine in patients with MVA. Although multiple studies have reported the effect of L-arginine on CBF and endothelial function improvement, there is limited knowledge about the favorable effect of L-arginine on exercise stress test and angina pectoris in MVA patients [91–93]. In a small study, Palloshi et al. reported the beneficial effect of L-arginine on exercise test and angina pectoris in hypertensive patients with angina and normal coronary angiograms [92]. Moreover, a trial conducted by Lerman et al. found that L-arginine supplementation for 6 months can have beneficial effects on endothelial function with improvement in symptoms and reduced endothelin concentrations [94]. Despite promising effects on endothelial function, there is currently insufficient evidence to support the use of L-arginine in the management of MVA.
4.3. Sildenafil
NO promotes muscle relaxation via the cyclic guanosine monophosphate (cGMP)-dependent mechanism [95]. Sildenafil as a phosphodiesterase type 5 (PDE-5) inhibitor prolongs NO bioavailability by blocking the nitric oxide degeneration [96].
The effect of sildenafil on improving endothelial dysfunction has been demonstrated frequently [97–100]. However, there are limited studies evaluating the effect of PDE-5 inhibitors on MVA. Denardo et al. noticed an acute improvement of CFR after PDE-5 inhibition by 100 mg oral sildenafil in MVA patients [101]. However, the long-term effect of sildenafil on CFR remains unclear. It seems that sildenafil can be a potential novel treatment for MVA, but more clinical trials are required to confirm the hypothesis. The inefficacy of nitrates compared to promising effects of PDE-5 inhibitor in MVA stems from the lack of nitrate-induced endothelial dysfunction, the transmural increase in coronary flow, and the less likelihood of developing coronary steal syndrome in sildenafil use in comparison with nitrates use [100]. In addition, sildenafil works specifically downstream of the endogenous NO pathway on the selective cGMP-induced vasodilation in affected regions and prevents unfavorable systemic effects of exogenous nitrate use [102].
4.4. Cilostazol
Cilostazol as a phosphodiesterase type 3 (PDE-3) inhibitor increases intracellular cyclic adenosine monophosphate with anti-inflammatory, antiplatelet, and vasodilatory effects [54]. The effects of cilostazol addition to CCBs and nitrates on vasospastic angina have been shown to be significant [103]. In addition, vasospastic angina refractory to amlodipine cases showed reduced angina frequency and intensity after administration of cilostazol [104].
4.5. Tetrahydrobiopterin (BH4)
Tetrahydrobiopterin is an essential cofactor of aromatic amino acid hydroxylases used in the biosynthesis of several neurotransmitters such as serotonin and catecholamines; in addition, it plays a critical role in nitric oxide production as a cofactor [105, 106].
Many studies have noted that BH4 causes significant improvement in the NO-mediated endothelial function [107–109]. Higashi et al. have indicated that BH4-ACh coinfusion acutely increases ACh-mediated vasodilation in 37 healthy subjects [110]. In addition, long-term administration of BH4 has been shown to restore the NO-mediated vasodilation [111]. Given BH4's favorable effect on endothelial function, it seems to be a promising therapy for MVA; however, its clinical efficacy remains to be determined.
4.6. Alpha-Blockers
Alpha-blockers have been proposed for MVA treatment due to their sympatholytic capacity which causes a reduction in the microvascular tone [112]. However, clinical trials have shown disappointing results.
Sixteen patients with MVA underwent a double-blind, placebo-controlled, crossover clinical trial for 10 weeks. Lastly, no difference in exercise duration, time to angina pectoris, and exercise time to 0.1 mV ST-segment depression has been noted between doxazosin-treated patients and placebo group [113]. In another study evaluating prazosin's effect on MVA patients, similar results have been reported [114].
In addition to the low efficacy of alpha-blockers, another limitation of their recommendation in MVA is tolerance which occurs in frequent administration [115].
5. Hormonal Drugs
5.1. Estrogen
The high prevalence of MVA in postmenopausal women implies that hormonal deficiency may be a critical factor in the development of endothelial dysfunction. Estrogen, by its property for accelerating reendothelialization and inhibiting endothelial cell apoptosis, certifies endothelial integrity [116, 117]. Furthermore, estrogen has anti-inflammatory and antioxidative properties and induces NO synthesis in human endothelial cells through a nongenomic estrogen receptor signaling [118–120].
Reduced number of chest pain episodes was observed in 17-beta-estradiol administration for postmenopausal women with cardiac syndrome X compared to placebo (3.7 episodes/10 days vs. 7.3 episodes/10 days, respectively) in a double-blind placebo-controlled study [121]. In another study, increased time to angina, time to 1 mm ST depression, total exercise time, and working capacity has been reported during 17-beta-estradiol treatment in patients with angina and no obstructive coronary artery lesions [122]. Finally, a trial was conducted on low-dose hormone therapy in postmenopausal women with no obstructive CAD. It concluded that low-dose hormone therapy could improve chest pain symptoms in addition to menopausal symptoms and increase the quality of life, despite not having an effect on ischemia or endothelial function [123].
Thus, estrogens are effective drugs in alleviating MVA symptoms; however, some pieces of evidence contradict with long-term prescription of estrogen because of safety concerns and reduction of benefits in long-term administration [119, 124].
5.2. Vitamin D
The inverse association of vitamin D and the renin-angiotensin-aldosterone system leads to increased vascular inflammation and endothelial dysfunction in vitamin D deficiency [125]. Increased parathormone level in response to insufficient serum vitamin D causes insulin resistance, which is a risk factor for endothelial dysfunction [126].
Studies have found significantly lower serum vitamin D levels in patients with MVA in comparison with the control group [127, 128]. Consistently, vitamin D replacement therapy in patients with MVA and low serum vitamin D3 has shown a significant improvement in the frequency of angina episodes (p = 0.003), exercise duration, maximal work capacity (p < 0.001), and maximal ST-segment depression (p = 0.001) [129].
Accordingly, recent studies suggest vitamin D as a novel and efficient medication for the treatment of MVA; however, more clinical trials are necessary to confirm the efficacy and safety of vitamin D supplementation therapy.
6. Novel Antianginal Drugs
6.1. Ranolazine
Increased inward late Na+ current which is observed in myocardial ischemia disturbs ion homeostasis through elevation of intracellular Na+ concentration with subsequent elevation of intracellular Ca2+. Ranolazine by inhibiting late sodium current in cardiomyocytes plays a significant role in relieving symptoms of myocardial ischemia [130]. A number of studies also noted ranolazine properties for endothelial function improvement [131].
Although favorable effects of ranolazine in the management of obstructive CAD were initially hopeful [132], there is a limited number of clinical trials to assess the effect of this drug in MVA patients. A randomized, double-blind, placebo-controlled trial investigating the effect of 4-week ranolazine therapy in 20 women with MVA showed physical functioning, angina stability, and quality of life improvement in the ranolazine-treated group, especially in those with CFR ≤ 3.0 [133]. Villano et al. also found ranolazine has a significant therapeutic effect on patients with nonobstructive CAD in combination with usual antiischemic therapies [134]. However, a more robust study on 128 patients with coronary microvascular dysfunction revealed no positive effect of 2-week ranolazine therapy on reported symptoms, myocardial perfusion reserve index, or diastolic filling rate and time [135]. This result changed in terms of significance after categorizing by baseline CFR. Rambarat et al. concluded that patients with CFR < 2.5 showed better myocardial perfusion and improvement in angina episodes after administration of ranolazine [136].
6.2. Ivabradine
Funny channels are highly expressed in sinoatrial (SA) node myocytes control If current, an important ionic current in charge of pacemaker activity of SA node. Ivabradine as a selective If current blocker lowers heart rate and subsequently reduces myocardial oxygen demand and improves oxygen supply [137].
Even though ivabradine's therapeutic effects on obstructive CAD have been demonstrated in several studies [138], there is insufficient evidence to determine its efficacy on MVA. Improved Seattle Angina Questionnaire items and EuroQoL scale have been observed in MVA patients who have undergone ivabradine treatment in comparison with the placebo group [134]. In addition, ranolazine has shown more potential advantages than ivabradine in symptomatic relief and treatment satisfaction in Villano et al.'s study [134]. Also, another randomized controlled trial demonstrated that ivabradine has effectiveness in reducing angina symptoms; however, it could not enhance microvascular function [134]. This may highlight its subsequent bradycardia effect in improving anginal symptoms rather than the microvascular effect.
Thus, ivabradine represents a novel and effective therapeutic modalities for MVA management; however, more clinical trials are warranted to empower such conclusions.
6.3. Fasudil
Fasudil, HA-1077, is an inhibitor of Rho-kinase, which mediates vascular smooth muscle, endothelial, and inflammatory cell function [139]. Preclinical studies demonstrated the substantial role of fasudil in inhibiting leukocyte-endothelial cell interactions via impaired neutrophil adhesion and chemotaxis [140]. In addition, it is reported that fasudil inhibits lipopolysaccharide (LPS)-triggered apoptosis of endothelial cells in pulmonary microvasculature by blocking c-Jun N-terminal Kinase (JNK) and mitogen-activated protein kinase (MAPK) pathway [141]. Rho-kinase inhibitors also diminish neutrophil-induced increase in endothelium permeability [142].
Even though the major therapeutic use of fasudil is pulmonary arterial hypertension treatment, a few studies report beneficial effects of this drug on vasospastic, microvascular, and stable effort angina. Fasudil therapy administered intracoronary (300 μg/min for 15 min) decreases pacing-induced angina symptoms, the magnitude of ST depression, and the lactate production [143]. Another study revealed that fasudil ameliorates ACh-induced myocardial infarction (p < 0.01) and lactate production (p = 0.0125) in patients with angina and normal coronary angiogram [144].
Taken together, even though there are reports of targeting Rho-kinase for the treatment of microvascular angina, further supporting evidence is mandatory.
7. Miscellaneous Medications
7.1. Trimetazidine
In low oxygen supply conditions, shifting from glucose oxidation to free fatty acid (FFA) beta-oxidation leads to greater oxygen consumption, intracellular acidosis, and ROS formation [145, 146]. Trimetazidine by inhibiting the long-chain of 3-ketoacyl coenzyme A thiolase (LC 3-KAT) and inducing pyruvate dehydrogenase activity suppresses FFA beta-oxidation and restores homeostasis between glucose oxidation and glycolysis [146, 147].
