Abstract
Acute Respiratory Distress Syndrome (ARDS) is common in COVID-19 patients and is associated with high mortality. The aim of this observational study was to describe patients’ characteristics and outcome, identifying potential risk factors for in-hospital mortality and for developing Long-COVID symptoms. This retrospective study included all patients with COVID-19 associated ARDS (cARDS) in the period from March 2020 to March 2021 who were invasively ventilated at the intensive care unit (ICU) of the University Hospital Dresden, Germany. Between October 2021 and December 2021 patients discharged alive (at minimum 6 months after hospital discharge—midterm survival) were contacted and interviewed about persistent symptoms possibly associated with COVID-19 as well as the quality of their lives using the EQ-5D-5L-questionnaire. Long-COVID was defined as the occurrence of one of the symptoms at least 6 months after discharge. Risk factors for mortality were assessed with Cox regression models and risk factors for developing Long-COVID symptoms by using relative risk (RR) regression. 184 Patients were included in this study (male: n = 134 (73%), median age 67 (range 25–92). All patients were diagnosed with ARDS according to the Berlin Definition. 89% of patients (n = 164) had severe ARDS (Horovitz-index < 100 mmHg). In 27% (n = 49) extracorporeal membrane oxygenation was necessary to maintain gas exchange. The median length of in-hospital stay was 19 days (range 1–60). ICU mortality was 51%, hospital mortality 59%. Midterm survival (median 11 months) was 83% (n = 55) and 78% (n = 43) of these patients presented Long-COVID symptoms with fatigue as the most common symptom (70%). Extreme obesity (BMI > 40 kg/m2) was the strongest predictor for in-hospital mortality (hazard ratio: 3.147, confidence interval 1.000–9.897) and for developing Long-COVID symptoms (RR 1.61, confidence interval 1.26–2.06). In-hospital mortality in severe cARDS patients was high, but > 80% of patients discharged alive survived the midterm observation period. Nonetheless, most patients developed Long-COVID symptoms. Extreme obesity with BMI > 40 kg/m2 was identified as independent risk factor for in-hospital mortality and for developing Long-COVID symptoms.
Trial registration DRKS-ID DRKS00027856.
Subject terms: Medical research, Respiratory distress syndrome
Introduction
According to WHO statistics, more than 500 million people globally were infected by SARS-CoV-2 and approximately up to 6 million people died by or with COVID-191. COVID-19 can cause severe acute respiratory distress syndrome (ARDS) with the need of mechanical ventilation (MV), and, for more severe cases, inhaled nitric oxide2 and extracorporeal membrane oxygenation (ECMO)3 are used as rescue therapies. The importance of ECMO therapy in SARS-CoV-2 ARDS is highlighted by a remarkable increase in the number of applications4. From the beginning in March 2020 till May 2021 the amount of ECMO applications in Europe raised from 68 to 43374.
Besides respiratory support, various pharmacological interventions for SARS-CoV-2 ARDS were tested—in particular during the early stage of the pandemic. Despite these efforts, ICU mortality remained high ranging from 40 to > 80%5–7. In addition to the infection and inflammatory damage to lung tissue, various mechanisms of hypercoagulopathy and fibrinolytic disorders have been described in patients infected by SARS-CoV-28–21 leading to high incidences of deep vein thrombosis and pulmonary embolism8,18,22,23. Compared to other types of ARDS, venous thromboembolism (VTE) rates of 20–58%8,18,22–24 are extremely high. Recent studies implicated a close connection between the occurrence of thromboembolic events and patients outcome17. As a consequence, strict anticoagulation recommendations were issued12,23 from the early stages of the pandemic. However, data on the optimal dosing of anticoagulant therapy are conflicting25–28 and the methodology of randomized trials addressing this topic suffered from major limitations and confounders.
Hyperinflammation or cytokine storm is often described as a common feature with high impact on COVID-19 morbidity and mortality29. Several pharmaceutical treatments were tested to prevent or treat hyperinflammation. Since the RECOVERY trial was published in July 2020—showing lower 28-day mortality in hospitalized COVID-19 patients with administration of dexamethasone30—institutional guidelines changed including glucocorticoid administration in all COVID-19 ARDS patients. Furthermore, later studies showed that, among critical ill COVID-19 patients the use of tocilizumab—a humanized monoclonal antibody against interleukin-6—is associated with lower in-hospital mortality31.
Finally, even for patients surviving the acute phase of severe SARS-CoV-2 infections or SARS-CoV-2 ARDS, increasing evidence suggests long-term sequelae for a large proportion of patients.
The term “Long-COVID” was first mentioned in May 2020 by Elisa Perego, who was experiencing prolonged symptoms after an infection with SARS-CoV-232. Based on the NICE-guideline, published in December 202033, Long-COVID is defined as newly occurring symptoms which were either not present during the acute phase of infection or persisted for longer than 4 weeks. In contrast, post-COVID should be considered when ongoing symptoms persist 3 months post-infection. In December 2021 the WHO Clinical Case Definition Working Group published a definition for post-COVID following a Delphi consensus. The five groups discussing the definition consisted of 61 patients, 18 patient-researchers, 138 external experts, 33 WHO staff, and 15 others. Items were evaluated using a nine-point Linkert scale. Items with a low rating in round one were later removed, while new items suggested by participants were added. The participants defined post-COVID as occurring “usually three months from the onset of COVID-19 with symptoms that last for at least two months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, and cognitive dysfunction […] and generally have an impact on everyday functioning”. There was no differentiation between persisting and newly occurring symptoms34.
The aim of this observational study was to describe characteristics and outcome of cARDS patients, discussing the role of potential risk factors for in-hospital mortality in these patients. Furthermore, patients discharged alive were evaluated for survival after minimum of 8 months—defined as midterm survival—and the prevalence of Long-COVID symptoms.
Methods
Study design
This was a single-center, retrospective observational study performed in a tertiary German university hospital specialized in lung diseases (University Hospital “Carl Gustav Carus” of Technical University of Dresden). All patients admitted to University hospital “Carl Gustav Carus” Dresden with polymerase chain reaction confirmed COVID-19 infection presenting with severe respiratory failure according to ARDS criteria35 (Horovitz-index < 300mHg), requiring invasive mechanical ventilation between March 2020 and March 2021 were enrolled in this study and mid-term outcome and the prevalence of Long-COVID were assessed by follow up > 6 months post discharge.
Data collection and outcome definitions
All patients’ data were recorded during the entire ICU stay. Primary outcome was defined as mortality during hospital stay. Secondary outcome was defined as occurrence of Long-COVID symptoms.
Sepsis was defined according to the International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)36, additional septic shock was defined as persistent hypotension with the need of catecholamine drugs to maintain mean arterial pressure ≥ 65 mmHg despite adequate volume substitution—and Serum lactate value > 2 mmol/l36,37. SOFA score and Charlson Comorbidity Index (CCI) score were calculated using standardized protocols at day of ICU admission.
All patients in our ICU were treated according to the same standard operating procedure (SOP) for anticoagulation therapy with consulting support by the department of internal medicine to identify patients at high risk for thrombosis at the time of ICU admission. On ICU admission, all patients were screened for venous thromboembolism (VTE) using complete compression ultrasound (cCUS) SOPs. Preexisting PE was detected by thoracic computed tomography pulmonary angiography (CTPA). Additional cCUS and CTPA were performed, if any clinical signs of venous or arterial thrombosis or embolism occurred. If PE was diagnosed, following cCUS was performed in every single case. Patients without venous or arterial thromboembolism received standard weight-based sub-therapeutic unfractionated heparin (target aPTT of 40–50 s) or intermediate doses of low molecular weight heparin (100 aXa units/kg/day). All patients with confirmed ATE/VTE received therapeutic weight-based unfractionated heparin (target aPTT of 60–80 s) or low molecular weight heparin (200 aXa units/kg/day). Patients with contraindications for full therapeutic anticoagulation received a patient specific therapy, according to benefit-risk assessments which included thrombus burden, bleeding risk or current bleeding intensity. Anticoagulant treatment target ranges for such patients were aPTT 50–60 s or LMWH dosages between 100 and 200 units/kg/day. Patients suffering from heparin-induced-thrombocytopenia (HIT) were treated with direct thrombin inhibitors according to guidelines.
All patients with refractory severe hypoxemia fulfilling the EOLIA criteria38 were screened for necessity of extracorporeal membrane oxygenation (ECMO). Individual decision was taken in multidisciplinary deliberation process. ECMO was performed as femoro-jugular veno-venous bypass using percutaneous ultrasound guided insertion of drainage and return cannula.
Laboratory analysis
Standard laboratory analyses including relative prothrombin time (PT in % of normal and INR), activated partial thromboplastin time (aPTT), fibrinogen, fibrin monomers and D-dimers on STA R Max3-Analyzers (STAGO Deutschland GmbH, Düsseldorf, Germany). PF 1 + 2 was analyzed applying LOCI-technology on an Atellica COAG 360 System (Siemens Healthcare GmbH, Erlangen, Germany).
Additional blood count analyses were performed using EDTA-tubes for hemoglobin concentration, white blood cell count and platelet count. A serum collecting tube was used for measurements of inflammatory parameters (CRP, Interleukin 2 and 6 (IL-2, IL-6) and Procalcitonin (PCT) and organ function monitoring (creatinine, bilirubin, and albumin)).
Every patient underwent VET and blood drawing for the laboratory analyses at the same time point each. Blood was drawn at least once daily for laboratory analysis. Laboratory parameters included into cox regression analysis for in-hospital mortality were selected due to clinical relevance and observations. Therefore, only values of d-dimers at admission to our ICU were included in regression analysis. Additional, maximum values of leucocytes, interleukin-6, procalcitonin, CRP, platelets as well as minimum values of platelets were included in further regression analysis. Thresholds were set according to clinical estimations.
