Abstract
A shortage of inpatient beds and nurses during the coronavirus disease 2019 pandemic has lent priority to safe same-day discharge after surgery. The minimally invasive nature of robotic surgery has allowed an increasing number of procedures to be done on an outpatient basis. Anesthetic management should be designed to complement the technical advantages of robotic surgery in facilitating early discharge.
Keywords: Robotic surgery, Outpatient/ambulatory surgery, Urologic surgery, Gynecologic surgery, Same-day discharge surgery
Key points
-
•
Robot-assisted surgical techniques are being used in an expanding number of procedures.
-
•
Generally good outcomes, or outcomes comparable with inpatient surgery, have been reported.
-
•
Known challenges and intraoperative management considerations include extremes of Trendelenburg positioning, CO2 insufflation, and having limited access to the patient after robot docking.
-
•
Risk factors associated with failure to achieve same-day discharge in robotic surgery include patient age, preexisting lung disease, occurrence of intraoperative complications, and surgery end time.
-
•
Awareness of risk factors is key to selecting patients for robotic surgery for same-day discharge.
Introduction: nature of the problem
Robotic surgery was initially developed in the 1990s as a military project that would allow for a remote surgeon to operate on wounded soldiers on a battlefield [1]. Since then, robotic surgery has gained popularity across a broad range of common surgical procedures: from general and colorectal surgery to urogynecologic procedures and mitral valve repairs [1]. In the field of general surgery, the use of robotic surgery accounted for only 1.8% of all procedures in 2012, and this increased to 15.1% by 2018 [2]. Robotic surgery is attractive for several reasons: like laparoscopic surgery, it offers smaller incisions, a lower risk of infection, a shorter hospital stay, and a shorter convalescence than its open counterpart [3]. In addition, unlike laparoscopic surgery, robotic surgery has the advantage of increasing surgical dexterity due to the increased degrees of freedom of the instruments. The increased dexterity allows for more precise dissection during the operation [4].
For the anesthesiologist, robotic surgery comes with the following challenges: steep Trendelenburg or reverse Trendelenburg to provide the best field of view for the surgeon, longer duration of pneumoperitoneum especially during the initial part of an operator’s learning curve, and limited access to the patient after robot docking [3,[5], [6], [7], [8]].
Despite the high initial costs of robotic surgery, the hope is that it will ultimately reduce health care costs by decreasing complications and reducing inpatient length of stay after surgery, thereby reducing hospital resource utilization and reducing risks of nosocomial infections [2,9], a benefit that has been highlighted by the recent coronavirus disease 2019 pandemic. The quick recovery associated with the minimally invasive nature of robotic surgery can allow for early discharge from the hospital, making it an attractive option for patients looking to reduce their time spent in health care facilities.
This article describes the role and challenges of anesthesia in promoting same-day discharge after robotic surgery: the indications, procedures, common complications, and management of postoperative care.
Indications/contraindications of ambulatory robotic surgery
To date, outpatient robotic surgery has been described for an expanding list of procedures as shown in Table 1 . Some procedures, such as colectomies, bariatric surgeries, and nephrectomies (including partial and living-related donor resections), have not hitherto been described in the peer review literature but are well established in select practices. In essence, the indications for ambulatory robot-assisted surgery will likely continue to grow rapidly with different institutions developing experience in various surgical procedures, and this is a list that is expected to evolve over time.
Table 1.
Robotic surgeries that have been described in the ambulatory setting
| General Surgery | Head and Neck | Gynecologic | Urologic | Orthopedic |
|---|---|---|---|---|
|
|
|
|
|
Commonly performed ambulatory robotic surgical procedures
General surgery: cholecystectomy [4], hernia repair [10], colectomy, bariatric surgery.
Head and neck surgery: facelift, thyroidectomy [11].
Gynecology: hysterectomy ± bilateral salpingo-oophorectomy, staging for endometrial or cervical cancer [[12], [13], [14], [15], [16]], salpingo-oophorectomy [13], tubal ligation [17], and sacrocolpopexy [18].
Urology: radical prostatectomy [19], nephrectomy (including partial and living-related donor resections) [20], pyeloplasty, adrenalectomy [21].
Pediatric surgery: Robotic outpatient pediatric surgery is mainly reported in urology in procedures such as pyeloplasty or extravesical ureteral reimplantation, but other procedures such as ureteroureterostomy and nephrectomy/heminephrectomy have been done as outpatients as well [22].
Orthopedic surgery: Orthopedic surgery such as total knee and hip arthroplasty: although not specifically described as outpatient procedures, successful discharge within 24 hours has been reported [23] and may move progressively toward quicker discharges.
Relative contraindications to robotic surgery
Although there are not any absolute contraindications to robotic surgery, one must review the suitability of the surgical goals and the ability of the patient to tolerate the hemodynamic changes associated with the pneumoperitoneum or extremes in positioning. As surgeons and anesthesia providers become more experienced and familiar with robot-assisted methods, however, the list of contraindications will also likely grow and dwindle over time.
