Abstract
Background
Life instability may be an important factor for HIV-related care outcomes in older adults living with HIV (OALWH). This study examined the degree to which an 11-item life instability index (LII) composed of individual- and community-level indicators was associated with HIV-related care outcomes—viral load, antiretroviral (ART) medication adherence, rates of detectable viral load, and HIV care appointment non-adherence among OALWH in the Miami area.
Methods
Six hundred twenty-three OALWH completed an interviewer-administered assessment (English or Spanish), which was matched with medical record data.
Results
Participants reported about six LII indicators each (M = 6.08, SD = 1.44). Greater index scores were associated with worse self-reported ART adherence (b = − 1.14, p = 0.03), lower observed appointment adherence (b = 0.02, p < 0.01), higher viral load (b = 0.09, p = 0.02), and greater odds of viral detection (OR = 1.22, p = 0.01). Regarding health behaviors, life instability was significantly associated with increased illicit substance use among participants and not associated with depression or anxiety. The association of life instability to ART adherence remained significant (although attenuated) when controlling for the significant effects of substance use (b = − 0.40, BSTP [− 0.87, − 0.09]).
Conclusion
This present study is the first to examine an additive life instability index and its association with HIV-related behavioral and biomedical health outcomes among a population of OALWH. Greater indicators of life instability among OALWH may lead to poorer HIV-related health outcomes above and beyond the net of the effects of depression, anxiety, and substance use.
Keywords: HIV/AIDS, Older adults, Life instability, Health disparities, Behavioral medicine
Introduction
Older adults—aged 50 years or older—living with HIV (OALWH) are a growing population with unique challenges. In 2018, about half (51%) of people living with HIV (PLWH) in the USA were over 50 years old, and 17% of new HIV diagnoses in 2018 were in this age bracket [1]. Projections indicate the number of PLWH over the age of 55 in the USA will more than double from 2013 to 2045 [2] with similar trends predicted worldwide as well [3].
OALWH are at increased risk for both negative mental and physical health comorbidities compared to both their non-HIV-infected age-matched peers and younger PLWH. Among OALWH, physical health issues (e.g., cardiovascular disease, cancer) and diminished physical functioning are becoming increasingly common at younger ages because of “accelerated aging” [4, 5]. Similarly, mental health comorbidities like depression, anxiety, and substance use challenges often affect OALH at inflated rates compared to their age-matched HIV-negative peers due to issues like stigma, health-related stressors, and challenges associated with managing a chronic illness across the aging continuum [6]. Furthermore, the presence of physical and mental health comorbidities on top of general effects of aging (e.g., poorer memory, increasing mobility issues) may complicate the delivery of HIV care treatment for OALWH.
Life chaos, or the perceived inability to plan or anticipate future events [7], may play an important role in the development and maintenance of poor HIV-related care outcomes among older adults. First theorized by developmental psychologists to explain adverse outcomes in the context of childhood [8], the notion of life chaos was adapted by HIV researchers to assess how life instability may negatively affect health behaviors and outcomes among PLWH [9]. Life chaos measures predict missed appointments and poor medication adherence among PLWH [7] and other chronic illnesses [10].
Stemming from the life chaos literature, the relevance of life instability to OALWH’s health and well-being has additional conceptual evidence. Consistent with the theories of social stability [11] and syndemics [12], factors of life instability may interact cyclically to create a nexus of risk that yields negative physical and mental health outcomes among PLWH. Previous research indicates that among PLWH, life destabilizing factors associated with poverty (e.g., food insecurity) contribute to HIV morbidity and mortality [13]. PLWH with incarceration histories or housing instability show more inconsistent engagement across the HIV care continuum [14], including lower viral suppression rates [15]. For PLWH living in more under-resourced communities, increased frequency of daily stressors and life disruptions make engaging in HIV-related care behaviors (e.g., ART and HIV care appointment adherence) more challenging [16–18]. These various life destabilizing factors may lead PLWH to develop a sense of “constrained rationality” in which the decision to engage in beneficial health behaviors cannot be separated from one’s daily stressors and narrow possibilities [19]. Despite these observations, few studies have taken a socioecological model approach [20] and explored how community and societal level indicators of life instability may synergistically affect HIV-related health outcomes, particularly among OALWH [13–15].
With the “greying of the epidemic,” it is important to understand the intersection of HIV, life instability, and aging [21]. Prior research has not examined the cumulative effect of individual- and community-level destabilizing factors on HIV-related health outcomes. This study sought to address gaps in the literature by investigating the frequency and cumulative impact of life instability among OALWH with respect to behavioral and objectively measured biomedical outcomes among patients in a US HIV epicenter [22].
Methods
Participants and Procedures
Six hundred twenty-three PLWH receiving care from a public, non-profit tertiary care hospital outpatient clinic in Miami that predominately serves low-income communities completed an interviewer-administered psychosocial assessment between April 2017 and April 2020. To maximize generalizability and inclusion in the parent study’s consent to contact database, eligible participants were those receiving HIV care at two public HIV care clinics and able to speak and understand English or Spanish. Since the current secondary analysis focused on exploring the experiences of OALWH living in Miami-Dade County, additional inclusion criteria were established, including requiring participants to be 50 years or older and to have a primary residence in Miami-Dade County. A 50-year age cutoff was used based on prior literature suggesting that PLWH over the age of 50 demonstrate poorer HIV-related care trajectories compared to their younger counterparts [1, 23]. Participants were excluded if: (1) they were unable to consent due to cognitive or emotional issues; or (2) they are currently incarcerated.
Participants were selected from a larger clinical survey/consent to contact database of PLWH focused on collecting data on psychosocial health factors and HIV-related care outcomes. Eligible participants were approached by study staff during their routine clinic visits and invited to participate in the study. All participants provided informed consent and all study procedures (e.g., ethical considerations, data safety and security) were approved by University of Miami institutional review board. All data for the current secondary analysis were from participants’ baseline assessments.
