Summary
Background
A growing number of studies have reported an increased risk of cardiovascular disease (CVD) and respiratory disease (RD) within hours after exposure to ambient air pollution or temperature. We assemble published evidence on the sub-daily associations of CVD and RD with ambient air pollution and temperature.
Methods
Databases of PubMed and Web of Science were searched for original case-crossover and time-series designs of English articles examining the intra-day effects of ambient air pollution [particulate matter with aerodynamic diameter ≤2.5 μm (PM2.5), ≤10 μm (PM10), 2.5–10μm (PM10-2.5), and < 7 μm (SPM), O3, SO2, NO2, CO, and NO] and temperatures (heat and cold) on cardiorespiratory diseases within 24 h after exposure in the general population by comparing with exposure at different exposure levels or periods. Meta-analyses were conducted to pool excess risks (ERs, absolute percentage increase in risk) of CVD and RD morbidities associated with an increase of 10 μg/m3 in particulate matters, 0.1 ppm in CO, and 10 ppb in other gaseous pollutants.
Findings
Final analysis included thirty-three papers from North America, Europe, Oceania, and Asia. Meta-analysis found an increased risk of total CVD morbidity within 3 h after exposure to PM2.5 [ER%: 2.65% (95% CI: 1.00% to 4.34%)], PM10-2.5 [0.31% (0.02% to 0.59%)], O3 [1.42% (0.14% to 2.73%)], and CO [0.41% (0.01% to 0.81%)]. The risk of total RD morbidity elevated at lag 7–12 h after exposure to PM2.5 [0.69% (0.14% to 1.24%)] and PM10 [0.38% (0.02% to 0.73%)] and at lag 12–24 h after exposure to SO2 [2.68% (0.94% to 4.44%)]. Cause-specific CVD analysis observed an increased risk of myocardial infarction morbidity within 6 h after exposure to PM2.5, PM10, and NO2, and an increased risk of out-of-hospital cardiac arrest morbidity within 12 h after exposure to CO. Risk of total CVD also increased within 24 h after exposure to heat.
Interpretation
This study supports a sudden risk increase of cardiorespiratory diseases within a few hours after exposure to air pollution or heat, and some acute and highly lethal diseases such as myocardial infarction and cardiac arrest could be affected within a shorter time.
Funding
The National Natural Science Foundation of China (Grant No. 42105165; 81773518), the High-level Scientific Research Foundation of Anhui Medical University (Grant No. 0305044201), and the Discipline Construction of Anhui Medical University (Grant No. 0301001836).
Keywords: Air pollution, Temperature, Cardiovascular, Respiratory, Sub-daily, Intra-day
Research in context.
Evidence before this study
Although mounting studies have shown that increased risk of cardiovascular or respiratory disease was associated with air pollution or temperature on the same day or few days of exposure, the existing evidence for daily association was insufficient to guide the prevention of acute and highly lethal cardiorespiratory diseases (e.g., myocardial infarction) which could be triggered within a few hours after exposure. In recent years, studies in many regions of the world have explored this association on an hourly scale, but with inconsistent and mixed findings. So, a synthesis of existing studies on the sub-daily association of cardiorespiratory disease with air pollution and temperature is required to identify hazardous exposure(s) and risk exposure windows, as well as cause-specific cardiorespiratory diseases affected.
Added value of this study
This global-scale systematic review provides robust evidence of an increased risk of cardiovascular or respiratory disease within 24 h after exposure to air pollution or temperature. Specifically, an increased risk of total cardiovascular disease was associated with exposure to high temperature and air pollution (PM2.5, PM10, PM10-2.5, O3, NO2, SO2, and CO), and an increased risk of respiratory disease was associated with exposure to PM2.5, PM10, and SO2. In addition, after exposure to air pollution, the risk of acute cause-specific cardiovascular disease such as myocardial infarction could increase within 6 h, and out-of-hospital cardiac arrest could increase within 12 h.
Implications of all the available evidence
Our findings suggest that potentially life-threatening cardiorespiratory disease risk from transient exposure to air pollution or hot weather needs to be considered by healthcare professionals including cardiologists and respiratory physicians as well as patient caregivers to avoid negative cardiorespiratory outcomes.
Introduction
Ambient air pollution and non-optimal temperatures (e.g., extreme heat and cold) lead to substantial cardiovascular (CVD) and respiratory disease (RD) burden worldwide.1,2 In past decades, numerous studies focused on the short-term associations between ambient air pollution and temperature and cardiorespiratory diseases and reported that the effects of exposures could occur on the same day of exposure or a few days later.3, 4, 5 However, cardiovascular or respiratory subclinical markers may change within a few hours after exposure to air pollution and temperature.6,7 For example, an increase in temperature was associated with a decrease in heart rate variability within 6 h of exposure.6 The airway inflammatory marker, nitric oxide in exhaled breath [FE(NO)], could be elevated within 12 h after exposure to PM2.5 (particulate matter with aerodynamic diameter ≤2.5 μm).7 The presence of these reactions may lead to an attack or worsening of the disease. As such, evidence of daily-level associations of cardiorespiratory diseases with ambient air pollution and non-optimal temperatures is less desirable for developing strategies to prevent acute and highly lethal cardiorespiratory diseases [e.g., myocardial infarction (MI)], because these diseases could be triggered within a few hours of exposure.8 Therefore, it is of public health and clinical importance to investigate the sub-daily effects of ambient air pollution and non-optimal temperatures on cardiorespiratory diseases.
Although sub-daily associations between ambient air pollution and temperature and cardiorespiratory diseases have been examined, findings were mixed and inconsistent. For example, a study in New York showed that PM2.5 exposure was associated with an increased risk of MI in the concurrent hour of exposure,9 while this association was not found in another study in Washington.10 Wang et al. (2021)11 observed an increased risk of out-of-hospital cardiac arrest (OHCA) within 16–18 h after heat exposure, whereas Dahlquist et al. (2016)12 did not observe this association. Therefore, a comprehensive review and synthesis of published evidence on the sub-daily effects of ambient air pollutants and temperature on the risk of cardiorespiratory diseases would be useful.
We conducted this comprehensive systematic review and meta-analysis to assemble the available evidence on the sub-daily associations between exposure to air pollution and non-optimal temperatures and risks of cardiorespiratory diseases, aiming to answer three key questions: (i) which air pollutant(s) or temperature exposure could increase risks of CVD or RD within 24 h? (ii) which cause-specific CVD or RD could be triggered within 24 h after exposure to air pollution or temperature? and (iii) what is the susceptible time window?
Methods
Data sources and search strategy
PubMed and Web of Science databases were searched from inception to October 31, 2021 for relevant studies investigating the risk of cardiovascular or respiratory diseases within 24 h after exposure to ambient air pollution or temperature. Two reviewers (MY and QYW) searched for relevant literature by screening the title and abstract, and eligible literature underwent a full-text review. In addition, the reference lists of the included literature were checked to ensure that all relevant papers could be included.
