Abstract
Background
Pulmonary vein isolation (PVI) ablation is a standard therapy for paroxysmal atrial fibrillation (PAF). Lesion Index (LSI) is a metric to guide radiofrequency (RF) ablation using the TactiCath Ablation Catheter, Sensor Enabled with the EnSite Cardiac Mapping System (Abbott).
Objective
This study (NCT-03906461) was designed to capture best practices using LSI-guided catheter ablation to treat PAF subjects in a real-world setting.
Methods
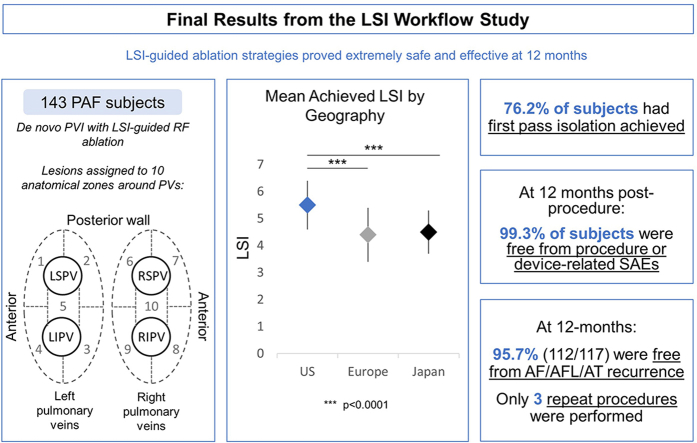
This prospective single-arm observational study enrolled 143 PAF subjects in the United States, Europe, and Japan undergoing de novo PVI with RF ablation. PVI lesions were assigned to 10 anatomically defined segments. Mean LSIs achieved for all lesions were analyzed. Follow-up was conducted between 3–6 months and 12 months after the procedure.
Results
Pulmonary veins were isolated in all subjects. The mean achieved LSI was 4.9, with lower values in Europe (4.4) and Japan (4.5) than the United States (5.5). First-pass success, defined as no gaps requiring touch-up ablation after 20 minutes post isolation, was achieved in 76.2% of subjects. Use of high LSI (≥5) resulted in shorter procedure, RF, and fluoroscopy times and fewer touch-up ablations compared to low LSI (<5). At 12 months, 99.3% of subjects were free from procedure- or device-related serious adverse events and 95.7% (112/117) (35.0% on antiarrhythmic drugs) were free from recurrence and/or a repeat ablation procedure for atrial fibrillation / atrial flutter / atrial tachycardia.
Conclusion
LSI-guided ablation strategies proved safe and effective despite differences in LSI workflows. Use of high LSI values resulted in shorter procedure, RF, and fluoroscopy times and fewer touch-up ablations compared to low LSI.
Keywords: Lesion Index, Atrial fibrillation, Radiofrequency catheter ablation, Pulmonary vein isolation, Contact force
Graphical abstract
Key Findings.
-
▪
Use of higher Lesion Index (LSI) values (≥5) resulted in shorter procedure and fluoroscopy time, fewer radiofrequency pulses required for pulmonary vein isolation, and fewer touch-up ablations (greater first-pass success).
-
▪
LSI-guided pulmonary vein isolation is safe.
-
▪
In this study, LSI-guided pulmonary vein isolation was reasonably effective through 12 months in treating paroxysmal atrial fibrillation despite differences in workflows.
Introduction
Measurement of contact force (CF) between the catheter tip and the target tissue can help guide mapping and ablation procedures. Data from several studies have shown that CF sensing is not only safe for use in pulmonary vein isolation (PVI) but is also associated with lower rates of gap and atrial fibrillation (AF) recurrence.1, 2, 3, 4, 5 The Force Time Integral (FTI™) is a linear calculation that combines CF and radiofrequency (RF) ablation time. The EFFICAS I and EFFICAS II studies established a minimum FTI threshold of 400 gram-seconds that was associated with significantly higher PVI success rates at 3 months.1,3
Key to a successful and durable ablation procedure is achieving well-formed, transmural lesions. Lesion size during cardiac ablation is impacted by multiple factors, including electrode tissue interface, power and current delivered, and ablation time. The relationship between power (P, watts), voltage (V, volts), and current (I, amps) is expressed as P = I × V. Building on the concept of CF and FTI, the Lesion Index (LSI™) is a proprietary index that combines CF, RF duration (S), and RF current into a single value: LSI = CF × S (RF Time) × I (Current). The LSI value expresses the gradual growth of lesion formation. All 3 subcomponents are proportional to ∼(1 − e−t/τ). Factors under an operator’s control during an ablation procedure include CF, duration of ablation, and the power (watts) setting of the RF generator. LSI combines these variables into a single value that is displayed in the EnSite™ CF module on the EnSite Velocity™, EnSite Precision™, or EnSite X Cardiac Mapping Systems (Abbott, Abbott Park, IL) and can be used (outside of the United States), along with FTI, as the AutoMark lesion color or size metric. The TactiCath™ Contact Force Ablation Catheter, Sensor Enabled™ (TactiCath SE; Abbott) is a CF-sensing catheter, which incorporates a magnetic sensor for tracking with the EnSite Precision Mapping System.
Use of an LSI >5.0 for ablation points has been associated with higher PV isolation success rates6 and freedom from recurrence at 1 year.7 Additional studies suggest that use of LSI provides a more uniform lesion delivery and that the addition of LSI to the CF recommendations may lead to a higher rate of durable PVI1,8 and long-term clinical success.9
The LSI Workflow Observational Study was intended to characterize the usage of LSI with the TactiCath SE catheter and the achieved LSI values for durable lesion formation in different anatomical regions around the pulmonary veins during RF ablation in subjects with paroxysmal atrial fibrillation in a real-world environment.
Methods
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committees at all participating sites. Every participating subject provided written informed consent.
