Abstract
Precipitating hydrophobic injectable liquid (PHIL; MicroVention, Aliso Viejo, CA, USA) and Squid (Balt, Irvine, CA, USA) are 2 newer liquid embolic agents used in endovascular embolization of cerebral arteriovenous malformation (AVM). This study aims to investigate and compare the effectiveness and safety profile of the 2 newer liquid embolic agents in the embolization of cerebral AVM. This is a retrospective study on all patients diagnosed with cerebral AVM undergoing endovascular embolization with liquid embolic agents PHIL and Squid admitted to the Division of Neurosurgery, Department of Surgery in Prince of Wales Hospital from January 2014 to June 2021. Twenty-three patients with cerebral AVM were treated with 34 sessions of endovascular embolization with either PHIL or Squid (17 sessions each) liquid embolic agents with a male to female ratio of 2.3:1 (male 16; female 7) and mean age of 44.6 (range, 12 to 67). The mean total nidus obliteration rate per session was 57% (range, 5% to 100%). Twenty-one patients (91.3%) received further embolization, stereotactic radiosurgery, or surgical excision after initial endovascular embolization. There were 2 morbidities (1 neurological and 1 non-neurological, 6%) and no mortalities (0%). All patients had static or improvement in modified Rankin Scale at 3 to 6 months at discharge. PHIL and Squid are effective and safe liquid embolic agents for endovascular embolization of cerebral AVM, achieving satisfactory nidal obliteration rates and patient functional outcomes.
Keywords: Intracranial arteriovenous malformation, Therapeutic embolization, Ethylene-vinyl alcohol copolymer, PHIL, Squid
INTRODUCTION
Endovascular embolization is one of the treatment modalities for managing cerebral arteriovenous malformation (AVM) [1]. It is indicated for reducing nidal size before surgical resection or radiosurgery, reducing hemorrhagic risk by eliminating high-risk areas such as intranidal aneurysm, or completely obliterating the nidus of AVM. Therefore, embolization can be curative with a single session or staged embolization, or as an adjunctive procedure followed by radiosurgery or surgical excision [2,3].
Precipitating hydrophobic injectable liquid (PHIL; MicroVention, Aliso Viejo, CA, USA), a dimethyl sulfoxide (DMSO)-based agent, consists of 2 copolymers (polylactide-co-glycolide and polyhydroxyethylmethacrylate) and covalently-bonded iodine [4-6]. Squid (Balt, Irvine, CA, USA) is an ethylene vinyl alcohol copolymer (EVOH)-based agent like Onyx (Medtronic, Irvine, CA, USA), but with smaller grains of tantalum powder [7]. With promising results in several preliminary cohorts that used PHIL and Squid in cerebral AVM, we incorporated them for the embolization stream in our AVM treatment protocol since 2014.
This study is the report on the effectiveness and safety profile of the 2 newer liquid embolic agents in the embolization of cerebral AVM.
Materials and Methods
This was a retrospective cohort study on patients who underwent embolization of cerebral AVM in the Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong, from January 2014 to June 2021. Endovascular embolization with liquid embolic agents of either PHIL or Squid was included. Patient demographics including age, sex, presenting symptoms, and past medical history were reviewed. Angiographic features of cerebral AVM including Spetzler-Martin (SM) grading, nidal size, presence of deep venous drainage, location, side, and eloquence of cerebral AVM were retrieved. Operation details of each endovascular embolization session including type and amount of liquid embolic agents, type of microcatheters used, complications, morbidity, and mortality were reviewed. The study was conducted in accordance with the declaration of Helsinki and was approved by the local ethics committee (Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee CREC Ref. No. 2022.191).