Assessment of long-COVID and Questionnaires
Between October 2021 and December 2021, all patients who consented to participate in the study were telephone-interviewed by a trained medical student with standardized questionnaires investigating specific persistent symptoms possibly associated with COVID-19 and the quality of their lives. The minimum interval between discharge and follow-up was defined as 6 months and varied between the patients. The questionnaires contain self-reported symptoms including fatigue, weakness, shortness of breath, cough, headache, and muscle or limb pain, smell disorder, sleep disorder, loss of hair, anxiety disorder or other neurological disorders. Furthermore, a standardized five-dimension five-level (EQ-5D-5L) questionnaire, and the EuroQol Visual Analogue Scale (EQ-VAS) was used to analyze quality of life. Participants were questioned to report symptoms (persistent or newly occurring) different than before COVID-19 at the time of the interview. The EuroQol is a validated questionnaire with two components. EQ-5D-5L, is a health state classification system with five different dimensions: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. Each dimension has to be rated ranging from 1—“no problems” to 5—“unable to/extreme problems” to classify severity of symptoms. The EQ-VAS is the individual self-assessment of overall health ranging from 0 to 100 considered as “the worst health you can imagine” to “the best health you can imagine”. Furthermore, participants were asked if they could return to work and if permanent oxygen support and renal replacement therapy is necessary. Long-COVID was defined as the occurrence of one of the self-reported symptoms occurring at least 6 months after discharge, in accordance with German Guidelines for diagnostic of Long-COVID syndrome39.
Statistical analyses
Statistical analyses were performed using the SPSS Statistics 27 software (IBM, Inc, Armonk, NY, U.S.) and R version 3.2.4. All categorical variables are described as absolute and relative frequencies; comparison between groups was done using Fisher's exact test. Continuous variables were presented as median and interquartile range (IQR 1st–3rd), group comparison was based on the Mann–Whitney U test. Cox regression analysis were performed to identify risk factors for mortality. In case of binary outcomes, we used robust Poisson regression40 for derivation of adjusted relative risks. Variables included in regression analysis were selected due to clinical estimations based on preexisting studies for ARDS (Tables 7 and 8). The Kaplan–Meier curves were constructed using R version 3.2.4 and group comparison were made using the log-rank test. The precision of relative risk (RR) estimates was quantified using 95%-confidence intervals (CIs). Significance level was set at 0.05.
Table 7.
Cox regressions for hospital mortality.
| Variable | Bivariate regressions | Known at admission | Full model | |||
|---|---|---|---|---|---|---|
| HR | CI | HR | CI | HR | CI | |
| n | 184 | 184 | 184 | |||
| Age | 1.042** | 1.020–1.064 | 1.051 | 1.023–1.080 | 1.092 | 1.053–1.132 |
| Male | 1.454 | 0.916–2.306 | 1.490 | 0.917–2.422 | 1.411 | 0.795–2.501 |
| BMI: 35–40 kg/m2 | 0.884 | 0.492–1.586 | 1.178 | 0.630–2.206 | 1.922 | 0.961–3.843 |
| BMI: > 40 kg/m2 | 1.084 | 0.499–2.353 | 1.718 | 0.715–4.128 | 3.380* | 1.085–10.533 |
| CCI | 1.035 | 0.958–1.118 | 0.949 | 0.852–1.058 | 0.915 | 0.809–1.034 |
| Septic shock at ICU admission | 1.891* | 1.145–3.121 | 1.258 | 0.728–2.174 | 1.692 | 0.906–3.161 |
| SOFA score at ICU admission | 1.125** | 1.055–1.201 | 1.129** | 1.037–1.230 | 1.084 | 0.957–1.227 |
| D-Dimers at ICU admission: > 4000 ng/ml | 1.524 | 0.997–2.331 | 1.621* | 1.011–2.599 | 0.919 | 0.515–1.639 |
| Logarithm of first Horovitz-index at ICU | 0.901 | 0.580–1.399 | 0.949 | 0.573–1.571 | 1.369 | 0.741–2.530 |
| Direct transfer to our ICU from other hospital | 1.393 | 0.931–2.084 | 1.166 | 0.708–1.920 | 1.248 | 0.732–2.130 |
| Intubated at ICU admission | 1.652* | 1.054–2.589 | 0.836 | 0.445–1.570 | 0.879 | 0.403–1.917 |
| Time from first symptom to admission to our ICU | 1.015 | 0.996–1.036 | 1.009 | 0.985–1.034 | 1.008 | 0.983–1.034 |
| ECMO | 1.542* | 1.032–2.303 | 2.268* | 1.193–4.311 | ||
| CRRT | 1.864** | 1.274–2.726 | 1.216 | 0.684–2.162 | ||
| NO inhalation | 2.086** | 1.425–3.055 | 2.434** | 1.422–4.165 | ||
| Prone position | 1.071 | 0.714–1.605 | 1.108 | 0.641–1.917 | ||
| PE | 1.212 | 0.813–1.806 | 0.832 | 0.477–1.449 | ||
| Pneumothorax | 0.837 | 0.481–1.457 | 0.465 | 0.187–1.161 | ||
| Lung emphysema | 1.042 | 0.630–1.725 | 2.411 | 0.881–6.600 | ||
| Mediastinal emphysema | 1.009 | 0.534–1.904 | 0.896 | 0.247–3.247 | ||
| Pleural effusion | 1.215 | 0.830–1.776 | 0.915 | 0.561–1.492 | ||
| Bacteremia | 1.141 | 0.776–1.677 | 0.645 | 0.375–1.109 | ||
| Logarithm of lowest Horovitz-index at ICU | 0.357** | 0.199–0.640 | 0.414* | 0.189–0.907 | ||
| Leucocytes maximum value: > 20 GPt/l | 1.393 | 0.952–2.036 | 0.805 | 0.485–1.337 | ||
| Interleukin 6 maximum value: > 150 pg/ml | 2.272** | 1.335–3.869 | 2.115 | 0.914–4.893 | ||
| PCT maximum value: > 2 ng/ml | 2.290** | 1.462–3.588 | 1.832 | 0.938–3.577 | ||
| CRP maximum value: > 400 mg/l | 1.435 | 0.746–2.760 | 0.394 | 0.138–1.123 | ||
| CRP maximum value: 200–400 mg/l | 1.685 | 0.978–2.902 | 0.508 | 0.220–1.178 | ||
| Platelets maximum value: > 350 GPt/l | 0.488** | 0.320–0.746 | 0.541* | 0.302–0.969 | ||
| Platelets minimum value: < 100 GPt/l | 1.661** | 1.135–2.430 | 0.921 | 0.512–1.657 | ||
| Fusion in lung | 1.383 | 0.767–2.494 | 0.871 | 0.430–1.766 | ||
| Mycosis | 1.079 | 0.714–1.631 | 1.071 | 0.626–1.832 | ||
| Catheter associated bacteremia | 0.753 | 0.439–1.291 | 0.703 | 0.354–1.397 | ||
| Tracheostomy | 0.660* | 0.449–0.970 | 0.402** | 0.243–0.664 | ||
| DVT | 1.184 | 0.796–1.760 | 1.253 | 0.730–2.152 | ||
Hazard ratios with 95%-confidence intervals for hospital mortality from bivariate Cox regression, Cox regression including covariates known at admission and Cox regression including all covariates (full model) (significance levels: * = 5%, ** = 1%).
BMI Body-Mass-Index, CCI Charlson ComorbidityIndex, CI Confidence interval, CRP C-reactive protein, CRRT Continuous renal replacement therapy, DVT Deep vein thrombosis, ECMO extracorporeal membrane oxygenation, HR Hazard ration, ICU Intensive care unit, NO Nitric oxide, PCT Procalcitonin, PE Pulmonary embolism.
Table 8.
Relative risk regressions for Long-COVID.
| Variable | Bivariate regressions | Adjusted for age and sex | ||
|---|---|---|---|---|
| RR | CI | RR | CI | |
| n | 55 | 55 | ||
| Age | 0.99 | 0.98–1.01 | ||
| Male | 1.11 | 0.80–1.56 | ||
| BMI: 35–40 kg/m2 | 1.32 | 1.00–1.76 | 1.37* | 1.04–1.79 |
| BMI: > 40 kg/m2 | 1.56** | 1.25–1.95 | 1.61** | 1.26–2.06 |
| CCI | 1.01 | 0.95–1.08 | 1.03 | 0.96–1.11 |
| Septic shock at ICU admission | 1.14 | 0.71–1.83 | 1.14 | 0.72–1.82 |
| SOFA-Score at ICU admission | 0.98 | 0.93–1.04 | 0.98 | 0.93–1.04 |
| ECMO | 1.17 | 0.84–1.62 | 1.14 | 0.79–1.64 |
| CRRT | 1.09 | 0.73–1.63 | 1.08 | 0.70–1.66 |
| Logarithm of lowest Horovitz-index at ICU | 1.04 | 0.75–1.44 | 1.06 | 0.74–1.53 |
| Logarithm of duration of mechanical ventilation at ICU | 1.11 | 0.90–1.37 | 1.09 | 0.87–1.36 |
| VTE during ICU stay | 1.11 | 0.83–1.48 | 1.13 | 0.84–1.54 |
| Direct transfer to our ICU from other hospital | 1.03 | 0.77–1.38 | 1.06 | 0.80–1.40 |
| DVT | 0.99 | 0.72–1.36 | 1.00 | 0.72–1.38 |
Relative risks with 95%-confidence intervals for Long-COVID from robust Poisson regressions (significance levels: * = 5%, ** = 1%).
BMI Body-Mass-Index, CCI Charlson ComorbidityIndex, CI Confidence interval, CRRT Continuous renal replacement therapy, DVT Deep vein thrombosis, ECMO extracorporeal membrane oxygenation, ICU Intensive care unit, RR Relative risk, VTE Thromboembolic complications.