Study design
This was a prospective, multicenter, single-arm postmarket observational study designed to understand the best practices using LSI from experienced operators.
Study population
Eligible patients were aged 18 years or older with plans to undergo a PVI procedure owing to symptomatic paroxysmal atrial fibrillation using RF ablation and were refractory or intolerant to at least 1 class I or class III antiarrhythmic drug (AAD). For the purposes of this study, the protocol defined “intolerant” to include subjects that were offered an AAD and refused to take it for any reason. Key exclusion criteria included a previous ablation or surgery in the left atria or an implantable cardiac defibrillator. Assessments at baseline included history of cardiovascular disease, arrhythmia history including documentation of paroxysmal atrial fibrillation diagnosis, complete physical examination within 30 days prior to enrollment, and AAD usage.
Ablation procedure
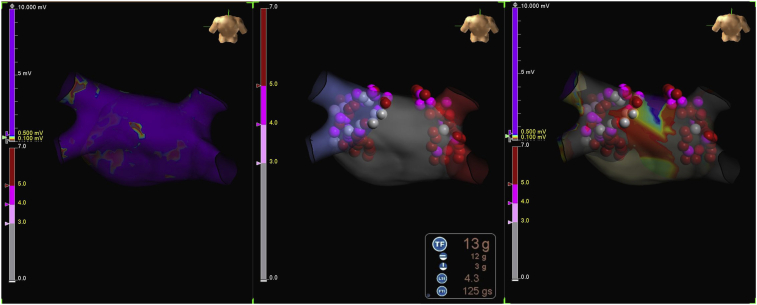
All subjects were prepped for an electrophysiology study per the site’s standard of care. If a thrombus was noted, the procedure was delayed until the thrombus was resolved or withdrawn within 30 days of the procedure. A standard mapping procedure was performed using a mapping catheter compatible with the EnSite system. Ablation procedures were performed with the TactiCath SE catheter and the EnSite AutoMark module. In the United States, operators chose their standard metrics for use with AutoMark. In Europe and Japan, LSI was used as the color metric for AutoMark (Figure 1). PVI was to be completed first. Then, subjects with documented atrial flutter (AFL), atrial tachycardia (AT), or other supraventricular tachycardia could undergo additional targeted ablation. Additional ablation of triggers or substrates was discouraged unless targeted for inducible and sustainable AT or flutters. PVI was verified with entrance block using a multipolar catheter at or beyond 20 minutes from the last ablation lesion for each vein. Use of adenosine and/or isoproterenol or cardioversion was acceptable.
Figure 1.
Lesion Index (LSI)–guided ablation using the EnSite System (Abbott). (Left) Voltage map created pre-ablation. (Middle) AutoMark lesions displayed using color settings according to achieved LSI (light pink ≥3.0, magenta ≥4.0, red ≥5.0). Real-time LSI is displayed on the EnSite system during ablation. (Right) Voltage map created post-ablation.
If acute success of PVI was not achieved, a new map of the pulmonary vein (PV) region was captured using the AutoMap module and the number and location of reconnections or gaps was noted. Touch-up ablations to close identified gaps were performed, if possible, to achieve isolation. AutoMark was utilized using the same guidelines as the initial lesion set.
Postablation monitoring and follow-up
Subjects were seen for clinic visits between 3 and 6 months post ablation procedure and at 12 months. After ablation there was a 90-day “blanking” period. Repeat ablations that occurred during this blanking period were not considered treatment failures. Repeat ablation procedures were required to follow the same device guidelines as the index procedure and otherwise standard practice of the site. At both visits, subjects were evaluated for arrhythmia recurrence using standard-of-care rhythm monitoring and procedure- or device-related serious adverse events (SAE). At the 12-month visit, subjects underwent 24-hour Holter monitoring.
LSI lesion analysis
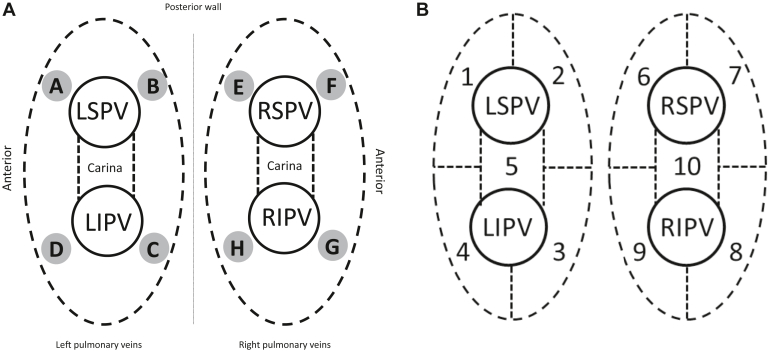
The PV antrum was divided into 10 regions for this study: anterior and posterior segments as well as superior and inferior segments and the carina areas. Reference points were used to define the location of the zone borders around the left and right pulmonary veins (Figure 2A). The lesion label numbers from the AutoMark system were recorded for each of the zone border locations if ablations were performed in the border areas. A custom algorithm leveraging fiducial points assigned to boundaries of anatomical regions of the left atria (eg, left-superior-anterior intersection) from AutoMark coordinate data was used to classify all other lesions into 1 of 10 zones around and between the left and right pulmonary veins (Figure 2B). Investigators indicated their minimum target LSI for each of the 10 regions based on their standard workflow. EnSite AutoMark exported an achieved LSI value for each lesion with a duration of at least 6 seconds.
Figure 2.