Embolization Techniques
Embolization sessions were performed under general anesthesia with a biplanar angiographic unit. A 6-French (6F) or 8-French (8F) arterial sheath was placed in the right femoral artery. Diagnostic cerebral angiography was performed, and a guiding catheter and/or intermediate catheter was then inserted in either an internal carotid artery or a dominant vertebral artery using a standard coaxial or triaxial technique. Two different types of microcatheters were used in different sessions, including conventional microcatheters (Headway Duo, MicroVention; Marathon, Medtronic) and microcatheters with detachable tips (Apollo, Medtronic; Sonic, Balt). The DMSO-compatible flow-directed microcatheter (either conventional or with detachable tip) was navigated to the nidus of AVM with an aid of a 0.008-inch or 0.007-inch guidewire. Angiography was reviewed to ensure that the feeding pedicle could be occluded retrogradely by the reflux of PHIL or Squid along the microcatheter of at least 2 cm. The microcatheter was flushed with DMSO, and the dead space of the microcatheter was slowly filled with PHIL or Squid for no less than 2 minutes (2 minutes was chosen to avoid the DMSO bolus reaching the cerebral circulation). PHIL or Squid was slowly and progressively injected into the nidus under continuous visual control using biplane subtracted fluoroscopy. As soon as reflux was noted along the microcatheter or early embolization of the draining vein was evident, the injection was stopped for 30 seconds to 2 minutes and then resumed. The maximum reflux tolerated was 2 cm for non-detachable catheters and 1.5 to 5 cm for detachable catheters. After embolization of AVM, the microcatheter was retrieved and the puncture site at the femoral artery was controlled with a percutaneous vascular closure device and/or manual compression. Xper-CT (Philips Healthcare, Andover, MA, USA) was performed to ensure no intracranial hemorrhage before termination of the procedure.
Intent of Embolization
Embolization procedures were either in a single session or staged, subsequently followed by radiosurgery with an interval of around 3 months, or followed by surgery during the same session or in stages with an interval of 2 to 6 weeks. Intent of embolization and subsequent treatments, either surgical resection or radiosurgery, were decided by the attending neurosurgeons, neurointerventionists, and radiation oncologists with the patient and family.
Primary Outcomes
The nidal obliteration rate was calculated by comparing the nidal size (A×B×C/2) from images of the pre- and post-embolization biplanar digital subtraction angiogram for each embolization session by an independent assessor. The number and intent of embolization sessions were recorded. The modified Rankin Scale (mRS) was recorded at baseline, at discharge, and after 3 to 6 months from discharge.
Statistical Analysis
Patient, angiographic, and operational factors associated with good functional outcome and morbidity were analyzed with an unpaired-t-test and χ2 test with a P-value <0.05 and 95% confidence interval (95% CI) as appropriate. Logistic regression was not performed because of the limited sample size. Statistical analysis was performed with SPSS version 28.0 (IBM Corp., Armonk, NY, USA).
Case Series
Basic Demographics
Twenty-three patients with cerebral AVM were treated with 34 sessions of endovascular embolization with liquid embolic agents of either PHIL or Squid (17 sessions each) with a male to female ratio of 2.3:1 (male 16; female 7) and a mean age of 44.6 (range, 12 to 67). Most patients presented with headache (34.8%) and seizure (21.7%), while the remaining presented with focal deficits or were asymptomatic. Twelve patients (52.2%) had ruptured cerebral AVM at admission. Over half of the patients had AVM on the right side (69.6%) and a SM grade 3 or above (56.5%). Basic demographic details were summarized in Table 1.
Table 1.
Basic demographics
| Variable | PHIL group (n=9) | Squid group (n=14) |
|---|---|---|
| Sex, male | 7 (77.8) | 9 (64.3) |
| Age | 46±11.5 (27–62) | 43.6±20.1 (12–67) |
| Presenting symptoms | ||
| Headache | 5 (56) | 3 (21) |
| Seizure | 1 (11) | 4 (29) |
| Focal deficits | 1 (11) | 2 (14) |
| Asymptomatic | 0 (0) | 2 (14) |
| Others | 2 (22) | 3 (21) |
| Ruptured, yes | 6 (66.7) | 6 (42.9) |
| Side of AVM, right | 5 (55.6) | 11 (78.6) |
| Spetzler-Martin grade | ||
| 1 | 2 (22) | 3 (21) |
| 2 | 1 (11) | 4 (29) |
| 3 | 5 (56) | 7 (50) |
| 4 | 1 (11) | 0 (0) |
| 5 | 0 (0) | 0 (0) |
| Nidal size | ||
| <3 cm | 4 (44) | 6 (43) |
| 3–6 cm | 5 (56) | 8 (57) |
| >6 cm | 0 (0) | 0 (0) |
| Deep venous drainage | 4 (44) | 3 (21) |
| Eloquent area | 5 (56) | 6 (43) |
| Microcatheters | ||
| Detachable tip | 13 (46) | 15 (54) |
| Conventional | 4 (67) | 2 (33) |
Values are presented as number (%) or mean±standard deviation (range).
