Abstract
Background
To reveal the function of protein tyrosine phosphatase‐L1 (PTPL1) in lung adenocarcinoma.
Methods
Lung cancer cell lines were transfected with short hairpin RNA against PTPL1 (shPTPL1 group) or negative control (shmock group). Quantitative real‐time polymerase chain reaction (qRT‐PCR) and western blotting were used to verify the transfection efficacy. Cell proliferation was analyzed by ethynyldeoxyuridine (EdU), Cell counting kit 8 (CCK8), and colony formation assay after PTPL1 or PTPL1 and yes‐associated protein (YAP1) knockdown. The effect of PTPL1 on tumor growth was examined in a xenograft lung cancer model.
Results
PTPL1 was downregulated in various types of lung cancer cell lines. The EdU, CCK8, colony formation assays and investigation using a xenograft lung cancer model indicated that PTPL1 knockdown increased the proliferation of lung cancer cells. Mechanistically, PTPL1 knockdown induced the activation of the Proto‐oncogene tyrosine‐protein kinase SRC (Src)/Extracellular regulated MAP kinase (ERK) pathway and thereby promoted yes‐associated protein (YAP1) nuclear translocation and activation.
Conclusions
In our study, PTPL1 played a crucial suppressive role in the pathogenesis of lung cancer potentially through counteracting the Src/ERK/YAP1 pathway.
Keywords: non‐small cell lung cancer, proliferation, PTPL1
Knockdown of PTPL1 increases the proliferation of lung cancer cells and induces the activation of the Src/MEK/ERK signaling pathway, and promoted nuclear translocation of Yes‐associated protein (YAP1).
INTRODUCTION
Despite the advances in medication and therapeutics, lung cancer remains the leading cause of cancer death worldwide. Non‐small cell lung cancer (NSCLC) is the most common subtype of lung cancer, accounting for 85% of all cases. 1 The overall 5‐year survival rate is less than 20%, and less significant improvement has been made over the past decades. 2 Recently, although recent targeted therapies and immunotherapy have improved the situation, the long‐term survival of advanced patients is still poor. 3 Since lung cancer is a molecularly heterogeneous disease and most patients are already in the advanced stage at the time of diagnosis, early diagnosis could remarkably reduce the mortality of patients. An in‐depth understanding of lung cancer pathogenesis may potentially lead to new effective treatments. 4
It is well known that protein tyrosine phosphorylation plays an important role in intracellular signaling and cell behaviors including proliferation, apoptosis, invasion, and drug resistance. 5 , 6 , 7 It has been determined that the deregulation of the aforementioned signal pathways is related to the development and progression of cancer. 6 In contrast to kinases that catalyze phosphorylation of the signal transducers, the roles of protein tyrosine phosphatases (PTPs) have not been clearly depicted in the past decades. So far, only a few known PTPs have been established as oncogenes or tumor suppressors. 8 , 9 Human genome sequencing identified 107 PTP genes, 81 of which encode active protein phosphatases. 10 PTP represents an enzyme superfamily subdivided into classical PTPs, dual‐specificity PTPs, and low molecular weight PTPs 13, 14. 11 The catalytic domains of the PTP superfamily are highly conserved. 12 In accordance with their critical involvement in orchestrating canonical intracellular signal pathways, PTP mutations have been frequently documented in various malignancies. 12 The classical PTPs are further grouped into receptor and nonreceptor PTPs. 12 Of the receptor PTPs, protein tyrosine phosphatase receptor type T (PTPRT)‐regulated signal transducer and activation of transcription 3 (STAT3) signaling and paxillin phosphorylation seem to be critical for head and neck or colorectal tumorigenesis. 13 Protein tyrosine phosphatase receptor type D (PTPRD) suppresses tumors of various tissue origins by inducing apoptosis of cells. 14 Protein tyrosine phosphatase receptor type K (PTPRK) can counteract HER2‐induced proliferation of breast cancer cells by inhibiting human epidermal growth factor receptor 2 (HER2) phosphorylation. 15 Protein tyrosine phosphatase receptor type M (PTPRM) is mainly involved in regulating the proliferation and migration of breast and brain cancer cells, while protein tyrosine phosphatase receptor type J (PTPRJ) may be a candidate colon tumor suppressor gene. Protein tyrosine phosphatase receptor type B (PTPRB) regulates endothelial cell polarity and vascular permeability through the dephosphorylation of substrates such as receptor‐2 during angiogenesis. 12
The mitogen‐activated protein kinases (MAPK) pathway has been documented as a regulatory target of PTPs. In particular, PTPN11 inactivates MAPK signaling through dephosphorylating Src or Sprouty, the inhibitor of reticular activating system (RAS). 16 Protein‐tyrosine phosphatase‐L1 (PTPL1, also known as tyrosine‐protein phosphatase nonreceptor type [PTPN13], fas‐associated phosphatase 1 [FAP‐1], protein‐tyrosine phosphatase‐Basophil [PTP‐BAS], protein tyrosine phosphatase 1E [PTP1E], and protein tyrosine phosphatase LE [PTPLE]) is a nonreceptor PTP with 2485 amino acid residues and has the largest molecular weight of the known PTPs. 10 PTPL1 is composed of several important domains including the kinase noncatalytic C‐leaf domain (KIND), the 4.1/ezrin/radixin/moesin (FERM) domain that usually exists in the peripheral membrane protein family, and the carboxy‐terminal catalytic domain. Five PSD‐95/CD large/ZO‐1 (PDZ) domains located between the FERM domain and the carboxy‐terminal catalytic domain. 17 As a protein/protein interaction domain, the PDZ domain catalyzes the dephosphorylation of different protein tyrosine sites and participates in the occurrence and development of various diseases. 18
