Abstract
Lactic acid bacteria (LAB) are believed to have health-promoting properties to the host and can be used in therapeutics interventions; intriguingly, they have the property to produce bio-preservatives substances. Therefore, this study aimed to mine probiotics and evaluate their safety, functional properties, and cholesterol-lowering capability. Seven potential probiotic strains were compared from 56 LAB strains isolated from traditional Chinese fermented milk. The results showed that all tested strains are tolerant to gastric acidity (45.5–83.26) and bile salts (11.92–92.91%) and have antibacterial activity against Staphylococcus aureus ATCC25923 and Escherichia coli ATCC25922. Likewise, it lowered the cholesterol levels in vitro by live cells (26.57–45.76%) and dead cells (29.53–50.97%) with remarkable aggregation ability (13.8–43.71%). Antioxidant properties and produce short chain fatty acids (SCFAs) were strain-dependent features. Upon assessment of the safety, Enterococcus faecium NWAFU-BIO-AS14 exhibited virulence factors genes (VFs) of (mur-2ed, odc, and tet(K)) and + hemolysis activity. While Enterococcus faecium NWAFU-BIO-A-B24 and Limosilactobacillus fermentum NWAFU-BIO-B-S6 have VFs of (odc, vanC2, and ant(6)-Ia). Limosilactobacillus fermentum NWAFU-BIO-D-B2 has only (odc). Thus, they are not considered as safe probiotics. In contrast, Lactiplantibacillus plantarum NWAFU-BIO-BS29, Companilactobacillus crustorum NWAFU-BIO-AS16, and Lactobacillus gallinarum NWAFU-BIO-D-S7 are the safest and best strains, respectively, due to the absence of 16 VFs and their sensitivity to antibiotics such as kanamycin, erythromycin, tetracycline, gentamycin, vancomycin, streptomycin, chloramphenicol, and ampicillin. Accordingly, these strains have a high potentiality to be used as starter cultures or safely applied as perfect probiotics in functionals food and feed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03403-z.
Keywords: Probiotics, Lactic acid bacteria, Virulence genes, Antioxidant, Hemolytic activity, Antibiotic resistance, SCFAs, Cholesterol-lowering
Introduction
Lactic acid bacteria are known to have a positive role in human and animal health which are recognized as safe (GRAS) and can be used as probiotics in food and feed. These microorganisms are ubiquitous in nature and essential in the production of fermented foods (Habib et al. 2022; Qian et al. 2018). The food and agriculture organization and world health organization (FAO/WHO 2002) defined probiotics as live microorganisms that, when administered in appropriate amounts, provide a health benefits on the host (Pinto et al. 2020; Raman et al. 2022; Sui et al. 2021). Therapeutic properties of LAB include: enhanced immune function, maintenance of antitumor activity, reduction of the population of harmful microorganisms, improvement of the balance of microflora in the human and animal guts, prevention against some intestinal infection, increased tolerance to lactose-containing foods, and possibly prevention of cancer, and cholesterol lowering (Todorov et al. 2017; Zheng et al. 2020). Seventeen million fatalities occur yearly in world’s due to cardiovascular diseases (CVDs) (WHO 2018). WHO report of 2015 mentioned that a 10% reduction in serum cholesterol could decrease the incidence of heart disease within 5 years by 50% in 40-year-old men (Bendali et al. 2017). Numerous attempts have been made to isolate cholesterol-reducing bacteria to study the relationship between intestinal microbiota and cholesterol metabolism. In 1974 Mann and Spoerry reported that LAB of dairy products are associated with lowering serum cholesterol in African Maasai warriors (Mendes et al. 2018). The gut microbiota metabolism can convert half of the dietary cholesterol into coprostanol as a non-absorbable sterol (metabolism of cholesterol) excreted in the feces. While the colonic bacteria metabolize all cholesterol arriving in the large intestine (Pan et al. 2011). In another direction, LAB could be a promising bio-preservatives because they can produce natural antimicrobial metabolites to kill or inhibit food-borne pathogens, which are considered a global concern to the end-user’s health. Those metabolic substances consist of organic acids (lactate, acetate, and butyrate), hydrogen peroxide, and bacteriocins which have an excellent contribution to food safety; they can replace antibiotics due to the emerging concern of antibiotic resistance by these pathogens (Li et al. 2020). Another mechanism eliminates the possible threats by the competition for nutrients or adhesion receptors with the harmful bacteria of gut microbiota (Kavitha et al. 2020). It is vital to ensure that LAB probiotics strains are safe for human and animal consumption and do not have antimicrobial resistance properties, hemolysis activity, or carried virulence factor genes that can potentially be transferred to the flora of humans, animals, or to the pathogenic bacteria temporarily residing in the hosts (spread virulence genes by horizontal transfers) (Klare et al. 2007). According to the EFSA, safe strains do not have one or more VFs to be applied as a food additive (Nami et al. 2015). To survive and colonize the gut by LAB, they must have adhere ability to the intestinal cells of the host by bacteria clumping aggregation of the same strain (auto-aggregation) or different strains (co-aggregation), which is importance to prevent pathogens colonization (Dlamini et al. 2019).
Additionally, antioxidants from bio-resources have received a great deal of attention in recent years; these substances have shown potential function to help the human body to reduce oxidative damage during cellular metabolism by delaying or preventing the oxidation of cellular substrates of reactive oxygen species (ROS) such as superoxide anion (O2-), hydrogen peroxide (H2O2), and hydroxyl radical (-OH) which play essential roles in cell signalling, apoptosis, gene expression, and ion transportation (Lü et al. 2010; Madjirebaye et al. 2022). Generating ROS molecules at a high or low level in cellular defenses can cause degenerative diseases like cancer, Alzheimer’s disease, and Parkinson’s disease (Afonso et al. 2007; Li et al. 2012). Evidence suggests that some LAB probiotic strains can exhibit substantial antioxidant activity to alleviate oxidative damage when host defense is weakened mainly by scavenging free radicals, chelating pro-oxidative metal ions, regulating the production of antioxidant enzymes to help remove ROS, and modulating the gut microbiota of the host (Feng and Wang 2020). Lactobacillus rhamnosus GG, Lactobacillus brevis BJ20, Lactobacillus casei, Lactobacillus plantarum 7FM10, Lactobacillus plantarum NCU001563, and Lactobacillus fermentum ME-3 were found to have important biological functions with antioxidant activity (Oh and Jung 2015).
Fermentation of carbohydrates by LAB produced short-chain fatty acids (SCFAs) such as acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid and isovaleric acid, which have impacts on the non-immune and immune intestinal cells and play an important role in the innate immunity as anti-inflammatory inhibitors; thus, administration of LAB can increase the expression of interleukins, thereby conferring protection against colitis (Kang et al. 2021; Zhang et al. 2020). Therefore, potential probiotic strains of LAB can be applied as bio-additives in many industries such as dairy, meat, pickle, feed, brewing industry, and functional foods to promote health benefits or improve the quality of the products (Chlebowska-Smigiel et al. 2017; Chlebowska-Śmigiel et al. 2019; Kieliszek et al. 2021).
The present study aims to mine good potential probiotics from Chinese traditional fermented milk and characterize their properties. In vitro investigation was conducted to assess the safety of these strains by testing the hemolysis activity, the susceptibility against 8 antibiotics, and detecting 16 genes related to the VFs. Furthermore, tolerance to acidic and bile salts, cholesterol-lowering, antibacterial activity, and aggregation activity were determined. In addition, SCFAs production and the antioxidant activity were evaluated which includes; scavenging activity against ABTS and hydroxyl, superoxide anion, 2,2-diphenyl-1 picrylhydrazyl (DPPH) free radicals, and their resistance to hydrogen peroxide. According to the findings of this study, we will identify a safe and good LAB to be used as a promising probiotic and has potential properties to promote health benefits and produce bio-preservative compounds.
Materials and methods
Sample collection and isolation of LAB strains
Traditional Chinese fermented milk of Gansu was collected from Gansu province, China in sterile sampling bottles, and transported at 4°C to the lab of bioresources at NWAFU. 10 mL of sample was diluted as ten-fold serial dilutions (1–6) on sterile peptone water, 200 µL was spread to MRS (LAND BRIDGE, China) plates which were incubated aerobically and unerotically at 37 °C and 45 °C for 84 h (Sengun et al. 2009). Subsequently, purified the LAB consider colonies that had morphological characteristics of gram positive and peroxidase negative. The strains have been stored at − 80 °C in MRS broth (LAND BRIDGE, China) with 40% (v/v) glycerol (KESHI Chemical, China).
LAB molecular identification
The DNAs of the potential probiotic strains were extracted by following the protocol of the purification kit (Sangon Biotech, Shanghai, China) (Fhoula et al. 2018), and then the polymerase chain reaction (PCR) amplification was carried out by using a set of primers of (F27: AGTTTGATCMTGGCTCAG, and R1492: GGTTACCTTGTTACGACTT), and thermocycler parameters of PCR (BIO-RAD T100™ Thermal Cycler, Singapore) are (94 °C for 5 min; 35 cycles of 94 °C for 30 s, 54 °C for 20 s and 72 °C for 1 min; 72 °C for 5 min). The PCR products have been sent to (Yangling Tianrun Aoke Biotechnology Co, Ltd), sequencing department for 16S rDNA sequences. BLAST tool on NCBI sequence database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to identify the obtained strain sequences at the level of species (Yusuf et al. 2020). Molecular Evolutionary Genetics Analysis (MEGA) 11.0 software (https://www.megasoftware.net/) was used to construct the phylogenetic tree by using neighbor-joining method (Saitou and Nei 1987).