PHIL, precipitating hydrophobic injectable liquid; AVM, arteriovenous malformation.
Intent of Embolization
The mean number of embolization sessions per patient was 1.5, ranging from 1 to 4 sessions per patient. Each embolization session lasted for an average of 167 minutes (range, 12 to 690 minutes±133 minutes). Twenty embolization sessions (59%) were intended for single or staged embolization. Among the 7 patients receiving staged embolization sessions (range, 2 to 4 sessions), 3 patients (42.9%) had another liquid embolic agent applied at the next stage. In these patients, Squid was initially used, followed by PHIL in the subsequent embolization sessions. Nine embolization sessions (26%) were followed by surgical excision, and 7 patients (78%) had embolization followed by surgical excision on the same day. Five patients (15%) had stereotactic radiosurgery or radiotherapy after embolization, of which 3 patients had hypofractionated stereotactic radiosurgery 7 Gy per session for 4 sessions, and 2 patients had single dose stereotactic radiosurgery (Table 2).
Table 2.
Nidal obliteration rates according to different intents of embolization
| Intent of embolization | Number of sessions | Nidal obliteration rate (%) |
|
|---|---|---|---|
| Squid | PHIL | ||
| Overall | 34 (100) | 57±26 | 57±32 |
| Embolization only | 4 (12) | 75±13 | 100±0 |
| Staged embolization | 16 (47) | 42±24 | 49±29 |
| Embolization before excision | 9 (26) | 90±14 | 60±34 |
| Embolization before SRS/SRT | 5 (15) | 56±23 | N/A |
Values are presented as number (%) or mean±standard deviation.
SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy; N/A, not applicable.
Nidal Obliteration Rate
The mean total nidus obliteration rate per session was 57% (range, 5% to 100%). Complete obliteration of cerebral AVM was achieved in 4 patients (17%). One patient achieved an angiographic cure using PHIL without subsequent treatment. The remaining 3 patients underwent surgical excision after embolization (2 patients underwent embolization with PHIL; 1 underwent embolization with Squid). The nidal obliteration rate varied with different intent of embolization. The overall mean obliteration rate was 57% in both Squid and PHIL groups. Around 40–50% of nidal obliteration was achieved for staged embolization. As for other intents of embolization, a greater than 50% nidal obliteration rate was achieved (Table 2).
Functional Outcome
The functional outcome was assessed by mRS. More than 80% of patients had an mRS less than or equal to 3 at 3 to 6 months after intervention. Among patients with unruptured cerebral AVM, no differences were shown in mRS before, at discharge, and at 3 to 6 months after intervention. Factors associated with good functional outcome (i.e., an mRS less than or equal to 2 at 3 to 6 months after intervention) included male sex (odds ratio, 5.778; P=0.078; 95% CI, 0.025–1.221), unruptured cerebral AVM (odds ratio, 10; P=0.054; 95% CI, 0.957–104.490), and right-sided cerebral AVM (odds ratio, 5.778; P=0.078; 95% CI, 0.819–40.760).
Morbidity and Mortality
There were no mortalities. There were 2 morbidities (6%), 1 neurological and 1 non-neurological. A 26-year-old male underwent partial embolization for an unruptured right occipital SM grade 2 cerebral AVM with Squid 18, complicated with a right occipital infarct resulting in visual field defect. A 67-year-old female underwent embolization for ruptured left temporal SM grade 2 cerebral AVM with Squid 18, complicated with acute dissection of right common femoral artery with an emergency operation for suturing of artery performed on the next day after embolization. Factors associated with morbidity including Squid liquid embolic agent (odds ratio, 3.664; P=0.320; 95% CI, 0.284–47.253) and embolization followed by surgical excision or stereotactic radiosurgery/radiotherapy (odds ratio, 5.088; P=0.204; 95% CI, 0.413–62.733) were not statistically significant.
