Abstract
Mucoadhesive polymers improve oral bioavailability of drugs by prolonging the duration of adhesion of drugs with mucosa. Various methods could be employed to address the problems of mucoadhesive polymers like weak adhesion forces. Chemical modification of polymers, such as the addition of a thiol group or thiolation, is another way for improving the polymers’ mucoadhesive properties that is studied in present research work. A novel thiomer of chitosan was prepared by attaching 2-mercaptobenzoic acid, a hydrophobic ligand onto it. The docking of thiomer and chitosan with mucin structure showed higher binding energy for former. The prepared thiomer was subjected to X-ray diffraction and DSC which established reduction in crystallinity and formation of a new compound through changes in glass transition, melting point and change in diffraction pattern. The NMR studies established conjugation of 2-mercapto benzoic acid to chitosan. The increased mucoadhesion in thiomer behaviour (2–3 fold) was confirmed through mucus glycoprotein assay as well as through texture analysis. The permeation enhancing the property of thiomer was established by demonstrating the permeation of phenol red across thiomer treated intestinal membrane. An in vitro cell toxicity assay was done to establish toxicity of chitosan and thiolated chitosan. Finally, the reduced water uptake of thiomer over chitosan proved that the increase in mucoadhesion is not contributed by swelling. Thus, a thiomer with improved mucoadhesion and enhanced permeation properties was prepared and characterized. Hence, all these properties render the newly synthesized polymer a better alternative to chitosan as an excipient for mucoadhesive drug delivery systems.
Keywords: Chitosan, Thiomer, Mucin, Mucoadhesion, Docking
Introduction
Mucoadhesive polymers have improved capabilities of numerous drug delivery systems. This has led to an increased interest in mucoadhesive polymers to improve oral bioavailability of drugs (Mythri et al. 2011; Roy et al. 2009). These polymers act as carriers to enhance the residence time of drugs in the body thus increasing contact time of the drugs with GI mucosa and in turn enhancing oral bioavailability (Boddupalli et al. 2010). Prolong residence time would lead to reduced dosing and would help in improving patient compliance. Chitosan is a naturally occurring polysaccharide obtained from deacetylation of chitin that exhibit mucoadhesive properties (Anjana Anil 2018; Sogias et al. 2008). In addition to mucoadhesive properties, chitosan displays non-toxicity and high biocompatibility. However, the mucoadhesive properties of chitosan are weak as they are attributed to electrostatic interactions between positively charged chitosan and negatively charged substructures of mucus (Agnihotri et al. 2004; Illum et al. 2001; Issa et al. 2005). The weak mucoadhesion reduces the residence time of dosage forms at the drug absorption site.
Hence, to overcome this drawback, chitosan is covalently attached with thiol bearing ligands. Thiols modify mucoadhesive properties of chitosan by formation of covalent bonds with subdomains of mucin glycoprotein. This modified chitosan, synthesized through disulfide exchange reaction, is called thiomer (Leichner et al. 2019; Sakloetsakun and Bernkop-Schnürch 2010). In addition to mucoadhesive properties, thiomers display enhanced permeation, PgP efflux inhibition, and enzyme inhibitory action. Thiomers have evolved as a potential drug delivery system by imitating the natural behaviour of secreted mucins that are also covalently anchored by disulfide bonds in the mucus (Gradauer et al. 2013; Shah et al. 2017).
In the present work, a novel lipophilic aromatic thiol ligand with higher reactivity was chosen to be covalently bound to chitosan to further enhance its mucoadhesion properties (Bernkop-Schnürch et al. 2003; Schmitz et al. 2008). Lipophilic thiol ligands increase residence time of the first generation of mucoadhesive polymers. Extensive literature can be found for hydrophilic ligands to develop mucoadhesive properties but much less literature is available for lipophilic ligands (Kafedjiiski et al. 2005; Maculotti et al. 2005; Ways et al. 2018). Therefore, there is an absolute need to explore mucoadhesive properties of lipophilic ligands in detail. Recently, 2-mercaptobenzoic acid-functionalized chitosan conjugate (Fig. 1), a novel thiomer has been synthesized (Marwaha et al. 2020). This thiomer exhibited enhanced mucoadhesion as compared to unmodified chitosan and other chitosan conjugates; therefore, in this study, the mucoadhesive property of this polymer has been further scrutinized.
Fig. 1.
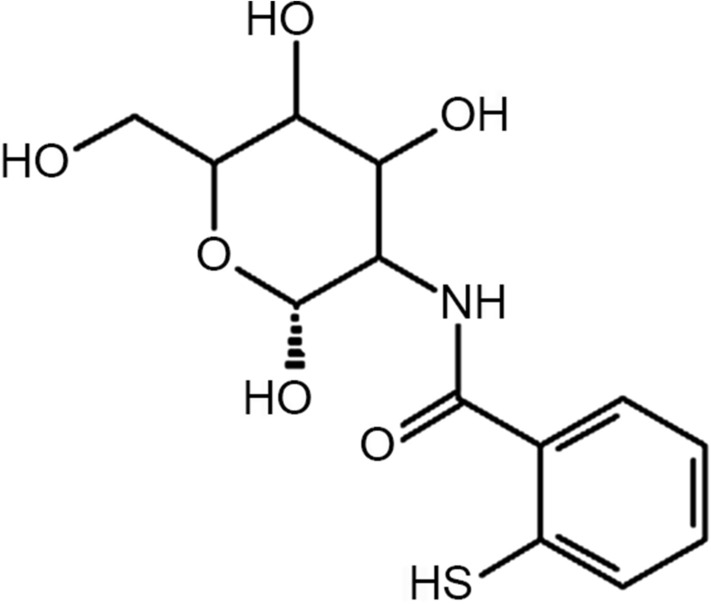
Structure of 2-mercaptobenzoic acid-functionalized chitosan
A distinctive approach to use molecular docking to determine interaction between chitosan mucin and modified chitosan mucin has been employed in this research work and the results are complemented by wet lab studies. Docking is a molecular simulation technique that uses scoring functions to assess the binding affinity between various ligands and target residues. This can minimize time and resources extensively used in search for a new excipient (Desai et al. 2012; Kaur et al. 2019). The aim of this research work is to further explore potential of novel thiolated chitosan polymer as an excipient for drug delivery system with a special focus on swelling behaviour, mucoadhesion, biocompatibility and permeability using unique methodology of molecular docking for determining interaction of mucin with thiolated chitosan and compare it with unmodified chitosan.