Ethics
The study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee from of the Technical University Dresden, Germany (BO-EK-374072021) and registered at the German Clinical Trials Registry (DRKS0027856). According to german law, informed consent was not required due to the retrospective and observational design of the study.
Results
Short-term outcome
Characteristics of the cohort
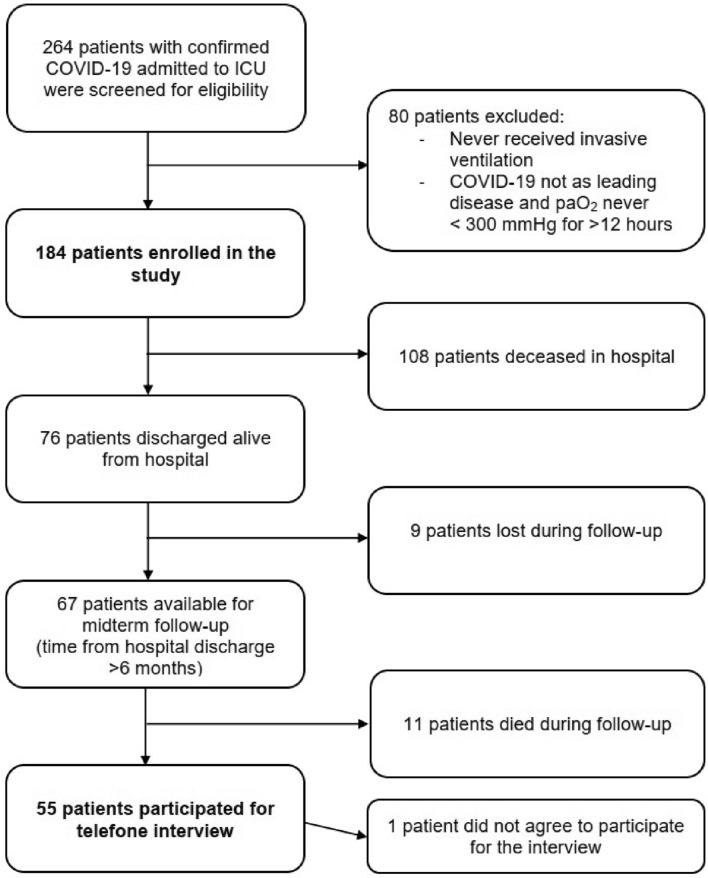
Flow of patients screening and enrollment is shown in Fig. 1. Between 03/2020 and 03/2021, 184 patients were treated for severe respiratory failure secondary to COVID-19 in our ICU and were included in this study. Median age was 67 years (range 25–92, IQR 61–73) and 73% of the patients were men (n = 134). All patients showed critical organ failure on the day of study enclosure with a median SOFA score of 12 points (range 4–19, IQR 10–13).
Figure 1.
Flow of patient screening and enrollment. ICU intensive care unit, ARDS acute respiratory distress syndrome, paO2 arterial oxygen partial pressure.
All patients were intubated and mechanically ventilated, with a median Horovitz-index at hospital admission of 130 (range 45–450, IQR 82.5–150). Patients without ECMO (n = 135) had a lowest daily median Horovitz-index of 60 mmHg (range 23–225, IQR 52.5–75.0) during ICU stay. Patients were treated in a prone position in 61% (n = 113) at minimum of 16 h/d, median rate was 4 cycles (range 1–14, IQR 2–6).
In 34% (n = 62) additional inhaled nitric oxide therapy was needed and in 27% (n = 49) veno-venous ECMO was necessary to maintain gas exchange. Continuous veno-venous hemodialysis (CVVH) was necessary in 34% (n = 67). Corticoid therapy was applied in 90% (n = 165) during ICU stay. 4 patients (2%) received Immunoglobulins, CytoSorb® therapy was used in 8 (4%), in 19% reconvalescence plasma therapy (n = 34) was used and 20 patients (11%) received remdesivir (Table 1).
Table 1.
ICU baseline characteristics during ICU stay.
| All patients | Range | |
|---|---|---|
| n | 184 | |
| Intubated at ICU admission | 133 (72.3%) | |
| ARDS mild at ICU admission | 14 (7.6%) | |
| ARDS moderate at ICU admission | 85 (46.2%) | |
| ARDS severe at ICU admission | 82 (44.6%) | |
| Septic shock at ICU admission | 24 (13.1%) | |
| First Horovitz-index at ICU | 108.8 (82.5; 150) | 45.0–450.0 |
| Lowest Horovitz-index at ICU | 60.0 (52.5; 75.0) | 22.5–225.0 |
| Pmean at admission [mbar] | 20 (17; 22) | 7–30 |
| PEEP at admission [mbar] | 14 (12; 15) | 6–20 |
| pH at admission | 7.38 (7.33; 7.44) | 6.81–7.62 |
| PaCO2 at admission [kPa] | 6.42 (5.64; 7.17) | 3.23–15.90 |
| SpO2 at admission [%] | 93 (90; 96) | 56–100 |
| SOFA score at ICU admission | 12 (10; 13) | 4–19 |
| D-dimers at ICU admission [ng/ml] | 5178 (2326; 8936) | 484–20,000 |
| Lactate at ICU admission [mmol/l] | 1.20 (0.90; 1.70) | 0.40–9.90 |
| Duration mechanical ventilation ICU [days] | 12 (7; 17) | 1–61 |
| Reintubation | 4 (2.2%) | |
| Prone position | 113 (61.4%) | |
| Cycles of prone position | 4 (2; 6) | 1–14 |
| Tracheostomy | 82 (44.6%) | |
| Days from intubation to tracheostomy | 12 (9; 15) | 3–26 |
| CRRT | 67 (36.4%) | |
| Duration CRRT [h] | 154.66 (31.51; 310.66) | 1.44–906.53 |
| ECMO | 49 (26.6%) | |
| Duration ECMO [h] | 274.66 (178.78; 353.04) | 16.78–1068.31 |
| Cytosorb | 8 (4.3%) | |
| Duration cytosorb [h] | 20.00 (17.17; 21.15) | 8.67–51.00 |
| Red cell transfusion | 6 (2; 12) | 1–40 |
| NO inhalation | 62 (33.7%) | |
| Corticosteroid | 165 (89.7%) | |
| Immunoglobulin | 4 (2.2%) | |
| Convalescent plasma | 34 (18.5%) | |
| Remdesivir | 20 (10.9%) | |
| Anticoagulation | 184 (100%) | |
| Argatroban at any time on ICU | 15 (8.2%) | |
| UFH at any time on ICU | 140 (76.1%) | |
| LMWH at any time on ICU | 115 (62.5%) | |
| Bacteremia | 92 (50%) | |
| Staph. aureus bacteremia | 12 (6.5%) | |
| Catheter associated bacteremia | 24 (13%) | |
| Antibiotics | 176 (95.7%) | |
| Antimycotics | 28 (15.2%) | |
| CRP maximum value [mg/l] | 261.2 (189.9; 342.1) | 31.4–618.0 |
| Interleukin 6 maximum value [pg/mL] | 359.5 (123.0; 755.5) | 8.6–792,732.0 |
| Leucocytes maximum value [GPt/L] | 19.06 (13.96; 25.91) | 3.14–63.87 |
| Leucocytes minimum value [GPt/L] | 7.22 (4.89; 9.46) | 0.20–22.47 |
| Procalcitonin [ng/ml] | 2.95 (0.91; 10.80) | 0.09–373.20 |
| Prothrombin fragment F1 + 2 [pmol/l] | 468 (272; 930) | 73–4948 |
| Platelets maximum value [GPt/L] | 315 (251; 418) | 48–989 |
| Platelets minimum value [GPt/L] | 124 (73; 198) | 1–469 |
Data are median (Interquartile range) or n (%).
ICU Intensive care unit, ARDS Acute respiratory distress syndrome, Pmean Mean pressure, PEEP Positive end-expiratory pressure, PaCO2 partial pressure of carbon dioxide, SpO2 Oxygen saturation, SOFA Sequential organ failure assessment, CRRT Continuous renal replacement therapy, NO Nitric oxide, UFH Unfractionated heparin, LMWH Low-molecular-weight heparin, Staph. Staphylococcus, CRP C-reactive protein, ECMO extracorporeal membrane oxygenation.
Duration between onset of symptoms and hospital admission was 5 days (range 0–23, IQR 0–7), for ICU admission 11 days (range 0–35, IQR 5–15) and for ECMO therapy 15 days (range 0–31, IQR 11–23). The majority of the patients had previous disease (97%, n = 179) with median Charlson Comorbidity Index of 3 points (range 0–12, IQR 2–5), while arterial hypertension (71%, n = 131), diabetes (43%, n = 79) and cardiovascular disease (25%, n = 45) were frequent and obesity was common in this cohort (median BMI 29, range 19–70, IQR 26–34). 14% (n = 26) presented obesity grade II (BMI 35–39.9 kg/m2) and 7% (n = 13) were noticed with severe obesity grade III (BMI ≥ 40 kg/m2) according to the WHO definition. Long-term drug intake was recorded frequently, mostly antihypertensive drugs were used in 52% (n = 95) of cases, beta blockers in 40% (n = 74), anti-platelet agents in 27% (n = 49) and oral anticoagulant drugs in 16% (n = 30). Only 7% (n = 12) were smokers (Table 2).
Table 2.