Case Illustration
Case 1
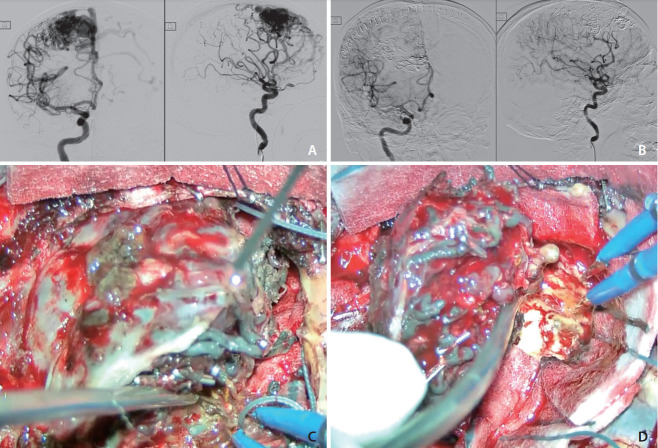
An early elderly patient presented with headache and left-sided weakness. Brain computed tomography (CT) showed an intracranial hemorrhage over the right frontal region. He was found to have a ruptured SM grade 1 AVM with a nidal size of 2 cm, arterial supply from cortical branches of the right anterior cerebral artery and right middle cerebral artery, and venous drainage to the superior sagittal sinus. Subsequently, a 64% nidal obliteration rate was achieved with 3 mL Squid 18 low density (LD) and 0.4 mL Squid 12 LD in the first session of embolization in March 2014. An 80% nidal obliteration was achieved with 3 mL Squid 18 and 4.5 mL Squid 18 LD in the second session of embolization in May 2014. A total of 92.8% nidal obliteration was achieved. Craniotomy for total excision of right frontal AVM was performed in the same session after the second embolization (Fig. 1).
Fig. 1.
(A) Digital subtraction angiogram (DSA) before embolization, anterioposterior (AP) and lateral views. (B) DSA after embolizations and surgical excision, AP and lateral views. (C) Embolized vessels by Squid appeared black due to the admixed tantalum powders. (D) Circumferential dissection during surgical excision.
Case 2
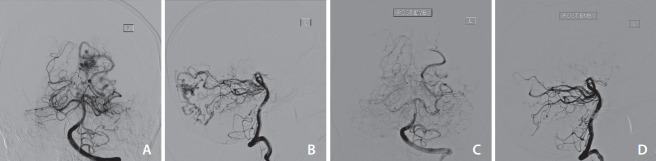
A middle-aged patient presented with sudden onset of severe headache, diplopia, and vomiting. Brain CT showed an intracranial hemorrhage and a subarachnoid hemorrhage over the medial left occipital region. He was found to have a ruptured SM grade 3 left occipital AVM with a nidal size of 1.6 cm×1.6 cm×2.5 cm, arterial drainage from left posterior cerebral artery, and venous drainage to straight sinus, left transverse sinus, and superior sagittal sinus. Embolization with 1 mL PHIL via left posterior cerebral artery feeder was performed in November 2019. A complete angiographic cure was achieved and his mRS improved from 2 (before the procedure) to 0 (3 to 6 months after the procedure). Progress magnetic resonance arteriography at 1 year after embolization showed no residual AVM (Fig. 2).
Fig. 2.
(A, B) Pre-embolization digital subtraction angiogram (DSA), anterioposterior (AP) and lateral views respectively. (C, D) Post-embolization DSA, AP/lateral views respectively.
DISCUSSION
Embolization of cerebral AVM with liquid embolic agent PHIL and Squid is effective in achieving a satisfactory nidal obliteration rate with a complete obliteration rate of 17% and a mean nidal obliteration rate per session of 57%. Embolization can be used in selected cases for embolization cure or as an adjunct to surgery or radiosurgery, as shown in around 40% of all the embolization sessions in this study [8,9].
