Abstract
We evaluated a less-sensitive enzyme immunoassay (3A11-LS) for its possible use for early diagnosis of human immunodeficiency virus type 1 (HIV-1) infection in infants. The results were compared with those from the immunoglobulin G-capture enzyme immunoassay. A total of 239 sera from 77 infants were tested. All 25 sera from the 10 infants born to seronegative mothers were found to be negative by both assays. Forty-one seroreverting infants showed a complete decay of maternal antibodies by 4 months by the 3A11-LS assay. However, the assay detected HIV antibodies in only 9 (36%) of 25 sera collected from infected infants between 4 and 6 months and in 27 (63%) of 43 sera collected after 6 months of age. Further analysis with alternative cutoff values indicated that the 3A11-LS had a sensitivity of 12 to 44% and a specificity of 90 to 100% for infants between 4–6 months of age. This data suggest that a diagnosis of HIV infection in some of the infants could be made after 4 months of age by the 3A11-LS assay, although a negative 3A11-LS test result may not rule out infection and may require a further followup.
Considerable efforts have been devoted to developing and assessing new approaches for the early diagnosis of human immunodeficiency virus (HIV) infection in infants (1, 6, 7, 12, 14, 17). Although it is generally agreed that molecular methods, such as the detection of viral RNA or proviral DNA, are the most sensitive methods for HIV type 1 (HIV-1) diagnosis in infants (7, 14), these methods involve complex and expensive technologies and thus have remained largely unavailable in resource-poor settings in the developing world, where the majority of pediatric HIV infections continue to occur (3, 5). While affordable intervention strategies are now available to prevent vertical transmission (4, 5, 8, 13, 16), early diagnosis of HIV infection in exposed infants remains a major obstacle and challenge both to assessing the efficacy of these strategies and to providing early appropriate care to infected infants. Identification of a simple and inexpensive laboratory tool for early diagnosis in infants would have great implications, especially in developing countries (11).
In 1998 Janssen et al. (9) described a modified, less-sensitive enzyme immunoassay (3A11-LS) which, when used in conjunction with the sensitive enzyme immunoassay (EIA), identifies recent HIV-1 infection and is useful for estimating incidence in a population. This method detects increasing antibody levels during the early phase of infection and thus is quite sensitive to various levels of HIV-1 antibodies. We postulated that this method may also be useful in diagnosing HIV infection in perinatally exposed infants who are making their own antibodies in the background of decaying maternal antibodies. Our previous work (15), using an immunoglobulin G-capture EIA (IgG-CEIA), had shown that the decay of maternal antibodies was observed over a period of 6 months and that most infected infants (>90%) developed their own HIV-specific antibodies which were detectable after 6 months. However, since the complete decay of maternal antibodies took at least 6 months by this approach, a definitive diagnosis of HIV infection in exposed infants could not be accomplished earlier than 6 months. Because the 3A11-LS assay is performed at a 1/20,000 dilution, it is likely to observe different antibody kinetics. If the time period for decay of maternal antibodies is shortened, this would allow detection of infants' own antibodies. Therefore, we used the same specimens to comparatively examine the 3A11-LS assay to assess whether the diagnosis of HIV infection can be accomplished with high sensitivity and specificity in infants younger than 6 months of age.
Infant serum specimens were collected from New York City hospitals as part of a multicenter perinatal HIV-1 transmission study, as described in detail elsewhere (1, 15). The study groups were composed of 115 longitudinal specimens from 41 uninfected, seroreverting infants; 99 specimens from 26 HIV-1-infected infants; and 25 specimens from 10 infants born to HIV-1-seronegative mothers (negative controls). The infection status of infants born to HIV-1-seropositive mothers was confirmed by PCR, serology, and clinical followup beyond 18 months of age or until death. All specimens were collected under a protocol approved by the institutional review board, and informed consents were obtained from mothers.
