Abstract
Joint trauma induces a presynaptic reflex inhibition termed arthrogenic muscle inhibition (AMI) that prevents complete activation of muscles. Reduced motor unit (MU) output is a hypothesized mechanism for persistent strength deficits. The objective of this study was to determine MU characteristics of thigh musculature and determine how they change with anterior cruciate ligament (ACL) injury compared to healthy controls. A randomized protocol of knee flexion/extension isometric contractions (10–50% maximal voluntary isometric contraction) was performed for each leg with surface EMG 5-pin array electrodes placed on the vastus medialis, vastus lateralis, semitendinosus, and biceps femoris. Longitudinal assessments for average rate coding, recruitment thresholds, and MU action potentials were acquired at 6-month intervals. With exception of the vastus medialis, all thigh musculature of ACL-injured demonstrated smaller MU action potential peak-to-peak amplitude. For average rate coding, ACL-injured demonstrated lower coding rates than Controls for the quadriceps (p<0.05) and higher rates than Controls for the hamstrings (p<0.05). These MU characteristics were different from Controls after ACL reconstruction up to 12 months post-surgery, yet maximal strength increased during this time frame. As thigh MU characteristics are known across phases of ACL rehabilitation, future studies can assess these patterns of motor control and its potential to determine risk of re-injury. Further, future rehabilitation can target specific intervention programs to restore motor control.
Keywords: rehabilitation, motor control, Biomechanics, Injury and Prevention
INTRODUCTION
Quadriceps strength deficits appear after anterior cruciate ligament (ACL) injury and reconstruction (ACLR) despite quadriceps-focused rehabilitation. The joint trauma induces a presynaptic reflex inhibition of the quadriceps, clinically termed arthrogenic muscle inhibition (AMI).(Hart, Pietrosimone, Hertel, & Ingersoll, 2010) This inhibition may be initially protective to the joint by prevention of anterior pull from the quadriceps; however, the persistent AMI prevents complete activation of the muscle and can impede recovery.(Hart et al., 2010) The mechanism for persistent strength deficits remains unclear, although reduced motor unit output has been suggested to reduce voluntary strength after ACLR.(Rice & McNair, 2010) Lowered strength production (i.e. asymmetrical limb strength and decreased hamstrings to quadriceps strength ratios) is an important factor in a return-to-sport (RTS) decision making and may contribute to increased risk of second ACL injury.(Kyritsis, Bahr, Landreau, Miladi, & Witvrouw, 2016) Therefore, it is critical to understand the physiological mechanism for impaired quadriceps strength after ACLR.
Voluntary force production is achieved by neural drive to the muscle, which can be assessed with electromyography (EMG) techniques; EMG measures the electrical activity of muscles and is a stochastic signal from observed motor unit action potentials (MUAPs). Previous work has shown that muscle force generation is proportional to both rate coding (measured in pulses per second; pps) and quantity of motor unit (MU) recruitment.(Maffiuletti et al., 2016) Thus, in order to achieve a desired force, neural activity adapts with a combination of rate coding and/or recruitment strategies. Previous work has utilized decomposition of surface EMG to investigate the relationship between rate coding and recruitment threshold during voluntary isometric contractions.(Carlo J De Luca & Hostage, 2010; Del Vecchio et al., 2019) In healthy subjects, MU recruitment threshold contributed to MU rate coding characteristics, characterized by an inverse linear relationship.(Carlo J De Luca & Hostage, 2010) Variable effects of interventions on rate coding and recruitment threshold have been reported in the literature. A four-week strength training program resulted in increased MU rate coding and decreased recruitment thresholds in healthy subjects, which may indicate the adaptability of neural control to achieve force production.(Del Vecchio et al., 2019) In addition, studies of healthy individuals have shown variable effects of strength, high intensity interval training, and endurance training programs on MU rate coding.(Martinez-Valdes et al., 2017; Vila-Chã, Falla, & Farina, 2010) While strength and high intensity interval training programs resulted in increased quadriceps rate coding, endurance training programs resulted in either no change or decreased rate coding.(Martinez-Valdes et al., 2017; Vila-Chã et al., 2010) In contrast, however, no differences were reported for rate coding and recruitment for thigh musculature at 50–80% maximal voluntary isometric contraction (MVIC) after resistance training programs.(Beck TW, DeFreitas JM, & MS, 2011; Stock & Thompson, 2014) While the exact effects of interventions on MU activity in a healthy population remain inconclusive, it is also unknown how injury and subsequent rehabilitation affect neural control of muscle force production.
The purpose of this study was to determine the MU characteristics of the thigh musculature and determine MU changes with ACL injury (both injured and uninjured limbs) across rehabilitation compared to healthy controls. It was hypothesized that MU average rate coding of ACL-injured would be lower compared to controls. Additionally, it was hypothesized that the size of the MU (measured indirectly via the peak-to-peak MU action potential(Hu, Rymer, & Suresh, 2013b)) would be lower for ACL-injured compared to controls. This information will be helpful to elucidate the motor control outcomes that occur with AMI following an ACL injury and determine means to address the motor control deficits in rehabilitation.
