Abstract
Light chain deposition disease (LCDD) is a form of monoclonal gammopathy of renal significance. The diagnosis is based on the immunofluorescence (IF) findings of linear monoclonal light chain staining of basement membranes throughout the kidney, which appear as non-organized, granular punctate to powdery electron dense deposits by electron microscopy (EM). Although “LCDD by IF only” without EM deposits has been well-described, LCDD identified by EM with negative IF is very rare and hardly mentioned in the literature. Herein we describe a case of lambda-type LCDD that appeared negative by IF and showed light microscopic findings of nodular glomerulosclerosis, which was initially attributed to the patient’s history of significant tobacco use and uncontrolled hypertension. However, EM later showed powdery electron dense material in focal glomerular and tubular basement membranes and mesangium. Subsequent bone marrow analysis revealed greater than 60% lambda-restricted plasma cells. We report this case to illustrate that within the differential diagnosis of nodular sclerosis, monoclonal immunoglobulin deposition disease (MIDD) should remain in the differential even if immunofluorescence appears negative as EM can prove to be crucial in identifying cases of MIDD.
Keywords: Monoclonal gammopathy of renal significance, Monoclonal immunoglobulin deposition disease, Nodular glomerulosclerosis, Multiple myeloma
Introduction
Monoclonal immunoglobulin deposition disease (MIDD) is a form of monoclonal gammopathy of renal significance (MGRS) characterized by monoclonal light and/or heavy chain deposits involving basement membranes in all renal compartments by immunofluorescence (IF) and non-organized punctate to powdery electron dense material by electron microscopy (EM). Light chain deposition disease (LCDD) is the most common form, comprising approximately 80–90% of MIDD cases, and are typically kappa-light chain type.
Discrepancies between the immunofluorescence and ultrastructural findings in LCDD have been reported, where the monotypic light chain deposits are demonstrated by IF but not by EM. These cases are referred to as “LCDD by IF only” and are often associated with light chain cast nephropathy (aka “myeloma cast nephropathy”), an entity which is now considered a myeloma-defining event [1, 2].
However, LCDD detected by EM but not IF is very rare and hardly described [3, 4]. Herein we report a case in which immunofluorescence failed to detect the pathologic light chain but was identified on electron microscopy.
Case report/case presentation
A 70-year-old woman with a history of significant tobacco use and uncontrolled hypertension presented to the hospital with progressive exertional dyspnea and lower extremity edema over a month and was found to have hypertensive emergency with blood pressure up to 200/105 mm Hg and acute kidney injury with a serum creatinine of 1.5 mg/dL (baseline 0.5 mg/dL). Further laboratory workup revealed anemia (hemoglobin 7.0 g/dL), thrombocytopenia (platelet count 140 × 103/µL), elevated lactate dehydrogenase (500 U/L), low haptoglobin (< 20 mg/dL), and significant hypocomplementemia (C3: 20 mg/dL, C4: 5 mg/dL). Peripheral blood smear showed presence of schistocytes. Bilirubin was normal. Urinalysis showed greater than 50 red blood cells and white blood cells per high-power field. Spot urine protein to creatinine ratio was 2.0 g. Initial concern was for thrombotic microangiopathy related to uncontrolled hypertension, but additional serologic work up revealed positive anti-nuclear antibody (titer 1:320), borderline elevated double-stranded DNA antibody (5 IU/mL), significant IgG lambda paraproteinemia, and a serum kappa to lambda free light chain ratio of 0.01 (kappa 1.5 mg/dL and lambda 111.0 mg/dL). Anti-neutrophil cytoplasmic antibody (ANCA), glomerular basement membrane antibody, and ADAMTS13 activity were unremarkable. Skeletal survey demonstrated osteolytic lesions. Given the concern for paraproteinemic-related kidney disease, a renal biopsy was performed.
