Abstract
Uncontrolled bleeding remains as a leading cause of death in surgical, traumatic, and emergency situations. Management of the hemorrhage and development of hemostatic materials are paramount for patient survival. Owing to their inherent biocompatibility, biodegradability and bioactivity, biopolymers such as polysaccharides and polypeptides have been extensively researched and become a focus for the development of next-generation hemostatic materials. The construction of novel hemostatic materials requires in-depth understanding of the physiological hemostatic process, fundamental hemostatic mechanisms, and the effects of material chemistry/physics. Herein, we have recapitulated the common hemostatic strategies and development status of biopolymer-based hemostatic materials. Furthermore, the hemostatic mechanisms of various molecular structures (components and chemical modifications) are summarized from a microscopic perspective, and the design based on them are introduced. From a macroscopic perspective, the design of various forms of hemostatic materials, e.g., powder, sponge, hydrogel and gauze, is summarized and compared, which may provide an enlightenment for the optimization of hemostat design. It has also highlighted current challenges to the development of biopolymer-based hemostatic materials and proposed future directions in chemistry design, advanced form and clinical application.
Keywords: Material design, Biopolymer, Hemostasis, Molecular structure, Form
Graphical abstract
Highlights
-
•
Biopolymers possess sound biocompatibility, biodegradability and bioactivity for the design of hemostatic materials.
-
•
Molecular structure designs including component and chemical modification are summarized from a microscopic perspective.
-
•
Design of various forms of hemostatic materials is discussed and compared synthetically from a macroscopic perspective.
1. Introduction
Massive hemorrhage is a leading cause of death, and excessive blood loss can cause serious complications including hypothermia, organ failure, shock, and even death [1]. As estimated, deaths caused by excessive bleeding accounts for 30–40% of trauma mortality. Conversely, 50% of mortalities may be avoided with efficient hemostatic techniques [[2], [3], [4]]. Even though the body possesses an effective coagulation system, in the face of massive bleeding, advanced hemostatic managements are still required, particularly for the pre-hospital care [5,6]. Biopolymers are substances derived from nature, and often contain multitudinous polysaccharides (e.g., chitosan, cellulose and alginate) and polypeptides (e.g., gelatin, silk and fibroin). Owing to their unique molecular structure and bioactivity, they have attracted much interests from the field of biomedicine and exhibited a great potential for clinical transformation [7]. For first-aid hemostasis, compared with synthetic polymer and inorganic hemostatic materials, biopolymers have shown sound biodegradability, reliable biocompatibility and non-exothermic reaction [8]. Various products based on biopolymers, e.g., Chitoflex®, HemCon® and Celox®, have become available and showed good hemostatic performance [9]. Drawing on the unique advantages, many studies have been carried out by global researchers to design and develop more advanced biopolymer-based hemostatic materials with various molecular structures and forms.
As a pivotal factor, the molecular structure of the hemostatic materials, including components and chemical modifications, can greatly influence the hemostasis effect. Notably, the biopolymers have shown precious inherent bioactivities such as pro-coagulation, pro-healing and anti-infection [10]. Though the connection by glycosidic bonds, the physical and chemical characteristics, e.g., molecular weight, solubility, functional groups, etc. of the polysaccharides are disparate, which can endow them with various hemostatic characteristics as detailed in the following sections. Similarly, polypeptides, which are abundant in tissue and blood, play a vital role in coagulation and can be utilized as the key point for hemostatic material design [11,12]. Furthermore, many functional groups have recently been introduced into the biopolymers to modify their physical and physiological properties, making them smart and multifunctional. In view of the increasingly prominent role in biopolymer functionalization, we have reviewed the modification of the polymers based on their functional designs including bio-adhesion, wettability, charge stimulation and addition of procoagulant functional groups and ions.
Even with the same component, hemostatic materials can be prepared into different forms, e.g., powder, sponge, gauze and hydrogel, which endowed them with different hemostatic mechanisms [13]. Under different circumstances (bleeding site, depth, environment, etc), such materials have shown particular advantages for hemostasis. For example, bleeding caused by gunshot are characterized by penetrating, deep and irregular wounds, for which ordinary gauze-type materials may not suffice. Incompressible bleeding wounds caused by brain, heart and other organ injuries often require the material to have bio-adhesion ability. As to moving sites, to avoid secondary bleeding caused by material movement and destruction, bio-adhesion and stretchability of hemostatic materials need to be considered. Hence, reasonable form design of the materials is crucial for attaining their hemostatic effect.