We used the following search terms: i) Time resolution (hourly, short-term); ii) Exposure variables (temperature, hot, heat, cold, air pollution, air pollutants, particulate matter, fine particle, PM2.5, PM10, gaseous pollutant, nitrogen dioxide, sulfur dioxide, nitrogen oxide, carbon monoxide, ozone, SO2, NO2, NO, O3, CO); iii) Outcome variables (cardiovascular, myocardial infarction, ischemic heart disease, heart failure, cardiac failure, cardiac arrest, heart arrest, atrial fibrillation, atrial flutter, arrhythmia, respiratory, asthma, bronchitis, pneumonia, chronic obstructive pulmonary disease, COPD, cardiorespiratory). All literature was limited to population-based studies published in English journal articles. Detailed searching strategies were shown in Supplement Table S1.
Inclusion and exclusion criteria
Because this review focused on short-term effects of exposures (i.e., air pollution and temperature) within 24 h exposure window, we included two common study designs in environmental health studies: time-stratified case-crossover and time-series designs. Time-stratified case-crossover design is a sub-type of case–control study that assesses exposure at an individual level base on a “case matching its own controls” approach. Traditionally, each event and its exposure in a case period (i.e., at the time of the event) were compared with events (and corresponding exposures) in control periods (e.g., the day of the week in the same month and year), whereby controlling for the day of the week effect, seasonal trends as well as individual characteristics (e.g., age and gender).13,14 In comparison, time-series design is an ecological study that applies group data to assess exposure-health variations at the population level, based on differences in event counts (e.g., number of hospital admissions or emergency department visits) and exposure levels at two specific time points and adjustment for confounding factors such as meteorological parameters.15
Additional criteria to consider the eligibility of a study included: i) general population as the population of interest; ii) ambient temperature or particular matters [aerodynamic diameter ≤2.5 μm (PM2.5), ≤10 μm (PM10), 2.5–10μm (PM10-2.5), or < 7 μm (SPM)] or gaseous pollutants [ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO), or nitrogen monoxide (NO)] as exposures of interest; iii) cardiorespiratory diseases as the outcome of interest. These included total or cause-specific CVD or RD such as MI, OHCA, asthma, and respiratory infection; iv) effect estimates of air pollution or temperature [odds ratio (OR), relative risk (RR), excess risk (ER), percent change] were reported or could be calculated from the included studies. Otherwise, the corresponding author was contacted by e-mail. If there was no response, we also sought to contact the first author or other authors to get the data. Exclusion criteria included: i) merely focused on the daily-level association between air pollution/temperature and cardiorespiratory disease; ii) did not report any effect estimates; iii) human or animal experiment; or iv) systematic review.
Data extraction and quality evaluation
Two reviewers (QYW and MY) extracted the information from each included literature separately, including the first author's last name, study country, study population and period, study design, the type of outcome, the onset timing of outcome, exposure, source of exposure, outcome, diagnostics, lag time (hours), effect estimates [i.e., OR, RR, ER, percent change and 95% confidence intervals (CIs)], and adjustment variables. Disagreement on data extraction was resolved through discussion with a third reviewer (JWT). Detailed information was presented in Supplement Table S2–S7.
Three reviewers (QYW, MY, and JWT) evaluated the quality of the case-crossover and time-series studies by referring to the method of the previous study,16 which was developed based on the Newcastle Ottawa17 and Cochrane risk of bias tool.18 The assessment method consists of three components [quality of exposure measurement (0–1 point), quality of outcome (0–1 point), and degree of adjustment for confounding (0–3 points)] (Supplement Table S8). If all three components received the highest score, the study was considered to be “high quality”; if one of the three components received the lowest score (i.e., 0), the study was considered to be “low quality”; otherwise, the study was considered to be “moderate quality”.
Data synthesis methods
To uniformly measure the effects of exposures on cardiorespiratory diseases, effect estimates of OR/RR/percent change reported in each study were transformed to ER using the formula: ER = OR/RR–1; ER = percent change∗100. Study-specific ER was then pooled for a standardized increment in air pollutant concentration as follows: 10 μg/m3 for particulate matters, 0.1 ppm (parts per million) for CO, and 10 ppb (parts per billion) for other gaseous pollutants.19
Two non-optimal temperature indicators were included in this review: heat and cold. We defined heat as the temperature exceeding a threshold such as the minimum morbidity temperature (MMT) or the average value of hourly temperature distribution, or extreme thermal condition compared to a reference temperature (e.g., 95th versus 50th).20,21 By contrast, cold referred to the temperature below a threshold or average value of hourly temperature distribution, or extreme cold condition compared to a reference temperature.20,21
The exposure-response association between air pollutants and cardiorespiratory disease in most prior studies was considered to be linear.22,23 If the study only reported effect estimates by comparing different air pollutant levels (e.g., 95th versus 5th of pollutant concentration or 90th versus 10th of pollutant concentration),11,24 we treated pollutant difference between the two pollution levels as the increment and transformed it to a standardized increment as mentioned above. If the study only reported effect estimates at multiple pollution levels (e.g., a fixed increase of air pollutant concentration at 50th and 95th),25 we first transformed the fixed value to a standardized increment, then used a restricted maximum likelihood (REML) fixed-effects meta-analysis model to obtain combined effects to reflect the overall effect.
To capture the effects of air pollutants on CVD and RD at different time windows within 24 h after exposure, we pooled existing effect estimates by aggregating and categorizing the exposure time windows available from original articles. Finally, exposure time windows for intra-day effects of air pollutants on total CVD in the meta-analysis were grouped into lag 0–3h (included lag 0, 1, 2, 3, 0–1, 0–2, 0–3, 1–2, and 1–3h), 0–6h, 0–12h, 0–24h, 7–12h, and 13–24h. Exposure time windows for MI included lag 0–6h, 0–12h, 0–24h, 7–12h, and 13–24h, and for OHCA included lag 0–3h, 0–6h, 0–12h, and 0–24h. Exposure time windows for the effects of PM2.5 and PM10 on total RD included lag 0–6h, 0–12h, 0–24h, 7–12h, and 13–24h, and exposure time windows for the effects of gaseous pollutants on total RD included lag 0–11h, 12–24h, 0–24h.
We obtained effect estimates of total CVD and RD using the following methods: i) If an effect estimate for total CVD or RD was given in the article, we included the effect estimate in the final meta-analysis; ii) If only one cause-specific CVD or RD was analyzed in the article, we also included the effect estimate in the final meta-analysis; iii) If multiple cause-specific CVD or RD were explored in the article, a fixed-effects meta-analysis model was used to pool all cause-specific CVD or RD estimates to obtain the overall effect estimates.20 Due to a lack of available studies, we only performed the meta-analysis of the cause-specific diseases, OHCA and MI.
Several studies reported effect estimates for multiple exposure time windows. We obtained effect estimates using the following methods: i) If the exposure time windows in a study did not overlap with each other (e.g., lag 0h, lag 1h, and lag 2–3h), a fixed-effects meta-analysis model would be used to pool all effect estimates within 3 h after exposure to obtain the estimate of lag 0–3h; ii) If the exposure time windows in a study partially overlapped with each other (e.g., lag 0h, lag 1h, and lag 1–3h), a fixed-effects meta-analysis model would be used to pool the overlapping (lag 1–3h) and non-overlapping (lag 0h) within 3 h after exposure to obtain the estimate of lag 0–3h; iii) If the exposure time windows in a study completely overlapped with each other (e.g., lag 0h, lag 1h, lag 0–3h), the overlapping window (lag 0–3h) was used in the final meta-analysis.26 Effect estimates for the final meta-analysis were shown in Supplement Tables S9–S12.