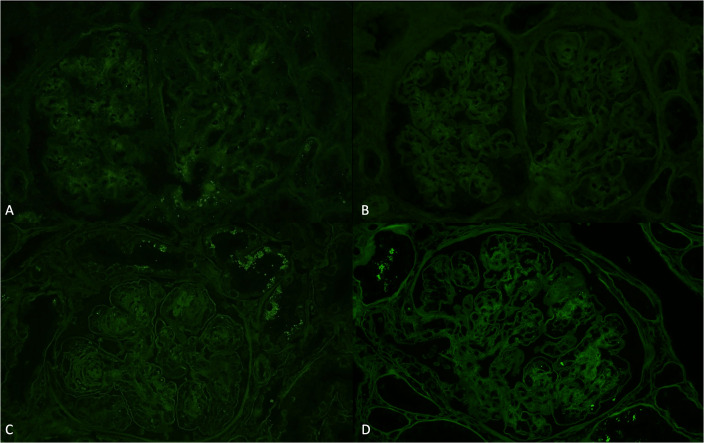
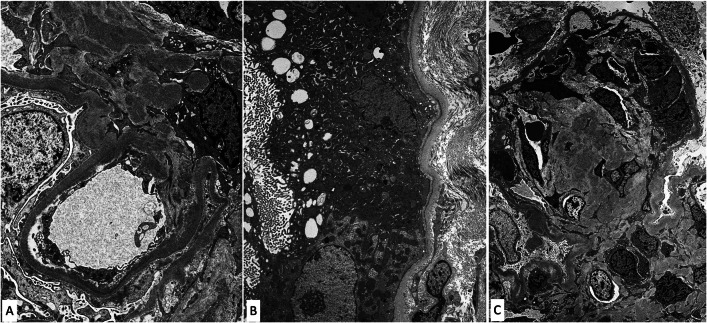
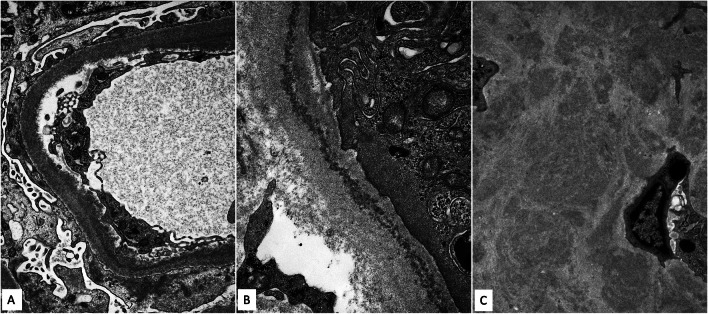
A total of 50 glomeruli were sampled for light microscopy, 10 of which were globally sclerotic. Glomeruli were enlarged with diffuse and global, moderate to severe mesangial sclerosis forming nodules, many of which were surrounded by capillary loop microaneurysms (Fig. 1). Some of the nodules were densely sclerotic and stained positively for periodic-acid Schiff, argyrophilic on Jones methenamine silver stain, and trichrome blue. Other nodules appeared hypercellular owing to the presence of multiple vascular spaces embedded within the mesangial matrix. The glomerular and tubular basement membranes were diffusely thickened by matrix material. There was only minimal tubular atrophy and interstitial fibrosis affecting less than 10% of the sampled cortex. Proximal tubules displayed patchy and diffuse degenerative changes. No atypical fractured or crystalline casts to suggest myeloma cast nephropathy were identified. Vessels showed mild arteriosclerosis. No fibrin thrombi were seen. Congo red stain showed no evidence of amyloid. Immunofluorescence was negative for all immunoglobulins and complement except for 2+ staining of the casts with both kappa and lambda (Fig. 2A, B). Immunofluorescence performed on paraffin-embedded tissue revealed negative kappa and weak trace lambda in all renal compartments which was initially thought to reflect possible non-specific background staining (Fig. 2C, D). There was also 3+ lambda in the distribution of the proximal tubular epithelial cell protein resorption droplets with weaker kappa, and no evidence of intracytoplasmic crystalline inclusions. By ultrastructural examination, focal granular punctate to powdery electron dense deposits were present along some of the tubular and glomerular basement membranes and mesangium (Figs. 3 and 4). Taken together with the patient’s clinical presentation, a diagnosis of monoclonal immunoglobulin deposition disease of lambda-light chain type with nodular sclerosing features was made.
Fig. 1.
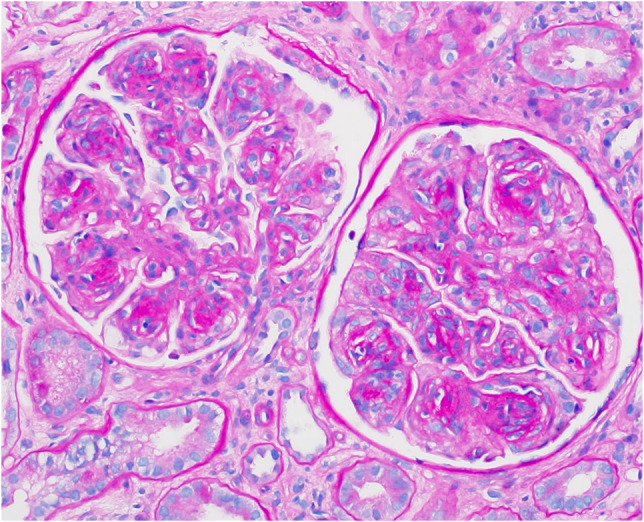
Glomeruli showing nodular glomerulosclerosis (periodic-acid Schiff, original magnification ×400)
Fig. 2.