Surgical factors that make robot-assisted surgery a poor choice will vary depending on the type of surgery. Relative contraindications to robot-assisted ventral hernia repairs, for instance, include strangulated or acutely incarcerated hernias, cirrhosis or ascites, prior open abdominal surgery, recurrent or complex ventral hernias, and redundant skin and soft tissue, although the degree to which each of these poses a contraindication to surgery will vary depending on surgical experience [24]. For colectomies and urologic and gynecologic procedures, these include a high likelihood of needing an open procedure, for instance, a laparotomy for tumor debulking or extensive adhesions [25].
Poor candidates for robotic surgery generally include patients who may not tolerate insufflation or extremes of positioning well, and this will vary based on surgical location. Certain cardiopulmonary conditions such as severe pulmonary hypertension, or congenital heart disease including single-ventricle physiology or intra/extracardiac shunts, may not tolerate a pneumoperitoneum well [26,27]. The presence of ascites may result in challenging port placement. In colectomies and gynecologic and urologic surgery, for instance, severe obesity or respiratory pathology such as chronic obstructive pulmonary disease (COPD) may lead to inadequate ventilation or oxygenation; the same considerations are less of a problem with bariatric surgery because of the reverse Trendelenburg positioning. However, this may result in decreased venous blood return and lead to hypotension that may be poorly tolerated in patients with severe carotid stenosis or cardiac disease [26,27]. Again, the extent to which these factors pose a limit to the use of a robotic approach depends on the severity of disease and surgical and anesthetic experience. Modifying, for instance, the speed of abdominal insufflation, or a shorter duration of Trendelenberg positioning with a faster surgeon, may enable surgery to proceed despite the aforementioned comorbidities.
Technique/procedure
Companies such as Medtronic, Johnson & Johnson, Stryker, Zimmer Biomet, Smith and Nephew, and NuVasive are currently manufacturing surgical robots. At present, Intuitive Surgical, which makes the da Vinci robot system, has the largest market share. For the purpose of describing the robot-assisted technique here, the authors refer to the da Vinci system.
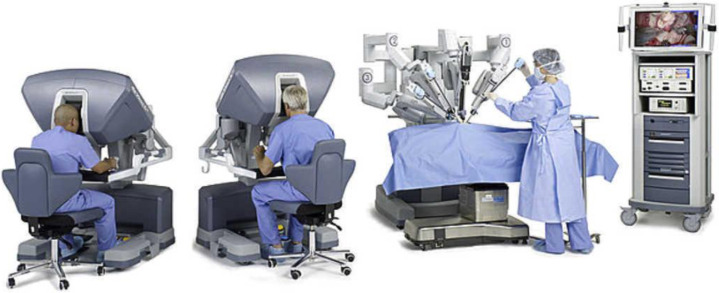
The da Vinci robot surgical system consists of 3 major components (https://www.intuitive.com/en-us/products-and-services/da-vinci/systems) (Fig. 1 ).
-
•
A console for the operating surgeon: where the surgeon sits to view the operating field and control the robot’s arms
-
•
A patient-side cart with 4 interactive robotic arms; also known as the surgical cart: instruments have 7 degrees of freedom
-
•
A vision cart including optical devices for the robotic camera (optical 3D vision tower): a viewscreen as well as computer equipment needed to integrate the optical channels and provide stereoscopic vision
Fig. 1.
The da Vinci robot surgical system consists of 1 or 2 surgical consoles, a patient cart, and a vision cart.
(From Pietrabissa A, et al. Cirugia robótica: controversias actuales y expectativas futuras.Cir Esp. 2013;91:67–71.)
Challenges for the anesthesiologist
As robotic surgery continues to gain popularity, the anesthesiologist is faced with challenges related to patient positioning and limited access to the patient after docking [28] (Tables 2 and 3 ), and pneumoperitoneum (Table 4 ).
Table 2.
Patient positioning: summary of challenges and management considerations
| Consideration | Consequence | Management |
|---|---|---|
| Heavy and bulky robot | Can contact the head of the patient if not positioned correctly | Make sure that the face of the patient is visible during surgery |
| Arms are tucked by the sides of patient | No access to arms once the robot is docked |
|
| Steep Trendelenburg: physiologic consequences |
|
|
| Steep Trendelenburg: physical consequences |
|
|
Abbreviations: ETT, endotracheal tube; IV, intravenous; V/Q, ventilation perfusion ratio.
Table 3.
Limited access to patient: summary of challenges and management considerations
| Consideration | Consequence | Management |
|---|---|---|
| Robot is over the chest and abdomen of the patient | If airway or cardiovascular events happen intraoperatively might delay intervention |
|
| Peripheral nerve and soft tissue injuries | Ensure proper pressure point padding |
Table 4.
