Abstract
As antiretroviral therapy becomes more affordable, valid, reliable, and inexpensive laboratory tests are also needed to monitor the progression of disease in people with human immunodeficiency virus (HIV) infection. The CD4+ T-cell counts estimated by Capcellia, an immunocapture method, and flow cytometry were compared and were correlated with HIV type 1 (HIV-1) load. There was a significant negative correlation between the HIV-1 load and CD4+ T-cell counts estimated by flow cytometry (r = −0.63, P = <0.001) as well as between the HIV-1 load and CD4+ T-cell counts estimated by Capcellia (r = −0.61, P = <0.001). Capcellia is a cost-effective, user-friendly assay that correlated well with HIV-1 load determinations for individuals both with and without treatment.
With dramatic developments taking place in the field of antiretroviral therapy, estimation of human immunodeficiency virus type 1 (HIV-1) load and CD4+ T-cell counts is required for decisions on treatment and analysis of response (12). The most important constraint other than the cost of the drugs is the cost of such investigations. Hence, it is important to have cost-effective monitoring tests. The technique of estimating CD4+ and CD8+ T-cell counts by an immunocapture method (Capcellia) was investigated earlier (9). In the present study, we investigated the correlation of CD4+ T-cell counts as estimated by the immunocapture method with HIV-1 load estimation and the performance of this assay in relation to flow cytometry.
Fifty-one HIV-infected individuals attending the infectious disease clinic of Christian Medical College Hospital, Vellore, India, or those who were directly referred to the Department of Clinical Virology from other general practitioners were recruited after informed consent was obtained. The study group included 38 males and 13 females; the age range was 19 to 63 years (mean and standard deviation [SD], 34 ± 10 years). None was on any antiretroviral therapy. These individuals were classified into category A (n = 33), B (n = 9), and C (n = 9) in accordance with the Centers for Disease Control and Prevention (CDC) classification system (2).
Samples were collected between 8:00 and 10:00 a.m. in two separate EDTA-containing tubes. Whole blood from one tube was used for the CD4+ and CD8+ T-cell count estimation, and plasma from the other tube was separated and stored at −60°C for the HIV-1 load estimation.
Estimation of CD4+ and CD8+ T-cell counts for all 51 samples was carried out with a Capcellia CD4/CD8 whole-blood kit (Bio-Rad, Hercules, Calif.) and by flow cytometry in parallel. The Capcellia CD4/CD8 whole-blood kit from Bio-Rad (previously manufactured and marketed by Sanofi Diagnostic Pasteur, Marnes-la-Coquette, France) is an immunocapture assay based on an enzyme-linked immunosorbent assay (ELISA); paramagnetic particles coated with anti-pan-T-cell antibodies (anti-CD2) are used to capture T lymphocytes (1, 9). The unbound cells are washed using a manual washing manifold connected to an electric vacuum pump. The T-cell subsets are estimated using peroxidase-labeled monoclonal antibodies specific for CD4+ and CD8+ T cells. The mean coefficients of variation for CD4+ and CD8+ T-cell counts were 6.5 and 12%, respectively, for samples obtained from healthy controls and tested in duplicate (9). Hence, although the kit manufacturer recommended that each sample be tested once, we tested all the samples in duplicate wells and took the average cell counts as CD4+ and CD8+ T-cell counts per microliter.
Flow cytometry analysis was carried out with a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.) with SimulSet software. All the samples were processed within 6 h in accordance with the manufacturer's instructions. CD45CD14, isotypic control, CD3CD4, and CD3CD8 monoclonal antibodies (DAKO A/S, Glostrup, Denmark) were used. To derive the absolute CD4+ and CD8+T-cell counts, total and differential white blood cell counts, as measured by Maxm or Status (Coulter Corporation, Hialeah, Fla.), were used.
The quantitation of HIV type 1 (HIV-1) RNA in plasma was carried out with an Amplicor HIV-1 monitor test, version 1.5 (Roche Diagnostics, Branchburg, N.J.); this method estimates ≥400 RNA copies/ml of plasma.
Additionally, samples from seven HIV-1-infected individuals were tested for CD4+ and CD8+ T cells by Capcellia and for viral load at baseline and while they were on antiretroviral therapy. These individuals were not part of the group of 51 individuals for whom the correlation of viral loads and T-cell counts is shown below, as flow cytometry was not carried out on these samples.