A Immunofluorescence microscopy shows negative staining for kappa. B Immunofluorescence microscopy shows negative staining for lambda. C Immunofluorescence microscopy on protease-digested paraffin-embedded tissue shows negative staining for kappa. D Immunofluorescence microscopy on protease-digested paraffin-embedded tissue shows weak trace staining for lambda
Fig. 3.
Electron microscopy shows powdery punctate electron dense deposits involving the glomerular capillary wall in a subendothelial distribution and mesangium (A), tubular basement membranes (B), and within nodular mesangial sclerosis (C)
Fig. 4.
Higher magnification of powdery punctate electron dense deposits involving the glomerular capillary wall in a subendothelial distribution (A), tubular basement membrane (B) and nodular mesangial sclerosis (C)
Bone marrow biopsy was performed and revealed hypercellular marrow with lambda-restricted plasma cells comprising greater than 60% of the cellularity. In light of those findings, she began treatment for multiple myeloma with cyclophosphamide, bortezomib and dexamethasone subsequently transitioning therapy to daratumumab, lenalidomide, bortezomib and dexamethasone.
Discussion/conclusion
MIDD is a rare disease associated with MGRS and multiple myeloma. The diagnosis requires the presence of linear monotypic renal parenchymal staining by light chains (LCDD), light and heavy chains (LHCDD), or heavy chains only (HCDD) on IF, and punctate to powdery electron dense material by EM. On light microscopy (LM), most cases display nodular mesangial sclerosis similar to diabetic nephropathy and amyloidosis but can have a heterogeneous morphology including normal-appearing glomeruli as well as mesangial proliferative and membranoproliferative patterns [5].
Although most cases of LCDD show both IF and EM findings typical of the disease, a small subset of cases of “LCDD by IF only” without EM deposits have been described. These cases have been reported frequently with light chain cast nephropathy and typically show minimal or no glomerular changes by LM [5, 6]. Some propose that these changes suggest an early form of the disease process. In a case series of LCDD in renal allograft biopsies, Nasr et al. were able to document the progression of LCDD detected on IF only to eventually both IF and EM, supporting the possibility that IF only is an earlier form of LCDD before it is detectable by EM [7].
In contrast, IF on frozen tissue in our case failed to initially detect the pathologic light chain but was eventually identified on EM. IF was also performed on paraffin-embedded tissue but showed very weak staining of the abnormal light chain that could be easily missed or discounted as non-specific artifactual staining [8]. Cases of MIDD with absent or equivocal IF immunoreactivity but positive EM findings is uncommon and has only been identified in a paucity of case studies [3, 4, 8]. Several possible explanations for this observation include the possibility that the antibody failed to bind to the deposited monoclonal lambda-light chains in our patient. Generic antibodies to kappa and lambda can detect most pathologic light chains in LCDD, but not in all cases[3]. This may be because a specific antibody to that pathologic light chain is required to detect it or a structural change in an antigenic site on the light chain masks epitope recognition by the antibody. Moreover, in diabetic patients, the frequent glycosylation of the abnormal light chains may also impair their detection [9]. Another possibility is that early manifestations of MIDD in some cases are detectable by EM before other diagnostic techniques. In a study of early MIDD with unusual or equivocal immunomorphology, most of the cases had very subtle IF findings that could have been easily dismissed as background staining or non-diagnostic [3]. In one case, IF was completely negative and another case was noted to have polyclonal staining for both light chains. EM by immunogold labeling was able detect the monoclonal immunoglobulins and help establish diagnosis in those cases [3, 8].
Confounding the diagnosis was the patient’s history of heavy smoking and uncontrolled hypertension, both of which are known to cause nodular glomerulosclerosis [10, 11]. While the majority of LCDD are kappa-related and present with a nodular sclerosing glomerulopathy, lambda-related LCDD is usually not associated with a nodular sclerosing pattern. Nodular glomerulosclerosis is also more commonly seen in advanced cases of MIDD. This may be due to the accumulation of the pathogenic light chains which appear to stimulate mesangial cells leading to increased transforming growth factor-beta and matrix protein production [12]. Given the weak to negative staining on IF and the presence of only focal deposits detected on EM, we favored the findings to likely represent an early form of lambda-related LCDD with nodular glomerulosclerosis related to both the pathologic light chains deposits and the patient’s history of significant tobacco use and uncontrolled hypertension.