MATERIALS AND METHODS
The Mayo Clinic Institutional Review Board approved the study (16–010600). Fifty-four subjects were recruited (see Table 1 for demographics) and completed written informed consent compliant with the latest revision of the Declaration of Helsinki. ACL injured subjects were recruited either prior to surgery (data capture the day prior to ACLR; n=24; ‘ACL Pre-Surg’) or at 6 months post-surgery (± 1 month; n=6). Of the ACL injured subjects, 7 (23%) had experienced a second ACL tear prior to recruitment, a percentage consistent with existing literature.(Paterno, Rauh, Schmitt, Ford, & Hewett, 2014; Schilaty et al., 2017) The ACL injured participants were followed longitudinally for testing intervals of 6 months (± 1 month), which could include 6 months post-surgery (n=14; ‘ACLR 6mo’) and/or 12 months post-surgery (n=12; ‘ACLR 12mo’). Among the ACL-injured, 73% received a bone-patellar-bone autograft, 24% received a hamstring autograft, and 3% received an allograft. Control subjects (n=25, ‘CTRL’) were also recruited and were followed longitudinally for 6 months after the initial visit. From initial recruitment, the overall attrition rate was 26%. Subject inclusion criteria were healthy, active individuals between the ages of 14 – 25. Exclusion criteria were lower extremity injury (other than ACL) or surgery in the past 6 months, neurological disorders, paralysis, neuromuscular disease, cardiovascular disease, exercise-induced injury, asthma, and pregnancy.
Table 1.
Population Demographics (divided by Sex and Group).
| Height (cm) | Mass (kg) | Age (yrs) | ACL Injured (n) | Non-contact Mechanism of Injury (n) | ||
|---|---|---|---|---|---|---|
| Sex | M (n=24) | 180.3 ± 5.6 | 81.6 ± 13.8 | 19.4 ± 2.9 | 13 | 10 (77%) |
| F (n=30) | 169.0 ± 7.2 | 66.4 ± 12.6 | 18.7 ± 3.2 | 17 | 13 (77%) | |
| p-value | 0.001 a | 0.001 a | 0.397 | 1.000 | 1.000 | |
| Group | Control (n=25) | 173.9 ± 8.5 | 67.6 ± 13.4 | 18.8 ± 3.1 | --- | --- |
| ACL Injured (n=29) | 173.8 ± 8.8 | 77.2 ± 15.1 | 19.1 ± 3.1 | --- | --- | |
| p-value | 0.948 | 0.016 b | 0.726 | --- | --- | |
Values reported as mean ± SD. Bolded values are significant.
indicates significant difference between sex (p<0.05).
indicates significant difference between groups (p<0.05).
All data collections were performed with the participants positioned in a dynamometer (HumacNORM; CSMi, Stoughton, MA, USA). A custom load cell apparatus (MLP-300; Transducer Techniques, Temecula, CA, USA) was affixed to the dynamometer torque arm to measure the subject’s force production required for the EMG decomposition software (dEMG Analysis; Delsys, Natick, MA, USA). For isometric knee extension testing, participants were positioned in a seated position with their leg at 80° flexed position (0° = full extension). Participants were secured with straps at the shoulder and waist to minimize whole body movement during testing. Surface 5-pin dEMG electrodes (Delsys) were placed on the muscle belly of both the vastus medialis (VM) and vastus lateralis (VL) muscles. Pair wise subtraction of voltages at the five detection surfaces was used to derive multi-channel surface EMG (sEMG) signals.(Nawab, Chang, & De Luca, 2010) For isometric knee flexion testing, subjects were positioned prone on the dynamometer with their leg positioned at 30° knee flexion (0° = full extension). Surface EMG electrodes were placed on the muscle belly of the biceps femoris (BF) and semitendinosus (ST) muscles. Prior to electrode placement, the skin was shaved and cleansed with an alcohol swab to ensure adequate skin-electrode contact. All electrodes were placed according to SENIAM standards.(Hermens, Freriks, Disselhorst-Klug, & Rau, 2000)
Testing Protocol
Three isometric knee flexion and extension MVICs were performed to determine 10%, 25%, 35% and 50% MVIC contraction levels for each leg. Subjects were verbally encouraged to push as hard as possible for three seconds and were permitted to view the computer monitor during the MVIC trials for visual feedback and motivation. A protocol was generated for each subject to determine test order; limb side (right vs. left), muscle group (hamstring vs. quadriceps), and order of trials (10%−50% MVIC) were all randomized. After the MVIC trials, the randomized protocol was followed. Each trial was repeated twice to ensure adequate MU data capture. Each trial consisted of following a trapezoidal waveform (three second ramp up, ten second sustained contraction at designated %MVIC, and three second ramp down). Each participant was instructed to follow the trapezoid as closely as possible, with real-time visual feedback displayed on a computer monitor.
EMG Signal Decomposition
The analog sEMG channels were band-pass filtered with cut-off frequencies of 20 and 1750 Hz. Each channel was then over-sampled at 20 kHz to avoid introduction of significant phase skew across channels. The digital sEMG signals were digitally filtered using a high-pass filter with a cutoff frequency of 50 Hz before decomposition.(Nawab et al., 2010) The signal decomposition algorithm first extracted action potential ‘templates’ of as many MUAPs as possible from the input sEMG signal. The algorithm then searched for signal regions where the extracted MUAP templates were superimposed with other identified MUAPs or with unidentified action potentials. The algorithm takes both constructive and destructive interference effects into account when analyzing such superpositions. Moreover, the algorithm requires that the unidentified action potentials account for less than 25% of the signal energy at the firing locations of the decomposed MUAP trains.(Nawab et al., 2010)
In order to verify the decomposed signal, the algorithm performed a Decompose-Synthesize-Decompose-Compare test.(C J De Luca & Contessa, 2012) The original signal was decomposed, as described in the preceding paragraph. Then, white noise with a root mean square error value equivalent to the residual of the non-decomposed signal was added to the decomposed signal and synthesized. The synthesized signal was then decomposed, as described above, and compared to the original signal decomposition. Only MUs with an accuracy of ≥90% were included in analysis for the current study. In addition to internal validation by the development group, the decomposition algorithm has been externally validated against spike triggered averaging techniques.(Hu, Rymer, & Suresh, 2013a; Nawab et al., 2010) and further details of the methodology can be found elsewhere.
