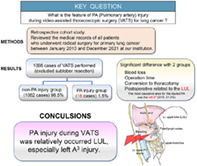
Abstract
Background
Bleeding from the pulmonary artery (PA) can be fatal in video‐assisted thoracoscopic surgery (VATS) for lung cancer. We evaluated intraoperative PA injury and assessed precautions for thoracoscopic anatomic pulmonary resection.
Methods
We retrospectively analyzed a total of 1098 patients who underwent radical surgery for lung cancer utilizing complete VATS from January 2010 to December 2021.
Results
A total of 16 patients (1.5%) had PA injury during VATS, while hemostasis was performed by conversion to thoracotomy in eight patients (50.0%). Although there was a significantly greater operation time and blood loss for patients in the PA injury group (318.4 vs. 264.9 min, p = 0.001; 550.3 vs. 60.5 g, p ≤ 0.001, respectively), there was no significant different for the chest tube insertion duration and length of postoperative hospital stay (4.9 vs. 7.8 days, p = 0.157; 10.6 vs. 9.9 days, p = 0.136, respectively). There was a significant difference observed for the surgical procedure related to the left upper lobectomy in the PA injury group (43.8 vs. 18.8%, p = 0.012), with the primary causative PA determined to be the left anterior segmental PA (A3) (31.3%).
Conclusions
VATS is both feasible and safe for lung cancer treatment provided the surgeon performs appropriate hemostasis, although fatal vascular injury could potentially occur during VATS. Surgeons need to be aware of the pitfalls regarding PA dissection management.
Keywords: left anterior segmental pulmonary artery (A3), pulmonary artery injury, vascular stapler, video‐assisted thoracoscopic surgery
Pulmonary artery (PA) injury during video‐assisted thoracoscopic surgery was relatively occurred left upper lobectomy (LUL), especially left A3 injury. Bleeding control in pulmonary resection is a fundamental challenge, and this retrospective study also confirmed that LUL is prone to accidents as per the surgeon's impression. It is a report as one of the data for building more clear evidence.
INTRODUCTION
With the recent increase in the use of video‐assisted thoracoscopic surgery (VATS) for patients with primary lung cancer, there has been marked progress in the development of associated medical devices related to thoracoscopic surgical systems, including automatic linear staplers and various hemostatic devices. 1 , 2 , 3 VATS for primary lung cancer is now widely accepted based on reports showing decreased duration of chest tube drainage, 4 , 5 , 6 fewer complications, 4 , 5 , 6 , 7 shorter hospital stay, 4 , 5 , 6 , 7 less postoperative pain, 4 improved patient reported outcomes, 6 and earlier recovery to preoperative activity level. 4
In contrast, there are some limitations for the thoracoscopic approach in terms of moving forceps and surgical view, in addition to requiring a much more restricted management of the vascular injury during thoracoscopic surgery as compared to that observed during a thoracotomy. For these reasons, concerns have been raised about the safety of VATS, especially regarding bleeding from the pulmonary arteries (PAs), which can often lead to a life‐threatening situation. Although several valuable reports have shown how to manage intraoperative massive bleeding during thoracoscopic pulmonary resection, 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 there are few reports that have assessed the pitfalls and precautionary management of PA injury.
The purpose of this retrospective study, which was based on our own experience, was to assess the surgical outcomes and evaluate the precautionary management of PA injury during VATS for patients with primary lung cancer.
METHODS
Patient selection
This retrospective cohort study reviewed the medical records of all patients who underwent radical surgery for primary lung cancer between January 2010 and December 2021 at Iwate Medical University Department of Thoracic Surgery. This study was approved by the Iwate Medical University Institutional Review Board. Informed consent was waived for this retrospective medical record review (permit number: MH2020‐075).
At our institution, surgical indications for VATS under monitor vision only require the presence of a thoracoscopically resectable lesion, and do not require bronchial plasty, angioplasty, and chest wall reconstruction, which comprises almost 90% of all of the surgical patients with primary lung cancer. Cases of sublobar resection were excluded. All patients with chronic obstructed pulmonary disease (COPD) were instructed to continue their inhaled medications preoperatively. In all patients, smoking was stopped for at least 8 weeks prior to the surgery. Antithrombotic medication was discontinued at the appropriate period before surgery, with cases heparinized where necessary, and then discontinued at 3 h before surgery. Antithrombotic medication in patients without any bleeding tendencies was started on the day after surgery.