Data analysis
REML random- or fixed-effects meta-analysis was used to quantify the intra-day effects of air pollutants on total and cause-specific CVD and RD. The heterogeneity of included studies was reflected by Cochran's Q and I2 statistics. Low, moderate, and high heterogeneity were considered when I2 was below 25%, between 25% and 75%, and above 75%, respectively.20 A fixed-effects meta-analysis model was employed in the case of p > 0.05 for Cochran's Q test; otherwise, a random-effects meta-analysis model was used. Publication bias was examined for the effect of air pollutants with at least 10 risk estimates by visually inspecting funnel plots and assessing them for asymmetry using Egger's test.27 Furthermore, a trim-and-fill method was used to evaluate the potential impacts of missing studies.28 To identify the source of heterogeneity, we also conducted a subgroup analysis based on the type of outcome. Sensitivity analyses were also conducted to explore the effects of article quality and adjustment variables. The differences between the two effect estimates were checked by Z-test.29
A narrative review was also performed for studies that were not included in the meta-analysis.30 All data analyses were conducted in R software (version 4.1.2) using the package “meta”. A two-sided p < 0.05 was considered statistically significant.
This systematic review was based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)31 and comply with the recommendations of Meta-analysis of Observational Studies in Epidemiology (MOOSE)32 and registered with PROSPERO (number: CRD42022312550).
Role of funders
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
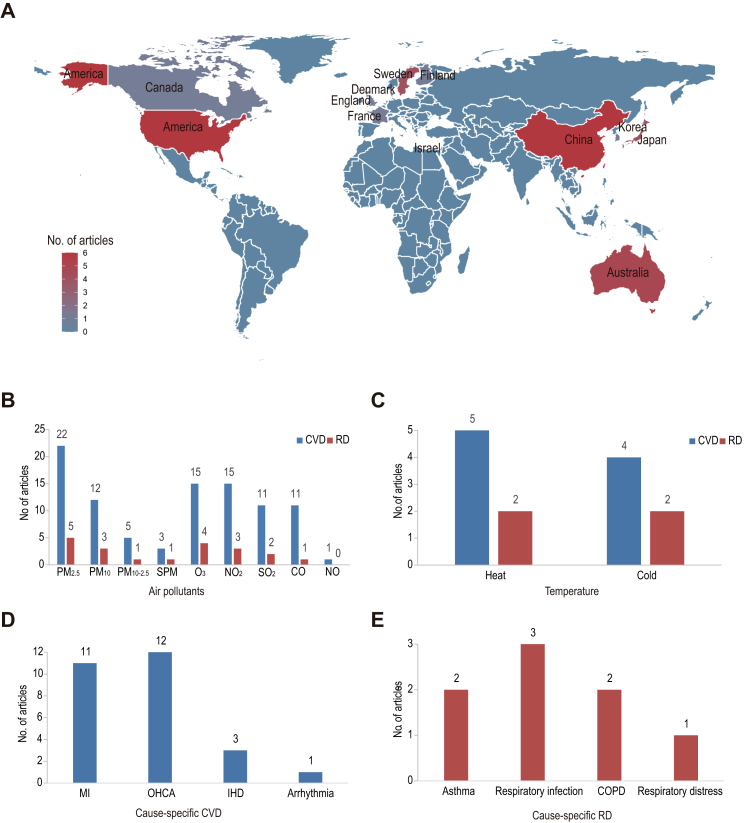
A total of 9241 articles were identified in the primary screening after removing duplications. After screening titles and abstracts, 47 articles underwent a full-text review. Finally, 33 articles8, 9, 10, 11, 12,24,25,33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 were included in this systematic review, and 26 articles were included in the meta-analysis (Supplement Fig. S1 and Tables S2–S7), 29 articles were evaluated as moderate-quality and 4 articles were evaluated as high-quality (Supplement Table S13). These studies were conducted in North America, Europe, Oceania, and Asia (Fig. 1a), with the majority of studies focusing on morbidity. PM2.5 was studied most often among the air pollutants (Fig. 1b) and heat exposure was studied most often among non-optimal temperatures (Fig. 1c). Cause-specific CVD with the majority of studies focusing on OHCA and MI (Fig. 1d). Cause-specific RD involved respiratory infection, asthma, chronic obstructive pulmonary disease (COPD), and respiratory distress (Fig. 1e).
Fig. 1.
The geographical distribution of eligible studies by country (a), and the number of studies that focused on air pollutants (b), temperature (b), cause-specific cardiovascular diseases (d), and cause-specific respiratory diseases (e). CVD, cardiovascular disease; RD, respiratory disease; MI, myocardial infarction; OHCA, out-of-hospital cardiac arrest; IHD, ischemic heart disease; COPD, chronic obstructive pulmonary disease.
Air pollutants and cardiovascular diseases
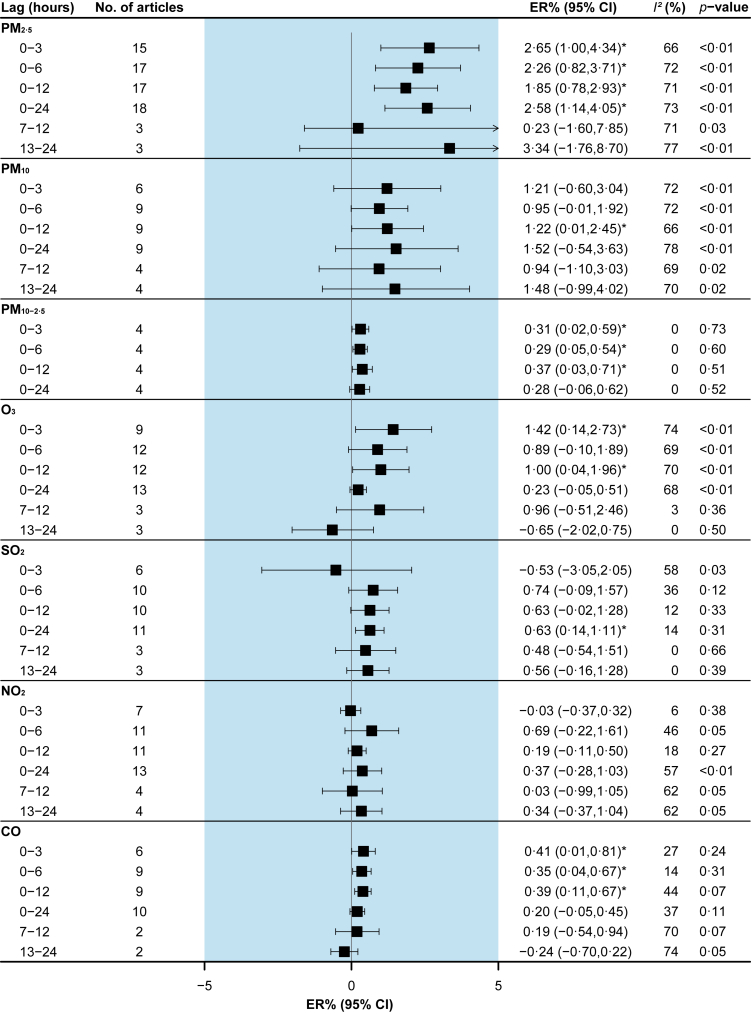
There was an increased risk of total CVD morbidity within 3 h after exposure to PM2.5 [2.65% (95%CI: 1.00% to 4.34%)], PM10-2.5 [0.31% (95%CI: 0.02% to 0.59%)], O3 [1.42% (95%CI: 0.14% to 2.73%)], and CO [0.41% (95%CI: 0.01% to 0.81%)], and PM10 and SO2 were associated with total CVD morbidity at lag 0–12h [1.22% (95%CI: 0.01% to 2.45%)] and lag 0–24h [0.63% (95%CI: 0.14% to 1.11%)], respectively (Fig. 2).