Pneumoperitoneum: summary of challenges and management considerations
| Consideration | Consequence | Management |
|---|---|---|
| Long continuous insufflation with cold gas | Hypothermia | Ensure that the patient has a warming air blanket before docking |
| Venous air embolism |
|
|
|
|
|
| Pneumoperitoneum: physiologic consequences |
|
|
| Hypercarbia and respiratory acidosis | Consider pressure-controlled ventilation |
Abbreviations: LV, left ventricular; MAP, mean arterial pressure; SVR, systemic vascular resistance
Robotic surgery depends on establishing and maintaining the best possible field of view for the surgeon, which is partly achieved by insufflation of carbon dioxide to create a pneumoperitoneum and partly by positioning the patient in such a way that noninvolved organs move away from the surgical site. For bariatric surgery, cholecystectomy, and other upper abdominal procedures this means elevating the head and lowering the feet (extreme reverse Trendelenburg position). For prostatectomy, hysterectomy, and other lower abdominal surgeries this means the opposite: for much of the procedure the patient will be head down (see Fig. 1). A steep Trendelenberg is defined as a 30° to 40° head down position, and most operating room tables can achieve a maximum of a 45° tilt; for gynecologic surgery some investigators have reported that an average Trendelenburg angle of 28° was sufficient for surgical visualization [29]. For colectomy, nephrectomy, and other midabdominal operations the bed may remain in a neutral position or the position may be changed from one extreme to the other throughout the operation. Extremes of lateral tilt to the right or left may also occur and may vary over the course of the surgical dissection.
Proper patient positioning should allow for safe docking of the robot and adequate access to the robot arms and ports for the surgical assistant [3,30]. Although each case is different, the footprint of the robot, the desired bed position, the anticipated length of the procedure, and the body habitus of the patient should all be taken into account when docking the robot. It is sometimes useful to move the patient and bed through the full range of possible positions before making the surgical incision, to determine the safe limits of the procedure and likely intraoperative pressure points; this is especially true when dealing with unusual circumstances such as a patient with high body mass index scheduled for bariatric surgery in extreme reverse Trendelenburg position.
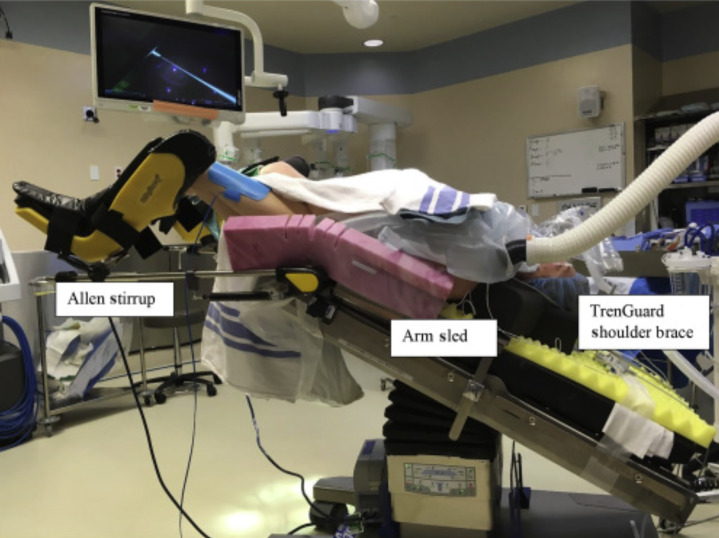
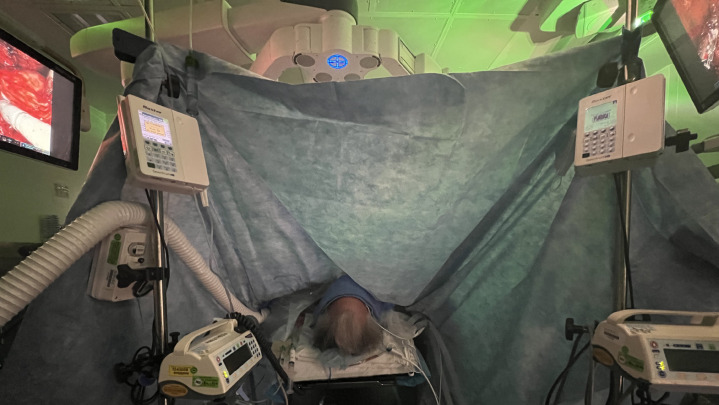
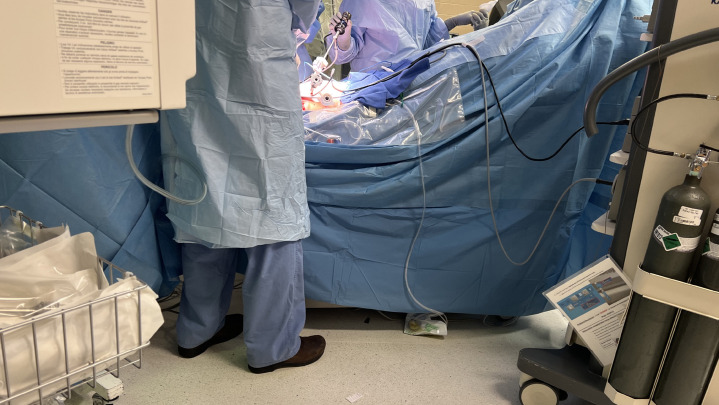
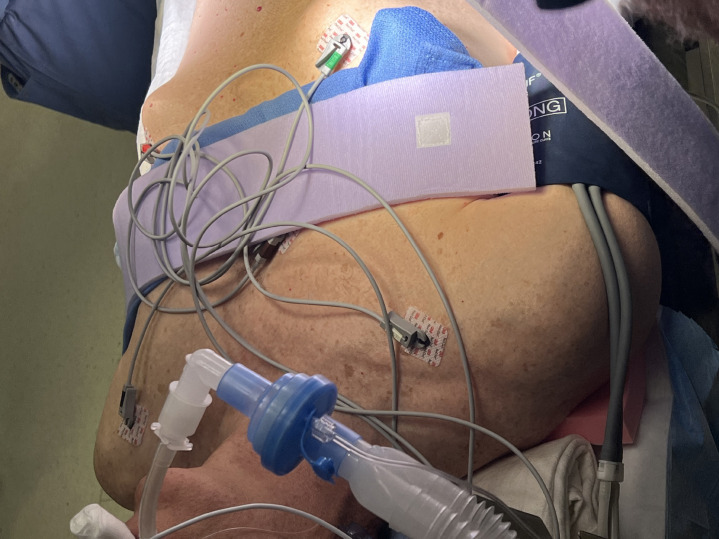
The robotic surgical equipment is heavy and bulky and can inadvertently place excess pressure on various parts of the body depending on the position of the surgery. For instance, when the surgery is in Trendelenburg position, it is important to monitor the face of the patient to avoid injury from the robot arms and to ensure that the height of surgical tables is adjusted to avoid impact with the patient when going into Trendelenburg or reverse Trendelenburg position (for instance, stands above the patient’s feet and steep head down positions) (Fig. 2 ). Furthermore, given the limited access to the patient and different spatial consideration of the operating room, the pressure points must be adequately padded before draping and docking the robot. A patient’s arms are often tucked by the side to facilitate robot docking, which means venous or arterial access cannot be easily monitored, for instance, for leaks or infiltration, and new access sites are not easily obtained once the robot is positioned (Fig. 3 ). The limited access to the patient also increases the risk of peripheral nerve and soft tissue injuries [31] and occult blood loss and can present challenges during emergent situations [3,28] (Fig. 4 ). Given the complexity of the room setup, it is crucial to have good communication between the team members.
Fig. 2.