Discordant results have been reported about the efficacy of trimetazidine in the treatment of MVA. Nalbantgil et al. examined the effect of trimetazidine on 35 patients with nonobstructive CAD in a placebo-controlled, double-blind study. They have found no change in heart rate, blood pressure at rest, peak exercise, and the time of 1 mm ST segment depression by trimetazidine intake; however, prolonged total exercise time and time to 1 mm ST depression have been noted in the trimetazidine-treated group compared with placebo [148]. Also, the study by Leonova et al. concluded that adding trimetazidine to standard therapy led to improved symptoms, quality of life, and exercise tolerance via increased myocardial perfusion and endothelial function [149]. Nevertheless, Leonardo et al. have noted no significant effects for trimetazidine in patients with MVA [35].
7.2. Proton Pump Inhibitors
According to a hypothesis, exposure of distal esophageal mucosa to gastric acid triggers esophagocardiac reflex, leading to coronary vasoconstriction [150]. The development of CAD symptoms with normal epicardial coronary artery angiogram in these patients shows that antiacid medications may be efficient in the treatment of MVA [151, 152].
Dietrich et al. conducted a study on 72 patients with MVA with gastroesophageal reflux disease (GERD) to assess the effect of proton pump inhibitor (PPI) on relieving angina. After eight weeks of PPI therapy in doubled standard dose, statistically significant improvement has been achieved in intensity, frequency, and duration of symptoms (p < 0.001) [153]. Although the study has shown the therapeutic effect of PPI on MVA, to understand the exact relation between GERD and MVA and to confirm PPI efficacy in the treatment of non-obstructive CAD in patients simultaneously suffering from acid reflux disease, further assessment is required.
7.3. Thiazolidinediones
Thiazolidinediones, a class of antidiabetic medications used in type-2 diabetes mellitus, have been demonstrated to be efficient in improving endothelial dysfunction through modulation of oxidative processes [154, 155]. A considerable reduction in the levels of C-reactive protein, endothelin-1, and ADMA and consequently flow-mediated dilatation have been reported in patients with metabolic syndrome after 8 weeks of rosiglitazone administration [156].
Although positive effects of thiazolidinediones on endothelial dysfunction have been determined in several studies; however, their effects on patients with MVA have not been examined.
7.4. Metformin
Metformin as an antidiabetic agent commonly prescribed for type-2 diabetes has been shown to be beneficial in reducing myocardial infarction in diabetic patients [157]. The role of metformin on endothelial function in diabetic patients has been investigated and shown to have significant improvement in acetylcholine-stimulated flows compared with placebo [158]. A randomized double-blind placebo-controlled study by Jadhav et al. assessed the effect of metformin in nondiabetic women patients with a normal coronary angiography but positive exercise tolerance test. It was concluded that metformin could improve vascular function and decrease myocardial ischemia [159]. However, the mechanism involved and larger trials with longer follow-ups need further research.
7.5. SGLT Inhibitors
Sodium-glucose cotransporter (SGLT) channel 2 inhibitors as an antidiabetic medication have proven to have beneficial effects on cardiovascular outcomes including cardiovascular death and heart failure in people with or without diabetes [160–162]. An in vitro study investigated the effects of these inhibitors on endothelium and suggested that observed cardiovascular benefits of them may be due to their action on endothelium other than mentioned myocardial and renal effects [163]. Further research assessing their effect on MVA in human studies is warranted.
7.6. Endothelin Receptor Antagonist
Endothelin (ET-1) increases vascular tone and causes vasoconstriction, especially in coronary arteries [164, 165]. ETA and ETb are two receptors mediating ET-1, from which ETA is involved in the coronary vasoconstriction [166, 167]. It has been shown that circulating ET-1 is increased in MVA patients which can have negative effects on vascular function and reduce CFR [15, 168].
Due to the possible effects of endothelin receptors antagonist on MVA, a randomized double-blind controlled trial investigated the effects of ET-1 receptor antagonist atrasentan on patients with multiple cardiovascular risk factors, nonobstructive CAD, and coronary endothelial dysfunction. It concluded that 6-month treatment with atrasentan could enhance coronary microvascular endothelial function [169]. The possible genetic role of ET-1 in the pathogenesis of coronary microvascular dysfunction has been investigated as well. It is reported that ET-1 dysregulation may be the cause of disease and opens up the opportunity for further research for precision medicine using the gene therapy [170].
7.7. Xanthine Derivatives
Adenosine, besides its role in blood flow regulation through vasodilatory effects, is the major mediator of ischemic pain perception [171, 172]. As mentioned, pain hypersensitivity is one of the pathophysiologic mechanisms proposed for the pathogenesis of MVA, which can be modulated by xanthine derivatives as adenosine receptor blockers [172]. Another illustrated mechanism for the antianginal effect of xanthine derivatives is redistribution of blood flow toward ischemic myocardial areas through constriction of nondysfunctional microvasculature induced by inhibition of the vasodilatory effect of the adenosine [173].
A double-blind crossover study has indicated beneficial effects on exercise-induced angina in MVA patients who had received oral aminophylline for three weeks. However, no difference between aminophylline-treated patients and placebo group in frequency of ST depression and peak exercise ST depression has been noted [174]. Afterward, another small study demonstrated that aminophylline infusion could lengthen the time before the occurrence of ischemia in patients with cardiac syndrome X during the treadmill exercise test, in addition to beneficial effects on exercise-induced chest pain [175]. A few clinical trials with a limited number of participants have reported improvements in exercise-induced angina and ischemic ECG changes in MVA patients who underwent xanthine derivative treatment [176–178]. These results reveal the need for further research on the management of MVA pain sensitivity via xanthine derivatives.
Allopurinol, a xanthine oxidase inhibitor, has established a positive role in myocardial function improvement in ischemic injury and endothelial dysfunction [179]. The study by Erdogan et al. demonstrated that lower serum uric acid is associated with higher CFR and better coronary microvascular function, suggesting the administration of allopurinol for MVA. A single trial on 19 patients receiving either high-dose allopurinol or placebo was conducted. Despite a reduced serum BNP level in the allopurinol group, there was no difference in maximum exercise time, coronary flow reserve, and flow-mediated vasodilatation of the brachial artery [180].
7.8. Potassium-Channel Opener (Nicorandil)
Hyperpolarization induced by the opening of K+ channels in cell membranes leads to arterioles' smooth muscle relaxation and subsequently blood flow augmentation in ischemic areas [181]. Additionally, nicorandil, a potassium-channel opener, causes vasodilation through its nitrate property [182].
Nicorandil treatment was significantly associated with longer total exercise time and prolonged time to 1 mm ST depression (p = 0.036 and 0.026, respectively) compared with placebo treatment [183]. It also improved coronary flow reserve in patients with angina and normal coronary arteriograms, although the study had no control group [184]. Finally, a recent meta-analysis on the effects of nicorandil on MVA patients showed its potential of improving angina symptoms, ECG, and endothelial dysfunction. However, due to low-quality evidence among analyzed studies, clinical benefits remained unclear [185].
Although larger studies with sufficient follow-up are required, potassium-channel openers appear to be highly potent antiangina drugs that can be used in the treatment of MVA.
7.9. Imipramine
Imipramine, a tricyclic antidepressant (TCA), has been shown to elevate the pain threshold through the inhibition of serotonin and adrenaline reuptake [186]. Imipramine analgesic effect might be helpful for MVA patients, particularly in the presence of enhanced painful perception.
In a randomized, double-blind, placebo-controlled trial, the effect of imipramine in patients with chest pain and normal coronary angiogram was tested, which resulted in a statistically significant reduction in angina episodes in imipramine-treated patients in comparison with the placebo group (1 ± 86 vs. 52 ± 25, p = 0.03) [187]. Cox et al. also reported significantly fewer chest pain episodes during treatment compared to placebo in patients with angina and normal epicardial coronary arteries (11 (3–22) vs. 21 (16–28)—median (interquartile range); p = 0.01). However, 83% of the treated group have shown imipramine side effects including dry mouth, dizziness, nausea, and constipation, and even three patients had to be withdrawn from the study [188].
Although imipramine causes a significant reduction in chest pain episodes in patients with angina and normal angiogram, especially in whom remain symptomatic despite conventional antianginal therapy, the high incidence of side effects limited its use in MVA management.
7.10. Omega-3
Omega-3 fatty acids as polyunsaturated fatty acids (PUFAs) have beneficial effects on vascular integrity and endothelial dysfunction by modifying inflammatory cytokine expression and inhibiting oxidative stress [189, 190].
Bozcali et al. evaluated the effect of 4-month treatment with omega-3 in MVA patients. They have reported a substantial increase in flow-mediated dilation (from 47 ± 48 to 104 ± 23%, p < 0.05) and nitroglycerin-mediated dilatation (from 51 ± 53 to 93 ± 35%, p < 0.05), and malondialdehyde, a marker for oxidative stress, significantly decreases (4.4 ± 0.86 to 3.35 ± 0.33 mmol/L, p = 0.012) in patients who had received omega-3. Significant improvement in MVA symptoms also has been noted in omega-3-treated group [191]. During stress echocardiography, MVA patients had fewer repetitions of ST-segment depression when pretreated with n-3 PUFAs [192].
Thus, omega-3 has shown to be a favorable treatment in patients with nonobstructive CAD. However, further assessment is warranted to qualify for omega-3 efficacy in the management of MVA.
7.11. Incretin
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that improves glycemic control through stimulating insulin secretion, inhibiting glucagon release and appetite suppression [193, 194]. Laboratory studies have proposed several positive cardiovascular effects for GLP-1. A 5 h incubation of human umbilical vein endothelial cell (HUVEC) cultures with liraglutide (0.1–100 μg/mL) a GLP-1 receptor agonist-induced endothelial NO synthase phosphorylation and subsequently increased NO production via a 5′AMP-activated protein kinase (AMPK)-dependent pathway [195]. Moreover, reduced ROS and vascular cell adhesion molecule-1 (VCAM-1) mRNA expression have been reported in HUVEC treated with GLP-1 (0.03 and 0.3 nmol/L for 4 h) following exposure to advanced glycation end products (100 μg/mL glycated bovine serum albumin) [196].
Clinical studies also demonstrated favorable effect of incretin on coronary and forearm blood flow. Basu et al. have shown enhancement in ACh (2–8 μg/100 mL)-induced forearm blood flow in healthy volunteers (n = 10) undergoing GLP-1 infusion (1.2 pmol/kg/min), whereas GLP-1 did not affect sodium nitroprusside–regulated blood flow (0.5–2 μg/100 mL) [197]. Furthermore, improved endothelial function after GLP-1 infusion (2 pmol/kg/min) has been noted in 12 fasted patients with type-2 diabetes mellitus and stable CAD [198]. In contrast, a trial in overweight participants showed no effect of intact GLP-1, protected from dipeptidyl-peptidase 4 mediated degradation on coronary microcirculation [199].
Although incretin seems to have therapeutic properties for MVA management, clinical studies are warranted to demonstrate its efficacy and safety in patients with MVA.