The correlation between the CD4+ and CD8+ T-cell counts estimated by the two methods and the correlation between the CD4+ T-cell counts and HIV-1 loads were analyzed by Pearson's correlation test. Comparisons of means of CD4+ and CD8+ T-cell counts and CD4/CD8 ratios obtained by the two methods and viral loads in different clinical categories were analyzed by Kruskal-Wallis one-way analysis of variance. Comparisons of means of CD4+ and CD8+ T-cell counts and CD4/CD8 ratios obtained by the two methods were analyzed by analysis of variance.
The means and SDs of CD4+ T-cell counts estimated by Capcellia for CDC categories A, B, and C were 548 ± 270, 285 ± 179, and 132 ± 71 cells/μl, respectively, and those of CD8+ T-cell counts were 993 ± 523, 752 ± 320, and 552 ± 255 cells/μl, respectively. The mean and SD estimated for the same samples by flow cytometry were 301 ± 150, 175 ± 165, and 65 ± 43 cells/μl for CD4+ T cells and 1,280 ± 586, 1,013 ± 570, and 558 ± 275 cells/μl for CD8+ T cells in the three categories, respectively. There were significant differences (Kruskal-Wallis) in the CD4+ (P = 0.000035) and CD8+ (P = 0.0185) T-cell counts and the ratio (P = 0.007) among the three groups with Capcellia. Significant differences in the CD4+ (P = 0.00002) and CD8+ (P = 0.0012) T-cell counts but not in the ratio (P = 0.051) were seen among the three groups with flow cytometry. The correlations between the immunocapture method and flow cytometry were significant for the estimation of CD4+ and CD8+ T-cell counts and the ratio. The correlation coefficients for these determinations were 0.70, 0.47, and 0.59, respectively.
The mean numbers of copies of viral RNA were 1.17 × 105, 1.95 × 105, and 3.85 × 105 copies of RNA/ml for CDC categories A, B, and C, respectively. The plasma HIV-1 RNA levels among the three groups were significantly different (Kruskal-Wallis; P = 0.00156).
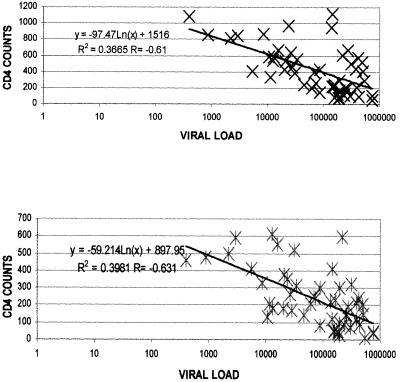
The negative correlation between the HIV-1 load and CD4+ T-cell count with flow cytometry (r = −0.63, P < 0.001) was significant, as was the viral load and CD4+ T-cell count with the immunocapture method (r = −0.61, P < 0.001). The scatter diagrams showing the correlations of CD4+ T-cell counts by both methods with HIV-1 loads are shown in Fig. 1.
FIG. 1.
Diagrams showing the correlations for CD4+ T-cell counts estimated by Capcellia (top) and flow cytometry (bottom) and HIV loads.
The CD4+ T-cell counts determined by Capcellia and the HIV loads for seven patients before and after treatment are shown in Table 1.
TABLE 1.
CD4+ T-cell counts and viral loads of seven HIV-1-infected individuals before and after treatment (>3 months) with antiretroviral drugs
| Patient identification no. | Values
|
Interval between samples (duration of treatment, in weeks) | |||
|---|---|---|---|---|---|
| Pretreatment
|
Posttreatmenta
|
||||
| CD4+ T-cells/μl | Viral load (RNA copies/ ml) | CD4+ T-cells/μl | Viral load (RNA copies/ ml) | ||
| CAP 97/99 | 354 | 411,275 | 248 | 76,287 | 16 |
| CAP 46/00 | 82 | 394,416 | 400 | <400 | 43 |
| CAP 61/00 | 280 | 35,879 | 497 | <400 | 59 |
| CAP 67/00 | 199 | 23,266 | 388 | <400 | 31 |
| CAP 136/00 | <30 | 90,213 | >1,445 | <400 | 23 |
| CAP 1/01 | 259 | 19,432 | 746 | <400 | 27 |
| CAP 24/01 | <30 | 162,901 | 354 | <400 | 14 |
Dual or triple antiretroviral drugs.