Materials and methods
Materials
Docking software
The 2D structure of chitosan and chitosan derivative, chit-2-mercapto benzoic acid was sketched and converted to 3D model using software Chem3D Ultra version 16.0 of Chem Office suite. The structure of mucin (Fig. 2) was procured from Protein Data Bank (PDB ID: 6BSC). Different modules of Schrödinger suite version 9 were used to perform docking.
Fig. 2.

Mucin structure
Chemicals and reagents
Chitosan (low molecular mass, 120 kDa; degree of deacetylation, 85–90%) was purchased from Analab Fine Chemicals, 2-mercapto benzoic acid, N-hydroxysuccinimide (NHS), ethyl (dimethylaminopropyl) carbodiimide (EDAC), dioxane, schiff’s reagent, sodium metabisulphite was purchased from Loba Chemicals Pvt. Ltd. (Mumbai), sodium borohydride was purchased from Baker&Baker, periodic acid was received as a gift sample from DNS Fine Chemicals and Lab Pvt. Ltd. (Mumbai). All other chemicals used were of analytical grades.
Methodology
In silico molecular docking studies
Ligand preparation
Three-dimensional structure of the ligands, unmodified chitosan and 2-mercapto benzoic acid, combined with chitosan were generated from PM3 semi-empirical calculations using Chem3D. The ligands were converted to mol format using LigPrep 2.3 module of Schrödinger suite. The molecule was subjected to optimization using merck molecular force field to achieve the lowest free energy state within the protein binding site (LigPrep 2009).
Protein preparation
The structure of mucin protein (PDB ID: 6BSC) was down-loaded from the Protein Data Bank (http://www.pdb.org). The structure was imported in protein preparation wizard of Maestro (9.0) and prepared by a multi-step process. It comprised of removal of water molecules that did not contribute to any interaction and addition of hydrogen atoms. After preparation, the structure was minimized and optimized using optimized potential for liquid simulations (OPLS-2005) force field of Schrödinger Suite Version 9 (Protein Preparation wizard 2009).
Docking
Glide application of Maestro was used to generate the receptor grid followed by docking of ligands into the protein using Glide docking protocol. The docked conformers were ranked according to the Glide score empirical scoring function which is based on combination of several parameters like electrostatic and van der Waal’s interactions (Glide 2009).
Synthesis of chitosan-2-mercapto benzoic acid conjugate
The synthesis of thiomer was carried out as described in detail earlier. Briefly, chitosan was allowed to swell in 0.1 M Acetic acid pH 3 overnight under continuous stirring to obtain 1% (w/v) polymer solution. To this, 0.5 g thiosalicylic acid dissolved in 50 mL dioxane was added slowly under continuous stirring. The pH was adjusted to 5 with 0.5 M NaOH. EDAC and NHS were added in a concentration of 25 mM each under vigorous stirring to the above solution. The reaction was allowed to proceed at pH 6 for 48 h at room temperature under continuous stirring. Sodium borohydride solution in concentration of 2% (w/v) in ethanol was added to this solution at pH 5 and incubated for 24 h at room temperature to reduce any disulfide bond formed during the reaction. The resultant reduced conjugated polymer was further dialyzed in tubing (mol mass cut off 12 kDa) first against 2.5 L demineralized water with 7 mM HCl, twice against 7 mM HCl containing 1% NaCl, once against 5 mM HCl and in the end against 1 mM HCl at 8 °C in the dark. The solution was further lyophilized and stored in refrigerator for further use (Marwaha et al. 2020).
Characterization
X-ray diffraction (XRD)
XRD analysis was carried out to investigate the crystalline nature and interactions of chitosan and thiolated chitosan. XRD studies were carried out using a Bruker D8 Advance X-ray Diffractometer. The wavelength of the X-radiation was 1.5403 Å and the diffraction pattern was recorded for 2θ values between 5° and 60° (Han et al. 2012).
Differential scanning calorimetry (DSC)
The thermal properties of chitosan and thiomer was determined using DSC(DSC 823, Mettler Toledo, Melbourne, Australia) using a water-cooling accessory and nitrogenas a purge gas with flow rate of 10 mL.min−1. Samples weighing 4 mg hermetically sealed in aluminium pans (Al-Crucibles, 40 Al) were subjected to analysis with a reference cell which consists of an empty hermetically sealed aluminium pan. The samples were initially heated from 30 °C to 200 °C at a heating rate of 10 °C.min−1 to remove any trace amount of solvent and trapped moisture, then the samples were cooled down to 30 °C at the cooling rate of –10 °C.min−1 to remove the thermal history of the samples. It was followed by reheating the samples to 250 °C at the same heating rate. The DSC results were analysed and the percent crystallinity of chitosan and thiomer was further calculated using Star software (Correlo et al. 2005; Marwaha et al. 2020).
Nuclear magnetic resonance (NMR)
As the polymer sample did not dissolve in any of the commonly used solvents for NMR, the sample was subjected to C13 Cross-Polarization Magic Angle Spinning (CPMAS) solid state NMR analysis. Solid-state 13C CPMAS NMR studies were carried out on a Jeol-JNM-ECX400II 400 MHz spectrometer at resonance frequencies of 100.6 and 399.78 MHz, respectively, and the chemical shifts for 13C spectra were referenced to Tetramethyl silane at 0 ppm (Kazemi et al. 2019).
In vitro cytocompatibility studies
HEK-293 cell line was used in this study. The cell line was subcultured in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% foetal bovine serum (FBS) and developed to confluency in a humidified environment at 37 °C and 5% CO2 in a CO2 incubator. The cells were split and utilised for experiments after the third passage. After reaching the confluency, the cells were trypsinized with 0.025% Trypsin in PBS/ 0.5 mM EDTA solution. The cell suspension was centrifuged for 3 min at 3000 rpm and then resuspended for further tests in the growth medium. The cell suspension was quantified using hemocytometry and further diluted with culture medium appropriately to a cell density of 1 × 105 cells/mL. Furthermore, the cells were placed into 96-well tissue culture plates at a density of 10,000 cells in each well and incubated for 24 h to assure 70–80% confluency.
Different concentrations of thiolated chitosan and unmodified chitosan were prepared (0–30 µg/mL) and added to wells in triplicate as 100 μL quantities. The blank wells constituted only 100 μL of medium. The plates were incubated for 24 h after which the supernatant and test material was removed. The cells were washed and fresh media along with 30 μL of MTT solution (MTT -5 mg/mL dissolved in PBS) was added. The plates were wrapped with aluminium foil for protection from light and incubated at 37 °C for 4 h. The MTT solution was removed and 100 μL of DMSO was added to each well to dissolve the formazan crystals formed. After incubating at room temperature for 30 min, the solution was centrifuged, and the absorbance was recorded on an ELISA microplate reader (ELISASCAN, ERBA) at 570 nm using DMSO as blank (Kazemi et al. 2019; Oliveira et al. 2013). Percentage viability was calculated as follows:
Percentage viability = (OD of Test/ OD of Control) × 100.