7.12. NAC
N-acetylcysteine (NAC), a thiol, is a prodrug to L-cysteine, which converts to the biologic antioxidant, and glutathione. Hence, NAC modifies NO half-life and potentiates the activity of NO by forming NO adducts [200–202].
Andrews et al. conducted a study on 16 patients undergoing cardiac catheterization (nine without obstructive lesion in epicardial coronary artery) testing the effect of NAC on ACh-mediated coronary vasodilation. They have shown 36 ± 11% (p = 0.02) elevation in coronary and femoral blood flow after NAC administration [203].
Taken together, NAC potentially is an effective medication to manage MVA, but more pieces of evidence are required to support its efficacy.
8. Recent Trials on MVA
Besides the mentioned treatments, there are some newly-introduced ones that should be considered and investigated in detail. A summary of treatment type, duration of treatment, and main findings in recently published trials on the management of MVA are available in Table 1. Mentioned trials are very insufficient to draw conclusions about the optimal drug in the treatment of MVA, and the need for new trials with larger sample sizes and investigation of different drugs is strongly recommended.
Table 1.
Recently-published articles on management of MVA.
| Study | Design | Treatment | Duration | Results | Ref |
|---|---|---|---|---|---|
| Henry et al. [206] | Pilot clinical trial | Autologous CD34+ stem cell therapy | 180 days | (i) Improved coronary flow reserve (2.08 ± 0.32 changed to 2.68 ± 0.79; p < 0.005) (ii) Decreased angina frequency (p < 0.004) (iii) Improved Canadian cardiovascular society class (p < 0.001) (iv) Improved quality of life (p ≤ 0.04) |
[206] |
|
| |||||
| Zhang et al. [55] | RCT | Group 1: fluvastatin (statin; 40 mg daily) Group 2: diltiazem (CCB; 90 mg daily) Group 3: statin and CCB |
90 days | (i) Improved coronary flow reserve (23.2%, 12.4%, and 29.1% in groups 1 to 3, respectively; p < 0.05) (ii) Increased time to 1 mm ST segment depression (241 ± 97 to 410 ± 140 s, p < 0.05 in group 1; 258 ± 91 to 392 ± 124 s, p < 0.05 in group 2, and 250 ± 104 to 446 ± 164 s, p < 0.05 in group 3) |
[55] |
|
| |||||
| Kabaklić et al. [207] | Pilot RCT | Atorvastatin (20 mg daily) | 90 and 180 days | (i) Improved flow-mediated dilation (p < 0.001 for 90 and 180 days) (ii) No difference in reactive hyperemia index (iii) Insignificant improvement in rate-normalized augmentation index (p = 0.077) |
[207] |
|
| |||||
| Makarewicz-Wujec et al. [208] | RCT | DASH diet | 12 months | (i) Insignificant reduction in RANTES (42.7 ± 21.1 to 38.1 ± 18.5, p = 0.134) (ii) Reduced CXCL4 (12.38 ± 4.1 to 8.36 ± 2.3, p < 0.001) |
[208] |
Ref: reference; RCT: randomized controlled trial; CCB: calcium channel blocker; DASH: dietary approaches to stop hypertension.
9. Conclusion
Although the heterogeneity in the pathophysiology of MVA makes it difficult to control the disease in a fixed pattern, traditional anti-ischemic medications are the first line of MVA pharmacologic management. Beta-blockers, statins, ACEI/ARBs, and CCBs are the most efficient classes of drugs and should still remain the first choice of MVA treatment. If single-drug therapy is not effective, a combination of beta-blockers or statins with CCBs can be proposed (Figure 2). Also, in patients with variable thresholds of exercise-induced angina, combination therapy can be the first choice. A notable finding that differentiates the treatment of MVA from other normal-angiogram anginas like vasospastic angina is that nitrates have not been shown to work effectively in patients with MVA, yet PDE-5 inhibitors including sildenafil had therapeutic efficacy in this group. In the case of insufficient control of symptoms with the first-line medications, other available drugs including nicorandil, estrogen, imipramine, PPIs, and fasudil adjusted to patients' characteristics, can be considered (Figure 2). Novel antianginal drugs such as ranolazine or ivabradine and xanthine derivatives have been represented as the last resources for pharmacologic treatment in patients with refractory MVA [204, 205] (Table 2). Some medications are anticipated to arise as highly effective drugs in controlling MVA, including omega-3 fatty acids and vitamin D. Besides, some medications like incretin have mechanistic rationale to improve MVA, but there is no clinical study to test their efficacy in the treatment of MVA. Proposed steps of treatment for MVA are illustrated in Figure 2. In addition, the classification of drugs for the management of MVA and their proposed mechanisms are available in Table 2.
Figure 2.

Proposed pharmacologic management algorithm for patients with microvascular angina.
Table 2.
Classification of drugs for management of microvascular angina and their proposed mechanisms; (a) first line therapy or synergistic combination therapy; (b) effective treatments with lower level of evidence; (c) less effective drugs.
(a) First-line therapy or synergistic combination therapy
| Medication class | Example | Mechanism of action |
|---|---|---|
| Beta-blockers | (i) Propranolol (ii) Atenolol (iii) Carvedilol (iv) Nebivolol |
(i) Lowering heart rate, myocardial contractility, blood pressure, and oxygen consumption (ii) Endothelium-dependent vasodilatory effects through increasing plasma NO (endothelium-dependent) |
|
| ||
| Statins | (i) Pravastatin (ii) Fluvastatin |
(i) Improving endothelial function through increasing NO bioavailability (endothelium-dependent and anatomical) |
|
| ||
| ACEI/ARBs | (i) Enalapril (ii) Quinapril (iii) Irbesartan (iv) Eplerenone |
(i) Vasoconstriction through increasing superoxide production by its effect on nicotinamide adenine dinucleotide phosphate -NADPH- and nicotinamide adenine dinucleotide (endothelium-dependent) (ii) Stimulating NO production by lowering bradykinin degradation |
|
| ||
| Calcium channel blockers | (i) Amlodipine (ii) Nifedipine (iii) Verapamil (iv) Diltiazem |
(i) Negative inotropic and vasodilatory effects (reducing microvascular tone and relieving spasm), therefore, reducing afterload (endothelium-dependent) (ii) Protecting endothelium against free radical injuries (endothelium-independent) (iii) Increasing nitrate |
|
| ||
| Antiplatelet | (i) Aspirin | (i) Inhibition of thromboxane A2 (TXA2) (endothelium-dependent) |
(b) Effective treatments with lower level of evidence
| Medication class | Example | Mechanism of action |
|---|---|---|
| Nitric oxide modulators | (i) L-arginine (ii) Sildenafil (iii) Cilostazol (iv) Tetrahydrobiopterin |
(i) Vasodilation induced by nitrates through activation of the guanylyl cyclase signaling pathway (endothelium-dependent) |
|
| ||
| Hormonal drugs | (i) Estrogen | (i) Accelerating reendothelialization (endothelium-independent) (ii) Inhibiting endothelial cell apoptosis |
| (ii) Vitamin D | (iii) Decreased vascular inflammation and improving endothelial function (endothelium-independent) | |
|
| ||
| Novel antianginal | (i) Ivabradine | (i) Lowering heart rate and reducing myocardial oxygen demand |
| (ii) Fasudil | (ii) Mediates vascular smooth muscle, endothelial, and inflammatory cell function | |
|
| ||
| Miscellaneous | (i) PPI | (i) Inhibiting esophagocardiac reflex which leads to coronary vasoconstriction (endothelium-dependent) |
| (ii) Metformin | (ii) Significant improvement in acetylcholine-stimulated flows (endothelium-dependent) | |
| (iii) SGLT inhibitors | (iii) Action on endothelium-not know yet (endothelium-dependent) | |
| (iv) Endothelin receptor antagonist | (iv) Decreasing vascular tone and causing vasodilation (endothelium-dependent) | |
| (v) Nicorandil | (v) Arterioles' smooth muscle relaxation (vi) Vasodilation through nitrate |
|
| (vi) Imipramine | (vii) Elevating pain threshold | |
| (vii) Omega-3 | (viii) Modifying inflammatory cytokine expression and inhibiting oxidative stress (endothelium-independent) | |
| (viii) Incretin | (ix) Endothelial nitric oxide synthase (eNOS) phosphorylation and increasing NO | |
| (ix) NAC | (xi) Modifying NO half-life and potentiates the activity of NO | |
(c) Less effective drugs
| Medication class | Example | Mechanism of action |
|---|---|---|
| Nitric oxide modulators | (i) Nitrates | (i) Vasodilation induced by nitrates through activation of the guanylyl cyclase signaling pathway (endothelium-dependent) |
|
| ||
| Alpha-blockers | (i) Doxazosin | (i) Sympatholytic capacity which causes a reduction in the microvascular tone (endothelium-dependent) |
|
| ||
| Novel antianginal | (i) Ranolazine | (i) Inhibiting late sodium current in cardiomyocytes (ii) Improving endothelial function (endothelium-dependent) |
|
| ||
| Miscellaneous | (i) Trimetazidine | (i) Inhibiting the long-chain of 3-ketoacyl coenzyme A thiolase |
| (ii) Thiazolidinediones | (i) Improving endothelial dysfunction through modulation of oxidative processes (endothelium-independent) | |
| (iii) Xanthine derivatives | (i) Vasodilation (endothelium-dependent) (ii) Mediating ischemic pain perception |
|
NO: nitric oxide; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blockers; NADPH: nicotinamide adenine dinucleotide phosphate; SGLT: sodium-glucose cotransporter; NAC: N-acetylcysteine.
To sum up, MVA seems to have received insufficient attention from clinicians and researchers up to now. However, according to our investigations, beta-blockers, statins, ACEI/ARBs, and CCBs in addition to risk factor control may be helpful in the management of MVA. The only way to assess the efficacy and safety of suggested treatments for MVA is through large clinical trials. We hope that future trials on the treatment of this tricky yet burdensome cardiovascular issue will yield definite therapeutic strategies.
Ethical Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflicts of Interest
All authors declare that they do not have any conflicts of interest.