Measures
Sociodemographic Information
Age, race/ethnicity, gender, education level, and sexual orientation were collected via self-report. Participant home addresses were geocoded using ArcGIS Pro to identify census tracts [24].
HIV Disease Severity Outcomes
Continuous log HIV RNA viral load (viral load) was extracted from electronic medical records via a two-step process. As per clinic procedure, laboratory samples analyzed from commercial or other private labs are first entered manually from laboratory reports that are scanned into the patients’ records. To carry out the current study, necessary electronic medical record data was then requested by study investigators and then imported directly from the clinic’s electronic medical record directly into a data repository. Unsuppressed viral load was defined as ≥ 200 copies/mL [25] and the log of the continuous HIV RNA viral load was calculated and analyzed.
HIV Care Appointment Adherence
The number of cancelled and missed treatment visits was divided by the total number of scheduled treatment visits for participants during the study period and analyzed continuously. Cancelled, missed, and completed HIV care appointments were extracted from electronic medical records via the same process described above.
ART Adherence
A composite score from Wilson and colleagues’ 3-item adherence measure indicated percentage of ART adherence for the past month [26]. Questions assessed missed dose days, frequency of optimal adherence (from 0 = never to 5 = always), and overall self-reported adherence (from 0 = very poor to 5 = excellent) in the past 30 days. Patient’s past month adherence was then converted into a continuous variable based on a composite score calculated on a scale from 0 (missed all doses) to 100% (perfect adherence). The Wilson and colleagues’ 3-item adherence measure exhibited strong internal consistency (α = 0.83) when assessing adherence to ART medications among a population of PLWH [18].
Life Instability Index
The LII is composed of 11 indicators: 7 at the individual level and 4 at the community level. Indicators were chosen because of either their theoretical relevance as an underpinning of life instability or prior independent associations with HIV disease outcomes (i.e., ART adherence, viral load, and history of missed/cancelled HIV care appointments). LII indicators were dichotomized (0 or 1) and summed (0–11) with higher LII scores indicating greater life instability indicators experienced.
Individual-level LII indicators
Individual-level LII indicators included the following self-reported participant information: (a) unstable housing if they reported past 12-month homelessness or temporary/transitional housing, (b) history of incarceration if they were ever incarcerated, (c) comorbid health conditions if they reported any other diagnosed health condition in addition to HIV (e.g., cancer, cardiovascular disease), (d) unstable work if they were neither working nor in school full time or were on disability, (e) relationship instability if single, divorced and/or separated, and/or widowed, (f) new US resident status if they had lived in the USA for less than 15 years, and (g) low educational attainment if they had less than a high school degree.
Community-level LII
Community-level LII indicators of life instability were assessed for individuals based on census tract. Communities are often represented by census tracts, statistical subdivisions of a county which generally have a population of 1,200 to 8,000 residents, and are the smallest geographic unit for which sociodemographic information is statistically reliable [27, 28]. Community-level data was accessed from the 2019 American Community Survey and U.S. Census Bureau via the Miami Matters website which offered data related to the following four indicators at the census tract level [29–31].
Participants were identified as living in a census tract reflecting three life instability indictors of poverty including (a) low income, residing in a census tract in which the percentage of people living below the poverty line was 12.4% or above; (b) single parent households, residing in a census tract in which the percentage of single parent households was 22.7% or above; and (c) use of public transportation to access work: residing in a census tract in which the percentage of people commuting to work via public transportation was 5.1% or above. Finally, participants were coded for the life expectancy indicator (reflecting community health) if they resided in a census tract in which the average life expectancy was 78.9 years or below.
Community-level indicators in the LII were dichotomized based on whether that indicator’s national average was higher or lower than the margin of error. Accordingly, certain community-level LII indicators (e.g., low income, single parent households) which were above the national average margin of error were scored positively for the presence of that LII indicator and indicators below the national average margin of error were scored negatively for the absence of that LII indicator. The opposite was true for the remaining community-level LII indicators (e.g., use of public transportation to work, life expectancy).
Depression
The 9-item Patient Health Questionnaire (PHQ-9) assessed depressive symptoms reflecting major depression diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders 5 [32, 33]. Participants reported frequencies of depressive symptoms on a 4-point scale from 0 (not at all) to 3 (nearly every day) with greater scores indicating greater symptoms. The PHQ-9 has been validated in both aging populations [34] and PLWH [35] has exhibited high reliability in validation studies (α = 0.89) [33].
Anxiety
The anxiety thermometer is a single-item self-report measure that assessed participants’ anxiety using a visual analog scale from 0 (no anxiety) to 10 (extreme anxiety) and was adapted from the National Comprehensive Cancer Network Distress (NCCND) Thermometer [36]. Prior studies have demonstrated high correlation between the NCCND Thermometer and the lengthier Behavioral Health Status index suggesting the validity the thermometer has in assessing distress within clinical populations [37].
Substance Use
To create a substance use variable, frequency of 30-day use was assessed for crack, cocaine, heroin, other opioids, amphetamines, hallucinogens, ecstasy/MDMA, sedatives/tranquilizers, and other drugs (0 = no use, 1 = 1 to 2 times a week, 2 = once a week, 3 = several times a week, 4 = about every day). Marijuana use was not included in this variable.
Statistical Analyses
Analyses were conducted using SPSS 25 [38]. First, we conducted descriptive statistics and log-transformed viral load to meet assumptions for regression analyses. To evaluate the effects of the life instability index on HIV-related health outcomes, we employed a series of simple linear (ART adherence, HIV care appointment adherence, viral load) and logistic regression models (rates of viral suppression). Because true mediation requires a sense of temporality as defined by VanderWeele (2016) [39], we instead analyzed whether a patients’ depression, anxiety, or substance use indirectly affects the relationship between life instability and our outcomes of interest using Hayes’s Mediation PROCESS macro [40]. This approach tests whether an intermediary variable (e.g., substance use) explains a significant amount of variance in the association between a predictor (e.g., life instability index) and outcome (e.g., ART adherence) of interest at one moment in time. Accordingly, we first established whether there were significant direct associations between both life instability indicators and outcomes of interest as well as measures of psychological distress and HIV-related health outcomes. For any relationship in which direct associations were observed between life instability and the main outcomes, depression, anxiety, and substance use were assessed to determine whether complete or partial indirect effects were present. Missing data was addressed using a maximum likelihood estimation approach [41].