Probiotics properties
Screening of LAB with antibacterial activity
Antibacterial activity of LAB strains has been tested by using agar well diffusion assay method (AWDA) as described by Sui et al. (2021) and Pinto et al. (2020) with some difference, in short, LAB were cultured in MRS broth (LAND BRIDGE, China) at 37 °C for 24 h, the Cell-free supernatants were collected by centrifugation at 6000 RPM at 4 °C. 200 µL of cell free supernatant was poured into the well made by an Oxford cup placed in double layer plates of 1.5% agar overlaid with semi-solid LB medium (0.75% agar) containing 106 cfu/mL of (gram+ and −) indicator strains (Staphylococcus aureus ATCC25923 and Escherichia coli ATCC25922), first the plates were put in refrigerator at 4 °C to allow diffusion of the supernatant for 1.5 h, and then incubated at 37 0C for 24 h. The diameter of the inhibition zone was measured.
Acid and bile salt tolerance
To simulate the acidic environment of the human stomach, the tolerance of the potential probiotics to acid and bile salts was evaluated in vitro according to the methods of (Zheng et al. 2020) and (Fhoula et al. 2018); briefly, LAB strains were cultured for 24 h, and then washed twice with sterile phosphate buffer saline (PBS) of pH 7.0, and suspended in MRS broth (LAND BRIDGE, China). The pH was adjusted to 3.0 by HCL (XILONG SCIENTIFIC, China) and incubated at 37 °C for 3 h. The absorbance was measured at 600 nm after 0 and 3 h. Survival rate (SR) was calculated according to Eq. (1):
| 1 |
Where A1 is the absorbance of LAB culture after treatment, and A0 is the absorbance before treatment.
The bile tolerance test was carried out in a similar manner as above, LAB strains were grown in MRS broth supplemented with 0.3%, 0.5%, and 1.0% oxgall bile salt (MACKLIN, China) and then incubated at 37 °C for 12 h. The absorbance was measured at 600 nm (Qian et al. 2018). SR was calculated according to the Eq. (1): A1 (absorbance of LAB culture), A0 (absorbance of blank sample).
Auto-aggregation, and co-aggregation ability
Auto-aggregation activity was measured as described by (Azat et al. 2016; Li et al. 2020; Pieniz et al. 2015) with some changes, overnight cultures of LAB strains were centrifuged at 8000 rpm, 4 °C for 10 min (Hc-3016, High speed refrigerated centrifuge, ZONKIA, China), followed by washing twice and resuspending in PBS. Equal volumes of each suspension were mixed with broth and incubated at 37 °C for 14 h. The absorbance of cell suspensions was determined at 600 nm (UNICO7200 SPECTROPHOTOMETER, Shanghai Instrument, China). The auto-aggregation ability (AA) was calculated using the Eq. (2):
| 2 |
Where: Ainitial and Atime are the absorbance before and after incubation, respectively.
A total of 1 ml cell suspension was mixed with an equal proportion of hydrocarbons (xylene).
The co-aggregation ability (CA) was assessed by resuspending 3 mL of LAB overnight culture and an equal volume/concentration of E. coli in (PBS). Equation (3) was used to computed (CA) by measuring the absorbance of suspensions directly (Am0), and again after incubated for 4 h at 37 °C (Am4) (Dlamini et al. 2019).
| 3 |
Cholesterol-lowering ability
Cholesterol degrading activity was assessed for live and dead cells of LAB in vitro as reported by (Bendali et al. 2017; Fhoula et al. 2018; Majeed et al. 2019; Yusuf et al. 2020) with some modification, first; 2 ml of pre-active culture was harvested at 8000 rpm, for 15 min at 4 °C (Hc-3016, High speed refrigerated centrifuge, ZONKIA, China), followed by washing twice the pellets with PBS. For dead cells, the suspensions were autoclaved (BOXUN, Shanghai Boxun Industry & Commerce, China) at 121 °C for 15 min. Second, MRS broth (LAND BRIDGE, China) containing 0.3% (w/v) oxgall Bile (MACKLIN, Shanghai Macklin Biochemical, China) and 100 mg/L of water-soluble cholesterol (SIGMA-Aldrich, Japan) was inoculated by (2% v/v) of bacterial suspension of live and dead cells as mentioned above and then incubated at 37 °C for 24 h. Nill MRS broth was used as a negative control. After that, the supernatant was collected by centrifugation the suspension at 8000 rpm, for 15 min at 4 °C, to measure the residual cholesterol 1 ml of supernatant have been mixed strongly with 1 mL of KOH (33% w/v) (Guangdong Guanghua Sci-Tech, China), 2 mL of absolute ethanol (KESHI Chemical, China), heated (KEWEI, China) at 37 °C for 15 min, and cooling to 25 °C. Subsequently, 5 mL hexane (GHTECH, Guangdong Guanghua Sci-Tech. China) and 2 mL distilled water (MOLECULAR H2O, China) were added to the mixture which was left at room temperature for 10 min to separate the hexane layer. The residues were immediately dissolved in 4 mL of o-phthalaldehyde (Aladdin, China) and 1 mL sulfuric acid, and then allowed to stand again for 10 min. The absorbance was measured at 560 nm similar for live and dead cells.
| 4 |
where Cholesterolresidual and Cholesterolintial are the absorbance of uninoculated and inoculated samples, respectively.
Assay of safety aspects
Antibiotic susceptibility test
Antibiotic susceptibility of LAB was assessed according to the Clinical and Laboratory Standards Institute Technical Guidelines (2018) as described by (CLSI 2018; Klare et al. 2007; Li et al. 2020) after slight alterations by spreading 100μL of bacterial culture on the surface of MRS agar with antibiotics, plates were incubated at 37 °C for 24 h. Then the colonies were accounted. The following eight antibiotics were tested in range of concentration given in parentheses (mg/L): Kanamycin (0.5–2.0), Tetracycline (0.02), Vancomycin (1.0) produced by (DIYIBIO, China), Ampicillin (0.005), Streptomycin (0.25–0.3), Erythromycin (0.005), Chloramphenicol (1.5–2.0), and Gentamycin (0.25–0.3) produced by (MP Biomedicals, France). The concentrations outside the test range of the corresponding antimicrobial are available at JAC Online (http://jac.oxfordjournals.org/).
Hemolytic activity
According to the method described by Pieniz et al. (2015), the hemolytic activity of seven LAB strains had been evaluated. Briefly, the strains were inoculated into blood agar (Qing Dao Hope Bio-Technology, China) supplemented with 5% v/v of defibrinated sheep blood (Beijing Landbridge Technology, China) and then incubated at 37 °C for 72 h. Evaluation of the hemolytic activity according to observing a clear zone of hydrolysis around the colonies, strains that display a clear zone are considered as positive hemolytic (b-hemolysis), while, the presence of a green-hued zone is considered to have partial-hemolytic (a-hemolysis), the negative strains will not show any zones around the colonies (g-hemolysis).
Detection of virulence and resistance genes
The presence of 16 virulence vectors (VFs) genes in the potential probiotics were screened. (VFs) are related to: virulence genes of (gelatinase (fsrA), aggregation (asa1), E. faecalis endocarditis (efaA), chemotactic factors (cpd), hyaluronidase (hyl), Enterococcus factors (surface protein) (mur-2ed), genetic exchange (Int)), Antibiotic resistance genes of (aminoglycosides gentamicin (aac(6′)-Ie-aph(2″)-Ia), glycopeptides vancomycin (vanC2), macrolides erythromycin (ermA), tetracyclines tetracycline (tet(K)), other chloramphenicol (catA), streptogramin (vat(E)), streptomycin (ant(6)-Ia), and Biogenic amines genes of (tyrosine (tdc), ornithine (odc), histidine (hdc1). The primer sequences are listed in Table S1 which have been synthesized in (Synthesis Laboratory, Yangling Tianrun Aoke Biotechnology, China). Method of (Dlamini et al. 2019; Moraes et al. 2012; Nami et al. 2015; Perin et al. 2014; Zhao et al. 2021) was used to detect the presence of (VFs) genes by using PCR; in brief, DNA has been extracted from LAB strains of (NWAFU-BIO) according to protocol of Sangon Biotech, China. 2 μL of each gDNA at a concentration (100–200 ng/μL) was mixed with 2 μL of forward and reverse primers, 12.5 μL 2 × Rapid Taq master Mix (GoTaq® Green Master Mix) and 6.5μL of nuclease-free water, the total volume of the mixture is 25 μL. The PCR reaction was performed on (BIO-RAD T100TM Thermal Cycler, Singapore) under the following conditions: initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 1 min, annealing of primers was set at a temperature of 46 °C for (hdc1), 48 °C for (fsrA), 50 °C for (asa-1), 52 °C for (odc), 55 °C for (vat(E), catA, int, mur-2ed, cpd, tet)K), ermA, and vanc2), and 56 °C for (hyl) all to 30 s, 72 °C extensions for 30 s, and final extension at a temperature of 72 °C for 10 min. PCR products were loaded on 3.0% agarose gel (TSINGKE, Biological Technology, China) stained by 0.005% (w/v) gel red (DIYIBIO, China) and transmission on 1X TAE solution at electrophoresis power supply (BIO-RAD PowerPac™ Basic, Singapore). Finally, the DNA bands were visualized under ultraviolet light (BIO-RAD Universal Hood II Gel Doc™ XR + , Molecular Imager, U.S.A).