Fig. 2.
Pooled associations between air pollutants and total cardiovascular disease morbidity. Excess risks (ERs) are for an increase of 10 μg/m3 of particulate matter, 0.1 ppm of CO, and 10 ppb of other gaseous pollutants. I2 statistic represents the proportion of total variation between effect estimates due to heterogeneity. The p-value is based on Cochran's Q test.
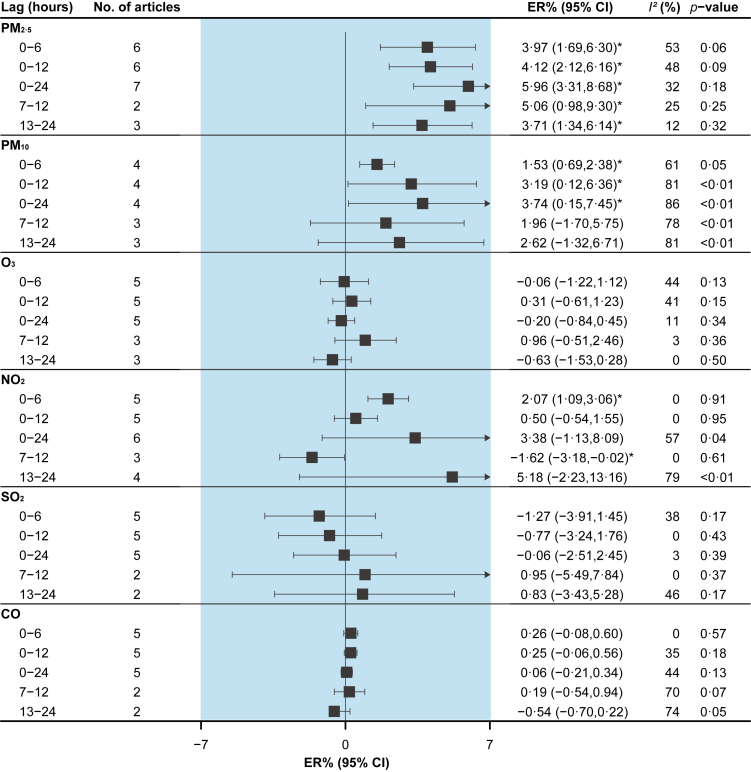
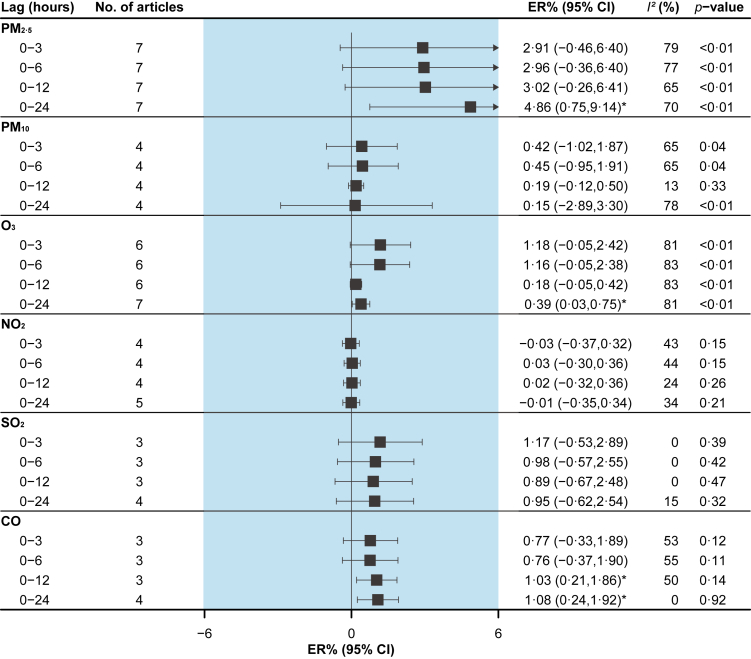
PM2.5 [3.97% (95%CI: 1.69% to 6.30%)], PM10 [1.53% (95%CI: 0.69% to 2.38%)] and NO2 [2.07% (95%CI: 1.09% to 3.06%)] were associated with an elevated risk of MI morbidity at lag 0–6h (Fig. 3). CO [1.03% (95%CI: 0.21% to 1.86%)] was associated with an increased risk of OHCA morbidity at lag 0–12h, and PM2.5 [4.86% (95%CI: 0.75% to 9.14%)] and O3 [0.39% (95%CI: 0.03% to 0.75%)] were associated with an increased risk of OHCA at lag 0–24h (Fig. 4).
Fig. 3.
Pooled associations between air pollutants and myocardial infarction morbidity. Excess risks (ERs) are for an increase of 10 μg/m3 of particulate matter, 0.1 ppm of CO, and 10 ppb of other gaseous pollutants. I2 statistic represents the proportion of total variation between effect estimates due to heterogeneity. The p-value is based on Cochran's Q test.
Fig. 4.
Pooled associations between air pollutants and out-of-hospital cardiac arrest morbidity. Excess risks (ERs) are for an increase of 10 μg/m3 of particulate matter, 0.1 ppm of CO, and 10 ppb of other gaseous pollutants. I2 statistic represents the proportion of total variation between effect estimates due to heterogeneity. The p-value is based on Cochran's Q test.
Relatively fewer studies focused on SPM37,52 and NO36 (Supplement Table S4). Studies conducted in Japan showed that SPM was associated with an increased risk of total CVD morbidity37 [1.92% (95%CI: 0.48% to 2.87%), per 20.6 μg/m3 increased, lag 0–6h] and MI mortality52 [13.00% (95%CI: 7.00% to 20.00%), 100–149 μg/m3 versus 0–99 μg/m3, lag 1h], but not associated with OHCA morbidity.38 NO was not associated with OHCA morbidity.36
Air pollution and respiratory diseases
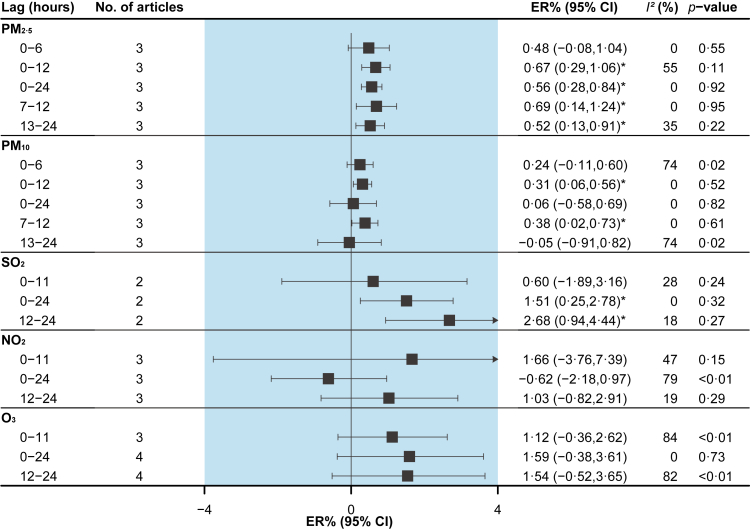
PM2.5 and PM10 were associated with an increased risk of total RD morbidity at lag 7–12h [0.69% (95%CI: 0.14% to 1.24%) and 0.38% (95%CI: 0.02% to 0.73%), respectively], and SO2 at lag 12–24h [2.68% (95%CI: 0.94% to 4.44%)] (Fig. 5).