A: Reference points for the location of zone borders around the left (shaded circles A–D) and right (shaded circles E–H) pulmonary veins (PVs). Circle A: LSPV roof and anterior border; circle B: LSPV roof and posterior border; circle C: LIPV floor and posterior border; circle D: LIPV floor and anterior border; circle E: RSPV roof and posterior border; circle F: RSPV roof and anterior border; circle G: RIPV floor and anterior border; circle H: RIPV floor and posterior border. B: Numbered anatomical zones around the PVs. LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
AutoMark normalization
The placement of AutoMark lesions is based on 3 user-defined settings: away time, minimum AutoMark time, and AutoMark spacing. Away time refers to the maximum amount of time the catheter can remain away from the AutoMark region before a new AutoMark will be placed. The minimum AutoMark time refers to the minimum amount of time the catheter tip must remain within the AutoMark region before an AutoMark lesion is placed. The AutoMark spacing parameter refers to the spatial catheter stability requirement for each AutoMark, which defines the AutoMark region. When the catheter moves farther than the AutoMark spacing for longer than the Away Time parameter, a new AutoMark is created. If the catheter returns to the same location, a new AutoMark is created and the data are not cumulative. Since different AutoMark parameters can influence the number of lesions, AutoMark parameters were normalized for certain analyses. To normalize, AutoMark parameters were set to default placement settings of Away time = 8.0 seconds, Min AutoMark Time = 3 seconds, AutoMark Spacing = 3.0 mm. EnSite AutoMark files were exported after parameter normalization and used for analyses.
Study outcomes
The primary endpoint was a summary of LSI values achieved for RF lesion formation in different anatomical regions of the left atrium around the pulmonary veins. Additional descriptive endpoints collected during this study included procedural data, ablation data, device- or procedure-related SAEs within 7 days and 12 months of index procedure, repeat ablation procedures, and 12-month freedom from recurrence. Ablation and mapping data were collected with the EnSite AutoMark and AutoMap features. Freedom from recurrence was defined as freedom from AF/AFL/AT with no documented episodes greater than 30 seconds with a 24-hour Holter.
Statistical methods
The primary endpoint and additional evaluation analyses were summarized descriptively. No formal hypothesis testing was performed.
Continuous variables were summarized as mean, standard deviation, and number of observations. Categorical variables were summarized as the proportion of observations, along with the corresponding numerator and denominator.
All P values were reported descriptively. P values for comparisons of continuous variables across 3 subgroups were generated via 1-way analysis of variance. P values for comparisons of categorical variables across 3 subgroups were generated via χ2 test. P values for comparisons of continuous variables across 2 subgroups were generated via t test. P values for comparisons of categorical variables across 2 subgroups were generated via Fisher exact test.
Results
A total of 143 subjects were enrolled at 9 investigational sites with 25 operators in the United States, Europe, and Japan between May 20, 2019, and March 10, 2020. A total of 56 subjects were enrolled in the United States, 57 in Europe, and 30 in Japan. Of the 143 subjects, 66.4% were male and the mean age was 62.2 ± 10.9 years old. Body mass index was significantly different between each geography, being highest in the United States and lowest in Japan (P < .0001). Forty subjects (28.0%) had a history of other arrhythmia, the most common being atrial flutter (20.3%). A small set of subjects also had other arrhythmias such as ventricular tachycardia and heart block, notably highest in the US cohort. There were 83 (58.0%) subjects with a history of hypertension and 120 (83.9%) had been on class I/III antiarrhythmic medications. Baseline characteristics and medical history are summarized in Table 1.
Table 1.
Subject characteristics and medical history
| US subjects (n = 56) | European subjects (n = 57) | Japanese subjects (n = 30) | All subjects (n = 143) | P value† | |
|---|---|---|---|---|---|
| Age, y, mean ± SD (n) | 63.4 ± 9.8 (56) | 60.7 ± 10.9 (57) | 62.6 ± 13.0 (30) | 62.2 ± 10.9 (143) | .4245 |
| Sex, % (n/N) | |||||
| Female | 48.2% (27/56) | 28.1% (16/57) | 16.7% (5/30) | 33.6% (48/143) | .0067 |
| Male | 51.8% (29/56) | 71.9% (41/57) | 83.3% (25/30) | 66.4% (95/143) | .0067 |
| BMI, mean ± SD (n) | 32.3 ± 6.7 (56) | 27.0 ± 3.6 (57) | 23.9 ± 3.1 (30) | 28.4 ± 6.0 (143) | <.0001 |
| History of other arrhythmia, % (n/N) | 42.9% (24/56) | 19.3% (11/57) | 16.7% (5/30) | 28.0% (40/143) | .0061 |
| Atrial flutter | 28.6% (16/56) | 15.8% (9/57) | 13.3% (4/30) | 20.3% (29/143) | .1362 |
| Atrial tachycardia | 5.4% (3/56) | 0.0% (0/57) | 0.0% (0/30) | 2.1% (3/143) | .0925 |
| Ventricular tachycardia | 10.7% (6/56) | 1.8% (1/57) | 0.0% (0/30) | 4.9% (7/143) | .0330 |
| Heart block or atrioventricular dysfunction | 12.5% (7/56) | 3.5% (2/57) | 3.3% (1/30) | 7.0% (10/143) | .1169 |
| History of hypertension, % (n/N) | 75.0% (42/56) | 43.9% (25/57) | 53.3% (16/30) | 58.0% (83/143) | .0030 |
| History of any procedure to occlude or close the left atrial appendage, % (n/N) | 0.0% (0/56) | 0.0% (0/57) | 0.0% (0/30) | 0.0% (0/143) | n/a |
| History of class I/III AAD, % (n/N) | 89.3% (50/56) | 84.2% (48/57) | 73.3% (22/30) | 83.9% (120/143) | .1581 |
| Pacemaker or implantable cardiac monitor, % (n/N) | 1.8% (1/56) | 1.8% (1/57) | 0.0% (0/30) | 1.4% (2/143) | .7639 |
| Left atrial diameter (cm) | 4.0 ± 0.6 (54) | 3.9 ± 0.8 (36) | 3.6 ± 0.6 (24) | 3.9 ± 0.7 (114) | .0317 |
AAD = antiarrhythmic drugs; BMI = body mass index; n/a = not applicable.