Previous studies have found that PTPL1 has mutations or low expression in a variety of tumors, including lung cancer, colon cancer, breast cancer, liver cancer, head and neck tumors. 19 , 20 , 21 , 22 , 23 Interestingly, it acts as a tumor suppressor gene in most tumor types. 21 PTPL1 directly dephosphorylates Src on the active protease 419 (Y419) to regulate the invasiveness of breast cancer cells, suggesting its function of suppressing breast cancer. 22 On the contrary, PTPL1 can protect pancreatic cancer cells from CD95‐mediated apoptosis. 24 In addition, PTPL1 can also induce chemoresistance in head and neck cancer. 23 Wang et al. found that PTPL1 inhibited the progression of ovarian cancer by dephosphorylating and stabilizing phospho‐inhibitor of kappa Balpha (IκBα) and thus impairing nuclear translocation of Nuclear factor kappaB NF‐κB. 25 The activation of STAT3 inhibits the expression of PTPN13 in squamous cell lung cancer by recruiting histone deacetylase 5 (HDAC5). 19 MicroRNA‐30e‐5p negatively regulates PTPL1 to promote the growth of lung adenocarcinoma cells, which eventually affects the survival and recurrence rate of patients. 26
The present study aimed to investigate the impact of PTPL1 on the proliferation of lung cancer cells. To explore the signaling responsible of its role in carcinogenesis, the activation of MAP kinse‐ERK kinase (MEK)/ERK pathway and the expression of yes‐associated protein (YAP1), a key oncogenic transcription factor, were examined in PTPL1 knockdown cells. Both in vivo and in vitro investigations have proved that the knockdown of PTPL1 increases the proliferation of lung cancer cells and induces the activation of the Src/ERK/YAP1 signaling pathway.
METHODS
Cell culture
Human NSCLC cell lines A549, NCI‐H1975(H1975), BEAS‐2B and human embryonic kidney cell HEK293T were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in growth medium (GM) consisting of Dulbecco's Modified Eagle Medium (DMEM, Gibco) and Roswell Park Memorial (RPMI‐ 1640 medium, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 μg/ml streptomycin and 100 U/ml penicillin under a humidified atmosphere of 5% CO2 at 37°C.
Reverse transcription‐polymerase chain reaction (RT‐PCR) and quantitative PCR analyses
Total RNA extraction was performed using a RNase kit (Yeasen), according to the manufacturer's protocol. RNA purity and concentration were assessed with the Nano Drop 1000 Spectrophotometer (Thermo Fisher Scientific). For each RT‐PCR reaction, first‐strand cDNA was generated from 1 μg of total RNA with the RT master mix (Takara). The SYBR Green Master mix (Roche) was used to perform real‐time PCR following instructions stipulated by the manufacture. All data were normalized to the expression levels of housekeeping genes glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) according to the (2−ΔΔCt ) method.
Western blot analysis
Total protein extraction mainly involved placing the treated cells in radioimmunoprecipitation assay (RIPA) lysis buffer (Solarbio) supplemented with ProtLytic protease and phosphatase inhibitor cocktail (NCM Biotech) for 30 min at 4°C. The cytoplasmic protein was extracted using a cytoplasmic protein extraction kit (Beyotime Biotechnology). Protein concentration was assessed using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific). Lysates were diluted in 5 × laemmli loading buffer, loaded onto 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gels, proteins were transferred to polyvinylidene fluoride membranes. The blots were placed in 5% BSA blocking solution for 1 h and incubated with primary antibodies at 4°C overnight. The primary antibodies used for western blotting included PTPL1 (Proteintech, 25 944‐1‐AP), Src (Abcam, ab109381), anti‐Src (phosphoY419) (Abcam, ab185617), Phospho‐p44/42 MAPK (Erk1/2) (CST, #4370), p44/42 MAPK (Erk1/2) (CST, #4695), MEK (CST, #4694), Phospho‐MEK1/2 (Ser217/221) (CST, #9154), YAP (CST, #14074), Phospho‐YAP (Ser127) (CST, #13008). Tris‐buffered saline plus tween 20 (TBST) was used to wash the membranes three times, and horseradish peroxidase (HRP)‐conjugated secondary antibody was incubated for 1.5 h at room temperature. TBST was used to wash the excess secondary antibody, and the western blot was visualized by electrogenerated chemiluminescent (ECL) solution, and finally the chemiluminescence imaging system was used for imaging (Tanon). Band densities were quantified by ImageJ software (Wayne Rasband, National Institutes of Health).
Cell counting kit‐8 assay
Cells were seeded into 96‐well dish at an initial density of 2 × 103 –3 × 103 cells/well. At 0, 24, 48, or 72 h post‐transfection, the culture medium was changed to serum‐free medium containing the same doses of CCK8 (10 μl/well) for an additional 2 h for 37°C. The absorbance at the wavelength of 450 nm using Thermo Multiskan GO (Thermo Scientific) was then recorded to assess cell proliferation.
Colony formation assay
Cells were collected in the logarithmic growth phase, and a suspension prepared with a concentration of 2000 cells/6‐well plate. The culture dish was placed in an incubator at 37°C and 5% carbon dioxide. After culturing in complete medium for 7 days, the cells were fixed with 4% paraformaldehyde for 30 min, and then stained with 0.1% crystal violet solution (Sigma‐Aldrich). After washing the excess dye with phosphate buffered saline (PBS), a microscope was used to detect the total number of colonies with a minimum of 80 cells, and the data recorded and analyzed.