Fig. 5.
Pooled associations between air pollutants and total respiratory disease morbidity. Excess risks (ERs) are for an increase of 10 μg/m3 of particulate matter, and 10 ppb of gaseous pollutants. I2 statistic represents the proportion of total variation between effect estimates due to heterogeneity. The p-value is based on Cochran's Q test.
Several studies also examined the effects of PM10-2.5, SPM, and CO (Supplement Table S5).49,50 However, only PM10-2.5 was marginally associated with an increased risk of asthma morbidity at lag 1–6h [5.00% (95%CI: 0.00% to 11.00%), per 16.7 μg/m3 increased].50
Temperature and cardiorespiratory diseases
Five articles examined the effect of heat on CVD morbidity (Supplement Table S6). Articles from China showed that extreme heat was associated with an increased risk of total CVD56 at lag 0h [0.40% (95%CI: 0.10% to 0.70%), 32.1 °C versus MMT], and OHCA11 at lag 16–18h [0.06% (95%CI: 0.01% to 0.11%), 32 °C versus 25 °C]. However, a Sweden study suggested a non-significant increase in the risk of OHCA when temperature increased by 5 °C during the warm interval (>16 °C at lag 1h, >12 °C at lag 1–24h).12 In addition, studies in Australia57 and England55 showed that heat was associated with an increased risk of MI.
Four articles explored the association between cold and CVD morbidity, with mixed findings (Supplement Table S6). A study from Sweden12 showed that the risk of OHCA increased by 7.00% (95%CI: 2.00% to 11.00%) for a 5 °C decrease in temperature during the cold interval (<16 °C) at lag 1h, but an increased risk of OHCA associated with extreme cold (15 °C versus 25 °C) was not observed in a Chinese study.11 A study in Australia57 showed that cold (5 °C) could lead to an increased risk of acute MI at lag 9h to lag 22h. However, for total CVD, a Chinese study56 found that cold may be associated with a risk reduction [−12.00% (95% CI: −20.00% to −4.00%), −2.5 °C versus MMT].
Two available studies from China examined the link between extreme temperature and total RD56 and respiratory distress11 morbidity within 24 h, but no significant associations were found (Supplement Table S7).
Heterogeneity and publication bias
The heterogeneity of results was mainly low to moderate, but also partially high, especially for the studies of O3, which were mostly at marginally moderate to high heterogeneity (Fig. 2, Fig. 3, Fig. 4, Fig. 5). Publication bias existed in the pooled analyses of the effects of most air pollutants (Supplement Table S14 and Fig. S2). Despite the publication bias, pooled effect estimates remained stable before and after using a trim-and-fill approach (p > 0.05 for the Z-test) (Supplement Table S15).
Subgroup and sensitivity analysis
Due to the limited number of included studies, we can only conduct a subgroup analysis by the emergency medical service (EMS) and hospital admission for CVD morbidity. Generally, the effect of air pollution remained positively associated with the risk of total CVD morbidity. For the association between O3 and total CVD morbidity, heterogeneity was low for hospital admission but high for EMS; for the effects of other pollutants, heterogeneity was mostly low to moderate (Supplement Table S16).
After removing high-quality articles, the effect of air pollution on CVD morbidity risk in moderate-quality studies was most stable and showed not statistically different from the overall effect estimates (p > 0.05 based on Z-test). Heterogeneity was generally low to moderate, except for studies for PM2.5 (lag 13–24h), PM10 (lag 13–24h), and O3 (lag 0–3h). Note that all other groups of O3 studies were only marginally moderate (I2>72%) (Supplement Table S17).
After removing articles with adjustments for uncommon factors (i.e., wind speed, air pressure, public holidays, or influenza), the effect of air pollution on CVD morbidity risk was only stable for PM2.5, O3, and CO, but all effect estimates were not statistically different from overall effect estimates (p > 0.05 based on Z-test). Studies for PM10 (lag 0–24h) with high heterogeneity and almost all groups for O3 were with high or marginally moderate heterogeneity (Supplement Table S18).
Discussion
Two findings from this systematic review and meta-analysis are noteworthy. Firstly, heat, PM2.5, PM10, PM10-2.5, O3, NO2, SO2, and CO were associated with increased risk of CVD morbidity, and PM2.5, PM10, and SO2 were associated with increased risk of RD morbidity within 24 h of exposure. Secondly, after exposure to air pollution, the risk of total CVD and MI morbidities may be increased within 6 h, the risk of OHCA morbidity may be increased within 12 h, and the risk of total RD morbidity may be increased within 7–24 h. Subgroup and sensitivity analyses showed the results to be generally stable.
Our meta-analysis findings provided evidence that CVD may occur within a few hours after exposure to air pollution. Although the underlying mechanisms have yet been well understood, pathophysiological responses associated with air pollutants have been postulated to be responsible for the increases in the risk of CVD morbidity. For example, heart rate variability was found to be decreased within 4 h of PM10, PM10-2.5, and SO2 exposure.59,60 Systolic blood pressure could decrease within a few hours of SO2 or CO exposure and increase within a few hours of O3 or NO2 exposure.61 It is worth noting that acute effects may also occur at the concurrent hour or 1 h after exposure.36,58 However, the limited number of available studies restricted to undertake of a more detailed analysis. Pooling results regarding an increased morbidity risk of RD within a few hours of air pollution exposure could be supported by physiology studies. For example, a decrease in peak expiratory flow (PEF) and an increase in FE(NO) was found with 12–24h and 10–12h PM2.5 exposure windows, respectively.7,62 Bronchial hyperresponsiveness occurred in asthmatics after SO2 exposure for 24h.63 Additionally, sputum eosinophil concentrations in asthmatics were significantly reduced after NO2 exposure for 6h,64 despite no significant association between NO2 and RD in our results. Furthermore, one study reported that PEF could decrease immediately after exposure to PM10,65 which indicates a possible more acute effect. Therefore, health professionals need to be made aware of the very acute effect of air pollutants on the risk of cardiorespiratory diseases, as they work at the frontline to treat the patients. Future studies are warranted to investigate the exposure to air pollution at finer time windows.
The existing body of evidence suggested that there was an intra-day association between heat and CVD morbidity. A physiological study showed that platelet counts and blood viscosity may increase within a few hours after exposure to heat,66 which could promote thrombosis and thus lead to sudden attacks such as MI. Interestingly, such phenomena may also occur after cold exposure.67 However, findings from different studies regarding cold and CVD morbidity were inconsistent, which may be attributable to regional differences such as climate characteristics and population adaptation.68 For example, a Sweden study found an association between every 5 °C reduction in the cold interval (<16 °C) and an increased risk of CVD, but a Chinese study showed a decreased risk in extremely cold conditions of −2.5 °C. Perhaps it is because in some extremely cold areas people actively take preventive measures, such as adding clothes and staying at home when the outdoor temperature drops to freezing.69 As climate change continues, more frequent and intense extreme heat events are expected to happen,70 posing a threat to CVD. Therefore, patients with a history of CVD as well as their caregivers and emergency medical services should be made aware of this risk posed by heat and take pre-emptive actions before hot days arrive.