Control and tests were done in triplicate and the results are expressed as mean ± SD, where n = 3 (Calculated using Microsoft Office Excel 2007).
Mucoadhesion studies in vitro
Five mucin standard solutions (0.1, 0.2, 0.3, 0.4 and 0.5 mg/mL) were prepared in three isoosmotic solutions differing in pH, namely simulated gastric fluid (SGF pH 1.2), 0.1 N sodium acetate buffer (pH 4.0) and simulated intestinal fluid (SIF pH 6.4). The mucin standard solutions prepared in different media were further diluted to obtain concentration of 0.1, 0.2, 0.3, 0.4 and 0.5 µg/mL. These standard solutions were used to plot standard calibration curves which were used for further studies (Juntapram et al. 2012).
Mucus glycoprotein assay Mucins, glycoproteins, and polysaccharides are analyzed qualitatively and quantitatively in tissues and cells by using the periodic acid Schiff (PAS) method. Both Schiff’s reagent and periodic acid reagent were freshly prepared. Periodic acid reagent 0.1 mL was added to mucin standard solutions prepared in different media. The solutions were incubated at 37 °C for 2 h followed by addition of 0.1 mL Schiff reagent and further incubated for 30 min at room temperature. The solutions were further subjected to colorimetric analysis using UV spectrophotometer and the absorbance was recorded at 555 nm. The free mucin content was calculated with reference to the standard calibration curves plotted.
Adsorption of mucin on chitosan and thiolated chitosan The free mucin content was determined using PAS colorimetric assay in order to estimate the amount of mucin adsorbed onto chitosan and its derivative. Mucin solution of 0.5% (w/v) concentration was prepared in three different solutions with pH 1.2, 4.0 and 6.4. Chitosan and its derivative were dispersed in the above three different media at a concentration of 20 mg/1.5 mL followed by shaking in orbital shaking incubator for 2 h at 37 °C. The dispersions were then centrifuged at 10,000 rpm for 5 min to sediment chitosan mucin and thiolated chitosan mucin complex. The supernatant obtained was separated and subjected to free mucin content determination with the help of UV spectrophotometer. The concentration of mucin was determined with reference to the calibration curve plot. The amount of mucin adsorbed on chitosan and its derivative was calculated as the difference between the total amount of mucin added and the free mucin content determined in the supernatant.
Mucoadhesion studies ex vivo
Mucoadhesion studies were carried out ex vivo using compressed discs of unmodified chitosan and freeze-dried chitosan thiosalicylic acid conjugate. Briefly, 50 mg of a weighed quantity of each was compressed into 13.0 mm diameter using a manual hydraulic pellet press (Model M-15, technosearch instruments, Mumbai, India). A constant compaction force of 4 Ton was maintained throughout the disc preparation (Severian and Andreas 2001).
Mucoadhesion strength with dry polymer compacts Mucoadhesion experiments were performed using a texture analyzer (CEB Texture Analyzer, Brookfield Engineering Labs, Inc., Model Texture Pro CT V1.4 Build 17) with thiomer and unmodified chitosan tablets (13 mm) on freshly excised goat intestinal mucosa. Using a double-sided adhesive tape, the tablet was attached to a 10 mm cylindrical probe (TA 3/100 probe). After attaching the mucosa to the cylinder, the probe was lowered at a steady speed of 1 mm/s to contact mucosa with 0.5 N compressive force and 1000 g load cell. The mucosa was pulled from the tablet at a speed of 1 mm/s after a certain contact time. At 60 s and 600 s hold time the hardness, total work of adhesion representing the overall positive area under the force-distance curve, adhesiveness representing the overall negative area and the maximum detachment force were determined.
Mucoadhesion studies with hydrated polymers To minimize the influence of adhesion by hydration the tensiometer studies were carried out with hydrated polymer. The tablet was attached to a 10 mm cylindrical probe (TA 3/100 probe) using a double-sided adhesive tape. The mucosa was attached to a cylinder dipped in a beaker containing 0.1 M phosphate buffer pH 6.8 at 37 °C. The test was performed as mentioned above.
Ex vivo drug permeation using non-everted rat intestinal sac method
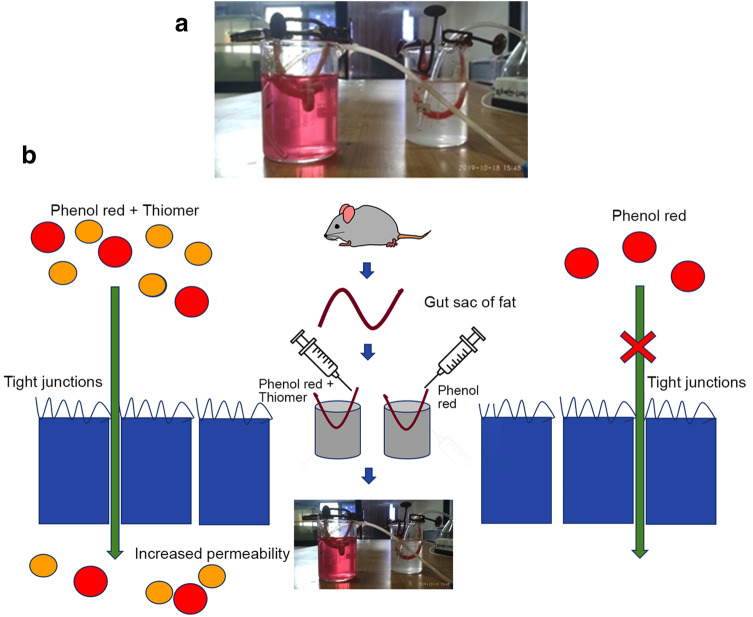
Two freshly excised rat intestinal (duodenum) segments were isolated and washed with Kreb’s-Ringer solution. The sac was created by tying non-everted tissue of 5 cm length at one end and filling it with 10% solution of phenol red solution. To the other segment, 50 mg thiomer was additionally inserted. The sacs were immersed in the Kreb’s-Ringer solution contained in the beakers. After 15 min, aliquots of Krebs solution in beakers were removed and analyzed to quantify phenol red permeated across intestinal segment (Da Silva et al. 2015; Swenson et al. 1994).