P-value from 1-way analysis of variance for continuous variables and χ2 test for categorical variables.
All 143 enrolled subjects completed the procedure. Of these 143 subjects, 141 completed the 6-month follow-up and 133 completed the 12-month follow-up. Four subjects were withdrawn from the study. One subject was withdrawn owing to a death that was not related to the study procedure or device, 2 subjects were lost to follow-up (2 consecutive missed visits), and 1 subject withdrew consent. The remaining subjects missed the 12-month follow-up visit.
Average initial PVI time was 55.6 ± 31.2 minutes with 19.3 ± 9.0 minutes of RF time. After PVI, 37.1% (53/143) of subjects had additional non-PVI ablations performed. Total procedure time was 142.5 ± 53.1 minutes with a total RF time of 22.1 ± 10.3 minutes. Shortest procedure and RF times were in the United States and longest procedure and RF times in Europe. Fluoroscopy was used in 127 of 143 subjects with an average of 23.7 ± 20.0 minutes, ranging from 12.0 ± 9.6 minutes in the United Stated to 44.1 ± 26.8 minutes in Japan.
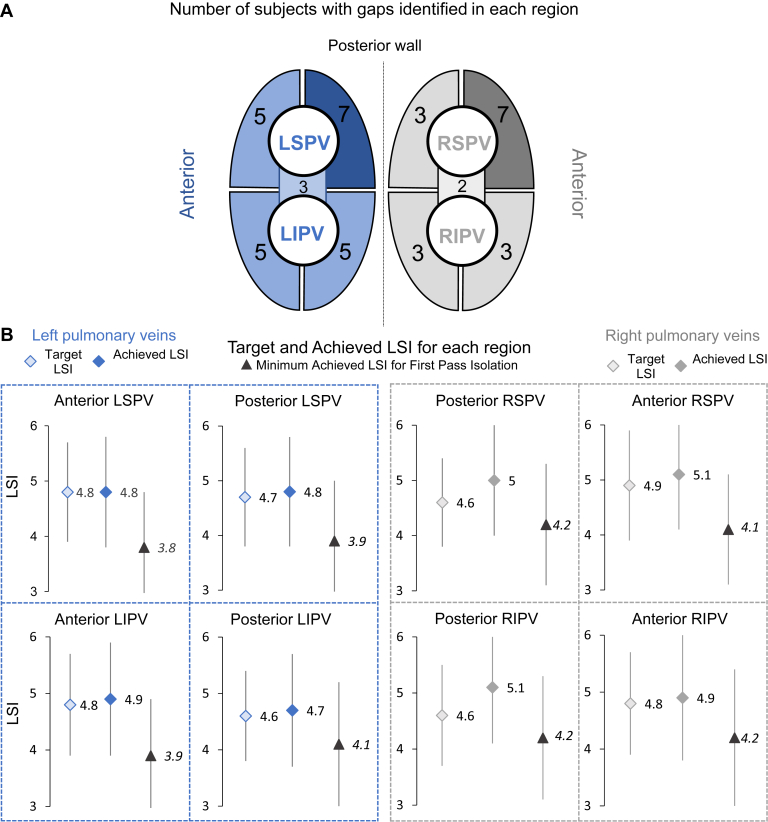
The mean minimum targeted LSI value across all PV regions was 4.7 ± 0.8. Target LSI values differed by operator and their standard of care, ranging from a minimum of 3.0 to 6.0 for each individual PV region. There was little variation in target LSI between any of the designated regions, but average target LSI was lower for posterior regions compared to anterior regions (Figure 3B).
Figure 3.
Identified gaps, target Lesion Index (LSI), and achieved LSI by anatomical region. A: The number of subjects with gaps identified in each defined pulmonary vein region is noted. Note that in 3 subjects gaps were identified but unable to be isolated. Subjects may have experienced a reconnection in more than 1 zone. B: For each defined pulmonary vein region displayed in panel A, the mean ± standard deviation target LSI, the mean ± standard deviation achieved LSI, and the mean ± standard deviation minimum achieved LSI per subject for first-pass isolation (no gaps identified in the zone) are displayed. Target LSI, the desired LSI value for each lesion, was defined by the operator for each region in each subject. Achieved LSI for each lesion was included in the AutoMark data. Postprocedure, each lesion was categorized into a defined region. Abbreviations as in Figure 2.