EdU incorporation assay
Ethynyldeoxyuridine (EdU) detection kit (Beyotime Biotechnology) was used to assess cell proliferation according to the manufacturer's instructions. Cells were cultured at 1 × 105 cells/well in a 6‐well dish. EdU‐labeled medium was added to the 6‐well dish, and then incubated for 2 h at 37°C and 5% carbon dioxide. Cells were fixed with 4% paraformaldehyde for 30 min at room temperature, followed by permeabilization in 0.5% Triton X‐100 at room temperature. Then, 500 μl of anti‐EdU working solution was added to each well and incubated for 30 min under light‐shading conditions. After washing three times with PBS, the nuclei were counterstained with Hoechst 333 42 for 10 min at room temperature. Finally, EdU‐labeled cells were observed by a fluorescence microscope (Bio‐Rad). The EdU incorporation rate was calculated as the ratio of EdU positive cells (red cells) to the total number of Hoechst 333 42 positive cells (blue cells).
shRNA lentiviral vector construction and transduction
To silence PTPL1 and YAP1, cells were transduced with short hairpin (shRNA) lentivirus targeting the human PTPN13 gene (Gene ID: 5783) and YAP1 (Gene ID: 10413) with lentivirus recombinant interfering plasmid (pGCSIL)‐green fluorescent protein (GFP) for transduction rate evaluation. Lentivirus lacking the shRNA insert was used as a control. Cells were seeded into a 6‐well dish at a density of 1 × 105 cells/well and transduced with shRNA‐PTPL1 (6 × 108 TU/ml) shRNA‐YAP1 (6 × 108 TU/ml) or shRNA‐mock lentivirus (6 × 108 TU/ml). After transfection for 48 h, the cells were imaged under a fluorescence microscope and further selection by puromycin. Seven days post‐infection, PTPL1 and YAP1 silencing was verified through qRT‐PCR analysis and western blotting.
Mouse xenografts
BALB/c mice were purchased from the Shanghai Slack Laboratory Animal Center. Fifteen 8‐week‐old nude mice were randomly assigned to three groups, and five mice in each group were independently reared in a 12‐h light/dark system to provide sufficient water and clean food. For tumorigenicity assays, 1 × 106 shmock, shPTPL1, or shPTPL1 + shYAP1 cells were resuspended in 100 μl of PBS. Underarm injections of 8 weeks old male or female nude mice as described previously. 27 The bodyweight and tumor size were measured every 3 days, and the tumor size was measured manually using calipers. All animals were used in compliance with the guidelines approved by the Institutional Animal Care and Use Committee. On the 30th day, the mice were anesthetized to obtain tumor tissue samples and stored in liquid nitrogen.
Tumor volume calculation formula: Tumor volume = 0.5 × length × width2 mm.
Statistical analysis
Statistical analysis was performed using GraphPad Prism8.0 (GraphPad Software, Inc.). Each experiment was repeated three times and all values are expressed as mean ± SD. Image J software was used for Western blot pattern gray‐scale scanning. A t‐test was used to compare the data between the two groups, and the analysis of variance was used to compare the data between multiple groups. p < 0.05 was considered statistically significant.
RESULTS
Silencing of PTPL1 promotes the proliferation of NLCSC cells
To examine the function of PTPL1 in lung cancer, we assessed its expression in a normal human bronchial epithelial cell line, Human Normal Lung Epithelial cells(BEAS‐2B), and NLCSC cell lines, H1299, H1650, PC‐9, H1975 and A549. We found that PTPL1 knockdown caused significantly improved Src activation and concurrently PTPL1 levels were significantly lower in NSCLC cells than in BEAS‐2B cells (Figure 1), indicating a potential role in NSCLC pathogenesis. We next infected cells with recombinant lentiviruses expressing PTPL1‐targeted short hairpin RNAs (sh‐PTPL1), and validated efficient PTPL1 knockdown via qRT‐PCR and Western blot (Figure 2a,b). Consequently, cell proliferation was enhanced by knockdown of PTPL1 in these cells, as revealed by CCK8 assay (Figure 3a). We also used clone formation and Ethynyldeoxyuridine (EdU) assays to investigate the role of PTPL1 in the proliferation of lung cancer cells. Compared with the control group, PTPL1 knockdown significantly increased the proliferation of cells (Figure 3b,c). Collectively, these results indicate that PTPL1 downregulation contributes to the over‐proliferation of NSCLC cells.
FIGURE 1.
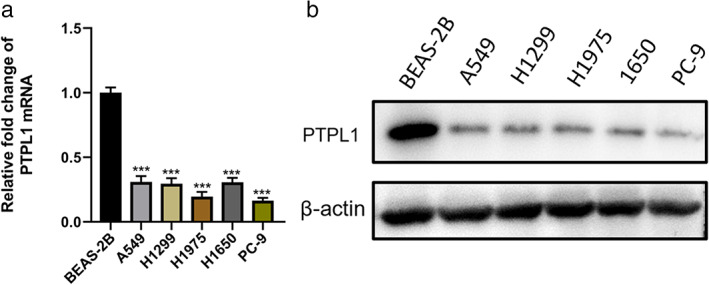
Expression of PTPL1 in lung cancer cell lines. (a) qRT‐PCR to detect the relative levels of mRNA in five NSCLC cell lines. (b) Western blot detection of protein levels of PTPL1 in various cell lines. All data are representative of three independent experiments and are shown as mean ± SD. ***p < 0.001. mRNA, messenger RNA; NSCLC, non‐small cell lung cancer; PTPL1, protein‐tyrosine phosphatase‐L1
FIGURE 2.