A marginally moderate to a high level of heterogeneity was found in the meta-analysis for some air pollutants, especially for O3; so, several subgroups and sensitivity analyses were conducted to explore the source of results heterogeneity. Specifically, by removing high-quality articles and articles with adjustments for uncommon factors (i.e., wind speed, air pressure, public holidays, or influenza), sensitivity analyses indicated a decrease in heterogeneity in some results; however, O3 studies remained high or marginally moderate heterogeneity. Furthermore, an obvious difference in heterogeneity was found in the associations between O3 and various CVD outcome types. This suggested that differences in outcome types, quality of articles, and adjusted variables could partly explain the heterogeneity. Other factors such as geographic regions, study design, the composition of air pollutants, and socio-demographic characteristics may also be sources of heterogeneity. Specifically, it is important to note that O3 levels could vary a lot in different seasons and baseline O3 levels over various regions were also extremely different.71 Furthermore, O3 could affect haze formations resulting in a huge difference in air pollutants pollution.72 As our studies regarding air pollution effects covered various regions and subpopulations, high heterogeneity could be from the above underlying causes and should be additionally explored in the future.
There are several knowledge gaps in the literature. Firstly, existing studies were diverse in the interpretation of the onset timing of the disease, mainly focusing on the timing of emergency ambulance calls,34,37 hospital admissions,42 and emergency department visits.46 However, these methods did not take into account the time elapsed on the way to the medical institution and the lag time between the onset of symptoms and seeking medical assistance. Some remedies are to consider the distance from the site of the health event to the hospital as well as the local traffic condition to approximate the incident onset time, or patients or their families report on the onset time of disease.9,44 Secondly, most of the included studies assessed exposure to air pollutants and temperature merely using the averaged values across the local fixed monitoring stations, which could lead to exposure measurement bias due to variation in exposures and have affected the accuracy of the risk assessment. Therefore, more advanced exposure assessment methods deserve to be explored in the future. Thirdly, temperature or air pollution was usually controlled as a confounder when examining the effects of air pollution or temperature,8,56 but their effects on health are not independent.73 Therefore, it is worthwhile to clarify the interaction between air pollution and temperature. Fourthly, existing studies lack a clear understanding of the effects of subgroups (e.g., age, economic status, urban and rural areas). The effects difference among subgroups should be considered, which may help to identify the at-risk groups and target health care resource allocation. Finally, it was suggested that for some acute CVD, such as MI,52 death may occur within a few hours of exposure to high levels of air pollutants, but there is a paucity of research. In addition, evidence is also limited about the effects of air pollution or temperature on other cause-specific diseases such as ischemic heart disease, asthma, respiratory infection, etc. So, more exploration is urgently needed to elucidate such an association.
This review has several limitations. Firstly, sub-daily associations between ambient air pollution and temperature and cardiorespiratory diseases have been explored only in a few countries, which restricted our further assessment of variations across countries or regions. Secondly, this review only included studies with time-stratified case-crossover and time-series designs, both could lead to some bias. For example, in a time-stratified case-crossover design, time intervals that are too long may have an impact on the results due to seasonal patterns of exposure and outcome or changes in conditions at different points, while time intervals that are too short may introduce bias because of autocorrelation between exposures.74 Time-series design cannot control for some individual-level confounders (e.g., socioeconomic status).75 Thirdly, we only explored the contribution of the outcome types, article quality, and adjustment variables to the heterogeneity and did not perform further subgroup analyses (e.g., age). Some sub-populations could be more vulnerable to air pollution and non-optimal temperatures. For instance, MI often happens among the elderly,8,44 and children are more susceptible to respiratory infections after exposure to air pollutants.50 Therefore, the source of heterogeneity should be explored in the future to obtain a precise assessment of the cardiorespiratory effects of air pollution and temperature. Finally, we did not pool the effect of temperature owing to the shortage of sufficient reports. So, the effects of temperature exposures such as extreme heat and cold were an area that requires intense investigation in the context of climate change nationally and regionally.
In conclusion, this systematic review supports an increased risk of cardiorespiratory morbidity within a few hours after exposure to ambient air pollution or heat. The risk of CVD, especially MI, may increase within 6 h after exposure to air pollutants, and the risk of RD may increase at lag 7–24h. Healthcare professionals and people with pre-existing CVD or RD should be made aware of the acute effects of exposure to air pollution or heat on the increased risk of CVD and RD.
Contributors
JC and ZWX conceived and designed the study. KYW and HCH searched the literature. MY, QYW and JWT extracted the data and assessed the quality of the articles. KYW, HCH, HS and CRH performed the analysis. HZ, WYZ, MZH, YQZ and KH contributed to the interpretation of the findings. KYW and HCH wrote the first draft of the manuscript. All authors contributed to the revision of the manuscript. JC and ZWX have verified the data and taken responsibility for the decision to submit the manuscript. All authors read and approved the final version of the manuscript, and ensure it is the case.
Data sharing statement
All the data supporting the conclusions of this article are included within the article and its supplementary materials.
Declaration of interests
All the authors declared no conflict of interests.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (Grant No. 42105165; 81773518), the High-level Scientific Research Foundation of Anhui Medical University (Grant No. 0305044201), and the Discipline Construction of Anhui Medical University (Grant No. 0301001836).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104327.
Contributor Information
Zhiwei Xu, Email: xzw1011@gmail.com.
Jian Cheng, Email: jiancheng_cchh@163.com.