Demographic and baseline characteristics of all patients on admission to our ICU.
| All patients | Range | |
|---|---|---|
| n | 184 | |
| Male | 134 (72.8%) | |
| Age [years] | 67 (61; 73) | 25–92 |
| Body-Mass-Index [kg/m2] | 29.22 (26.04; 33.60) | 18.94–70.31 |
| Time from first symptom to hospital admission [days] | 5 (0; 7) | 0–23 |
| Time from first symptom to admission to our ICU [days] | 11 (5; 15) | 0–35 |
| Time from first symptom to ECMO therapy [days] | 15 (11; 23) | 0–31 |
| Direct transfer to our ICU from other hospital | 121 (65.8%) | |
| External tracheostomy | 14 (7.6%) | |
| External intubation | 133 (72.3%) | |
| Invasive mechanical ventilation before admission to our ICU [days| | 2 (0; 7) | 0–20 |
| NIV before admission to our ICU [days| | 2 (1; 4) | 1–22 |
| Charlson ComorbidityIndex | 3 (2; 5) | 0–12 |
| Arterial Hypertension | 131 (71.2%) | |
| Cardiovascular disease | 45 (24.5%) | |
| Neurovascular symptoms | 18 (9.8%) | |
| Coronary artery disease | 31 (16.8%) | |
| Thrombembolic events in medical history | 11 (6.0%) | |
| Chronic arrhythmias | 37 (20.1%) | |
| COPD | 13 (7.1%) | |
| Other pulmonary disease | 11 (6.0%) | |
| Nicotine abuse | 12 (6.5%) | |
| Diabetes mellitus | 79 (42.9%) | |
| Previous organ or bone marrow transplantation | 9 (4.9%) | |
| Chronic renal failure | 28 (15.2%) | |
| Chronic need of renal replacement therapy | 8 (4.3%) | |
| Admission with trauma | 8 (4.3%) | |
| ACE inhibitors | 14 (7.6%) | |
| AT2 receptor blocker | 85 (46.2%) | |
| Beta blocker | 82 (44.6%) | |
| Antithrombotic drug | 49 (26.8%) | |
| DOAC | 30 (16.4%) | |
| Corticosteroids | 21 (11.5%) | |
| Immunosuppressive drugs | 10 (5.5%) | |
| Nosocomial infection | 19 (10.3%) |
Data are median (Interquartile range) or n (%).
ICU Intensive care unit, ECMO extracorporeal membrane oxygenation, NIV non-invasive ventilation, COPD chronic obstructive pulmonary disease, ACE angiotensin-converting enzyme, AT2 Angiotensin II, DOAC Direct oral anticoagulants.
Short-term survival and thromboembolic complications
Median in-hospital stay was 19 days (range 1–60, 14; 28) and end-of-treatment follow-up was 100% complete. 90 of 184 patients (49%) could be discharged alive from the anesthesiology ICU. 32 patients (17.4%) could be discharged to rehabilitation and the other alive patients were transferred to another ICU (n = 38; 20.7%) or to regular ward (n = 11; 6.7%) within the clinic (Table 3). Overall hospital mortality was 59% (n = 108). Non-survivors were at median 68 years (IQR 63–75) and significantly older than survivors (median 64 years, IQR 58–70, Table 4).
Table 3.
Patients outcome all.
| All patients | Range | |
|---|---|---|
| n | 184 | |
| Duration of hospital stay [days] | 19 (14; 28) | |
| Duration of ANE-ICU stay [days] | 13 (8.5; 19) | |
| Duration of stay at UKD [days] | 17 (12; 24.5) | |
| VTE during ICU stay | 85 (46.2%) | |
| DVT | 58 (31.5%) | |
| Catheter associated thrombosis | 5 (2.7%) | |
| PE | 57 (31.0%) | |
| ATE | 11 (6.0%) | |
| VTE before ICU admission | 17 (9.2%) | |
| Pneumothorax | 22 (12.0%) | |
| Lung emphysema | 9 (4.9%) | |
| Mediastinal emphysema | 13 (7.1%) | |
| Subcutaneous emphysema | 17 (9.3%) | |
| Pleural effusion | 81 (44.3%) | |
| Fusion in lung | 15 (8.2%) | |
| Status on day of discharge | ||
| Death | 95 (51.6%) | |
| Regular ward | 11 (6.0%) | |
| Other ICU | 38 (20.7%) | |
| Rehabilitation clinic | 32 (17.4%) | |
| Other hospital | 8 (4.3%) | |
| Withdraw of care by patients will | 105 (57.1%) | |
| Hospital survival | 76 (41.3%) | |
| ICU survival | 77 (41.8%) | |
| ANE-ICU survival | 90 (48.9%) | |
Data are median (Interquartile range) or n (%).
ICU Intensive care unit, ANE-ICU Intensive care unit of the Department of Anesthesiology and Critical Care Medicine, UKD University hospital Dresden, DVT Deep vein thrombosis, VTE Thromboembolic complications, PE Pulmonary embolism.
Table 4.
Patients characteristics survival.
| Survivors | Range | Non-survivors | Range | p | |
|---|---|---|---|---|---|
| n | 90 | 94 | |||
| Male | 60 (66.7%) | 74 (78.7%) | |||
| Age [years] | 64 (58;70) | 25–83 | 68 (63; 75) | 33–92 | < 0.05 |
| Body-Mass-Index [kg/m2] | 30.45 (26.12; 34.26) | 20.81–52.47 | 27.78 (25.48; 33.14) | 18.94–70.31 | |
| Time from first symptom to hospital admission [days] | 5 (0; 7) | 0–50 | 4.5 (0; 8) | 0–23 | |
| Time from first symptom to admission to our ICU [days] | 10 (5; 14) | 0–28 | 11 (5; 16) | 0–35 | |
| Time from first symptom to ECMO therapy [days] | 16 (13; 22) | 4–25 | 15 (11; 23) | 0–31 | |
| Direct transfer to our ICU from other hospital | 55 (61.1%) | 66 (70.2%) | |||
| External tracheostomy | 6 (6.7%) | 8 (8.5%) | |||
| External intubation | 60 (66.7%) | 73 (77.7%) | |||
| Invasive mechanical ventilation before admission to our ICU [days] | 2 (0; 5) | 0–20 | 3 (0; 7) | 0–16 | |
| Non-invasive mechanical ventilation before admission to our ICU [days] | 2 (1; 3) | 1–18 | 2 (1; 4) | 1–22 | |
| Charlson ComorbidityIndex | 3 (2; 5) | 0–11 | 3 (2; 6) | 0–12 | |
| Arterial Hypertension | 66 (73.3%) | 65 (69.1%) | |||
| Cardiovascular disease | 21 (23.3%) | 24 (25.5%) | |||
| Neurovascular symptoms | 9 (10%) | 9 (9.6%) | |||
| Coronary artery disease | 15 (16.7%) | 16 (17.0%) | |||
| Thromboembolic events in medical history | 4 (4.4%) | 7 (7.4%) | |||
| Chronic arrhythmias | 14 (15.6%) | 23 (24.5%) | |||
| COPD | 8 (8.9%) | 5 (5.3%) | |||
| Other pulmonary disease | 3 (3.3%) | 8 (8.5%) | |||
| Nicotine abuse | 8 (8.9%) | 4 (4.3%) | |||
| Diabetes mellitus | 42 (46.7%) | 37 (39.4%) | |||
| Previous organ or bone marrow transplantation | 4 (4.4%) | 5 (5.3%) | |||
| Chronic renal failure | 12 (13.3%) | 16 (17.0%) | |||
| Chronic need of renal replacement therapy | 1 (1.1%) | 7 (7.4%) | |||
| Admission with trauma | 6 (6.7%) | 2 (2.1%) | |||
| ACE inhibitors | 26 (28.9%) | 17 (18.3%) | |||
| AT2 receptor blocker | 23 (25.6%) | 29 (31.2%) | |||
| Beta blocker | 36 (40.0%) | 38 (40.9%) | |||
| Antithrombotic drug | 24 (26.7%) | 25 (26.9%) | |||
| DOAC | 13 (14.4%) | 17 (18.3%) | |||
| Corticosteroids | 11 (12.2%) | 10 (10.8%) | |||
| Immunosuppressive Drugs | 4 (4.4%) | 6 (6.5%) | |||
| Nosocomial infection | 8 (8.9%) | 11 (11.7%) |
Data are median (Interquartile range) or n (%).
Significant values are in [bold].
ICU Intensive care unit, ECMO extracorporeal membrane oxygenation, NIV Non-invasive ventilation, COPD Chronic obstructive pulmonary disease, ACE Angiotensin-converting enzyme, AT2 Angiotensin II, DOAC Direct oral anticoagulants.
Overall, the incidence of venous thromboembolic complications was high, affecting 46% (n = 84) of all patients. VTE manifested as deep vein thrombosis in 32% (n = 58), pulmonary embolism (PE) in 31% (n = 57) and catheter associated thrombosis in 3% (n = 5). Arterial thromboembolic events (myocardial infarction, stroke, systemic embolism or acute arterial thrombosis in peripheral or mesenterial arteries) affected 6% (n = 11).
Notable, 92 patients (50%) presented treatment-worthy bacteremia in blood culture next to sepsis. Septic shock at ICU admission was significantly more frequent in non-Survivors (19.4% vs 6.7%, Table 5). Besides, deceased patients showed amongst others higher need of additional supportive treatment of RRT, iNO and ECMO (Table 5). Non-survivors presented significantly higher rates of pleural effusion with the need of drainage (53% vs 36%, Table 6).
Table 5.