Characteristics of PHIL and Squid
PHIL and Squid are newer non-adhesive liquid embolic agents for embolization of cerebral AVM. Squid has smaller-sized grains of tantalum powder that enhance the homogeneity in radiopacity, allow slower precipitation, and improve visibility during a longer injection time [10]. PHIL has a constant radiopacity over time due to the covalently-bonded iodine copolymer when compared with Squid or Onyx, in which their sedimentation can disturb the visibility during a longer injection time. Squid will have a lava-like filling of vessels in which the opacified blood vessels may still be centrally perfused, while PHIL completely occluded the vessels in the column [4].
Imaging Artifacts, Visibility, and Viscosity of PHIL and Squid
Imaging artifacts are one of the obstacles for liquid embolic agents in embolization of cerebral AVM, which may hinder the detection of periprocedural hemorrhage and subsequent planning of radiosurgery. Both Onyx and Squid are EVOH-based copolymers using tantalum powder as a source of radiopacity on imaging. The higher atomic number of tantalum (atomic number 73) in Onyx and Squid compared to iodine (atomic number 53) in PHIL leads to the generation of more imaging artifacts. However, Squid has a smaller grain size of micronized tantalum powder compared with Onyx which improves the distribution of tantalum particles within a defined volume, thus enhancing homogeneity in radiopacity and reducing X-ray diffraction and imaging artifacts. Also, Squid has a prolonged suspension time due to smaller tantalum particles, which may improve visibility during a longer injection time. According to a 3-dimensional in vitro AVM model resembling partially embolized AVM, iodine-based liquid embolic agent PHIL 25% produces fewer imaging artifacts compared to tantalum-based Onyx 18 and Squid 18 in CT and cone-beam CT [11]. Apart from the use of PHIL and Squid, metal artifact reduction (MAR) software has also shown a significant reduction in imaging artifacts by more than half of the density unit (DU) (38 DU without MAR vs. 18 DU with MAR) in both in vitro and in vivo analysis for embolization with Onyx 18 [12]. Viscosity is another determining factor in embolization performance. Optimal fluoroscopic visualization, less volume, and shorter embolization time were demonstrated with PHIL low viscosity compared with Squid 12 in the in vivo swine rete model [13].
Neurological Complications Associated with PHIL and Squid Embolization
The multicenter randomized controlled trial a randomized trial of unruptured brain arteriovenous malformations (ARUBA) comparing medical treatment and interventional treatment on unruptured cerebral AVM demonstrated that the primary outcome of composite death of all causes or symptomatic stroke was as high as 30.7% in the interventional group when compared with the medical management group (10.1%) [14]. Half of the patients in the interventional group received embolization alone or multimodality treatment including embolization. However, it was not certain how much of the poor outcome was due to embolization alone or other treatment modalities or was due to delayed hemorrhage of cerebral AVM. A recent meta-analysis on embolization for cerebral AVM showed a lower rate of poor neurological outcome of 5.2% and 6.8% for glue and Onyx, respectively [15]. The permanent morbidity after embolization with glue and Onyx in the literature ranges from 1.6% to 11% [16-20], which was 3% in our cohort.
PHIL and Squid are safe alternative options of liquid embolic agents aside from glue and Onyx for embolization of cerebral AVM. Lower morbidity was shown in a case series using PHIL and Squid for embolization. A Bulgarian cohort of 27 patients with embolization using PHIL showed a complication rate of 22.2% and permanent morbidity of 3.7% due to intracranial hemorrhage requiring surgical evacuation [21]. A Turkish series of 28 patients undergoing embolization with Squid showed a complication rate of 12.5% and permanent morbidity of 6.3%, also due to intracranial hemorrhage requiring surgical evacuation [7]. In the present series, the total neurological and non-neurological morbidity was 6%, in which no hemorrhagic complications were reported. Only 1 case of permanent morbidity (3%) with visual field defect occurred due to occipital lobe infarct after embolization. Although both cases of morbidity involved Squid embolization of cerebral AVM, there was no statistically significant difference between PHIL and Squid embolization in terms of morbidity.
