Summary
The use of spectroscopy to characterize electrocatalytic processes is vital to the understanding and continuing the development of new catalysts for clean energy transformations. Electron paramagnetic resonance spectroscopy (EPR), which allows for the study of unpaired electron spins, shows great fundamental promise for the study of electrocatalysts, but was previously hindered by design limitations. Recently, several groups have demonstrated that these limitations can be overcome, providing valuable understandings of electrocatalyst function that other techniques are less suitable for. In this review, we summarize these findings across a range of experimental approaches and systems and describe the importance of EPR to each of these studies. By providing outlines for how these studies were able to overcome experimental design challenges, we hope to provide insight into potentially interested users.
Subject areas: Chemistry, Materials science, Materials chemistry
Graphical abstract

Chemistry; Materials science; Materials chemistry
Introduction
Energy storage is a key aspect in the implementation of green energy as it allows for carbon-neutral electricity to be kept and utilized at will. Electrochemical processes figure prominently in the energy storage solutions, such as artificial fuels and batteries. Toward the development of new materials and devices, spectroscopy plays an important role as it allows for phenomena to be correlated with specific physical events. One such technique is electron paramagnetic resonance (EPR) spectroscopy, which is used to study unpaired electron spins. EPR has so far been used in studying operando electrolyte phenomena in redox flow batteries1, 2, 3 or fuel cell membranes,4,5 but its utility in studying conductive solid-state materials such as electrocatalysts remains limited.
EPR can be challenging to perform for electrochemical studies in the presence of conductors and high-dielectric electrolytes such as water, which both can affect the quality of the spectroscopic signal. Although these contradictions can prove debilitating to data collection, demand for new spectroscopic understandings of catalytic systems has fueled interest in how such challenges can be overcome. In this review, we provide an overview of EPR, the potential benefits of using EPR for characterizing electrocatalysts, how challenges in carrying out solid-state electrochemical EPR have been successfully overcome, and some of the recent insights that EPR has provided toward electrocatalytic materials in several key systems.
Overview of solid-state electron paramagnetic resonance spectroscopy
EPR spectroscopy is used to study the nature of unpaired (paramagnetic) electrons in a given system, based on transitions between their spin states. Since electrons are charged particles, their natural spins (often denoted as +1/2 (high spin) and –½ (low spin)) generate magnetic fields. Electrons can transition between spin states with the application of energy. Furthermore, the interaction of an electron’s magnetic field with an external magnetic field can change the energy required to induce a spin transition, a principle known as the Zeeman Effect (Figure 1), named after Pieter Zeeman who first observed that the absorption spectrum of a sodium flame splits in the presence of a magnetic field. EPR spectroscopy enables the user to measure the energy required to induce spin transitions under a given magnetic field strength, with the required energy being characteristic of a specific paramagnetic species.
Figure 1.
The Zeeman effect
As the strength of a magnetic field is increased, the energy required to transition between spin states is also increased. The listed band frequencies are the operating frequencies under which EPR at each band is usually undertaken.
EPR is often compared to its more well-known cousin, nuclear magnetic resonance (NMR) spectroscopy, which is focused on resolving nuclear spins. The relative sensitivities of the two techniques can be compared using the Larmor equation:
Where ν is the frequency of the photon released (or absorbed), γ is the native gyromagnetic ratio of the particle of interest, and B0 is the magnetic field. Since an atom’s gyromagnetic ratio is directly proportional to the energy of the released (or absorbed) photon, and γ for an electron is three orders of magnitude greater than that of most nuclei, EPR is much more sensitive than NMR. This sensitivity makes EPR ideal for studying species at low concentrations such as in surface studies,6 which would include solid-state electrocatalysis.
As with NMR, the Larmor equation also points to increased magnetic field strength as another source of improved spectral resolution and sensitivity. However, unlike the gyromagnetic ratio, which is an innate property of a particle, magnetic field strength can be achieved with increasingly advanced infrastructure. As shown in Figure 1, the four most commonly used bands of microwave radiation for EPR are the S, X, Q, and W bands, with typical working frequencies ranging from 2 GHz (S-band) up to 110 GHz (W-band). Most published EPR studies are carried out using standard X-band spectrometers.
In practice, EPR spectrometers measure emitted microwaves as the applied magnetic field is varied. By measuring the magnetic field at which spin transitions take place, EPR can be used to calculate the g-factor, a unitless value that is characteristic of the paramagnetic oxidation state. For example, free electrons have an ideal isotropic g-factor of 2.0023.7 However, the EPR spectrum of any paramagnetic species undergoes variation due to the interaction of the electron with the surrounding environment. The primary source of variation is the nuclei with which the paramagnetic electron is associated with, allowing EPR to be used for the specific identification of oxidation states. In solid-state systems, where rotational degrees of freedom are finite, EPR spectra are further split into different g tensors, which are often described by a 3 × 3 matrix representing a three-dimensional coordinate system. The tensors arise as a result of interactions between the orientations of electron spins with the physically constrained solid crystal field as well as the applied magnetic field. Sometimes the EPR spectra of electrocatalysts in the solid state remain isotropic, often when the environment of the paramagnetic species retains a highly symmetric crystal field (i.e., tetrahedral, octahedral species with highly equivalent ligands). However, if the crystal field is elongated in one direction (i.e., axial), the spectra will split into perpendicular (g⊥) and parallel tensors (gװ). Finally, if the tensors have low symmetry, the spectrum will split into three separate tensors (i.e., rhombic) denoted by their coordinate direction (gx, gy, gz). The separation distance of the tensors in the EPR spectrum gives rise to the symmetry factor, which describes the overall symmetry of the paramagnetic center. For reference, a list of g-factors in electrocatalytic systems discussed in this review is provided in Table 1.
Table 1.
Common g-factors found in electrocatalysts as discussed in this review
The next important feature of EPR spectra is their line widths, which originate from the relaxation times of the electron spins. Relaxation time refers to the time that it takes for electron spins to reach equilibrium upon entering a state of disequilibrium. There are two types of relaxation time in EPR: spin-lattice or longitudinal relaxation (T1), and spin-spin or transverse relaxation (T2). T1 refers to the time that electron spins take to relax from a higher energy spin state back to the ground state upon removal of an applied magnetic field. While in the magnetic field, spins are aligned parallel to the magnetic field (or z direction), but upon removal of the magnetic field, these spins gradually return to a lower energy spin state, with the excess energy dissipating through the surrounding lattice structure. T2 originates from exchanges between nearby electrons with opposite spins; these spins can exchange their spins with each other. The time that it takes for these spins to exchange constitutes T2. These spins are not aligned with the applied magnetic field and hence are referred to as transverse as they exist within the transverse plane of the magnetic field (the x/y directions). For both types of relaxations, shorter relaxation times translate into broader linewidths due to the increase in uncertainty of the electron spin state. Solid-state EPR spectra are significantly broader than liquid-state spectra due to the increased proximity and interactions with neighboring spins; as a result, T2 is much shorter, resulting in linewidth broadening.
Electrons can also interact with spin-active nuclei, resulting in hyperfine coupling of a spectrum. The magnitude of a hyperfine interaction is quantifiable; furthermore, since each tensor has a vector component, different magnitudes of interaction can be applied to each tensor. The ultimate result is that, if the hyperfine interactions are large enough, the tensor of interest will undergo broadening and ultimately splitting. Dipole-dipole interactions between different unpaired electrons can result in another type of splitting known as zero-field splitting. Zero-field splitting takes place as a result of the presence of multiple, interacting unpaired electrons around a single nucleus, resulting in the existence of an additional, field-independent energy level that can be represented in a Zeeman diagram. Spins can transition between this field-independent energy level as well as the field-dependent energy levels, resulting in new splittings in an EPR spectrum. However, zero-field splitting is often extremely broad and difficult to detect, except at low temperatures. Furthermore, in the examples discussed here, there have been no demonstrated examples of zero-field splitting as the spectra arising from paramagnetic species have been more detectable and useful to electrocatalyst studies thus far.
Given these complications, a common misconception about g-factors is that they are constant. Indeed, our inclusion of Table 1 seems to encourage this belief. However, although the value of g-factors is heavily dominated by the associated paramagnetic oxidation state that they are associated with, the presence of the variables discussed above means that significant variation can take place. The primary example is how tensor splitting results in an ordinarily isotropic g-factor splitting into multi-dimensional tensors, but even linewidth broadening or the presence of multiple paramagnetic species can appear to vary the positions of collected EPR data. To assist in the interpretation and modeling of potentially complex data, open source tools such as EasySpin (a MATLAB toolbox) and SimLabel (a user-friendly interface version of EasySpin for non-MATLAB users),17 can be quickly learned even by inexperienced users to model output signals with ease.18 Realistically, given the challenges of interpreting EPR data, simulations are the ideal benchmark for analyzing and comparing EPR data. Finally, since this review is directed toward non-users, we recommend the introductory text by Chechik et al.19
Experimental setups for studying electrocatalysts by electron paramagnetic resonance spectroscopy
Paramagnetic species are central to catalysis due to the outsized role that unpaired electrons have in determining chemical reactivity.20 Since electrochemistry often involves the direct generation of charged species by sequential electron transfer, EPR and electrocatalysis can be quite complimentary. As with any liquid-based EPR system, practical applications are complicated by the absorption of microwaves by high-dielectric electrolytes. Electrolytes by definition have high-dielectric constants; traditionally, this issue could be overcome by the use of flat cells to minimize the cross-section of the electrolyte, minimizing dielectric loss.21, 22, 23 Electrochemical intermediates could then be studied by placing two electrodes on opposite ends of the cell, outside the cavity, such that generated species diffuse through the cavity during analysis. This setup is often used to study liquid-phase paramagnetic electrochemical intermediates (Figure 2A).