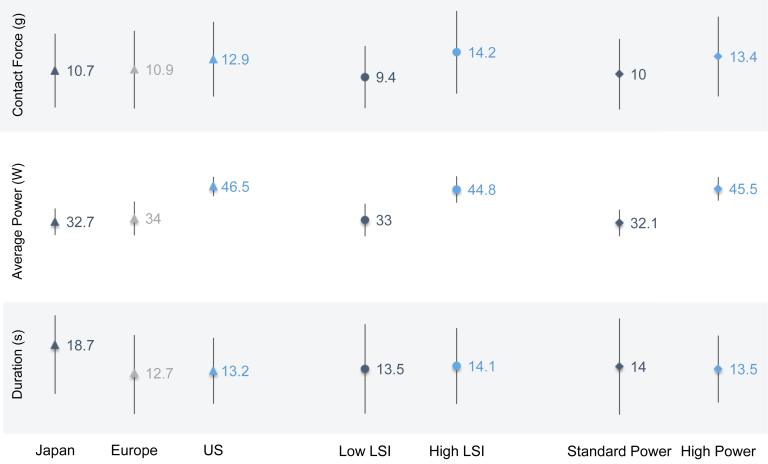
Achieved LSI across all PV regions ranged from 1.2 to 8.9, with an average value of 4.9 ± 1.0. Looking at the achieved LSI for each PV region, the highest mean achieved LSI was 5.1 ± 1.0 in both the posterior region of the right inferior PV (RIPV) and the anterior region of the right superior PV (RSPV). The lowest mean achieved LSI of 4.7 ± 1.0 was in the posterior region of the left inferior PV (LIPV) (Figure 3B). Achieved LSI was not consistently lower for posterior regions compared to anterior regions. Significantly higher achieved LSI values were seen in US subjects (5.5 ± 0.9) compared to both Japanese (4.5 ± 0.8) and European subjects (4.4 ± 1.0) (P < .0001). A breakdown of lesion parameters by CF, average RF power delivered, and duration found significant differences (P < .0001) in workflow by geography (Figure 4). Average CF was highest in the United States (12.9 g; median [interquartile range; IQR]: 11.0 [8.0,16.0] g) and lowest in Japan (10.7 g; median [IQR]: 9.0 [6.0,14.0] g). This corresponded to the average RF power delivered, which was highest in the United States (46.5 W; median [IQR]: 47.0 [46.0,49.0] W) and lowest in Japan (32.7 W; median [IQR]: 33.0 [29.0,38.0] W). Average CF in Europe was 10.9 g (median [IQR]: 9.0 [6.0, 14.0] g) with 34.0 W (median [IQR]: 34.0 [30.0,35.0] W) average RF power delivered. Likewise, average duration was 13.2 seconds (median [IQR]: 12.0 [9.0,15.0] seconds) in the United States, 12.7 seconds (median [IQR]: 10.0 [7.0,16.0] seconds) in Europe, and 18.7 seconds (median [IQR]: 17.0 [11.0,24.0] seconds) in Japan.
Figure 4.
Lesion-level characteristics. The mean and standard deviation for contact force, average power, and duration are included for each subgroup analyzed. LSI = Lesion Index.
AutoMark parameters differed widely by geography, with the AutoMark lesion spacing parameter set to 8.9 ± 1.0 mm in Japan, 5.6 ± 1.1 mm in the United States, and 4.7 ± 1.0 mm in Europe. Differences were also seen in the AutoMark away time parameter, which was set to 8.1 ± 0.4 seconds in Japan, 6.5 ± 1.4 seconds in the United States, and 7.2 ± 2.8 seconds in Europe. Finally, the AutoMark minimum lesion time parameter was 2.6 ± 0.6 seconds in Japan, 2.7 ± 0.5 seconds in the United States, and 3.9 ± 0.8 seconds in Europe. With these AutoMark parameters, there were significantly more PVI lesions per subject in Europe (103.1 ± 51.8 lesions) compared to the United States (88.6 ± 30.2 lesions) and Japan (64.0 ± 25.7 lesions) (P = .0002). However, when AutoMark parameters were normalized and only lesions with LSI were considered, the United States had the highest number of PVI lesions (86.4 ± 29.9 lesions), followed by Europe (78.6 ± 27.5 lesions) and Japan (65.5 ± 28.7 lesions). With AutoMark parameters normalized, there was an average of 78.8 ± 29.5 PVI lesions per subject.
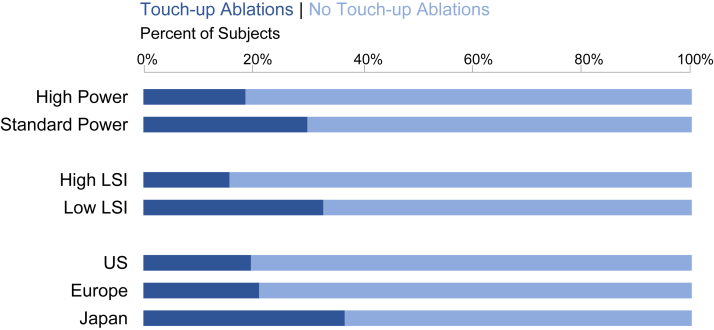
Isolation of the PVs was achieved in all subjects and verified at least 20 minutes after the completion of the initial isolation lesion set in 99.3% (142/143) of subjects. Following this waiting period, 76.2% (109/143) of subjects had no gaps identified and achieved first-pass isolation. Touch-up ablations were performed in 19.6% (11/56) of US subjects, 21.1% (12/57) of European subjects, and 36.7% (11/30) of Japanese subjects (Figure 5).
Figure 5.
Touch-up ablations. The number of subjects with pulmonary vein touch-up ablations performed during the index procedure, evaluated for each subgroup: by geography (bottom), by mean achieved Lesion Index (LSI) (middle), and by high or standard average power (top).
The mean minimum LSI for each region in subjects that achieved first-pass isolation are shown in Figure 3B. In subjects without gaps, the mean minimum LSI ranged from 3.8 in the anterior region of the left superior pulmonary vein (LSPV) to 4.2 in the posterior region of the RSPV, the anterior region of the RIPV, and the posterior region of the RIPV. For comparison, in subjects with gaps, the mean minimum LSI was 4.5 in the anterior region of the LSPV (P = .130), 3.9 in the posterior region of the RSPV (P = .622), and 4.0 in the posterior region of the RIPV (P = .682). In subjects with gaps, the mean minimum LSI ranged from 3.3 in the posterior LIPV to 4.5 in the anterior LSPV and anterior RIPV. There were no significant differences in the mean minimum LSI for any of the reconnection zones in subjects with or without gaps.
Overall, there were a low number of subjects with gaps in all anatomical regions. The highest number of gaps (7) was identified in both the posterior region of the LSPV and the anterior region of the RSPV (Figure 3A). The lowest number of gaps was identified in the right-side carina. There were 3 gaps in the left carina and 2 gaps in the right carina. There were 3 subjects in whom the operator was unable to isolate the gap. There were no statistically significant differences in location of gaps. At 12 months post procedure, excluding the 90-day blanking period, 95.7% (112/117) (35.0% on AADs) of all subjects were free from recurrence of AF/AFL/AT. Additionally, only 3 subjects (1 each from United States, Europe, and Japan) had a repeat ablation up to 12 months post index ablation procedure (excluding the 90-day blanking period). All repeat procedures occurred after the blanking period; no subjects had repeat procedures during the blanking period.