Postoperative complications in this study were evaluated as pulmonary and cardiac complications as defined by Clavien–Dindo classification grade II or higher complications. 17 Pulmonary complications included pneumonia, prolonged air leak, interstitial pneumonitis, atelectasis, bronchopleural fistula, bronchial asthma, atelectasis, and acute respiratory distress syndrome. 18 Prolonged air leak was defined as air leakage lasting 7 days or more. 19 Cardiac complications included atrial fibrillation, hypotension, and bradycardia. 20 Other complications, such as surgical site infection, drug eruption, and mental disorder, were excluded in this study.
General surgical procedures
VATS lobectomy was performed under general anesthesia using a double‐lumen endotracheal tube for single‐lung ventilation. The affected lung was deflated as soon as the pleural space was opened, with this deflation then maintained throughout most of the operative period. The patient was placed in the lateral decubitus position. After first creating a 3‐cm incision in the sixth intercostal space at the midaxillary line, 5 mm of a flexible thoracoscope (Endoeye Flex, Olympus) was inserted. General exploratory thoracoscopy was performed, with an additional intercostal incision made on the anterior axillary line in the third intercostal space and posterior auscultation triangle in the sixth intercostal space. VATS lobectomy was performed via a three‐port method under monitor vision alone. Although the energy devices primarily used included LigaSure™ Blunt Tip (Medtronic) and an automatic linear stapler that utilized the Endo GIA Reload with Tri‐Staple Technology™ (Medtronic), other energy devices as well as vascular and bronchial staplers were allowed at the surgeon's discretion. Large vessels and bronchi were divided with a stapler. Relatively small‐caliber vessels were divided using an energy device or scissors after the ligation. The specimen was placed in an endoscopic tissue collection bag and retrieved through the sixth intercostal access port after completing the pulmonary resection. If necessary, the access incision was lengthened depending on the size of the specimen. A systematic complete hilar and mediastinal lymph node dissection was performed in all cases. Following completion of the procedure, a sealing test was performed prior to closing the wound. The sealing test was confirmed by documentation of the reinflation of the lung on the affected side, with a chest tube (Blake™, 19 Fr, Ethicon) placed from the fifth intercostal trocar to the apex.
Management of intraoperative bleeding from PA
PA injury is defined as significant bleeding from the PA that requires advanced treatment such as compression hemostasis or more in our institute. When bleeding from the PA was identified, the bleeding point was initially gently compressed with lung parenchyma or a cotton stick for several minutes to achieve pressure hemostasis. If the bleeding could not be controlled, a thrombostatic sealant (TachoSil™, CSL Behring), which is a collagen patch coated with human fibrinogen and thrombin, was cut to the appropriate size and subsequently introduced into the thorax through a port and then attached to the bleeding point for a few minutes in conjunction with sponge compression. If two attempts with the TachoSil™ were unsuccessful, or total blood loss reached over 600 ml, then the VATS approach was converted to a thoracotomy, with the operating surgeon determining whether vascular clamps were required to control the massive bleeding. Once the thoracotomy began and the bleeding point was exposed, this was followed by direct suturing of the injured vessels with 5–0 prolene™ (Ethicon) when needed after proximal and distal clamping of the injured PA. The primary cause of the PA injury was verified by three surgeons reviewing the intraoperative video recording postoperatively.
Postoperative management
In general, patients were extubated at the end of the operation and transferred to the ward after a brief stay in the recovery area. In chest tube insertion cases, the tube was placed under −5 cm H2O suction on the morning of postoperative day 1. Chest X‐rays were obtained daily. Chest tube withdrawal criteria included absence of air leakage through the chest tube at the time of evaluation, pleural fluid drainage <200 ml/24 h, and a postoperative chest X‐ray showing no pneumothorax. The morning after chest tube withdrawal, a chest X‐ray was performed to rule out pneumothorax. Routine postoperative pain management was administered in all patients from both groups. Briefly, oral analgesia was started 6 h after surgery, which typically included LOXOPROFEN (60 mg, three times per day) or sometimes a diclofenac suppository (25 mg, one to two times per day, as needed). If no complications occurred during this perioperative period, patients were discharged when convenient. Our institutional standard protocol is to follow all patients every 3–6 months after surgery for 5 years.