The 3A11-LS assay is a modified version of the Abbott 3A11 HIV-1 (viral lysate) EIA for HIV-1 antibodies. The assay protocol was described earlier (9) and included dilution of specimens at 1/20,000 and reduced incubation times. An external Calibrator (CAL) and low positive control were added to the assay for increased consistency and were run in triplicate on every plate. Specimens were run in duplicate. Standardized optical density (SOD) values were calculated to normalize the optical density (OD) values and to minimize the interassay variability by using the formula (mean specimen OD − mean negative control OD)/(mean CAL OD). SOD values versus infant age were plotted. Data were analyzed by using alternative cutoffs ranging from 0.5 to 1.5 SOD. IgG-CEIA results were taken from previously published work (15) for comparative analysis. The method has been described in detail earlier.
All 25 specimens from the 10 HIV-unexposed uninfected infants were found to be negative by both the 3A11-LS assay with a mean SOD of −0.019 and the IgG-CEIA with a mean OD of 0.066 (not shown).
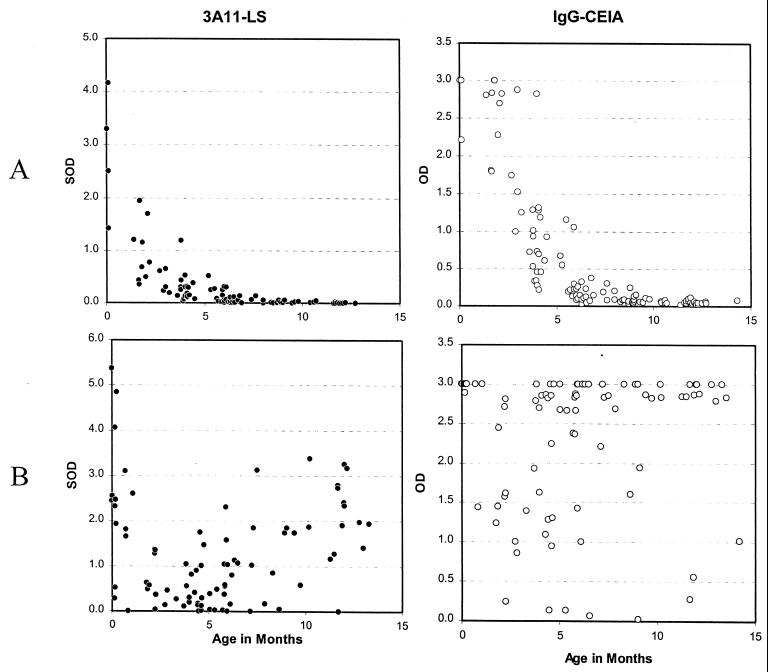
The comparative results of the 3A11-LS assay and the IgG-CEIA on the 115 specimens from 41 seroreverting infants are shown in Fig. 1A (upper panels). Decay of maternal antibodies was observed in specimens taken soon after birth by the 3A11-LS assay and reached below the threshold of 0.75 SOD by 4 months of age. All 90 specimens collected after 4 months of age were below an SOD of 0.75, while 88 (98%) of these 90 specimens had SOD values of <0.5. In contrast, the observed decay of maternal antibodies by the IgG-CEIA was delayed initially by about 2 months and reached a background level (<0.5 OD) by 6 months. Thus, all 69 specimens from 39 seroreverting infants collected after 6 months of age were negative. Interestingly, the decay kinetics of maternal antibodies were quite similar by both assays, with parallel slopes and half-lives (t1/2) of 28 to 30 days (15).
FIG. 1.
HIV-1 antibody detected in seroreverting infants (A, upper panels) or in HIV-1-infected infants (B, lower panels) with respect to their ages. SOD values obtained by the 3A11-LS assay (●, left panels) or OD values by IgG-CEIA (○, right panels) are shown.
The comparative results of the 3A11-LS assay and the IgG-CEIA on 99 specimens from the 26 infected infants are shown in Fig. 1B (lower panels). As before, the 3A11-LS assay demonstrated an early, rapid decay of maternal antibodies, followed by an increase reflecting synthesis of infant's own antibodies. The distribution showed a clear pattern of decline and rise in HIV antibodies. However, the rise in detectable antibodies observed by the 3A11-LS assay following decay of the maternal antibodies was slower than that detected by the IgG-CEIA.