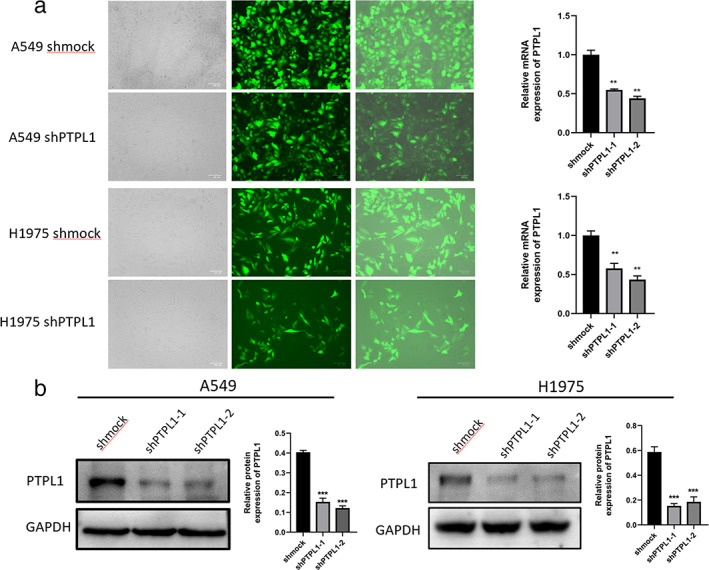
Knockdown of PTPL1 in various cells. (a) The infection efficiency of recombinant lentiviruses was assessed under fluorescence microscope and by qRT‐PCR. (b) Knockdown of PTPL1 was validated via western blot analyses. Data shown are mean ± SD (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001. GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; mRNA, messenger RNA; PTPL1, protein‐tyrosine phosphatase‐L1; shmock, negative control; shPTPL1, short hairpin RNA against PTPL1
FIGURE 3.
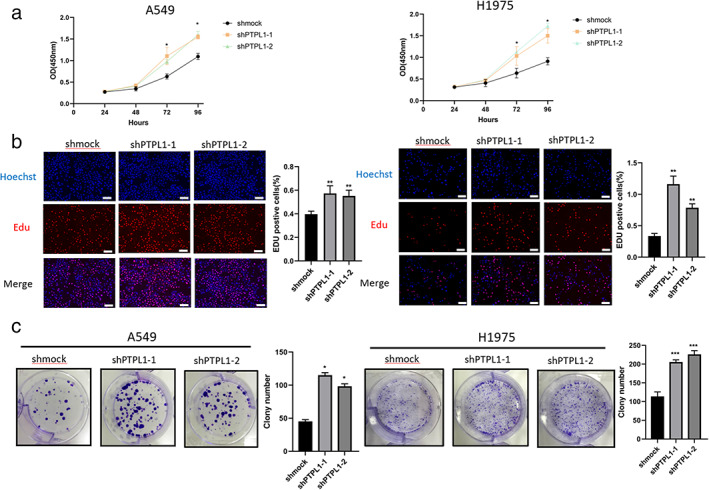
PTPL1 knockdown facilitates NSCLC cell proliferation in vitro. (a) CCK8 assays of control and PTPL1 knockdown cells. Cells (2 × 103 were seeded in 96‐well plates, and the OD values were measured per 24 h for 4 days. *p < 0.05 compared with control group. (b) Cell proliferation was analyzed by immunofluorescence staining for EdU. (c) Cell proliferation was analyzed by colony formation assay. Knockdown of PTPL1 expression significantly promoted cell proliferation in NSCLC cell. Scale bar = 20 μm or (EdU) 100 μm (colony). Data shown are mean ± SD (n = 5). (*p < 0.05, **p < 0.01, ***p < 0.001). CCK8, Cell counting kit‐8 assay; EdU, ethynyldeoxyuridine; NSCLC, non‐small cell lung cancer; OD, optical density; PTPL1, protein‐tyrosine phosphatase‐L1
PTPL1 attenuates phosphorylation of ERK and inactivates YAP1 in NSCLC cells
PTPL1 was reported to repress cell proliferation through dephosphorylating Src, a non‐receptor tyrosine kinase known to activate canonical signal pathways including MEK/ERK, and YAP1 is a key oncogenic transcription factor regulated by various signaling such as MEK/ERK pathway. 28 We thus investigated the effects exerted by PTPL1 on the phosphorylation status of Src, MEK, ERK and YAP1 in NSCLC cells. We observed that PTPL1 knockdown A549 cells exhibited consistently increased levels of phosphorylated Src and ERK when cells were exposed to serum or EGF (Figure 4a). In parallel, with the increased phosphorylation of Src and MEK, nuclear YAP1 levels were significantly upregulated after knockdown of PTPL1 in A549 cells (Figure 4b). Thus, PTPL1 knockdown promotes YAP1 nuclear translocation and activation possibly via MEK/ERK pathway in NSCLC cells.
FIGURE 4.