Statistical analysis
JMP 12.2.0 (SAS Institute) statistical software was used for statistical analysis. Groups were compared using the Pearson chi‐square test or Wilcoxon's rank sum test. Differences between groups were considered significant at p < 0.05. Continuous data were expressed as mean ± standard deviation, while the categorical data were expressed as counts and proportions.
RESULTS
This retrospective review study enrolled 1098 patients who underwent radical surgery for primary lung cancer. Table 1 summarizes the clinical characteristics for both the non‐PA injury (n = 1082) and PA injury (n = 16) groups during this study period. There were no significant differences between the groups in terms of age, gender, body mass index, Brinkman index, incidence of COPD and interstitial pneumonia (IP), histological type of lung cancer, tumor size, and population of nodal metastasis. PA injury during VATS was observed in 16 patients (1.5%), and hemostasis was done by conversion to thoracotomy in eight patients (50.0%). As compared with the non‐PA injury group, although the PA injury group exhibited a significantly greater difference for the operation time, blood loss, and postoperative cardiovascular system complications, such as arrhythmia (318.4 ± 102.4 vs. 264.9 ± 72.1 min, p = 0.011; 550.3 ± 1317.9 vs. 60.5 ± 67.0 ml, p = 0.001; 18.8 vs. 5.5%, p = 0.022, respectively), there were no significant differences observed for the duration of chest tube insertion and length of postoperative hospital stay (4.9 ± 1.3 vs. 4.8 ± 2.6 days, p = 0.157; 10.6 ± 5.9 vs. 9.9 ± 8.9 days, p = 0.136, respectively). There were no morbidities related to intraoperative bleeding. In the PA injury group, there was a significant difference observed for the surgical procedure related to the left upper lobectomy (LUL) compared to the non‐PA injury group (43.8 vs. 18.8%, p = 0.012).
TABLE 1.
Clinical background of all patients who underwent radical surgery for primary lung cancer
| Non‐PA injury | PA injury | p value | |
|---|---|---|---|
| (n = 1082) | (n = 16) | ||
| Age (years) | 69.2 ± 9.3 | 71.8 ± 9.1 | 0.199 |
| Gender | |||
| Male | 648 (59.9) | 9 (56.3) | 0.768 |
| Female | 434 (40.1) | 7 (43.7) | |
| Body mass index (kg/m2) | 23.3 ± 3.4 | 23.5 ± 2.5 | 0.762 |
| Brinkman index | 497.7 ± 588.2 | 356.6 ± 443.4 | 0.294 |
| Underlying disease | |||
| COPD | 276 (25.5) | 5 (31.3) | 0.726 |
| Interstitial pneumonia | 105 (9.7) | 0 (0) | 0.190 |
| Surgical procedure | |||
| RUL | 329 (30.4) | 5 (31.3) | 0.942 |
| RML | 70 (6.5) | 2 (12.5) | 0.334 |
| RLL | 262 (24.2) | 2 (12.5) | 0.277 |
| LUL | 203 (18.8) | 7 (43.8) | 0.012 a |
| LLL | 175 (16.2) | 0 (0) | 0.080 |
| Bilobectomy | 23 (2.1) | 0 (0) | 0.556 |
| Pneumonectomy | 20 (1.8) | 0 (0) | 0.583 |
| Histology | |||
| Adenocarcinoma | 810 (74.9) | 12 (75.0) | 0.990 |
| Squamous cell carcinoma | 186 (17.2) | 3 (18.8) | 0.870 |
| Other | 86 (7.9) | 1 (6.3) | 0.803 |
| Tumor size (mm) | 26.8 ± 14.8 | 25.7 ± 11.7 | 0.513 |
| Nodal metastasis (positive) | 222 (20.5) | 4 (25.0) | 0.603 |
| Operation time (min) | 264.9 ± 72.1 | 318.4 ± 102.4 | 0.011 a |
| Blood loss (g) | 60.5 ± 67.0 | 550.3 ± 1317.9 | <0.001 a |
| Conversion to thoracotomy | 41 (3.8) | 8 (50.0) | <0.001 a |
| Duration of chest tube drainage (days) | 4.8 ± 2.6 | 4.9 ± 1.3 | 0.157 |
| Postoperative hospital stay (days) | 9.9 ± 8.9 | 10.6 ± 5.9 | 0.136 |
| Postoperative complication | |||
| Respiratory system | 144 (13.3) | 3 (18.8) | 0.526 |
| Cardiovascular system | 59 (5.5) | 3 (18.8) | 0.022 a |
| Other | 48 (4.4) | 0 (0) | 0.389 |
| 30 days mortality | 1 (0.09) | 0 (0) | 0.903 |
Abbreviations: COPD, RLL, RML, RUL, LLL, LUL,
p < 0.05 versus short period group.