Antioxidant activity analysis
Antioxidants are defined chemically as reducing agents, which are molecules that can receive electrons and/or donate hydrogen (Griffiths 2016). The 2,2-diphenyl-1-picrylhydrazyl Free Radical (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), hydroxyl radical scavenging activity (HR), superoxide anion scavenging activity (SA), and resistance to hydrogen peroxide (RHP), these assays are widely used to characterize the antioxidant properties of biological mixtures such as culture extracts (Sujarwo and Keim 2019).
DPPH radical scavenging activity
The DPPH assay is based on reduction of the free radical by an antioxidant, whereby the DPPH radical is converted by reduction into a colorless compound (Mfotie Njoya 2021). Method of (Azat et al. 2016; Li et al. 2012; Sui et al. 2021; Wang et al. 2020, 2017) was used to assess the LAB DPPH with minor modification as below; 2 mL of fresh bacterial culture was harvested at 4000 × g for 10 min at 4 °C, washed twice with (PBS), resuspended in 1 mL (PBS), subsequently, mixed with 2 mL of 0.05 mM DPPH contains 10–20% Benzene purchased from (TCI (Shanghai) Development. China) in 95% ethanol, and then incubated at 37 °C in the dark for 30 min. The absorbance of solutions was measured at 517 nm. A blank sample was used as a control negative.
| 5 |
where Asample is the absorbance of suspension, Ablank is the absorbance of only cells and ethanol, and Acontrol is the absorbance of the deionized water and DPPH solutions.
ABTS radical scavenging activity
The (ABTS) test is an electron transfer-based assay to evaluate the antioxidant activity of both hydrophilic and hydrophobic compounds by determining metmyoglobin and hydrogen peroxide induced by the ABTS* + radical cation formation (Dasgupta and Klein 2014). A method of Wang et al. (2017), and Wang et al. (2020) was used to conduct it. In brief, the reaction mixture was prepared from 7 mM ABTS (Solarbio, China) in water with 2.45 mM potassium persulfate (Guangdong Guanghua Sci-Tech. China), it was placed in the dark for 16 h at room temperature, and then the solution was diluted by ethanol (SUN, Tianjin ZhiYuan Reagent, China) to an absorbance of 0.70 at 734 nm, after that, 2 mL of sample cultures was added into 4 mL of ABTS solution, followed by incubation for 5 min in dark. The absorbance was measured directly at 734 nm. Ascorbic acid was used as a positive control (GHTECH, Guangdong Guanghua Sci-Tech, China). Equation number (6) was used to calculate the ABTS radical scavenging activity:
| 6 |
where Ax is the blank absorbance by replacing EPS solution with water, Ab: sample solution, A0: sample control.
Hydroxyl radical scavenging activity
(HR) radical scavenging is one of the potent oxygen reactive. Sulfosalicylic acid method was used to determine scavenging activity as described by (Azat et al. 2016; Li et al. 2012; Wang et al. 2017). Briefly, 1.0 ml of reaction mixture which contain salicylic acid (KESHI, China), FeSO4 (Guangdong Guanghua Sci-Tech. China), and H2O2 (3.0%, w/v) (GHTECH, Guangdong Guanghua Sci-Tech. China) at (6, 2, and 6 mmol/L), respectively, was added to 1.0 ml of strains culture or cell-free extract, and then incubated at room temperature for 20 min. The absorbance was determined at 510 nm on (UNICO7200 SPECTROPHOTOMETER, Shanghai Instrument, China). Equation (7) was used to calculate the HR scavenging activity.
| 7 |
where As is the absorbance of the strains, Ab is the absorbance without H2O2, and Ac is the absorbance of the control.
Superoxide anion scavenging activity
The SA scavenging ability of the potential probiotics was measured as described by Sui et al. (2021) with some modification, 1.0 mL of strain cultures were mixed with Tris–HCl buffer (MP Biomedicals, France) (0.05 M, pH 8) and then incubated at 25 °C for 10 min. After that, 200 µL of pyrogallol (Kernel, China) 30 mM was added to the mixture which was pre-heated at 25 °C and kept at room temperature for 4 min. Subsequently, (0.5 mL) concentrated HCl (AR, Sichuan Xilong Chemical. China) was added. The absorbance was measured at 329 nm and for calculate the (SA) formula (8) was used. A sample without pyrogallol was used as a control.
| 8 |
where Acontrol: absorbance of the control reaction, and Asample: absorbance of the LAB culture.
Resistance to hydrogen peroxide
The method of S. Li et al. (2012) was used with some modifications to assess the survival ability in the presence of hydrogen peroxide. LAB strains were cultured overnight 1% (v/v) in MRS broth containing (0.2, 0.4, 0.6, 0.8, 1.0, and 2.0 mmol L−1) hydrogen peroxide (GHTECH, China). The growth was measured as optical density (OD) by using spectrophotometer (UV Spectrophotometer 7225, Shanghai precision science instruments, China) at 600 nm. The survival ability of the strains was calculated according to Eq. (9).
| 9 |
Short-chain fatty acids (SCFAs) analysis
The levels of acetic acid, propionic acid, butyric acid, isobutyric acid, and valeric acid in the fermented broth were measured by using gas chromatography (GC-2014C Shimadzu corporation, Japan). The extraction and measurement of SCFAs were performed according to methods described by Kang et al. (2021), and Zhang et al. (2020) with slight modifications. Briefly, approximately 800 µL of cultured strains were extracted with 50% hydrochloric acid (H2SO4) and 1000 µL ether. The mixtures were centrifuged at 10,000 × g for 15 min; the organics phase was subjected to (GC). The samples were separated using a DB-FFAP column (30 m × 0.25 µm × 0.25 μm, Agilent Technologies, Palo Alto, CA, USA).
Statistical analysis
All data were analyzed in triplicate with Statistic 5.5 software. One-way analysis of variance (ANOVA). Values of P < 0.05 were considered statistically significant. Experimental data were presented as the mean ± standard deviation of the mean. The variable differences among means were detected by paired Student’s test. Evolutionary analysis by MEGA11 and timetree online by (http://www.timetree.org/search/goto_pairwise). Spearman’s correlation matrix by GraphPad prism 8.0.
Results
Molecular identification of LAB
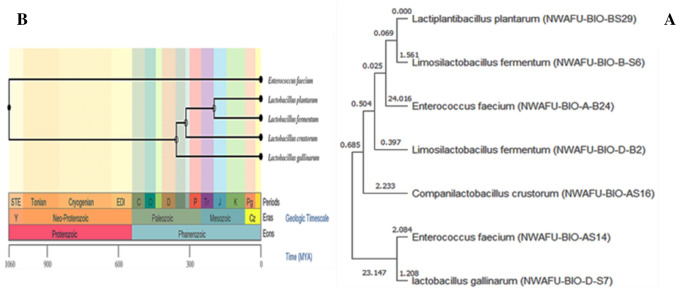
The Chinese conventionally fermented dairy product could be considered a valuable bioresource for screening probiotics. Fifty-six isolates were identified as LAB strains (data not shown), among them seven isolates were selected to identify at molecular level by 16S rRNA sequencing according to their good antimicrobial activity and designated as following: Enterococcus faecium NWAFU-BIO-AS14, Companilactobacillus crustorum NWAFU-BIO-AS16, Enterococcus faecium NWAFU-BIO-A-B24, Lactiplantibacillus plantarum NWAFU-BIO-BS29), Limosilactobacillus fermentum NWAFU-BIO-B-S6, Lactobacillus gallinarum NWAFU-BIO-D-S7, and Limosilactobacillus fermentum NWAFU-BIO-D-B2. The 16S rRNA nucleotide sequences (S2) have been deposited in NCBI data base under GenBank accession number(s): ON340621, ON340622, ON340623, ON340624, ON340625, ON340627, and ON340628. See figure (Fig. 1A and B) for evolutionary analysis and timetree, respectively. The evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa–Kishino–Yano model.
Fig. 1.
A Evolutionary analysis by Maximum Likelihood method. B Time tree (http://www.timetree.org/search/goto_pairwise). Note: The evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa-Kishino-Yano model. The tree with the highest log likelihood (− 13,623.92) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. This analysis involved 7 nucleotide sequences. There were a total of 1477 positions in the final dataset. Evolutionary analyses were conducted in MEGA11 (www.megasoftware.net)
Probiotics properties
Screening of LAB with antibacterial activity
Antibacterial activity of (CFS) against indicator strains of Staphylococcus aureus ATCC25923 and Escherichia coli ATCC25922 showed that seven isolates have largest inhibition zones ranged (17–25) and (16–21) mm, respectively, so were selected for further characterization. Lactiplantibacillus plantarum NWAFU-BIO-BS29 showed the highest activity. See figure (Fig. 2A).