Steep Trendelenberg positioning. Surgical tables are often placed over the patient’s head (or legs) and have to be adjusted to ensure they do not cause any injury to patients.
(From Lim PC, Kang E. How to prepare the patient for robotic surgery: before and during the operation. Best Pract Res Clin Obstet Gynaecol 2017 Nov;45:32-47.)
Fig. 3.
Limited access to patient’s head and airway.
Fig. 4.
Limited access to patients. Arms are tucked, venous/arterial access sites cannot be easily monitored for integrity, increases the risk of peripheral nerve, soft tissue injuries and occult blood loss, thus creating a challenge during emergencies.
To improve visualization for pelvic and lower abdominal surgeries, the patient is often required to be in a steep Trendelenburg with or without exaggerated lithotomy position [3,8,32]. Upper abdominal surgery requires a reverse Trendelenburg position with the robot docked above the head of the patient (Fig. 4). This position requires the head of the bed to be rotated away from the anesthesia machine further increasing the risk of injury to head and neck [28]. The physiologic effect seen in this position is mainly a consequence of venous pooling, which can lead to resistant hypotension [33]. Prolonged reverse Trendelenburg positioning, along with increased abdominal pressures from insufflation, has also been associated with a higher deep vein thrombosis (DVT) risk due to venous pooling in the legs, and attention should be paid to proper DVT prophylaxis.
The physiologic consequences associated with steep Trendelenburg position include downward movement of the diaphragm by abdominal contents. Together with the increased abdominal pressure from pneumoperitoneum, this leads to a decrease in pulmonary compliance and subsequently tidal volumes, a decrease in functional residual capacity and vital capacity, increase in peak and plateau airway pressures, and exacerbation of ventilation perfusion ratio mismatch [32]. Some studies have found that pressure-controlled ventilation will allow for lower peak airway and plateau pressures as well as greater pulmonary compliance [5].
Steep Trendelenburg positioning and pneumoperitoneum can also lead to an increase in intracranial pressure, cerebral blood flow, and intraocular pressure [6]. Some investigators have reported erroneous pulse oximeter readings resulting from high venous pressures in the ear lobe induced by Trendelenburg positioning, and suggest that if possible, the pulse oximeter should be placed on a finger instead [7]. Prolonged time spent in steep Trendelenburg may also lead to upper airway, periorbital, and brain edema [8]. The cephalad movement of the diaphragm can lead to the displacement of the endotracheal tube and subsequent mainstem intubation. If not well secured in the bed, the patient may shift position during the course of the surgery, creating a risk for friction burns to the skin, nerve injury to the extremities, and even fall from the operating room table [34].
Pneumoperitoneum
Pneumoperitoneum for robotic surgery has similar consequences to that of laparoscopic surgery (see Table 4). However, especially during the early parts of a surgical team’s learning curve, the patient is exposed to these consequences for longer periods given the longer duration of the cases. For instance, the risk of hypothermia is increased secondary to insufflation with cold gas (CO2) for a prolonged period [34,35].
Fluid shifts, changes in venous return (preload), and afterload may lead to hemodynamic compromise in patients with preexisting cardiopulmonary disease. Factors that contribute to a decrease in cardiac output include [34]:
-
•
Decrease in renal, splanchnic, and portal flow
-
•
Increased systemic vascular resistance
-
•
Increased mean arterial pressure: both from direct compression and from an increase in renin-angiotensin system activation that ultimately results in increased circulating vasopressin levels [34]
Prolonged carbon dioxide (CO2) insufflation may result in postoperative hypercarbia and respiratory acidosis especially in patients with COPD in whom the efficiency of CO2 elimination is decreased [32].