Table 2 presents the reasons for the conversion to thoracotomy from VATS. During this study period, the whole conversion rate was 4.5% (49/1098 cases), with the most common cause found to be hilar lymphadenopathy, which was observed in 30.6% (15/49 cases). In contrast, emergency conversion to thoracotomy was performed when vascular injury occurred, which was the most common reason for PA injury in 16.3% (8/49 cases).
TABLE 2.
Reasons for converting to thoracotomy from complete VATS
| Reason for conversion | Cases (%) n = 49 |
|---|---|
| Vascular injury | |
| Pulmonary artery | 8 (16.3) |
| Pulmonary vein | 1 (2.0) |
| Azygos vein | 1 (2.0) |
| Miss fire of stapler | 1 (2.0) |
| Bronchial injury | 1 (2.0) |
| Hilar lymphadenopathy | 15 (30.6) |
| Fused fissure | 7 (14.3) |
| Sever intrathoracic adhesion | 7 (14.3) |
| Concomitant resection of thoracic wall | 2 (4.1) |
| Others | 6 (12.2) |
Table 3 shows the details of the PA injury group (n = 16), which includes the non‐conversion cases. The PA bleeding‐related conversion ratio was 0.7% (8/1098 cases). The primary causative area for the injured PA was the left anterior segmental PA (A3) (31.3%, 5/16 cases). The primary cause of the injuries was technical insufficiency (50.0%). In this case series, the use of thrombostatic sealant as a hemostatic method was performed in 62.5% (10/16 cases), with the recovery approach for converting thoracotomy account for 50.0%.
TABLE 3.
Details of pulmonary artery injury and management for hemostasis
| Variable | Cases (%) n = 16 |
|---|---|
| Injury point | |
| Right | |
| A1 + 3 | 2 (12.5) |
| A2 | 1 (6.3) |
| A5 | 2 (12.5) |
| A6 | 2 (12.5) |
| A7 | 1 (6.3) |
| A8 | 1 (6.3) |
| Left | |
| A1 + 2 | 1 (6.3) |
| A3 | 5 (31.3) |
| A4 | 1 (6.3) |
| Cause of injury | |
| Technical insufficiency | 8 (50.0) |
| Adherence lymph nodes | 4 (25.0) |
| Stapling failure | 3 (18.8) |
| Tumor direct invasion | 1 (6.3) |
| Hemostatic procedure | |
| Compression | 2 (12.5) |
| Thrombostatic sealant | 10 (62.5) |
| Ligation | 1 (6.3) |
| Suture | 3 (18.8) |
| Recovery approach | |
| Thoracoscopy | 8 (50.0) |
| Thoracotomy | 8 (50.0) |
DISCUSSION
Hemorrhage, when caused by pulmonary vascular injuries, is a common and fundamental problem during lung surgery. 8 This is especially the case for bleeding from the PA, which can lead to a life‐threatening crisis during VATS radical surgery in patients with lung cancer. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 This retrospective study evaluated 1098 patients who underwent radical surgery for lung cancer utilizing VATS at our institute. The results showed that PA injury during VATS was observed in 1.5% of cases, with hemostasis done by conversion to thoracotomy in eight patients (50.0%, 8/16 cases). There was a significant difference observed for the surgical procedure related to the LUL in the PA injury group (43.8%, p = 0.012), and the primary causative PA was found to be the left A3 (31.3%). In this current study, we reviewed our previous cases during VATS in patients with primary lung cancer.