Evaluation of the swelling behavior
The swelling behavior of thiomer was determined in terms of weight gain by the tablet. The unmodified chitosan and thiomer were submerged in a petri dish containing 0.1 M phosphate buffer pH 6.8 at 37 °C. At specific time intervals, the tablets were taken out of the medium and weighed (Shargel et al. 2012).
Statistical data analysis
The statistical data analysis was carried out using the t test with p < 0.05 as the minimum level of significance.
Result and discussion
Docking
Docking of chitosan and thiomer with mucin
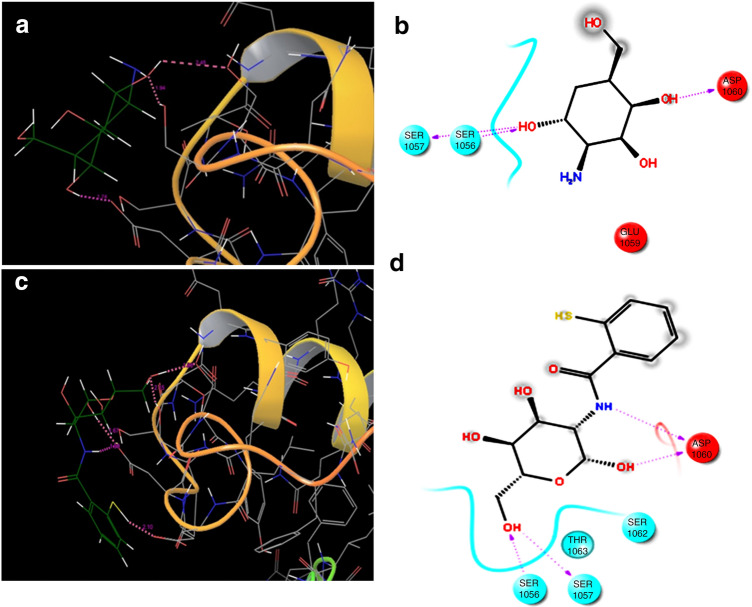
A distinctive approach of molecular docking has been used as a comparative tool in this research work to study mucoadhesion of chitosan and thiolated chitosan with mucin. Extensive literature search exhibited a need for molecular docking approach in the field of formulation and development. This is an upcoming field which is being explored that helps to bring down the wet lab studies (Kaur et al. 2018). However, the limitation of the implemented in silico molecular docking approach to study binding affinity of thiolated chitosan with mucin is the presence of rigid receptor that acts as a key constraint of molecular docking (Sethi et al. 2019). A strong interaction exists between mucin and thiolated chitosan that result in improved mucoadhesion. Molecular docking was performed to study and compare mucoadhesion of mucin with chitosan with the prepared thiomer. In this study, protein structure of mucin was docked with 3D structures of chitosan and thiolated chitosan. Four parameters were considered to rank the ligands viz; Glide score, Glide energy, Emodel and number of hydrogen bond formation. The docking results and docked conformations are illustrated in Table 1 and Fig. 3. The minimum values of Glide score, Glide energy and E model depict the enhanced binding affinity of ligand with the protein.
Table 1.
Docking results of chitosan and thiomer with mucin
| Ligand | Ligand atom | Protein residue | H-bond length (Ao) | Glide score (kcal/mol) | Glide energy (kcal/mol) | Emodel (kcal/mol) |
|---|---|---|---|---|---|---|
| Chitosan | H | SER 1056 (O) | 2.45 | − 1.711 | − 3.882 | − 19.7 |
| O | SER 1057 (H) | 1.94 | ||||
| H | ASP 1060 (O) | 1.74 | ||||
| Thiomer | O | SER 1056 (H) | 2.10 | − 2.512 | − 4.179 | − 34.093 |
| H | SER 1057 (O) | 1.93 | ||||
| H | ASP 1060 (O) | 1.67 | ||||
| H | ASP 1060 (O) | 1.99 | ||||
| H | GLU 1059 (O) | 2.10 |
Fig. 3.
Docked complex of mucin and chitosan: a dashed red line indicate hydrogen interaction in between target residue and ligand, b structural view; red dotted lines represent hydrogen bond side chain, c docked complex of mucin and thiomer with dashed red line indicate hydrogen interaction in between target residue and ligand, and d structural view with red dotted lines represent hydrogen bond side chain
Characterization of chitosan-2-mercaptobenzoic acid
Thiomer was synthesized by grafting 2-mercaptobenzoic acid on chitosan backbone using EDAC and NHS as coupling agents. The synthesis was carried out by activating carboxylic groups of 2-mercaptobenzoic acid with EDAC/NHS followed by their conjugation to primary amine in the chitosan backbone. The synthesized thiomer was light yellow in color, odourless and fibrous in nature. It was non-dissolvable in water and organic liquids. The novel conjugate exhibited solubility in acidic aqueous solution.
Evaluation
XRD
Powder X-ray diffraction patterns of chitosan and thiomer were measured to investigate the change in crystalline nature of chitosan after conjugation. XRD is used frequently to identify or confirm amorphous, semi-crystalline, or crystalline nature of polymer and their conjugates. It has proved to be a useful tool to study arrangements of crystal lattice and provide significant information on degree of crystallinity. X-ray diffractograms of chitosan and thiomer are demonstrated in Fig. 4. The XRD pattern of pure chitosan showed two sharp diffractions at 21.3°, 29.8° and one broad peak at 13.4°. The high intensity peak of pure chitosan at 21.38o attributes to its crystalline nature. This peak becomes weak and shifts to 24.1o in thiomer that corresponds to alteration in crystal structure which is probably due to the lower amount of free amino groups. Introduction of thiol groups disrupts the crystalline structure of chitosan, particularly through the loss of intermolecular hydrogen bonding. The width of the X-ray diffraction peak depends on the crystallite size; imperfect crystals usually display broad peaks. The semi-crystalline peak displayed by pure chitosan at 13.4° is shifted to 10.1° in thiomer, it increases in intensity and becomes broader. This broad peak indicates disturbance in crystalline form that may have occurred due to the formation of thiolated chitosan derivative. The presence of two more weak peaks in thiomer at 28.7° and 33.2o confirmed the conjugation and formation of a new polymer. The peak area corresponds to the reduced percent crystallinity in thiomer thus imparting partial amorphous character, which may improve its mucoadhesive properties and biodegradability.
Fig. 4.