Data Analysis
Electromyography decomposition (dEMG) was performed to obtain MU rate coding (initial, average, and terminal), defined as MU rate coding per second.(Carlo J De Luca, Adam, Wotiz, Gilmore, & Nawab, 2006) Average rate coding during the 10 second sustained contraction was computed with a low-pass filter applied to the MUAPs with a unit-area Hanning window of 1-second duration. Additional details on the filtering procedure can be found elsewhere.(C. J. de Luca, LeFever, McCue, & Xenakis, 1982)
All statistical analyses were performed with JMP Pro 14 (SAS Institute Inc., Cary, NC, USA). As MU strategies are non-linear,(Franklin & Wolpert, 2011; Gorassini, Yang, Siu, & Bennett, 2002) log (MUAP peak-to-peak amplitude) and cube root (Recruitment Threshold) transformations were utilized, as appropriate, to provide parametric data for linear regressions. MUAP data were normalized to mass for statistical analysis as MU sizes are dependent upon muscle size which is dependent on mass. Descriptive statistics were calculated for both sex and group via Student’s t-test and Fisher’s Exact test and reported as mean (SD) or n values (Table 1). MVIC value comparisons utilized the Wilcoxon test for nonparametric force data. The number of MUs and MU signal amplitude were reported as median and quantiles (25th, 75th) and compared with Wilcoxon tests when appropriate. Rate coding and MUAP were compared between groups with standard least squares regression and least squares means ANOVA with Tukey’s HSD post-hoc comparisons, when appropriate. As there were minimal (if any) differences between values of the CTRL group, both 6-month interval data was combined when appropriate and used for analysis compared to ACL injured. Significance was set a priori at p<0.05.
RESULTS
Motor Unit Characteristics
A total of 41,473 MUs were identified from this cohort; 17,451 MUs for CTRL participants and 24,022 MUs were identified for the ACL-injured participants (11,673 MUs ACLR Pre-Surg; 6,921 for ACLR 6mo, and 5,428 for ACLR 12mo). The median number of MUs identified with each contraction for quadriceps were 11 [5, 17] for 10% MVIC and 13 [6,20] MUs for 25, 35, and 50% MVIC; for the hamstrings, 9 [5, 15] for 10% MVIC, 11 [6, 17] for 25% MVIC, and 11 [6, 18] for 35 and 50% MVIC. Table 2 provides the initial, average, and terminal FR for each trial and muscle. The initial, average, and terminal FR for all muscles combined were 6.5 (2.3) pulses per second (pps), 16.6 (4.3) pps, and 8.4 (3.1) pps, respectively.
Table 2. Summary of Motor Unit rate coding by Muscle and Trial.