Conversion to thoracotomy
Among the treatment options available for massive bleeding during VATS lung surgery, urgent conversion to thoracotomy is the most commonly used. An expert consensus in the literature 8 has cited a report from six European centers that provided details on 3076 cases of VATS major pulmonary resection. 21 The analysis of these cases demonstrated that intraoperative bleeding due to vascular injury occurred in 2.3% of cases, with 5.5% converted to open surgery. 21 A recent study of the Italian VATS lobectomy registry that enrolled 1679 cases from 10 high‐volume centers found that the bleeding‐related conversion rate was 2.6% among all patients. 22 Several previous studies have reported that the conversion ratio, which took into account all of the reasons for conversion, was almost 4–6%, 9 , 11 , 12 , 16 with a PA injury observed during VATS in 1.4%–6.0%. 9 , 11 , 12 , 16 In our current study, our case series demonstrated that the conversion ratio, which took into account all reasons for the conversion, was 4.5%. The observed PA injury during the VATS was 1.5% (with a thoracotomy need for hemostasis of 50.0%), while the bleeding‐related conversion was 0.7% for all the cases of VATS for primary lung cancer. This rate is almost the same as that in previous reports. 9 , 11 , 12 , 16 In addition, despite the longer operation times and greater blood loss in the non‐PA injury group, there was no mortality related to intraoperative bleeding observed in our current evaluation. These results indicate that patients with PA injuries had uneventful postoperative courses, with the longer operation time and greater intraoperative blood loss having no significant effect on postoperative hospitalization or mortality rate. It is therefore important to adequately manage significant intraoperative bleeding, even though these procedures can take more time and result in an additional slight blood loss. Thus, there should be no hesitation in changing to thoracotomy from VATS, as an emergency conversion does not result in an unsuccessful operation. Furthermore, to avoid progressing to a fatal situation, it is important to appropriately treat intraoperative massive bleeding. Thus, surgeons should be ready for the primary closure using a proximal vascular clamp, which may be necessary depending on the situation encountered.
Cause of PA injury
The PA usually travels along the same path as that for the bronchus. 8 However, several inflammatory diseases, such as COPD, IP, and other chronic infectious diseases, can cause chronic inflammation, calcification of hilar lymph nodes, and dense adhesions between the PA and bronchus. These are difficult to surgically separate and thus can increase the dissection‐related vascular injury. Several studies have reported that most patients with pulmonary arterial injury had severe fibrosis around the bronchus or vessels, as well as having dense hilar adhesions. 11 , 12 , 13 , 16 In addition, PA injuries often occur due to improper endo‐stapler angulation adjustments. In our current study, the reasons for the PA injury were adherent lymph node cases (25.0%), technical insufficiency (50.0%), and inadequate PA stapling (18.8%). Although these issues are unavoidable when converting to thoracotomy due to PA fixation of a swollen fibrotic lymph node, surgeons need to be reminded that it may be possible to avoid issues associated with PA injury due to improper use of a vascular stapler.
Location of PA injury
There are few studies that have examined the location of PA injury during VATS lobectomy, but some reports have demonstrated that this is likely to occur in the LUL. 9 , 12 , 16 Yamashita et al. reported a predominant tendency in LUL (30.1%, 8/26 cases) for PA injury. 12 Sawada et al. reported a high tendency for conversion from VATS to open thoracotomy in LUL (45.8%, 11/24 cases), and conversion due to PA injury during LUL was 29.2% (7/24 cases). 16 In particular, life‐threatening bleeding is generally from the proximal end of the PA, with left A3 being typical for LUL. The cause is not clear, but there has been speculation that the left A3 is the first thick branch of the left main PA, which fixation of lymph nodes is likely to have occurred. 23 In this retrospective study, the results show that PA injury during VATS was relatively occurred LUL, especially left A3 injury. Although there are some reports of intraoperative management for preventing PA injury, 24 it is necessary to pay attention for any vascular treatment during VATS lobectomy.