References
- 1.Kemp H. G. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. The American Journal of Cardiology . 1973;32(3):375–376. doi: 10.1016/S0002-9149(73)80150-X. [DOI] [PubMed] [Google Scholar]
- 2.Ong P., Camici P. G., Beltrame J. F., et al. International standardization of diagnostic criteria for microvascular angina. International Journal of Cardiology . 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 3.Reis S. E., Holubkov R., Conrad Smith A. J., et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. American Heart Journal . 2001;141(5):735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 4.Patel M. R., Peterson E. D., Dai D., et al. Low diagnostic yield of elective coronary angiography. New England Journal of Medicine . 2010;362(10):886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran D., Young R., Adlam D., et al. Coronary microvascular dysfunction in patients with stable coronary artery disease: the CE-MARC 2 coronary physiology sub-study. International Journal of Cardiology . 2018;266:7–14. doi: 10.1016/j.ijcard.2018.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masoudkabir F., Vasheghani-Farahani A., Hakki E., et al. Novel scoring system for prediction of cardiac syndrome X in women with typical angina and a positive exercise tolerance test. Texas Heart Institute Journal . 2018;45(1):5–10. doi: 10.14503/THIJ-16-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safdar B., D'Onofrio G., Dziura J., Russell R. R., Johnson C., Sinusas A. J. Prevalence and characteristics of coronary microvascular dysfunction among chest pain patients in the emergency department. European Heart Journal Acute Cardiovascular Care . 2020;9(1):5–13. doi: 10.1177/2048872618764418. [DOI] [PubMed] [Google Scholar]
- 8.Gulati M., Cooper-DeHoff R. M., McClure C., et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Archives of Internal Medicine . 2009;169(9):843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutledge T. Chest Pain with Normal Coronary Arteries . Springer; 2013. Impaired quality of life–causes, assessment and management; pp. 277–285. [Google Scholar]
- 10.Parsyan A., Pilote L. Cardiac syndrome X: mystery continues. Canadian Journal of Cardiology . 2012;28(2):S3–S6. doi: 10.1016/j.cjca.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Bottcher M., Botker H. E., Sonne H., Nielsen T. T., Czernin J. Endothelium-dependent and -independent perfusion reserve and the effect of L-arginine on myocardial perfusion in patients with syndrome X. Circulation . 1999;99(14):1795–1801. doi: 10.1161/01.CIR.99.14.1795. [DOI] [PubMed] [Google Scholar]
- 12.Zeiher A. M., Krause T., Schächinger V., Minners J., Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation . 1995;91(9):2345–2352. doi: 10.1161/01.CIR.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 13.Egashira K., Inou T., Hirooka Y., Yamada A., Urabe Y., Takeshita A. Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. New England Journal of Medicine . 1993;328(23):1659–1664. doi: 10.1056/NEJM199306103282302. [DOI] [PubMed] [Google Scholar]
- 14.Kaski J. C., Cox I. D., Crook J. R., et al. Differential plasma endothelin levels in subgroups of patients with angina and angiographically normal coronary arteries. American Heart Journal . 1998;136(3):412–417. doi: 10.1016/S0002-8703(98)70214-5. [DOI] [PubMed] [Google Scholar]
- 15.Cox I. D., Bøtker H. E., Bagger J. P., Sonne H. S., Kristensen B. Ø., Kaski J. C. Elevated endothelin concentrations are associated with reduced coronary vasomotor responses in patients with chest pain and normal coronary arteriograms. Journal of the American College of Cardiology . 1999;34(2):455–460. doi: 10.1016/S0735-1097(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 16.Ford T. J., Rocchiccioli P., Good R., et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. European Heart Journal . 2018;39(46):4086–4097. doi: 10.1093/eurheartj/ehy529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong P., Athanasiadis A., Borgulya G., Mahrholdt H., Kaski J. C., Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries: the ACOVA Study (abnormal coronary vasomotion in patients with stable angina and unobstructed coronary arteries) Journal of the American College of Cardiology . 2012;59(7):655–662. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Aziz A., Hansen H. S., Sechtem U., Prescott E., Ong P. Sex-related differences in vasomotor function in patients with angina and unobstructed coronary arteries. Journal of the American College of Cardiology . 2017;70(19):2349–2358. doi: 10.1016/j.jacc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Schroder J., Zethner-Moller R., Bové K. B., et al. Protein biomarkers and coronary microvascular dilatation assessed by rubidium-82 PET in women with angina pectoris and no obstructive coronary artery disease. Atherosclerosis . 2018;275:319–327. doi: 10.1016/j.atherosclerosis.2018.06.864. [DOI] [PubMed] [Google Scholar]
- 20.Tritto I., Zuchi C., Ambrosio G. Chest Pain with Normal Coronary Arteries . Springer; 2013. Microvascular angina in different clinical conditions: diabetes and the metabolic syndrome; pp. 137–148. [Google Scholar]
- 21.Cosín-Sales J., Pizzi C., Brown S., Kaski J. C. C-reactive protein, clinical presentation, and ischemic activity in patients with chest pain and normal coronary angiograms. Journal of the American College of Cardiology . 2003;41(9):1468–1474. doi: 10.1016/S0735-1097(03)00243-2. [DOI] [PubMed] [Google Scholar]
- 22.Vancheri F., Longo G., Vancheri S., Henein M. Coronary microvascular dysfunction. Journal of Clinical Medicine . 2020;9(9):p. 2880. doi: 10.3390/jcm9092880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaski J. C. Overview of gender aspects of cardiac syndrome X. Cardiovascular Research . 2002;53(3):620–626. doi: 10.1016/S0008-6363(01)00460-6. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H., Takeyama Y., Koba S., Suwa Y., Katagiri T. Small vessel pathology and coronary hemodynamics in patients with microvascular angina. International Journal of Cardiology . 1994;43(2):139–150. doi: 10.1016/0167-5273(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 25.Mosseri M., Yarom R., Gotsman M., Hasin Y. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation . 1986;74(5):964–972. doi: 10.1161/01.CIR.74.5.964. [DOI] [PubMed] [Google Scholar]
- 26.Pasceri V., Lanza G. A., Buffon A., Montenero A. S., Crea F., Maseri A. Role of abnormal pain sensitivity and behavioral factors in determining chest pain in syndrome X. Journal of the American College of Cardiology . 1998;31(1):62–66. doi: 10.1016/S0735-1097(97)00421-X. [DOI] [PubMed] [Google Scholar]
- 27.Lanza G. A., Giordano A., Pristipino C., et al. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I] metaiodobenzylguanidine myocardial scintigraphy. Circulation . 1997;96(3):821–826. doi: 10.1161/01.CIR.96.3.821. [DOI] [PubMed] [Google Scholar]
- 28.Koren W., Koldanov R., Peleg E., Rabinowitz B., Rosenthal T. Enhanced red cell sodium-hydrogen exchange in microvascular angina. European Heart Journal . 1997;18(8):1296–1299. doi: 10.1093/oxfordjournals.eurheartj.a015441. [DOI] [PubMed] [Google Scholar]
- 29.Hill J. M., Zalos G., Halcox J. P., et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New England Journal of Medicine . 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 30.Potts S. G., Bass C. Chest Pain with Normal Coronary Angiograms: Pathogenesis, Diagnosis and Management . Springer; 1999. Chest pain with normal coronary arteries: psychological aspects; pp. 13–32. [Google Scholar]
- 31.Khuddus M. A., Pepine C. J., Handberg E. M., et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Journal of Interventional Cardiology . 2010;23(6):511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frishman W. H. β-Adrenergic blockade in cardiovascular disease. Journal of Cardiovascular Pharmacology and Therapeutics . 2013;18(4):310–319. doi: 10.1177/1074248413484986. [DOI] [PubMed] [Google Scholar]
- 33.Yusuf S., Wittes J., Friedman L. Overview of results of randomized clinical trials in heart disease: I. Treatments following myocardial infarction. JAMA . 1988;260(14):2088–2093. doi: 10.1001/jama.1988.03410140100032. [DOI] [PubMed] [Google Scholar]
- 34.Bugiardini R., Borghi A., Biagetti L., Puddu P. Comparison of verapamil versus propranolol therapy in syndrome X. The American Journal of Cardiology . 1989;63(5):286–290. doi: 10.1016/0002-9149(89)90332-9. [DOI] [PubMed] [Google Scholar]
- 35.Leonardo F., Fragasso G., Rossetti E., et al. Comparison of trimetazidine with atenolol in patients with syndrome X: effects on diastolic function and exercise tolerance. Cardiologia . 1999;44(12):1065–1069. [PubMed] [Google Scholar]
- 36.Lanza G. A., Colonna G., Pasceri V., Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. The American Journal of Cardiology . 1999;84(7):854–856. doi: 10.1016/S0002-9149(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 37.Erbil M. K., Poyraz F., Okyay K., Turfan M., Cemri M. Nebivolol therapy improves endothelial function and increases exercise tolerance in patients with cardiac syndrome X. Anatolian Journal of Cardiology . 2009;9(5) [PubMed] [Google Scholar]
- 38.Togni M., Vigorito F., Windecker S., et al. Does the beta-blocker nebivolol increase coronary flow reserve? Cardiovascular Drugs and Therapy . 2007;21(2):99–108. doi: 10.1007/s10557-006-0494-7. [DOI] [PubMed] [Google Scholar]
- 39.Kaski J. C., Rodriguez-Plaza L., Brown J., Maseri A. Efficacy of carvedilol (BM14, 190), a new beta-blocking drug with vasodilating properties, in exercise-induced ischemia. The American Journal of Cardiology . 1985;56(1):35–40. doi: 10.1016/0002-9149(85)90562-4. [DOI] [PubMed] [Google Scholar]
- 40.Kalinowski L., Dobrucki L. W., Szczepanska-Konkel M., et al. Third-generation β-blockers stimulate nitric oxide release from endothelial cells through ATP efflux. Circulation . 2003;107(21):2747–2752. doi: 10.1161/01.CIR.0000066912.58385.DE. [DOI] [PubMed] [Google Scholar]
- 41.Maffei A., Di Pardo A., Carangi R., et al. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension . 2007;50(4):652–656. doi: 10.1161/HYPERTENSIONAHA.107.094458. [DOI] [PubMed] [Google Scholar]
- 42.Neglia D., De Maria R., Masi S., et al. Effects of long-term treatment with carvedilol on myocardial blood flow in idiopathic dilated cardiomyopathy. Heart . 2007;93(7):808–813. doi: 10.1136/hrt.2006.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason R. P., Kalinowski L., Jacob R. F., Jacoby A. M., Malinski T. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation . 2005;112(24):3795–3801. doi: 10.1161/CIRCULATIONAHA.105.556233. [DOI] [PubMed] [Google Scholar]