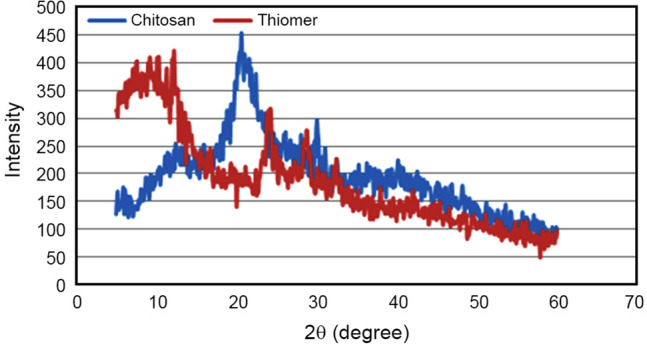
XRD results of chitosan and thiolated chitosan (thiomer)
DSC
The DSC results of chitosan and thiolated chitosan are shown in Table 2. The DSC curve shows melting of crystalline part of polymer. The melting area has been integrated to obtain heat flow. The glass transition temperature of chitosan and thiomer was observed to be 70 °C and 115 °C, respectively. The increase in Tg can be attributed to restricted movement of polymer chain due to crosslinking with thiol moiety thus indicating an increase in molecular weight of chitosan. A sharp endothermic peak was observed at 175 °C in chitosan graph, attributed to its melting point. The melting temperature (Tm) and heat of fusion (∆Hm) of chitosan and thiomer were determined from the maximum melting peak and area of the melting peak, respectively (Marwaha et al. 2020). The heat of fusion is the integral of heat flow with respect to time. It was observed that thiomer exhibited reduced heat of fusion and degree of crystallinity as compared to unmodified chitosan which may be due to crosslinking of thiol moiety on polymer backbone. The decrease in crytallinity can be attributed to hydrogen bond interactions between –NH2 groups of chitosan and –COOH groups of thiols. Such interactions take place in amorphous state, thus suppressing the crystallinity of chitosan. The results obtained established a good correlation with the XRD results. The glass transition temperature (Tg), melting temperature (Tm), heat of fusion (∆Hm) and percentage of crystallinity (χc ) of chitosan and thiolated chitosan are summarized in Table 2.
Table 2.
DSC results of chitosan and thiomer
| Polymer | Tg (oC) | Tm (oC) | ∆Hm (J/g) | χc (%) |
|---|---|---|---|---|
| Chitosan | 70 | 175 | 58.46 | 100.01 |
| Thiomer | 115 | 220 | 10.07 | 82.82 |
The heat and cool cycles were used to determine glass transition temperature (Tg) and melting temperature (Tm) through endothermic and exothermic reactions, respectively.
NMR
For solid samples, C13 Magic Angle Spinning solid state NMR analysis was performed as it helps to understand chemical functionality. It is a very important technique for understanding the structure of material at molecular level and confirming the attachment of ligand to the polymer backbone. C13NMR data provided evidence that thiol ligand is attached to chitosan backbone.
Figure 5 shows the NMR spectra of pure chitosan and thiomer. The solid state C13NMR spectra of chitosan showed signal at 24.12 ppm corresponding to the C8 methyl group in acetamido moiety. The C2 and C6 ring signal appeared at 58.06 and 61.55 ppm, respectively, the C3 ring signal appears at 76.04 ppm, the C4-C5 ring signal appears at 83.46 ppm, C1 ring signal appears at 105.74 ppm and the carbonyl group signal belonging to acetamido moiety appeared at 175.89 ppm displaying less intensity. Thus, we can conclude from this that the assigned signals agree with NMR data in the literature.
Fig. 5.

C13NMR spectra of chitosan and thiomer
The spectrum shows additional peaks between 110 and 175 ppm which can be assigned to aromatic ring carbon and a new absorption peak observed at 156.351 ppm related to amide carbonyl group reveal the graft polymerization of mercaptobenzoic acid onto chitosan. Thus, the peaks observed indicate that the thiol ligand was incorporated into the chitosan matrix.
Cytotoxicity assay
The biocompatibility of newly synthesized thiomer was compared with unmodified chitosan using MTT assay in HEK293 cell line. For its functional use in drug delivery systems, determining the toxicity profile of this newly synthesized excipient is significant. Work has been reported on this cell line to evaluate the toxicity of a newly synthesized polymer and to evaluate biocompatibility of drug delivery systems. MTT assay is a colorimetric test based on viable cells’ selective ability to reduce MTT in purple colored formazan crystals that show absorbance maxima at 570 nm. The absorption of blank wells comprising of cells in the medium was considered as that corresponding to 100% proliferation and the relative proliferation of the cells in the treated wells was measured. The absorption produced is directly proportional to the number of cells that are viable. A graph of percentage viability of cells versus concentration of chitosan and thiomer in μg/mL was plotted based on this as shown in Fig. 6.
Fig. 6.

Percentage viability of cells versus concentration of chitosan and thiomer; indicated values are means (± SD) of at least three experiments
A concentration-dependent cytotoxicity profile of thiomer and chitosan as measured by MTT has been found in the present study. At low concentrations, thiomer showed lower cytotoxicity as compared to chitosan which can be attributed to the amide groups and sulfhydryl groups that are commonly found functional groups in the body making the polymer more accepted as compared to unmodified native polymer. For cells in contact with higher concentrations of both polymers, cell proliferation decreased. From the results, thiolation is seen to have a positive impact on the biocompatibility properties of the chitosan and can contribute to safer derivatives of polymer that are already considered safe for use as drug delivery vehicle.
Mucoadhesion studies in vitro
Mucus glycoprotein assay
In this study, we used mucus glycoprotein assay to assess the mucoadhesive behaviour of chitosan and its derivative. The PAS colorimetric assay was used to determine free mucin concentration which in turn can be used to evaluate the amount of mucin adsorbed onto the chitosan and its derivative. In this method, periodic acid is used as oxidising agent to oxidize compounds having free hydroxyl or amino groups resulting in formation of dialdehydes. These dialdehydes are then exposed to Schiff’s reagent resulting in formation of an insoluble purple-magenta complex. Porcine mucin type II was selected for this study as it is widely used in mucoadhesive assays due to its lower batch-to-batch variability and higher reproducibility. Mucin should become adsorbed onto chitosan surface and its derivative spontaneously owing to the strong interaction between mucin and chitosan or its derivatives. Thus, the mucoadhesive behaviour of chitosan and thiomer was analyzed spectrophotometrically that allowed the measurement of even much diluted mucin solutions. A linear relationship was obtained between quantity of mucin and the absorbance at 555 nm using linear regression equations for all three different pH media solutions. The results indicate that as the mucin concentration increases, so does the amount of mucin adsorbed.