There were several limitations to our current study. First, this was a retrospective study that used data from a single institution. Thus, the small number of samples for the objective group could have affected the statistical accuracy. Second, the experience of the surgeons was not taken into account. As our hospital is a teaching hospital with several different staff surgeons and trainees, the outcomes could differ depending on the person actually performing the procedure.
CONCLUSIONS
In this retrospective study, the results showed that PA injury during VATS was relatively occurred LUL, especially left A3 injury. The frequency of intraoperative PA bleeding in the present study was consistent with that reported in other studies, with the patient outcomes also similar in terms of safety. VATS is both feasible and safe for lung cancer treatment provided the surgeon performs appropriate hemostasis, although fatal vascular injury could potentially occur during VATS. Surgeons need to be aware of the pitfalls regarding PA dissection management.
AUTHOR CONTRIBUTIONS
Conception and design: Makoto Tomoyasu. Collection and assembly of data: Makoto Tomosyasu, Hiroyuki Deguchi, Satoshi Kudo, Wataru shigeeda, Ryuichi Yoshimura, Yuka Kanekoand Hironaga Kanno. Data analysis and interpretation: Makoto Tomoyasu and Hajime Saito. Manuscript writing: All authors. Final approval of manuscript: All authors.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to declare.
ACKNOWLEDGMENTS
The authors thank Dr Tatsuo Tanita for his valuable suggestions.
Tomoyasu M, Deguchi H, Kudo S, Shigeeda W, Kaneko Y, Yoshimura R, et al. Evaluation of pulmonary artery bleeding during thoracoscopic pulmonary resection for lung cancer. Thorac Cancer. 2022;13(21):3001–3006. 10.1111/1759-7714.14649
Contributor Information
Makoto Tomoyasu, Email: tomoyasu@iwate-med.ac.jp.
Hiroyuki Deguchi, Email: hdeguchi@iwate-med.ac.jp.
Satoshi Kudo, Email: sevenstarsgitanes12@yahoo.co.jp.
Wataru Shigeeda, Email: shigeeda@iwate-med.ac.jp.
Yuka Kaneko, Email: ukaneko@iwate-med.ac.jp.
Ryuichi Yoshimura, Email: andromeda8008@gmail.com.
Hironaga Kanno, Email: kahirona@iwate-med.ac.jp.
Hajime Saito, Email: hasaito@iwate-med.ac.jp.
REFERENCES
- 1. Deguchi H, Tomoyasu M, Shigeeda W, Kaneko Y, Kanno H, Saito H. Reduction of air leakage using linear staple device with bioabsorbable polyglycolic acid felt for pulmonary lobectomy. Gen Thorac Cardiovasc Surg. 2020;68:266–72. [DOI] [PubMed] [Google Scholar]
- 2. Shigeeda W, Deguchi H, Tomoyasu M, Kaneko Y, Kanno H, Tanita T, et al. The utility of the stapler with PGA sheet for pulmonary wedge resection: a propensity score‐matched analysis. J Thorac Dis. 2019;11:1546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deguchi H, Tomoyasu M, Shigeeda W, Kaneko Y, Kanno H, Maeda E, et al. Usefulness of a suction ball coagulation probe for hemostasis in complete VATS lobectomy for patients with non‐small cell lung cancer. Surg Today. 2019;49:580–6. [DOI] [PubMed] [Google Scholar]
- 4. Cao C, Manganas C, Ang SC, Yan TD. A meta‐analysis of unmatched and matched patients comparing video‐assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg. 2012;1:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Wang S, Qu J. Video‐assisted thoracic surgery (VATS) compares favorably with thoracotomy for the treatment of lung cancer: a five‐year outcome comparison. World J Surg. 2009;33:1857–61. [DOI] [PubMed] [Google Scholar]
- 6. Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early‐stage non‐small cell lung cancer: a systematic review of the video‐assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–16. [DOI] [PubMed] [Google Scholar]