Fig. 2.
The inhibition diameters of LAB cell free supernatant against gram + and pathogens, B Tolerance of strains to acid conditions, C Tolerance of strains to Bile conditions. Note: 1 is Enterococcus faecium NWAFU-BIO-AS14, 2; Companilactobacillus crustorum NWAFU-BIO-AS16, 3; Enterococcus faecium NWAFU-BIO-A-B24, 4; Lactiplantibacillus plantarum NWAFU-BIO-BS29, 5; Limosilactobacillus fermentum NWAFU-BIO-B-S6, 6; Lactobacillus gallinarum NWAFU-BIO-D-S7, and 7; Limosilactobacillus fermentum NWAFU-BIO-D-B2
Acid and bile salt tolerance
As shown in Fig. 3, the acid tolerance for the tested strains has been ranged from 45.5 to 83.26%, Companilactobacillus crustorum NWAFU-BIO-AS16 showed the highest acid tolerance Fig. 2B. Likewise, for the bile tolerance ranged between (92.91–74.96%), (59.36–15.95%), and (30.54–11.92%) at presence of 0.3, 0.5, and 1% of bile salt, respectively, Fig. 2C, while, the best tolerance at these concentrations were observed from Enterococcus faecium NWAFU-BIO-A-B24, lactobacillus gallinarum NWAFU-BIO-D-S7, and Limosilactobacillus fermentum NWAFU-BIO-B-S6, respectively.
Fig. 3.
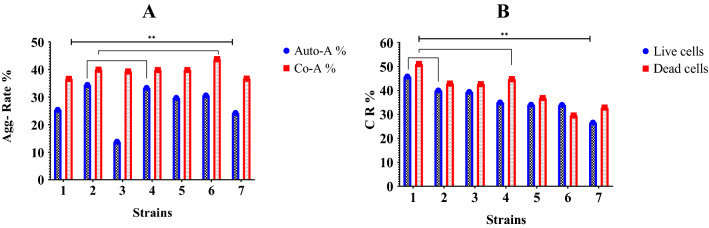
A Auto-aggregation, and Co-aggregation ability. B Cholesterol removal ability of the potential probiotic strains. Note: The key of strain names from (1–7) as describe above
Auto-aggregation, and co-aggregation abilities
All tested strains showed a high increased auto-aggregation activity at 12 h ranging from 13.8 to 34.4%, see figure (Fig. 3A). Enterococcus faecium NWAFU-BIO-A-B24 and Companilactobacillus crustorum NWAFU-BIO-AS16 had the lowest and highest activity, respectively. While co-aggregation at 4 h was ranged from 36.61 to 43.71%, Limosilactobacillus fermentum NWAFU-BIO-D-B2 and lactobacillus gallinarum NWAFU-BIO-D-S7 had the lowest and highest activity, respectively.
Cholesterol-lowering ability
For further characterization, a comparison between seven lactic acid bacteria strains was conducted to screen their cholesterol-lowering ability in vitro. All of them were found to had ability to reduce the cholesterol levels by more than 26.57% (Live cells), and 29.53 (Dead cells) % in vitro (Fig. 3 B). Enterococcus faecium NWAFU-BIO-AS14 had the best cholesterol reduction ability in vitro (45.76% live cells) and (50.97% dead cells).
In vitro safety evaluation
Antibiotic susceptibility test
According to Guidance of EFSA standards cut-off values of using microorganisms as feed additives or as functional organisms (Feed 2018) the antibiotic susceptibility test revealed that all the strains had high sensibility against the tested antibiotics see (Table 1). The strains were categorized as: susceptible when their growth is inhibited at a concentration of antibiotics equal to or lower than the cut-off value. resistant, if it grows at a concentration higher than the established cut-off value. Interpretation of the susceptibility categories are the resistance (R), susceptibility (S), and intermediate (I) according to CLSI 2018 (Li et al. 2020). (Data were expressed as the mean ± SD from three replicates).
Table 1.
Antibiotic classification, MIC (mg/ml) profiles to inhibit LAB strains, and susceptibility type
| Antibiotic classification (Zheng et al. 2020) | Antibiotic | Strains | Cut-off values (mg/L) | Susceptibility type |
|---|---|---|---|---|
| Aminoglycoside | Kanamycin | 1, 3 | 1024 | S |
| 2, 4, 5, 7 | 64 | S | ||
| 6 | 16 | S | ||
| Gentamycin | 1, 3 | 32 | S | |
| 2, 4, 5, 7 | 16 | S | ||
| 6 | 4 | S | ||
| Beta-lactams | Ampicillin | 1, 3, 4, 5, 7 | 2 | S |
| 2 | 4 | S | ||
| 6 | 1 | S | ||
| Protein synthesis inhibitor | Tetracycline | 1, 3 | 4 | S |
| 2, 5, 7 | 8 | S | ||
| 4 | 32 | S | ||
| 6 | 2 | S | ||
| Cyclic peptides | Streptomycin | 1, 3 | 128 | S |
| 2 | 32 | S | ||
| 4 | n.r | S | ||
| 5, 7 | 64 | S | ||
| 6 | 8 | S | ||
| Glycopeptide | Vancomycin | 1, 3, 6 | 4 | S |
| 2, 4, 5, 7 | n.r | S | ||
| Macrolides | Erythromycin | 1, 3 | 4 | S |
| 2, 4, 5, 6, 7 | 1 | S | ||
| Amphenicols | Chloramphenicol | 1, 3 | 16 | S |
| 2, 5, 6, 7 | 4 | S | ||
| 4 | 8 | S |
The key of strain names from (1–7) as describe above
n.r. not required, S sensitive, R resistant, I intermediate to the antibiotics tested
Hemolytic activity
The evaluation of Hemolytic activity was showed that, Enterococcus faecium NWAFU-BIO-AS14 has a green-hued zone around the colonies (a-hemolysis). See Table 2, while other strains did not display any zones around the colonies on the blood plates.
Table 2.
Evaluation of hemolytic activity of the potential probiotic strains
| LAB | Haemolytic activity |
|---|---|
| Enterococcus faecium NWAFU-BIO-AS14 | a |
| Companilactobacillus crustorum NWAFU-BIO-AS16 | g |
| Enterococcus faecium NWAFU-BIO-A-B24 | g |
| Lactiplantibacillus plantarum NWAFU-BIO-BS29 | g |
| Limosilactobacillus fermentum NWAFU-BIO-B-S6 | g |
| Lactobacillus gallinarum NWAFU-BIO-D-S7 | g |
| Limosilactobacillus fermentum NWAFU-BIO-D-B2 | g |
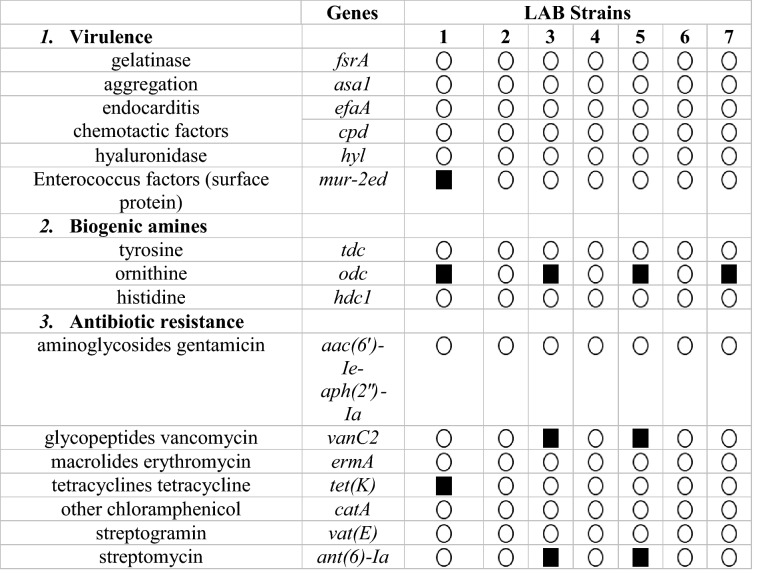
Detection of virulence and resistance genes
The safety assessment of LAB strains to be use as probiotics was evaluated by screening the presence of (VFs) in genomic DNA. Enterococcus faecium NWAFU-BIO-AS14, Enterococcus faecium NWAFU-BIO-A-B24, Limosilactobacillus fermentum NWAFU-BIO-B-S6, and Limosilactobacillus fermentum NWAFU-BIO-D-B2 were showed presence of 3, 3, 3, and 1 (VFs), respectively. See (Table 3), while, Companilactobacillus crustorum (NWAFU-BIO-AS16), Lactiplantibacillus plantarum NWAFU-BIO-BS29, and lactobacillus gallinarum NWAFU-BIO-D-S7 were found negative for all tested (VFs).