The data is represented in average pulses per second (pps) and standard deviation. (FR = firing rate)
| Initial FR (SD) | Average FR (SD) | Terminal FR (SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %MVIC | 10 | 25 | 35 | 50 | 10 | 25 | 35 | 50 | 10 | 25 | 35 | 50 | |
| Quads |
VL (n=11,656) |
5.8 (2.7) | 6.2 (2.2) | 6.3 (1.7) | 6.3 (1.6) | 14.7 (4.8) | 15.6 (3.8) | 15.9 (3.1) | 15.6 (3.4) | 7.7 (3.8) | 8.0 (2.9) | 8.3 (2.6) | 8.2 (2.3) |
|
VM (n=10,048) |
7.2 (3.5) | 6.7 (2.5) |
6.8 (2.2) | 6.7 (1.9) | 17.3 (5.8) | 16.7 (4.2) | 16.7 (4.0) | 16.3 (3.9) | 9.2 (4.3) | 8.6 (3.4) | 8.7 (3.0) | 8.9 (2.8) | |
| Hams |
BF (n=9,364) |
6.1 (3.0) | 6.0 (2.3) | 6.4 (2.0) | 6.5 (1.9) | 15.4 (5.1) |
16.3 (3.9) | 16.8 (3.9) | 16.4 (4.3) | 7.7 (3.9) | 8.0 (3.0) | 8.4 (2.8) | 8.3 (2.4) |
|
ST (n=10,405) |
7.0 (2.8) | 6.9 (2.4) | 6.9 (2.2) | 6.9 (2.1) | 17.7 (4.9) | 18.0 (4.2) | 18.0 (4.2) |
17.4 (4.7) | 8.4 (3.6) | 8.5 (2.9) | 8.8 (2.9) | 9.0 (2.7) | |
Average Rate Coding
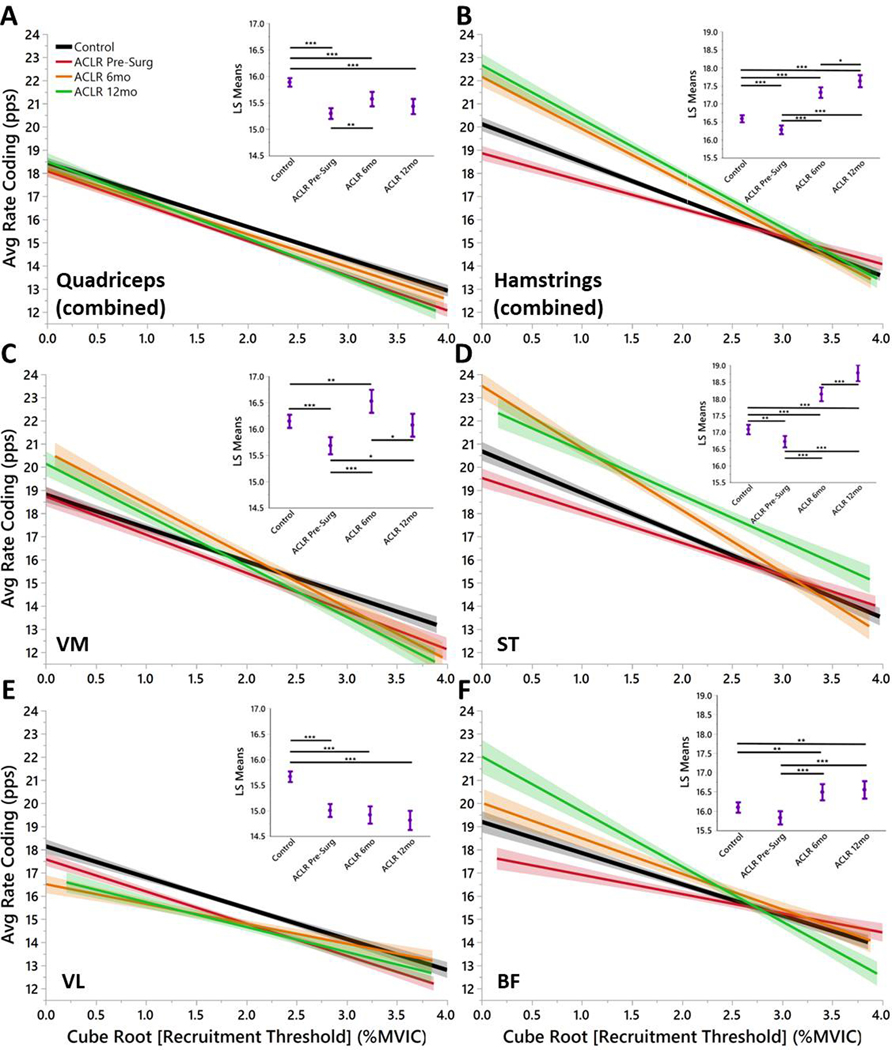
The overall regression model of average rate coding by recruitment threshold for the quadriceps (R2=0.09) demonstrated that all time points of ACL injured had lower average rate coding across recruitment levels (Fig. 1A, C, E) with the exception of the VM of the ACLR 6mo and ACLR 12mo groups (Fig. 1C). The overall regression model of average rate coding by recruitment threshold for the hamstrings (R2=0.12) demonstrated that ACLR Pre-Surg had lower average rate coding than CTRL (Fig. 1B, D, F). However, the rate coding for the hamstrings increased for ACLR 6mo and ACLR 12mo compared to CTRL (Fig. 1B, D, F).
Figure 1. Quadriceps and Hamstrings Average Rate Coding by Recruitment Threshold Separated by Group.
Once a motor unit is recruited, the average firing rate represents the overall performance of the motor unit. Insets) Least Squares Means ANOVA demonstrates significant differences of average rate coding between Groups. (* denotes p<0.05; ** denotes p<0.01; *** denotes p<0.001; line shading and error bars denote 95% confidence intervals of the mean.)
Motor Unit Action Potential
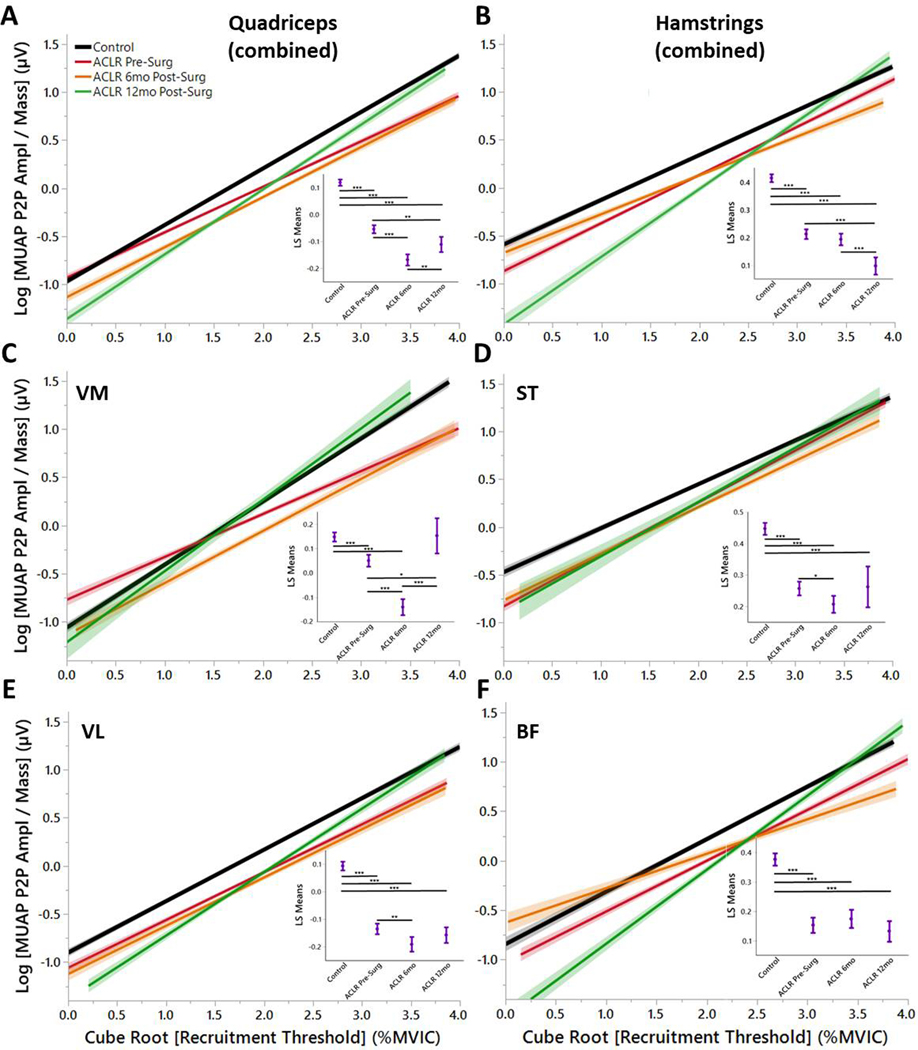
The overall regression model of MUAP peak-to-peak amplitude by recruitment threshold for the quadriceps (R2=0.40) demonstrated that all time points of ACL injured had lower MUAP across recruitment levels (Fig. 2A, C, E) with the sole exception of the VM of the ACLR 12mo group (Fig. 2C). The overall regression model of MUAP peak-to-peak amplitude by recruitment threshold for the hamstrings (R2=0.34) demonstrated that all time points of ACL injured had lower MUAP across recruitment levels (Fig. 2B, D, F).