In this review, we have highlighted the key factors which may affect the hemostasis and the physiological and physical mechanisms of biopolymer-based hemostatic materials. Based on this, the current situation and principles for the design of molecular structure and form are summarized and thoroughly discussed, in addition with the shortcomings and challenges to the currently available biopolymer-based hemostatic materials.
2. Hemostatic mechanism
Understanding the hemostatic mechanism of the materials, especially how they interact with cells, proteins and other substances in the blood is important. To optimize the design of hemostatic materials, the physiological process of coagulation is first described in detail. In addition, the process that the material can regulate, namely the designable point, is highlighted. Thereafter, based on the existing studies, we have classified the hemostatic mechanisms as physical and physiological hemostasis according to their participation in the body's coagulation process.
2.1. The physiological process of hemostasis
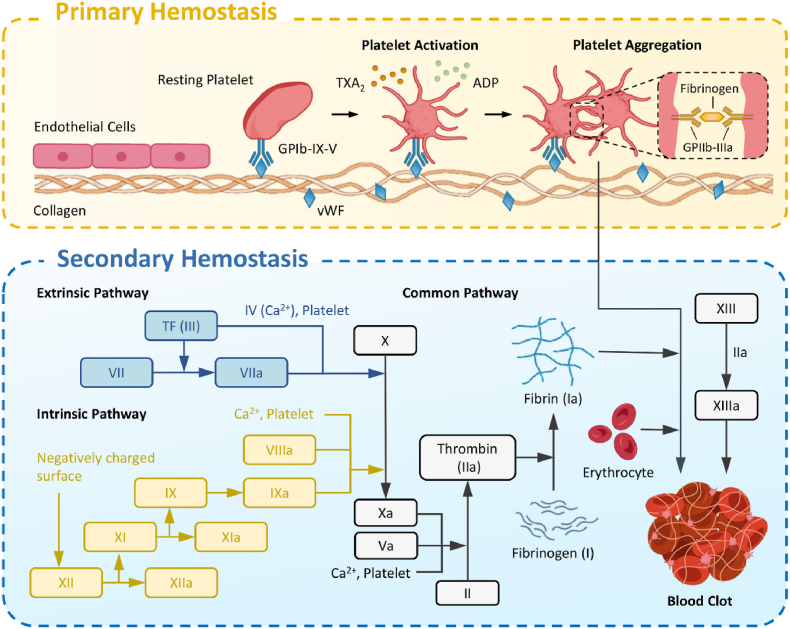
Under normal circumstances, bleeding from a small blood vessel will stop spontaneously within a few minutes owning to physiological hemostasis at the injury site, which mainly involves primary hemostasis (vascular contraction and platelet thrombosis) and secondary hemostasis (blood coagulation) (Fig. 1) [14].
Fig. 1.
Schematic illustration of the physiologic hemostasis process: primary and secondary hemostasis.
When bleeding occurs, the damaged blood vessel will firstly constrict to slow and reduce the blood flow. In the presence of von Willebrand factor (vWF), platelets will adhere to the exposed subcutaneous tissue (comprised mainly type I, III and IV collagen) via glycoprotein Ib-IX-V (GPIb-IX-V). The adherent platelets are activated via stimulation of the intracellular signaling pathways and release of endogenous adenosine diphosphate (ADP) and thromboxane A2 (TXA2), both of which can cause platelet aggregation [15,16]. Following the activation of platelets, the most abundant glycoprotein on the platelet membrane, namely GPIIb-IIIa, will undergo a conformational change, increasing the affinity to fibrinogen. Bridged by fibrinogen and Ca2+, platelet thrombus could form to block the wound and achieve primary hemostasis. The adhesion, release, aggregation and contraction of the platelets during the primary hemostasis are collectively referred as platelet activation process [17].
During secondary hemostasis, the coagulation factors are activated in sequence to produce thrombin, and fibrinogen (solvable) will subsequently convert to fibrin (insolvable), resulting in blood coagulation. The coagulation process can be divided into three steps: (1) formation of the prothrombinase complex; (2) activation of the thrombin, and (3) generation of the fibrin. The formation of prothrombinase complex can be divided into intrinsic and extrinsic pathways. For the intrinsic pathway, all coagulation factors are derived from the blood, and is usually triggered by contact of the blood with negatively-charged foreign bodies such as collagen. The coagulation factors involved mainly include factors XII, XI, IX and IV (Ca2+). The extrinsic pathway is a process initiated by exposure of tissue factor (TF), a transmembrane glycoprotein from outside of the blood, and the coagulation factors involved mainly include TF, Ca2+ and factor VII [18]. The two pathways will eventually join together to form a common process. With the help of Ca2+, factor Xa (a is short for activated) and factor Va form a factor Xa-factor Va-Ca2+-phospholipid complex on the surface of phospholipid membrane, which is named as prothrombinase complex. Under its action, the prothrombin is activated into thrombin, which in turn will convert fibrinogen into fibrin monomer and promote its aggregation [19]. Blood coagulation will then be completed with the synergy of fibrin, factor XIIIa, and blood cells. Of note, although the coagulation process includes two stages, they are overlapped and could synergize with each other.