Table 3.
Detection of 16 (VFs) genes related to virulence, biogenic amines, and antibiotic resistance
 ; not detect,
; not detect,  ; detect. The key of strain names from (1–7) as describe above
; detect. The key of strain names from (1–7) as describe above
Antioxidant activity analysis
The antioxidant activities of the potential probiotics were evaluated in vitro, the seven strains exhibited various radical scavenging capacities.
The ABTS assay the strains showed scavenging activity ranged between (90.97–62.43%) see Fig. 4A, while the strains that indicate the highest and lowest activity are belonging to lactobacillus gallinarum NWAFU-BIO-D-S7 and Limosilactobacillus fermentum NWAFU-BIO-D-B2.
Fig. 4.

A DPPH radical scavenging activity, B ABTS radical scavenging activity, C Hydroxyl radical scavenging activity. D Superoxide anion scavenging activity of potential probiotics. Note: The key of strain names from (1–7) as describe above
The DPPH assay of the CFS of selected strains had scavenging activity range between (94.05–75.78%) as shown in Fig. 4B. Lactiplantibacillus plantarum NWAFU-BIO-BS29 and Enterococcus faecium NWAFU-BIO-AS14 had the highest and lowest values, respectively.
(HR) scavenging activity of all potential probiotics were showed high ranges between (61.44–54.26%), thus, no significant difference among the seven strains (Fig. 4C), Enterococcus faecium NWAFU-BIO-AS14 showed the highest hydroxyl radical scavenging activity followed by Lactiplantibacillus plantarum NWAFU-BIO-BS29.
All the strains had showed superoxide anion scavenging activity above (50%) see figure (Fig. 4D), based on these results, the highest and lowest activity were observed in Companilactobacillus crustorum NWAFU-BIO-AS16 and Limosilactobacillus fermentum NWAFU-BIO-B-S6 at a range between (53.23–76.09%), respectively.
Indeed, all the tested strains were showed a high ability to grow in the presence of different concentrations of hydrogen peroxide at ranges of (99.9–95.29%), (90.66–84.38%), (20.34–14.98%), (9.8–7.72%) at concentration of hydrogen peroxide (0.2, 0.6, 1.0, and 2.0 mM, respectively. See (Fig. 5). The strains which have exhibited high RHP capability were Limosilactobacillus fermentum NWAFU-BIO-D-B2 (0.2 mM), Companilactobacillus crustorum NWAFU-BIO-AS16 (0.6, and 2.0 mM), and lactobacillus gallinarum NWAFU-BIO-D-S7 at 1.0 mM hydrogen peroxide.
Fig. 5.
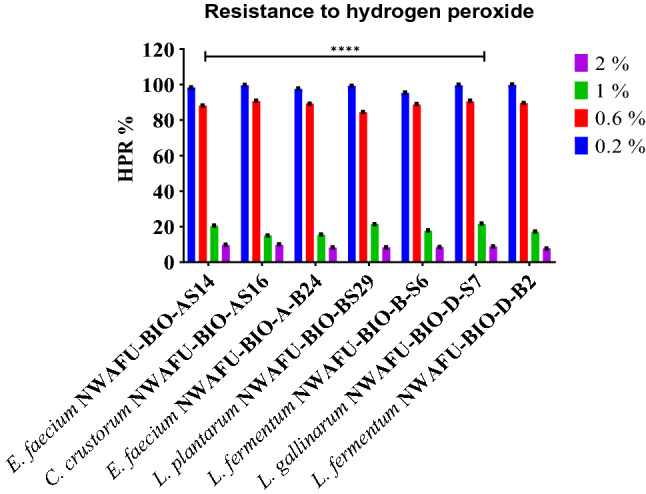
Evaluation the resistance to hydrogen peroxide of the potential probiotic strains
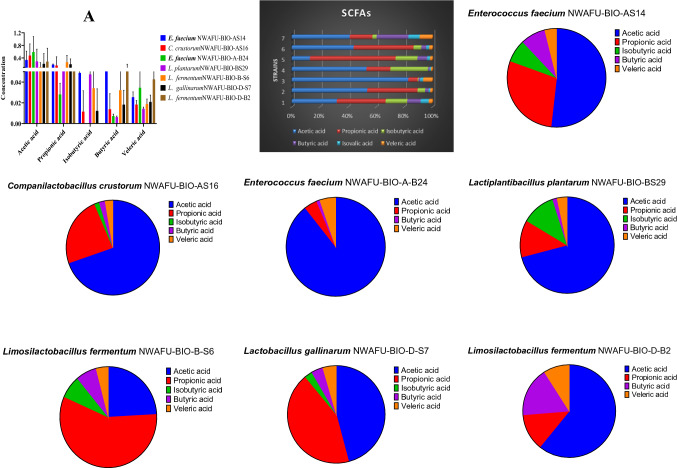
SCFAs present in culture medium
SCFAs are produce during fermentation of carbohydrates by lactic acid bacteria, acetic acid was the most abundant SCFAs (0.590–0.112 μm/ml), and then propionic acid (0.267–0.028 μm/ml). The SCFAs profile of tested strains and their concentrations were presented in figure (Fig. 6).
Fig. 6.
SCFAs profile present in culture medium (µm/ml). Note: The key of strain names from (1–7) as describe above
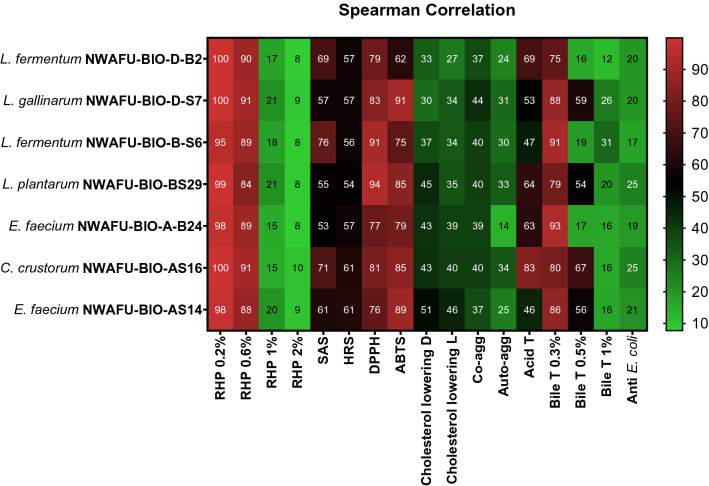
Correlation
Correlation coefficient were used to evaluate establish of the relationship between different parameters of probiotics properties (antibacterial and antioxidant activity, acid and Bile salt tolerance, aggregation capability, and Cholesterol-lowering ability). Spearman’s correlation matrix was used to determine if there are correlation between each other (Fig. 7).
Fig. 7.
Spearman’s correlation matrix
Discussion
In this study, LAB strains were isolated from fermented milk samples, and their probiotic properties were investigated. According to their antibacterial activity, seven strains with vigorous activity were selected as potential probiotics; they had inhibition zones ranged between (16–21) and (17–25) mm against Escherichia coli ATCC25922 and Staphylococcus aureus ATCC25923, respectively, which is a promising result to use these strains as bio-preservatives. Lactiplantibacillus plantarum NWAFU-BIO-BS29 displayed the highest activity; the same result reported by Azat et al. (2016) LAB strains have an inhibition zone ranging between (6.3–10.5) and (4.0–8.2 mm) in diameter, against Escherichia coli and Staphylococcus aureus, respectively. This activity could be explained by the production of antimicrobial compounds that might inhibit or reduce the growth of pathogens, such as lactic acid, acetic acid, diacetyl, fatty acids, aldehydes, bacteriocins, and other compounds.
The gastric and intestinal fluids have high acidic pH and high bile salts concentration, tolerant to these conditions are the most common methods to detect the viability and activity of probiotics. The survival rate at pH 3.0 was evaluated, where all the strains could survive with a survival percentage ranging between 45.5 and 83.26% which are considered acid tolerant; Companilactobacillus crustorum NWAFU-BIO-AS16 had the highest acid tolerance; the same findings were outlined by Azat et al. (2016). LAB had acid tolerance ranged 66.50 to 85.25%, while L. plantarum E680 had the highest acid tolerance, as well as, Lactobacillus plantarum YS2 (LP-YS2) was found to have an acid tolerance of 68.05% (Qian et al. 2018), and 100% for Lactobacillus pentosus KF923750 (Bendali et al. 2017). The concentration of bile in the human gastrointestinal tract is about 0.3%; good probiotic strains can grow and metabolize in this concentration (Azat et al. 2016). In vitro assessment was shown all the strains had remarkably bile salts resistant with a proportion of growth between (92.91–74.96%), (59.36–15.95%), and (30.54–11.92%) at 0.3, 0.5, and 1% bile salt, respectively. In contrast, Qian et al. (2018) reported that Lactobacillus plantarum YS2 (LP-YS2) was found to have a percentage of bile salt resistant of (19.85%, 15.01%, and 8.35%) in the presence of 0.3%, 0.5%, and 1.0% bile salt. Otherwise, Lactobacillus pentosus KF923750 showed resistant to bile (0.1% [98.42%], 0.3% [88.52%], 0.5% [75.60%] and 1% [71.15%]) (Bendali et al. 2017), and L. plantarum E680 which displayed survival rate of 80.79% (Zheng et al. 2020). Also Azat et al. (2016) investigated six LAB strains that had growth above 65%.