Results
Table 1 contains the descriptive statistics of relevant data for the full sample (N = 623) and the descriptive results of each of the 11 LII indicators. Participants were predominantly cis-gender men (60.8%), heterosexual (80.6%), and Black non-Hispanic/Latino (65.9%) and had a mean age of 58 years. Table 2 provides a summary of all analyses. Figure 1 outlines the various bivariate associations between study variables and Fig. 2 shows the distribution of LII scores among the entire sample with participants reporting an average of just over six LII items each (M = 6.08, SD = 1.44).
Table 1.
Participant characteristics (N = 623)
| Mean (SD) or n (%) | |
|---|---|
| Age (years) | 57.99 (5.90) |
| Race/ethnicity | |
| White Non-Hispanic/Latinx | 22 (3.6%) |
| Black Non-Hispanic/Latinx | 406 (65.9%) |
| Hispanic/Latinx (any race) | 170 (27.6%) |
| Asian/Pacific-Islander/Indigenous Persons | 7 (1.1%) |
| Multiracial | 9 (1.5%) |
| Gender | |
| Male | 379 (60.8%) |
| Female | 239 (38.4%) |
| Other | 5 (0.80%) |
| Sexual orientation | |
| Heterosexual | 502 (80.6%) |
| Sexual minority (gay, lesbian, bisexual, other identity) | 116 (19.4%) |
| Patient Health Questionnaire-9 depression score (0–27) | 4.88 (5.26) |
| Anxiety thermometer score (0–10) | 3.55 (3.35) |
| Frequency of substance use 30 days (0 = no use, 1 = 1 to 2 times, 2 = about once a week, 3 = several times a week, 4 = about every day) | 0.24 (0.80) |
| Continuous log RNA viral load | 1.10 (1.38) |
| Viral suppression (undetectable viral load < 200 copies/mL) | 496 (79.6%) |
| HIV care appointment adherence (0–100%) | 77.3% (20.6%) |
| Antiretroviral therapy adherence in past 30 days (0–100%) | 93.9% (18.3%) |
| Life instability (0–11) | 6.08 (1.44) |
| History of incarceration | 348 (56.1%) |
| New immigrant to USA (<15 years) | 24 (3.90%) |
| Instable housing over past 12 months | 116 (18.8%) |
| Not in a relationship (single, widowed, divorced, separated) | 155 (25.1%) |
| Less than a high school education/GED | 248 (39.8%) |
| Lacking full-time employment/education/or on disability | 470 (75.4%) |
| Comorbid health condition (N = 224) | 192 (85.7%) |
| Poverty community indicator | 573 (92.0%) |
| Single parent households community indicator | 595 (95.5%) |
| Use of public transportation to work/school community indicator | 489 (78.5%) |
| Life expectancy community indicator | 445 (71.4%) |
Table 2.
Direct and indirect effects of life instability on HIV-related health outcomes via behavioral health outcomes (i.e., depression, anxiety, and substance use)
| b | SE | p-value [C.I.] | |
|---|---|---|---|
| Direct effects of life instability on intermediary variables Life instability on depression | 0.16 | 0.15 | p = 0.27, [−0.13, 0.46] |
| Life instability on anxiety | 0.17 | 0.09 | p = 0.07, [−0.02, 0.36]* |
| Life instability on substance use | 0.08 | 0.02 | p < 0.01, [0.04, 0.13]** |
| Effects of life instability on continuous log RNA viral load controlling for behavioral health outcomes | 0.09 | 0.04 | p = 0.02, [0.02, 0.17]** |
| Effects of life instability on ART adherence controlling for behavioral health outcomes | −1.14 | 0.52 | p = 0.03, [−2.15, −0.12]** |
| Via substance use | −0.40 | 0.20 | [−0.87, −0.09]** |
| Effects of life instability on HIV-care appointment adherence controlling for behavioral health outcomes | −0.02 | 0.01 | p < 0.01, [−0.03, −0.01]** |
| OR/b | SE | p-value [C.I.] | |
| Effects of life instability on viral suppression controlling for behavioral health outcomes | OR = 1.22 | 0.46 | p < 0.01, [1.06, 1.40]** |
p < .10
p < .05
Fig. 1.