There was no protocol requirement to discontinue AADs after the blanking period, but AAD use was collected at the follow-up visits. AAD usage at 12 months was 39.1% (52/133) on average. This varied across geographies, with 53.2% of US subjects, 33.3% of European subjects, and 27.6% of Japanese subjects taking AADs at 12 months. At 12 months post procedure, excluding the 90-day blanking period, 60.7% (71/117) of subjects were free from both AADs and documented AF/AFL/AT recurrence.
Only 1 procedure- and device-related SAE occurred, a cardiac tamponade with onset during the procedure that resolved without sequelae. This equates to a 0.7% (1/143) SAE rate at 7 days. No further procedure- or device-related SAE occurred through 12 months post index procedure.
Subgroup analysis: Achieved LSI
Subjects were divided into 2 subgroups based on their average achieved LSI values. Subgroups were determined based on the distribution of achieved LSI values (Supplemental Figure S1), ensuring an even distribution of subjects and significantly different mean achieved LSI of all lesions (P < .0001). A total of 137 of 143 subjects were included in this subgroup analysis. The low-LSI subgroup included 67 subjects with an average LSI less than 5. The high-LSI subgroup included 70 subjects with an average LSI greater than or equal to 5.
Corresponding to average LSI, average CF was significantly higher in the high-LSI subgroup (14.2 ± 8.0 g; median [IQR]: 12.0 [9.0,18.0] g) than the low-LSI subgroup (9.4 ± 6.0 g; median [IQR]: 8.0 [5.0,12.0] g) (P < .0001) (Figure 4). Similarly, average RF power delivered in the high-LSI subgroup (44.8 ± 5.1 W; median [IQR]: 46.0 [43.0,48.0] W) was significantly higher than the low-LSI subgroup (33.0 ± 6.3 W; median [IQR]: 33.0 [29.0,35.0] W) (P < .0001). Average duration for each RF application was significantly longer in the high-LSI subgroup (14.1 ± 7.9 seconds; median [IQR]: 12.0 [9.0,18.0] seconds) compared to the low-LSI subgroup (13.5 ± 9.3 seconds; median [IQR]: 11.0 [7.0,17.0] seconds) (P = .0001). Although the low-LSI subgroup had a greater number of PVI lesions per subject than the high-LSI subgroup, there was no significant difference (P = .071). When AutoMark parameters were normalized and only lesions with LSI were considered, there was still no significant difference in the total number of PVI lesions per subject between subgroups (P = .506).
Procedure times varied between the achieved LSI groups as well (Table 2). Significant differences between the groups were seen for total RF time for the initial PVI (P < .0001) as well as the RF time for the entire procedure (P < .0001), with highest times in the low-LSI subgroup. RF time for the initial PVI in the low-LSI subgroup was 23.1 ± 10.4 minutes, compared to 15.9 ± 5.5 minutes for the high-LSI subgroup. Similarly, significant differences between the subgroups were seen for total fluoroscopy time used up to the end of the first-pass PVI (P < .0001) and total fluoroscopy time (P < .0001). The fluoroscopy used for the first-pass PVI was 22.9 ± 16.5 minutes for the low-LSI subgroup and 10.8 ± 9.6 minutes for the high-LSI subgroup (P < .0001).
Table 2.
Procedure times evaluated for the mean achieved Lesion Index and average power subgroups
| Procedure detail | Low-LSI subjects (n = 67) | High-LSI subjects (n = 70) | P value | Standard-power subjects (n = 67) | High-power subjects (n = 70) | P value | All subjects (n = 137) |
|---|---|---|---|---|---|---|---|
| Total RF time for initial PV isolation in minutes | 23.1 ± 10.4 (67) | 15.9 ± 5.5 (70) | <.0001 | 22.7 ± 10.5 (67) | 16.2 ± 5.9 (70) | <.0001 | 19.4 ± 9.0 (137) |
| Total RF time for entire procedure in minutes | 26.2 ± 11.6 (67) | 18.3 ± 7.2 (70) | <.0001 | 25.5 ± 11.3 (67) | 18.9 ± 8.2 (70) | .0002 | 22.1 ± 10.3 (137) |
| Total procedure time in minutes | 153.1 ± 55.8 (67) | 134.6 ± 50.0 (70) | .0432 | 153.2 ± 52.8 (67) | 134.5 ± 53.0 (70) | .0407 | 143.7 ± 53.5 (137) |
| Total fluoroscopy time in minutes | 30.5 ± 23.0 (65) | 16.1 ± 13.1 (56) | <.0001 | 32.2 ± 22.9 (65) | 14.1 ± 10.6 (56) | <.0001 | 23.8 ± 20.3 (121) |
Data are presented as mean ± SD (n).
LSI = Lesion Index; PV = pulmonary vein; RF = radiofrequency.
After a 20-minute waiting period, PV reconnection and gap identification was significantly greater in the low-LSI subgroup (32.8%) than the high-LSI subgroup (15.7%) (P = .019).