Figure 2.
EPR cell configurations used for electrochemical studies described in this review
In order to study a solid electrocatalyst material by EPR, however, the electrode itself must be placed within the cavity in the direct path of the magnetic field. As conductors, electrode materials reflect electromagnetic radiation including microwaves. In effect, a shielded sample cannot be locked onto by the spectrometer, and spectra cannot be obtained. Conductive EPR is only possible if the amount of sample in the spectrometer is minimized such that the shielding is not overwhelming. Hence the earliest studies of conductive, paramagnetic metals were performed by dispersing microparticles in a low dielectric medium,24 or reducing the cross-section of the sample in the cavity.25,26 In electrochemical settings, the combination of both spatially constrained electrodes and electrolytes further creates diffusion limitations that vastly increase the resistance of the cell, requiring the user to use both a good potentiostat and carefully mind cell design. There are also reports of placing wire electrodes within the cavity to study reactions of soluble intermediates at or between electrodes.27,28 The success of these studies arises from the use of thin wire electrodes, which have a minimal cross-section, but for studying solid-state catalysts, the lack of material presence in the cavity would ultimately minimize already weak signal.
Consequently, ex situ measurements, where a reaction is quenched before analysis, have utility for EPR assuming the active state can be successfully trapped (Figure 2B). However, ex situ electrochemical EPR studies of aqueous-based catalysts where freeze-quenching is utilized, benefit from the fact that ice does not absorb microwaves, greatly reducing electrolyte dielectric loss. As a result, cell design is less stringent and standard quartz EPR tubes can be used for analysis. Furthermore, EPR is more sensitive at lower temperatures due to the longer relaxation time of paramagnetic species. On the other hand, in situ measurements can be carried out under experimental applied potentials in non-operational electrolytes (such as organic solvents) particularly in reactions where water (a high-dielectric electrolyte) is involved (Figure 2C).29 Organic electrolytes have recently demonstrated great value in studying aqueous reactions specifically as they do not interact with catalyst materials under operational potentials, making it possible to study electrocatalysts in only the presence of an applied potenital.8,30,31 The primary downside of organic electrolytes is their poorer conductivities, making it difficult to apply current without overloading the potentiostat. To circumvent higher electrolyte resistance, higher concentrations of the electrolyte can be used, and the reference electrode should be positioned as close to the working electrode as possible without being in the cavity. Finally, operando measurements are in theory the most ideal measurements as they capture a system in its natural state (Figure 2D). However, they also tend to be the most challenging for aqueous-based reactions for the reasons described above. Fortunately, the high conductivity of aqueous electrolytes often means that the electrochemical setup is often easier than for in situ measurements. As a summary, the general characteristics of each setup are listed in Table 2.
Table 2.
Comparison of different setups of EPR
| Setup | Ex situ | In situ | Operando |
|---|---|---|---|
| Sensitivity | Highest | Moderate | Lowest |
| Captures catalyst behavior? | Variable | Partial | Full |
| Ease of setup (electrochemical) | Easiest | Hardest | Moderate |
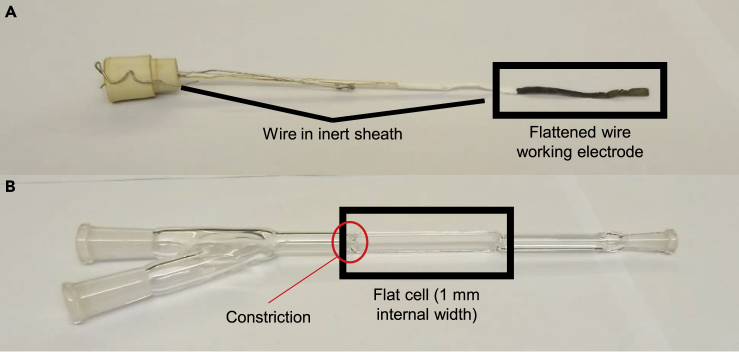
The two most important parts of any design are the cell that will go inside the cavity and the working electrode that will go inside the cell. To maximize signal while minimizing dielectric loss, the electrode must have as large an area as possible while being as thin as possible. For example, flat metal wires or thin films sputtered on flat EPR-silent substrates can both be effective. The flat section of the electrode must then fit inside the cell, which itself will go within the cavity of the instrument. The other parts of the electrode should be sheathed with inert material, i.e. glass, Teflon, solvent-resistant epoxy, to prevent interactions between the electrode and electrolyte.
For the cell, the most important component is the section that fits within the spectrometer. In situ and operando measurements are best carried out in flat cells but may not be needed depending on the electrolyte. The goal of the flat cell is, like with the flattened wire, to reduce the cross-section of the electrolyte in the EPR cavity. Additional ports attached to the flat cell are also needed to interface counter and reference (if needed) electrodes, but since these will be outside the cavity, their specific design is less stringent as long as they follow basic electrochemical principles, i.e. allowing for relative proximity to the working electrode. For ex situ aqueous measurements, cell design is less relevant as ice has a low microwave absorption profile.
Since flat cells are generally the most difficult part to home-make, oftentimes it is most convenient to acquire one commercially from EPR cell vendors such as Wilmad Labglass (Figure 3). The common electrochemical cell, which is designed for liquid-phase measurements, can be easily used for solid-state measurements as well. However, the commercial version has a constriction between the flat cell and the top of the port, which can hinder electrocatalytic experiments as diffusion from the electrode within the flat cell to the reference electrode is constricted. This constriction can be avoided in a custom cell to improve conductivity.
Figure 3.
Cell design considerations for operando and in situ EPR
The two most important components, as they fit within the cavity, are marked and labeled with a black rectangle.
(A) The working electrode for EPR measurements, here consisting of a gold wire flattened on one end to fit within a 30 mm quartz flat cell.
(B) Wilmad-LabGlass quartz electrochemical EPR cell used for cavity experiments in conjunction with a flat wire.
Electron paramagnetic resonance spectroscopy compared to other techniques
Given these complications, it is important to emphasize why EPR is still valuable in providing information on solid-state electrocatalysts. As discussed above, the unique properties of EPR make it notably available, sensitive, and informative in the study of paramagnetic atoms (Table 3). Two other techniques that are often utilized to provide similar information are X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS). Although both techniques can be used to study non-paramagnetic species, they also have higher limits of detection in the single-digit atomic percentage range. Both techniques also collect element-specific data in a sample and can therefore be limited in their abilities to study oxidation states of interest. EPR in contrast has ppm level sensitivity and is focused on paramagnetic oxidation states.
Table 3.
EPR compared to other techniques
| Technique | EPR | XPS | XAS |
|---|---|---|---|
| Sensitivity | ppm | % | % |
| Availability | Relatively common |
Ex situ – common In situ/Operando – rare |
Limited mostly to synchrotron facilites |
| Target atoms | Paramagnetic atoms or vacancies on atoms | All elements | All elements |
However, while the relative sensitivity of EPR is impressive, it is ultimately the availability of EPR that greatly distinguishes it from the other two. XAS is typically limited to synchrotron facilities, and the time constraints in such facilities often means that specialized, surface-sensitive configurations are less accessible to common users. XPS is surface-sensitive, but using it for in situ or operando studies requires complicated equipment configurations to achieve ambient conditions.32 In contrast, in the combined experiences of the authors across institutions around the world, standard X-band EPR spectrometers are more accessible than competing techniques and faster to measure due to its lower cost and easier infrastructure requirements.
On a final note, EPR has the capability to study the coordination of paramagnetic atoms that other techniques would find difficult, such as vacancies or protons. Vacancies in particular have notable EPR spectra since the loss of a coordinating atom automatically leaves a free electron on a paramagnetic center—something that not all techniques are effective at.33 Considering that vacancies are where reactants are often coordinated to catalytic centers, EPR has a unique role in providing basic catalyst characterization.
Electron paramagnetic resonance spectroscopy studies of battery and supercapacitor electrodes
EPR techniques have proven successful in studying battery systems via both in situ and operando setups,34 which is unsurprising given that the paramagnetic metals that serve as anodes have long been studied by EPR.24,35, 36, 37, 38, 39, 40 Since EPR can be used to study the coordination environment of paramagnetic atoms, it can be used as a measure of the porosity of alkali metal deposits in batteries that contribute to battery degradation35, 36, 37 or how Li interacts with electrode materials.39,40 Transition metal oxidation states common in electrode materials are also good targets for EPR, and paramagnetic species have been reported for Ru-,41 V-,42,43 Co-,44,45 Mo-,46 Mn/Ni-,47, 48, 49, 50, 51 and Cu-containing52,53 electrodes. EPR can also be used to examine the dissolution of transition metal components of battery electrodes during operating conditions.54,55
The use of EPR for studying batteries has been more prolific than for electrocatalysts because the electrolyte is typically confined into a semi-solid thin film within the EPR cavity, minimizing dielectric loss. Similarly, studies of carbon-based supercapacitors minimize dielectric loss under operando conditions through the use of an electrode setup that fits into a 1 mm normal quartz EPR capillary.56, 57, 58 Supercapacitor studies are benefited as they mostly study free electrons; the broader nature of paramagnetic species in transition metal electrocatalysts requires greater surface area. Electrolyte confinement can be utilized for electrocatalysts through the use of membrane-based EPR cells,4,59 which have to this point mostly been used to study membrane behavior. It remains to be seen whether or not an operando fuel cell-style cell could be of use to study the catalysts themselves under operando conditions, or if the presence of decomposition products from the membrane would overwhelm potential signals. However, these cells have the advantage that catalysts can be studied under ultimate commercial conditions.