It is noteworthy to mention that our patient presented with clinical features of thrombotic microangiopathy and significant hypocomplementemia, raising a differential diagnosis that also includes atypical hemolytic uremic syndrome (aHUS) or C3 glomerulopathy related to abnormal regulation of the alternative complement pathway. However, immunofluorescence was negative for complement C3 and electron microscopy did not show the typical features of TMA including subendothelial widening by electron lucent “fluff,” fibrin tactoids, or schistocytes, providing evidence against renal involvement by aHUS or C3 glomerulopathy. That said, we cannot completely exclude the possibility of a concurrent abnormality of the alternative complement pathway in this patient as monoclonal paraproteins have been shown to have the capacity to interfere with the alternative complement pathway [13, 14].
This case illustrates that within the differential for nodular sclerosis, MIDD should always be in the differential even if immunofluorescence appears negative. Although IF is the most sensitive technique in detecting monoclonal immunoglobulins, rare cases can evade antibody detection. Our case emphasizes the importance of EM in correlation with clinical and laboratory evidence of an underlying monoclonal gammopathy in identifying cases of MIDD that can be easily missed.
Acknowledgements
The authors are grateful to Karen Vanderbilt and Tracy Fontaine-Matteson for excellent technical support.
Author contributions
AS and HYC wrote the first draft of the manuscript. RG, FP, BG critically revised the work. All authors read and approved the final manuscript.
Funding
None.
Declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Attempts to obtain informed consent was unsuccessful as the patient had moved away and was lost to follow-up at our institution. Personal details and dates and other information which identify the patient are omitted to ensure that there is no breach of confidentiality.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leung N, Bridoux F, Batuman V, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15(1):45–59. doi: 10.1038/s41581-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin J, Markowitz GS, Valeri AM, et al. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. 2001;12(7):1482–1492. doi: 10.1681/ASN.V1271482. [DOI] [PubMed] [Google Scholar]
- 3.Herrera GA, Sanders PW, Reddy BV, Hasbargen JA, Hammond WS, Brooke JD. Ultrastructural immunolabeling: a unique diagnostic tool in monoclonal light chain-related renal diseases. Ultrastruct Pathol. 1994;18(4):401–416. doi: 10.3109/01913129409023211. [DOI] [PubMed] [Google Scholar]
- 4.Pozzi C, D'Amico M, Fogazzi GB, et al. Light chain deposition disease with renal involvement: clinical characteristics and prognostic factors. Am J Kidney Dis. 2003;42(6):1154–1163. doi: 10.1053/j.ajkd.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Gokden N, Cetin N, Colakoglu N, et al. Morphologic manifestations of combined light-chain deposition disease and light-chain cast nephropathy. Ultrastruct Pathol. 2007;31(2):141–149. doi: 10.1080/01913120701376139. [DOI] [PubMed] [Google Scholar]
- 6.Nasr SH, Valeri AM, Cornell LD, et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7(2):231–239. doi: 10.2215/CJN.08640811. [DOI] [PubMed] [Google Scholar]
- 7.Nasr SH, Leung N, Heybeli C, Cornell LD, Alexander MP. Evidence for transition from light chain deposition disease by immunofluorescence-only to classic light chain deposition disease. Kidney Int Rep. 2021;6(5):1469–1474. doi: 10.1016/j.ekir.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera GA, Turbat-Herrera EA. Ultrastructural immunolabeling in the diagnosis of monoclonal light-and heavy-chain-related renal diseases. Ultrastruct Pathol. 2010;34(3):161–173. doi: 10.3109/01913121003672873. [DOI] [PubMed] [Google Scholar]
- 9.Jennette JC, D’Agati VD, Olson JL, Silva FG. Heptinstall's pathology of the kidney. Philadelphia: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 10.Nasr SH, D'Agati VD. Nodular glomerulosclerosis in the nondiabetic smoker. J Am Soc Nephrol. 2007;18(7):2032–2036. doi: 10.1681/ASN.2006121328. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz GS, Lin J, Valeri AM, Avila C, Nasr SH, D'Agati VD. Idiopathic nodular glomerulosclerosis is a distinct clinicopathologic entity linked to hypertension and smoking. Hum Pathol. 2002;33(8):826–835. doi: 10.1053/hupa.2002.126189. [DOI] [PubMed] [Google Scholar]
- 12.Zhu L, Herrera GA, Murphy-Ullrich JE, Huang ZQ, Sanders PW. Pathogenesis of glomerulosclerosis in light chain deposition disease. Role for transforming growth factor-beta. Am J Pathol. 1995;147(2):375–385. [PMC free article] [PubMed] [Google Scholar]
- 13.Ravindran A, Go RS, Fervenza FC, Sethi S. Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int. 2017;91(3):691–698. doi: 10.1016/j.kint.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Ravindran A, Fervenza FC, Smith RJH, Sethi S. C3 glomerulopathy associated with monoclonal Ig is a distinct subtype. Kidney Int. 2018;94(1):178–186. doi: 10.1016/j.kint.2018.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]