The data were analyzed for sensitivity and specificity, by age, using a cutoff of 0.5 OD for IgG-CEIA (15) and variable cutoffs of 0.5 to 1.5 SOD for the 3A11-LS assay (Table 1). The results demonstrate that the IgG-CEIA had poor specificity during the infants' early months. However, in specimens taken from infants after 6 months of age, this assay had sensitivities ranging from 88 to 94% in different age groups, with specificities of 100% in each age group analyzed. The 3A11-LS assay had excellent specificity, ranging from 90 to 100% in specimens from infants after 4 months of age due to the lack of detection of maternal antibodies. However, the sensitivity of the assay in detecting HIV antibodies in infected infants was 12 to 44% between 4 and 6 months of age and increased to 20 to 60% between 6 and 8 months depending on the cutoffs used. Using the cutoffs of 0.5 or 1.0 SOD, the highest sensitivity of 78% and specificity of 100% was achieved only after the infants were >10 months old.
TABLE 1.
Sensitivity and specificity of IgG-CEIA and 3A11-LS assay at various cutoffs, grouped by age
| Method and cutoff (SOD) | %SE and %SPa at various cutoffs in infants at age (mo):
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–2
|
2–4
|
4–6
|
6–8
|
8–10
|
10–12
|
>12
|
||||||||
| %SE | %SP | %SE | %SP | %SE | %SP | %SE | %SP | %SE | %SP | %SE | %SP | %SE | %SP | |
| IgG-CEIA | 100 | 0 | 92 | 13 | 92 | 43 | 90 | 100 | 88 | 100 | 89 | 100 | 94 | 100 |
| 3A11-LS EIA | ||||||||||||||
| 0.5 | 83 | 20 | 38 | 67 | 44 | 90 | 60 | 100 | 63 | 100 | 78 | 100 | 71 | 100 |
| 1.0 | 67 | 30 | 31 | 87 | 28 | 100 | 50 | 100 | 38 | 100 | 78 | 100 | 71 | 100 |
| 1.5 | 67 | 60 | 0 | 93 | 12 | 100 | 20 | 100 | 38 | 100 | 56 | 100 | 69 | 100 |
%SE, percent sensitivity; %SP, percent specificity.
In spite of this limitation, our results suggest that the 3A11-LS may potentially be useful in detecting HIV antibodies in some of the infected infants by as early as 4 to 6 months of age (sensitivity = 36% and specificity = 100% using a cutoff of 0.75). Therefore, a positive 3A11-LS result after 4 months of age would be highly indicative of infection. However, a negative 3A11-LS test result would not rule out infection and will require a further followup. It is possible that the infants can subsequently be tested by IgG-CEIA after 6 months of age when it has better sensitivity and specificity (90 and 100%, respectively). Thus, an algorithm involving these two serologic assays can help diagnose HIV infection in most perinatally exposed infants by 4 to 6 months of age.
Since the 3A11-LS assay is performed at 1/20,000 dilution, only high-titered antibodies are detected. The limited sensitivity (25 to 35%) of the 3A11-LS assay can possibly be explained in that all infected infants did not make high-titered antibodies at the early time points. In contrast, >90% of infected infants had detectable antibodies as determined by the IgG-CEIA after 6 months of age. Since this assay reflects the proportion of HIV-specific antibody in the sera, it appears to be more sensitive in this setting.
It was interesting that the two different approaches, one that measures levels of antibodies indirectly by HIV antibody titer and the other that measures the proportion of HIV-specific antibodies showed similar decay characteristics of maternal antibodies in seroreverting infants. Since the dynamic ranges of the two assays are somewhat different, the 3A11-LS assay showed decay soon after birth, while the IgG-CEIA showed decay after about 2 months of age. This shift was also reflected in the time it took for residual maternal antibodies to reach background levels; 0 to 4 months for the 3A11-LS assay and 2 to 6 months for the IgG-CEIA. Unfortunately, the sensitivity of the 3A11-LS assay was not adequate to detect antibodies in all infected infants. Our previous work (15) has shown that the synthesis of infant antibodies in significant amounts begins only after about 3 months of age, but high-titer antibodies may not be present until much later. Our results show that most (>90%) HIV-infected infants have a significant proportion of HIV-specific antibodies, detectable by the IgG-CEIA, by 6 months of age, but the titers of these antibodies are not high enough (>1/20,000) to be detectable by the 3A11-LS assay. Other techniques used to detect early HIV IgG in the infected infants include the in vitro antibody production assay (2) and the enzyme-linked immunospot assay (12). Both of these procedures detect HIV-specific antibodies secreted by infants' lymphocytes but have had limited success for use in the early period after birth. Secretion of small amounts of infant HIV IgG, in the background of maternal antibodies, cannot be detected by either of the two assays described here.