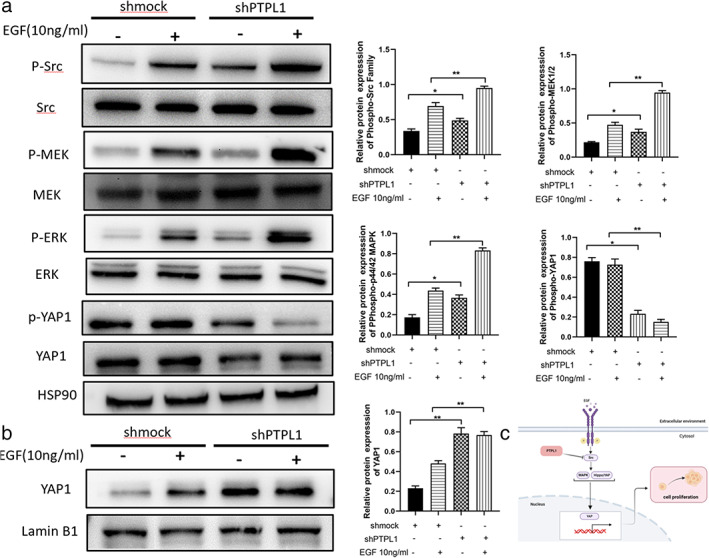
Knockdown of PTPL1 activates Src/ERK/YAP1 signaling in lung cancer cells. (a) A549 cells were treated with growth factor (EGF; 10 ng/ml) for 10 min. Cells were then lysed and the proteins was separated by gel electrophoresis on SDS‐PAGE, followed by detection of the effects of PTPL1 on the expression of Src, p‐Src, MEK, p‐MEK, ERK1/2, p‐ERK1/2, YAP1 and p‐YAP1. (b) The effects of PTPL1 knockdown on the expression of YAP1 in A549 nuclei was evaluated by western blot. Data shown are mean ± SD. (*p < 0.05, **p < 0.01, ***p < 0.001). ERK1/2, p44/42 MAPK; MEK, MAP kinse‐ERK kinase; p‐ERK1/2, phospho‐p44/42 MAPK; p‐MEK, phospho‐MAP kinse‐ERK kinase; p‐Src, phospho‐proto‐oncogene tyrosine‐protein kinase SRC; PTPL1, protein‐tyrosine phosphatase‐L1; p‐YAP1, phospho‐yes‐associated protein; SDS‐PAGE, sodium dodecyl sulfate – polyacryla mide gel electrophoresis; Src, Proto‐oncogene tyrosine‐protein kinase SRC; YAP1, yes‐associated protein
YAP1 silencing reversed the hyperproliferation of NSCLC cells after PTPL1 knockdown
To examine the potential role of YAP1 in the pathogenesis of NSCLC and figure out whether there is an interaction relationship between YAP1 and PTPL1. Next, we infected cells with recombinant lentivirus expressing YAP1 targeting short hairpin RNA (sh‐YAP1) and verified the efficient knockout of YAP1 by qRT PCR and Western blot (Figure 5). Therefore, as shown by CCK8 assay, knockdown of YAP1 in these cells reversed cell proliferation in the short hairpin RNA against PTPL1 (shPTPL1 group) (Figure 6a). We also used clonogenic and EdU analysis to investigate the role of YAP1 in lung cancer cell proliferation. Compared with shmock, PTPL1 knockdown significantly increased cell proliferation, while the excessive proliferation of cells was reversed after YAP1 knockdown (Figure 6b,c). Taken together, these results indicate that YAP1 downregulation reverses the hyperproliferation of shPTPL1 cells.
FIGURE 5.
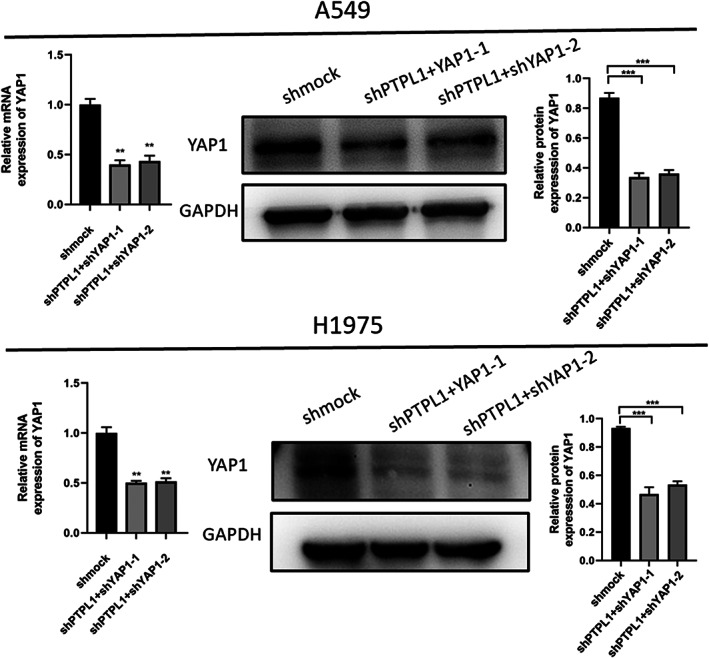
Knockdown of YAP1 in various NSCLC cells. The infection efficiency of recombinant lentiviruses was validated via western blot analyses and by qRT‐PCR. Data shown are mean ± SD (n = 5). (*p < 0.05, **p < 0.01, ***p < 0.001). GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; NSCLC, non‐small cell lung cancer; shmock, negative control; shYAP1, short hairpin RNA against YAP1l; YAP1, yes‐associated protein
FIGURE 6.

PTPL1 inhibits growth of lung cancer in vitro through downregulation of YAP1. (a) Cell counting kit‐8 (CCK8) assays of control and YAP1 knockdown cells. *p < 0.05 compared with control group. (b) Cell proliferation was analyzed by immunofluorescence staining for EdU. (c) Cell proliferation was analyzed by colony formation assay. Knockdown of YAP1 expression alleviates the inhibition of cell proliferation by PTPL1 in NSCLC cells. Scale bar = 20 μm or (EdU) 100 μm (colony). Data shown are mean ± SD (n = 5). (*p < 0.05, **p < 0.01, ***p < 0.001). EdU, ethynyldeoxyuridine; NSCLC, non‐small cell lung cancer; PTPL1, protein‐tyrosine phosphatase‐L1; shmock, negative control; shPTPL1, short hairpin RNA against PTPL1; shmock, negative control; shPTPL1, short hairpin RNA against PTPL1; shYAP1, short hairpin RNA against YAP1; YAP1, yes‐associated protein
PTPL1 and YAP1 dictate tumor growth in a xenograft lung cancer model
We next evaluated whether PTPL1 and downstream signaling played a role in in vivo lung cancer development. Nude mice were subcutaneously injected with control A549 cells or those subjected to PTPL1 or concurrent YAP1 knockdown. Within 30 days, all mice developed visible subaxillary solid tumors and exhibited comparable bodyweights (Figure 7a,b) While PTPL1 knockdown significantly promoted the growth of xenograft tumors, further silencing of YAP1 counteracted the effect of PTPL1 knockdown on tumor development (Figure 7b,c). These data suggest that PTPL1 represses in vivo development of lung cancer via downregulation of YAP1.