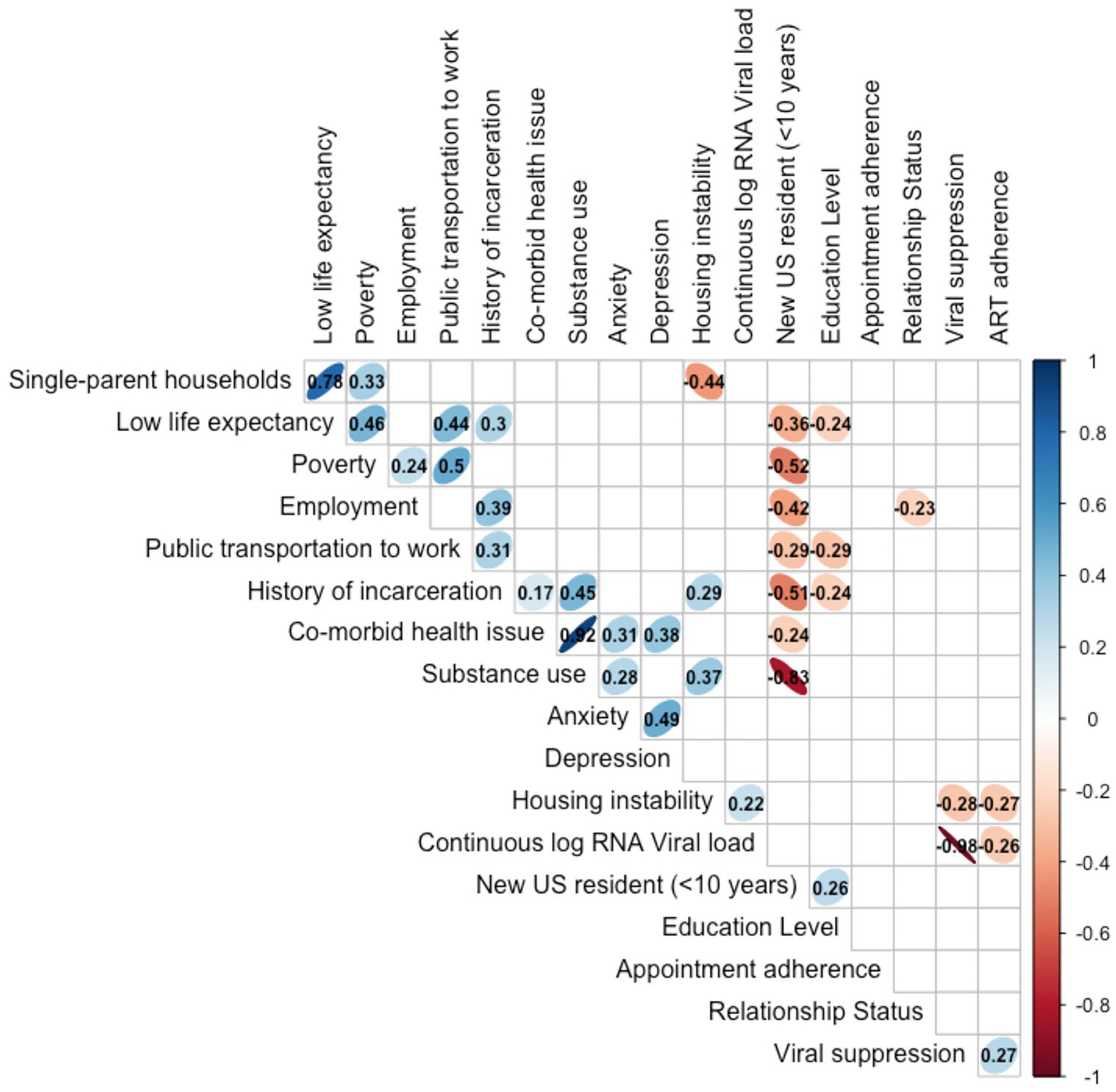
Bivariate associations between study variables.
*Ellipses represent significant correlations between variables at an alpha level of 0.05. The more narrow the ellipses, the greater the strength of the association. Statistically significant R-correlation statistics can be found within the cell of the table
Fig. 2.
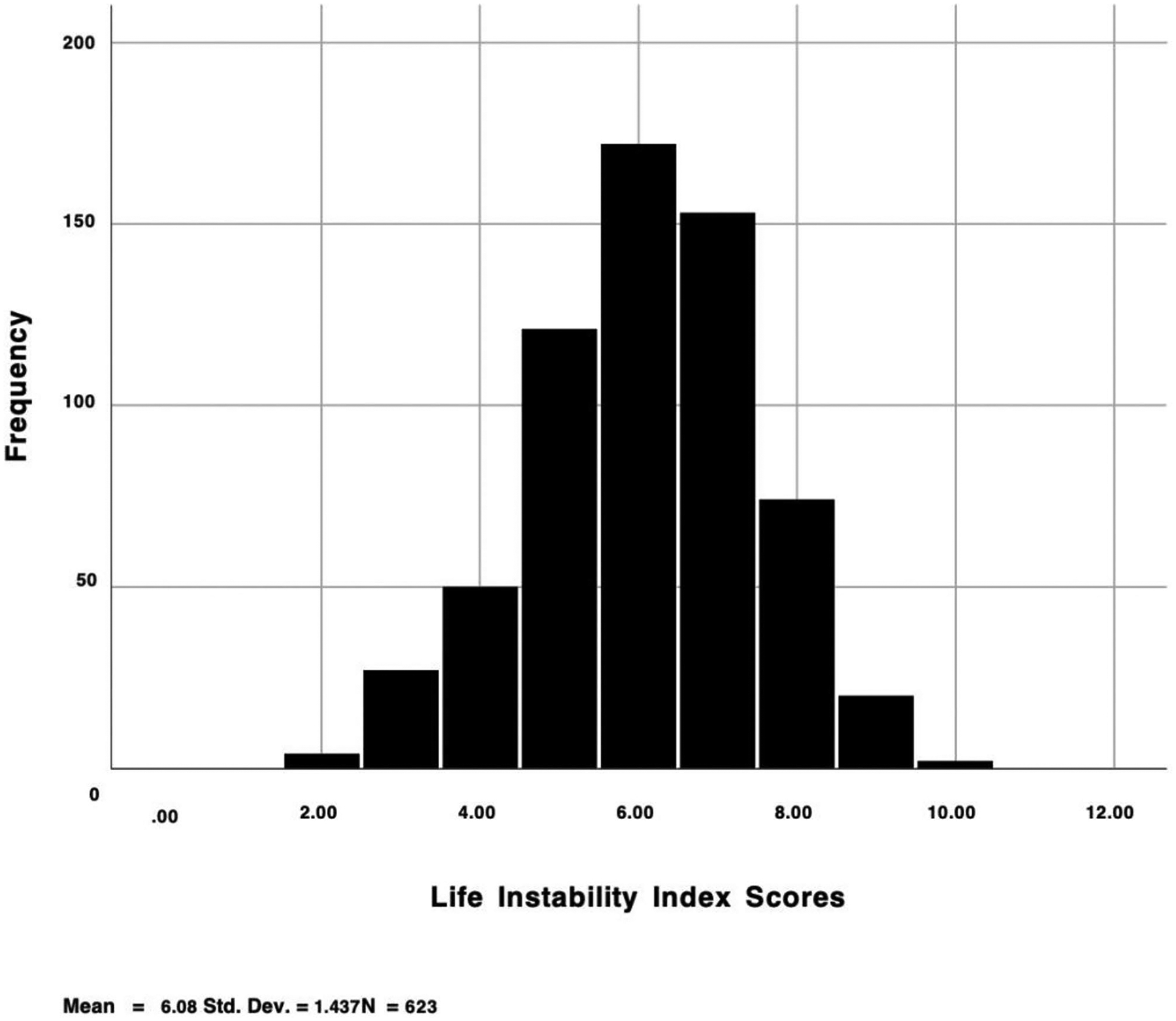
Distribution of life instability scores
Life Instability and HIV-related Health Outcomes: Direct Effects
For every additional life instability variable endorsed, there was a 1.14 percentage reduction in art adherence (b = − 1.14, SE = 0.52, C.I. [− 2.15, − 0.12], p = 0.03), a two-percentage point decrease in HIV care appointment adherence (b = − 0.02, SE = 0.01, C.I. [− 0.03, − 0.01], p < 0.01), and a 0.09 increase in viral load (b = 0.09, SE = 0.04, C.I. [0.02, 0.17], p = 0.02). Furthermore, for every additional factor of life instability experienced, the likelihood of having an unsuppressed viral load increased by 22% (OR = 1.22, SE = 0.46, C.I. [1.06, 1.40], p = 0.01). Results are further summarized in Table 2.
Life Instability and Behavioral Health Outcomes: Direct Effects
First, the relationship between life instability and depression was not significant (b = 0.16, SE = 0.15, C.I. [− 0.13, 0.46], p = 0.27). Anxiety trended towards significance, with each additional life instability indicator being non-significantly associated with a 0.17 unit increase in anxiety symptoms (b = 0.17, SE = 0.09, C.I. [− 0.02, 0.36], p = 0.07). Substance use was significant, with each additional life instability variable associated with a 0.08 increase in substance use (b = 0.08, SE = 0.02, C.I. [0.04, 0.13], p < 0.01).
Life Instability and HIV-Related Health Outcomes: Controlling for Behavioral Health Outcomes
In a multiple linear regression model of life instability, depression, anxiety, substance use, and ART adherence, the effects of life instability on ART adherence remained significant after adjusting for mental health symptoms and substance use (b = − 1.14, SE = 0.52, bootstrap (BTSP) confidence interval [− 2.15, − 0.12]). Similarly, life instability remained significantly associated with HIV care appointment adherence after adjusting for mental health symptoms and substance use (b = − 0.02, SE = 0.01, BSTP [− 0.028, − 0.005]). Furthermore, life instability remained significantly associated with viral load after adjusting for mental health symptoms and substance use (b = 0.08, SE = 0.04, BSTP [0.001, 0.161], p = 0.048). Finally, the relationship between life instability and viral suppression remained significant after adjusting for mental health symptoms and substance use (b = 0.25, SE = 0.08, BSTP [0.10, 0.40], p < 0.01). The indirect effect of life instability on viral suppression via all three measures of psychological functioning (i.e., depression, anxiety, and substance use) was not significant.
The only significant indirect effect observed was for substance use on the relationship between the life instability index and ART adherence (b = − 0.40, SE = 0.20, BSTP [− 0.87, − 0.09]), suggesting substance use partially explains the association between life instability and ART adherence even when controlling for the other psychological distress variables.