2.2. Common mechanisms of hemostasis
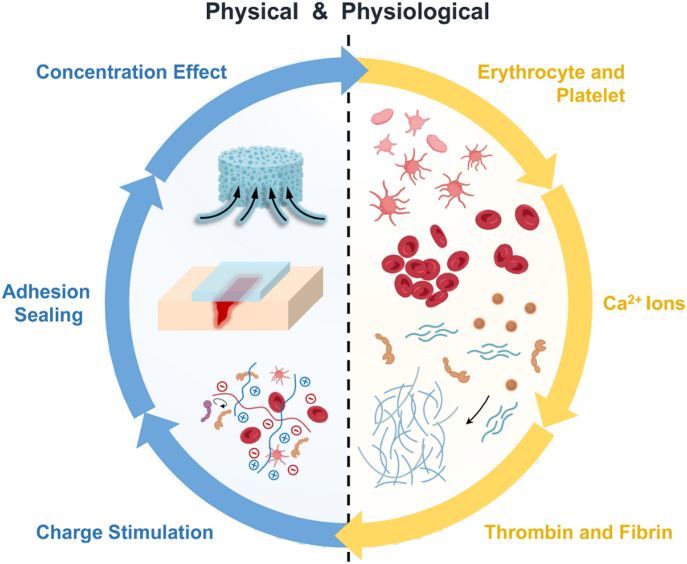
The process of hemostasis is complex and involves a variety of cells, proteins, ions and external factors. On the whole, the hemostatic mechanism of the materials could be viewed from physical and physiological aspects (Fig. 2). From the physical perspective, the hemostatic mechanisms are summarized by concentration effect, adhesion sealing and charge stimulation. From the physiological perspective, as the key players in hemostasis, the platelet, erythrocyte, fibrin, thrombin and Ca2+ ions have become the focus of attention. Of note, advanced materials often employ a variety of hemostatic mechanisms to achieve the hemostasis in a synergistic manner.
Fig. 2.
Schematic illustration of common hemostatic mechanisms from the physical and physiological aspects.
2.2.1. Physical mechanisms
-
i)
Concentration Effect
External means to increase the concentration of procoagulant components in the blood, e.g., platelets, erythrocytes, coagulation factors, etc., is an effective and frequently used method for hemostasis [20]. Hence, the concentration effect is most extensively used in inorganic hemostatic materials. The earliest studies have started from zeolite for its advantages including low cost, stability, abundant source and no potential risk of disease transmission. Owing its unique porous structure, it can quickly absorb water and concentrate the erythrocytes, platelets and coagulation factors to halt hemorrhage. In 2002, zeolite-based QuickClot™ was approved by the FDA and recommended by the Committee on Tactical Combat Casualty Care (CoTCCC) for use in bleeding emergencies [2,21]. However, its clinical use has been restricted due to severe exothermic reaction. Gelatin sponge was another commercially available hemostatic porous material, and can attain the hemostatic effect by raising the concentration of platelets and coagulation factors [22]. It has been claimed that conventional cotton gauze can lead to unnecessary blood loss due to water absorption [23]. Therefore, the relationship between hydrophilicity and hydrophobicity needs to be considered for the design of hemostatic materials, which will be addressed further in the following sections.
-
ii)
Adhesion and Sealing
Tissue adhesives can tightly adhere the wound, adapt to the irregular shape, and be easy to use, making them particularly suitable for battlefield and first aid. These properties also endow them with a wide application in hemostasis [24,25]. Many hemostatic sealants have been developed and can mainly be divided into two categories. One type is biological materials such as the first generation tissue adhesives, e.g., fibrin glue. Comprising thrombin and virus-inactivated fibrinogen, it could accelerate formation of insoluble fibrin clot at the bleeding site and plug the vessel. However, due to its poor binding strength with the tissue, the fibrin glue is incompetent for applications requiring resistance to high mechanical loads and blood pressure. Moreover, such biological products are potentially immunogenic [26]. The other type is chemosynthetic adhesive. A common one is cyanoacrylates-based adhesive, which has shown excellent adhesion performance with the tissue owing to the isocyanate structure. Unfortunately, the thermal damage during the polymerization and the toxic effect of degradation products including formaldehyde and alkyl cyanoacetate should raise concern [27]. Facing the harsh condition of dampness and even gushing bleeding, the materials are supposed to achieve wet adhesion. Furthermore, customized hemostatic adhesives for various tissues should be designed according to the diverse physiological conditions [28,29]. Facing such needs, the development of advanced and intelligent hemostatic tissue adhesive is now in full bloom.