ICU characteristics survival.
| Survivors | Range | Non-survivors | Range | p | |
|---|---|---|---|---|---|
| n | 90 | 94 | |||
| Intubated at ICU admission | 30 (33.3%) | 21 (22.3%) | |||
| ARDS mild at ICU admission | 7 (7.8%) | 7 (7.4%) | |||
| ARDS moderate at ICU admission | 41 (45.6%) | 44 (46.8%) | |||
| ARDS severe at ICU admission | 39 (43.3%) | 43 (45.7%) | |||
| Septic shock at ICU admission | 6 (6.7%) | 18 (19.4%) | < 0.05 | ||
| First Horovitz-index at ICU | 112.5 (83; 165) | 52.5–450 | 105 (75; 142.5) | 45–262.5 | |
| Lowest Horovitz-index at ICU | 75 (52.5; 90) | 22.5–225 | 52.5 (45; 67.5) | 22.5–135 | |
| Pmean at admission [mbar] | 19 (16; 22) | 7–28 | 20 (18; 22) | 8–30 | |
| PEEP at admission [mbar] | 13 (12; 15) | 6–20 | 14 (12; 15) | 6–20 | |
| pH at admission | 7.40 (7.36; 7.46) | 7.17–7.62 | 7.37 (7.31; 7.42) | 6.81–7.59 | |
| PaCO2 at admission [kPa] | 6.29 (5.38; 6.82) | 3.23–9.86 | 6.64 (5.83; 7.52) | 4.42–15.90 | |
| SpO2 at admission [%] | 94 (91; 96) | 56–100 | 93 (89; 96) | 64–100 | |
| SOFA score at ICU admission | 11 (8; 13) | 5–16 | 12 (11; 14) | 4–19 | < 0.05 |
| D-dimers at ICU admission [ng/ml] | 4000 (1808; 7638) | 484–20,000 | 6128 (4114; 10,994) | 495–20,000 | < 0.05 |
| Lactate at ICU admission [mmol/L] | 1.10 (0.85; 1.40) | 0.40–3.30 | 1.30 (0.90; 1.90) | 0.50–9.90 | < 0.05 |
| Duration mechanical ventilation ICU [days] | 10 (6; 17) | 2–56 | 13 (8; 17) | 1–61 | |
| Reintubation | 2 (2.2%) | 2 (2.1%) | |||
| Prone position | 48 (53.3%) | 65 (69.1%) | < 0.05 | ||
| Cycles of prone position | 3 (2; 4) | 1–14 | 4 (3; 7) | 1–11 | < 0.05 |
| Tracheostomy | 41 (45.6%) | 41 (43.6%) | |||
| Days from intubation to Tracheostomy | 13 (10; 15) | 3–26 | 11 (8; 15 | 3–21 | |
| CRRT | 15 (16.7%) | 52 (55.3%) | < 0.05 | ||
| Duration CRRT [hours} | 337.01 (100.66; 483.67) | 17.33–788.74 | 138.10 (30.17; 239.08) | 1.44–906.53 | < 0.05 |
| ECMO | 14 (15.6%) | 35 (37.2%) | < 0.05 | ||
| Duration ECMO [hours] | 312.34 (208.30; 479.50) | 70.78–1068.31 | 253.80 (163.27; 347.93) | 16.78–577.63 | |
| Cytosorb | 0 | 8 (8.5%) | < 0.05 | ||
| Duration Cytosorb [hours] | 20.00 (17.17; 21.15) | 8.67–51.00 | |||
| Red Cell Transfusion | 5 (1; 8) | 1–36 | 7 (3; 13) | 1–40 | |
| NO inhalation | 12 (13.3%) | 50 (53.2%) | < 0.05 | ||
| Corticosteroid | 73 (81.1%) | 92 (97.9% | < 0.05 | ||
| Immunoglobulin | 2 (2.2%) | 2 (2.1%) | |||
| Convalescent plasma | 20 (22.2%) | 14 (14.9%) | |||
| Remdesivir | 14 (15.6%) | 6 (6.4%) | < 0.05 | ||
| Anticoagulation | 90 (100%) | 94 (100%) | |||
| Argatroban at any time on ICU | 8 (8.9%) | 7 (7.4%) | < 0.05 | ||
| UFH at any time on ICU | 51 (56.7%) | 89 (94.7%) | < 0.05 | ||
| LMWH at any time on ICU | 72 (80.0%) | 43 (45.7%) | < 0.05 | ||
| Bacteremia | 36 (40.0%) | 56 (59.6%) | < 0.05 | ||
| Staph. aureus bacteremia | 3 (3.3%) | 9 (9.6%) | |||
| Catheter associated bacteremia | 12 (13.3%) | 12 (12.8%) | |||
| Antibiotics | 84 (93.3%) | 92 (97.9%) | |||
| Antimycotics | 12 (13.3%) | 16 (17.0%) | |||
| CRP maximum value [mg/l] | 229.9 (144.4; 302.2) | 31.4–584.7 | 305.5 (231.2; 373.5) | 81.8–618.0 | < 0.05 |
| Interleukin 6 maximum value [pg/mL] | 152.0 (80.5; 398.0) | 8.6–21,728.0 | 674 (254; 2345) | 15.9–792,732.0 | < 0.05 |
| Leucocytes maximum value [GPt/L] | 17.19 (13.11; 22.43) | 7.06–63.87 | 20.82 (16.75; 27.17) | 3.14–63.64 | < 0.05 |
| Leucocytes minimum value [GPt/L] | 7.22 (5.00; 9.39) | 0.51–15.84 | 7.20 (4.63; 9.74) | 0.20–22.47 | |
| Procalcitonin [ng/ml] | 1.31 (0.43; 6.02) | 0.09–373.20 | 7.39 (2.20; 15.60) | 0.15–148.40 | < 0.05 |
| Prothrombin fragment F1 + 2 [pmol/l] | 393 (231; 780) | 98.0–4948 | 541 (339; 1001) | 73–4948 | |
| Platelets maximum value [GPt/L] | 355 (284; 461) | 103–989 | 286 (219; 357) | 48–617 | < 0.05 |
| Platelets minimum value [GPt/L] | 170 (110; 219) | 4–469 | 96 (47; 139) | 1–414 | < 0.05 |
Data are median (Interquartile range) or n (%).
Significant values are in [bold].
ICU Intensive care unit, ARDS Acute respiratory distress syndrome, Pmean Mean pressure, PEEP Positive end-expiratory pressure, PaCO2 partial pressure of carbon dioxide, SpO2 Oxygen saturation, SOFA Sequential organ failure assessment, CRRT Continuous renal replacement therapy, NO Nitric oxide, UFH Unfractionated heparin, LMWH Low-molecular-weight heparin, Staph. Staphylococcus, CRP C-reactive protein, ECMO extracorporeal membrane oxygenation.
Table 6.
ICU outcome survival.
| Survivors | Non-survivors | p | |
|---|---|---|---|
| n | 90 | 94 | |
| Duration of hospital stay [days] | 19 (14; 27) | 19 (14; 29) | |
| Duration of ANE-ICU stay [days] | 14 (8; 19) | 13 (9; 18) | |
| Duration of stay at UKD [days] | 22 (15; 30) | 14 (9; 20) | |
| VTE during ICU stay | 37 (41.1%) | 48 (51.1%) | |
| DVT | 24 (26.7%) | 34 (36.2%) | |
| Catheter associated thrombosis | 2 (2.2%) | 3 (3.2%) | |
| PE | 25 (27.8%) | 32 (34.0%) | |
| VTE before ICU admission | 8 (8.9%) | 9 (9.6%) | |
| Pneumothorax | 8 (8.9%) | 14 (15.1%) | |
| Lung emphysema | 2 (2.2%) | 7 (7.5%) | |
| Mediastinal emphysema | 3 (3.3%) | 10 (10.8%) | |
| Subcutaneous emphysema | 6 (6.7%) | 11 (11.8%) | |
| Pleural effusion | 32 (35.6%) | 49 (52.7%) | < 0.05 |
| Fusion in lung | 2 (2.2%) | 13 (13.8%) |
Data are median (Interquartile range) or n (%).
Significant values are in [bold].
ICU Intensive care unit, ANE-ICU Intensive care unit of the Department of Anesthesiology and Critical Care Medicine, UKD University hospital Dresden, DVT Deep vein thrombosis, VTE Thromboembolic complications, PE Pulmonary embolism.
The estimated probability of 30 days survival in patients with the need of ECMO therapy was 22% (SE 6.7%) and worse than in patients without ECMO-therapy with 40% (SE 5.3%, p < 0.05, Fig. 2).
Figure 2.
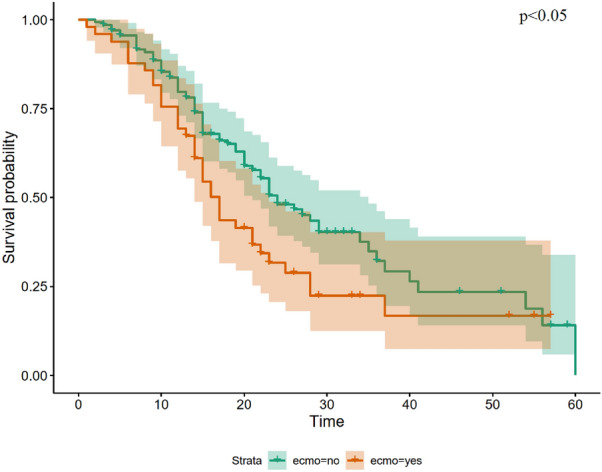
Kaplan–Meier Curves comparing ECMO therapy for COVID-19 ARDS. ARDS acute respiratory distress syndrome, ECMO extracorporeal membrane oxygenation. Time is indicated in days. Group comparison were performed using Log Rank test.
Risk factors associated with in-hospital mortality in regression analysis
In bivariate regression analysis the following variables were associated with higher in-hospital mortality: higher age, septic shock at ICU admission, higher SOFA score at ICU admission, d-dimer greater than 4000 ng/ml at ICU admission, invasive ventilation at ICU admission, need of RRT during ICU stay, need of inhaled nitric oxide therapy, need of ECMO therapy, lowest paO2 during ICU stay, maximum IL-6 values greater than 150 pg/ml during ICU stay, maximum PCT greater than 2 ng/ml during ICU stay, maximum values of platelets lower than 350 GPt/l, lowest value of platelets lower than 100 GPt/l and not conducting tracheotomy during ICU stay (Table 7).