Adsorption of mucin on chitosan and thiolated chitosan
The mucins which belong to the family of highly glycosylated proteins are the main component of mucus. The mucus function varies with mucin characteristics and environmental conditions owing to the repulsive electrostatic forces that appear on mucins because of adverse charge on mucin surface at pH > 2. The pKa values for sialic acid (saccharide acid), mucin (glycoprotein), 2-mercapto benzoic acid and chitosan are 2.6, 3–5, 5.8 and 6.5, respectively. The amount of mucin adsorbed onto chitosan and thiomer varied with pH, because degree of ionization of sialic acid and mucin are affected by changes in environmental pH.
It was observed from the results depicted in Fig. 7 that amount of mucin adsorbed on chitosan and thiomer was less in pH above 6 and increased with decrease in pH. It can be due to the electrostatic and hydrophobic effects on mucoadhesion of chitosan and its derivative that are prominent in acidic pH. In acidic media due to the electrostatic effect, the remaining NH3+ moieties on chitosan backbone interact with COO− or SO3− groups on mucin carbohydrate side chain. Whereas in hydrophobic effect, the –CH2 moieties of 2-mercaptobenzoic acid interact with the –CH3 groups on the mucin side chain that results in high mucoadhesive adsorption. However, a significant increase in mucin adsorption on thiomer as compared to unmodified chitosan was observed in acetate buffer and SGF. It might be due to the presence of thiol bearing side chains in the thiolated polymer that form covalent disulfide bonds with cysteine-rich subdomains of mucus glycoproteins. The greater the thiol group density covalently attached to copolymer, the greater the mucin content that would bind onto the polymer. In SIF, the quantity of mucin adsorbed was higher in chitosan relative to thiomer, which was likely due to the reduction in thiomer solubility in pH above 6. The estimation of mucin adsorption ability can be used to determine mucoadhesive character of chitosan and its derivative. At low pH range, thiomer showed significantly higher mucoadhesion as compared to chitosan. This might be due to change in pKa of the synthesized thiomer.
Fig. 7.
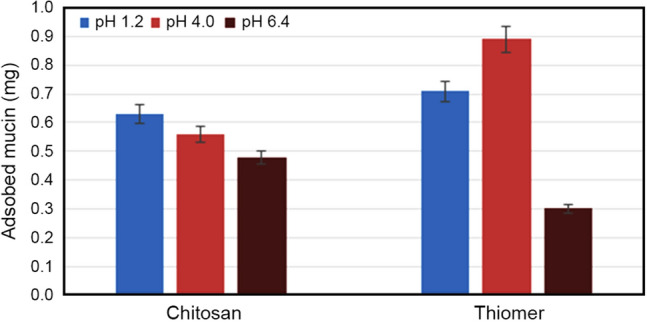
Adsorption of mucin on chitosan and thiomer. Data are shown as mean ± SD and are derived from three independent repeats
Mucoadhesion studies
The mucoadhesive strength of the polymer depends on several factors such as the molecular weight of the polymer, polymer concentration, water uptake, swelling and contact time with the mucosal membrane. The mucoadhesion analysis was performed using texture analyzer.
Texture analyzer was used to determine the mechanical properties of chitosan and thiomer such as hardness, adhesiveness and adhesive force using goat intestinal mucosa. In comparison to the unmodified chitosan, the thiolated polymer displayed greater mucoadhesive strength. Chitosan’s mucoadhesive characteristics are due to formation of hydrogen bonds with mucin, whereas thiolated polymers exhibit mucoadhesive properties due to formation of covalent disulfide bonds with cysteine-rich subdomains of mucus glycoprotein. The hydrogen bonds display higher bond strength in comparison to the bond strength of covalent bonds. Thus, higher mucoadhesive strength of thiolated polymers is due to its higher bond strength.
Hardness is the force needed to achieve a certain deformation. For a polymer to attach itself to the mucosa reduced hardness and greater flexibility are desired. The two important variables used to determine mucoadhesive characteristics are adhesive force and adhesiveness. The results are illustrated in Table 3. The mucoadhesive strength is defined as the maximum force needed to separate the tablet from the mucosal surface and adhesiveness is the work required to overcome attractive forces between the tablet surface and the mucosa. Though initially the adhesiveness of chitosan was more as compared to thiomer however, in thiomer an increase in adhesive strength is noted over time, while the adhesiveness of unmodified chitosan is lowered over time. Thus, thiomer provides stronger mucoadhesive force for longer period, whereas the mucoadhesive strength of chitosan fades with time resulting in mucoadhesion for a shorter period. Thiomer has exhibited a two-fold increase in mucoadhesive strength in dry polymer analysis and a three-fold increase in hydrated polymer analysis as compared to unmodified chitosan based on the results of texture analyzer.
Table 3.
Mechanical and mucoadhesive properties of chitosan and modified chitosan
| Time (s) | Dry polymer | Hardness (g) | Adhesive force (g) | Adhesiveness (mJ) |
|---|---|---|---|---|
| 60 | Chitosan | 46.40 ± 0.011 | 2.60 ± 0.013 | 0.33 ± 0.022 |
| Thiomer | 47.30 ± 0.001 | 5.00 ± 0.021 | 0.17 ± 0.003 | |
| 600 | Chitosan | 49.10 ± 0.089 | 2.72 ± 0.002 | 0.24 ± 0.081 |
| Thiomer | 47.80 ± 0.024 | 6.10 ± 0.005 | 0.57 ± 0.005 |
| Time (s) | Hydrated polymer | Hardness (g) | Adhesive force (g) | Adhesiveness (mJ) |
|---|---|---|---|---|
| 60 | Chitosan | 32.65 ± 0.005 | 2.45 ± 0.009 | 0.35 ± 0.003 |
| Thiomer | 41.25 ± 0.003 | 7.05 ± 0.126 | 0.27 ± 0.021 | |
| 600 | Chitosan | 37.01 ± 0.006 | 2.58 ± 0.007 | 0.21 ± 0.007 |
| Thiomer | 48.20 ± 0.002 | 8.54 ± 0.008 | 0.62 ± 0.002 |
A good correlation is exhibited by the results obtained through docking studies with the results of tensile studies which indicate values of means (± SD) of at least three experiments.