Appendix A. Supplementary data
References
- 1.Cheng J., Xu Z., Bambrick H., Su H., Tong S., Hu W. Impacts of exposure to ambient temperature on burden of disease: a systematic review of epidemiological evidence. Int J Biometeorol. 2019;63(8):1099–1115. doi: 10.1007/s00484-019-01716-y. [DOI] [PubMed] [Google Scholar]
- 2.Manisalidis I., Stavropoulou E., Stavropoulos A., Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faustini A., Stafoggia M., Colais P., et al. Air pollution and multiple acute respiratory outcomes. Eur Respir J. 2013;42(2):304–313. doi: 10.1183/09031936.00128712. [DOI] [PubMed] [Google Scholar]
- 4.Michelozzi P., Accetta G., De Sario M., et al. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med. 2009;179(5):383–389. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- 5.Seposo X., Ueda K., Sugata S., Yoshino A., Takami A. Short-term effects of air pollution on daily single- and co-morbidity cardiorespiratory outpatient visits. Sci Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138934. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman E.B., Zareba W., Utell M.J., et al. Acute changes in ambient temperature are associated with adverse changes in cardiac rhythm. Air Qual Atmos Health. 2014;7(3):357–367. doi: 10.1007/s11869-014-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mar T.F., Jansen K., Shepherd K., Lumley T., Larson T.V., Koenig J.Q. Exhaled nitric oxide in children with asthma and short-term PM2.5 exposure in Seattle. Environ Health Perspect. 2005;113(12):1791–1794. doi: 10.1289/ehp.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhaskaran K., Hajat S., Armstrong B., et al. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. BMJ. 2011;343:d5531. doi: 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner B., Ling F., Hopke P.K., et al. Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: a case-crossover study. Part Fibre Toxicol. 2014;11:1. doi: 10.1186/1743-8977-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan J., Sheppard L., Schreuder A., Ishikawa N., Siscovick D., Kaufman J. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology. 2005;16(1):41–48. doi: 10.1097/01.ede.0000147116.34813.56. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y.C., Sung F.C., Chen Y.J., Cheng C.P., Lin Y.K. Effects of extreme temperatures, fine particles and ozone on hourly ambulance dispatches. Sci Total Environ. 2021;765 doi: 10.1016/j.scitotenv.2020.142706. [DOI] [PubMed] [Google Scholar]
- 12.Dahlquist M., Raza A., Bero-Bedada G., et al. Short-term departures from an optimum ambient temperature are associated with increased risk of out-of-hospital cardiac arrest. Int J Hyg Environ Health. 2016;219(4–5):389–397. doi: 10.1016/j.ijheh.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Janes H., Sheppard L., Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 2005;16(6):717–726. doi: 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- 14.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 15.Fung K.Y., Krewski D., Chen Y., Burnett R., Cakmak S. Comparison of time series and case-crossover analyses of air pollution and hospital admission data. Int J Epidemiol. 2003;32(6):1064–1070. doi: 10.1093/ije/dyg246. [DOI] [PubMed] [Google Scholar]
- 16.Mustafic H., Jabre P., Caussin C., et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307(7):713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 17.Wells G., Shea B., O'Connell D., Peterson J., et al. 2011. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 18.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah A.S., Langrish J.P., Nair H., et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382(9897):1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phung D., Thai P.K., Guo Y., Morawska L., Rutherford S., Chu C. Ambient temperature and risk of cardiovascular hospitalization: an updated systematic review and meta-analysis. Sci Total Environ. 2016;550:1084–1102. doi: 10.1016/j.scitotenv.2016.01.154. [DOI] [PubMed] [Google Scholar]
- 21.Sun Z., Chen C., Xu D., Li T. Effects of ambient temperature on myocardial infarction: a systematic review and meta-analysis. Environ Pollut. 2018;241:1106–1114. doi: 10.1016/j.envpol.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 22.Çapraz Ö., Deniz A., Doğan N. Effects of air pollution on respiratory hospital admissions in İstanbul, Turkey, 2013 to 2015. Chemosphere. 2017;181:544–550. doi: 10.1016/j.chemosphere.2017.04.105. [DOI] [PubMed] [Google Scholar]
- 23.Dastoorpoor M., Sekhavatpour Z., Masoumi K., et al. Air pollution and hospital admissions for cardiovascular diseases in Ahvaz, Iran. Sci Total Environ. 2019;652:1318–1330. doi: 10.1016/j.scitotenv.2018.10.285. [DOI] [PubMed] [Google Scholar]
- 24.Peters A., Dockery D.W., Muller J.E., Mittleman M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 25.Nirel R., Levy I., Adar S.D., et al. Concentration-response relationships between hourly particulate matter and ischemic events: a case-crossover analysis of effect modification by season and air-mass origin. Sci Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143407. [DOI] [PubMed] [Google Scholar]
- 26.Cheng J., Xu Z., Bambrick H., et al. Cardiorespiratory effects of heatwaves: a systematic review and meta-analysis of global epidemiological evidence. Environ Res. 2019;177 doi: 10.1016/j.envres.2019.108610. [DOI] [PubMed] [Google Scholar]
- 27.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 29.Altman D.G., Bland J.M. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziou M., Tham R., Wheeler A.J., Zosky G.R., Stephens N., Johnston F.H. Outdoor particulate matter exposure and upper respiratory tract infections in children and adolescents: a systematic review and meta-analysis. Environ Res. 2022;210 doi: 10.1016/j.envres.2022.112969. [DOI] [PubMed] [Google Scholar]
- 31.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 33.Nirel R., Adar S.D., Dayan U., et al. Fine and coarse particulate matter exposures and associations with acute cardiac events among participants in a telemedicine service: a case-crossover study. Environ Health Perspect. 2018;126(9) doi: 10.1289/EHP2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal F.S., Carney J.P., Olinger M.L. Out-of-hospital cardiac arrest and airborne fine particulate matter: a case-crossover analysis of emergency medical services data in Indianapolis, Indiana. Environ Health Perspect. 2008;116(5):631–636. doi: 10.1289/ehp.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straney L., Finn J., Dennekamp M., Bremner A., Tonkin A., Jacobs I. Evaluating the impact of air pollution on the incidence of out-of-hospital cardiac arrest in the Perth Metropolitan Region: 2000-2010. J Epidemiol Community Health. 2014;68(1):6–12. doi: 10.1136/jech-2013-202955. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal F.S., Kuisma M., Lanki T., et al. Association of ozone and particulate air pollution with out-of-hospital cardiac arrest in Helsinki, Finland: evidence for two different etiologies. J Expo Sci Environ Epidemiol. 2013;23(3):281–288. doi: 10.1038/jes.2012.121. [DOI] [PubMed] [Google Scholar]
- 37.Yorifuji T., Suzuki E., Kashima S. Cardiovascular emergency hospital visits and hourly changes in air pollution. Stroke. 2014;45(5):1264–1268. doi: 10.1161/STROKEAHA.114.005227. [DOI] [PubMed] [Google Scholar]
- 38.Yorifuji T., Suzuki E., Kashima S. Outdoor air pollution and out-of-hospital cardiac arrest in Okayama, Japan. J Occup Environ Med. 2014;56(10):1019–1023. doi: 10.1097/JOM.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 39.Raza A., Dahlquist M., Jonsson M., et al. Ozone and cardiac arrest: the role of previous hospitalizations. Environ Pollut. 2019;245:1–8. doi: 10.1016/j.envpol.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 40.Dennekamp M., Straney L.D., Erbas B., et al. Forest fire smoke exposures and out-of-hospital cardiac arrests in melbourne, Australia: a case-crossover study. Environ Health Perspect. 2015;123(10):959–964. doi: 10.1289/ehp.1408436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pradeau C., Rondeau V., Lévèque E., et al. Air pollution and activation of mobile medical team for out-of-hospital cardiac arrest. Am J Emerg Med. 2015;33(3):367–372. doi: 10.1016/j.ajem.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Sahlén A., Ljungman P., Erlinge D., et al. Air pollution in relation to very short-term risk of ST-segment elevation myocardial infarction: case-crossover analysis of SWEDEHEART. Int J Cardiol. 2019;275:26–30. doi: 10.1016/j.ijcard.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 43.Evans K.A., Hopke P.K., Utell M.J., et al. Triggering of ST-elevation myocardial infarction by ambient wood smoke and other particulate and gaseous pollutants. J Expo Sci Environ Epidemiol. 2017;27(2):198–206. doi: 10.1038/jes.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J., Liu C., Cheng Y., et al. Association between ambient particulate matter air pollution and ST-elevation myocardial infarction: a case-crossover study in a Chinese city. Chemosphere. 2019;219:724–729. doi: 10.1016/j.chemosphere.2018.12.094. [DOI] [PubMed] [Google Scholar]