Our data show that the 3A11-LS assay may be useful for early diagnosis of HIV infection in infants after 4 months of age but that its sensitivity is limited. The 3A11-LS assay and IgG-CEIA could possibly be used in an algorithm to diagnose most infected infants between 4 and 6 months of age; however, practical implementation of such an approach involving two assays may not be easy. Since diagnosis of HIV-exposed infants will continue to pose a major challenge in developing countries for years to come, it will be important to develop a sensitive and specific, yet simple and less-expensive assay. Recently, Ledergerber et al. (10) described a boosted enzyme-linked immunosorbent assay for p24 antigen detection that had comparable sensitivity to viral RNA measurements in predicting progression to AIDS and survival in HIV-1-infected adults. The application of such an assay for early diagnosis in infants should be evaluated and could prove to be extremely valuable. Alternatively, the development of an inexpensive, qualitative HIV-1 RNA or DNA detection method should be a high priority. The development of such diagnostic method(s) will be highly valuable in efforts to assess strategies to prevent vertical transmission and for early treatment of HIV-1-infected infants.
Acknowledgments
We gratefully acknowledge the assistance of other members of the New York City Perinatal HIV Transmission Collaborative Study (Tina Alford, Mahrukh Bamji, Joanna Dobrosycki, Louise Kuhn, Ellie Schoenbaum, Donald Thea, and Jeremy Weedon) and of the members of the Perinatal AIDS Collaborative Transmission Studies (PACTS) at the Centers for Disease Control and Prevention, Atlanta, Ga. (April Bell, Joanne Ethier-Ives, Mary Glenn Fowler, Marcia Kalish, Margaret Lampe, Sherry Orloff, R. J. Simonds, and Jeffrey Wiener).
REFERENCES
- 1.Abrams E J, Matheson P, Thomas P, Thea D M, Krasinski K, Lambert G, Shaffer N, Bamji M, Hutson D, Grimm K. Neonatal predictors of infection status and early death among 332 infants at risk of HIV-1 infection monitored prospectively from birth. Pediatrics. 1995;96:451–458. [PubMed] [Google Scholar]
- 2.Amadori A, DeRossi A, Chieco-Bianchi L, Giaquitto C, De Maria A, Ades A E. Diagnosis of human immunodeficiency virus 1 infection in infants: in vitro production of virus-specific antibody in lymphocytes. Pediatr Infect Dis J. 1990;9:26–30. doi: 10.1097/00006454-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bulterys M, Fowler M G. Prevention of HIV infection in children. Pediatr Clin North Am. 2000;47:241–260. doi: 10.1016/s0031-3955(05)70203-0. [DOI] [PubMed] [Google Scholar]
- 4.Dabis F, Msellati P, Meda N, Welffens-Ekra C, You B, Manigart O, Leroy V, Simonon A, Cartoux M, Combe P, Ouangré A, Ramon R, Ky-Zerbo O, Montcho C, Salamon R, Rouzioux C, Van de Perre P, Mandelbrot L. 6-Month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d'Ivoire and Burkina Faso : a double blind placebo-controlled multicentre trial. Lancet. 1999;353:786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 5.DeCock K M, Fowler M G, Mercier E, Vincenzi I, Saba J, Hoff E, Alnwick D J, Rogers M, Shaffer N. Prevention of mother-to-child HIV transmission in resource-poor settings: translating research into policy and practice. JAMA. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 6.Derossi A, Ades A E, Mammano F, Mistro A D, Amado A, Giaquinto C, Chieco-Bianchi L. Antigen detection, virus culture, polymerase chain reaction, and in vitro antibody production in the diagnosis of vertically transmitted HIV-1 infection. AIDS. 1991;5:15–20. doi: 10.1097/00002030-199101000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Dunn D T, Simonds R J, Bulterys M, Kalish K A, Moye J, de Maria A, Kind C, Rudin C, Denamur E, Krivine A, Loveday C, Newell M L. Interventions to prevent vertical transmission of HIV-1: effect on viral detection rate in early infant samples. AIDS. 2000;14:1421–1428. doi: 10.1097/00002030-200007070-00016. [DOI] [PubMed] [Google Scholar]