There are different methods available for the estimation of T-cell counts and viral loads (1, 3–5, 8,11). Flow cytometry, the standard method for the estimation of CD4+ and CD8+ T-cell counts, is available only in a very few centers in developing countries. An earlier study showed that the Capcellia method can be used as an alternative to flow cytometry for the quantitation of CD4+ and CD8+ T-cell counts in less well-equipped laboratories (9). The study showed a significant difference in CD4+ and CD8+ T-cell counts and the ratio measured by Capcellia between asymptomatic and symptomatic HIV-infected individuals. The findings in the present study are in concordance with the earlier findings.
There are a few reports in which the performances of flow cytometry and Capcellia have been compared and found to have a good correlation (1, 4,5). Our study also showed a significant correlation for CD4+ and CD8+ T-cell counts and the ratio estimated by Capcellia and flow cytometry for HIV-infected patients. The r values for these three variables were 0.70 (P < 0.001), 0.47 (P < 0.001), and 0.59 (P < 0.001), respectively.
The differences in cell counts between Capcellia and flow cytometry were significant for category A (P = 0.00001) and category C (P = 0.03) but not for category B. For CD8+ T cells, this difference was significant only for category A (P = 0.04). The median, 10th, and 90th percentile differences between the absolute CD4+ T-cell counts and CD8+ T-cell counts determined by flow cytometry and Capcellia are shown in Table 2. It was previously shown that a count of CD4+ T cells of ≤628 by Capcellia could be considered a significant reduction (9). Using this cutoff, 100% of category B (n = 9) and category C (n = 9) members were identified as CD4+ T-cell lymphopenic. The cell counts estimated by Capcellia could not be used in the same way as the CDC criteria to categorize HIV-infected individuals. Hence, the use of Capcellia is possible if the baseline levels for both CD4+ and CD8+ T-cell populations in normal healthy individuals in a given geographical region are determined for interpretation.
TABLE 2.
Median, 10th, and 90th percentiles of the CD4+ and CD8+ T-cell counts estimated by both Capcellia and flow cytometry for the three CDC categories of patients
| CDC category | Median or percentile | Valuea determined for the indicated cells by:
|
|||
|---|---|---|---|---|---|
| Capcellia
|
Flow cytometry
|
||||
| CD4+ | CD8+ | CD4+ | CD8+ | ||
| A | 10th percentile | 243 | 453 | 154 | 688 |
| 90th percentile | 932 | 1,695 | 516 | 2,083 | |
| Median | 525 | 869 | 260 | 1,160 | |
| B | 10th percentile | 157 | 463 | 82 | 552 |
| 90th percentile | 591 | 934 | 303 | 1,440 | |
| Median | 219 | 739 | 120 | 795 | |
| C | 10th percentile | 77 | 279 | 34 | 252 |
| 90th percentile | 207 | 861 | 100 | 904 | |
| Median | 107 | 572 | 50 | 494 | |
In cells per microliter.
There should be a negative correlation between the plasma RNA level and the CD4+ T-cell count in an HIV-1-infected individual. However, this feature is not always absolute, as some individuals with a high CD4+ T-cell count may have a high viral load and vice versa (7, 10). The present study also showed a significant negative correlation between the cell count and the plasma RNA level. The negative correlation was almost the same for Capcellia (r = −0.61) and flow cytometry (r = −0.63). This result was also observed for seven patients who were on antiretroviral drugs. Six (86%) of them clearly showed an increase in the CD4+ T-cell count and a significant decrease in the viral load after the initiation of treatment. This finding emphasizes the usefulness of Capcellia as an alternative to flow cytometry for CD4+ and CD8+ T-cell count estimation in conjunction with viral load estimation to monitor the level of immune deterioration and as a prognostic test in individuals who are on antiretroviral treatment.
New antiretroviral formulations continue to come to the market and, following a price war between companies, the prices have fallen to such a level that many persons in developing countries can consider having treatment. Hence, it is important to have affordable monitoring tests as well.