Subgroup analysis: Average power
Subjects were divided into standard average power (<40 W; n = 67) and high average power (≥40 W; n = 70) subgroups (Supplemental Figure S2 contains histograms of achieved power values and average power per subject). Average RF power delivered for the standard-power group was 32.1± 5.2 W (median [IQR]: 33.0 [29.0,35.0] W), with an average RF power delivered of 45.5 ± 4.6 W (median [IQR]: 47.0 [44.0,48.0] W) for the high-power group (Figure 4). There were 11 operators (3 in Europe, 8 in the United States) in the high-power group and average power delivered across all PVI lesions was calculated per high-power operator. Average power for PVI lesions was <42 W for 3 operators, average power was between 45 W and 47 W for 6 operators, and average power was >47 W for 2 operators. Average CF was higher in the high-power group (13.4 ± 7.7; median [IQR]: 12.0 [8.0,17.0]) compared to the standard-power group (10.0 ± 6.8; median [IQR]: 9.0 [5.0,13.0]) (P < .0001). Average duration for each RF application was slightly longer with standard power (14.0 ± 10.0 seconds; median [IQR]: 11.0 [7.0,18.0] seconds) compared to 13.5 ± 7.0 seconds (median [IQR]: 12.0 [9.0,16.0] seconds) in the high-power group (P = .002). However, both groups used relatively short RF pulse durations. There were a greater number of PVI lesions in subjects with standard power (93.7 ± 52.9) compared to the high-power group (84.9 ± 27.7), but this difference was not statistically significant (P = .232). Further, when AutoMark parameters were normalized and only lesions with LSI were considered, there was still no significant difference in the number of PVI lesions per subject between groups (P = .321).
Procedural times were longer with lower power, as expected (Table 2). Total RF time for initial PVI was significantly higher in the standard-power group (22.7 ± 10.5 vs 16.2 ± 5.9; P < .0001). Similarly, total RF time for the entire procedure was 25.5 ± 11.3 minutes for the Standard-power group and 18.9 ± 8.2 minutes for the high-power group (P = .0002). Total procedure time was also significantly longer in the standard-power group (153.2 ± 52.8 minutes vs 134.5 ± 53.0 minutes; P = .041). Finally, total fluoroscopy time was significantly higher in the standard-power group as well (32.2 ± 22.9 minutes compared to 14.1 ± 10.6 minutes) (P < .0001). After a 20-minute waiting period, PV touch-up ablations were performed in 29.9% (20/67) of subjects in the standard-power group, compared to 18.6% (13/70) of the high-power group, which was not significantly different (P = .123).
Discussion
The LSI workflow study found LSI-guided PVI to be safe and effective, with 95.7% (35.0% on AADs) of subjects free from recurrence at 12 months and only a 2% (3/143) rate of repeat procedures. Compared to the 2017 consensus document, which reports overall 12-month success rates from 59% to 89% and repeat procedure rates between 15% and 50%, the success of LSI-guided PVI is evident.10 Higher LSI values (>5) have previously been associated with a lack of AF recurrence.7,9 However, we found no significant differences in acute or 12-month outcomes based on LSI values, suggesting the use of a workflow that incorporates LSI may allow for more consistent lesion formation and lesion quality.
Previous studies have also demonstrated an association between low LSI values and gaps or acute reconnections after PVI.1,11 Overall, there were very low numbers of gaps identified, and thus a high rate of first-pass isolation, since no more than 5% of subjects had a gap in each of the 10 anatomical regions. In this study, the highest number of gaps or acute reconnections were in subjects with lower mean LSI values, significantly more than in subjects with higher mean LSI values. Consequently, there were significantly greater number of subjects that needed touch-up ablations performed in the low LSI group. This indicated a significant difference in first-pass success between those workflows that incorporated high LSI vs low LSI. To understand the minimum LSI required to achieve first-pass isolation, the mean minimum achieved LSI was reported for each region in subjects without gaps. In general, these values hovered around an LSI of 4 with a mean minimum achieved LSI of 4.1–4.2 in the right pulmonary veins and a mean minimum achieved LSI of 3.8–4.1 in the left pulmonary veins. In subjects with gaps, there was a wider spread of mean minimum achieved LSI, with 3.5–4.5 in the right pulmonary veins and a mean minimum achieved LSI of 3.3–4.5 in the left pulmonary veins. Unfortunately, statistical analysis did not reveal a significant difference in the mean minimum achieved LSI for those subjects with and without gaps in any of the regions owing to the low number of subjects with gaps in each region. For each region, there were only between 3 and 7 subjects with a gap. However, this study reveals that achieving, on average, a high LSI ≥5 is significantly associated with higher acute durability. Interestingly, while there were more gaps in subjects with lower power compared to higher power, the difference was not significant (P = .094). As expected, there was overlap between the low-LSI group and the standard-power group, but there was also some crossover from the low-LSI group into the high-power group and vice versa. This crossover and the low overall number of gaps may have contributed to the lack of significance.
The rate of recurrence and repeat procedures was also extremely low in this study, so it was not possible to evaluate the long-term durability of lesions related to specific achieved LSI values. Although the average LSI of all lesions was 5.5 in the United States and significantly lower in Europe and Japan, there were no statistically significant differences seen between higher and lower average LSI values for either acute procedure success or 12-month effectiveness.
Low achieved LSI values (defined as <5) were associated with a difference in certain lesion-level characteristics and procedural details. While lesions with a low LSI had a significantly lower CF, duration, and average power delivered, subjects with low achieved LSI had significantly longer RF time for the initial PVI and the entire procedure, more fluoroscopy used during the first-pass PVI, and longer total fluoroscopy time. This was despite a larger (but not significantly) number of PVI lesions and total number of lesions per subject.
Similarly, subjects with standard average power (defined as <40 W) had significantly longer RF time for the initial PVI and in the entire procedure and more fluoroscopy used. There was also no significant difference in the number of PVI lesions or total number of lesions per subject between the standard- and high-power groups. Since power is a component of LSI, it was expected that low LSI and standard power would have the same trend. As expected, there was also overlap between the 2 groups, with only 7 subjects in the low-LSI group that were in the high-power group and 7 subjects in the high-LSI group in the standard-power group.