Discussion
This study is the first to show that an additive life instability index at both the individual and community levels is associated with HIV-related health outcomes among a population of OALWH 50 years or older. We found that this 11-item life instability index was associated with poorer HIV-related health outcomes among OALWH. Overall, for every additional life instability indicator that OALWH were experiencing, participants faced a reduction in monthly ART adherence by approximately one percentage point and were less adherent to their scheduled HIV care visits by about two percentage points. These findings support previous findings suggesting PLWH who experience life chaos are more likely to have both inconsistent ART adherence [7] and more missed medical appointments [9] compared to their peers with less life chaos.
OALWH in our study with higher amounts of life instability displayed worse HIV RNA viral load outcomes. The data showed significant positive relationships between life instability and both continuous log RNA viral load and having a detectable viral load. OALWH faced a 22% increase in odds of having a detectable viral load for every additional life instability indicator experienced.
A consistent pattern of results for the indirect effects of behavioral health (depression, anxiety, substance use) on HIV-related outcomes as hypothesized did not emerge. Although life instability was not associated with depression in our study despite being significantly positively related to substance use and trending towards significance in relation to anxiety, the LII was associated with the HIV-related health outcomes of interest even when controlling for these three measures of psychological distress. It is possible that we were unable to determine whether anxiety serves as a protective or risk factor of this relationship due to the null findings observed in this study, possibly because anxiety serves as a risk factor for some, and a protective factor for others—a significant pattern for partial indirect effects through substance use did emerge in our models. Future research could further disentangle these relationships among OALWH.
Although the literature has consistently cited a relationship between depression, substance use, and poor ART medication adherence among PLWH [42–44], it is possible that because OALWH in this study had low levels of overall psychological distress, we were unable to observe any significant indirect effects. One possible reason for this may be due to the age-related positivity effect which reflects a cognitive preference for positive over negative stimuli among older adults [45]. According to this theory, older adults are more inclined to focus on the “positives” in their life rather than the “negatives” which may in turn lead them to report less psychological distress compared to their younger peers [46]. Future studies should examine the potential impact of the age-related positivity effect among OALWH to better understand how this phenomenon may influence the relationship between life instability, psychological distress, and HIV-related health outcomes.