It has been reported already that one of the plasma activation markers, such as neoptrin, soluble tumor necrosis factor receptor II (TNF-RII), or soluble interleukin-2 receptor, can be used along with the CD4+ T-cell count instead of the plasma viral load to determine the prognosis of the disease (6). In developing countries such as India, it is extremely difficult to establish expensive equipment such as flow cytometry for CD4+ T-cell estimation and viral load measurements for care giving at the lower end of health care systems. The capital investment for flow cytometry varies from U.S. $0.1 to 0.2 million and requires costly reagents and well-trained personnel to carry out the test. For Capcellia, the kit price is about U.S. $500, and by testing patient samples in batches, 18 samples can be analyzed if the samples are run in duplicate (U.S. $30 per sample). It is possible to have facilities for ELISAs in low-technology settings. With some amount of technical support, centers can be provided with facilities for CD4+ and CD8+ T-cell estimation with the immunocapture technique and detection of a plasma marker with the ELISA. This study for the first time also establishes the relationship between Capcellia and HIV-1 load estimates. Capcellia for CD4+ and CD8+ T-cell counts is a cost-effective, user-friendly assay which provides counts that correlate well with HIV-1 load measurements.
REFERENCES
- 1.Carriere D, Vendrell J P, Fontaine C. Whole blood Capcellia CD4/CD8 immunoassay for enumeration of CD4+ and CD8+ peripheral T lymphocytes. Clin Chem. 1999;45:92–97. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1993 revised classification system for adolescents and adults. Morb Mortal Wkly Rep. 1993;41(RR-17):1–19. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Revised guidelines for performing CD4+ T-cell determinations in persons infected with human immunodeficiency virus (HIV) Morb Mortal Wkly Rep. 1997;46(RR-2):1–29. [PubMed] [Google Scholar]
- 4.Diaghbouga S, Durand G, Sanou P T, Dahourou H, Ledru E. Evaluation of quantitative determination of CD4 and CD8 molecules as an alternative to CD4+ and CD8+ T lymphocyte counts in Africans. Trop Med Int Health. 1999;4:79–84. doi: 10.1046/j.1365-3156.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 5.Didier J M, Kazatchkine M D, Demouchy C, Moat C, Diagbouga S, Sepulveda C, Lonardo A M D, Weiss L. Comparative assessment of five alternative methods for CD4+ T-lymphocyte enumeration for implementation in developing countries. J Acquir Immune Defic Syndr. 2001;26:193–195. doi: 10.1097/00042560-200102010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Fahey J I, Taylor J M, Manna B, Nishanian P, Aziz N, Giorgi J V, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load, and CD4 T-cell measurements. AIDS. 1998;14:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Felipe G, Carmen V, Jose M G, Jose M M, Alex S, Tomas P. Viral load in asymptomatic patients with CD4+ lymphocyte counts above 500 × 106/l. AIDS. 1997;11:53–57. doi: 10.1097/00002030-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Frank L, Nugel E, Docke W D, Porstmann T. Quantitative determination of CD4/CD8 molecules by a cell marker ELISA. Clin Chem. 1994;40:38–42. [PubMed] [Google Scholar]
- 9.Kannangai R, Prakash K J, Ramalingam S, Abraham O C, Mathews K P, Jesudason M V, Sridharan G. Peripheral CD4+/CD8+ T-lymphocyte counts estimated by an immunocapture method in the normal healthy south Indian adults and HIV seropositive individuals. J Clin Virol. 2000;17:101–108. doi: 10.1016/s1386-6532(00)00080-9. [DOI] [PubMed] [Google Scholar]
- 10.Ledergerber B, Flepp M, Boni J, Tomasik Z, Cone R W, Luthy R, Schupbach J. Human immunodeficiency virus type 1 p24 concentration measured by boosted ELISA of heat-denatured plasma correlates with decline in CD4 cells, progression to AIDS, and survival: comparison with viral RNA measurement. J Infect Dis. 2000;181:1280–1288. doi: 10.1086/315366. [DOI] [PubMed] [Google Scholar]
- 11.Robert W C, Patricia R. Use of plasma HIV-1 RNA to assess prognosis and monitor therapy in HIV-1 infection. In: Merigan T C Jr, Bartlett J G, Bolognesi D, editors. Textbook of AIDS medicine. 2nd ed. Baltimore, Md: Williams & Wilkins Publishers; 1999. pp. 673–688. [Google Scholar]
- 12.Serchuck L K, Welles L, Yarchoan R. Antiretroviral treatment for HIV infection. In: Merigan T C Jr, Bartlett J G, Bolognesi D, editors. Textbook of AIDS medicine. 2nd ed. Baltimore, Md: Williams & Wilkins Publishers; 1999. pp. 780–806. [Google Scholar]