- 7. Miller DL, Roy S, Kassis ES, Yadalam S, Ramisetti S, Johnston SS. Impact of powered and tissue‐specific endoscopic stapling technology on clinical and economic outcomes of video‐assisted thoracic surgery lobectomy procedures: a retrospective, observational study. Adv Ther. 2018;35:707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu L, Mei J, He J, Demmy TL, Gao S, Li S, et al. International interest group on bleeding during VATS lung surgery. International expert consensus on the management of bleeding during VATS lung surgery. Ann Transl Med. 2019;7(23):712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Igai H, Kamiyoshihara M, Yoshikawa R, Ohsawa F, Yazawa T, Matsuura N. Algorithm‐based troubleshooting to manage bleeding during thoracoscopic anatomic pulmonary resection. J Thorac Dis. 2019;11:4544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez‐Rivas D, Stupnik T, Fernandez R, et al. Intraoperative bleeding control by uniportal video‐assisted thoracoscopic surgery†. Eur J Cardiothorac Surg. 2016;49(Suppl 1):i17–24. [DOI] [PubMed] [Google Scholar]
- 11. Miyazaki T, Yamasaki N, Tsuchiya T, Matsumoto K, Hatachi G, Kitamura Y, et al. Management of unexpected intraoperative bleeding during thoracoscopic pulmonary resection: a single institutional experience. Surg Today. 2016;46:901–7. [DOI] [PubMed] [Google Scholar]
- 12. Yamashita S, Tokuishi K, Moroga T, Abe S, Yamamoto K, Miyahara S, et al. Totally thoracoscopic surgery and troubleshooting for bleeding in non‐small cell lung cancer. Ann Thorac Surg. 2013;95:994–9. [DOI] [PubMed] [Google Scholar]
- 13. Mei J, Pu Q, Liao H, Ma L, Zhu Y, Liu L. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc. 2013;27:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang C, Wen H, Guo Y, Shi B, Tian Y, Song Z, et al. Severe intraoperative complications during VATS lobectomy compared with thoracotomy lobectomy for early stage non‐small cell lung cancer. J Thorac Dis. 2013;5:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flores RM, Ihekweazu U, Dycoco J, Rizk NP, Rusch VW, Bains MS, et al. Video‐assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg. 2011;142:1412–7. [DOI] [PubMed] [Google Scholar]
- 16. Sawada S, Komori E, Yamashita M. Evaluation of video‐assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg. 2009;36:487–90. [DOI] [PubMed] [Google Scholar]
- 17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118:317–34. [DOI] [PubMed] [Google Scholar]
- 19. Rivera C, Bernard A, Falcoz PE, Thomas P, Schmidt A, Bénard S, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg. 2011;92:1062–8. [DOI] [PubMed] [Google Scholar]
- 20. Uramoto H, Nakanishi R, Fujino Y, Imoto H, Takenoyama M, Yoshimatsu T, et al. Prediction of pulmonary complications after a lobectomy in patients with non‐small cell lung cancer. Thorax. 2001;56:59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Decaluwe H, Petersen RH, Hansen H, Piwkowski C, Augustin F, Brunelli A, et al. Major intraoperative complications during video‐assisted thoracoscopic anatomical lung resections: an intention‐to‐treat analysis. Eur J Cardiothorac Surg. 2015;48:588–98. [DOI] [PubMed] [Google Scholar]
- 22. Bertolaccini L, Davoli F, Pardolesi A, Brandolini J, Argnani D, Bertani A, et al. Conversion due to vascular injury during video‐assisted thoracic surgery lobectomy: a multicentre retrospective analysis from the Italian video‐assisted thoracic surgery group registry. Eur J Surg Oncol. 2019;45:857–62. [DOI] [PubMed] [Google Scholar]
- 23. Edwards JP, Balderson SS, D'Amico TA. Management of pulmonary arterial bleeding in the post induction setting. J Vis Surg. 2016;16:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tomoyasu M, Deguchi H, Shigeeda W, Saito H. Pitfall of left anterior segmental pulmonary artery (A3) dissection in left upper lobectomy. A technical note. Asian J Surg. 2020;43:853–4. [DOI] [PubMed] [Google Scholar]