Finally, consequences such as venous air embolisms [36], subcutaneous emphysema [37], pneumothorax, pneumomediastinum, and pneumopericardium are rare but may result in hemodynamic collapse [38,39]. Providers should remain vigilant for any of these potential complications and should have plans in place to deal with each.
Management goals
Preoperative assessment
In addition to the standard preoperative assessment, providers should have a good understanding of the ability of the patient to tolerate consequences related to steep Trendelenburg positioning and pneumoperitoneum. For instance, patients with underlying lung disease or morbid obesity might be difficult to ventilate and therefore poor candidates for outpatient robotic surgery [3,8]. Special attention should be paid to patients with a history of glaucoma who are scheduled for lower abdominal procedures, given the potential increase in intraocular pressure [5]. Patients with congenital heart disease or significant cardiovascular disease may not tolerate changes in preload or afterload caused by extreme positioning and pneumoperitoneum [6,8].
Intraoperative
In addition to proper patient selection, intraoperative anesthetic management for planned outpatients must ensure timely emergence and recovery in the Post Anesthesia Care Unit (PACU) [10,12,13,18,23].
Once a standard induction is achieved and the airway is secured, obtaining additional intravenous access is often useful because access to patient’s arms will be extremely limited once the robot is docked [3,6,8]. An invasive arterial line may be helpful as well, if suggested by comorbidities or the potential for fluid volume shifts; direct arterial pressure monitoring may also be helpful in larger patients where a noninvasive cuff may not fit or work well. Subsequently providers should also pay attention to ensuring that all access sites are working after the arms have been tucked and the robot docked. Similarly, it is important to ensure proper padding of pressure points (ie, elbow, axilla, back, and shoulders) given the limited access to the patient during the case. Transitioning to Trendelenburg position can lead to the patient shifting down the table: several adjuncts such as shoulder braces, leg suspension, and iliac support have been associated with neuropathic injury and therefore are not recommended [40,41]. Placing the patient on an antiskid material (egg-crate pink foam) has shown minimal shift of the patient without evidence of skin or neurologic injuries [42] (Fig. 5 ).
Fig. 5.
Pink foam to minimize patient skidding during steep Trendelenberg, reverse Trendelenberg, or lateral tilts.
Given the known physiologic changes associated with Trendelenburg and pneumoperitoneum, plans should be made to achieve adequate ventilation and for judicious fluid administration. Trendelenburg positioning alone and in combination with pneumoperitoneum can lead to hypercarbia, or the need for high airway pressures, which may run the risk of barotrauma and subsequent lung injury [32,37]. Some investigators have reported that in patients in whom ventilation or oxygenation is challenging, a pressure-controlled ventilation mode might allow for larger tidal volumes and lower peak and plateau pressures [37]. Muscle relaxation is typically maintained throughout the case to facilitate pneumoperitoneum and avoid the catastrophic consequences associated with patient movement while the robot is docked [3].
A carefully designed fluid strategy balancing hemodynamic goals individual to the patient and minimizing facial and airway edema is important. If the patient can tolerate it, a restrictive fluid management strategy may allow for reduction of edema and, in some urologic procedures, improve surgical visualization by reducing urine output [43].
Pain management should complement the gains offered by robotic surgery by thus allowing for safe and expedient discharge. A multimodal approach to analgesia, including regional blocks where appropriate, can help minimize postoperative opioid use, nausea and vomiting, and ileus, and forms a key part of any ambulatory surgery [44]. Although epidural analgesia may promote quicker return of bowel function by minimizing narcotics, its use should be balanced by the fact that dosing the epidural in the Trendelenburg position might lead to a high block [45]. In addition, epidural-associated hemodynamic changes might contribute to greater fluid loading. Furthermore, the patient will not be eligible for discharge home until all block-related muscle weakness has resolved.
Ultrasound-guided transversus abdominis plane or quadratus lumborum blockade may be performed after induction or placed under laparoscopic guidance by the surgical team to provide postoperative analgesia. This approach is a common adjunct for pain relief after robotic surgery, especially in cases wherein a larger incision will be needed for hand assist or specimen removal, such as in the case of nephrectomies [46,47]. This approach might be favorable to decrease the length of stay in the hospital postoperatively. [48], and has been reported by some groups to decrease opioid requirement and the time to ambulation and bowel function recovery [47,48], and even decrease the development of chronic pain [46].
Postoperative
In most ambulatory patients, the use of standard postanesthesia discharge criteria such as the Post Anesthetic Discharge Scoring System (PADSS) [49] is commonly implemented. The PADSS discharge criteria includes ensuring that vital signs are within 20% of preoperative baseline, that the patient ambulates with a steady gait, and that there is good control of nausea/vomiting, pain, and surgical bleeding. Criteria should be developed and systematically applied for both patients going home on the day of surgery and “23-hour” patients scheduled to leave the next morning.
Because the ability to select patients for rapid discharge is not perfect, and cannot account for intraoperative surprises, any facility performing outpatient robotic procedures must have an established relationship with an inpatient facility, and an organized protocol for transferring care when needed.