- 44.Parker J. D., Parker J. O. Stable angina pectoris: the medical management of symptomatic myocardial ischemia. The Canadian Journal of Cardiology . 2012;28(2):S70–S80. doi: 10.1016/j.cjca.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Mak I. T., Boehme P., Weglicki W. B. Protective effects of calcium channel blockers against free radical-impaired endothelial cell proliferation. Biochemical Pharmacology . 1995;50(9):1531–1534. doi: 10.1016/0006-2952(95)02039-X. [DOI] [PubMed] [Google Scholar]
- 46.Mak I. T., Boehme P., Weglicki W. B. Antioxidant effects of calcium channel blockers against free radical injury in endothelial cells. Correlation of protection with preservation of glutathione levels. Circulation Research . 1992;70(6):1099–1103. doi: 10.1161/01.RES.70.6.1099. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X., Hintze T. H. Amlodipine releases nitric oxide from canine coronary microvessels an unexpected mechanism of action of a calcium channel–blocking agent. Circulation . 1998;97(6):576–580. doi: 10.1161/01.CIR.97.6.576. [DOI] [PubMed] [Google Scholar]
- 48.McLvor M. E., Undemir C., Lawson J., Reddinger J. Clinical effects and utility of intracoronary diltiazem. Catheterization and Cardiovascular Diagnosis . 1995;35(4):287–291. doi: 10.1002/ccd.1810350402. [DOI] [PubMed] [Google Scholar]
- 49.Nishigaki K., Inoue Y., Yamanouchi Y., et al. Prognostic effects of calcium channel blockers in patients with vasospastic angina--a meta-analysis. Circulation Journal . 2010;74(9):1943–1950. doi: 10.1253/circj.CJ-10-0292. [DOI] [PubMed] [Google Scholar]
- 50.Cannon R. O., Watson R. M., Rosing D. R., Epstein S. E. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. The American Journal of Cardiology . 1985;56(4):242–246. doi: 10.1016/0002-9149(85)90842-2. [DOI] [PubMed] [Google Scholar]
- 51.Özçelik F., Altun A., Özbay G. Antianginal and anti-ischemic effects of nisoldipine and ramipril in patients with syndrome X. Clinical Cardiology . 1999;22(5):361–365. doi: 10.1002/clc.4960220513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Held C., Iqbal R., Lear S. A., et al. Physical activity levels, ownership of goods promoting sedentary behaviour and risk of myocardial infarction: results of the INTERHEART study. European Heart Journal . 2012;33(4):452–466. doi: 10.1093/eurheartj/ehr432. [DOI] [PubMed] [Google Scholar]
- 53.Sütsch G., Oechslin E., Mayer I., Hess O. M. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. International Journal of Cardiology . 1995;52(2):135–143. doi: 10.1016/0167-5273(95)02458-9. [DOI] [PubMed] [Google Scholar]
- 54.Bairey Merz C. N., Pepine C. J., Shimokawa H., Berry C. Treatment of coronary microvascular dysfunction. Cardiovascular Research . 2020;116(4):856–870. doi: 10.1093/cvr/cvaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X., Li Q., Zhao J., et al. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coronary Artery Disease . 2014;25(1):40–44. doi: 10.1097/MCA.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 56.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation . 2004;109(23_suppl_1) doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 57.Tousoulis D., Antoniades C., Vassiliadou C., et al. Effects of combined administration of low dose atorvastatin and vitamin E on inflammatory markers and endothelial function in patients with heart failure. European Journal of Heart Failure . 2005;7(7):1126–1132. doi: 10.1016/j.ejheart.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Anderson T. J., Meredith I. T., Yeung A. C., Frei B., Selwyn A. P., Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. The New England Journal of Medicine . 1995;332(8):488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 59.Nanayakkara P. W., Van Guldener C., Ter Wee P. M., et al. Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on carotid intima-media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: results from the Anti-Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Archives of Internal Medicine . 2007;167(12):1262–1270. doi: 10.1001/archinte.167.12.1262. [DOI] [PubMed] [Google Scholar]
- 60.Mäki-Petäjä K. M., Booth A. D., Hall F. C., et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. Journal of the American College of Cardiology . 2007;50(9):852–858. doi: 10.1016/j.jacc.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 61.Reriani M. K., Dunlay S. M., Gupta B., et al. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. European Journal of Cardiovascular Prevention and Rehabilitation . 2011;18(5):704–716. doi: 10.1177/1741826711398430. [DOI] [PubMed] [Google Scholar]
- 62.Fábián E., Varga A., Picano E., Vajo Z., Rónaszéki A., Csanády M. Effect of simvastatin on endothelial function in cardiac syndrome X patients. The American Journal of Cardiology . 2004;94(5):652–655. doi: 10.1016/j.amjcard.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 63.Kayikcioglu M., Payzin S., Yavuzgil O., Kultursay H., Can L. H., Soydan I. Benefits of statin treatment in cardiac syndrome-X. European Heart Journal . 2003;24(22):1999–2005. doi: 10.1016/S0195-668X(03)00478-0. [DOI] [PubMed] [Google Scholar]
- 64.Rajagopalan S., Kurz S., Münzel T., et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. Journal of Clinical Investigation . 1996;97(8):1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griendling K. K., Minieri C. A., Ollerenshaw J. D., Alexander R. W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation Research . 1994;74(6):1141–1148. doi: 10.1161/01.RES.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 66.Hornig B., Landmesser U., Kohler C., et al. Comparative effect of ACE inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease. Circulation . 2001;103(6):799–805. doi: 10.1161/01.CIR.103.6.799. [DOI] [PubMed] [Google Scholar]
- 67.Wassmann S., Hilgers S., Laufs U., Böhm M., Nickenig G. Angiotensin II type 1 receptor antagonism improves hypercholesterolemia-associated endothelial dysfunction. Arteriosclerosis, Thrombosis, and Vascular Biology . 2002;22(7):1208–1212. doi: 10.1161/01.ATV.0000022847.38083.B6. [DOI] [PubMed] [Google Scholar]
- 68.Chen J.-W., Hsu N.-W., Wu T.-C., Lin S.-J., Chang M.-S. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. The American Journal of Cardiology . 2002;90(9):974–982. doi: 10.1016/S0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 69.Taddei S., Virdis A., Ghiadoni L., Mattei P., Salvetti A. Effects of angiotensin converting enzyme inhibition on endothelium-dependent vasodilatation in essential hypertensive patients. Journal of Hypertension . 1998;16(4):447–456. doi: 10.1097/00004872-199816040-00006. [DOI] [PubMed] [Google Scholar]
- 70.Higashi Y., Sasaki S., Nakagawa K., et al. A comparison of angiotensin-converting enzyme inhibitors, calcium antagonists, beta-blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: a multicenter study. Journal of the American College of Cardiology . 2000;35(2):284–291. doi: 10.1016/S0735-1097(99)00561-6. [DOI] [PubMed] [Google Scholar]
- 71.Koifman B., Topilski I., Megidish R., et al. Effects of Losartan \+ l-Arginine on Nitric Oxide Production, Endothelial Cell Function, and Hemodynamic Variables in Patients With Heart Failure Secondary to Coronary Heart Disease. The American Journal of Cardiology . 2006;98(2):172–177. doi: 10.1016/j.amjcard.2006.01.085. [DOI] [PubMed] [Google Scholar]
- 72.Pauly D. F., Johnson B. D., Anderson R. D., et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE) American Heart Journal . 2011;162(4):678–684. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masuda D., Nohara R., Tamaki N., et al. Evaluation of coronary blood flow reserve by 13N-NH3 positron emission computed tomography (PET) with dipyridamole in the treatment of hypertension with the ACE inhibitor (cilazapril) Annals of Nuclear Medicine . 2000;14(5):353–360. doi: 10.1007/BF02988695. [DOI] [PubMed] [Google Scholar]
- 74.Kawata T., Daimon M., Hasegawa R., et al. Effect on coronary flow velocity reserve in patients with type 2 diabetes mellitus: comparison between angiotensin-converting enzyme inhibitor and angiotensin II type 1 receptor antagonist. American Heart Journal . 2006;151(4):798.e9–798.e15. doi: 10.1016/j.ahj.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Russell S. J., Di Stefano E. M., Naffati M. T., Brown O., Saltissi S. The effects of the angiotensin II receptor (type I) antagonist irbesartan in patients with cardiac syndrome X. Heart . 2007;93(2):253–254. doi: 10.1136/hrt.2006.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bavry A. A., Handberg E. M., Huo T., et al. Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: an ancillary study of the national heart, lung, and blood institute-sponsored women's ischemia syndrome evaluation. American Heart Journal . 2014;167(6):826–832. doi: 10.1016/j.ahj.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Handberg E. M., Merz C. N. B., Cooper-Dehoff R. M., et al. Rationale and design of the Women's Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. American Heart Journal . 2021;237:90–103. doi: 10.1016/j.ahj.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 78.Nossaman V. E., Nossaman B. D., Kadowitz P. J. Nitrates and nitrites in the treatment of ischemic cardiac disease. Cardiology in Review . 2010;18(4):190–197. doi: 10.1097/CRD.0b013e3181c8e14a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torfgård K. E., Ahlner J. Nitrates Updated . Springer; 1997. Mechanisms of action of nitrates; pp. 21–56. [DOI] [Google Scholar]
- 80.Harrison D. G., Bates J. N. The nitrovasodilators. New ideas about old drugs. Circulation . 1993;87(5):1461–1467. doi: 10.1161/01.CIR.87.5.1461. [DOI] [PubMed] [Google Scholar]
- 81.Wu M., Villano A., Russo G., et al. Poor tolerance and limited effects of isosorbide-5-mononitrate in microvascular angina. Cardiology . 2015;130(4):201–206. doi: 10.1159/000370027. [DOI] [PubMed] [Google Scholar]
- 82.Russo G., Di Franco A., Lamendola P., et al. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovascular Drugs and Therapy . 2013;27(3):229–234. doi: 10.1007/s10557-013-6439-z. [DOI] [PubMed] [Google Scholar]
- 83.Kaski J. C., Rosano G. M., Collins P., Nihoyannopoulos P., Maseri A., Poole-Wilson P. A. Cardiac syndrome X: clinical characteristics and left ventricular function: long-term follow-up study. Journal of the American College of Cardiology . 1995;25(4):807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 84.Thomas G. R., DiFabio J. M., Gori T., Parker J. D. Once Daily Therapy With Isosorbide-5-Mononitrate Causes Endothelial Dysfunction in Humans: Evidence of a Free-Radical-Mediated Mechanism. Journal of the American College of Cardiology . 2007;49(12):1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 85.Oelze M., Knorr M., Kröller-Schön S., et al. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress and a marked increase in vascular endothelin-1 expression. Free Radical Biology & Medicine . 2011;51:p. S161. doi: 10.1016/j.freeradbiomed.2011.10.223. [DOI] [PubMed] [Google Scholar]