Taking only variables into account, which were known at ICU admission, d-dimers > 4000 ng/ml (HR 1.641, CI 1.641–2.633), higher values of SOFA score (HR 1.129, CI 1.037–1.230) and higher age (HR 1.051, CI 1.023–1.080) showed the highest predictive value for in-hospital mortality (Table 7).
In multivariate full model regression analysis, morbid obesity with BMI > 40 kg/m2 was the strongest predictor for in-hospital mortality (HR 3.147, CI 1.000–9.897). Furthermore, higher age, need of inhaled nitric oxide therapy, need of ECMO therapy, maximum values of platelets lower than 350 GPt/l, lowest paO2 during ICU stay and not performing tracheotomy were associated with higher in-hospital mortality (Table 7).
Midterm outcome and the prevalence of long-COVID
Characteristics of the cohort
Midterm follow-up was complete for 88% (n = 67) of the 76 patients discharged alive from hospital. Nine patients (12%) were lost during follow-up. At time of the telephone follow-up, 83% (56) of patients were alive and 55 patients participated in the survey, whereas 11 patients died during midterm follow-up. The midterm follow-up intervals varied from 8 to 20 months with median 11 months (IQR 10–11). The estimated probability of 8 months survival (midterm survival) after SARS-CoV-2 ARDS was 32.8% (SE 3.6%) in our cohort.
Of the survivors, 78% (n = 43) reported symptoms of Long-COVID associated with discomfort. The most common symptoms were fatigue (70%), shortness of breath (57%), impaired mental concentration (50%) and limb or muscle pain (50%). Long-COVID symptoms lead to hospital admission in 37% of all patients. Permanent home oxygen support was necessary in 11% and 6% remained on renal replacement therapy. Additionally, 15% needed outpatient care and 26% stayed in nursing homes or other comparable institutions.
Following discharge from our hospital, all patients were treated in rehabilitation institutions with a median stay of 56 days (range 14–246, IQR 28–98). The majority of our patients (n = 33; 60%) was already retired at the time of SARS-CoV-2 ARDS, but reintegration into work life was successful in 50% of all patients working before ICU stay (n = 11). The median EQ-VAS was 60 points (range 0–100; IQR 45–75).
Risk factors for developing long-COVID
In multivariate analysis for patients discharged alive from hospital, only obesity was associated with increased probability of developing Long-COVID symptoms. Thereby, the relative risk was higher in patients with BMI > 40 kg/m2 (RR 1.61, CI 1.26–2.06) than in patients with BMI between 35 and 40 kg/m2 (RR 1.37, CI 1.04–1.79, Table 8).
Discussion
This study reported short-term and mid-term outcome of cARDS patients with the need of invasive ventilation and specialized ICU treatment and provided new insights in an area where data are still scarce.
Short-term outcome
Data on short-term outcome for hospitalized COVID-19 patients as well as patients on ICU have been widely reported and large cohort studies are available, demonstrating hospital mortalities ranging from 42%6 to 73.7%7. However, COVID-19 can lead to ARDS making invasive ventilation and in severe cases ECMO support necessary4,41,42. In this context, the reported ICU mortality of 51% and in-hospital mortality of 59% in our ARDS cohort falls into the lower range of expectations, especially since we are a referral center where often the most critically ill patients are transferred from community hospitals. This referral bias limits our data to more severe ARDS cases and patients with non-invasive ventilation are not represented in this study. At the same time, this selection pattern puts our mortality rate into a favorable perspective, which is also demonstrated by a median initial SOFA score of 12 points at ICU admission, already predicting mortality rates up to 95%43–45. Other studies reported far different results for hospital mortality, mostly dependent on the number of invasively ventilated patients or the severity of ARDS. The more severe ARDS patients were included in the study, the higher the number of reported deaths leading to ICU mortality up to 84.6%5 and 85.7% for ECMO patients46.
Aim of this analysis was also to identify risk factors for inferior outcome. Our study suggests, that in particular BMI > 40 kg/m2 and the amount of d-dimers at ICU admission could be used to identify patients at increased risk for unfavorable outcomes close to admission. Of note, both parameters could causally be connected, since patients with increased BMI have been demonstrated to present with higher levels of plasminogen activator inhibitor 1 (PAI-1). Visceral fat has been reported to be the main physiological storage for PAI-147 and higher PAI-1 values have been shown in obese patients. PAI-1 is released from infected, activated endothelial cells, adipocytes and platelets in septic patients48 and high PAI-1 levels are associated with worse outcome in COVID-19 patients49. PAI-1, emitted by monocytes, is a strong inhibitor of fibrinolysis50. Ranucci et al. showed that COVID-19 patients with worse outcome had up to sixfold higher PAI-1 levels compared to survivors49. In consequence of high plasma levels of PAI-1, fibrinolysis mediated by tissue plasminogen activator (tPA) and urokinase plasminogen-activator (uPA) may be severely reduced51 and could lead to a fibrinolytic shutdown, which is frequently seen in COVID-19 patients52–55. This could also explain why many of the critically ill COVID-19 patients are obese, or vice versa, why many obese patients develop more severe stages of COVID-19. It should be noteworthy, that BMI > 40 kg/m2 was shown as a strong risk factor for in-hospital mortality as well as the prevalence of Long-COVID symptoms. Similar results were found in a series of 3615 patients with COVID-19 from New York, US, those under 60 years of age with a BMI ranging from 30 to 34 kg/m2 had a 1.8-fold increase in the probability of ICU admission compared to patients with a BMI < 30 kg/m2. This likelihood increased to 3.6-fold among patients with a BMI ≥ 35 kg/m233. Moreover, COVID-19 patients in ICUs had higher BMI than non-ICU patients (BMI, median 30.5 kg/m2 vs 28.77 kg/m256. Furthermore, Salinas-Aguirre et al. reported an 1.88 fold increased mortality in patients with obesity > 30 kg/m2, investigating on 17.479 patients from Mexico57. A meta-analysis published by Yang et al. showed, that obesity > 30 kg/m2 is associated with increased risk of hospitalization, admission to ICU, need for invasive mechanical ventilation and mortality among COVID-19 patients58.
However, the only risk associated with the development of Long-COVID was obesity with BMI > 40 kg/m2 (RR 1.61, CI 1.26–2.06). While some studies likewise suggest obesity to be a possible risk for the development of post-COVID59, female sex is mentioned more often as a risk factor for the development of post-COVID60,61, which could not be confirmed in our study.
Complications during ICU stay were high in survivors and non-survivors. The occurrence of thromboembolic complications was up to 50% in our cohort but had no significant influence on patient’s outcome. This is surprising compared to other studies17. We can only hypothesize, that our consistent screening at ICU admission helped to early identify patients with ATE/VTE and subsequent increased anticoagulation therapy protected from inferior outcome. Noteworthy, the high VTE rates observed in our and many other COVID studies are not caused by ARDS itself, since VTE rates in patients with severe influenza ARDS were demonstrated to be considerably lower at 3%62.
As one would expect, patients in our cohort with inhaled nitric oxide therapy (iNO) and/or ECMO-therapy showed significant worse outcomes. Concomitantly, this subgroup showed higher SOFA-score and lower Horovitz-indices. Additional to MV and prone position iNO was regularly applied for treatment of severe hypoxemia in ARDS patients preliminary or instead (in cases, considered unsuitable for) of ECMO support. According to current recommendations, ECMO support is suggested as rescue therapy38,63. Complications related to ECMO therapy and mortality remain high3,41. Recent studies reported mortality for COVID-19 patients after ECMO support ranging from 22% in a very small cohort (9 patients) from Zurich64 up to 86% in other small series (7 patients) from Munich46 and 39% in the preliminary data from the ELSO-registry study42. A recent germanwide study did not recommend liberally ECMO use in COVID-19 ARDS (cARDS) patients and summarizes that the unconditional use of ECMO therapy in COVID-19 must be carefully considered and advanced age should be considered as a relative contraindication65. Indication for ECMO support should be critically discussed for every individual patient, considering structural lung damage, comorbidities, multi-organ failure and acceptable potential patients’ outcome. Taking the high number of critical ill patients into account, the limited number of available ECMO-devices, there could be an additional bias towards more conservative decision making.
Bacteremia and sepsis in the course of COVID-19 infection were frequent in our cohort, requiring antibiotic therapy necessary in 95% of all cases. However, proof of bacteremia was only possible in 50%. The other patients received calculated antibiotic therapy considering impaired organ function accompanied by elevated inflammatory parameters, e.g. procalcitonin. Another recently published study highlights the importance for IL-6 and PCT measurement as predictive biomarkers for COVID-19 severity66. Septic shock was treated in our department in accordance to national guidelines37, with fluid and catecholamine support as well as renal replacement therapy in case of acute kidney injury KDIGO stage 367, metabolic acidosis, hyperkalemia or volume overload. Special approaches, like clearing inflammatory cytokines with CytoSorb filters, were only used in a small number of patients as a rescue therapy because of lack of evidence68, especially in patient with cARDS67,69,70. Hospital mortality in our patients who presented with septic shock exceeded the one reported in Non-COVID patients (40–60%)71.
Midterm outcome and the prevalence of long-COVID
In addition to in-hospital outcomes, we reported mid-term outcomes of our ARDS patients after a minimum of eight months after hospital discharge. 83% of all patients (56/67) discharged from hospital were alive. Considering the whole cohort, this results in a probability of 8 months survival after admission to ICU for cARDS limited to 32.8%, which highlights the life threatening severity of COVID-19. Additional, 78% of our patients with available midterm follow-up reported symptoms of Long-COVID with median EQ-VAS of only 60 points.