Figure 2. Quadriceps and Hamstrings Motor Unit Action Potential (MUAP) Peak Amplitude by Group.
MUAPs are a surrogate for MU size. The linear plots demonstrate the Henneman Size Principle in which smaller units are recruited first, followed by larger units later. Insets) Least Squares Means ANOVA demonstrates significantly lower MUAPs for ACLR Pre-Surg, ACLR 6mo, and ACLR 12mo compared to Controls with the exception of the VM for ACLR 12mo. (* denotes p<0.05; ** denotes p<0.01; *** denotes p<0.001; line shading and error bars denote 95% confidence intervals of the mean.)
DISCUSSION
The main findings of this study demonstrate three important concepts of MU characteristics with ACL injury and rehabilitation: 1) overall average rate coding was lower for quadriceps musculature for ACL-injured across rehabilitation compared to CTRLs (with the exception of the VM), 2) compared to CTRLs, average rate coding for hamstring musculature was initially lower at ACLR Pre-Surg but then was increased with rehabilitation and at time of RTS, 3) MU size were smaller across the entire range of recruitment for ACL-injured compared to CTRLs and this did not recover at RTS. Further evaluation of the slopes of the linear regressions demonstrate that ACL-injured favored a higher rate coding with earlier recruited MUs.(Stock, Beck, & Defreitas, 2012) From these results, the hypotheses that MU average rate coding of ACL-injured would be lower compared to CTRLs and that MU size would be lower for ACL-injured compared to CTRLs were both supported.
The MU rate coding are similar to that which have been reported previously, such as VL demonstrating a lower rate coding than the VM (Table 2).(De Souza, Cabral, Fernandes De Oliveira, & Martins Vieira, 2018) Across rehabilitation, the current evidence demonstrates that ACL-injured are dramatically different from CTRLs in their overall rate coding strategy. This may indicate that ACL-injured have inhibited larger MUs and thus favor the lower threshold MUs and utilize higher rate coding strategies with these smaller MUs. There are differences of reports with changes of recruitment thresholds with training.(Beck TW et al., 2011; Del Vecchio et al., 2019; Fukunaga, Johnson, Nicholas, & McHugh, 2018) A shift towards higher recruitment could indicate improved efficiency of lower threshold units, or a shift to lower recruitment thresholds could indicate increased use of slow-twitch motor units or increased MU synchronization.(McHugh, 2003) Either way, changes in recruitment thresholds indicate potential adaptations of motor control strategies and recruitment thresholds have not previously been reported for major orthopedic injuries.
A previous study showed no difference in recruitment threshold between VM and VL MUs.(De Souza et al., 2018) Our data similarly demonstrates no difference in recruitment threshold between the VM and VL (data not shown), however, in the current study there are differences between these muscles for average rate coding by recruitment threshold wherein the VL underperformed CTRL at all time points of rehabilitation whereas the VM demonstrated increased average firing rate during early recruitment (Fig. 1C, E). Although the VM appears to recover over ACLR rehabilitation with the least squares mean ANOVA, the motor control scheme is different from CTRL rate coding patterns with the initial recruited MUs firing at higher rates (Fig. 1C). This could lead to early fatigue for this muscle or demonstrate inability of the larger, later recruited units to control muscular demands of certain athletic tasks. Average VL rate coding (for all participants and all trials) in the current study was slightly lower than previously reported for healthy male subjects during a 50% MVIC contraction (15.7 vs 20.5 pps).(Stock & Thompson, 2014) Differences in average rate coding may be attributed to different subject demographics (i.e. age, sex), isometric testing setup, or inherent error of MU identification. Similar to De Luca et al., all muscle MUs were derecruited at higher force levels than which they were recruited for all trials (Table 2).(Carlo J De Luca & Hostage, 2010)
The observed MU control followed the previously outlined scheme in which an inverse relationship was observed between MU recruitment thresholds and average rate coding.(Carlo J De Luca & Contessa, 2015) This relationship allows for biomechanically and energy efficient force production across a range of contraction intensities. This inverse relationship has previously been demonstrated for isometric contractions in healthy subjects across a range of muscles and effort levels but demonstrated inconsistent quantitative changes dependent on the muscle and target force.(Charntikov et al., 2013) With known inter-individual variability of neural control schemes in a healthy population, it was further warranted to determine how an ACL injury affects this motor control scheme.