Electron paramagnetic resonance spectroscopy for solid-state electrocatalyst characterization
Solid-state spectroscopy of electrocatalytic materials is a relatively new field of study that has been necessitated by recent interest in electrochemical-based fuel systems, especially those reactions critical to fuel cells and electrolyzers. In the following section, we discuss a number of approaches that have been reported for the characterization of electrocatalytic systems via EPR with a focus on three general topics—the characterization of paramagnetic oxidation states, the local environment of a given paramagnetic oxidation state, and the quantification of vacancies (Figure 4). These three topics are ideal for EPR as they rely on the strengths of EPR in order to function properly. In the case of the first two topics, the species of interest must be paramagnetic, whereas vacancies by nature are paramagnetic. Although limiting, this drawback does not necessarily stop the useful collection of data by EPR.
Figure 4.
Conceptual diagram of three topics that EPR is excellent for studying in solid-state electrocatalysts
Examination of catalytic paramagnetic oxidation states – Co O2 evolution reaction catalysts
Co-containing electrodes are of interest to electrochemistry not only as battery cathodes but also as water oxidation catalysts. Amorphous electrodeposited Co oxide films deposited from phosphate (CoPi) and borate (CoBi) electrolytes display some of the highest reported activities for the O2 evolution reaction (OER) for non-noble metals.60,61 As these films lack any long-range structural order that could be the basis for activity, studies into the molecular origins for their catalytic activity are important not only for fundamental reasons but also for the design of new catalyst materials. Initial ex situ EPR studies of these films were aided by the assumed persistence of Co oxidation states during freeze-quenching, allowing thick films of prepared material to be manually delaminated and loaded into EPR tubes.12,13 In both studies, Co2+ species were found to disappear in favor of Co4+ prior to the onset of the OER. The recognized signal for high-spin Co2+ is a broad transition ∼ g = 5, whereas Co4+ exhibited a narrower transition ∼ g = 2.3 (Figure 5A), which was consistent with model compounds. No other species, for example, high-spin Co3+ (a paramagnetic configuration of an otherwise EPR-silent species) were detected. However, the Co4+ signal was severely attenuated with increasingly anodic potentials,13 which often takes place when paramagnetic centers become too concentrated in a sample, due to excessive hyperfine interactions.62 This attenuation seems exclusive to Co4+ in the Co system, as no attenuation was found when the catalyst was dominantly (if not almost entirely) Co2+.
Figure 5.
EPR characterization of CoPi electrocatalysts for the OER
(A) EPR spectra of CoPi catalyst deposited at 1.03 V vs. NHE (blue curve), 1.14 V vs. NHE (red curve), and 1.34 V (black curve) vs. NHE. Reprinted with permission from McAlpin et al.12 Copyright 2010 American Chemical Society.
(B) Comparison of the reported transition point between Co2+ and Co4+ in three studies discussed in this review. The thermodynamic OER potential is shown for comparison and all values (if needed) were corrected to RHE.
Although catalyst delamination was used, the same behavior prevails on the catalyst that is directly quenched under operating potentials.63 In this subsequent study, Co4+ formation was found to be roughly coincidental with the onset of the OER (+/− 100 mV). Across all three studies, the same trend emerges—formation of Co4+ from Co2+ takes place around, or slightly beyond the onset of the OER. This trend is especially apparent once the results are normalized for pH (Figure 5B). It is likely then that Co4+ is the resting state of the catalyst, which then proceeds with O2 evolution once the thermodynamic potential is reached. In all three cases, it is apparent that Co4+ formation at the expense of Co2+ is the most significant electrochemical event prior to OER activity. Most significantly for experimental design, the catalyst can be analyzed on a conductive electrode without the need for the destruction of the original sample, greatly facilitating future electrochemical measurements.
An opposite upfield shift was reported in a mixed Co-Cu oxide system.16 To acquire operando spectra, this study was performed using a carbon sheet with dispersed catalyst as the working electrode. Unlike the amorphous Co oxide films, the CoCu oxides are nanoparticles with a bulk rock-salt structure, meaning that it is antiferromagnetic and as a result, EPR silent (except for uncoordinated surface sites), possibly also explaining the high background of the EPR spectra.64 These surface states disappeared with longer exposures to 0.1 M KOH, possibly due to coordination arising from hydroxide groups according to the Pourbaix diagram of Co.65 Assessing the nature of the new species is difficult without more information, but the final spectrum acquired during the OER seems to suggest that a change in catalyst structure as a result of Cu dissolution, which was also demonstrated by EPR. This study, therefore, highlights how EPR is useful in tracing the ability of dopants to change the behavior of new catalysts compared to parent systems.
Examination of mixed metal catalysts – Mn-Ni O2 evolution reaction catalysts
Like Co, pure Mn oxides show promise for OER catalysis, albeit at lower activities. Mn is the OER catalyst of nature, found as part of the Mn4Ca complex of Photosystem II. EPR has been an important tool for understanding this biologically important system. For example, the discovery of a characteristic ≥18 hyperfine line spectrum was attributed to a Mn4Ca cluster with Mn nuclei in the 3 + or 4 + oxidation states.66 Paramagnetic Mn species with specific oxidation states (2+, 4+) display characteristic EPR spectra due to the nuclear spin of Mn (+5/2), resulting in spectra with significant hyperfine coupling for both Mn2+ and Mn4+.67, 68, 69 Similarly to Photosystem II, OER-active Mn-based catalysts contain Mn3+ and Mn4+,70 and much work has gone into exploring the unique EPR spectra characteristic of Mn3+/4+-based clusters both in natural and artificial systems.71, 72, 73 Mn oxides stand out from first-row transition metal oxide OER catalysts for their better stabilities in neutral and acidic conditions, even if they are less active.74,75 These traits (better stability, wide oxidation state range, lower activity) suggest that Mn atoms could better be used as a source of oxidation state control in other, oxidation-state dependent catalysts.
Since unique paramagnetic centers have unique g-factors, EPR shows promise for studying the presence of multiple oxidation states within a given catalyst. Oftentimes, the mixing of transition metals in a catalyst will lead to the synergy between neighboring metal centers, allowing for the novel activity of the mixed catalyst. For example, the incorporation of Mn in nominally NiO oxide nanosheets (rock-salt structure) can be used to stabilize Ni3+ at ground state, resulting in superior OER activities compared to pure Mn2O3 or NiO.15 Mn2+ was not observed in the EPR spectrum, suggesting that Mn was mostly present as Mn3+ (Figures 6A and 6C). Ordinarily, Ni3+ formation from Ni2+ takes place during a one electron transfer prior to the OER as a result of the Ni2+/3+ redox couple taking place prior to the thermodynamic OER potential,76 possibly contributing to sluggish OER activity of pure Ni oxides based on their large Tafel slopes compared to other transition metal oxides.77 The stabilization of Ni3+ prior to the OER possibly explains the activity of Mn-doped NiO as Ni oxidation step is not necessary during anodic polarization.
Figure 6.
Mn-Ni mixed oxides for the OER studied by EPR
(A) X-band EPR spectrum of ground state Mn-doped NiO nanosheets, with the g-factor of Ni3+ labeled. Reprinted with permission from Tian et al.15 Copyright 2018 American Chemical Society.
(B) X-band EPR spectra of the emergence of low-spin Mn4+ species under OER potentials from an electrode of Ni-doped Mn3O4 nanoparticles. Mn2+ spectrum is visible in the top row; starting at 1.1 V vs. NHE, the low-spin Mn4+ species is observed. Modeled data, generated using EasySpin, is provided as the dotted red spectra. Reprinted with permission from Park et al.14 Copyright 2020 Springer Nature.
(C and D) Depictions of how the dopant atom added to the base material in both cases yields EPR-visible paramagnetic species Ni3+ and low-spin Mn4+.
Alternatively, Ni doping into spinel Mn3O4 nanoparticles also can result in a more active Mn-Ni oxide OER catalyst.14 By tracing the behavior of the catalyst on polarization using ex situ freeze-quenching, it was observed that a new, low-spin Mn4+ species was found to emerge, leading the authors to conclude that Ni stabilized low-spin Mn4+ in Mn3O4 within a compressed crystal field (Figures 6B and 6D). Notably, at no time were any EPR spectra for Ni3+ observed, demonstrating that the oxidation state of Ni remained Ni2+. Although the catalyst electrode was composed of the doped nanoparticles on a NiO support, OER activity was nonetheless greater than the support alone. Therefore, it is reasonable to argue that the new low-spin state of Mn is responsible for conferring OER activity, and not the presence of dopant Ni atoms.