Nidal Obliteration Rates
Since the first report of embolization of cerebral AVM in 1960 by Luessenhop and Spence with the use of methylmethacrylate embolospheres, and later with the wide use of N-butyl cyanoacrylate (NBCA) in the 1990s, embolization was used as a pre-operative adjunct to facilitate cerebral AVM resection [22]. After the introduction of EVOH Onyx in 2004, there was an improvement in the nidal obliteration rate. Therefore, there were more indications for embolization such as pre-radiosurgery volume reduction, treatment of nidal aneurysm, and curative intent of the AVM. A meta-analysis of 103 eligible studies demonstrated a higher complete nidal obliteration rate for the EVOH group when compared with the NBCA group (30.5% vs. 13.7% respectively) and an improvement in obliteration rate over time [15]. It was also similarly shown to achieve a 33% complete angiographic cure by embolization with Onyx in our center [23]. Among the retrospective studies on liquid embolic agents PHIL and Squid, the complete obliteration rate ranged from 0–60%, which had a wide variability [5-7, 21]. In one of the case series on preliminary experience on PHIL, 3 patients underwent partial embolization with a nidal obliteration rate of 70–90% followed by surgical excision on the same day, achieving a satisfactory outcome [5]. The difference in nidal obliteration rate could be attributed to the different intents of embolization and different angiographic features of cerebral AVM as well as more surgical treatments in selected ruptured SM grade 1 cerebral AVMs. In the current cohort with options of multi-modality treatment, the complete nidal obliteration rate was 17% with embolization alone, and the overall nidal obliteration rate at last follow-up was 90%. A literature review of embolization of cerebral AVM with PHIL and Squid is summarized in Table 3 [4-6,14].
Table 3.
Literature review of embolization of cerebral AVM with PHIL and Squid liquid embolic agents
| Study | Patient (n) | Agent | Complete obliteration rate | Complication rate |
|---|---|---|---|---|
| Sirakov et al. [21] (2019) | 27 | PHIL 25 | 52% | 22.2% |
| Koçer et al. [5] (2016) | 3 | PHIL | 0% (mean obliteration 80%) | 0% |
| Akmangit et al. [7] (2014) | 16 | Squid 18 | 37.5% | 12.5% (intracranial hemorrhage) |
| Squid 12 | ||||
| Samaniego et al. [6] (2016) | 5 | PHIL | 60% | 0% |
AVM, arteriovenous malformation; PHIL, precipitating hydrophobic injectable liquid.
Study Limitations
First, the sample size of this study was small but comparable with the case series on PHIL or Squid liquid embolic agents in the literature [7,21]. Second, different intents of embolization (either pre-surgical or curative) and selection of liquid embolic agents could affect the nidal obliteration rate. Third, the use of different types of microcatheters, either the conventional type or those with a detachable tip, may affect the injection of liquid embolic agents and thus the nidal obliteration rate. According to a case series using Apollo detachable microcatheters for embolization of cerebral AVM with NBCA or Onyx, microcatheter tip detachment was a significant factor associated with a high obliteration rate [24]. Fourth, the pressure cooker technique was not applied in this series although a case series has reported a complete angiographic cure rate of up to 96% in selected cases [25,26]. Fifth, the measurement of nidal volume of cerebral AVM was not a precise 3-dimensional volume measurement with multiple independent assessors, causing potential information bias. Further studies with a larger number of patients and adjustment of potential biases are required to investigate these aspects.
In conclusion, PHIL and Squid are effective and safe liquid embolic agents for endovascular embolization of cerebral AVM, achieving satisfactory nidal obliteration rates and functional outcomes.
Footnotes
Fund
None.