CTRL subjects recruited larger MUs at similar recruitment levels compared to ACL-injured for all thigh musculature with exception of the VM at ACLR 12mo (Fig. 2). This supports the neural inhibition hypothesis after ACL injury.(Rice & McNair, 2010) Of particular interest, MU inhibition occurs for both the quadriceps and hamstrings. This indicates that the joint trauma affects both the flexors and extensors for up to one year following injury. Both groups increased MU size with later recruitment thresholds (following Henneman’s size principle),(Ertas, Stålberg, & Falck, 1995) but ACL-injured exhibit smaller units than healthy CTRLs across all phases of rehabilitation. The hamstring musculature appears to compensate for the smaller MUs (Fig. 2B, D, F) with increased average rate coding (Fig. 1B, D, F). However, the quadriceps musculature remains inhibited for both MU size (Fig. 2A, C, E) and average rate coding (Fig. 2B, D, F). These differences between ACL-injured subjects across all phases of rehabilitation, particularly the change in average rate coding and MU size recruitment post-surgery may provide insight into the underlying differences that persist throughout rehabilitation manifested with altered motor output.(Nagai, Bates, Hewett, & Schilaty, 2018; Nagai, Schilaty, Strauss, Crowley, & Hewett, 2018)
After ACL injury, it is known that quadriceps atrophy and overall strength is reduced.(Strandberg, Lindstrom, Wretling, Aspelin, & Shalabi, 2013) This was observed in the current study wherein the injured quadriceps had lower force values than the uninjured (data not shown), with the lowest force production at 6 months post-surgery. It has been previously documented that bilateral effects have been observed in patients with isolated ACL injuries, but to our knowledge, has only been described for the quadriceps.(Rice & McNair, 2010) The differences observed in rate coding, recruitment, and MU size demonstrates that ACL injury has a sensorimotor effect on the ability for ACL-injured to activate MUs as observed with CTRLs.(Balshaw, Pahar, Chesham, Macgregor, & Hunter, 2017) Further, the observed imbalance between VM and VL motor control could lead to progression of patellar tracking issues that lead to patellofemoral pain and chondromalacia patella post-ACLR.(Lee, Yeom, Kim, Kim, & Kim, 2018)
Clinical Relevance
The findings from this study provide evidence towards understanding the physiological mechanism of motor control and voluntary force output after ACL injury relative to healthy CTRLs. Historically, RTS after ACLR was permitted around 6 months post-surgery; recently however, due to the increased risk of second injury, a delayed RTS has been suggested as graft healing and neuromotor adaptations are still in progress.(Nagelli & Hewett, 2016; Sundaram et al., 2019) Findings in the current study support delayed RTS beyond the conventional 6 to 9 months; even at 12 months post-ACLR, there are significant neuromechanical deficits of the majority of thigh musculature. Some of the improvements observed for the 6- and 12-month time points for the ACL-injured (hamstrings and VM) may be attributed to intensive physical therapy rehabilitation visits between 6 and 12 months. It is possible that cessation of targeted rehabilitation at RTS could lead to further motor control deficits that lead to the high occurrence of second injury.(Paterno et al., 2014; Schilaty et al., 2017) Previous work has suggested that RTS be delayed even until two years due to a host of biological and biomechanical factors.(Nagelli & Hewett, 2016) The findings from the current study support delayed RTS and suggest that rehabilitation after ACLR should continue until at least 12 months post-ACLR to continue restoration of normal MU activity. Further, the information gained from this longitudinal assessment of thigh musculature MU characteristics will allow researchers and clinicians to improve targeted rehabilitation to return MU characteristics to normal.
The results of this study will allow for innovation into informed individualized rehabilitation and intervention strategies. With the continued development and improvement of sEMG wearable decomposition technology, future work may allow clinicians to monitor patients’ neural strategies to achieve targeted forces and dynamic stabilization during rehabilitative activities. Similar to the ACL-injured groups, if an injured patient demonstrates impaired or altered recruitment, MU size, or rate coding strategies, artificially intelligent algorithms could be developed that allow clinicians to focus rehabilitation strategies to address these deficits. Specific exercises that restore specific MU characteristics remain to be determined and should be the focus of future work. In addition, while ACL injury is known to disrupt and ACLR fails to fully restore afferent signaling, rehabilitation that incorporates sensory stimulation could prove beneficial to improve efferent signals. Previous work has reported moderately increased quadriceps voluntary force production and dampened H-reflex decline after transcutaneous electrical nerve stimulation, possibly attributed to decreased pain or altered sensory pathways.(Hopkins & Ingersoll, 2000) Further, in a study of artificially-induced AMI quadriceps function (measured by central activation ratio), improvements occurred after whole-body vibration in the simulated knee-pathology model.(Blackburn, Pamukoff, Sakr, Vaughan, & Berkoff, 2014) These studies show the positive effect of mechanical or electrical stimulation on mechanoreceptors and motor output and provide a basis for future incorporation of dEMG into rehabilitation programs to mitigate AMI effects.
Strengths and Limitations
The isometric testing protocol performed in this study, although not equivalent to dynamic tasks of regular athletic play, assessed the thigh muscles in the functional range of activity and allowed for analysis of more than maximum effort. In rehabilitation protocols, strength measures are often focused on maximum voluntary isometrics or normalization to artificial maximum.(Farquhar, Chmielewski, & Snyder-Mackler, 2005)
Force output was different among groups and between limbs, making it difficult to compare or establish normative values. Consequently, future research could perform similar analyses with standardized torque/force values. Additionally, no thigh girth measurements were taken to quantify volumetric atrophy of the thigh musculature. As the adolescent population (14–25 years of age) were in the process of growth / development, this could have led to increased strength values with a longitudinal design due to muscle (bulk) and skeletal growth (longer levers).
EMG decomposition at low levels of contraction is difficult to measure due to lower signal-to-noise ratios. Contrarily, it is possible that low-threshold MUs are not well-represented in the decomposed signal for greater effort-level trials, due to superposition of higher-threshold MUs. However, as indicated above, the decomposition algorithm considers superposition of MUAP trains. While there has been recent debate about different decomposition techniques, the decomposition algorithm used in the current study has demonstrated valid rate coding and MUAP shapes.(Enoka, 2019; Hu et al., 2013a; Nawab et al., 2010) The sEMG signal measures electrical activity of the whole muscle, and as previously described, the signal decomposition technique used in the current study identifies individual MUAP trains contained in the sEMG signal.