FIGURE 7.
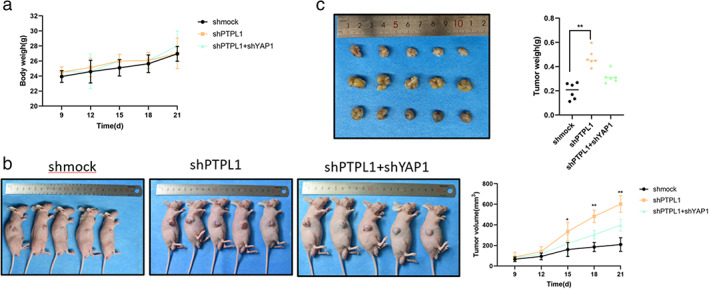
PTPL1 inhibits growth of xenograft lung cancer in vivo through downregulation of YAP1. (a) Mice were subcutaneously challenged by parental A549 cells, those subjected to PTPL1 or concurrent YAP1 knockdown (n = 5), and the bodyweights of mice were monitored. (a–c) A549 cells treated with sh‐mock lentivirus were subcutaneously inoculated to the flanks, sh‐PTPL1 and shPTPL1 + shYAP1 lentivirus were treated to the flanks of nude mice. The tumor growth curves showed that compared with the sh‐mock group, the sh‐PTPL1 group inhibited tumor growth. (b) The tumor volumes of nude mice described in (a) measured and plotted. (c) Mice in (a) were sacrificed 28 days after inoculation, and tumors were isolated and weighed. The displayed data is the average ± SD (n = 5). (*p < 0.05, **p < 0.01, ***p < 0.001). PTPL1, protein‐tyrosine phosphatase‐L1; shmock, negative control; shPTPL1, short hairpin RNA against PTPL1; shYAP1, short hairpin RNA against YAP1; YAP1, yes‐associated protein
DISCUSSION
In this study, we demonstrated that the expression of the PTP, PTPL1, was reduced in lung cancer cells compared with normal lung epithelial cells. Moreover, we evaluated the impact of PTPL1 in lung cancer cell proliferative using in vitro and animal models and dissected the molecular mechanism responsible for this effect. We found that PTPL1 impaired the proliferation of lung cancer cells, and silencing of PTPL1 led to increased tumor growth in athymic mice. These results suggest that PTPL1 functions as a potent suppressor in the occurrence of NSCLC. This is also consistent with previous reports that PTPs plays an important role in inhibiting or controlling growth as a tumor suppressor. 29
Although it is largely uncharacterized how PTPL1 fine‐tunes intracellular signaling that controls mitosis, this has been addressed by several previous studies. PTPL1 was found to regulate the cell cycle and upregulate genes related to invasion in prostate cancer cells. 30 PTPL1 acts as a negative regulator of the insulin‐like growth factor (IGF)‐1R/IRS‐1/Akt pathway in breast cancer cells and inhibits fat formation. 31 The loss of PTPL1 in prostate cancer cells may contribute to apoptosis resistance and tumor progression. 32 Depletion of PTPL1 in mice caused increased incidence of breast cancer and accelerated tumor growth in vivo. 33 These findings unraveled a multifaceted role of PTPL1 in suppressing tumorigenesis, highlighting the importance of identifying novel players in PTPL1‐regulated network in the context of vital malignancies like lung cancer.
The Hippo pathway is one of the most frequently altered signaling pathways in human cancer. Targeting the core YAP1/PDZ‐binding motif‐TEA domain family member 1(TAZ‐TEAD) complex in this pathway has the potential to remarkably suppress tumorigenesis. 34 TEAD activity is necessary for maintaining cell proliferation and renewal, and the blockade of YAP1/TAZ binding to TEAD leads to the activation of the Kruppel‐like factor 4 (KLF4) transcription network. Furthermore, KLF4 knockout mice showed an increased incidence of cancer. 35 YAP1 has been established to function as a critical oncoprotein in NSCLC. 36 Here, the phenotypic results of the double knockdown cell lines showed that YAP1 promoted the proliferation of NSCLC cells. In vitro experiments showed that YAP1 knockdown reduced the tumorigenic ability of nude mice. Previous studies have also shown that YAP1 was related to the proliferation of various cell types. 37 The N6‐methyladenosine demethylase AlkB homolog5 (ALKBH5) inhibited tumor growth by reducing YTH domain‐containing‐proteins (YTHDFs)‐mediated YAP1 expression and inhibiting miR‐107/large tumor suppressor kinase 2 (LATS2)‐mediated YAP1 activity in NSCLC. 38 The long non‐coding RNA, MALAT1, can upregulate YAP1 through sponging miR‐194‐3p, and thereby induce drug resistance of NSCLC. 39 YAP/TAZ was also found to play an important role in regulating alveolar regeneration and alleviation of lung inflammation. 40 In line with these observations, we found that YAP1 could be upregulated via MEK/ERK signaling in response to PTPL1 deficiency. Although the detailed mechanisms remain elusive, ERK has been documented to serve as a crucial regulator of YAP1. 41 , 42 Since YAP1 has also been reported to activate the MEK/ERK pathway, 43 it is likely that they form a feedback regulatory loop to reinforce oncogenic signaling. Finally, our findings do not rule out the probability that other mediators also play indispensable roles in the downstream of PTPL1/ERK signaling in NSCLC. Nonetheless, we currently describe a new mechanism by which PTPL1 inhibits pulmonary tumorigenesis, and thus provide candidate targets for clinical therapy and novel biomarkers for prognostic assessment of lung cancer.