Ethics Statement
The study was conducted in accordance with the declaration of Helsinki and was approved by the local ethics committee (Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee CRE Ref. No. 2022.191). Written informed consent was waived. We anonymized the patient information that may identify the patient because informed consent for publication is unavailable.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: GKCW. Analysis and interpretation: EYHC and GKCW. Data collection: EYHC. Writing the article: EYHC. Critical revision of the article: EYHC, RYTN, SCHY, JTFZ, and GKCW. Final approval of the article: EYHC, RYTN, SCHY, JTFZ, and GKCW. Statistical analysis: EYHC. Obtained funding: GKCW. Overall responsibility: GKCW.
REFERENCES
- 1.Pierot L, Cognard C, Herbreteau D, Fransen H, van Rooij WJ, Boccardi E, et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: results of a prospective, multicentre study (BRAVO) Eur Radiol. 2013;23:2838–2845. doi: 10.1007/s00330-013-2870-6. [DOI] [PubMed] [Google Scholar]
- 2.Lawton MT, Rutledge WC, Kim H, Stapf C, Whitehead KJ, Li DY, et al. Brain arteriovenous malformations. Nat Rev Dis Primers. 2015;1:15008. doi: 10.1038/nrdp.2015.8. [DOI] [PubMed] [Google Scholar]
- 3.Chan DYC, Chan DTM, Zhu CXL, Kan PKY, Ng AY, Hsieh YS, et al. Awake craniotomy for excision of arteriovenous malformations? A qualitative comparison study with stereotactic radiosurgery. J Clin Neurosci. 2018;51:52–56. doi: 10.1016/j.jocn.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Vollherbst DF, Chapot R, Bendszus M, Möhlenbruch MA. Glue, onyx, squid or PHIL? Liquid embolic agents for the embolization of cerebral arteriovenous malformations and dural arteriovenous fistulas. Clin Neuroradiol. 2022;32:25–38. doi: 10.1007/s00062-021-01066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koçer N, Hanımoğlu H, Batur Ş, Kandemirli SG, Kızılkılıç O, Sanus Z, et al. Preliminary experience with precipitating hydrophobic injectable liquid in brain arteriovenous malformations. Diagn Interv Radiol. 2016;22:184–189. doi: 10.5152/dir.2015.15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samaniego EA, Kalousek V, Abdo G, Ortega-Gutierrez S. Preliminary experience with precipitating hydrophobic injectable liquid (PHIL) in treating cerebral AVMs. J Neurointerv Surg. 2016;8:1253–1255. doi: 10.1136/neurintsurg-2015-012210. [DOI] [PubMed] [Google Scholar]
- 7.Akmangit I, Daglioglu E, Kaya T, Alagoz F, Sahinoglu M, Peker A, et al. Preliminary experience with squid: a new liquid embolizing agent for AVM, AV fistulas and tumors. Turk Neurosurg. 2014;24:565–570. doi: 10.5137/1019-5149.JTN.11179-14.0. [DOI] [PubMed] [Google Scholar]
- 8.Tam AKY, Chan DYC, Lim K, Poon D, Kam M, Cheung M, et al. Long term treatment efficacy & complications of hypofractionated stereotactic radiosurgery in brain arteriovenous malformations. J Clin Neurosci. 2020;82(Pt B):241–246. doi: 10.1016/j.jocn.2020.10.057. [DOI] [PubMed] [Google Scholar]
- 9.Wong GK, Kam MK, Chiu SK, Lam JM, Leung CH, Ng DW, et al. Validation of the modified radiosurgery-based arteriovenous malformation score in a linear accelerator radiosurgery experience in Hong Kong. J Clin Neurosci. 2012;19:1252–1254. doi: 10.1016/j.jocn.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Mason JR, Dodge C, Benndorf G. Quantification of tantalum sedimentation rates in liquid embolic agents. Interv Neuroradiol. 2018;24:574–579. doi: 10.1177/1591019918773443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt N, Floca RO, Paech D, El Shafie RA, Neuberger U, Bendszus M, et al. Imaging artifacts of nonadhesive liquid embolic agents in conventional and cone-beam CT in a novel in vitro AVM model. Clin Neuroradiol. 