-
iii)
Charge Stimulation
The proteins and cells in the blood are known to have negatively charged surfaces [30]. Electrostatic interactions of the materials with procoagulant components can capture and activate them, speeding up the clotting process. When a positive-charged hemostatic material is applied to the wound, plentiful blood cells will gather on it [31]. Some researchers have shown that the hemostatic activity of nanoparticles with positive charges may significantly surpass the negatively charged nanoparticles by targeting the injured sites through opsonization with fibrinogen [32]. Nevertheless, many studies have still indicated that negatively-charged materials could also promote coagulation. For intrinsic pathway, factor XII is activated to factor XIIa after contacting the negative-charged substances. Negatively charged substances such as alginate can induce the activation of factor XII, thereby accelerating the hemostasis [33,34]. Hence, both positive and negative charges can promote clotting through different hemostatic mechanisms, and the hemostatic effect will depend on the particular material and bleeding scenario.
2.2.2. Physiology-related mechanisms
-
i)
Platelet:
Platelet activation and aggregation are closely related to the two stages of physiological hemostasis, and play a pivotal role in the process [35]. During primary hemostasis, the 5-hydroxytryptamine and TXA2 released by activated platelets can enhance blood vessel contraction. The release of coagulation factors (fibrinogen and factor V) from the α-granules of the activated platelets can accelerate the clotting process. Within in the blood clots, the pseudopodia of the platelets will bind to fibrin through activated GPIIb-IIIa [12]. Therefore, various strategies based on the platelets have been applied for developing the hemostatic materials. By using the platelet membrane, a nanoplateletsome was constructed, which could target the damaged blood vessels and control massive bleeding [36]. Synthetic platelet substitutes can effectively stop bleeding and have the advantage of large-scale production. For instance, vWF-binding peptides (CBP and VBP) and fibrinogen mimicking peptides (FMP) have been used to decorate the surface of liposomes to mimic platelet adhesion and aggregation, which demonstrated an enhanced hemostatic effect [37]. Furthermore, the platelets are also involved in the immune system of the host and are of great value for tissue regeneration [38,39].
-
ii)
Erythrocyte:
The particular effect of the erythrocytes on the hemostasis has gradually been recognized, which is reflected by platelet activation, thrombin generation and clot contraction. Erythrocytes can enhance the platelet adhesion and aggregation by the release of ADP and TXA2. The interaction between the erythrocytes and fibrinogen can affect the structure, mechanical properties, and lytic resistance of blood clots [[40], [41], [42]]. Studies have shown that the erythrocytes will undergo a series of time-dependent shape change during the coagulation process. Those in the wound thrombus will lose their normal biconcave-disk shape and become densely polyhedral, which is beneficial to establish a tight interface between the materials and the bleeding tissue [43]. This is in keeping with the study by Litvinov et al. on pulmonary emboli, when the erythrocytes have changed from biconcave to compressed polyhedral, forming polyhedron-erythrocytes [44].
-
iii)
Thrombin and Fibrin:
Many coagulation factors are involved in the secondary hemostasis, which will ultimately motivate the formation of thrombin. The latter in turn will accelerate transformation of fibrinogen into fibrin to achieve blood coagulation. Thrombin has been added as a drug to the materials and showed an outstanding hemostatic performance [45]. Currently, fibrin and thrombin are commercially available as coagulation components, and have been extensively used in the clinics. The long-standing drawbacks are immunogenicity and the risk for viral contamination, as such ingredients have derived from animals (cattle, pigs) or human blood [46]. In addition, their pre-hospital application, e.g., in battlefield and emergency treatments, is limited by the time-consuming process of lyophilized powder dissolution and mixing. As a result, how to activate the coagulation cascade and induce more thrombin and fibrin production may be considered as a design point [47]. On the other hand, the inhibition of fibrinolytic system activation has also attracted much attention. Many antifibrinolytic drugs have been put into clinical use. This is represented by tranexamic acid (TXA), a life-saving drug with proven benefit, which can competitively inhibit the activation of plasminogen to plasmin, preventing degradation of the fibrin clot [48,49].