- 45.Raza A., Bellander T., Bero-Bedada G., et al. Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm. Eur Heart J. 2014;35(13):861–868. doi: 10.1093/eurheartj/eht489. [DOI] [PubMed] [Google Scholar]
- 46.Cheng J., Tong S., Su H., Xu Z. Hourly air pollution exposure and emergency department visit for acute myocardial infarction: vulnerable populations and susceptible time window. Environ Pollut. 2021;288 doi: 10.1016/j.envpol.2021.117806. [DOI] [PubMed] [Google Scholar]
- 47.Wu P.C., Cheng T.J., Kuo C.P., et al. Transient risk of ambient fine particulate matter on hourly cardiovascular events in Tainan City, Taiwan. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0238082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ai S., Wang C., Qian Z.M., et al. Hourly associations between ambient air pollution and emergency ambulance calls in one central Chinese city: implications for hourly air quality standards. Sci Total Environ. 2019;696 doi: 10.1016/j.scitotenv.2019.133956. [DOI] [PubMed] [Google Scholar]
- 49.Yorifuji T., Suzuki E., Kashima S. Hourly differences in air pollution and risk of respiratory disease in the elderly: a time-stratified case-crossover study. Environ Health. 2014;13:67. doi: 10.1186/1476-069X-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J., Kim H., Kweon J. Hourly differences in air pollution on the risk of asthma exacerbation. Environ Pollut. 2015;203:15–21. doi: 10.1016/j.envpol.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 51.Cheng J., Su H., Xu Z. Intra-day effects of outdoor air pollution on acute upper and lower respiratory infections in Australian children. Environ Pollut. 2021;268(Pt A) doi: 10.1016/j.envpol.2020.115698. [DOI] [PubMed] [Google Scholar]
- 52.Murakami Y., Ono M. Myocardial infarction deaths after high level exposure to particulate matter. J Epidemiol Community Health. 2006;60(3):262–266. doi: 10.1136/jech.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wichmann J., Folke F., Torp-Pedersen C., et al. Out-of-hospital cardiac arrests and outdoor air pollution exposure in Copenhagen, Denmark. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0053684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao J., Brauer M., Wei J., McGrail K.M., Johnston F.H., Henderson S.B. Sub-daily exposure to fine particulate matter and ambulance dispatches during wildfire seasons: a case-crossover study in British columbia, Canada. Environ Health Perspect. 2020;128(6) doi: 10.1289/EHP5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhaskaran K., Armstrong B., Hajat S., Haines A., Wilkinson P., Smeeth L. Heat and risk of myocardial infarction: hourly level case-crossover analysis of MINAP database. BMJ. 2012;345 doi: 10.1136/bmj.e8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui Y., Ai S., Liu Y., et al. Hourly associations between ambient temperature and emergency ambulance calls in one central Chinese city: call for an immediate emergency plan. Sci Total Environ. 2020;711 doi: 10.1016/j.scitotenv.2019.135046. [DOI] [PubMed] [Google Scholar]
- 57.Cheng J., Su H., Xu Z.W., Tong S.L. Extreme temperature exposure and acute myocardial infarction: elevated risk within hours? Environ Res. 2021:202. doi: 10.1016/j.envres.2021.111691. [DOI] [PubMed] [Google Scholar]
- 58.Ensor K.B., Raun L.H., Persse D. A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation. 2013;127(11):1192–1199. doi: 10.1161/CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- 59.Lipsett M.J., Tsai F.C., Roger L., Woo M., Ostro B.D. Coarse particles and heart rate variability among older adults with coronary artery disease in the Coachella Valley, California. Environ Health Perspect. 2006;114(8):1215–1220. doi: 10.1289/ehp.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Routledge H.C., Manney S., Harrison R.M., Ayres J.G., Townend J.N. Effect of inhaled sulphur dioxide and carbon particles on heart rate variability and markers of inflammation and coagulation in human subjects. Heart. 2006;92(2):220–227. doi: 10.1136/hrt.2004.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi Y.J., Kim S.H., Kang S.H., et al. Short-term effects of air pollution on blood pressure. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-56413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma H., Liu F.C., Yang X.L., et al. Association of short-term fine particulate matter exposure with pulmonary function in populations at intermediate to high-risk of cardiovascular disease: a panel study in three Chinese cities. Ecotoxicol Environ Saf. 2021:220. doi: 10.1016/j.ecoenv.2021.112397. [DOI] [PubMed] [Google Scholar]
- 63.Taggart S.C., Custovic A., Francis H.C., et al. Asthmatic bronchial hyperresponsiveness varies with ambient levels of summertime air pollution. Eur Respir J. 1996;9(6):1146–1154. doi: 10.1183/09031936.96.09061146. [DOI] [PubMed] [Google Scholar]
- 64.Witten A., Solomon C., Abbritti E., et al. Effects of nitrogen dioxide on allergic airway responses in subjects with asthma. J Occup Environ Med. 2005;47(12):1250–1259. doi: 10.1097/01.jom.0000177081.62204.8d. [DOI] [PubMed] [Google Scholar]
- 65.Zuurbier M., Hoek G., Oldenwening M., Meliefste K., van den Hazel P., Brunekreef B. Respiratory effects of commuters' exposure to air pollution in traffic. Epidemiology. 2011;22(2):219–227. doi: 10.1097/EDE.0b013e3182093693. [DOI] [PubMed] [Google Scholar]
- 66.Keatinge W.R., Coleshaw S.R., Easton J.C., Cotter F., Mattock M.B., Chelliah R. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med. 1986;81(5):795–800. doi: 10.1016/0002-9343(86)90348-7. [DOI] [PubMed] [Google Scholar]
- 67.Keatinge W.R., Coleshaw S.R., Cotter F., Mattock M., Murphy M., Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J. 1984;289(6456):1405–1408. doi: 10.1136/bmj.289.6456.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng J., Xu Z., Bambrick H., Su H., Tong S., Hu W. Impacts of heat, cold, and temperature variability on mortality in Australia, 2000-2009. Sci Total Environ. 2019;651(Pt 2):2558–2565. doi: 10.1016/j.scitotenv.2018.10.186. [DOI] [PubMed] [Google Scholar]
- 69.Barnett A.G., Hajat S., Gasparrini A., Rocklöv J. Cold and heat waves in the United States. Environ Res. 2012;112:218–224. doi: 10.1016/j.envres.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 70.WHO . 2018. Heat and Health.https://www.who.int/news-room/fact-sheets/detail/climate-change-heat-and-health [Google Scholar]
- 71.Zhu Q., Bi J., Liu X., et al. Satellite-based long-term spatiotemporal patterns of surface ozone concentrations in China: 2005–2019. Environ Health Perspect. 2022;130(2) doi: 10.1289/EHP9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li K., Jacob D.J., Liao H., et al. Ozone pollution in the North China Plain spreading into the late-winter haze season. Proc Natl Acad Sci USA. 2021;118(10) doi: 10.1073/pnas.2015797118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsu W.H., Hwang S.A., Kinney P.L., Lin S. Seasonal and temperature modifications of the association between fine particulate air pollution and cardiovascular hospitalization in New York state. Sci Total Environ. 2017;578:626–632. doi: 10.1016/j.scitotenv.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu Y., Zeger S.L. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics. 2007;8(2):337–344. doi: 10.1093/biostatistics/kxl013. [DOI] [PubMed] [Google Scholar]
- 75.Gasparrini A., Armstrong B., Kenward M.G. Distributed lag non-linear models. Stat Med. 2010;29(21):2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.