Ex vivo drug permeation studies
The in situ single pass intestinal perfusion technique was used to investigate the intestinal permeability of thiomer in rat duodenum. A single layer of epithelial cells separates the intestinal lumen from the underlying lamina propria in the human intestinal epithelium. Tight junctions seal the area between these cells and regulate the permeability of the intestinal barrier (Ulluwishewa et al. 2011). Phenol red acts as a non-absorbable marker compound and due to its size cannot permeate through the mucosal membrane. Due to the tightness of the junction, the membrane electrical resistance is high, and the paracellular migration of phenol red across the monolayer of intestinal epithelium is restricted. Therefore, in our study, the absence of phenol red in the receiver compartment of standard confirmed intactness of mucosal membrane as shown in Fig. 8. Presence of phenol red in the receiver compartment of the test confirmed the opening of tight junctions and hence increased permeation by synthesized thiolated chitosan (Mateer et al. 2016; Tariq et al. 2015).
Fig. 8.
Ex vivo permeation study for thiomer (test) and chitosan (standard) using a non- everted rat intestinal sac method and b Scheme for ex-vivo permeation
Swelling behaviour
It is one of the significant parameters to be considered while evaluating mucoadhesive polymers. When mucoadhesive polymer comes in contact with mucosa it wets and swells up due to capillary effect and thus it adheres to the mucosa. This prolongs the residence time, in turn, increases absorption of drugs. The cohesive and adhesive behaviour of the polymer can be determined with the help of swelling studies. Overhydration of polymer due to excessive water uptake can lead to decreased adhesiveness. Therefore, covalent attachment of chitosan to lipophilic ligand thiosalicylic acid results in imparting hydrophobic characteristic to chitosan thus reducing its swelling properties and increasing adhesive properties. Water uptake studies are shown in Fig. 9. The water uptake in the graph is expressed in milligram over a period of 420 min. This behaviour is attributed to the lipophilic nature of the ligand.
Fig. 9.
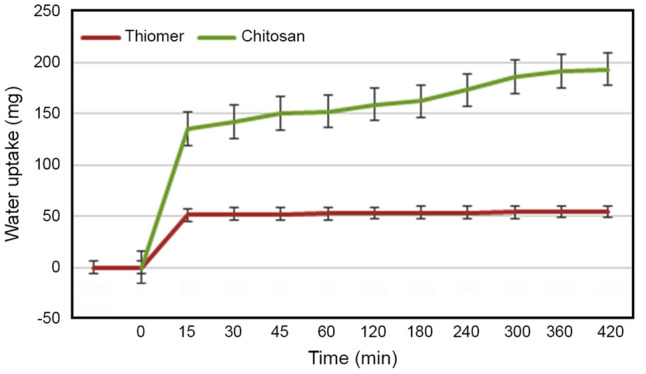
Swelling behaviour evaluation of chitosan and thiomer; with indicated values which are means (± SD) of at least three experiments
Conclusion
In the present work, we have made an effort to utilize in silico technique to gauge the interaction of chitosan and thiomer with mucin to explore their mucoadhesive properties that was further confirmed by wet lab studies. The characterization of thiomer was done for its swelling behaviour, permeability, including toxicity in cell line studies. The thiolated chitosan has high mucoadhesion and has capability to improve permeation by opening tight junctions of the cell wall. The low swelling behaviour of the thiomer was responsible for prolonged mucoadhesion. Due to the conjugation of aromatic hydrophobic moiety to chitosan, the polymer was prone to less water absorption that led to enhanced mucoadhesive property. Moreover, the cytocompatibility and permeability of the chitosan was improved after modification with 2-mercaptobenzoic acid. Thus, the thiolated chitosan synthesized can prove to be good excipient in drug delivery of BCS class III and IV drugs. Therefore, this novel polymer can be further explored to develop better pharmaceutical formulations that will contribute to future needs.
Acknowledgements
The authors gratefully acknowledge the assistance provided by the Department of Biotechnology, Savitribai Phule Pune University for carrying out cytotoxicity studies. Authors are also thankful to University Grants Commission for the financial assistance provided by them to undertake this research work. We also express sincere thanks to Principal, Dr. Ashwini Madgulkar, AISSMS College of Pharmacy, Pune for providing necessary facilities to carry out this research work.
Abbreviations
- PDB
Protein data bank
- DSC
Differential scanning calorimetry
- NMR
Nuclear magnetic resonance
- XRD
X-ray diffraction
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- DMSO
Dimethyl sulphoxide
- NHS
N-Hydroxysuccinimide
- EDAC
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
- PAS
Periodic acid schiff
- Thiomer
Thiolated chitosan
Funding
The authors have no relevant financial or non-financial interests to disclose.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The Institutional Research Ethics Committee has confirmed that no ethical approval is required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. In J Contr Rel. 2004;100(1):5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Anjana Anil PS. Mucoadhesive polymers: a review. J Pharm Res. 2018;17:47–55. [Google Scholar]
- Bernkop-Schnürch A, Hornof M, Zoidl T. Thiolated polymers—Thiomers: synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int J Pharma. 2003;260(2):229–237. doi: 10.1016/S0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- Boddupalli BM, Mohammed ZNK, Nath AR, Banji D. Mucoadhesive drug delivery system: an overview. In J Adv Pharma Technol Res. 2010;1(4):381–387. doi: 10.4103/0110-5558.76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correlo VM, Boesel LF, Bhattacharya M, Mano JF, Neves NM, Reis RL. Properties of melt processed chitosan and aliphatic polyester blends. Mat Sci Eng A. 2005;403:57–68. doi: 10.1016/j.msea.2005.04.055. [DOI] [Google Scholar]
- Desai N, Mahto MK, Alekhya B, Naveen CR, Bhaskar M. Comparative docking studies of Estrogen Receptor inhibitors and their binding interaction analysis. Int J Pharm Sci Rev Res. 2012;16(1):91–95. [Google Scholar]
- Glide (5.5). (2009). Schrödinger Suite, LLC