Outcomes
Most published studies evaluating the feasibility of outpatient robotic procedures have been done in the urology and gynecologic fields. Most of the studies have observed low rates of complications and readmissions, although the longest follow-up period of available studies is 6 weeks postoperatively [12,14]. Several groups have tried to identify factors that contribute to successful discharge and patient outcomes. In a study of patients undergoing robotic hysterectomies, older age, preoperative lung disease, and later surgical end time were risk factors for requiring a prolonged hospital stay [16]. Another study in robotic hysterectomies found that the need for abdominal hysterectomy, older age, Medicare insurance, ethnicity, higher number of comorbidities, and concomitant procedures were associated with the need for inpatient stay [15].
Current controversies/future considerations
Successful outpatient robotic surgery depends on the following key factors: appropriate patient selection and identification of risks to patients (Table 5 ), good surgical technique, and skilled anesthetic management.
Table 5.
Risks to patients undergoing abdominal or pelvic robotic surgery and management goals
| Cardiovascular |
|
|
| Respiratory |
|
|
| Airway |
|
|
| Nervous system |
|
|
| Positioning |
|
|
Abbreviations: ERV, expiratory reserve volume; FRC, functional residual capacity; ICP, itracranial pressure; IOP, intraoccular pressure ; MAP, mean arterial pressure; PEEP, positive end expiratory pressure.
At present, there are only limited studies describing readmission rates or short-term safety outcomes after outpatient robotic surgery. Further data about which patients have consistently good or poor outcomes after outpatient robotic surgery and what types of procedures are most appropriate might be useful. There is conflicting data on whether the use of robot-assisted techniques in outpatient surgery increases or decreases costs [13,16,50]; facility fees for outpatient procedures are lower than for inpatient ones, but the costs for the robot and associated disposables may outweigh this benefit.
Future studies are needed to better characterize the risk-benefit ratios of ambulatory robotic surgery in different patient populations, and to better capture the economic or financial costs of expanding the various indications for outpatient robotic surgery.
Summary
Patient selection, surgical technique, and prudent perioperative anesthetic management are important to reduce surgical duration and the risk of complications. More robust data are required to support the use of ambulatory robotic surgery in various surgical and patient populations.
Clinics care points
-
•
Careful patient selection is an important factor insuring safe discharge after ambulatory surgery.
-
•
Steep trendelenberg position used in pelvic and urologic surgery leads to physiological changes which may or may not be tolerated by every patient.
-
•
As the lenght of the surgery decreases, there should be less risks of severe positional injuries seen with prolonged procedure.
Disclosure
The authors have nothing to disclose.
References
- 1.George E.I., Brand T.C., LaPorta A., et al. Origins of robotic surgery: from skepticism to standard of care. JSLS. 2018;22(4) doi: 10.4293/JSLS.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheetz K.H., Claflin J., Dimick J.B. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3(1):e1918911. doi: 10.1001/jamanetworkopen.2019.18911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J.R. Anesthetic considerations for robotic surgery. Korean J Anesthesiol. 2014;66(1):3–11. doi: 10.4097/kjae.2014.66.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman R.M., Umer A., Bozzuto B.J., et al. Surgical value of elective minimally invasive gallbladder removal: a cost analysis of traditional 4-port vs single-incision and robotically assisted cholecystectomy. J Am Coll Surg. 2016;222(3):303–308. doi: 10.1016/j.jamcollsurg.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Oğurlu M., Küçük M., Bilgin F., et al. Pressure-controlled vs volume-controlled ventilation during laparoscopic gynecologic surgery. J Minim Invasive Gynecol. 2010;17(3):295–300. doi: 10.1016/j.jmig.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Molloy B.L. Implications for postoperative visual loss: steep trendelenburg position and effects on intraocular pressure. Aana j. 2011;79(2):115–121. [PubMed] [Google Scholar]
- 7.Ludbrook G., Sutherland P. Erroneous pulse oximetry readings during robotic prostatectomy. Anaesth Intensive Care. 2007;35(1):144–145. [PubMed] [Google Scholar]
- 8.Phong S.V., Koh L.K. Anaesthesia for robotic-assisted radical prostatectomy: considerations for laparoscopy in the Trendelenburg position. Anaesth Intensive Care. 2007;35(2):281–285. doi: 10.1177/0310057X0703500221. [DOI] [PubMed] [Google Scholar]