Overall, findings associated with both the prevalence of life instability and their effects on HIV-related health outcomes among OAWLH are particularly notable given that participants lived in Miami-Dade County, an epicenter of the US HIV epidemic. Existing structural barriers may exacerbate life instability particularly among older adults who possess further marginalized identities (e.g., persons with immigration histories, persons who use substances). Almost 3% of older adults over the age of 65 in Miami-Dade County do not have any access to health insurance—more than double the rate of uninsured older adults in Florida and triple the rate of uninsured older adults in the nation at large [47]. Lack of comprehensive access to health insurance may create inconsistent access to care for PLWH as they enter and progress through older adulthood. Additionally, Miami-Dade County’s limited public transportation system in comparison to other cities of its size (e.g., Boston, Washington D.C., Chicago) may create added barriers to care for OALWH who may not have the resources, financial, or otherwise, to get themselves to clinic appointments or lab work [14, 48]. Furthermore, Florida state and federal laws (e.g., HIV criminalization laws [49]; strict immigration laws [50]) may further contribute to life instability, and in turn, poorer HIV-related care outcomes experienced by multiply marginalized OALWH.
This study may have implications for strengthening coordinated HIV care models by providing stakeholders with greater insight into how indicators of life instability may affect OALWH across the aging process. Currently, clinics in major cities such as Los Angeles and New York City have employed person-centered and holistic coordinated HIV care models to retain PLWH in care and improve HIV care treatment outcomes among this marginalized population [51–53]. PLWH who received a more coordinated care model for their HIV treatment demonstrated greater engagement in care [52] as well as rapid improvements in both short-term [51, 52] and long-term [53] viral suppression. Our findings suggesting that OALWH experience significant life instability and that those with more life instability face poorer HIV-related care outcomes complement those by Li et al. who found that the PLWH in their study with housing instability, stimulant use, or multiple comorbid conditions had more trouble achieving viral suppression compared to their peers with none of those destabilizing factors [51]. By incorporating cognitive-behavioral therapy focused interventions that utilize problem solving, we can further strengthen these coordinated models of care and support OALWH facing life instability.
Furthermore, these findings suggest a greater need to bolster legislative policies and federal programs focused on supporting OALWH. At the federal level, the Older Americans Act (OAA) is the largest vehicle for the provision and delivery of services for aging populations; however, only a minority of these resources are allocated for OALWH or for those who identify as LGBTQ + and or Black, Indigenous, or People of Color [54]. To support these marginalized groups, public health practitioners should continue to demand reauthorization and funding for the OAA, but also explicitly center OALWH as a population with some of the greatest social needs. Similarly, in the last 10 years, programs such as the Ryan White HIV/AIDS program have seen close to a 15% increase in the number of OALWH—many of whom face significant life instability—seeking supportive services [55]. With the number of uninsured OAWLH expected to grow over the next few years, expanding resources through federal programs is key.
There are some limitations of note to this study. First, mediation could not be analyzed due to the study’s cross-sectional design, but it can be tested in future studies as longitudinal data continue to be collected. Furthermore, the relationship between life instability and poorer psychological functioning, specifically substance use, is likely bi-directional. Therefore, future longitudinal research should aim to clarify these associations. Second, although data suggests that simulants (i.e., methamphetamines, cocaine) are the class of drug most closely linked to difficulties with HIV disease management, the substance use measure employed in this analysis evaluated frequency of use of any drug over the past 30 days. Future research should specifically evaluate how different classes of drugs may affect HIV care outcomes differently among OALWH. Third, although the life instability index created for this study consisted of relevant indicators of life instability as outlined in the literature, other factors such as food insecurity [56], social support [57], and even community-level crime rates would further strengthen the index. Finally, this study included OALWH, aged 50 years or over, receiving care from two public HIV clinics, and living in Miami-Dade County, limiting generalizability of our findings to individuals with similar profiles (e.g., racially/ethnically diverse OALWH living in a large metropolitan area with significant income inequality). However, because Miami, Florida, is the city with one of the highest incidence and prevalence of HIV in the USA [25], our findings may be applied to describe the life experiences of OALWH in other HIV epicenters across the USA.
Investigating the role of life instability on HIV-related health outcomes among OALWH is a necessary step to bolstering the health and well-being of the largest growing demographic of PLWH in the USA. In this large sample of racial, sexual, and gender diverse OALWH receiving HIV care services in Miami, Florida—one of the cities with the highest incidence and prevalence of HIV [25]—we observed that additive indicators of life instability at both the individual and community levels are significantly associated with HIV disease severity and health-related adherence behaviors. Few prior studies have incorporated life instability into models of health and HIV; therefore, this life instability index moves the field forward by demonstrating how indicators of life instability at both the individual and the community levels affect ART adherence, HIV care appointment adherence, viral load, and viral suppression among OALWH above and beyond the net effects of depression, anxiety, and substance use.
Funding
Data collection for this study was supported by National Institute of Mental Health grant P30 MH116867 (Safren). Additional research support was provided by National Institute of Allergy and Infectious Disease grant P30 AI073961 (Pahwa). Some of the author time was supported by National Institute of Minority Health Disparities grants 5K23MD015690 (Harkness) and F31MD015988 (Shrader) as well as National Institute of Drug Abuse grant K24DA040489 (Safren). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Ethics Approval “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed Consent “Informed consent was obtained from all individual participants included in the study.”
Conflict of Interest Dr. Safren receives royalties from Oxford University Press, Guilford Publications, and Springer/Humana presss for books on cognitive behavioral therapy. Other authors declare no competing interests.
References
- 1.CDC. HIV and Older Americans. Centers for Disease Control and Prevention Published September 14, 2020. Accessed 29 Jan 2021. https://www.cdc.gov/hiv/group/age/olderamericans/index.html.