AUTHOR CONTRIBUTIONS
JW and SHL performed the experiments, XJZ and RY analyzed data and help conduct partial experiment. NZ, LD and CWL provided helpful discussion and reviewed the manuscript. WG, JWX, JL, DBL and XNL provided guidance on experimental technology and gave suggestions. YZZ and YYZ supervising the study. Shengqing Li designed this study and supervised the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This study was supported by the grants from the National Natural Science Foundation of China (no. 81970048), Research Foundation for Huashan Hospital Affiliated to Fudan University (no. 2017QD078) and the National Science and Technology Major Project (2020DQ056).
Wang J, Li S, Zhang X, Zhu N, Yiminniyaze R, Dong L, et al. Protein tyrosine phosphatase PTPL1 suppresses lung cancer through Src/ERK/YAP1 signaling. Thorac Cancer. 2022;13(21):3042–3051. 10.1111/1759-7714.14657
Jing Wang, Shuanghui Li, Xiujuan Zhang contributed equally to this work.
Funding information National Natural Science Foundation of China, Grant/Award Number: 81970048; National Science and Technology Major Project, Grant/Award Number: 2020DQ056; Research Foundation for Huashan Hospital Affiliated to Fudan University, Grant/Award Number: 2017QD078
REFERENCES
- 1. Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non‐small‐cell lung cancer. Nat Rev Dis Primers. 2015. May;21(1):15009. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. May;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3. Yang CJ, Gu L, Shah SA, Yerokun BA, D'Amico TA, Hartwig MG, et al. Long‐term outcomes of surgical resection for stage IV non‐small‐cell lung cancer: a national analysis. Lung Cancer. 2018. Jan;115:75–83. [DOI] [PubMed] [Google Scholar]
- 4. Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction‐evidence, pit falls and future perspectives. Nat Rev Clin Oncol. 2021. Mar;18(3):135–51. [DOI] [PubMed] [Google Scholar]
- 5. Huang Y, Zhang Y, Ge L, Lin Y, Kwok HF. The roles of protein tyrosine phosphatases in hepatocellular carcinoma. Cancers (Basel). 2018. Mar 20;10(3):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bollu LR, Mazumdar A, Savage MI, Brown PH. Molecular pathways: targeting protein tyrosine phosphatases in cancer. Clin Cancer Res. 2017. May 1;23(9):2136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiede F, Lu KH, Du X, Zeissig MN, Xu R, Goh PK, et al. PTP1B is an intracellular checkpoint that limits T‐cell and CAR T‐cell antitumor immunity. Cancer Discov. 2022. Mar 1;12(3):752–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jobe F, Patel B, Kuzmanovic T, Makishima H, Yang Y, Przychodzen B, et al. Deletion of Ptpn1 induces myeloproliferative neoplasm. Leukemia. 2017. May;31(5):1229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antony J, Zanini E, Kelly Z, Tan TZ, Karali E, Alomary M, et al. The tumour suppressor OPCML promotes AXL inactivation by the phosphatase PTPRG in ovarian cancer. EMBO Rep. 2018. Aug;19(8):e45670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004. Jun 11;117(6):699–711. [DOI] [PubMed] [Google Scholar]
- 11. Wu W, Hale AJ, Lemeer S, den Hertog J Differential oxidation of protein‐tyrosine phosphatases during zebrafish caudal fin regeneration. Sci Rep 2017. Aug 16;7(1):8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao S, Sedwick D, Wang Z. Genetic alterations of protein tyrosine phosphatases in human cancers. Oncogene. 2015. Jul 23;34(30):3885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang X, Guo A, Yu J, Possemato A, Chen Y, Zheng W, et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci U S A. 2007. Mar 6;104(10):4060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solomon DA, Kim J‐S, Cronin JC, Sibenaller Z, Ryken T, Rosenberg SA, et al. Mutational inactivation of PTPRD in glioblastoma multiforme and malignant melanoma. Cancer Res. 2008;68:10300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucci MA, Orlandi R, Triulzi T, Tagliabue E, Balsari A, Villa‐Moruzzi E. Expression profile of tyrosine phosphatases in HER2 breast cancer cells and tumors. Cell Oncol. 2010;32:361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu X, Qu CK. Protein tyrosine phosphatase SHP‐2 (PTPN11) in hematopoiesis and leukemogenesis. J Signal Transduct. 2011;195239; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abaan OD, Toretsky JA. PTPL1: a large phosphatase with a split personality. Cancer Metastasis Rev. 2008. Jun;27(2):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bilder D. PDZ proteins and polarity: functions from the fly. Trends Genet. 2001. Sep;17(9):511–9. [DOI] [PubMed] [Google Scholar]
- 19. Han XJ, Xue L, Gong L, Zhu SJ, Yao L, Wang SM, et al. Stat3 inhibits PTPN13 expression in squamous cell lung carcinoma through recruitment of HDAC5. Biomed Res Int. 2013;2013:468963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Tu Y, Zhao J, Chen K, Wu C. Reversion‐induced LIM interaction with src reveals a novel src inactivation cycle. J Cell Biol. 2009. Mar 23;184(6):785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeh SH, Wu DC, Tsai CY, Kuo TJ, Yu WC, Chang YS, et al. Genetic characterization of fas‐associated phosphatase‐1 as a putative tumor suppressor gene on chromosome 4q21.3 in hepatocellular carcinoma. Clin Cancer Res. 2006. Feb 15;12(4):1097–108. [DOI] [PubMed] [Google Scholar]