A similar study from Spain showed a 5.2% mortality (5 out of 97 patients) 6 months after ICU release. The study was performed using data from 7 different ICUs72. Of the 92 surviving patients 91 were interviewed regarding their life-quality following the EQ-5D-3L. 61 (67%) patients reported a decreased quality of life, most commonly impeded were mobility (56%), pain (48%) and anxiety or depression (46%)72. Likhvantsev et al. reported 16 (7.2%) patients deceased out of 222 patients discharged from ICU73 although as many as 34 patients were lost to follow-up. Of the 125 patients which completed the survey, 68% reported serious problems regarding physical health while 48% reported serious problems regarding mental health73. Another recently published study including 41 patients with an average ICU stay of only 8.42 days concentrates on the psychological impairments. 12.2% had moderate depression, 2.4% severe depression. 14.6% of patients suffered from mild to moderate anxiety, 12.2% severe anxiety. 29.3% reported acute PTSD74.
In summary, short and midterm outcome of patients with COVID-19 developing severe ARDS was not satisfying. The high prevalence of Long-COVID shows the long healing path of severe COVID-19 ARDS patients, which goes far beyond the discharge from hospital. Obesity seems to be a serious risk factor associated with increased in-hospital mortality and the occurrence of Long-COVID.
Study limitations
As this is a retrospective study, it faces all the limitations associated with this type of analyses. We have observed different variations in patient characteristics and quantities that are likely to influence the prognosis. The main bias in this study is the inhomogeneous disease stage, caused by a high number of patients admitted from other hospitals or ICUs. Despite the fact that some statistics must be interpreted with caution, the key findings of this study reflect our clinical observations. Therapeutic approaches changed during the time period, some medications, e.g. corticoids, became standard treatment, while others could not reach significant improving effect in recently published studies and were not further used.
Conclusion
ARDS in COVID-19 patients is characterized by high morbidity and mortality. Complications during ICU stay are frequent. Midterm survival was acceptable with > 80%, but most of the patients developed Long-COVID symptoms associated with discomfort. To identify patients at high risk, laboratory parameters for inflammation and d-dimers can be helpful. Especially patients with BMI > 40 kg/m2 are at high risk for inferior short-term outcome and prevalence of Long-COVID.
Acknowledgements
The authors thank Volkmar Franz, Stephanie May and the staff of the ICU.
Abbreviations
- aPTT
Activated partial thromboplastin time
- ARDS
Acute respiratory distress syndrome
- ATE/VTE
Thromboembolic complications
- AWMF
Association of the Scientific Medical Societies in Germany
- aXa
Anti-Xa activity
- BMI
Body-Mass-Index
- cARDS
COVID-19 associated acute respiratory distress syndrome
- CAT
Catheter associated thrombosis
- CCI
Charlson ComorbidityIndex
- cCUS
Complete compression ultrasound
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- CRP
C-reactive protein
- CTPA
Computed tomography pulmonary angiography
- DIC
Disseminated intravascular coagulation
- DVT
Deep vein thrombosis
- ECMO
Extracorporeal membrane oxygenation
- EDTA
Ethylene diamine tetraacetic acid
- e.g.
Exempli gratia
- ELSO
European extracorporeal life support organization
- EOLIA
ECMO to rescue acute lung injury in severe ARDS
- EQ-VAS
EuroQol visual analogue scale
- HIT
Heparin-induced-thrombocytopenia
- HR
Hazard ratio
- ICU
Intensive care unit
- IL
Interleukin
- IQR
Interquartile range
- iNO
Inhaled nitric oxide
- INR
International normalized ratio
- KDIGO
Kidney Disease: Improving Global Outcomes
- MV
Mechanical ventilation
- NICE
National Institute for Health and Care Excellence
- NIV
Non-invasive ventilation
- PaO2
Partial pressure of oxygen
- PCT
Procalcitonin
- PE
Pulmonary embolism
- PEEP
Positive end-expiratory pressure
- PF 1 + 2
Prothrombin fragment 1 + 2
- P/F ratio
Horovitz-index
- PT
Prothrombin time
- PTSD
Post-traumatic stress disorder
- RR
Relative risk
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SE
Standard error
- SOFA
Sequential organ failure assessment
- SOP
Standard operating procedure
- VET
Viscoelastic testing
- VT
Venous thrombosis
- VTE
Venous thromboembolism
- WHO
World Health Organization
Author contributions
L.H.—study design, conducting research, drafting the paper including critical revisions. P.L.P.—collection of clinical data, drafting the paper. A.G.—conducting research, clinical management, revising the paper. L.B.—collection of clinical data, drafting the paper. M.R.—clinical management, critical contributions. M.M. drafting the paper, critical contributions. A.R.—clinical management, critical contributions. O.T.—critical contributions. J.B.-W.—critical contributions. M.R.—conducting research, revising the paper. J.S.—critical contributions. T.K.—critical contributions, revising the paper. P.M.S.—supervision, drafting and revising the paper. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets are not publicly available due to data sharing protocols but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020. https://covid19.who.int/ (last cited: [26.04.2022]).
- 2.Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388(10058):2416–2430. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N. Engl. J. Med. 2011;365(20):1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 4.EuroELSO: EuroELSO Survey on ECMO use in Adult COVID-19 Patients in Europe. https://www.euroelsonet/covid-19/covid-19-survey/. Accessed 16 March 2021.
- 5.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75(10):1340–1349. doi: 10.1111/anae.15201. [DOI] [PubMed] [Google Scholar]
- 6.Richards-Belle A, Orzechowska I, Gould DW, Thomas K, Doidge JC, Mouncey PR, Christian MD, Shankar-Hari M, Harrison DA, Rowan KM, et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46(11):2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ñamendys-Silva SA, Gutiérrez-Villaseñor A, Romero-González JP. Hospital mortality in mechanically ventilated COVID-19 patients in Mexico. Intensive Care Med. 2020;46(11):2086–2088. doi: 10.1007/s00134-020-06256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Samkari, H., Gupta, S., Leaf, R.K., Wang, W., Rosovsky, R.P., Brenner, S.K., Hayek, S.S., Berlin, H., Kapoor, R., Shaefi, S. et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann. Internal Med. M20-6739 (2021). [DOI] [PMC free article] [PubMed]
- 10.Kruse JM, Magomedov A, Kurreck A, Münch FH, Koerner R, Kamhieh-Milz J, Kahl A, Gotthardt I, Piper SK, Eckardt KU, et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit. Care (Lond. Engl.) 2020;24(1):676. doi: 10.1186/s13054-020-03401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortus JR, Manek SE, Brubaker LS, Loor M, Cruz MA, Trautner BW, Rosengart TK. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw. Open. 2020;3(6):e2011192. doi: 10.1001/jamanetworkopen.2020.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluge, S., Janssens, U., Welte, T., Weber-Carstens, S., Schälte, G., Salzberger, B., Gastmeier, P., Langer, F., Welper, M., Westhoff, M. et al. Recommendations for treatment of critically ill patients with COVID-19: Version 3 S1 guideline. Der Anaesthesist. 1–11 (2020). [DOI] [PMC free article] [PubMed]
- 13.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 15.Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N, Douair A, Barbin E, Fraix A, Bouchot O, et al. Pulmonary embolism in COVID-19 patients: A French multicentre cohort study. Eur. Heart J. 2020;41(32):3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamed MFH, Al-Shokri SD, Shunnar KM, Mohamed SF, Najim MS, Ibrahim SI, Elewa H, Abdalla LO, El-Bardissy A, Elshafei MN, et al. Prevalence of venous thromboembolism in critically ill COVID-19 patients: Systematic review and meta-analysis. Front. Cardiovasc. Med. 2021;7:598846. doi: 10.3389/fcvm.2020.598846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, Y., Cai, J., Wang, C., Jin, J., Qu, L. The incidence, prognosis and laboratory indicators of venous thromboembolism in hospitalized patients with COVID-19: A Systematic review and meta-analysis. J. Vasc. Surg. Venous Lymphat. Disord. (2021). [DOI] [PMC free article] [PubMed]
- 18.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachler, M., Bösch, J., Stürzel, D.P., Hell, T., Giebl, A., Ströhle, M., Klein, S.J., Schäfer, V., Lehner, G.F., Joannidis, M, et al. Impaired fibrinolysis in critically ill COVID-19 patients. Br. J. Anaesthesia. (2020). [DOI] [PMC free article] [PubMed]
- 20.Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, David JS, Bonnet A, Negrier C, Dargaud Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020;18(9):2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, Arabi YM, Loeb M, Ng Gong M, Fan E, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: First update. Crit. Care Med. 2021;49(3):e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 26.Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, Leavitt AD, Lee AYY, Macbeth F, Morgan RL, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, Davila J, DeSancho MT, Diuguid D, Griffin DO, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musoke N, Lo KB, Albano J, Peterson E, Bhargav R, Gul F, DeJoy R, 3rd, Salacup G, Pelayo J, Tipparaju P, et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb. Res. 2020;196:227–230. doi: 10.1016/j.thromres.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rad F, Dabbagh A, Dorgalaleh A, Biswas A. The relationship between inflammatory cytokines and coagulopathy in patients with COVID-19. J. Clin. Med. 2021;10(9):2020. doi: 10.3390/jcm10092020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern. Med. 2021;181(1):41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callard F, Perego E. How and why patients made Long COVID. Soc. Sci. Med. 2021;268:113426. doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sivan M, Taylor S. NICE guideline on long COVID. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 34.Soriano, J.B., Murthy, S., Marshall, J.C., Relan, P., Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. (2021). [DOI] [PMC free article] [PubMed]
- 35.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 36.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leinlinie. AdWMFA-SK: S3-Leitlinie Sepsis—Prävention, Diagnose, Therapie und Nachsorge. Online Ressource Verfügbar. https://www.awmforg/uploads/tx_szleitlinien/079-001k_S3_Sepsis-Praevention-Diagnose-Therapie-Nachsorge_2020-02pdf (Zugriff am 150621) 2018.