- 9.Lanfranco A.R., Castellanos A.E., Desai J.P., et al. Robotic surgery: a current perspective. Ann Surg. 2004;239(1):14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escobar Dominguez J.E., Ramos M.G., Seetharamaiah R., et al. Feasibility of robotic inguinal hernia repair, a single-institution experience. Surg Endosc. 2016;30(9):4042–4048. doi: 10.1007/s00464-015-4717-5. [DOI] [PubMed] [Google Scholar]
- 11.Terris D.J., Singer M.C., Seybt M.W. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope. 2011;121(8):1636–1641. doi: 10.1002/lary.21832. [DOI] [PubMed] [Google Scholar]
- 12.Penner K.R., Fleming N.D., Barlavi L., et al. Same-day discharge is feasible and safe in patients undergoing minimally invasive staging for gynecologic malignancies. Am J Obstet Gynecol. 2015;212(2):186–188. doi: 10.1016/j.ajog.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Rivard C., Casserly K., Anderson M., et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015;22(2):219–226. doi: 10.1016/j.jmig.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahas S., Feigenberg T., Park S. Feasibility and safety of same-day discharge after minimally invasive hysterectomy in gynecologic oncology: A systematic review of the literature. Gynecol Oncol. 2016;143(2):439–442. doi: 10.1016/j.ygyno.2016.07.113. [DOI] [PubMed] [Google Scholar]
- 15.Borahay M.A., Patel P.R., Kilic C.H., et al. Outpatient robotic hysterectomy: clinical outcomes and financial analysis of initial experience. Int J Med Robot. 2014;10(2):244–250. doi: 10.1002/rcs.1565. [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio S., Li Y., Song C., et al. The shift from inpatient to outpatient hysterectomy for endometrial cancer in the United States: trends, enabling factors, cost, and safety. Int J Gynecol Cancer. 2021;31(5):686–693. doi: 10.1136/ijgc-2020-002192. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers A.K., Goldberg J.M., Hammel J.P., et al. Tubal anastomosis by robotic compared with outpatient minilaparotomy. Obstet Gynecol. 2007;109(6):1375–1380. doi: 10.1097/01.AOG.0000264591.43544.0f. [DOI] [PubMed] [Google Scholar]
- 18.Kisby C.K., Polin M.R., Visco A.G., et al. Same-day discharge after robotic-assisted sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2019;25(5):337–341. doi: 10.1097/SPV.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 19.Wilson C.A., Aminsharifi A., Sawczyn G., et al. Outpatient extraperitoneal single-port robotic radical prostatectomy. Urology. 2020;144:142–146. doi: 10.1016/j.urology.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Bernhard J.C., Robert G., Ricard S., et al. Day-case robotic-assisted partial nephrectomy: feasibility and preliminary results of a prospective evaluation (UroCCR-25 AMBU-REIN study) World J Urol. 2022;40(6):1351–1357. doi: 10.1007/s00345-020-03283-z. [DOI] [PubMed] [Google Scholar]
- 21.Abaza R., Murphy C., Bsatee A., et al. Single-port robotic surgery allows same-day discharge in majority of cases. Urology. 2021;148:159–165. doi: 10.1016/j.urology.2020.08.092. [DOI] [PubMed] [Google Scholar]
- 22.Neheman A., Kord E., VanderBrink B.A., et al. Outpatient robotic surgery in pediatric urology: assessment of feasibility and short-term safety. J Urol. 2022;207(4):894–900. doi: 10.1097/JU.0000000000002362. [DOI] [PubMed] [Google Scholar]
- 23.Sephton B.M., De la Cruz N., Shearman A., et al. Achieving discharge within 24 h of robotic unicompartmental knee arthroplasty may be possible with appropriate patient selection and a multi-disciplinary team approach. J Orthop. 2020;19:223–228. doi: 10.1016/j.jor.2020.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loor M., Liang M. In: Uptodate. Chen W., editor. 2022. Robotic ventral hernia repair.www.uptodate.com/contents/robotic-ventral-hernia-repair Waltham, MA. (Accessed July 2022) [Google Scholar]
- 25.Schmitt J.J., Occhino J.A., Weaver A.L., et al. Vaginal versus robotic hysterectomy for commonly cited relative contraindications to vaginal hysterectomy. J Minim Invasive Gynecol. 2017;24(7):1158–1169. doi: 10.1016/j.jmig.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Aceto P., Beretta L., Cariello C., et al. Joint consensus on anesthesia in urologic and gynecologic robotic surgery: specific issues in management from a task force of the SIAARTI, SIGO, and SIU. Minerva Anestesiol. 2019;85(8):871–885. doi: 10.23736/S0375-9393.19.13360-3. [DOI] [PubMed] [Google Scholar]
- 27.Corcione A., Angelini P., Bencini L., et al. Joint consensus on abdominal robotic surgery and anesthesia from a task force of the SIAARTI and SIC. Minerva Anestesiol. 2018;84(10):1189–1208. doi: 10.23736/S0375-9393.18.12241-3. [DOI] [PubMed] [Google Scholar]
- 28.Pathirana S., Kam P. Anaesthetic issues in robotic-assisted minimally invasive surgery. Anaesth Intensive Care. 2018;46(1):25–35. doi: 10.1177/0310057X1804600105. [DOI] [PubMed] [Google Scholar]