Local environments by electron paramagnetic resonance spectroscopy – Mo-based systems for the H2 Evolution Reaction (HER)
In our own experiences, EPR has proven helpful in analyzing less-stable oxidation states of Mo that can be stabilized or trapped under various conditions as well as their local environments. Mo is a d6 element with multiple oxidation states under ambient conditions, and Mo-based catalysts are some of the most-active noble metal-free catalysts for the HER.78, 79, 80 Existing non-EPR studies of Mo-containing catalysts during the HER generally agree that a reduced state of Mo is responsible (i.e., <Mo6+),81,82 but the variety of possibly involved oxidation states makes it difficult to pinpoint a specific charge. Theoretical mechanistic studies suggest that multiple Mo oxidation states participate in the HER,83 but our interest has been in identifying the active species, most likely a hydride- or H∗-containing species, that resting state of the catalyst. The earliest ex situ EPR study of amorphous Mo sulfide (a-MoSx) utilized Mo sulfide nanoparticles reduced by sodium dithionite in lieu of cathodic potentials to suggest the presence of Mo5+ (which became more intense after reduction).10 Since no electrodes were involved in the EPR part of the study, this system allowed for the quick isolation and study of Mo sulfides without the need for a hybrid EPR/electrochemical setup.
In our initial study of Mo in NiMo alloys, Mo3+ was found to be present during in situ (cathodic potential) EPR in organic electrolyte (Figure 7A). Lacking a reducing agent or reasonable means that could be used for ex situ studies, the catalyst electrode was electrochemically reduced in organic electrolyte. In situ conditions were necessary due to the instability of the Mo3+ state; the catalyst would re-oxidize upon the loss of applied potential. Furthermore, the use of THF as organic electrolyte lowered the microwave absorption profile for better signal resolution. The kinetics of the underlying reaction could also be slowed to the point that specific oxidation states could be electrochemically resolved.30 To demonstrate the connection between aqueous and organic systems, cyclic voltammograms of the catalyst in organic electrolyte with increasing amounts of water were used to show how the onset of the HER related to reduction events. Meanwhile, attempts to capture the same species by XAS,84 or by ex situ or operando EPR proved unable to resolve the species of interest. The EPR spectrum of Mo3+ also provided insight into its local environment due to the presence of axial symmetry, demonstrating that it had a distorted crystal field. Quantitative EPR was therefore used to compare the number of spins to the total amount of Mo (acquired using inductively coupled plasma measurements), finding that only a minority of the Mo species—likely only a surface layer—formed Mo3+. Therefore, the anisotropy implies that Mo3+ is surface bound, also highlighting the remarkable sensitivity of EPR.
Figure 7.
EPR for characterization of Mo during the HER
(A) Cyclic voltammograms of NiMo in 0.2 M Bu4N PF6/THF before (black) and after (green) electrolysis in 0.1 M KOH. Inset, EPR spectra of Mo3+ signal before (red), during (blue) and after (yellow) the NiMo electrode was held at −2.5 V vs. Ag/Ag+. Reprinted with permission from Bau et al.8 Copyright 2020 American Chemical Society.
(B) EPR spectrum of trapped Mo3+ hydride in a-MoSx (light green). The spectrum of Mo3+ formed from NiMo under organic cycling in (a) is shown for comparison (blue trace in (a), dark green). A simulated spectrum for a-MoSx is provided in red.
(C) EPR spectra of a-MoSx deposited with a cathodic final potential (blue, similar to 6b) and an anodic potential (red) with annotated characteristic g-factors corresponding to Mo5+ signals. Figures (b) and (c) reprinted with permission from Bau et al.85 Copyright 2022 Springer Nature; (c) was compiled and re-annotated with data from both references.
We later used a similar approach to re-examine a-MoSx.85 While in situ electrochemical measurements could (and were) used, the Mo3+ signal could also be trapped ex situ by ending the electrochemical deposition (by cycling) protocol for a-MoSx suggested by Merki et al.86 at H2-evolving potentials. The resulting catalyst exhibited a Mo3+ signal that was both broadened and isotropic compared to that found in NiMo (Figure 7B). The isotropy suggests that active sites are present throughout the entirety of the porous catalyst and oriented randomly. However, it was the broadening (31 G for a-MoSx vs. 15-19 G for NiMo or a-MoSx reduced in organic, aprotic electrolyte) that proved interesting as it illustrated the presence of hyperfine coupling from a neighboring atom. As the most terrestrially abundant form of S is not spin-active, the only possible remaining candidate was H, which could be confirmed by EasySpin simulations. By other means, the presence of a metal hydride was confirmed. EPR was able to provide direct experimental evidence for a Mo3+-H active site in a-MoSx. EPR has also demonstrated that both a-MoSx and NiMo behave similarly in electrochemical conditions, suggesting that a common mechanism between Mo catalysts exists for the HER.
Although differences in spectra exist between ex situ reduced Mo sulfide nanoparticles and electrochemically reduced Mo electrocatalysts, the results are not necessarily contradictory as Mo5+ was found in a-MoSx when the deposition was ended on the anodic edge (Figure 7C). The electrode-based system that we utilized allowed us to capture both species, allowing for the elucidation of a complete process.
Electron paramagnetic resonance spectroscopy for the examination of the roles of vacancies in electrocatalysis – NiFe O2 evolution reaction catalysts
Amongst the most well-studied multi-metallic electrocatalyst systems is Fe-containing Ni oxide, generally recognized as one of the most active earth-abundant OER catalysts.87, 88, 89 Although Ir- or Ru-based are still more active, the orders of magnitude abundance of Ni and Fe over noble metals makes them more realistic for practical implementation. The composition and amount of Fe dissolved in the Ni oxide structure is considered critical to high activity, and Fe is recognized as being important to carrying out the OER at low overpotential.90, 91, 92, 93 Like for Co oxides, amorphous NiFe oxides register high OER activities.77
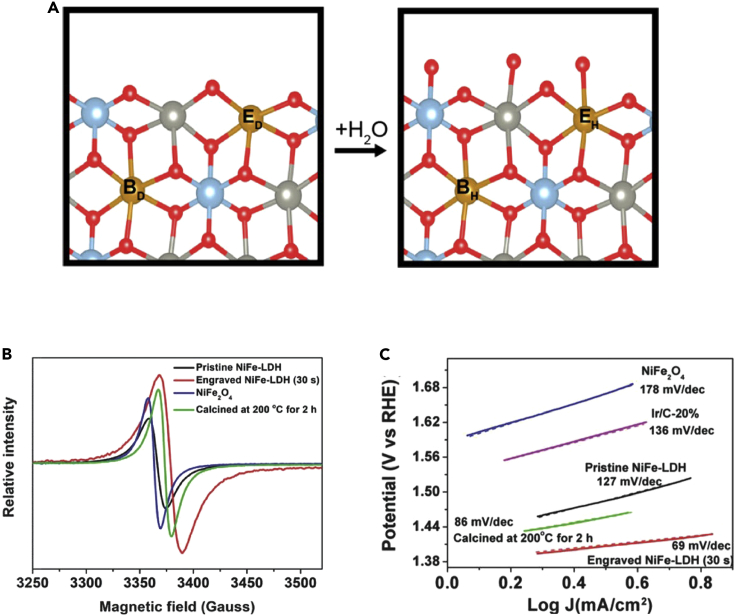
The oxidation states of NiFe oxides during the OER are potentially plentiful,30,90 meaning that a good experimental setup would be needed for proper EPR studies. However, like Co4+, NiFe systems suffer from internal magnetic interference arising from local interactions at high concentrations, making in situ and operando mechanistic studies with EPR difficult. Fortunately, EPR has been useful in exposing another aspect of NiFe oxide function—the presence and role of vacancies.94, 95, 96, 97 The vacancies seem to arise from the facile nature by which water associates and dissociates with surface Ni and Fe centers, as demonstrated by Sayler et al. on layered double hydroxides of Zn and Al doped with Ni and Fe atoms at low concentrations (Figure 8A).98 Both of Ni- and Fe-doped ZnAl LDH particles exhibit EPR peaks at the free electron value when dried under vacuum which disappears on rehydration, suggesting that these spectra arise from uncoordinated vacancies on the metal centers.
Figure 8.
EPR studies of NiFe oxide
(A) Dehydrated and hydrated forms of Ni/Fe LDH (orange) surrounded by Al (blue), Zn (gray) and O (red) atoms. E, edge sites; B, bulk sites. Reprinted with permission from Sayler et al.98 Copyright 2020 American Chemical Society.
(B) EPR spectra of NiFe LDHs as prepared (black), after reducing flame treatment (red), and calcined at 200°C for 2 h (green). NiFe2O4 powder is studied as a control (blue).
(C) Tafel plots of the same catalysts for the OER reaction in 0.1 M KOH. Color scheme is the same as (b), with the exception of Ir/C (purple) which was added for comparison. Reprinted with permission from Zhou et al.94 Copyright 2018 American Chemical Society.
Furthermore, different groups have demonstrated that the size of the free electron peak arising from vacancies can be related to OER activity. For example, Zhou et al. found that NiFe catalysts of varying activities can be prepared by carefully changing the heat treatment used post-preparation, postulating that each heat treatment introduces different amounts of oxygen vacancies into the structure (Figures 8B and 8C).94 The benefit of studying NiFe oxides with EPR is that no particular setups are needed, as long as the catalysts are consistently prepared, such as by pre-drying before measurement. The free electron is also not affected by the concentration of paramagnetic species, so EPR can be used to compare the presence of vacancies to OER activity even if other features of the sample are unmeasurable by EPR.