- 38.Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N. Engl. J. Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 39.Koczulla AR, Ankermann T, Behrends U, Berlit P, Böing S, Brinkmann F, Franke C, Glöckl R, Gogoll C, Hummel T, et al. S1 guideline post-COVID/long-COVID. Pneumologie. 2021;75(11):869–900. doi: 10.1055/a-1551-9734. [DOI] [PubMed] [Google Scholar]
- 40.Zou G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 41.Broman, L.M., Eksborg, S., Coco, V.L., De Piero, M.E., Belohlavek, J., Lorusso, R. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir. Med. (2021). [DOI] [PMC free article] [PubMed]
- 42.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, Bartlett RH, Tonna JE, Hyslop R, Fanning JJ, et al. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 44.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit. Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder I, Scharf C, Zoller M, Wassilowsky D, Frank S, Stecher S-S, Stemmler J, Kneidinger N, Peterß S, Zwißler B, et al. Charakteristika und Outcome von 70 beatmeten COVID-19-Patienten. Anaesthesist. 2021;70(7):573–581. doi: 10.1007/s00101-020-00906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaji H. Adipose tissue-derived plasminogen activator inhibitor-1 function and regulation. Compr. Physiol. 2016;6(4):1873–1896. doi: 10.1002/cphy.c160004. [DOI] [PubMed] [Google Scholar]
- 48.Guo J, Xu QH. The correlation of plasma thrombomodulin plasminogen activator inhibitor-1 and endothelial cell injury in septic patients. Zhonghua Nei Ke Za Zhi. 2021;60(2):143–146. doi: 10.3760/cma.j.cn112138-20200330-00319. [DOI] [PubMed] [Google Scholar]
- 49.Ranucci, M., Sitzia, C., Baryshnikova, E., Di Dedda, U., Cardani, R., Martelli, F., Corsi Romanelli, M. COVID-19-associated coagulopathy: biomarkers of thrombin generation and fibrinolysis leading the outcome. J. Clin. Med. 9(11) (2020). [DOI] [PMC free article] [PubMed]
- 50.Robbie LA, Dummer S, Booth NA, Adey GD, Bennett B. Plasminogen activator inhibitor 2 and urokinase-type plasminogen activator in plasma and leucocytes in patients with severe sepsis. Br. J. Haematol. 2000;109(2):342–348. doi: 10.1046/j.1365-2141.2000.02057.x. [DOI] [PubMed] [Google Scholar]
- 51.Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, Ledot S, Morgan C, Passariello M, Price S, et al. Pulmonary angiopathy in severe COVID-19: Physiologic, imaging, and hematologic observations. Am. J. Respir. Crit. Care Med. 2020;202(5):690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Creel-Bulos, C., Auld, S.C., Caridi-Scheible, M., Barker, N., Friend, S., Gaddh, M., Kempton, C.L., Maier, C., Nahab, F., Sniecinski, R. Fibrinolysis shutdown and thrombosis in a COVID-19 ICU. Shock. (2020). [DOI] [PMC free article] [PubMed]
- 53.Ibañez, C., Perdomo, J., Calvo, A., Ferrando, C., Reverter, J.C., Tassies, D., Blasi, A. High D dimers and low global fibrinolysis coexist in COVID19 patients: What is going on in there? J. Thromb. Thrombolysis. 1–5 (2020). [DOI] [PMC free article] [PubMed]
- 54.Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC., Jr Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J. Am. Coll. Surg. 2020;231(2):193–203.e191. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zátroch I, Smudla A, Babik B, Tánczos K, Kóbori L, Szabó Z, Fazakas J. Procoagulation, hypercoagulation and fibrinolytic “shut down” detected with ClotPro® viscoelastic tests in COVID-19 patients. Orv. Hetil. 2020;161(22):899–907. doi: 10.1556/650.2020.31870. [DOI] [PubMed] [Google Scholar]
- 56.Yu W, Rohli KE, Yang S, Jia P. Impact of obesity on COVID-19 patients. J. Diabetes Complications. 2021;35(3):107817. doi: 10.1016/j.jdiacomp.2020.107817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salinas-Aguirre JE, Sánchez-García C, Rodríguez-Sanchez R, Rodríguez-Muñoz L, Díaz-Castaño A, Bernal-Gómez R. Clinical characteristics and comorbidities associated with mortality in patients with COVID-19 in Coahuila (Mexico) Rev. Clin. Esp. 2022;222(5):288–292. doi: 10.1016/j.rce.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Wang L, Liu J, Fu S, Zhou L, Wang Y. Obesity or increased body mass index and the risk of severe outcomes in patients with COVID-19: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2022;101(1):e28499. doi: 10.1097/MD.0000000000028499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández-de-Las-Peñas C, Torres-Macho J, Elvira-Martínez CM, Molina-Trigueros LJ, Sebastián-Viana T, Hernández-Barrera V. Obesity is associated with a greater number of long-term post-COVID symptoms and poor sleep quality: A multicentre case-control study. Int. J. Clin. Pract. 2021;75(12):e14917. doi: 10.1111/ijcp.14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munblit D, Bobkova P, Spiridonova E, Shikhaleva A, Gamirova A, Blyuss O, Nekliudov N, Bugaeva P, Andreeva M, DunnGalvin A, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin. Exp. Allergy. 2021;51(9):1107–1120. doi: 10.1111/cea.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asadi-Pooya AA, Akbari A, Emami A, Lotfi M, Rostamihosseinkhani M, Nemati H, Barzegar Z, Kabiri M, Zeraatpisheh Z, Farjoud-Kouhanjani M, et al. Risk factors associated with long COVID syndrome: A retrospective study. Iran. J. Med. Sci. 2021;46(6):428–436. doi: 10.30476/ijms.2021.92080.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit. Care Med. 2010;38(4 Suppl):e91–97. doi: 10.1097/CCM.0b013e3181c92eeb. [DOI] [PubMed] [Google Scholar]
- 63.Fichtner F, Moerer O, Weber-Carstens S, Nothacker M, Kaisers U, Laudi S. Clinical guideline for treating acute respiratory insufficiency with invasive ventilation and extracorporeal membrane oxygenation: Evidence-based recommendations for choosing modes and setting parameters of mechanical ventilation. Respiration. 2019;98(4):357–372. doi: 10.1159/000502157. [DOI] [PubMed] [Google Scholar]
- 64.Sromicki J, Schmiady M, Maisano F, Mestres CA. ECMO therapy in COVID-19: An experience from Zurich. J. Card. Surg. 2021;36(5):1707–1712. doi: 10.1111/jocs.15147. [DOI] [PubMed] [Google Scholar]
- 65.Friedrichson, B., Kloka, J.A., Neef, V., Mutlak, H., Old, O., Zacharowski, K., Piekarski, F. Extracorporeal membrane oxygenation in coronavirus disease 2019: A nationwide cohort analysis of 4279 runs from Germany. Eur. J. Anaesthesiol. (2022). [DOI] [PubMed]
- 66.Tang J, Lin J, Zhang E, Zhong M, Luo Y, Fu Y, Yang Y. Serum IL-6 and procalcitonin are two promising novel biomarkers for evaluating the severity of COVID-19 patients. Medicine (Baltimore) 2021;100(22):e26131. doi: 10.1097/MD.0000000000026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ricci Z, Romagnoli S. Acute kidney injury: Diagnosis and classification in adults and children. Contrib. Nephrol. 2018;193:1–12. doi: 10.1159/000484956. [DOI] [PubMed] [Google Scholar]
- 68.Goetz, G., Hawlik, K., Wild, C. Extracorporeal cytokine adsorption therapy as a preventive measure in cardiac surgery and as a therapeutic add-on treatment in sepsis: An updated systematic review of comparative efficacy and safety*. Crit. Care Med. 49(8) (2021). [DOI] [PubMed]
- 69.Supady A, Weber E, Rieder M, Lother A, Niklaus T, Zahn T, Frech F, Müller S, Kuhl M, Benk C, et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): A single centre, open-label, randomised, controlled trial. Lancet Respir. Med. 2021;9(7):755–762. doi: 10.1016/S2213-2600(21)00177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song, T., Hayanga, J., Durham, L., Garrison, L., McCarthy, P., Barksdale, A., Smith, D., Bartlett, R., Jaros, M., Nelson, P. et al. CytoSorb therapy in COVID-19 (CTC) patients requiring extracorporeal membrane oxygenation: A multicenter, retrospective registry. Front. Med. 8 (2021). [DOI] [PMC free article] [PubMed]
- 71.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 72.Taboada M, Moreno E, Cariñena A, Rey T, Pita-Romero R, Leal S, Sanduende Y, Rodríguez A, Nieto C, Vilas E, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br. J. Anaesth. 2021;126(3):e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Likhvantsev, V., Landoni, G., Perekhodov, S., Chaus, N., Kadantseva, K., Ermokhina, L., Baeva, A., Yadgarov, M., Berikashvili, L., Kuzovlev, A. et al. Six-month quality of life in COVID-19 intensive care unit survivors. J. Cardiothorac. Vasc. Anesth. (2021). [DOI] [PMC free article] [PubMed]
- 74.Chadli A, Haraj NE, El Aziz S, Laidi S, Mounir A, Bensbaa S, Mjabber A, Barrou L, Hamidi EKEC, Nsiri A, et al. COVID-19: Patient care after discharge from the Intensive Care Unit. Int. J. Clin. Pract. 2021;75(9):e14270. doi: 10.1111/ijcp.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are not publicly available due to data sharing protocols but are available from the corresponding author on reasonable request.