- 8.Guay L A, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Ducar C, Deseyve M, Emel L, Mirochnick M, Fowler M G, Mofenson L, Miotti P, Dransfield K, Bray D, Mmiro F, Jackson J B. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 9.Janssen R S, Satten G A, Stramer S L, Rawal B D, O'Brien T R, Weiblen B J, Hecht F M, Jack N, Cleghorn F R, Kahn J O, Chesney M A, Busch M P. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Ledergerber B, Flepp M, Boni J, Tomasik Z, Cone R W, Lüthy R, Schüpbach J. Human immunodeficiency virus type 1 p24 concentration measured by boosted ELISA of heat-denatured plasma correlates with decline in CD4 cells, progression to AIDS, and survival: comparison with viral RNA measurement. J Infect Dis. 2000;181:1280–1288. doi: 10.1086/315366. [DOI] [PubMed] [Google Scholar]
- 11.Lepage P, Spira R, Kalibala S, Pillay K, Giaquinto C, Castetbon K, Osborne C, Courpotin C, Dabis F. Care of human immunodeficiency virus-infected children in developing countries. Pediatr Infect Dis J. 1998;17:581–586. doi: 10.1097/00006454-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Nesheim S, Lee F, Sawyer M, Jones D, Lindsay M, Slade B, Shaffer N, Holmes R, Ashby R, Grimes V, Rogers M, Czerkinsky C, Nahmias A. Diagnosis of human immunodeficiency virus infection by enzyme-linked immunospot assay in a prospectively followed cohort of infants of human immunodeficiency virus-seropositive women. Pediatr Infect Dis J. 1992;11:635–639. [PubMed] [Google Scholar]
- 13.Shaffer N, Chuachoowong R, Mock P A, Bhadrakom W, Young N L, Chotpitayasunodh T, Chearskul S, Roongpisuthipong A, Chinayon P, Karon J, Mastro T D, Simonds R J. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 14.Parekh B S, Shaffer N, Coughlin R, Hung C H, Krasinski K, Abrams E, Bamji M, Thomas P, Hutson D, Lambert G, Schochetman G, Rogers M, George J R. Human immunodeficiency virus 1-specific IgA capture enzyme immunoassay for early diagnosis of human immunodeficiency virus 1 infection in infants. Pediatr Infect Dis J. 1993;12:908–913. doi: 10.1097/00006454-199311000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Parekh B S, Shaffer N, Coughlin N, Hung C H, Krasinski K, Abrams E, Thomas P, Hutson D, Schochetman G, Rogers M, George J R. Dynamics of maternal IgG antibody decay and HIV-specific antibody synthesis in infants born to seropositive mothers. AIDS Res Hum Retrovir. 1993;9:907–912. doi: 10.1089/aid.1993.9.907. [DOI] [PubMed] [Google Scholar]
- 16.Wiktor S Z, Ekpini E, Karon J M, Nkengasong J, Maurice C, Severin S T, Roels T H, Kouassi M K, Lackritz E M, Coulibaly I, Greenberg A E. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d'Ivoire: a randomised trial. Lancet. 1999;353:781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 17.Young N L, Shaffer N, Chaowanachan T, Chotpitayasunodh T, Vanaparapar N, Mock P A, Waranawat N, Chokephaibulkit K, Chuachoowong R, Wasinrapee P, Mastro T D, Simonds R J. Early diagnosis of HIV-1-infected infants in Thailand using RNA and DNA PCR assay sensitive to non-B subtypes. J Acquir Immune Defic Syndr. 2000;24:401–407. doi: 10.1097/00126334-200008150-00001. [DOI] [PubMed] [Google Scholar]