- 2.Hood JE, Golden MR, Hughes JP, et al. Projected demographic composition of the United States population of people living with diagnosed HIV. AIDS Care. 2017;29(12):1543–50. 10.1080/09540121.2017.1308466. [DOI] [PubMed] [Google Scholar]
- 3.Harris TG, Rabkin M, El-Sadr WM. Achieving the fourth 90: healthy aging for people living h HIV. AIDS. 2018;32(12):1563–9. 10.1097/QAD.0000000000001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpiak SE, Havlik R. Are HIV-Infected Older Adults Aging Differently? HIV and Aging. 17;42:11–27. 10.1159/000448539. [DOI] [PubMed] [Google Scholar]
- 5.Khoury AL, Morey MC, Wong TC, et al. Diminished physical function in older HIV-infected adults in the Southeastern U.S. despite successful antiretroviral therapy. PLoS One. 2017;12(6):e0179874. 10.1371/journal.pone.0179874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care. 2013;25(4):451–8. 10.1080/09540121.2012.712669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalichman SC, Kalichman MO. HIV-Related Stress and Life Chaos Mediate the Association Between Poverty and Medication Adherence Among People Living with HIV/AIDS. J Clin Psychol Med Settings. 2016;23(4):420–30. 10.1007/s10880-016-9481-8. [DOI] [PubMed] [Google Scholar]
- 8.Matheny AP, Wachs TD, Ludwig JL, Phillips K. Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. J Appl Dev Psychol. 1995;16(3):429–44. 10.1016/0193-3973(95)90028-4. [DOI] [Google Scholar]
- 9.Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The Association Between Life Chaos, Health Care Use, and Health Status Among HIV-Infected Persons. J GEN INTERN MED. 2007;22(9):1286–91. 10.1007/s11606-007-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zullig Leah L, Shaw Ryan J, Crowley Matthew J et al. Association Between Perceived Life Chaos and Medication Adherence in a Postmyocardial Infarction Population. Circ Cardiovasc Qual Out 2013;6(6):619–625. 10.1161/CIRCOUTCOMES.113.000435. [DOI] [PubMed] [Google Scholar]
- 11.German D, Latkin CA. Social Stability and HIV Risk Behavior: Evaluating the Role of Accumulated Vulnerability. AIDS Behav. 2012;16(1):168–78. 10.1007/s10461-011-9882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389(10072):941–50. 10.1016/S0140-6736(17)30003-X. [DOI] [PubMed] [Google Scholar]
- 13.Jarrett OD, Wanke CA, Ruthazer R, Bica I, Isaac R, Knox TA. Metabolic Syndrome Predicts All-Cause Mortality in Persons with Human Immunodeficiency Virus. AIDS Patient Care STDS. 2013;27(5):266–71. 10.1089/apc.2012.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wawrzyniak AJ, Rodríguez AE, Falcon AE, et al. The Association of Individual and Systemic Barriers to Optimal Medical Care in People Living with HIV/AIDS (PLWHA) in Miami-Dade County. J Acquir Immune Defic Syndr. 2015;69(1):S63–S72. 10.1097/QAI.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim S, Nash D, Hollod L, Harris TG, Lennon MC, Thorpe LE. Influence of Jail Incarceration and Homelessness Patterns on Engagement in HIV Care and HIV Viral Suppression among New York City Adults Living with HIV/AIDS. PLoS One. 2015;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aiello AE, Simanek AM, Galea S. Population Levels of Psychological Stress, Herpesvirus Reactivation and HIV. AIDS Behav. 2010;14(2):308–17. 10.1007/s10461-008-9358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS Treatment Nonadherence: A Review and Meta-analysis: JAIDS Journal of Acquired Immune Deficiency Syndromes. Published online 2011:1. 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siefried KJ, Mao L, Kerr S et al. Socioeconomic factors explain suboptimal adherence to antiretroviral therapy among HIV-infected Australian adults with viral suppression. PLoS One. 2017;12(4). 10.1371/journal.pone.0174613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angel RJ, Lein L, Henrici J. Poor Families in America’s Health Care Crisis. Cambridge University Press; 2006. [Google Scholar]
- 20.Bronfenbrenner U Toward an experimental ecology of human development. Am Psychol. 1977;32(7):513–31. 10.1037/0003-066X.32.7.513. [DOI] [Google Scholar]
- 21.Relf MV, Oakes M, Granadino E, Blake BJ, Vance D. Are We Ready for the Graying of the HIV Epidemic? J Assoc Nurses AIDS Care. 2019;30(1):1–3. 10.1097/JNC.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 22.Volume 31 | HIV Surveillance | Reports | Resource Library | HIV/AIDS | CDC. Published May 7, 2020. Accessed 05 May 2021. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-31/index.html.
- 23.Blanco JR, Jarrín I, Vallejo M, et al. Definition of Advanced Age in HIV Infection: Looking for an Age Cut-Off. AIDS Res Hum Retroviruses. 2012;28(9):800–6. 10.1089/aid.2011.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GIS Software | ArcGIS Products for the Cloud, Mobile Apps & Desktop. Accessed 21 May 2021. https://www.esri.com/en-us/arcgis/products/index.
- 25.HIV Treatment as Prevention | HIV Risk and Prevention | HIV/AIDS | CDC. Published February 22, 2021. Accessed December 27, 2021. https://www.cdc.gov/hiv/risk/art/index.html.
- 26.Wilson IB, Lee Y, Michaud J, Fowler FJ, Rogers WH. Validation of a New Three-Item Self-Report Measure for Medication Adherence. AIDS Behav. 2016;20(11):2700–8. 10.1007/s10461-016-1406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bureau UC. Geography Program. The United States Census Bureau. Accessed 21 May 2021. https://www.census.gov/geography. [Google Scholar]
- 28.Spielman SE, Folch D, Nagle N. Patterns and causes of uncertainty in the American Community Survey. Appl Geogr. 2014;46:147–57. 10.1016/j.apgeog.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Census Bureau. American Community Survey 1-year estimates. Census Reporter Profile page for Miami-Dade County, FL Published 2018. Accessed 15 Apr 2020. http://censusreporter.org/profiles/05000US12086-miami-dade-county-fl/.