There are inherent complexities that exist with MU analysis. MU behavior is non-linear in nature;(Franklin & Wolpert, 2011) each contraction incorporates multiple MUs that initiate rate coding at independent recruitment thresholds which then accelerate and decelerate in rate coding throughout the demands of the muscle contraction. Consequently, the relationships between groups can be difficult to parse through as a breakdown of singular MU characteristics (i.e. average rate coding, recruitment threshold, etc.) will often yield similar characteristics between groups due to effect of averaging. Further, with the quantity of MUs identified, the averaged values may be significant due to sample size but are clinically insignificant as the values are very similar (e.g. average rate coding 15.9 vs 16.0). However, when the MU characteristics are assessed in a multivariate form as we performed herein, a different pattern emerges in which MU strategies become evident. This is evident in y-intercept, slope,(Stock et al., 2012) and least squares means which adjusts the average based on the multivariate approach.
Conclusion
The current findings indicate that quadriceps and hamstring MU activity are significantly different after ACL injury, post-ACLR, and throughout rehabilitation up to 12 months post-surgery compared to healthy CTRLs. As thigh MU characteristics are now known across the longitudinal phases of ACL rehabilitation, future studies can assess these patterns of motor control and its potential to determine risk of re-injury. Further, future rehabilitation can target specific intervention programs to restore motor control.
Highlights:
Motor unit strategies of arthrogenic muscle inhibition is characterized for the first time via decomposed EMG.
Motor unit deficits of thigh musculature persist throughout all phases of ACL rehabilitation, even after return-to-sport.
After ACL injury, motor unit sizes at similar recruitment thresholds were smaller than those of healthy controls.
ACKNOWLEDGEMENTS
The authors acknowledge the support of NIH grants R01AR056259 [NAB], K12HD065987 [NDS], L30AR070273 [NDS], and the Mayo Clinic Ultrasound Research Center. This publication was also made possible by CTSA Grant Number UL1TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Fellowship funding supported by the Mayo Clinic Graduate School of Biomedical Sciences [ALM].
Footnotes
DECLARATION OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- Balshaw TG, Pahar M, Chesham R, Macgregor LJ, & Hunter AM (2017). Reduced firing rates of high threshold motor units in response to eccentric overload. Physiological Reports, 5(2), 1–12. 10.14814/phy2.13111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck TW, DeFreitas JM, & MS S (2011). The Effects of a Resistance Training Program on Average Motor Unit Firing Rates. . Clinical Kinesiology, 65(1)(1), 1–8. [Google Scholar]
- Blackburn JT, Pamukoff DN, Sakr M, Vaughan AJ, & Berkoff DJ (2014). Whole body and local muscle vibration reduce artificially induced quadriceps arthrogenic inhibition. Archives of Physical Medicine and Rehabilitation, 95(11), 2021–2028. 10.1016/j.apmr.2014.07.393 [DOI] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, … Bevins RA (2013). Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology, 75, 138–144. 10.1016/j.neuropharm.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca CJ, LeFever RS, McCue MP, & Xenakis AP (1982). Behaviour of human motor units in different muscles during linearly varying contractions. The Journal of Physiology, 329(1), 113–128. 10.1113/jphysiol.1982.sp014293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, & Contessa P (2012). Hierarchical control of motor units in voluntary contractions. Journal of Neurophysiology, 107(1), 178–195. 10.1152/jn.00961.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca De, Carlo J, Adam A, Wotiz R, Gilmore LD, & Nawab SH (2006). Decomposition of surface EMG signals. J Neurophysiol, 96(3), 1646–1657. 10.1152/jn.00009.2006.This [DOI] [PubMed] [Google Scholar]
- Luca De, Carlo J, & Contessa P (2015). Biomechanical benefits of the onion-skin motor unit control scheme. Journal of Biomechanics, 48(2), 195–203. 10.1016/j.jbiomech.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca De, Carlo J, & Hostage EC (2010). Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. Journal of Neurophysiology, 104(2), 1034–1046. 10.1152/jn.01018.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza LML, Cabral HV, Fernandes De Oliveira L, & Martins Vieira T (2018). Motor units in vastus lateralis and in different vastus medialis regions show different firing properties during low-level, isometric knee extension contraction. Human Movement Science, 58, 307–314. 10.1016/j.humov.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Del Vecchio A, Casolo A, Negro F, Scorcelletti M, Bazzucchi I, Enoka R, … Farina D (2019). The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. Journal of Physiology, 7, 1873–1887. 10.1113/JP277250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM (2019). Physiological Validation of the Decomposition of Surface EMG Signals. Journal of Electromyography and Kinesiology, 46(March), 70–83. 10.1016/j.jelekin.2019.03.010 [DOI] [PubMed] [Google Scholar]
- Enoka RM, & Duchateau J (2017). Rate coding and the control of muscle force. Cold Spring Harbor Perspectives in Medicine, 7(10). 10.1101/cshperspect.a029702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertas M, Stålberg E, & Falck B (1995). Can the size principle be detected in conventional emg recordings? Muscle & Nerve, 18(4), 435–439. 10.1002/mus.880180410 [DOI] [PubMed] [Google Scholar]
- Farquhar SJ, Chmielewski TL, & Snyder-Mackler L (2005). Accuracy of predicting maximal quadriceps force from submaximal effort contractions after anterior cruciate ligament injury. Muscle and Nerve, 32(4), 500–505. 10.1002/mus.20366 [DOI] [PubMed] [Google Scholar]