The capability of the potential probiotics to auto and co-aggregation was assessed in vitro as functional characteristics. The results indicated that the majority had a high potential ability to adhere with themselves and with E. coli (Entero-pathogens) with auto and co-aggregation ability ranging between (13.8–34.4%) and (36.61–43.71%), respectively, Companilactobacillus crustorum NWAFU-BIO-AS16 had the highest percentage of adhere. M. Li et al. (2020) have reported that L. rhamnosus R4 and L. salivarius M2-71 had auto-aggregating of 45.83% and 95.6%, respectively, at 24 h with E. coli and S. typhimurium. In contrast, Weissella confusa F80, Weissella halotolerans FAS23, FAS3, and F99 had aggregation percentages of 72, 66.5, 64.6, and 64.1% after 1 h, respectively, with with co-aggregation ability more than 30% (Fhoula et al. 2018), as well as, Lactobacillus reuteri ZJ625, Lactobacillus reuteri VB4, Lactobacillus salivarius ZJ614, and Streptococcus salivarius NBRC13956 were showed abilities of auto- and co-aggregations ranging from (60 to 70%) and from (45 to 56%) after 4 h, respectively (Dlamini et al. 2019).
To further investigation of functional characteristics, the cholesterol lowering ability was assessed. All the strains were able to reduce the cholesterol levels at range of 45.76 -26.57% and 50.97–29.53% by live and dead cells, respectively. Enterococcus faecium NWAFU-BIO-AS14 displayed the highest level of cholesterol reduction, which was significantly higher than other strains have been reported. Lactobacillus kefiri, Lactobacillus fermentum NF4, and Lactobacillus rhamnosus were showed considerable ability to reduce cholesterol from the media, ranging at 68.7, 55.8, and 22%, respectively (Sui et al. 2021; Yusuf et al. 2020). Likewise, Weissella halotolerans F99 from camel feces and L. pentosus KF923750 were able to reduce it in vitro by 49, and 62.4%, respectively (Bendali et al. 2017; Fhoula et al. 2018), as well as, L. plantarum LIP-1 isolated from koumiss had assimilated 71.47 μg/mL of cholesterol in vitro (Zheng et al. 2020). It’s interesting in this study to note that the intact cells were able to reduce more cholesterol compared to the supernatant of the bacterial cells. This result implies may be due to the activity of certain components in the supernatant of the bacterial culture, in addition to those existing in the live cells, which is consistent with data from Azat et al. (2016).
The safety assessment of probiotics is the most critical factor in using these strains safely. Screening the presence of virulence factors showed that Enterococcus faecium NWAFU-BIO-AS14 had VFs of (mur-2ed, odc, and tet(K)), Enterococcus faecium NWAFU-BIO-A-B24 had VFs of (odc, vanC2, and ant(6)-Ia), Limosilactobacillus fermentum NWAFU-BIO-B-S6 had VFs of (odc, vanC2, and ant(6)-Ia), and Limosilactobacillus fermentum NWAFU-BIO-D-B2 had just one VFs of (odc). While Companilactobacillus crustorum NWAFU-BIO-AS16, Lactiplantibacillus plantarum NWAFU-BIO-BS29, Lactobacillus gallinarum NWAFU-BIO-D-S7 do not have any virulence genes, thus, these isolates may not have safety concerns. To our knowledge, numerous studies on lactobacilli species have not detected the presence of virulence factors such as E. faecium CM33 strain, which was found negative for all tested virulence genes of (vanA, vanB and vanC2) and other VFs of collagen adhesion (acm), aggregation substance (agg), cytolysin (cylA), and gelatinase (gelE) (Dlamini et al. 2019). Also, E. durans LAB18s was found negative for the following VFs: aggregation substances (agg and asa), adhesion collagen protein (ace), bopB (beta-phosphoglucomutase), bopC (aldose 1-epimerase), bopA (putative glycosyltransferase), and bopD (sugar-binding transcriptional regulator) and resistance genes of (vanA, vanC1 and vanC2/3) (Pieniz et al. 2015). On the other side, L. reuteri VB4 and S. salivarius NBRC13956 showed the presence of resistance genes vanC 2/3 and vanC1, respectively (Dlamini et al. 2019). Likewise, Lb. plantarum ST8Sh showed presence of 13 of 50 virulence genes related to (sex pheromones, aggregation substance, adhesion of collagen, tetracycline, gentamicin, chloramphenicol, and erythromycin (Todorov et al. 2017), and high frequencies of VFs were observed for (asa1, esp, efaA, and ace) in Lactococcus and Enterococcus isolates (Perin et al. 2014), Meanwhile, Klare et al. (2007) reported the prevalence of (tet) genes in Lactobacillus isolates like tet(M) which had been detected in L. plantarum, L. curvatus, Lactobacillus casei, L. acidophilus, L. gasseri and L. crispatus, and tet(W) gene in strains of L. crispatus, L. johnsonii, and L. reuteri. The presence of erm(B) Erythromycin resistance gene has been detected in many species like L. reuteri, L. fermentum, L. casei, L. plantarum, L. acidophilus, L. gasseri, L. rhamnosus and L. johnsonii.54 (Klare et al. 2007). According to the EFSA safe strains should not present any virulence factors which can be transferred to the host or pathogenic bacteria, in particular, vancomycin resistance which is the most significant global concern since it’s the last antibiotic that works effectively against multidrug-resistant pathogens (de Jesus et al. 2021; Ogier and Serror 2008). In many cases, the presence of resistance and virulence genes does not guarantee gene expression and there is a high possibility that these genes are not expressed or only weakly expressed (not transferrable) (Nami et al. 2015).
Another safety aspect that must be considered is evaluating the hemolytic activity of probiotics and antibiotic resistance, which indicated that Enterococcus faecium NWAFU-BIO-AS14 had a green-hued zone around the colonies (a-hemolysis). See Table 2); while other strains did not display any zone around the colonies on the blood plates, also the same species E. faecium isolate (GEn27) was reported to have positive α-hemolysis (Perin et al. 2014). The assessment of probiotics susceptibility to antibiotics was performed against (Kanamycin, Gentamycin, Ampicillin, Tetracycline, Streptomycin, Vancomycin, Erythromycin, and Chloramphenicol) antibiotics to recognize isolates with acquired antibiotic resistance. EFSA cut-off values were used to distinguish strains with acquired resistance from susceptible strains. Overall, our finding indicated that all the strains had a high susceptibility to all tested antibiotics almost identical to a broad range of LAB. Some species, such as E. durans LAB18s, exhibited sensibility to antibiotics commonly used in animal feed, like erythromycin, tetracycline, vancomycin, gentamicin, and penicillin (Pieniz et al. 2015). Another study by Klare et al. (2007) tested 12 LAB species against 13 antibiotics, as general, the vast majority of LAB were susceptible to penicillin, ampicillin, ampicillin/sulbactam, quinupristin/dalfopristin, chloramphenicol, linezolid, trimethoprim, trimethoprim/sulfamethoxazole, vancomycin, teicoplanin, and fusidic acid, while three Lactobacillus strains were highly resistant to one or more of streptomycin, erythromycin, clindamycin, and oxytetracycline, and E. faecium M6-29 strain was characterized as resistant to eleven antibiotics (Li et al. 2020), as well as, Enterococcus faecium CM33 strain was found to be susceptible to vancomycin and unsusceptible to gentamycin, erythromycin, tetracycline, and rifampicin (Nami et al. 2015). According to the FEEDAP panel, resistant bacteria to antibiotics of human and veterinary importance should not be used as food and feed additives unless proven that it results from a chromosomal mutation(s) (Klare et al. 2007). Upon assessment of the susceptibility, MRS agar can show high MICs of the aminoglycoside’s, gentamicin, and streptomycin. However, our strains showed intrinsic low resistance to vancomycin, which does not present a potential risk for horizontal gene transfer (Fhoula et al. 2018).