- 29.Gould C., Cull T., Wu Y.X., et al. Blinded measure of Trendelenburg angle in pelvic robotic surgery. J Minim Invasive Gynecol. 2012;19(4):465–468. doi: 10.1016/j.jmig.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Jara R.D., Guerrón A.D., Portenier D. Complications of robotic surgery. Surg Clin North Am. 2020;100(2):461–468. doi: 10.1016/j.suc.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Bjøro B., Mykkeltveit I., Rustøen T., et al. Intraoperative peripheral nerve injury related to lithotomy positioning with steep Trendelenburg in patients undergoing robotic-assisted laparoscopic surgery - A systematic review. J Adv Nurs. 2020;76(2):490–503. doi: 10.1111/jan.14271. [DOI] [PubMed] [Google Scholar]
- 32.Carry P.Y., Gallet D., François Y., et al. Respiratory mechanics during laparoscopic cholecystectomy: the effects of the abdominal wall lift. Anesth Analg. 1998;87(6):1393–1397. doi: 10.1097/00000539-199812000-00035. [DOI] [PubMed] [Google Scholar]
- 33.Knight D.J., Mahajan R.P. Patient positioning in anaesthesia. Continuing Educ Anaesth Crit Care Pain. 2004;4(5):160–163. [Google Scholar]
- 34.Hsu R.L., Kaye A.D., Urman R.D. Anesthetic challenges in robotic-assisted urologic surgery. Rev Urol. 2013;15(4):178–184. [PMC free article] [PubMed] [Google Scholar]
- 35.Dean M., Ramsay R., Heriot A., et al. Warmed, humidified CO(2) insufflation benefits intraoperative core temperature during laparoscopic surgery: A meta-analysis. Asian J Endosc Surg. 2017;10(2):128–136. doi: 10.1111/ases.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong J.Y., Kim J.Y., Choi Y.D., et al. Incidence of venous gas embolism during robotic-assisted laparoscopic radical prostatectomy is lower than that during radical retropubic prostatectomy. Br J Anaesth. 2010;105(6):777–781. doi: 10.1093/bja/aeq247. [DOI] [PubMed] [Google Scholar]
- 37.Miller R.D., Eriksson L.I., Fleisher L.A., et al. Vol. 1647. Elsevier Churchill Livingstone; Philadelphia: 2005. (Miller’s anesthesia). [Google Scholar]
- 38.Kim A., Geynisman-Tan J., Lewicky-Gaupp C. Pneumothorax after laparoscopic robotic-assisted supracervical hysterectomy and sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2017;23(3):e22–e24. doi: 10.1097/SPV.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 39.Huang T.J., Ahmed A., D'Souza D., et al. Delayed diagnosis of contralateral tension pneumothorax during robotic lung wedge resection. J Clin Anesth. 2018;45:30–31. doi: 10.1016/j.jclinane.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Barnett J.C., Hurd W.W., Rogers R.M., Jr., et al. Laparoscopic positioning and nerve injuries. J Minim Invasive Gynecol. 2007;14(5):664–672. doi: 10.1016/j.jmig.2007.04.008. [Quiz: 673] [DOI] [PubMed] [Google Scholar]
- 41.Irvin W., Andersen W., Taylor P., et al. Minimizing the risk of neurologic injury in gynecologic surgery. Obstet Gynecol. 2004;103(2):374–382. doi: 10.1097/01.AOG.0000110542.53489.c6. [DOI] [PubMed] [Google Scholar]
- 42.Klauschie J., Wechter M.E., Jacob K., et al. Use of anti-skid material and patient-positioning to prevent patient shifting during robotic-assisted gynecologic procedures. J Minim Invasive Gynecol. 2010;17(4):504–507. doi: 10.1016/j.jmig.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Danic M.J., Chow M., Alexander G., et al. Anesthesia considerations for robotic-assisted laparoscopic prostatectomy: a review of 1,500 cases. J Robot Surg. 2007;1(2):119–123. doi: 10.1007/s11701-007-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richebé P., Brulotte V., Raft J. Pharmacological strategies in multimodal analgesia for adults scheduled for ambulatory surgery. Curr Opin Anaesthesiol. 2019;32(6):720–726. doi: 10.1097/ACO.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 45.Irvine M., Patil V. Anaesthesia for robot-assisted laparoscopic surgery. Continuing Educ Anaesth Crit Care Pain. 2009;9(4):125–129. [Google Scholar]
- 46.Covotta M., Claroni C., Costantini M., et al. The effects of ultrasound-guided transversus abdominis plane block on acute and chronic postsurgical pain after robotic partial nephrectomy: a prospective randomized clinical trial. Pain Med. 2020;21(2):378–386. doi: 10.1093/pm/pnz214. [DOI] [PubMed] [Google Scholar]
- 47.Rogers T., Bhat K.R.S., Moschovas M., et al. Use of transversus abdominis plane block to decrease pain scores and narcotic use following robot-assisted laparoscopic prostatectomy. J Robot Surg. 2021;15(1):81–86. doi: 10.1007/s11701-020-01064-9. [DOI] [PubMed] [Google Scholar]
- 48.Damadi A.A., Lax E.A., Smithson L., et al. Comparison of therapeutic benefit of bupivacaine hcl transversus abdominis plane (TAP) block as part of an enhanced recovery pathway versus traditional oral and intravenous pain control after minimally invasive colorectal surgery: a prospective, randomized, double-blind trial. Am Surg. 2019;85(12):1363–1368. [PubMed] [Google Scholar]
- 49.Barash P.G.C.B. 7th edition. Lippincott Williams & Wilkins; Philadelphia: 2013. Clinical anesthesia. [Google Scholar]
- 50.Schwaitzberg S.D. Financial modeling of current surgical robotic system in outpatient laparoscopic cholecystectomy: how should we think about the expense? Surg Endosc. 2016;30(5):2082–2085. doi: 10.1007/s00464-015-4457-6. [DOI] [PubMed] [Google Scholar]