The role of vacancies in electrochemical CO2 reduction on Cu metal-organic frameworks
CO2 electroreduction is a promising field of study for the recycling of waste CO2 into useful hydrocarbon products, but its study by EPR is further complicated (in addition to the other challenges discussed above) by poor CO2 solubility in water, and only ex situ setups in the absence of CO2 have so far been reported. Nonetheless, as with NiFe systems in the OER, EPR is useful in these providing insight into the role that vacancies play in CO2 reduction. For example, the amount of uncoordinated Cu2+ (as measured by the free electron peak) in the Cu-MOF HKUST-1 can be increased by thermal treatment, leading to improved Faradaic efficiency (FE) for ethylene production.99 By tracking the deterioration of free electrons, Kang et al. also demonstrated that the FE for formic acid production in another Cu-MOF was directly correlated with the amount of remaining uncoordinated Cu2+ sites.100 The loss of such sites was due to the reduction of Cu2+ to Cu+. Similarly, anodized Ti has the activity for electrochemical CO2 to methanol conversion dependent on the amount of Ti3+ and oxygen vacancies formed during Ti anodization.101 The authors had been unable to properly study these vacancies using XPS,33 underlining the value of EPR over other techniques for studying paramagnetic, uncoordinated materials. Based on these studies, it is likely that, much as how water coordinates to vacancies in NiFe to carry out the OER, vacancies in these materials serve as coordination sites for dissolved CO2.
Outlook
In this review, we have sought to provide an overview of research seeking to utilize EPR to characterize the behavior of solid-state electrocatalysts with an eye to the experimental details that make such characterization possible. Given the role and range of transition metal oxidation states present in a variety of electrocatalysts, the local environments of these atoms, and the role of vacancies in catalyzing energy reactions of interest, EPR retains great potential as a spectroscopic tool. To complement some of these early discoveries, the development of several topics is important for future methodologies.
-
1.
Substrate and electrode materials for the electrode of interest—liquid-phase EPR measurements are not a novel concept and indeed are well-established through the use of quartz flat cells; however, the combination of electrodes and liquids within the EPR cavity adds significant complications to acquiring spectra. Although EPR-silent electrode materials can minimize or completely eliminate shielding effects (i.e., doped semiconductors such as indium-doped tin oxide102), the fact that micron-thick metal foils can still give rise to the meaningful signal under in situ conditions suggests that the overall dimensions of the material are just as important. Furthermore, some doped semiconductors may not be adequately stable in the acidic or alkaline electrolytes used for electrochemical studies. Sputtered noble metal electrodes are more commonly found and can provide adequate conductivity with ∼50 nm films. An EPR-silent substrate material such as uncontaminated mica sputtered with metal on both sides could effectively be used in the same manner as a foil with minimal shielding losses.
-
2.
New systems in which solid-state electrochemical EPR would have specific value—a major theme of the EPR studies so far reported is that the ideal catalysts to be studied have dilute active sites as samples with concentrated paramagnetic species can yield attenuated spectra, albeit depending on the species. Nonetheless, catalysts with dilute active sites—namely, single-site catalysts—have recently been of interest for their unique reactivities and high activities per weight loading. Many of these catalysts have transition metal centers that undergo changes in oxidation state under catalytic conditions. These characteristics make EPR a potentially unique technique for its characterization.
Gas-phase reactions (especially CO2 and N2 reduction) have also lately been of interest to the electrochemical community and catalysts could be more thoroughly explored using EPR. Such gas-consuming reactions studied in the liquid state would require the combination of flow systems such as those utilized for fuel cell studies4 (to minimize diffusion limitations and allow for longer-term operation) with the electrochemical considerations discussed here. To allow for the study of such systems, a standard electrochemical cell could be modified to allow for separate pumping and degassing functions (Figure 9). By substituting quartz with EPR-silent polymers, the production and testing of such cells could be significantly sped up.59,103
Figure 9.

Diagram of a combined flow/electrochemical EPR cell for studying catalyst behavior for reactions where a gas is a reactant
Another group of reactions that have so far been unexplored using EPR is organic electrochemical reactions. Although aqueous reactions currently constitute an outsized portion of electrochemical studies due to the role of water as a proton and oxygen source in H2 and O2 evolution, fuel cell reactions, and CO2 reduction, organic electrochemical transformations have become a topic of interest. Given the lower dielectric constants of non-aqueous electrolytes, solid-state EPR could be valuable in demonstrating how and why different metal catalysts have different reactivities for different reactions. As an added bonus, EPR could simultaneously be used to study the mechanism of the reactions themselves.
EPR for solid-state electrochemical characterization is still in its infancy, but it is apparent that material selection and cell design are important to any future developments, especially for valuable in situ and operando studies. However, the minimal requirements described here are commonly found throughout the chemistry and materials science departments. We conclude that such applications might be readily applied in the future to provide unprecedented and highly available insights into the reactions that will be critical to the economies of the future.
Acknowledgments
The authors acknowledge the King Abdullah University of Science and Technology for supporting this research.
Author contributions
J.A.B.: original draft preparation, figure and table conceptualization, review, and editing. A.-H. E.: original draft preparation, review, and editing. M.R.: review, editing, and supervision. All authors reviewed and approved the final article.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Jeremy A. Bau, Email: jeremy.bau@kaust.edu.sa.
Magnus Rueping, Email: magnus.rueping@kaust.edu.sa.
References
- 1.Zhao E.W., Jónsson E., Jethwa R.B., Hey D., Lyu D., Brookfield A., Klusener P.A.A., Collison D., Grey C.P. Coupled in situ NMR and EPR studies reveal the electron transfer rate and electrolyte decomposition in redox flow batteries. J. Am. Chem. Soc. 2021;143:1885–1895. doi: 10.1021/jacs.0c10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li M., Odom S.A., Pancoast A.R., Robertson L.A., Vaid T.P., Agarwal G., Doan H.A., Wang Y., Suduwella T.M., Bheemireddy S.R., et al. Experimental protocols for studying organic non-aqueous redox flow batteries. ACS Energy Lett. 2021;6:3932–3943. doi: 10.1021/acsenergylett.1c01675. [DOI] [Google Scholar]
- 3.Kulikov I., Panjwani N.A., Vereshchagin A.A., Spallek D., Lukianov D.A., Alekseeva E.V., Levin O.V., Behrends J. Spins at work: probing charging and discharging of organic radical batteries by electron paramagnetic resonance spectroscopy. Energy Environ. Sci. 2022;15:3275–3290. doi: 10.1039/D2EE01149B. [DOI] [Google Scholar]
- 4.Danilczuk M., Coms F.D., Schlick S. Visualizing chemical reactions and crossover processes in a fuel cell inserted in the ESR resonator: detection by spin trapping of oxygen radicals, nafion-derived fragments, and hydrogen and deuterium atoms. J. Phys. Chem. B. 2009;113:8031–8042. doi: 10.1021/jp901597f. [DOI] [PubMed] [Google Scholar]
- 5.Panchenko A., Dilger H., Kerres J., Hein M., Ullrich A., Kaz T., Roduner E. In-situ spin trap electron paramagnetic resonance study of fuel cell processes. Phys. Chem. Chem. Phys. 2004;6:2891–2894. doi: 10.1039/B404253K. [DOI] [Google Scholar]
- 6.Chiesa M., Giamello E., Che M. EPR characterization and reactivity of surface-localized inorganic radicals and radical ions. Chem. Rev. 2010;110:1320–1347. doi: 10.1021/cr800366v. [DOI] [PubMed] [Google Scholar]