The antioxidant activity of the potential probiotics was evaluated in vitro. Significant positive correlations were demonstrated among antioxidant assays with a correlation coefficient (R) ranged from 0.11 to 1. All these strains exhibited various degrees of potent scavenging activities ranged between (90.97–62.43%) ABTS, (94.05–75.78%) DPPH, (61.44–54.26%) Hydroxyl radical scavenging activity, (53.23 -76.09%) Superoxide anion scavenging activity, and as we can be seen these strains had resistance to hydrogen peroxide ranges of (99.9–95.29%), (90.66–84.38%), (20.34–14.98%), (9.8–7.72%) at concentrations of hydrogen peroxide (0.2, 0.6, 1.0, and 2.0 mM, respectively. Previous studies stated that Lactobacillus plantarum KX041 had ABTS, DPPH, hydroxyl, and superoxide free radical scavenging of 0.2, 1.4, 1.7, and 5.6 mg/mL, respectively (Wang et al. 2017), and L. plantarum C88, L. rhamnosus R4, and E. hirae K4-8 showed scavenging activity of (53.05%), (53.78%), and (32.11%), respectively, while L. plantarum C88 and L. rhamnosus R4 showed hydroxyl radical scavenging activity of 44.31% and 45.79%, respectively (Azat et al. 2016; Li et al. 2012). Azat et al. (2016) reported that, the intact cells of L. rhamnosus R4 were the most effective in removing of the DPPH radicals. The scavenging activities of the intact cells of L. helveticus S4, E. hirae H4, and R5 exhibited better behavior for scavenging the DPPH radicals than those of cell-free extracts, indicating that cell integrity was a factor that affected scavenging activity; thus, the intact cells exhibited higher hydroxyl radical scavenging activity than the cell-free extracts; this is probably due to their extracellular antioxidant components, such as polysaccharides, peptidoglycan, and teichoic acid. These basic cell-wall components play an essential role when the host suffers oxidative damage from free radicals. We inferred that some intracellular enzymes such as NADH-oxidase, NADH-peroxidase, and superoxide dismutase (SOD) were likely to be obtained after breaking up the bacterial cells into cell-free extracts that indicated the antioxidant activity of lactic acid bacteria (Azat et al. 2016).
The SCFA profile showed that L. fermentum NWAFU-BIO-D-B2 had significantly a higher ability to produce Butyric acid and Valeric acid (0.081 and 0.042 μm/ml), respectively, than those of other strains. While, E. faecium NWAFU-BIO-A-B24, L. fermentum NWAFU-BIO-B-S6, L. plantarum NWAFU-BIO-BS29 for the high production of acetic acid, valeric acid, acetic acid, propionic acid, and isobutyric acid (0.590, 0.267, and 0.047 μm/mL), respectively. SCFAs can suppress the growth of pathogenic intestinal bacteria and modulate lipid metabolism and the immune system. They also lower intestinal pH and promote the bioavailability of minerals such as magnesium and calcium. Propionic acid has been reported to reduce the activity of Escherichia coli and Salmonella spp. and has an anti-cholesterolemic effect (Kang et al. 2021). All strains are a butyrate-producer which has recently been shown to induce antioxidases to suppress hepatic oxidative stress in rats. Furthermore, SCFAs play an essential role in human immune function (Zhang et al. 2020). This result suggested that they can be use in therapeutics interventions to regulated the levels of antioxidant metabolites in the host.
Accordingly, these results indicated that all the strains have cholesterol-lowering capability by live and dead cells and are tolerant to acidic and bile salt conditions simulated to the gastro, with antibacterial activity and remarkable aggregation ability to prevent form adhere of pathogens. The safety investigation revealed Enterococcus faecium NWAFU-BIO-AS14, Enterococcus faecium NWAFU-BIO-A-B24, Limosilactobacillus fermentum NWAFU-BIO-B-S6, and Limosilactobacillus fermentum NWAFU-BIO-D-B2 were not considered as safe and good probiotics, while Lactiplantibacillus plantarum NWAFU-BIO-BS29, Companilactobacillus crustorum NWAFU-BIO-AS16, and lactobacillus gallinarum NWAFU-BIO-D-S7, respectively, exhibited a high potential to be used as starter cultures which can be applied safely in functional foods with a promising feature to produce bio-preservatives compounds. Among them, Lactiplantibacillus plantarum NWAFU-BIO-BS29 showed desirable antimicrobial activity against Staphylococcus aureus ATCC25923 and Escherichia coli ATCC25922 with the largest inhibition diameter zones (21–25 mm) and the highest activity of DPPH (94.05%) and auto-aggregation (39.94%), it also showed cholesterol lowering of 34.95% and 44.66% by live and dead cells, respectively, so it can be ideally used as a probiotic as it is believed to have health-promoting properties and a potential property to produce bio-preservative substances.
Conclusion
Consumers’ awareness of healthy and natural food has increased in the present decades, so we need to discover new probiotic strains. The work aimed to screen and characterize the features of lactic acid bacteria isolated from Gansu traditional fermented milk which is considered a functional food containing beneficial probiotics. In conclusion, the seven strains showed potentiality to be used as potential probiotic strains due to their high capacity to growth at low acidity (pH 3) with different survival rates, as well as their supernatants having the ability to inhibit pathogens, the presence of these LAB microorganisms in food products might influence the reduced survival of pathogens and therefore may have potential application in food preservation. Moreover, these LAB can produce SCFAs and have auto-aggregation ability (cells adherence) which is essential to be effective in the gut flora, while the co-aggregation ability (adhere to pathogens) plays a vital role in enabling it to form a barrier that prevents colonization of harmful enteric pathogens. Other important features are the ability to lower cholesterol in vitro and antioxidant activity; as seen from the results, all our tested strains are able to reduce the cholesterol and have a high antioxidant activity. Four isolates had exhibited one or more VFs, which can lead to low health risks, while one strain was showed a positive hemolytic activity; from these points, the best and safer strains can be used as probiotics in food and feed or as starter cultures for the dairy industry. A future study will be conducted on the mice model to show the effects of these probiotics on the health and their interaction with the gut microbiota.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are gratefully acknowledging the following financial supporters; Agro-Scientific Research in the Public Interest (Grant No. 201503135), and the Doctoral Program of Chinese scholarship council (SCS No. 2018SLJ018739) for their important contributions to funding of this research.
Data Availability
All data generated or analysed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Contributor Information
Mohamedelfatieh Ismael, Email: Alfagold2008@windowslive.com.
Yaxin Gu, Email: yaxingu@nwafu.edu.cn.
Yanlong Cui, Email: 1024923026@qq.com.
Tao Wang, Email: 2017@163.com.
Fangfang Yue, Email: 462034927@qq.com.
Qin Yantin, Email: 364611964@qq.com.
Xin Lü, Email: xinlu@nwsuaf.edu.cn.
References
- Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Jt Bone Spine. 2007;74(4):324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Azat R, Liu Y, Li W, Kayir A, Lin DB, Zhou WW, Zheng XD. Probiotic properties of lactic acid bacteria isolated from traditionally fermented Xinjiang cheese. J Zhejiang Univ Sci B. 2016;17(8):597–609. doi: 10.1631/jzus.B1500250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendali F, Kerdouche K, Hamma-Faradji S, Drider D. In vitro and in vivo cholesterol lowering ability of Lactobacillus pentosus KF923750. Benef Microbes. 2017;8(2):271–280. doi: 10.3920/bm2016.0121. [DOI] [PubMed] [Google Scholar]
- Chlebowska-Smigiel A, Gniewosz M, Kieliszek M, Bzducha-Wrobel A. The effect of pullulan on the growth and acidifying activity of selected stool microflora of human. Curr Pharm Biotechnol. 2017;18(2):121–126. doi: 10.2174/1389201017666161229154324. [DOI] [PubMed] [Google Scholar]
- Chlebowska-Śmigiel A, Kycia K, Neffe-Skocińska K, Kieliszek M, Gniewosz M, Kołożyn-Krajewska D. Effect of pullulan on physicochemical, microbiological, and sensory quality of yogurts. Curr Pharm Biotechnol. 2019;20(6):489–496. doi: 10.2174/1389201020666190416151129. [DOI] [PubMed] [Google Scholar]
- CLSI . Performance standards for antimicrobial susceptibility testing. 28. Wayne: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- Dasgupta A, Klein K. Chapter 2: methods for measuring oxidative stress in the laboratory. In: Dasgupta A, Klein K, editors. Antioxidants in food, vitamins and supplements. San Diego: Elsevier; 2014. pp. 19–40. [Google Scholar]
- de Jesus LCL, de Jesus Sousa T, Coelho-Rocha ND, Profeta R, Barroso FAL, Drumond MM, Azevedo V. Safety evaluation of Lactobacillus delbrueckii subsp. lactis CIDCA 133: a Health-Promoting Bacteria. Probiot Antimicrob Prot. 2021;13:1–14. doi: 10.1007/s12602-021-09826-z. [DOI] [PubMed] [Google Scholar]
- Dlamini ZC, Langa RLS, Aiyegoro OA. Safety evaluation and colonisation abilities of four lactic acid bacteria as future probiotics. Probiot Antimicrob Prot. 2019;11(2):397–402. doi: 10.1007/s12602-018-9430-y. [DOI] [PubMed] [Google Scholar]