Lesion-level characteristics such as CF, RF power delivered, and duration also varied widely by geography. The United States favored a higher power, whereas Japan used a lower power and corresponding longer durations. While CF was also higher in the US subjects, average CF was low across the board. The majority of lesions were well below the previous target of 20 g, with averages between 10 and 13 g. These lower contact forces led to durable, safe lesion formation.
While target LSI values varied widely by operator, ranging between 3.0 and 6.0, and posterior regions tended to have a slightly lower target LSI, this did not translate to achieved LSI. Thus, achieved LSI was not consistently lower in the posterior regions and in some cases was higher on average. However, in all regions, mean achieved LSI was equal to or higher than mean target LSI. This is contrary to a recent multicenter prospective registry that concluded that target Ablation Index was not achieved in a significant proportion of subjects during PVI and subsequently associated with acute reconnections.12, 13 Comparatively, first-pass isolation was also much lower at 59.5%, compared to 76.2% in this study.
This study is the first multicenter, multinational, prospective study on the use of the Lesion Index to guide RF ablation in the treatment of paroxysmal atrial fibrillation. This study evaluated the best practices and outcomes related to the use of LSI by experienced operators in a real-world setting and demonstrated the excellent safety profile and efficacy when LSI was used to guide ablations. The effectiveness was demonstrated, regardless of the achieved LSI values, by extremely low 12-month recurrence rates and number of repeat ablations. The use of high LSI (≥5) was advantageous in terms of procedural efficiencies and first pass success.
Limitations
The main limitation of this prospective, multicenter study is its observational nature and lack of a control group. All endpoints were descriptive and there was no comparison to a group that did not use LSI. This study was designed to better understand standard-of-care usage and outcomes related to LSI and thus was observational in nature and used a large number of sites and operators to encompass a wide variety of workflows across different geographies. This limited the number of subjects able to be enrolled at each center, which may limit the generalizability of these data. Additionally, episodes of recurrence may have been missed owing to a relatively low intensity, consistent with an observational study, of assessment, with recurrence assessed by a 24-hour Holter at the 12-month visit.
Conclusion
In this study, LSI-guided ablation strategies were reasonably effective through 12 months despite differences in workflows and achieved LSI. However, use of higher LSI values (≥5) resulted in shorter procedure, RF, and fluoroscopy times and fewer touch-up ablations. This study confirms that LSI-guided PVI is safe and effective in treating paroxysmal atrial fibrillation.
Acknowledgments
Funding Sources
This work was supported by Abbott.
Disclosures
C. Woods is a consultant with Abbott. MG has received speaker honorarium from Abbott Medical Japan. JA has received consulting fees from Abbott. IK has received an educational grant from Abbott. C. Williams, JT, LZV, and AS are employees of Abbott. JA has received consulting fees from Abbott. Abbott was the sponsor of this study and was responsible for the data analysis (C. Williams, JT, AS, LZV). KVP and LZV wrote the majority of the manuscript with input, review, and approval from all authors.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients provided written informed consent.
Ethics Statement
The research reported in this study was conducted according to the principles of the Declaration of Helsinki. The study was approved by the ethics committees at all participating sites.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2022.06.004.
Appendix. Supplementary data
References
- 1.Kautzner J., Neuzil P., Lambert H., et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015;17:1229–1235. doi: 10.1093/europace/euv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuck K.H., Reddy V.Y., Schmidt B., et al. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm. 2012;9:18–23. doi: 10.1016/j.hrthm.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil P., Reddy V.Y., Kautzner J., et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013;6:327–333. doi: 10.1161/CIRCEP.113.000374. [DOI] [PubMed] [Google Scholar]
- 4.Reddy V.Y., Shah D., Kautzner J., et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012;9:1789–1795. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Kautzner J., Natale A., Michaud G., et al. Segmental variability in lesion size is controlled using contact force during pulmonary venous isolation. EP Europace. 2013;15:ii56–ii78. [Google Scholar]
- 6.Reddy V.Y., Dukkipati S.R., Neuzil P., et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) study. Circulation. 2015;132:907–915. doi: 10.1161/CIRCULATIONAHA.114.014092. [DOI] [PubMed] [Google Scholar]
- 7.Dello Russo A., Fassini G.M., Casella M., et al. Lesion index: a novel guide in the path of successful pulmonary vein isolation. J Interv Card Electrophysiol. 2019;55:27–34. doi: 10.1007/s10840-018-0487-z. [DOI] [PubMed] [Google Scholar]
- 8.Sundaram S., Choe W., Jordan J.R., et al. Two year, single center clinical outcome after catheter ablation for paroxysmal atrial fibrillation guided by lesion index. J Atr Fibrillation. 2018;11:1760. doi: 10.4022/jafib.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattia L., Crosato M., Indiani S., et al. Prospective evaluation of lesion index-guided pulmonary vein isolation technique in patients with paroxysmal atrial fibrillation: 1-year follow-up. J Atr Fibrillation. 2018;10:1858. doi: 10.4022/jafib.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Interv Card Electrophysiol. 2017;50:1–55. doi: 10.1007/s10840-017-0277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamori N., Kato T., Sakagami S., et al. Optimal lesion size index to prevent conduction gap during pulmonary vein isolation. J Cardiovasc Electrophysiol. 2018;29:1616–1623. doi: 10.1111/jce.13727. [DOI] [PubMed] [Google Scholar]
- 12.Gasimova N., Kropotkin E., Ivanitsky E., et al. The role of a difference between target and actual ablation index values for first-pass point-by-point pulmonary vein isolation: results from a multicenter prospective registry. EP Europace. 2021;23 euab116.255. [Google Scholar]
- 13.Gasimova N.Z., Nechepurenko A.A., Kropotkin E.B., et al. Performance of the ablation index during pulmonary vein isolation: periprocedural data from a multicenter registry. J Interv Card Electrophysiol. 2022 doi: 10.1007/s10840-022-01242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.