- 7.Davies M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods. 2016;109:21–30. doi: 10.1016/j.ymeth.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Bau J.A., Kozlov S.M., Azofra L.M., Ould-Chikh S., Emwas A.-H., Idriss H., Cavallo L., Takanabe K. Role of oxidized Mo species on the active surface of Ni–Mo electrocatalysts for hydrogen evolution under alkaline conditions. ACS Catal. 2020;10:12858–12866. doi: 10.1021/acscatal.0c02743. [DOI] [Google Scholar]
- 9.Prior C., Webster L.R., Ibrahim S.K., Wright J.A., Alghamdi A.F., Oganesyan V.S., Pickett C.J. EPR detection and characterisation of a paramagnetic Mo(III) dihydride intermediate involved in electrocatalytic hydrogen evolution. Dalton Trans. 2016;45:2399–2403. doi: 10.1039/C5DT04432D. [DOI] [PubMed] [Google Scholar]
- 10.Tran P.D., Tran T.V., Orio M., Torelli S., Truong Q.D., Nayuki K., Sasaki Y., Chiam S.Y., Yi R., Honma I., et al. Coordination polymer structure and revisited hydrogen evolution catalytic mechanism for amorphous molybdenum sulfide. Nat. Mater. 2016;15:640–646. doi: 10.1038/nmat4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busetto L., Vaccari A., Martini G. Electron spin resonance of paramagnetic species as a tool for studying the thermal decomposition of molybdenum trisulfide. J. Phys. Chem. 1981;85:1927–1930. doi: 10.1021/j150613a030. [DOI] [Google Scholar]
- 12.McAlpin J.G., Surendranath Y., Dinca M., Stich T.A., Stoian S.A., Casey W.H., Nocera D.G., Britt R.D. EPR evidence for Co(IV) species produced during water oxidation at neutral pH. J. Am. Chem. Soc. 2010;132:6882–6883. doi: 10.1021/ja1013344. [DOI] [PubMed] [Google Scholar]
- 13.Gerken J.B., McAlpin J.G., Chen J.Y.C., Rigsby M.L., Casey W.H., Britt R.D., Stahl S.S. Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: the thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc. 2011;133:14431–14442. doi: 10.1021/ja205647m. [DOI] [PubMed] [Google Scholar]
- 14.Park S., Jin K., Lim H.K., Kim J., Cho K.H., Choi S., Seo H., Lee M.Y., Lee Y.H., Yoon S., et al. Spectroscopic capture of a low-spin Mn(IV)-oxo species in Ni–Mn 3 O 4 nanoparticles during water oxidation catalysis. Nat. Commun. 2020;11:5230. doi: 10.1038/s41467-020-19133-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian T., Gao H., Zhou X., Zheng L., Wu J., Li K., Ding Y. Study of the active sites in porous nickel oxide nanosheets by manganese modulation for enhanced oxygen evolution catalysis. ACS Energy Lett. 2018;3:2150–2158. doi: 10.1021/acsenergylett.8b01206. [DOI] [Google Scholar]
- 16.Hollmann D., Rockstroh N., Grabow K., Bentrup U., Rabeah J., Polyakov M., Surkus A.-E., Schuhmann W., Hoch S., Brückner A. From the precursor to the active state: monitoring metamorphosis of electrocatalysts during water oxidation by in situ spectroscopy. Chemelectrochem. 2017;4:2117–2122. doi: 10.1002/celc.201700142. [DOI] [Google Scholar]
- 17.Etienne E., Le Breton N., Martinho M., Mileo E., Belle V. SimLabel: a graphical user interface to simulate continuous wave EPR spectra from site-directed spin labeling experiments. Magn. Reson. Chem. 2017;55:714–719. doi: 10.1002/mrc.4578. [DOI] [PubMed] [Google Scholar]
- 18.Stoll S., Schweiger A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006;178:42–55. doi: 10.1016/j.jmr.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Chechik V., Carter E., Murphy D. Electron Paramagnetic Resonance. Oxford University Press; 2016. [Google Scholar]
- 20.Bracci M., Bruzzese P.C., Famulari A., Fioco D., Guidetti A., Liao Y.-K., et al. Paramagnetic species in catalysis research: a unified approach towards (the role of EPR in) heterogeneous, homogeneous and enzyme catalysis. Royal Society of Chemistry; 2020. pp. 1–46. [Google Scholar]
- 21.Hyde J.S. A new principle for aqueous sample cells for EPR. Rev. Sci. Instrum. 1972;43:629–631. doi: 10.1063/1.1685709. [DOI] [Google Scholar]
- 22.den Hartog S., Neukermans S., Samanipour M., Ching H.V., Breugelmans T., Hubin A., Ustarroz J. Electrocatalysis under a magnetic lens: a combined electrochemistry and electron paramagnetic resonance review. Electrochim. Acta. 2022;407:139704. doi: 10.1016/j.electacta.2021.139704. [DOI] [Google Scholar]
- 23.Bard A.J., McKinney T.M., Goldberg I.B. In: Foundations of Modern EPR. Eaton Gareth R., Eaton Sandra S., Salikhov Kev M., editors. World Scientific; 1998. EPR and electrochemistry; pp. 278–294. [Google Scholar]
- 24.Griswold T.W., Kip A.F., Kittel C. Microwave spin resonance absorption by conduction electrons in metallic sodium. Phys. Rev. 1952;88:951–952. doi: 10.1103/PhysRev.88.951. [DOI] [Google Scholar]
- 25.Feher G., Kip A.F. Electron spin resonance absorption in metals. I. Experimental. Phys. Rev. 1955;98:337–348. doi: 10.1103/PhysRev.98.337. [DOI] [Google Scholar]
- 26.Pifer J.H., Magno R. Conduction-electron spin resonance in a lithium film. Phys. Rev. B. 1971;3:663–673. doi: 10.1103/PhysRevB.3.663. [DOI] [Google Scholar]
- 27.Toybenshlak M., Carmieli R. A new and robust method for in-situ EPR electrochemistry. Isr. J. Chem. 2019;59:1020–1026. doi: 10.1002/ijch.201900074. [DOI] [Google Scholar]
- 28.Liu Y., Shi B., Liu Z., Gao R., Huang C., Alhumade H., Wang S., Qi X., Lei A. Time-resolved EPR revealed the formation, structure, and reactivity of N-centered radicals in an electrochemical C(sp3)–H arylation reaction. J. Am. Chem. Soc. 2021;143:20863–20872. doi: 10.1021/jacs.1c09341. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y., Wang J., Chu H., Chu Y.-C., Chen H.M. In situ/operando studies for designing next-generation electrocatalysts. ACS Energy Lett. 2020;5:1281–1291. doi: 10.1021/acsenergylett.0c00305. [DOI] [Google Scholar]
- 30.Hunter B.M., Thompson N.B., Müller A.M., Rossman G.R., Hill M.G., Winkler J.R., Gray H.B. Trapping an iron(VI) water-splitting intermediate in nonaqueous media. Joule. 2018;2:747–763. doi: 10.1016/j.joule.2018.01.008. [DOI] [Google Scholar]
- 31.Yoon Y., Yan B., Surendranath Y. Suppressing ion transfer enables versatile measurements of electrochemical surface area for intrinsic activity comparisons. J. Am. Chem. Soc. 2018;140:2397–2400. doi: 10.1021/jacs.7b10966. [DOI] [PubMed] [Google Scholar]
- 32.Han Y., Zhang H., Yu Y., Liu Z. In situ characterization of catalysis and electrocatalysis using APXPS. ACS Catal. 2021;11:1464–1484. doi: 10.1021/acscatal.0c04251. [DOI] [Google Scholar]
- 33.Idriss H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021;712:121894. doi: 10.1016/j.susc.2021.121894. [DOI] [Google Scholar]
- 34.Nguyen H., Clément R.J. Rechargeable batteries from the perspective of the electron spin. ACS Energy Lett. 2020;5:3848–3859. doi: 10.1021/acsenergylett.0c02074. [DOI] [Google Scholar]
- 35.Dutoit C.-E., Tang M., Gourier D., Tarascon J.-M., Vezin H., Salager E. Monitoring metallic sub-micrometric lithium structures in Li-ion batteries by in situ electron paramagnetic resonance correlated spectroscopy and imaging. Nat. Commun. 2021;12:1410. doi: 10.1038/s41467-021-21598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szczuka C., Ackermann J., Schleker P.P.M., Jakes P., Eichel R.-A., Granwehr J. Transient morphology of lithium anodes in batteries monitored by in operando pulse electron paramagnetic resonance. Commun. Mater. 2021;2:20–27. doi: 10.1038/s43246-021-00126-1. [DOI] [Google Scholar]
- 37.Wandt J., Marino C., Gasteiger H.A., Jakes P., Eichel R.-A., Granwehr J. Operando electron paramagnetic resonance spectroscopy – formation of mossy lithium on lithium anodes during charge–discharge cycling. Energy Environ. Sci. 2015;8:1358–1367. doi: 10.1039/C4EE02730B. [DOI] [Google Scholar]
- 38.Wandt J., Jakes P., Granwehr J., Gasteiger H.A., Eichel R.-A. Singlet oxygen formation during the charging process of an aprotic lithium–oxygen battery. Angew. Chem. Int. Ed. Engl. 2016;128:7006–7009. doi: 10.1002/ange.201602142. [DOI] [PubMed] [Google Scholar]
- 39.Wandt J., Jakes P., Granwehr J., Eichel R.-A., Gasteiger H.A. Quantitative and time-resolved detection of lithium plating on graphite anodes in lithium ion batteries. Mater. Today. 2018;21:231–240. doi: 10.1016/j.mattod.2017.11.001. [DOI] [Google Scholar]