- Gradauer K, Dünnhaupt S, Vonach C, Szöllösi H, Pali-Schöll I, Mangge H, Jensen-Jarolim E, Bernkop-Schnürch A, Prassl R. Thiomer-coated liposomes harbor permeation enhancing and efflux pump inhibitory properties. J Control Rel. 2013;165(3):207–215. doi: 10.1016/j.jconrel.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Wei Y, Jia X, Xu J, Li G. Correlation of the structure, properties, and antimicrobial activity of a soluble thiolated chitosan derivative. J Appl Polym Sci. 2012 doi: 10.1002/app.36548. [DOI] [Google Scholar]
- Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51(1–3):81–96. doi: 10.1016/S0169-409X(01)00171-5. [DOI] [PubMed] [Google Scholar]
- Issa MM, Köping-Höggård M, Artursson P. Chitosan and the mucosal delivery of biotechnology drugs. Drug Disco Today: Technol. 2005;2(1):1–6. doi: 10.1016/j.ddtec.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Juntapram K, Praphairaksit N, Siraleartmukul K, Muangsin N. Synthesis and characterization of chitosan-homocysteine thiolactone as a mucoadhesive polymer. Carbohyd Polym. 2012;87(4):2399–2408. doi: 10.1016/j.carbpol.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Kafedjiiski K, Krauland AH, Hoffer MH, Bernkop-Schnürch A. Synthesis and in vitro evaluation of a novel thiolated chitosan. Biomaterials. 2005;26(7):819–826. doi: 10.1016/j.biomaterials.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Kaur T, Madgulkar A, Bhalekar M, Asgaonkar K. Molecular docking in formulation and development. Curr Drug Discov Technol. 2018;16(1):30–39. doi: 10.2174/1570163815666180219112421. [DOI] [PubMed] [Google Scholar]
- Kaur T, Madgulkar A, Bhalekar M, Asgaonkar K. Molecular docking in formulation and development. Curr Drug Discov Technol. 2019;16(1):30–39. doi: 10.2174/1570163815666180219112421. [DOI] [PubMed] [Google Scholar]
- Kazemi MS, Mohammadi Z, Amini M, Yousefi M, Tarighi P, Eftekhari S, Rafiee Tehrani M. Thiolated chitosan-lauric acid as a new chitosan derivative: Synthesis, characterization and cytotoxicity. Int J Bio Macromol. 2019;136(1):823–830. doi: 10.1016/j.ijbiomac.2019.06.132. [DOI] [PubMed] [Google Scholar]
- Leichner C, Jelkmann M, Bernkop-Schnürch A. Thiolated polymers: bioinspired polymers utilizing one of the most important bridging structures in nature. Adv Drug Deliv Rev. 2019;151–152:191–221. doi: 10.1016/j.addr.2019.04.007. [DOI] [PubMed] [Google Scholar]
- LigPrep (2.3). (2009). Schrödinger Suite, LLC
- Maculotti K, Genta I, Perugini P, Imam M, Bernkop-Schnürch A, Pavanetto F. Preparation and in vitro evaluation of thiolated chitosan microparticles. J Microencap. 2005;22(5):459–470. doi: 10.1080/02652040500162220. [DOI] [PubMed] [Google Scholar]
- Marwaha TK, Madgulkar A, Bhalekar M, Asgaonkar K. Molecular docking, synthesis, and characterization of chitosan-graft-2-mercaptobenzoic acid derivative as potential drug carrier. J Appl Polym Sci. 2020 doi: 10.1002/app.49551. [DOI] [Google Scholar]
- Mateer SW, Cardona J, Marks E, Goggin BJ, Hua S, Keely S. Ex vivo intestinal sacs to assess mucosal permeability in models of gastrointestinal disease. JoVE (j Visual Exp) 2016;2016(108):e53250. doi: 10.3791/53250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mythri G, Kavitha K, Kumar MR, Jagadeesh Singh SD. Novel mucoadhesive polymers—A review. J Appl Pharmac Sci. 2011;1(8):37–42. [Google Scholar]
- Oliveira AV, Silva AP, Bitoque DB, Silva GA, Rosa Da Costa AM. Transfection efficiency of chitosan and thiolated chitosan in retinal pigment epithelium cells: a comparative study. J Pharma Bioal Sci. 2013;5(2):111–118. doi: 10.4103/0975-7406.111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protein Preparation Wizard (Epik version 2.0). (2009). Schrödinger Suite, LLC.
- Roy S, Pal K, Anis A, Pramanik K, Prabhakar B. Polymers in mucoadhesive drug-delivery systems: A brief note. Desig Monom Polym. 2009;12(6):483–495. doi: 10.1163/138577209X12478283327236. [DOI] [Google Scholar]
- Sakloetsakun D, Bernkop-Schnürch A. Thiolated chitosans. In J Drug Deliv Sci Technol. 2010;20(1):63–69. doi: 10.1016/S1773-2247(10)50007-8. [DOI] [Google Scholar]
- Schmitz T, Grabovac V, Palmberger TF, Hoffer MH, Bernkop-Schnürch A. Synthesis and characterization of a chitosan-N-acetyl cysteine conjugate. Int J Pharma. 2008;347(1–2):79–85. doi: 10.1016/j.ijpharm.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Sethi A, Joshi K, Sasikala K, Alvala M. Molecular docking in modern drug discovery: principles and recent applications. Drug Discov Devel- New Adv. 2019 doi: 10.5772/INTECHOPEN.85991. [DOI] [Google Scholar]
- Severian D, Andreas B. Polymeric Biomaterials. 2. New York: Marcel & Dekker; 2001. [Google Scholar]
- Shah KU, Shah SU, Dilawar N, Khan GM, Gibaud S. Thiomers and their potential applications in drug delivery. In Exp Opi Drug Deliv. 2017;14(5):601–610. doi: 10.1080/17425247.2016.1227787. [DOI] [PubMed] [Google Scholar]
- Shargel L, Yu, Wu-Pong S (2012). Modified-Release Drug Products. In Appl Biopharma Pharma, 6th ed (Shargel, Applied Biopharmaceuticals & Pharmacokinetics), Sixth Edition.
- Da Silva LC, Da Silva TL, Antunes AH, Rezende KR. A sensitive medium-throughput method to predict intestinal absorption in humans using rat intestinal tissue tegments. J Pharma Sci. 2015;104:2807–2812. doi: 10.1002/JPS.24372. [DOI] [PubMed] [Google Scholar]
- Sogias IA, Williams AC, Khutoryanskiy VV. Why is chitosan mucoadhesive? Biomacromol. 2008 doi: 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- Swenson ES, Milisen WB, Curatolo W. Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharmac Res. 1994;11:1132–1142. doi: 10.1023/A:1018984731584. [DOI] [PubMed] [Google Scholar]
- Tariq M, Alam MA, Singh AT, Iqbal Z, Panda AK, Talegaonkar S. Biodegradable polymeric nanoparticles for oral delivery of epirubicin: In vitro, ex vivo, and in vivo investigations. Coll Surf. B, Biointer. 2015;128:448–456. doi: 10.1016/J.COLSURFB.2015.02.043. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. The J Nutr. 2011;141:769–776. doi: 10.3945/JN.110.135657. [DOI] [PubMed] [Google Scholar]
- Ways TMM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 2018;10(3):267. doi: 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.