- 86.Daiber A., Münzel T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxidants & Redox Signaling . 2015;23(11):899–942. doi: 10.1089/ars.2015.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larsen W., Mandleco B. Chest pain with angiographic clear coronary arteries: a provider's approach to cardiac syndrome X. Journal of the American Academy of Nurse Practitioners . 2009;21(7):371–376. doi: 10.1111/j.1745-7599.2009.00425.x. [DOI] [PubMed] [Google Scholar]
- 88.Lu T. M., Lee T. S., Lin S. J., Chan W. L., Hsu C. P. The prognostic value of asymmetric dimethylarginine in patients with cardiac syndrome X. PLoS One . 2017;12(12, article e0188995) doi: 10.1371/journal.pone.0188995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piatti P., Fragasso G., Monti L. D., et al. Acute intravenous L-arginine infusion decreases endothelin-1 levels and improves endothelial function in patients with angina pectoris and normal coronary arteriograms: correlation with asymmetric dimethylarginine levels. Circulation . 2003;107(3):429–436. doi: 10.1161/01.CIR.0000046489.24563.79. [DOI] [PubMed] [Google Scholar]
- 90.Porro B., Eligini S., Veglia F., et al. Nitric oxide synthetic pathway in patients with microvascular angina and its relations with oxidative stress. Oxidative Medicine and Cellular Longevity . 2014;2014:9. doi: 10.1155/2014/726539.726539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gellman J., Hare J. M., Lowenstein C. J., et al. L-arginine ameliorates the abnormal sympathetic response of the dysfunctional human coronary microvasculature. Angiology . 2004;55(1):1–8. doi: 10.1177/000331970405500101. [DOI] [PubMed] [Google Scholar]
- 92.Palloshi A., Fragasso G., Piatti P., et al. Effect of oral L-arginine on blood pressure and symptoms and endothelial function in patients with systemic hypertension, positive exercise tests, and normal coronary arteries. The American Journal of Cardiology . 2004;93(7):933–935. doi: 10.1016/j.amjcard.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 93.Egashira K., Hirooka Y., Kuga T., Mohri M., Takeshita A. Effects ofl-Arginine supplementation on endothelium-dependent coronary vasodilation in patients with angina pectoris and normal coronary arteriograms. Circulation . 1996;94(2):130–134. doi: 10.1161/01.CIR.94.2.130. [DOI] [PubMed] [Google Scholar]
- 94.Lerman A., Burnett J. C., Jr., Higano S. T., McKinley L. J., Holmes D. R., Jr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation . 1998;97(21):2123–2128. doi: 10.1161/01.CIR.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 95.Serio R., Zizzo M. G., Mulè F. Nitric oxide induces muscular relaxation via cyclic GMP-dependent and -independent mechanisms in the longitudinal muscle of the mouse duodenum. Nitric Oxide . 2003;8(1):48–52. doi: 10.1016/S1089-8603(02)00144-1. [DOI] [PubMed] [Google Scholar]
- 96.Kimura M., Higashi Y., Hara K., et al. PDE5 inhibitor sildenafil citrate augments endothelium-dependent vasodilation in smokers. Hypertension . 2003;41(5):1106–1110. doi: 10.1161/01.HYP.0000068202.42431.CC. [DOI] [PubMed] [Google Scholar]
- 97.Mandosi E., Giannetta E., Filardi T., et al. Endothelial dysfunction markers as a therapeutic target for sildenafil treatment and effects on metabolic control in type 2 diabetes. Expert Opinion on Therapeutic Targets . 2015;19(12):1617–1622. doi: 10.1517/14728222.2015.1066337. [DOI] [PubMed] [Google Scholar]
- 98.McLaughlin K., Lytvyn Y., Luca M. C., Liuni A., Gori T., Parker J. D. Repeated daily dosing with sildenafil provides sustained protection from endothelial dysfunction caused by ischemia and reperfusion: a human in vivo study. American Journal of Physiology. Heart and Circulatory Physiology . 2014;307(6):H888–H894. doi: 10.1152/ajpheart.00215.2014. [DOI] [PubMed] [Google Scholar]
- 99.Gori T., Sicuro S., Dragoni S., Donati G., Forconi S., Parker J. D. Sildenafil prevents endothelial dysfunction induced by ischemia and reperfusion via opening of adenosine triphosphate–sensitive potassium channels a human in vivo study. Circulation . 2005;111(6):742–746. doi: 10.1161/01.CIR.0000155252.23933.2D. [DOI] [PubMed] [Google Scholar]
- 100.Gillies H. C., Roblin D., Jackson G. Coronary and systemic hemodynamic effects of sildenafil citrate: from basic science to clinical studies in patients with cardiovascular disease. International Journal of Cardiology . 2002;86(2-3):131–141. doi: 10.1016/S0167-5273(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 101.Denardo S. J., Wen X., Handberg E. M., et al. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a Women's Ischemia Syndrome Evaluation (WISE) ancillary study. Clinical Cardiology . 2011;34(8):483–487. doi: 10.1002/clc.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kukreja R. C. Cardiovascular protection with sildenafil following chronic inhibition of nitric oxide synthase. British Journal of Pharmacology . 2007;150(5):538–540. doi: 10.1038/sj.bjp.0707132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoo S. Y., Song S. G., Lee J. H., et al. Efficacy of cilostazol on uncontrolled coronary vasospastic angina: a pilot study. Cardiovascular Therapeutics . 2013;31(3):179–185. doi: 10.1111/j.1755-5922.2012.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shin E. S., Lee J. H., Yoo S. Y., et al. A randomised, multicentre, double blind, placebo controlled trial to evaluate the efficacy and safety of cilostazol in patients with vasospastic angina. Heart . 2014;100(19):1531–1536. doi: 10.1136/heartjnl-2014-305986. [DOI] [PubMed] [Google Scholar]
- 105.Sakai N., Kaufman S., Milstein S. Tetrahydrobiopterin is required for cytokine-induced nitric oxide production in a murine macrophage cell line (RAW 264) Molecular Pharmacology . 1993;43(1):6–10. [PubMed] [Google Scholar]
- 106.Davis M. D., Kaufman S. Products of the tyrosine-dependent oxidation of tetrahydrobiopterin by rat liver phenylalanine hydroxylase. Archives of Biochemistry and Biophysics . 1993;304(1):9–16. doi: 10.1006/abbi.1993.1315. [DOI] [PubMed] [Google Scholar]
- 107.Heitzer T., Brockhoff C., Mayer B., et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers. Circulation Research . 2000;86(2):e36–e41. doi: 10.1161/01.RES.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 108.Heitzer T., Krohn K., Albers S., Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type II diabetes mellitus. Diabetologia . 2000;43(11):1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 109.Stroes E., Kastelein J., Cosentino F., et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. Journal of Clinical Investigation . 1997;99(1):41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Higashi Y., Sasaki S., Nakagawa K., et al. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis . 2006;186(2):390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 111.Cosentino F., Hürlimann D., Gatti C. D., et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart . 2008;94(4):487–492. doi: 10.1136/hrt.2007.122184. [DOI] [PubMed] [Google Scholar]
- 112.Nash D. Alpha-adrenergic blockers: mechanism of action, blood pressure control, and effects on lipoprotein metabolism. Clinical Cardiology . 1990;13(11):764–772. doi: 10.1002/clc.4960131104. [DOI] [PubMed] [Google Scholar]
- 113.Bøtker H. E., Sonne H. S., Schmitz O., Nielsen T. T. Effects of doxazosin on exercise-induced angina pectoris, ST-segment depression, and insulin sensitivity in patients with syndrome X. The American Journal of Cardiology . 1998;82(11):1352–1356. doi: 10.1016/S0002-9149(98)00640-7. [DOI] [PubMed] [Google Scholar]
- 114.Galassi A. R., Kaski J. C., Pupita G., Vejar M., Crea F., Maseri A. Lack of evidence for alpha-adrenergic receptor-mediated mechanisms in the genesis of ischemia in syndrome X. The American Journal of Cardiology . 1989;64(5):264–269. doi: 10.1016/0002-9149(89)90517-1. [DOI] [PubMed] [Google Scholar]
- 115.Vincent J., Dachman W., Blaschke T. F., Hoffman B. B. Pharmacological tolerance to alpha 1-adrenergic receptor antagonism mediated by terazosin in humans. Journal of Clinical Investigation . 1992;90(5):1763–1768. doi: 10.1172/JCI116050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spyridopoulos I., Sullivan A. B., Kearney M., Isner J. M., Losordo D. W. Estrogen-receptor–mediated inhibition of human endothelial cell apoptosis. Circulation . 1997;95(6):1505–1514. doi: 10.1161/01.CIR.95.6.1505. [DOI] [PubMed] [Google Scholar]
- 117.Iwakura A., Luedemann C., Shastry S., et al. Estrogen-mediated, endothelial nitric oxide synthase–dependent mobilization of bone marrow–derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation . 2003;108(25):3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 118.Simoncini T., Genazzani A. R., Liao J. K. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation . 2002;105(11):1368–1373. doi: 10.1161/hc1102.105267. [DOI] [PubMed] [Google Scholar]
- 119.WsHIS C. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women's Health Initiative Randomized Controlled Trial. ACC Current Journal Review . 2004;13(6):p. 19. doi: 10.1016/j.accreview.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 120.Yu J., Eto M., Kozaki K., Akishita M., Okabe T., Ouchi Y. Raloxifene analogue LY117018 suppresses oxidative stress-induced endothelial cell apoptosis through activation of ERK1/2 signaling pathway. European Journal of Pharmacology . 2008;589(1-3):32–36. doi: 10.1016/j.ejphar.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 121.Ross G. M., Peters N. S., Lefroy D., et al. 17-beta-estradiol therapy lessens angina in postmenopausal women with syndrome X. Journal of the American College of Cardiology . 1996;28(6):1500–1505. doi: 10.1016/S0735-1097(96)00348-8. [DOI] [PubMed] [Google Scholar]
- 122.Albertsson P. A., Emanuelsson H., Milsom I. Beneficial effect of treatment with transdermal estradiol-17-β on exercise- induced angina and ST segment depression in syndrome X. International Journal of Cardiology . 1996;54(1):13–20. doi: 10.1016/0167-5273(96)02560-0. [DOI] [PubMed] [Google Scholar]
- 123.Merz C. N., Olson M. B., McClure C., et al. A randomized controlled trial of low-dose hormone therapy on myocardial ischemia in postmenopausal women with no obstructive coronary artery disease: results from the National Institutes of Health/National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) American Heart Journal . 2010;159(6):987.e1–987.e7. doi: 10.1016/j.ahj.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jhund P. S., Dawson N., Davie A. P., et al. Attenuation of endothelin-1 induced vasoconstriction by 17β estradiol is not sustained during long-term therapy in postmenopausal women with coronary heart disease. Journal of the American College of Cardiology . 2001;37(5):1367–1373. doi: 10.1016/S0735-1097(01)01168-8. [DOI] [PubMed] [Google Scholar]