- 40.Wang B., Le Fevre L.W., Brookfield A., McInnes E.J.L., Dryfe R.A.W. Resolution of lithium deposition versus intercalation of graphite anodes in lithium ion batteries: an in situ electron paramagnetic resonance study. Angew. Chem. Int. Ed. Engl. 2021;60:21860–21867. doi: 10.1002/anie.202106178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sathiya M., Leriche J.-B., Salager E., Gourier D., Tarascon J.-M., Vezin H. Electron paramagnetic resonance imaging for real-time monitoring of Li-ion batteries. Nat. Commun. 2015;6:6276. doi: 10.1038/ncomms7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao Y., Li C., Lou X., Hu X., Ning Y., Yuan F., Chen B., Shen M., Hu B. Carbon-coated Li3V2(PO4)3 derived from metal-organic framework as cathode for lithium-ion batteries with high stability. Electrochim. Acta. 2018;271:608–616. doi: 10.1016/j.electacta.2018.03.100. [DOI] [Google Scholar]
- 43.Li C., Shen M., Lou X., Hu B. Unraveling the redox couples of VIII/VIV mixed-valent Na3V2(PO4)2O1.6F1.4 cathode by parallel-mode EPR and in situ/ex situ NMR. J. Phys. Chem. C. 2018;122:27224–27232. doi: 10.1021/acs.jpcc.8b09151. [DOI] [Google Scholar]
- 44.Niemöller A., Jakes P., Eichel R.-A., Granwehr J. In operando EPR investigation of redox mechanisms in LiCoO2. Chem. Phys. Lett. 2019;716:231–236. doi: 10.1016/j.cplett.2018.12.022. [DOI] [Google Scholar]
- 45.Zhecheva E., Stoyanova R., Alcántara R., Lavela P., Tirado J.L. EPR studies of Li deintercalation from LiCoMnO4 spinel-type electrode active material. J. Power Sources. 2006;159:1389–1394. doi: 10.1016/j.jpowsour.2005.11.088. [DOI] [Google Scholar]
- 46.González J.R., Alcántara R., Tirado J.L., Fielding A.J., Dryfe R.A.W. Electrochemical interaction of few-layer molybdenum disulfide composites vs sodium: new insights on the reaction mechanism. Chem. Mater. 2017;29:5886–5895. doi: 10.1021/acs.chemmater.7b01245. [DOI] [Google Scholar]
- 47.Niemöller A., Jakes P., Eurich S., Paulus A., Kungl H., Eichel R.-A., Granwehr J. Monitoring local redox processes in LiNi0.5Mn1.5O4 battery cathode material by in operando EPR spectroscopy. J. Chem. Phys. 2018;148:014705. doi: 10.1063/1.5008251. [DOI] [PubMed] [Google Scholar]
- 48.Marinova D.M., Kukeva R.R., Zhecheva E.N., Stoyanova R.K. Selective sodium intercalation into sodium nickel–manganese sulfate for dual Na–Li-ion batteries. Phys. Chem. Chem. Phys. 2018;20:12755–12766. doi: 10.1039/C8CP01667D. [DOI] [PubMed] [Google Scholar]
- 49.Tang M., Dalzini A., Li X., Feng X., Chien P.-H., Song L., Hu Y.-Y. Operando EPR for simultaneous monitoring of anionic and cationic redox processes in Li-rich metal oxide cathodes. J. Phys. Chem. Lett. 2017;8:4009–4016. doi: 10.1021/acs.jpclett.7b01425. [DOI] [PubMed] [Google Scholar]
- 50.Geng F., Yang Q., Li C., Hu B., Zhao C., Shen M., Hu B. Operando EPR and EPR imaging study on a NaCrO2 cathode: electronic property and structural degradation with Cr dissolution. J. Phys. Chem. Lett. 2021;12:781–786. doi: 10.1021/acs.jpclett.0c03327. [DOI] [PubMed] [Google Scholar]
- 51.Liu H., Zhao C., Qiu Q., Hu B., Geng F., Li J., Tong W., Hu B., Li C. What triggers the voltage hysteresis variation beyond the first cycle in Li-rich 3d layered oxides with reversible cation migration? J. Phys. Chem. Lett. 2021;12:8740–8748. doi: 10.1021/acs.jpclett.1c02185. [DOI] [PubMed] [Google Scholar]
- 52.Strauss F., Rousse G., Alves Dalla Corte D., Ben Hassine M., Saubanère M., Tang M., Vezin H., Courty M., Dominko R., Tarascon J.-M. Electrochemical activity and high ionic conductivity of lithium copper pyroborate Li6CuB4O10. Phys. Chem. Chem. Phys. 2016;18:14960–14969. doi: 10.1039/C6CP01581F. [DOI] [PubMed] [Google Scholar]
- 53.Eguia-Barrio A., Castillo-Martínez E., Klein F., Pinedo R., Lezama L., Janek J., Adelhelm P., Rojo T. Electrochemical performance of CuNCN for sodium ion batteries and comparison with ZnNCN and lithium ion batteries. J. Power Sources. 2017;367:130–137. doi: 10.1016/j.jpowsour.2017.09.033. [DOI] [Google Scholar]
- 54.Szczuka C., Eichel R.-A., Granwehr J. Exploring the solvation sphere and spatial accumulation of dissolved transition-metal ions in batteries: a case study of vanadyl ions released from V2O5 cathodes. ACS Appl. Energy Mater. 2022;5:449–460. doi: 10.1021/acsaem.1c02979. [DOI] [Google Scholar]
- 55.Huang D., Engtrakul C., Nanayakkara S., Mulder D.W., Han S.-D., Zhou M., Luo H., Tenent R.C. Understanding degradation at the lithium-ion battery cathode/electrolyte interface: connecting transition-metal dissolution mechanisms to electrolyte composition. ACS Appl. Mater. Interfaces. 2021;13:11930–11939. doi: 10.1021/acsami.0c22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang B., Fielding A.J., Dryfe R.A.W. In situ electrochemical electron paramagnetic resonance spectroscopy as a tool to probe electrical double layer capacitance. Chem. Commun. 2018;54:3827–3830. doi: 10.1039/C8CC00450A. [DOI] [PubMed] [Google Scholar]
- 57.Wang B., Fielding A.J., Dryfe R.A. Electron paramagnetic resonance as a structural tool to study graphene oxide: potential dependence of the EPR response. J. Phys. Chem. C. 2019;123:22556–22563. doi: 10.1021/acs.jpcc.9b04292. [DOI] [Google Scholar]
- 58.Wang B., Likodimos V., Fielding A.J., Dryfe R.A. In situ electron paramagnetic resonance spectroelectrochemical study of graphene-based supercapacitors: comparison between chemically reduced graphene oxide and nitrogen-doped reduced graphene oxide. Carbon. 2020;160:236–246. doi: 10.1016/j.carbon.2019.12.045. [DOI] [Google Scholar]
- 59.Niemöller A., Jakes P., Kayser S., Lin Y., Lehnert W., Granwehr J. 3D printed sample holder for in-operando EPR spectroscopy on high temperature polymer electrolyte fuel cells. J. Magn. Reson. 2016;269:157–161. doi: 10.1016/j.jmr.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Kanan M.W., Nocera D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+ Science. 2008;321:1072–1075. doi: 10.1126/science.1162018. [DOI] [PubMed] [Google Scholar]
- 61.Esswein A.J., Surendranath Y., Reece S.Y., Nocera D.G. Highly active cobalt phosphate and borate based oxygen evolving catalysts operating in neutral and natural waters. Energy Environ. Sci. 2011;4:499–504. doi: 10.1039/C0EE00518E. [DOI] [Google Scholar]
- 62.Carretta P., Mariani M., Azzoni C.B., Mozzati M.C., Bradarić I., Savić I., Feher A., Šebek J. Mesoscopic phase separation in Na $ _x $ CoO $ _2 $($0.65\leq x\leq 0.75$) Phys. Rev. B. 2004;70:024409. doi: 10.1103/PhysRevB.70.024409. [DOI] [Google Scholar]
- 63.Kutin Y., Cox N., Lubitz W., Schnegg A., Rüdiger O. In situ EPR characterization of a cobalt oxide water oxidation catalyst at neutral pH. Catalysts. 2019;9:926. doi: 10.3390/catal9110926. [DOI] [Google Scholar]
- 64.Deori K., Deka S. Morphology oriented surfactant dependent CoO and reaction time dependent Co 3 O 4 nanocrystals from single synthesis method and their optical and magnetic properties. CrystEngComm. 2013;15:8465–8474. doi: 10.1039/C3CE41502C. [DOI] [Google Scholar]
- 65.Pourbaix M. Pergamon Press; 1966. Atlas of Electrochemical Equilibria in Aqueous Solutions. [Google Scholar]
- 66.Krewald V., Retegan M., Cox N., Messinger J., Lubitz W., DeBeer S., Neese F., Pantazis D.A. Metal oxidation states in biological water splitting. Chem. Sci. 2015;6:1676–1695. doi: 10.1039/C4SC03720K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reed G.H., Markham G.D. In: Berliner L.J., Reuben J., editors. Vol. 6. Springer US; 1984. EPR of Mn(II) complexes with enzymes and other proteins; pp. 73–142. (Biological Magnetic Resonance). [DOI] [Google Scholar]
- 68.Duboc C. Determination and prediction of the magnetic anisotropy of Mn ions. Chem. Soc. Rev. 2016;45:5834–5847. doi: 10.1039/C5CS00898K. [DOI] [PubMed] [Google Scholar]
- 69.Haque M.A., Gandi A.N., Mohanraman R., Weng Y., Davaasuren B., Emwas A., Combe C., Baran D., Rothenberger A., Schwingenschlögl U., et al. A 0D lead-free hybrid crystal with ultralow thermal conductivity. Adv. Funct. Mater. 2019;29:1809166. doi: 10.1002/adfm.201809166. [DOI] [Google Scholar]
- 70.Tian L., Zhai X., Wang X., Li J., Li Z. Advances in manganese-based oxides for oxygen evolution reaction. J. Mater. Chem. A Mater. 2020;8:14400–14414. doi: 10.1039/D0TA05116K. [DOI] [Google Scholar]
- 71.Haddy A. EPR spectroscopy of the manganese cluster of photosystem II. Photosynth. Res. 2007;92:357–368. doi: 10.1007/s11120-007-9194-9. [DOI] [PubMed] [Google Scholar]
- 72.Rapatskiy L., Ames W.M., Pérez-Navarro M., Savitsky A., Griese J.J., Weyhermüller T., Shafaat H.S., Högbom M., Neese F., Pantazis D.A., Cox N. Characterization of oxygen bridged manganese model complexes using multifrequency 17O-hyperfine EPR spectroscopies and density functional theory. J. Phys. Chem. B. 2015;119:13904–13921. doi: 10.1021/acs.jpcb.5b04614. [DOI] [PubMed] [Google Scholar]