- 22. Révillion F, Puech C, Rabenoelina F, Chalbos D, Peyrat JP, Freiss G. Expression of the putative tumor suppressor gene PTPN13/PTPL1 is an independent prognostic marker for overall survival in breast cancer. Int J Cancer. 2009. Feb 1;124(3):638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wieckowski E, Atarashi Y, Stanson J, Sato TA, Whiteside TL. FAP‐1‐mediated activation of NF‐kappaB induces resistance of head and neck cancer to Fas‐induced apoptosis. J Cell Biochem. 2007. Jan 1;100(1):16–28. [DOI] [PubMed] [Google Scholar]
- 24. Ungefroren H, Kruse ML, Trauzold A, Roeschmann S, Roeder C, Arlt A, et al. FAP‐1 in pancreatic cancer cells: functional and mechanistic studies on its inhibitory role in CD95‐mediated apoptosis. J Cell Sci. 2001. Aug;114(Pt 15):2735–46. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Li M, Huang T, Li J. Protein tyrosine phosphatase L1 inhibits high‐grade serous ovarian carcinoma progression by targeting IκBα. Onco Targets Ther. 2018. Oct;30(11):7603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhuang L, Shou T, Li K, Gao CL, Duan LC, Fang LZ, et al. MicroRNA‐30e‐5p promotes cell growth by targeting PTPN13 and indicates poor survival and recurrence in lung adenocarcinoma. J Cell Mol Med. 2017. Nov;21(11):2852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lucero MY, Chan J. Photoacoustic imaging of elevated glutathione in models of lung cancer for companion diagnostic applications. Nat Chem. 2021. Dec;13(12):1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V. αE‐catenin inhibits a Src‐YAP1 oncogenic module that couples tyrosine kinases and the effector of hippo signaling pathway. Genes Dev. 2016. Apr 1;30(7):798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Julien SG, Dubé N, Hardy S, Tremblay ML. Inside the human cancer tyrosine phosphatome. Nat Rev Cancer. 2011. Jan;11(1):35–49. [DOI] [PubMed] [Google Scholar]
- 30. Castilla C, Flores ML, Conde JM, Medina R, Torrubia FJ, Japón MA, et al. Downregulation of protein tyrosine phosphatase PTPL1 alters cell cycle and upregulates invasion‐related genes in prostate cancer cells. Clin Exp Metastasis. 2012. Apr;29(4):349–58. [DOI] [PubMed] [Google Scholar]
- 31. Glondu‐Lassis M, Dromard M, Chavey C, Puech C, Fajas L, Hendriks W, et al. Downregulation of protein tyrosine phosphatase PTP‐BL represses adipogenesis. Int J Biochem Cell Biol. 2009. Nov;41(11):2173–80. [DOI] [PubMed] [Google Scholar]
- 32. Castilla C, Chinchón D, Medina R, Torrubia FJ, Japón MA, Sáez C. PTPL1 and PKCδ contribute to proapoptotic signalling in prostate cancer cells. Cell Death Dis. 2013. Apr 4;4(4):e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamyeh M, Bernex F, Larive RM, Naldi A, Urbach S, Simony‐Lafontaine J, et al. PTPN13 induces cell junction stabilization and inhibits mammary tumor invasiveness. Theranostics. 2020. Jan 1;10(3):1016–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dey A, Varelas X, Guan KL. Targeting the hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020. Jul;19(7):480–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yuan Y, Park J, Feng A, Awasthi P, Wang Z, Chen Q, et al. YAP1/TAZ‐TEAD transcriptional networks maintain skin homeostasis by regulating cell proliferation and limiting KLF4 activity. Nat Commun. 2020. Mar 19;11(1):1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vichas A, Riley AK, Nkinsi NT, Kamlapurkar S, Parrish PCR, Lo A, et al. Integrative oncogene‐dependency mapping identifies RIT1 vulnerabilities and synergies in lung cancer. Nat Commun. 2021. Aug 9;12(1):4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Q, Wang H, Li Z, Li F, Liang L, Zou Y, et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J Hepatol. 2022. Jan;76(1):135–47. [DOI] [PubMed] [Google Scholar]
- 38. Jin D, Guo J, Wu Y, Yang L, Wang X, du J, et al. m6A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs‐mediated YAP expression and inhibiting miR‐107/LATS2‐mediated YAP activity in NSCLC. Mol Cancer. 2020. Feb 27;19(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jin D, Guo J, Wu Y, Du J, Yang L, Wang X, et al. m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1‐miR‐1914‐3p‐YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol. 2021. Feb 23;14(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. LaCanna R, Liccardo D, Zhang P, Tragesser L, Wang Y, Cao T, et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest. 2019. Apr 15;129(5):2107–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aharonov A, Shakked A, Umansky KB, Savidor A, Genzelinakh A, Kain D, et al. ERBB2 drives YAP activation and EMT‐like processes during cardiac regeneration. Nat Cell Biol. 2020. Nov;22(11):1346–56. [DOI] [PubMed] [Google Scholar]
- 42. Leach JDG, Vlahov N, Tsantoulis P, Ridgway RA, Flanagan DJ, Gilroy K, et al. Oncogenic BRAF, unrestrained by TGFβ‐receptor signalling, drives right‐sided colonic tumorigenesis. Nat Commun. 2021. Jun 8;12(1):3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y, Wang Y, Zhou D, Wang K, Wang X, Wang X, et al. Radiation‐induced YAP activation confers glioma radioresistance via promoting FGF2 transcription and DNA damage repair. Oncogene. 2021. Jul;40(27):4580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


