To the Editor:
Classification of Cutaneous Lupus Disease Area and Severity Index (CLASI) scores provides a framework for disease severity in patients with cutaneous lupus erythematosus (CLE). One prior study classified CLASI activity scores using a small cohort; however, no studies have been performed on CLASI damage (CLASI-D) scores.1 Our objective was to establish CLASI-D strata for mild, moderate, and severe disease using a large, heterogeneous cohort and compare characteristics between categories.
This is a cross-sectional analysis of patients with CLE recruited prospectively at outpatient dermatology clinics at the University of Texas Southwestern and Parkland Hospital in Dallas, Texas, from April 2009 to January 2020. This study was approved by the University of Texas Southwestern institutional review board. CLASI-D and Physicians’ Global Assessment (PGA) of damage were scored. PGA scores served as anchoring measures to classify patients into mild, moderate, and severe categories. Receiver operating characteristic curves were used to evaluate strata. Patients were classified into activity categories on the basis of CLASI activity strata published previously.1 Patient characteristics were compared using χ2 and Kruskal-Wallis tests.
Table I provides patient characteristics (N = 270). Based on PGA scores, 49% of patients had mild, 35% moderate, and 16% severe disease damage, with examples shown in Supplementary Fig 1 (available via Mendeley at https://data.mendeley.com/datasets/jmrbvdw32n/1 ). CLASI-D scores of 0 to 5, 6 to 16, and 17 to 56 corresponded to mild [sensitivity 86%, specificity 96%, and correctly classified 95%], moderate [sensitivity 73%, specificity 83%, and correctly classified 69%], and severe [sensitivity 65%, specificity 90%, and correctly classified 56%] damage, respectively. Among the patients with CLASI-D scores of 0 to 5, 95% had mild disease, and among those with CLASI-D scores between 6 and 16, 69% had moderate disease. Among patients with a CLASI-D score of ≥17, 56% had severe disease damage.
Table I.
Patient clinical and demographic characteristics (N = 270)
| Patient characteristic | Mean or N | SD or % |
|---|---|---|
| Age at visit, y | 45.74 | 14.04 |
| Sex | ||
| Male | 40 | 14.81% |
| Female | 230 | 85.19% |
| Race/ethnicity | ||
| Caucasian | 84 | 31.11% |
| Black | 140 | 51.85% |
| Hispanic | 30 | 11.11% |
| Asian | 10 | 3.70% |
| Other | 6 | 2.22% |
| PGA scores* | ||
| PGA-A | 7.58 | 1.93 |
| PGA-D | 7.18 | 2.31 |
| CLASI scores | ||
| CLASI-A | 5.9 | 6.60 |
| CLASI-D | 8.6 | 8.10 |
| Disease severity by PGA-A | ||
| Mild | 153 | 57% |
| Moderate | 87 | 32% |
| Severe | 29 | 11% |
| Disease Severity by PGA-D | ||
| Mild | 132 | 49% |
| Moderate | 95 | 35% |
| Severe | 43 | 16% |
| Predominant CLE subtype | ||
| Acute CLE | 25 | 9.26% |
| Bullous LE | 3 | 1.11% |
| Subacute CLE | 33 | 12.22% |
| Chronic CLE | 212 | 78.52% |
| Discoid LE | 179 | 62.96% |
| Chilblains LE | 3 | 1.11% |
| LE Tumidus | 26 | 9.63% |
| LE Panniculitis | 4 | 1.48% |
| Systemic lupus involvement | ||
| Present | 149 | 55.19% |
| Absent | 121 | 44.81% |
| Smoking status† | ||
| Nonsmoker | 135 | 50.00% |
| Current smoker | 46 | 17.04% |
| Past smoker | 88 | 32.59% |
Italicized terms refer to detailed subdivisions of CLE subtypes, ie, Bullous LE is a subtype of Acute CLE and Discoid LE is a subtype of Chronic CLE.
CLASI, Cutaneous Lupus Erythematosus Disease Severity and Activity Index; CLASI-A, Cutaneous Lupus Erythematosus Disease Severity and Activity Index-Activity Score; CLASI-D, Cutaneous Lupus Erythematosus Disease Severity and Activity Index-Damage Score; CLE, Cutaneous Lupus Erythematosus; LE, Lupus Erythematosus; PGA, Physician’s Global Assessment; PGA-A, Physician’s Global Assessment of Activity; PGA-D, Physician’s Global Assessment of Damage.
One patient had a missing PGA-A score.
One patient had missing smoking status.