- 73.Najafpour M.M., Hołyńska M., Shamkhali A.N., Kazemi S.H., Hillier W., Amini E., Ghaemmaghami M., Jafarian Sedigh D., Nemati Moghaddam A., Mohamadi R., et al. The role of nano-sized manganese oxides in the oxygen-evolution reactions by manganese complexes: towards a complete picture. Dalton Trans. 2014;43:13122–13135. doi: 10.1039/C4DT01367K. [DOI] [PubMed] [Google Scholar]
- 74.Huynh M., Bediako D.K., Nocera D.G. A functionally stable manganese oxide oxygen evolution catalyst in acid. J. Am. Chem. Soc. 2014;136:6002–6010. doi: 10.1021/ja413147e. [DOI] [PubMed] [Google Scholar]
- 75.Schnegg A., Nehrkorn J., Singh A., Calafell I.A., Bonke S.A., Hocking R.K., Lips K., Spiccia L. Probing the fate of Mn complexes in nafion: a combined multifrequency EPR and XAS study. J. Phys. Chem. C. 2016;120:853–861. doi: 10.1021/acs.jpcc.5b10451. [DOI] [Google Scholar]
- 76.Hall D.S., Bock C., MacDougall B.R. The electrochemistry of metallic nickel: oxides, hydroxides, hydrides and alkaline hydrogen evolution. J. Electrochem. Soc. 2013;160:F235–F243. doi: 10.1149/2.026303jes. [DOI] [Google Scholar]
- 77.Smith R.D.L., Prévot M.S., Fagan R.D., Zhang Z., Sedach P.A., Siu M.K.J., Trudel S., Berlinguette C.P. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science. 2013;340:60–63. doi: 10.1126/science.1233638. [DOI] [PubMed] [Google Scholar]
- 78.Zhuang Z., Huang J., Li Y., Zhou L., Mai L. The holy grail in platinum-free electrocatalytic hydrogen evolution: molybdenum-based catalysts and recent advances. Chemelectrochem. 2019;6:3570–3589. doi: 10.1002/celc.201900143. [DOI] [Google Scholar]
- 79.Hua W., Sun H.-H., Xu F., Wang J.-G. A review and perspective on molybdenum-based electrocatalysts for hydrogen evolution reaction. Rare Met. 2020;39:335–351. doi: 10.1007/s12598-020-01384-7. [DOI] [Google Scholar]
- 80.Ledendecker M., Mondschein J.S., Kasian O., Geiger S., Göhl D., Schalenbach M., Zeradjanin A., Cherevko S., Schaak R.E., Mayrhofer K. Stability and activity of non-noble-metal-based catalysts toward the hydrogen evolution reaction. Angew. Chem. Int. Ed. Engl. 2017;56:9767–9771. doi: 10.1002/anie.201704021. [DOI] [PubMed] [Google Scholar]
- 81.Soriaga M.P., Baricuatro J.H., Cummins K.D., Kim Y.-G., Saadi F.H., Sun G., McCrory C.C., McKone J.R., Velazquez J.M., Ferrer I.M., et al. Electrochemical surface science twenty years later: expeditions into the electrocatalysis of reactions at the core of artificial photosynthesis. Surf. Sci. 2015;631:285–294. doi: 10.1016/j.susc.2014.06.028. [DOI] [Google Scholar]
- 82.Lassalle-Kaiser B., Merki D., Vrubel H., Gul S., Yachandra V.K., Hu X., Yano J. Evidence from in situ X-ray absorption spectroscopy for the involvement of terminal disulfide in the reduction of protons by an amorphous molybdenum sulfide electrocatalyst. J. Am. Chem. Soc. 2015;137:314–321. doi: 10.1021/ja510328m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J., Yoshizawa K. Computational evidence for hydrogen generation by reductive cleavage of water and α-H abstraction on a molybdenum complex. Angew. Chem. Int. Ed. Engl. 2011;50:11972–11975. doi: 10.1002/anie.201102917. [DOI] [PubMed] [Google Scholar]
- 84.Bau J.A., Haspel H., Ould-Chikh S., Aguilar-Tapia A., Hazemann J.-L., Idriss H., Takanabe K. On the reconstruction of NiMo electrocatalysts by operando spectroscopy. J. Mater. Chem. 2019;7:15031–15035. doi: 10.1039/C9TA04494A. [DOI] [Google Scholar]
- 85.Bau J.A., Emwas A.-H., Nikolaienko P., Aljarb A.A., Tung V., Rueping M. Mo3+ hydride as the common origin of H2 evolution and selective NADH regeneration in molybdenum sulfide electrocatalysts. Nat. Catal. 2022;5:397–404. doi: 10.1038/s41929-022-00781-8. [DOI] [Google Scholar]
- 86.Merki D., Fierro S., Vrubel H., Hu X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2011;2:1262–1267. doi: 10.1039/C1SC00117E. [DOI] [Google Scholar]
- 87.Dionigi F., Strasser P. NiFe-based (Oxy)hydroxide catalysts for oxygen evolution reaction in non-acidic electrolytes. Adv. Energy Mater. 2016;6:1600621. doi: 10.1002/aenm.201600621. [DOI] [Google Scholar]
- 88.Gong M., Dai H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015;8:23–39. doi: 10.1007/s12274-014-0591-z. [DOI] [Google Scholar]
- 89.Yu M., Budiyanto E., Tüysüz H. Principles of water electrolysis and recent progress in cobalt-nickel-and iron-based oxides for the oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 2022;61:e202103824. doi: 10.1002/anie.202103824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Görlin M., Ferreira de Araújo J., Schmies H., Bernsmeier D., Dresp S., Gliech M., Jusys Z., Chernev P., Kraehnert R., Dau H., Strasser P. Tracking catalyst redox states and reaction dynamics in Ni–Fe oxyhydroxide oxygen evolution reaction electrocatalysts: the role of catalyst support and electrolyte pH. J. Am. Chem. Soc. 2017;139:2070–2082. doi: 10.1021/jacs.6b12250. [DOI] [PubMed] [Google Scholar]
- 91.Trotochaud L., Young S.L., Ranney J.K., Boettcher S.W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014;136:6744–6753. doi: 10.1021/ja502379c. [DOI] [PubMed] [Google Scholar]
- 92.Bau J.A., Luber E.J., Buriak J.M. Oxygen evolution catalyzed by nickel–iron oxide nanocrystals with a nonequilibrium phase. ACS Appl. Mater. Interfaces. 2015;7:19755–19763. doi: 10.1021/acsami.5b05594. [DOI] [PubMed] [Google Scholar]
- 93.Hunter B.M., Winkler J.R., Gray H.B. Iron is the active site in nickel/iron water oxidation electrocatalysts. Molecules. 2018;23:903. doi: 10.3390/molecules23040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou D., Xiong X., Cai Z., Han N., Jia Y., Xie Q., Duan X., Xie T., Zheng X., Sun X., Duan X. Flame-engraved nickel–iron layered double hydroxide nanosheets for boosting oxygen evolution reactivity. Small Methods. 2018;2:1800083. doi: 10.1002/smtd.201800083. [DOI] [Google Scholar]
- 95.Li X., Zha Q., Ni Y. Ni–Fe phosphate/Ni foam electrode: facile hydrothermal synthesis and ultralong oxygen evolution reaction durability. ACS Sustain. Chem. Eng. 2019;7:18332–18340. doi: 10.1021/acssuschemeng.9b03711. [DOI] [Google Scholar]
- 96.Chen M., Lu S., Fu X.-Z., Luo J.-L. Core–shell structured NiFeSn@NiFe (Oxy)Hydroxide nanospheres from an electrochemical strategy for electrocatalytic oxygen evolution reaction. Adv. Sci. 2020;7:1903777. doi: 10.1002/advs.201903777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asnavandi M., Yin Y., Li Y., Sun C., Zhao C. Promoting oxygen evolution reactions through introduction of oxygen vacancies to benchmark NiFe–OOH catalysts. ACS Energy Lett. 2018;3:1515–1520. doi: 10.1021/acsenergylett.8b00696. [DOI] [Google Scholar]
- 98.Sayler R.I., Hunter B.M., Fu W., Gray H.B., Britt R.D. EPR spectroscopy of iron- and nickel-doped [ZnAl]-layered double hydroxides: modeling active sites in heterogeneous water oxidation catalysts. J. Am. Chem. Soc. 2020;142:1838–1845. doi: 10.1021/jacs.9b10273. [DOI] [PubMed] [Google Scholar]
- 99.Nam D.-H., Bushuyev O.S., Li J., De Luna P., Seifitokaldani A., Dinh C.-T., García de Arquer F.P., Wang Y., Liang Z., Proppe A.H., et al. Metal–organic frameworks mediate Cu coordination for selective CO2 electroreduction. J. Am. Chem. Soc. 2018;140:11378–11386. doi: 10.1021/jacs.8b06407. [DOI] [PubMed] [Google Scholar]
- 100.Kang X., Li L., Sheveleva A., Han X., Li J., Liu L., Tuna F., McInnes E.J.L., Han B., Yang S., Schröder M. Electro-reduction of carbon dioxide at low over-potential at a metal–organic framework decorated cathode. Nat. Commun. 2020;11:5464. doi: 10.1038/s41467-020-19236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teh W.J., Piqué O., Low Q.H., Zhu W., Calle-Vallejo F., Yeo B.S. Toward efficient tandem electroreduction of CO2 to methanol using anodized titanium. ACS Catal. 2021;11:8467–8475. doi: 10.1021/acscatal.1c01725. [DOI] [Google Scholar]
- 102.Neukermans S., Samanipour M., Vincent Ching H.Y., Hereijgers J., Van Doorslaer S., Hubin A., Breugelmans T. A versatile in-situ electron paramagnetic resonance spectro-electrochemical approach for electrocatalyst research. Chemelectrochem. 2020;7:4578–4586. doi: 10.1002/celc.202001193. [DOI] [Google Scholar]
- 103.Neukermans S., Hereijgers J., Vincent Ching H.Y., Samanipour M., Van Doorslaer S., Hubin A., Breugelmans T. A continuous in-situ EPR electrochemical reactor as a rapid in-depth mechanistic screening tool for electrocatalysis. Electrochem. Commun. 2018;97:42–45. doi: 10.1016/j.elecom.2018.10.010. [DOI] [Google Scholar]