- 125.el Maaty M. A., Gad M. Z. Vitamin D deficiency and cardiovascular disease: potential mechanisms and novel perspectives. Journal of Nutritional Science and Vitaminology . 2013;59(6):479–488. doi: 10.3177/jnsv.59.479. [DOI] [PubMed] [Google Scholar]
- 126.Tarcin O., Yavuz D. G., Ozben B., et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. The Journal of Clinical Endocrinology & Metabolism . 2009;94(10):4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 127.Caglar F. T., Ungan I., Ksanski V., et al. Evaluation of serum vitamin D levels in patients with X syndrome. European Review for Medical and Pharmacological Sciences . 2016;20(6):1155–1160. [PubMed] [Google Scholar]
- 128.Güler G. B., Güler E., Hatipoğlu S., et al. Assessment of 25-OH vitamin D levels and abnormal blood pressure response in female patients with cardiac syndrome X. Anatolian Journal of Cardiology/Anadolu Kardiyoloji Dergisi. . 2016;16(12):961–966. doi: 10.14744/AnatolJCardiol.2016.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Andishmand A., Ansari Z., Soltani M., Mirshamsi H., Raafat S. Vitamin D replacement therapy in patients with cardiac syndrome X. Perfusion . 2015;30(1):60–63. doi: 10.1177/0267659114526629. [DOI] [PubMed] [Google Scholar]
- 130.Hasenfuss G., Maier L. Mechanism of action of the new anti-ischemia drug ranolazine. Clinical Research in Cardiology . 2008;97(4):222–226. doi: 10.1007/s00392-007-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lamendola P., Nerla R., Pitocco D., et al. Effect of ranolazine on arterial endothelial function in patients with type 2 diabetes mellitus. Atherosclerosis . 2013;226(1):157–160. doi: 10.1016/j.atherosclerosis.2012.10.051. [DOI] [PubMed] [Google Scholar]
- 132.Keating G. M. Ranolazine. Drugs . 2008;68(17):2483–2503. doi: 10.2165/0003495-200868170-00006. [DOI] [PubMed] [Google Scholar]
- 133.Mehta P. K., Goykhman P., Thomson L. E., et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC: Cardiovascular Imaging . 2011;4(5):514–522. doi: 10.1016/j.jcmg.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Villano A., Di Franco A., Nerla R., et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. The American Journal of Cardiology . 2013;112(1):8–13. doi: 10.1016/j.amjcard.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 135.Bairey Merz C. N., Handberg E. M., Shufelt C. L., et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. European Heart Journal . 2016;37(19):1504–1513. doi: 10.1093/eurheartj/ehv647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rambarat C. A., Elgendy I. Y., Handberg E. M., et al. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction: Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction ancillary study. International Journal of Cardiology . 2019;276:8–13. doi: 10.1016/j.ijcard.2018.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sulfi S., Timmis A. Ivabradine–the first selective sinus node If channel inhibitor in the treatment of stable angina. International Journal of Clinical Practice . 2006;60(2):222–228. doi: 10.1111/j.1742-1241.2006.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Borer J. S., Fox K., Jaillon P., Lerebours G., Ivabradine Investigators Group Antianginal and antiischemic effects of ivabradine, an IfInhibitor, in stable angina. Circulation . 2003;107(6):817–823. doi: 10.1161/01.CIR.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 139.Tawara S., Shimokawa H. Progress of the study of rho-kinase and future perspective of the inhibitor. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan . 2007;127(3):501–514. doi: 10.1248/yakushi.127.501. [DOI] [PubMed] [Google Scholar]
- 140.Wang J., Xu J., Zhao X., Xie W., Wang H., Kong H. Fasudil inhibits neutrophil-endothelial cell interactions by regulating the expressions of GRP78 and BMPR2. Experimental Cell Research . 2018;365(1):97–105. doi: 10.1016/j.yexcr.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 141.Liu H., Chen X., Han Y., et al. Rho kinase inhibition by fasudil suppresses lipopolysaccharide-induced apoptosis of rat pulmonary microvascular endothelial cells via JNK and p38 MAPK pathway. Biomedicine & Pharmacotherapy . 2014;68(3):267–275. doi: 10.1016/j.biopha.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 142.Breslin J. W., Yuan S. Y. Involvement of RhoA and Rho kinase in neutrophil-stimulated endothelial hyperpermeability. American Journal of Physiology. Heart and Circulatory Physiology . 2004;286(3):H1057–H1062. doi: 10.1152/ajpheart.00841.2003. [DOI] [PubMed] [Google Scholar]
- 143.Fukumoto Y., Mohri M., Inokuchi K., et al. Anti-ischemic effects of fasudil, a specific Rho-kinase inhibitor, in patients with stable effort angina. Journal of Cardiovascular Pharmacology . 2007;49(3):117–121. doi: 10.1097/FJC.0b013e31802ef532. [DOI] [PubMed] [Google Scholar]
- 144.Mohri M., Shimokawa H., Hirakawa Y., Masumoto A., Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. Journal of the American College of Cardiology . 2003;41(1):15–19. doi: 10.1016/S0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 145.Kantor P. F., Lucien A., Kozak R., Lopaschuk G. D. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circulation Research . 2000;86(5):580–588. doi: 10.1161/01.RES.86.5.580. [DOI] [PubMed] [Google Scholar]
- 146.Fantini E., Demaison L., Sentex E., Grynberg A., Athias P. Some biochemical aspects of the protective effect of trimetazidine on rat cardiomyocytes during hypoxia and reoxygenation. Journal of Molecular and Cellular Cardiology . 1994;26(8):949–958. doi: 10.1006/jmcc.1994.1116. [DOI] [PubMed] [Google Scholar]
- 147.Banach M., Okonski P., Rysz J., Drozdz J., Zaslonka J. The role of trimetazidine in the treatment of heart diseases . Poznan: Termedia Publishing House; 2006. [Google Scholar]
- 148.Nalbantgil S., Altintig A., Yilmaz H., Nalbantgil İ., Önder R. The effect of trimetazidine in the treatment of microvascular angina. International Journal of Angiology . 1999;8(1):40–43. doi: 10.1007/BF01616842. [DOI] [PubMed] [Google Scholar]
- 149.Leonova I. A., Boldueva S., Zakharova O., Gaykovaya L. P887Trimetazidine improves symptoms and reduces microvascular dysfunction in patients with microvascular angina. European Heart Journal . 2017;38(suppl_1) doi: 10.1093/eurheartj/ehx501.P887. [DOI] [Google Scholar]
- 150.Chauhan A., Petch M., Schofield P. Cardio-oesophageal reflex in humans as a mechanism for ‘linked angina’. European Heart Journal . 1996;17(3):407–413. doi: 10.1093/oxfordjournals.eurheartj.a014873. [DOI] [PubMed] [Google Scholar]
- 151.Kaski J. C. Pathophysiology and management of patients with chest pain and normal coronary arteriograms (cardiac syndrome X) Circulation . 2004;109(5):568–572. doi: 10.1161/01.CIR.0000116601.58103.62. [DOI] [PubMed] [Google Scholar]
- 152.Wong W. M. Use of proton pump inhibitor as a diagnostic test in NCCP. Journal of Gastroenterology and Hepatology . 2005;20(s3):S14–S17. doi: 10.1111/j.1440-1746.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- 153.Dietrich C. G., Laupichler S., Stanzel S., et al. Origin of and therapeutic approach to cardiac syndrome X: results of the proton pump inhibitor therapy for angina-like lingering pain trial (PITFALL trial) World Journal of Gastroenterology . 2008;14(42):6506–6512. doi: 10.3748/wjg.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Watanabe Y., Sunayama S., Shimada K., et al. Troglitazone improves endothelial dysfunction in patients with insulin resistance. Journal of Atherosclerosis and Thrombosis . 2000;7(3):159–163. doi: 10.5551/jat1994.7.159. [DOI] [PubMed] [Google Scholar]
- 155.Garg R., Kumbkarni Y., Aljada A., et al. Troglitazone reduces reactive oxygen species generation by leukocytes and lipid peroxidation and improves flow-mediated vasodilatation in obese subjects. Hypertension . 2000;36(3):430–435. doi: 10.1161/01.HYP.36.3.430. [DOI] [PubMed] [Google Scholar]
- 156.Wang T.-D., Chen W.-J., Cheng W.-C., Lin J.-W., Chen M.-F., Lee Y.-T. Relation of Improvement in Endothelium-Dependent Flow-Mediated Vasodilation After Rosiglitazone to Changes in Asymmetric Dimethylarginine, Endothelin-1, and C-Reactive Protein in Nondiabetic Patients With the Metabolic Syndrome. The American Journal of Cardiology . 2006;98(8):1057–1062. doi: 10.1016/j.amjcard.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 157.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) The Lancet . 1998;352(9131):854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 158.Mather K. J., Verma S., Anderson T. J. Improved endothelial function with metformin in type 2 diabetes mellitus. Journal of the American College of Cardiology . 2001;37(5):1344–1350. doi: 10.1016/S0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 159.Jadhav S., Ferrell W., Greer I. A., Petrie J. R., Cobbe S. M., Sattar N. Effects of Metformin on Microvascular Function and Exercise Tolerance in Women With Angina and Normal Coronary Arteries: A Randomized, Double-Blind, Placebo- Controlled Study. Journal of the American College of Cardiology . 2006;48(5):956–963. doi: 10.1016/j.jacc.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 160.McMurray J. J. V., Solomon S. D., Inzucchi S. E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. The New England Journal of Medicine . 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 161.Perkovic V., Jardine M. J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. The New England Journal of Medicine . 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 162.Zelniker T. A., Wiviott S. D., Raz I., et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet . 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 163.Venu V. K., El-Daly M., Saifeddine M., et al. Minimizing hyperglycemia-induced vascular endothelial dysfunction by inhibiting endothelial sodium-glucose cotransporter 2 and attenuating oxidative stress: implications for treating individuals with type 2 diabetes. Canadian Journal of Diabetes . 2019;43(7):510–514. doi: 10.1016/j.jcjd.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 164.Lüscher T. F., Richard V., Tschudi M., Yang Z. H., Boulanger C. Endothelial control of vascular tone in large and small coronary arteries. Journal of the American College of Cardiology . 1990;15(3):519–527. doi: 10.1016/0735-1097(90)90619-Z. [DOI] [PubMed] [Google Scholar]
- 165.Toyo-oka T., Aizawa T., Suzuki N., et al. Increased plasma level of endothelin-1 and coronary spasm induction in patients with vasospastic angina pectoris. Circulation . 1991;83(2):476–483. doi: 10.1161/01.CIR.83.2.476. [DOI] [PubMed] [Google Scholar]