- Franklin DW, & Wolpert DM (2011). Computational mechanisms of sensorimotor control. Neuron, 72(3), 425–442. 10.1016/j.neuron.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Johnson CD, Nicholas SJ, & McHugh MP (2018). Muscle hypotrophy, not inhibition, is responsible for quadriceps weakness during rehabilitation after anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy, 27(2), 573–579. 10.1007/s00167-018-5166-1 [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, & Bennett DJ (2002). Intrinsic activation of human motoneurons: Possible contribution to motor unit excitation. Journal of Neurophysiology, 87(4), 1850–1858. 10.1152/jn.00024.2001 [DOI] [PubMed] [Google Scholar]
- Hart JM, Pietrosimone B, Hertel J, & Ingersoll CD (2010). Quadriceps activation following knee injuries: a systematic review. Journal of Athletic Training, 45(1), 87–97. 10.4085/1062-6050-45.1.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, & Rau G (2000). Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology, 10(5), 361–374. 10.1016/S1050-6411(00)00027-4 [DOI] [PubMed] [Google Scholar]
- Hopkins JT, & Ingersoll CD (2000). Arthrogenic muscle inhibition: A limiting factor in joint rehabilitation. Journal of Sport Rehabilitation, 9(2), 135–159. [Google Scholar]
- Hu X, Rymer WZ, & Suresh NL (2013a). Assessment of validity of a high-yield surface electromyogram decomposition. Journal of NeuroEngineering and Rehabilitation, 10(1), 2–13. 10.1186/1743-0003-10-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Rymer WZ, & Suresh NL (2013b). Motor unit pool organization examined via spike-triggered averaging of the surface electromyogram. Journal of Neurophysiology, 110(5), 1205–1220. 10.1152/jn.00301.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis P, Bahr R, Landreau P, Miladi R, & Witvrouw E (2016). Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. British Journal of Sports Medicine, bjsports-2015–095908. 10.1136/bjsports-2015-095908 [DOI] [PubMed]
- Lee DW, Yeom CH, Kim DH, Kim TM, & Kim JG (2018). Prevalence and predictors of patellofemoral osteoarthritis after anterior cruciate ligament reconstruction with hamstring tendon autograft. CiOS Clinics in Orthopedic Surgery, 10(2), 181–190. 10.4055/cios.2018.10.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, & Duchateau J (2016). Rate of force development: physiological and methodological considerations. European Journal of Applied Physiology, 116(6), 1091–1116. 10.1007/s00421-016-3346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Valdes E, Negro F, Laine CM, Falla D, Mayer F, & Farina D (2017). Tracking motor units longitudinally across experimental sessions with high-density surface electromyography. Journal of Physiology, 595(5), 1479–1496. 10.1113/JP273662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MP (2003). Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports, 13(2), 88–97. 10.1034/j.1600-0838.2003.02477.x [DOI] [PubMed] [Google Scholar]
- Nagai T, Bates NA, Hewett TE, & Schilaty ND (2018). Effects of localized vibration on knee joint position sense in individuals with anterior cruciate ligament reconstruction. Clinical Biomechanics, 55, 40–44. 10.1016/j.clinbiomech.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Schilaty ND, Strauss JD, Crowley EM, & Hewett TE (2018). Analysis of Lower Extremity Proprioception for Anterior Cruciate Ligament Injury Prevention: Current Opinion. Sports Medicine, 48(6), 1303–1309. 10.1007/s40279-018-0889-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelli CV, & Hewett TE (2016). Should Return to Sport be Delayed Until 2 Years After Anterior Cruciate Ligament Reconstruction? Biological and Functional Considerations. Sports Medicine. 10.1007/s40279-016-0584-z [DOI] [PMC free article] [PubMed]
- Nawab SH, Chang S-S, & De Luca CJ (2010). High-yield decomposition of surface EMG signals. Clinical Neurophysiology, 121(10), 1602–1615. 10.1016/j.clinph.2009.11.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterno MV, Rauh MJ, Schmitt LC, Ford KR, & Hewett TE (2014). Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. The American Journal of Sports Medicine, 42(7), 1567–1573. 10.1177/0363546514530088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DA, & McNair PJ (2010). Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Seminars in Arthritis and Rheumatism, 40(3), 250–266. 10.1016/j.semarthrit.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Schilaty ND, Nagelli CV, Bates NA, Sanders TL, Krych AJ, Stuart MJ, & Hewett TE (2017). Incidence of Second Anterior Cruciate Ligament Tears and Identification of Associated Risk Factors From 2001 to 2010 Using a Geographic Database. Orthop J Sports Med, 5(8), 1–8. 10.1177/0363546517694026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock MS, Beck TW, & Defreitas JM (2012). Effects of fatigue on motor unit firing rate versus recruitment threshold relationships. Muscle and Nerve, 45(1), 100–109. 10.1002/mus.22266 [DOI] [PubMed] [Google Scholar]
- Stock MS, & Thompson BJ (2014). Effects of barbell deadlift training on submaximal motor unit firing rates for the vastus lateralis and rectus femoris. PLoS ONE, 9(12), 1–18. 10.1371/journal.pone.0115567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg S, Lindstrom M, Wretling M-L, Aspelin P, & Shalabi A (2013). Muscle morphometric effect of anterior cruciate ligament injury measured by computed tomography: Aspects on using non-injured leg as control. BMC Musculoskeletal Disorders, 14(150), 1–9. 10.1186/1471-2474-14-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Hughes RL, Peterson E, Müller-Oehring EM, Brontë-Stewart HM, Poston KL, … Schulte T (2019). Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson’s disease. Neuroscience and Biobehavioral Reviews, 103(January), 305–315. 10.1016/j.neubiorev.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Chã C, Falla D, & Farina D (2010). Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. Journal of Applied Physiology, 109(5), 1455–1466. 10.1152/japplphysiol.01213.2009 [DOI] [PubMed] [Google Scholar]