- Feed E, Panel O. A. P. O. S. U. I. A Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018;16(3):e05206. doi: 10.2903/j.efsa.2018.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes. 2020;12(1):1801944. doi: 10.1080/19490976.2020.1801944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fhoula I, Rehaiem A, Najjari A, Usai D, Boudabous A, Sechi LA, Hadda-Imene O. Functional probiotic assessment and in vivo cholesterol-lowering efficacy of Weissella sp. associated with arid lands living-hosts. Biomed Res Int. 2018;2018:1654151. doi: 10.1155/2018/1654151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization/World Health Organization (2002) “Guidelines for the Evaluation of Probiotics in Foods”, Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization: Geneva, Switzerland
- Griffiths HR. Antioxidants: characterization and analysis. In: Caballero B, Finglas PM, Toldrá F, editors. Encyclopedia of food and health. Oxford: Academic Press; 2016. pp. 221–226. [Google Scholar]
- Habib B, Vaid S, Bangotra R, Sharma S, Bajaj BK. Bioprospecting of probiotic lactic acid bacteria for cholesterol lowering and exopolysaccharide producing potential. Biologia. 2022;77(7):1931–1951. doi: 10.1007/s11756-022-01058-y. [DOI] [Google Scholar]
- Kang C-H, Kim J-S, Park HM, Kim S, Paek N-S. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech. 2021;11(5):217. doi: 10.1007/s13205-021-02767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitha S, Harikrishnan A, Jeevaratnam K. Characterization and evaluation of antibacterial efficacy of a novel antibiotic-type compound from a probiotic strain Lactobacillus plantarum KJB23 against food-borne pathogens. LWT. 2020;118:108759. doi: 10.1016/j.lwt.2019.108759. [DOI] [Google Scholar]
- Kieliszek M, Pobiega K, Piwowarek K, Kot AM. Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules. 2021 doi: 10.3390/molecules26071858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klare I, Konstabel C, Werner G, Huys G, Vankerckhoven V, Kahlmeter G, Goossens H. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J Antimicrob Chemother. 2007;59(5):900–912. doi: 10.1093/jac/dkm035. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao Y, Zhang L, Zhang X, Huang L, Li D, Wang Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012;135(3):1914–1919. doi: 10.1016/j.foodchem.2012.06.048. [DOI] [PubMed] [Google Scholar]
- Li M, Wang Y, Cui H, Li Y, Sun Y, Qiu HJ. Characterization of lactic acid bacteria isolated from the gastrointestinal tract of a wild boar as potential probiotics. Front Vet Sci. 2020;7:49. doi: 10.3389/fvets.2020.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14(4):840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjirebaye P, Xiao M, Mahamat B, Xiong S, Mueed A, Wei B, Peng Z. In vitro characteristics of lactic acid bacteria probiotics performance and antioxidant effect of fermented soymilk. Food Biosci. 2022 doi: 10.1016/j.fbio.2022.101952. [DOI] [Google Scholar]
- Majeed M, Majeed S, Nagabhushanam K, Arumugam S, Beede K, Ali F. Evaluation of the in vitro cholesterol-lowering activity of the probiotic strain Bacillus coagulans MTCC 5856. Int J Food Sci Technol. 2019;54(1):212–220. doi: 10.1111/ijfs.13926. [DOI] [Google Scholar]
- Mendes MCS, Paulino DS, Brambilla SR, Camargo JA, Persinoti GF, Carvalheira JBC. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J Gastroenterol. 2018;24(18):1995–2008. doi: 10.3748/wjg.v24.i18.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mfotie Njoya E. Chapter 31: medicinal plants, antioxidant potential, and cancer. In: Preedy VR, Patel VB, editors. Cancer. 2. San Diego: Academic Press; 2021. pp. 349–357. [Google Scholar]
- Moraes PM, Perin LM, Todorov SD, Silva A, Jr, Franco BDGM, Nero LA. Bacteriocinogenic and virulence potential of Enterococcus isolates obtained from raw milk and cheese. J Appl Microbiol. 2012;113(2):318–328. doi: 10.1111/j.1365-2672.2012.05341.x. [DOI] [PubMed] [Google Scholar]
- Nami Y, Haghshenas B, Haghshenas M, Yari Khosroushahi A. Antimicrobial activity and the presence of virulence factors and bacteriocin structural genes in Enterococcus faecium CM33 isolated from ewe colostrum. Front Microbiol. 2015;6:782. doi: 10.3389/fmicb.2015.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier J-C, Serror P. Safety assessment of dairy microorganisms: the Enterococcus genus. Int J Food Microbiol. 2008;126(3):291–301. doi: 10.1016/j.ijfoodmicro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Oh YJ, Jung DS. Evaluation of probiotic properties of Lactobacillus and Pediococcus strains isolated from Omegisool, a traditionally fermented millet alcoholic beverage in Korea. LWT Food Sci Technol. 2015;63(1):437–444. doi: 10.1016/j.lwt.2015.03.005. [DOI] [Google Scholar]
- Pan DD, Zeng XQ, Yan YT. Characterisation of Lactobacillus fermentum SM-7 isolated from koumiss, a potential probiotic bacterium with cholesterol-lowering effects. J Sci Food Agric. 2011;91(3):512–518. doi: 10.1002/jsfa.4214. [DOI] [PubMed] [Google Scholar]
- Perin LM, Miranda RO, Todorov SD, Franco BDGDM, Nero LA. Virulence, antibiotic resistance and biogenic amines of bacteriocinogenic lactococci and enterococci isolated from goat milk. Int J Food Microbiol. 2014;185:121–126. doi: 10.1016/j.ijfoodmicro.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Pieniz S, de Moura TM, Cassenego APV, Andreazza R, Frazzon APG, Camargo FADO, Brandelli A. Evaluation of resistance genes and virulence factors in a food isolated Enterococcus durans with potential probiotic effect. Food Control. 2015;51:49–54. doi: 10.1016/j.foodcont.2014.11.012. [DOI] [Google Scholar]
- Pinto A, Barbosa J, Albano H, Isidro J, Teixeira P. Screening of bacteriocinogenic lactic acid bacteria and their characterization as potential probiotics. Microorganisms. 2020;8(3):393. doi: 10.3390/microorganisms8030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Long X, Pan Y, Li G, Zhao X. Isolation and identification of lactic acid bacteria (Lactobacillus plantarum YS2) from yak yogurt and its probiotic properties. Biomed Res (india) 2018;29:815–820. doi: 10.4066/biomedicalresearch.29-17-3418. [DOI] [Google Scholar]
- Raman J, Kim JS, Choi KR, Eun H, Yang D, Ko YJ, Kim SJ. Application of lactic acid bacteria (LAB) in sustainable agriculture: advantages and limitations. Int J Mol Sci. 2022 doi: 10.3390/ijms23147784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sengun IY, Nielsen DS, Karapinar M, Jakobsen M. Identification of lactic acid bacteria isolated from Tarhana, a traditional Turkish fermented food. Int J Food Microbiol. 2009;135(2):105–111. doi: 10.1016/j.ijfoodmicro.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Sui Y, Liu J, Liu Y, Wang Y, Xiao Y, Gao B, Zhu D. In vitro probiotic characterization of Lactobacillus strains from fermented tangerine vinegar and their cholesterol degradation activity. Food Biosci. 2021;39:100843. doi: 10.1016/j.fbio.2020.100843. [DOI] [Google Scholar]
- Sujarwo W, Keim AP. Chapter 27: Spondiaspinnata (L. f.) Kurz. (Anacardiaceae): profiles and applications to diabetes. In: Watson RR, Preedy VR, editors. Bioactive food as dietary interventions for diabetes (second edition) London: Academic Press; 2019. pp. 395–405. [Google Scholar]
- Todorov SD, Perin LM, Carneiro BM, Rahal P, Holzapfel W, Nero LA. Safety of Lactobacillus plantarum ST8Sh and its bacteriocin. Robiot Antimicrob Prot. 2017;9(3):334–344. doi: 10.1007/s12602-017-9260-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Shao C, Liu L, Guo X, Xu Y, Lü X. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int J Biol Macromol. 2017;103:1173–1184. doi: 10.1016/j.ijbiomac.2017.05.118. [DOI] [PubMed] [Google Scholar]
- Wang T, Liu L, Rakhmanova A, Wang X, Shan Y, Yi Y, Lü X. Stability of bioactive compounds and in vitro gastrointestinal digestion of red beetroot jam: effect of processing and storage. Food Biosci. 2020;38:100788. doi: 10.1016/j.fbio.2020.100788. [DOI] [Google Scholar]
- WHO . Global health estimates 2016: deaths by cause, age, sex, by country and by region, 2000–2016. Geneva: World Health Organization; 2018. [Google Scholar]
- Yusuf D, Nuraida L, Dewanti-Hariyadi R, Hanafi D. In vitro characterization of lactic acid bacteria from indonesian kefir grains as probiotics with cholesterol-lowering effect. J Microbiol Biotechnol. 2020;30(5):726–732. doi: 10.4014/jmb.1910.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Fan L, Zhao H. Rapid detection of short-chain fatty acids in biological samples. Chromatographia. 2020;83(2):305–310. doi: 10.1007/s10337-019-03824-8. [DOI] [Google Scholar]
- Zhao X, Lv Y, Adam FEA, Xie Q, Wang B, Bai X, Yang Z. Comparison of antimicrobial resistance, virulence genes, phylogroups, and biofilm formation of Escherichia coli isolated from intensive farming and free-range sheep. Front Microbiol. 2021;12:699927–699927. doi: 10.3389/fmicb.2021.699927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZY, Cao FW, Wang WJ, Yu J, Chen C, Chen B, Ren DX. Probiotic characteristics of Lactobacillus plantarum E680 and its effect on hypercholesterolemic mice. BMC Microbiol. 2020;20(1):239. doi: 10.1186/s12866-020-01922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.