2021;31:1141–1148. doi: 10.1007/s00062-021-01013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt N, Weyland CS, Wucherpfennig L, Sommer CM, Bendszus M, Möhlenbruch MA, et al. The impact of software-based metal artifact reduction on the liquid embolic agent onyx in cone-beam CT: a systematic in vitro and in vivo study. J Neurointerv Surg. 2022;14:832–836. doi: 10.1136/neurintsurg-2021-018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samaniego EA, Derdeyn CP, Hayakawa M, Hasan D, OrtegaGutierrez S. In vivo evaluation of the new PHIL low viscosity in a swine rete mirabile model. Interv Neuroradiol. 2018;24:706–712. doi: 10.1177/1591019918784915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. international ARUBA investigators Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsenousi A, Aletich VA, Alaraj A. Neurological outcomes and cure rates of embolization of brain arteriovenous malformations with n-butyl cyanoacrylate or onyx: a meta-analysis. J Neurointerv Surg. 2016;8:265–272. doi: 10.1136/neurintsurg-2014-011427. [DOI] [PubMed] [Google Scholar]
- 16.Jayaraman MV, Marcellus ML, Hamilton S, Do HM, Campbell D, Chang SD, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol. 2008;29:242–246. doi: 10.3174/ajnr.A0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor CL, Dutton K, Rappard G, Pride GL, Replogle R, Purdy PD, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810–812. doi: 10.3171/jns.2004.100.5.0810. [DOI] [PubMed] [Google Scholar]
- 18.Ledezma CJ, Hoh BL, Carter BS, Pryor JC, Putman CM, Ogilvy CS. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58:602–611. doi: 10.1227/01.NEU.0000204103.91793.77. discussion 602-611. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann A, Pile-Spellman J, Stapf C, Sciacca RR, Faulstich A, Mohr JP, et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33:1816–1820. doi: 10.1161/01.str.0000020123.80940.b2. [DOI] [PubMed] [Google Scholar]
- 20.Haw CS, terBrugge K, Willinsky R, Tomlinson G. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104:226–232. doi: 10.3171/jns.2006.104.2.226. [DOI] [PubMed] [Google Scholar]
- 21.Sirakov SS, Sirakov A, Minkin K, Hristov H, Ninov K, Penkov M, et al. Initial experience with precipitating hydrophobic injectable liquid in cerebral arteriovenous malformations. Interv Neuroradiol. 2019;25:58–65. doi: 10.1177/1591019918798808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luessenhop AJ, Spence WT. Artificial embolization of cerebral arteries. Report of use in a case of arteriovenous malformation. J Am Med Assoc. 1960;172:1153–1155. doi: 10.1001/jama.1960.63020110001009. [DOI] [PubMed] [Google Scholar]
- 23.Wong GK, Yu SC, Zhu XL, Kam MK, Poon WS. Use of onyx (a patented ethylene-vinyl alcohol copolymer formulation) embolisation of cerebral arteriovenous malformations in Hong Kong: initial experience. Hong Kong Med J. 2009;15:359–364. [PubMed] [Google Scholar]
- 24.Flores BC, See AP, Weiner GM, Jankowitz BT, Ducruet AF, Albuquerque FC. Use of the Apollo detachable-tip microcatheter for endovascular embolization of arteriovenous malformations and arteriovenous fistulas. J Neurosurg. 2018;130:963–971. doi: 10.3171/2017.9.JNS17397. [DOI] [PubMed] [Google Scholar]
- 25.Mendes GAC, Kalani MYS, Iosif C, Lucena AF, Carvalho R, Saleme S, et al. Transvenous curative embolization of cerebral arteriovenous malformations: a prospective cohort study. Neurosurgery. 2018;83:957–964. doi: 10.1093/neuros/nyx581. [DOI] [PubMed] [Google Scholar]
- 26.Koyanagi M, Mosimann PJ, Nordmeyer H, Heddier M, Krause J, Narata AP, et al. The transvenous retrograde pressure cooker technique for the curative embolization of high-grade brain arteriovenous malformations. J Neurointerv Surg. 2021;13:637–641. doi: 10.1136/neurintsurg-2020-016566. [DOI] [PubMed] [Google Scholar]