- 30.Matters M-D. Miami-Dade Matters : Demographics : County : Miami-Dade : Ethnicity. Accessed 10 Mar 2021. http://www.miamidadematters.org/demographicdata?id=414§ionId=941.
- 31.U.S. Census Bureau. U.S. Census Bureau QuickFacts Published 2019. Accessed 17 Dec 2020. https://www.census.gov/quickfacts/.
- 32.American Psychiatric Association, ed. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Am Psych Assoc; 2013. [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16(9):606–13. 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kok RM, Reynolds CF III. Management of Depression in Older Adults: A Review. JAMA. 2017;317(20):2114–22. 10.1001/jama.2017.5706. [DOI] [PubMed] [Google Scholar]
- 35.Crane PK, Gibbons LE, Willig JH, et al. Measuring depression levels in HIV-infected patients as part of routine clinical care using the nine-item Patient Health Questionnaire (PHQ-9). AIDS Care. 2010;22(7):874–85. 10.1080/09540120903483034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network. NCCN Distress Thermometer and Problem List for Patients. Published online 2016. https://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf.
- 37.Cutillo A, O’Hea E, Person S, Lessard D, Harralson T, Boudreaux E. NCCN Distress Thermometer: Cut off Points and Clinical Utility. Oncol Nurs Forum. 2017;44(3):329–36. 10.1188/17.ONF.329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.IBM SPSS Statistics for Windows, Version 25.0 IBM Corp; 2017 [Google Scholar]
- 39.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 16;37(1):17–32. 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 40.Hayes AF. Process: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper]. Published online 2012. http://www.afhayes.com/public/process2012.pdf.
- 41.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–76. 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez JS, Hendriksen ES, Collins EM, Durán RE, Safren SA. Latinos and HIV/AIDS: Examining Factors Related to Disparity and Identifying Opportunities for Psychosocial Intervention Research. AIDS Behav. 2008;13(3):582. 10.1007/s10461-008-9402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinn KG, Voisin DR. ART Adherence Among Men Who Have Sex with Men Living with HIV: Key Challenges and Opportunities. Curr HIV/AIDS Rep. 2020;17(4):290–300. 10.1007/s11904-020-00510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11(3):291–307. 10.1007/s11904-014-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychol Sci. 2004;15(3):208–14. 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- 46.Reed AE, Carstensen LL. The Theory Behind the Age-Related Positivity Effect. Front Psychol. 2012;3. 10.3389/fpsyg.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matters M-D. Miami-Dade Matters Demographics County Miami-Dade Adults 65+ Without Health Insurance. Accessed 10 May 2021. https://www.miamidadematters.org/indicators/index/view?indicatorId=4655&localeTypeId=39&comparisonId=7227.
- 48.Ward MK, de la Cruz Y, Fernandez SB, et al. Provider Perceptions of Barriers to HIV Care Among Women with HIV in Miami-Dade County, Florida, and Possible Solutions: A Qualitative Study. J Int Assoc Provid AIDS Care. 2021;20:23259582211053520. 10.1177/23259582211053520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasenbush A HIV Criminalization in Florida: Penal Implications for People Living with HIV/AIDS. Published online October 1, 2018. Accessed 21 May 2021. https://escholarship.org/uc/item/4rn9t5s7.
- 50.Page KR, Dolwick Grieb S, Nieves-Lugo K, et al. Enhanced Immigration Enforcement in the US and the Transnational Continuity of HIV Care for Latinx Migrants in Deportation Proceedings. Lancet HIV. 2018;5(10):e597–604. 10.1016/S2352-3018(18)30074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li MJ, Su E, Garland WH, et al. Trajectories of Viral Suppression in People Living With HIV Receiving Coordinated Care: Differences by Comorbidities. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2020;84(4):387–95. 10.1097/QAI.0000000000002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irvine MK, Chamberlin SA, Robbins RS, Kulkarni SG, Robertson MM, Nash D. Come as You Are: Improving Care Engagement and Viral Load Suppression Among HIV Care Coordination Clients with Lower Mental Health Functioning, Unstable Housing, and Hard Drug Use. AIDS Behav. 2017;21(6):1572–9. 10.1007/s10461-016-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robertson MM, Penrose K, Irvine MK, et al. Impact of an HIV Care Coordination Program on Durable Viral Suppression. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2019;80(1):46–55. 10.1097/QAI.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams M, Tax AD. Assessing and Meeting the Needs of LGBT Older Adults via the Older Americans Act. LGBT Health. 2017;4(6):389–93. 10.1089/lgbt.2016.0171. [DOI] [PubMed] [Google Scholar]
- 55.Older Adult Clients: HRSA’s Ryan White HIV/AIDS Program, 2019: Population Fact Sheet. hab.HRSA.gov. Updated June, 2021. Accessed 15 Nov 2021. https://hab.hrsa.gov/sites/default/files/hab/Publications/factsheets/population-factsheet-older-adults.pdf.
- 56.Kalichman SC, Hernandez D, Kegler C, Cherry C, Kalichman MO, Grebler T. Dimensions of Poverty and Health Outcomes Among People Living with HIV Infection: Limited Resources and Competing Needs. J Community Health. 2015;40(4):702–8. 10.1007/s10900-014-9988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viswanath H, Wilkerson JM, Breckenridge E, Selwyn BJ. Life Chaos and Perceived Social Support Among Methamphetamine-Using Men Who Have Sex With Men Engaging in Transactional Sexual Encounters. Subst Use Misuse. 2017;52(1):100–7. 10.1080/10826084.2016.1222620. [DOI] [PMC free article] [PubMed] [Google Scholar]