- 166.Kyriakides Z. S., Kremastinos D. T., Bofilis E., Tousoulis D., Antoniadis A., Webb D. J. Endogenous endothelin maintains coronary artery tone by endothelin type A receptor stimulation in patients undergoing coronary arteriography. Heart . 2000;84(2):176–182. doi: 10.1136/heart.84.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Halcox J. P., Nour K. R., Zalos G., Quyyumi A. A. Coronary vasodilation and improvement in endothelial dysfunction with endothelin ET(A) receptor blockade. Circulation Research . 2001;89(11):969–976. doi: 10.1161/hh2301.100980. [DOI] [PubMed] [Google Scholar]
- 168.Kaski J. C., Elliott P. M. Angina pectoris and normal coronary arteriograms: clinical presentation and hemodynamic characteristics. The American Journal of Cardiology . 1995;76(13):35d–42d. doi: 10.1016/S0002-9149(99)80490-1. [DOI] [PubMed] [Google Scholar]
- 169.Reriani M., Raichlin E., Prasad A., et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation . 2010;122(10):958–966. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ford T. J., Corcoran D., Padmanabhan S., et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. European Heart Journal . 2020;41(34):3239–3252. doi: 10.1093/eurheartj/ehz915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wilson R. F., Wyche K., Christensen B. V., Zimmer S., Laxson D. D. Effects of adenosine on human coronary arterial circulation. Circulation . 1990;82(5):1595–1606. doi: 10.1161/01.CIR.82.5.1595. [DOI] [PubMed] [Google Scholar]
- 172.Crea F., Pupita G., Galassi A. R., et al. Role of adenosine in pathogenesis of anginal pain. Circulation . 1990;81(1):164–172. doi: 10.1161/01.CIR.81.1.164. [DOI] [PubMed] [Google Scholar]
- 173.Minamino T., Kitakaze M., Morioka T., et al. Bidirectional effects of aminophylline on myocardial ischemia. Circulation . 1995;92(5):1254–1260. doi: 10.1161/01.CIR.92.5.1254. [DOI] [PubMed] [Google Scholar]
- 174.Elliott P. M., Krzyzowska-Dickinson K., Calvino R., Hann C., Kaski J. C. Effect of oral aminophylline in patients with angina and normal coronary arteriograms (cardiac syndrome X) Heart . 1997;77(6):523–526. doi: 10.1136/hrt.77.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yeşildağ O., Yazici M., Yilmaz O., Uçar R., Sağkan O. The effect of aminophylline infusion on the exercise capacity in patients with syndrome X. Acta Cardiologica . 1999;54(6):335–337. [PubMed] [Google Scholar]
- 176.Emdin M., Picano E., Lattanzi F., l'Abbate A. Improved exercise capacity with acute aminophylline administration in patients with syndrome X. Journal of the American College of Cardiology . 1989;14(6):1450–1453. doi: 10.1016/0735-1097(89)90380-X. [DOI] [PubMed] [Google Scholar]
- 177.Lanza G., Gaspardone A., Pasceri V., et al. Effects of bamiphylline on exercise testing in patients with syndrome X. Giornale Italiano di Cardiologia . 1997;27(1):50–54. [PubMed] [Google Scholar]
- 178.Radice M., Giudici V., Pusineri E., et al. Different effects of acute administration of aminophylline and nitroglycerin on exercise capacity in patients with syndrome X. The American Journal of Cardiology . 1996;78(1):88–90. doi: 10.1016/S0002-9149(96)00231-7. [DOI] [PubMed] [Google Scholar]
- 179.Okafor O. N., Farrington K., Gorog D. A. Allopurinol as a therapeutic option in cardiovascular disease. Pharmacology & Therapeutics . 2017;172:139–150. doi: 10.1016/j.pharmthera.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 180.Lim T. K., Noman A., Choy A. M. J., Khan F., Struthers A. D., Lang C. C. The APEX trial: effects of allopurinol on exercise capacity, coronary and peripheral endothelial function, and natriuretic peptides in patients with cardiac syndrome X. Cardiovascular Therapeutics . 2018;36(1) doi: 10.1111/1755-5922.12311.e12311 [DOI] [PubMed] [Google Scholar]
- 181.Andersson K. E. Clinical pharmacology of potassium channel openers. Pharmacology & Toxicology . 1992;70(4):244–254. doi: 10.1111/j.1600-0773.1992.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 182.Nakae I., Matsumoto T., Horie H., et al. Effects of intravenous nicorandil on coronary circulation in humans: plasma concentration and action mechanism. Journal of Cardiovascular Pharmacology . 2000;35(6):919–925. doi: 10.1097/00005344-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 183.Chen J. W., Lee W. L., Hsu N. W., et al. Effects of short-term treatment of nicorandil on exercise-induced myocardial ischemia and abnormal cardiac autonomic activity in microvascular angina. The American Journal of Cardiology . 1997;80(1):32–38. doi: 10.1016/S0002-9149(97)00279-8. [DOI] [PubMed] [Google Scholar]
- 184.Yamabe H., Namura H., Yano T., et al. Effect of nicorandil on abnormal coronary flow reserve assessed by exercise 201Tl scintigraphy in patients with angina pectoris and nearly normal coronary arteriograms. Cardiovascular Drugs and Therapy . 1995;9(6):755–761. doi: 10.1007/BF00879868. [DOI] [PubMed] [Google Scholar]
- 185.Jia Q., Shi S., Yuan G., et al. The effect of nicorandil in patients with cardiac syndrome X: a meta-analysis of randomized controlled trials. Medicine . 2020;99(37, article e22167) doi: 10.1097/MD.0000000000022167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sindrup S. H., Jensen T. S. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain . 1999;83(3):389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 187.Cannon R. O., Quyyumi A. A., Mincemoyer R., et al. Imipramine in patients with chest pain despite normal coronary angiograms. The New England Journal of Medicine . 1994;330(20):1411–1417. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 188.Cox I., Hann C., Kaski J. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. European Heart Journal . 1998;19(2):250–254. doi: 10.1053/euhj.1997.0615. [DOI] [PubMed] [Google Scholar]
- 189.Ye D., Zhang D., Oltman C., Dellsperger K., Lee H.-C., VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. The Journal of Pharmacology and Experimental Therapeutics . 2002;303(2):768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- 190.Abeywardena M. Y., Head R. J. Longchain n− 3 polyunsaturated fatty acids and blood vessel function. Cardiovascular Research . 2001;52(3):361–371. doi: 10.1016/S0008-6363(01)00406-0. [DOI] [PubMed] [Google Scholar]
- 191.Bozcali E., Babalik E., Himmetoglu S., Mihmanli I., Toprak S. ω-3 fatty acid treatment in cardiac syndrome X: a double-blind, randomized, placebo-controlled clinical study. Coronary Artery Disease . 2013;24(4):328–333. doi: 10.1097/MCA.0b013e32835f3005. [DOI] [PubMed] [Google Scholar]
- 192.Gaibazzi N., Ziacchi V. Reversibility of stress-echo induced ST-segment depression by long-term oral n-3 PUFA supplementation in subjects with chest pain syndrome, normal wall motion at stress-echo and normal coronary angiogram. BMC Cardiovascular Disorders . 2004;4(1):p. 1. doi: 10.1186/1471-2261-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Haffner S. M., Lehto S., Rönnemaa T., Pyörälä K., Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. The New England Journal of Medicine . 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 194.Killilea T. Long-term consequences of type 2 diabetes mellitus: economic impact on society and managed care. The American Journal of Managed Care . 2002;8(16 Suppl):S441–S449. [PubMed] [Google Scholar]
- 195.Hattori Y., Jojima T., Tomizawa A., et al. RETRACTED ARTICLE: a glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia . 2010;53(10):2256–2263. doi: 10.1007/s00125-010-1831-8. [DOI] [PubMed] [Google Scholar]
- 196.Ishibashi Y., Matsui T., Takeuchi M., Yamagishi S.-i. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochemical and Biophysical Research Communications . 2010;391(3):1405–1408. doi: 10.1016/j.bbrc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 197.Basu A., Charkoudian N., Schrage W., Rizza R. A., Basu R., Joyner M. J. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. American Journal of Physiology. Endocrinology and Metabolism . 2007;293(5):E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 198.Nyström T., Gutniak M. K., Zhang Q., et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. American Journal of Physiology. Endocrinology and Metabolism . 2004;287(6):E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 199.Nilsson M., Bové K. B., Suhrs E., et al. The effect of DPP-4-protected GLP-1 (7-36) on coronary microvascular function in obese adults. IJC Heart & Vasculature . 2019;22:139–144. doi: 10.1016/j.ijcha.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stamler J. S., Slivka A. Biological chemistry of thiols in the vasculature and in vascular-related disease. Nutrition Reviews . 1996;54(1):1–30. doi: 10.1111/j.1753-4887.1996.tb03770.x. [DOI] [PubMed] [Google Scholar]
- 201.Feelisch M., Te Poel M., Zamora R., Deussen A., Moncada S. Understanding the controversy over the identity of EDRF. Nature . 1994;368(6466):62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- 202.Stamler J. S., Jaraki O., Osborne J., et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proceedings of the National Academy of Sciences . 1992;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Andrews N. P., Prasad A., Quyyumi A. A. N-acetylcysteine improves coronary and peripheral vascular function. Journal of the American College of Cardiology . 2001;37(1):117–123. doi: 10.1016/S0735-1097(00)01093-7. [DOI] [PubMed] [Google Scholar]
- 204.Lanza G. A., Crea F. Primary coronary microvascular dysfunction. Circulation . 2010;121(21):2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 205.Montalescot G., Sechtem U., Achenbach S., et al. 2013 ESC guidelines on the management of stable coronary artery disease. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Revista Española de Cardiología . 2014;67(2):p. 135. doi: 10.1016/j.rec.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 206.Henry T. D., Bairey Merz C. N., Wei J., et al. Autologous CD34+ stem cell therapy increases coronary flow reserve and reduces angina in patients with coronary microvascular dysfunction. Circulation. Cardiovascular Interventions . 2022;15(2, article e010802) doi: 10.1161/CIRCINTERVENTIONS.121.010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kabaklić A., Fras Z. Moderate-dose atorvastatin improves arterial endothelial function in patients with angina pectoris and normal coronary angiogram: a pilot study. Archives of Medical Science . 2017;13(4):827–836. doi: 10.5114/aoms.2017.68238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Makarewicz-Wujec M., Henzel J., Kruk M., et al. The effect of intensive dietary intervention on the level of RANTES and CXCL4 chemokines in patients with non-obstructive coronary artery disease: a randomised study. Biology . 2021;10(2):p. 156. doi: 10.3390/biology10020156. [DOI] [PMC free article] [PubMed] [Google Scholar]