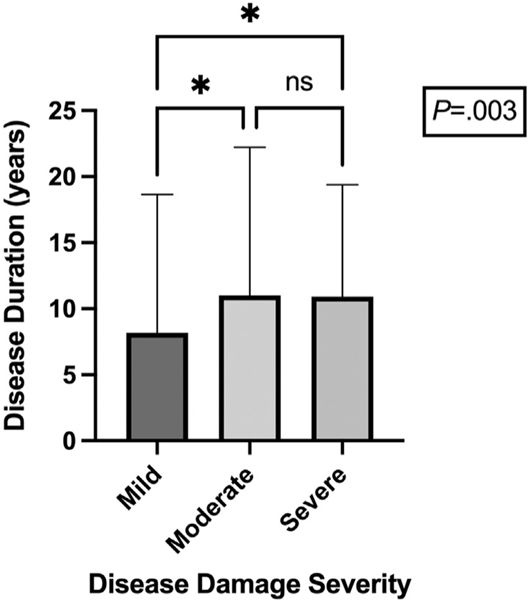
Patients with moderate and severe disease damage experienced longer disease duration than patients with mild damage (mild: 8.19 ± 10.48 years; moderate: 11.01 ± 11.21 years; severe: 10.93 ± 8.46 years, P =.003) (Fig 1). The correlation of disease damage with disease duration may be secondary to patients with longer disease duration experiencing increased damage from prior active flares.2 Patients with severe disease damage were more likely to have discoid lupus erythematosus (DLE) (100% DLE vs 0% subacute CLE vs 0% acute CLE, P <.0001). DLE is characterized by significant disease damage3 and may predispose patients to worse disease damage independent of disease activity. Disease activity severity did not correlate with disease subtype (Supplementary Fig 1, available via Mendeley at https://data.mendeley.com/datasets/jmrbvdw32n/1 ).
Fig 1.
The mean disease duration (years) of patients with mild, moderate, and severe disease damage. This bar graph demonstrates that the mean disease duration (±SD) of patients with moderate disease damage (11.01 ± 11.21 years) and of patients with severe disease damage (10.93 ± 8.46 years) was significantly greater than that of patients with mild damage (8.19 ± 10.48 years) (P =.003). The asterisk represents a P value of <.05. ns, Not significant.
We have introduced CLASI-D severity strata using a large, diverse cohort and highlighted characteristics associated with different disease damage categories. This work proposes an anchor-based technique for quantifying disease damage instead of relying on individualized, subjective descriptions of damage severity. This standardization can help providers contextualize CLE damage severity in their patients, explore the impact on their quality of life, and guide treatment decisions.2,4 In addition, the identification of characteristics associated with worse disease damage severity, specifically longer disease duration and DLE subtype, improves our understanding of disease course in patients with CLE. Limitations include single-center, single-rater, cross-sectional design, relatively low percentages of severe disease, and subjective nature of PGA scores. Future multicenter, larger studies will help validate our data to define disease severity strata in CLE.
Supplementary Material
Acknowledgments
The authors would like to thank the participants of the University of Texas Southwestern Cutaneous Lupus Erythematosus Registry for their contributions to lupus research.
Funding sources:
Supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR061441. The content is solely the responsibility of the authors and does not necessarily represent the official views of the University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Research Resources, and the National Institutes of Health.
Footnotes
IRB approval status: Approved by University of Texas Southwestern Medical Center IRB (STU# 082010-241).
Conflicts of interest
Dr Chong is an investigator for Daavlin Corporation, Biogen Incorporated, and Pfizer Incorporated and a consultant for Bristol Meyers Squibb, EMD Serono, Horizon Therapeutics, and Biogen Incorporated. Drs Abbas and Nandy have no conflicts of interest to declare.
REFERENCES
- 1.Klein R, Moghadam-Kia S, LoMonico J, et al. Development ofthe CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol. 2011;147(2):203–208. 10.1001/archdermatol.2010.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creadore A, Watchmaker J, Maymone MBC, Pappas L, Vashi NA, Lam C. Cosmetic treatment in patients with autoimmune connective tissue diseases: best practices for patients with lupus erythematosus. J Am Acad Dermatol. 2020; 83(2):343–363. 10.1016/j.jaad.2020.03.123 [DOI] [PubMed] [Google Scholar]
- 3.Szczęch J, Rutka M, Samotij D, Zalewska A, Reich A. Clinicalcharacteristics of cutaneous lupus erythematosus. Postepy Dermatol Alergol. 2016;33(1):13–17. 10.5114/pdia.2014.44031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joseph AK, Abbas LF, Chong BF. Treatments for diseasedamage in cutaneous lupus erythematosus: a narrative review. Dermatol Ther. 2021;34(5):e15034. 10.1111/dth.15034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.