-
iv)
Ca2+ Ion:
As the only non-protein clotting factor, Ca2+ ions are ubiquitously involved in the coagulation process and play an important role at all stages [50]. In the absence of Ca2+ ions, platelet surface activated GPIIb-IIIa cannot bind with fibrinogen and aggregate. Intracellular Ca2+ ions can lead to exposure of phosphatidylserine on the surface of platelets, which can provide more sites for the aggregation of coagulation complexes and significantly promote thrombin formation. In addition, prothrombinase complex, as an indispensable activator for the formation of thrombin, must be formed by the action of Ca2+ ions. Calcium-containing biomaterials have been verified for attaining hemostasis effectively. Calcium-modified oxidized microporous starch has been proved to activate the coagulation cascade and induce platelet adhesion, so as to effectively control the bleeding in rabbit liver and femoral artery injuries [51]. Likewise, mesoporous silica nanoparticles modified by tannic acid, silver nanoparticles, and calcium ions were prepared by Chen et al. Among these, Ca2+ ions were released from the materials when exposed to blood, thereby promote the activation and circulation of coagulation cascade [52]. Inorganic materials containing Ca2+ ions like whitlockite can release the Ca2+ ions to initiate the coagulation cascade [53].
3. Molecular structure design of the hemostasis materials
The hemostatic properties of the materials may be greatly affected by their molecular structures including compositions and chemical modifications. The potential of hemostatic materials varies with their main components. Here, polysaccharide and polypeptide-derived hemostatic materials are emphatically recapitulated (Table 1). Notably, chemical modification could also endow the hemostatic materials with desired bioactivity and multifunction. Merits of chemical modification of the hemostatic materials in recent years are discussed below, with a focus on the adhesion and sealing, wettability, charge stimulation, procoagulant functional groups and ions (Table 2).
Table 1.
Component design for biopolymer-based hemostatic materials.
| Materials | Hemostatic mechanism | Characteristic applications | Limitation |
|---|---|---|---|
| Polysaccharide-derived Hemostatic Materials | |||
| Chitosan and its derivatives | Positively charged amino; Erythrocyte aggregation; Platelet adhesion | Hemostasis of rat liver and femoral artery trauma [62]; Bio-adhesion on rat liver and beating heart, hemostasis of rat liver hemorrhage [60] | Poor solubility |
| Cellulose and its derivatives | Concentration effect; Negatively charged carboxyl; Complexation with Fe3+; Platelet activation and aggregation | Hemostasis of rabbit liver and ear artery hemorrhage [70]; Hemostasis of rat liver and Swine femoral artery injury [68] | Weak pro-healing bioactivity; Potential acidosis risk |
| Alginate and Hyaluronic Acid | Concentration effect; Negatively charged carboxyl; Activating intrinsic pathway | Hemostasis of rat liver hemorrhage, shape-recovery ability, wound healing [74]; Hemostasis of liver hemorrhage in normal and hemophilia mice, prevention of abdominal adhesion [77] | Low hemostatic ability; Insufficient chemical stability |
| Polypeptide-derived Hemostatic Materials | |||
| Collagen | Platelet activation; Activating intrinsic pathway | Promotive capacity of blood cell adhesion, hemostasis of rabbit liver injury [82]; Minimal proinflammatory cytokine production, platelet adhesion and activation [83] | Heterogeneity; Immunogenicity risk |
| Silk fibroin | Platelet aggregation and adhesion; Enhanced binding of platelets and fibrinogen | Wet tissue adhesion, Hemostasis of rabbit ear artery, liver, cardiac puncture, and femoral artery injury, hemostasis of rat liver, heart and tail bleeding [95] | Insufficient chemical stability |
| Keratin | Platelet adhesion; Activating intrinsic and extrinsic pathways; Promoting fibrin clotting | Hemostasis of rat liver puncture and tail amputation injury [101]; Hemostasis of rat liver puncture and femoral artery injury [104] | Heterogeneity; Complex keratin-related proteins |
Table 2.
Design of chemical modification for biopolymer-based hemostatic materials.
| Design strategies | Hemostatic mechanism | Reference | |
|---|---|---|---|
| Adhesion and sealing | Mussel-inspired design | Physical interactions (hydrogen bonds, metal coordination, π-π, π-cation and hydrophobic interactions); Chemical bonds (Michael addition and Schiff base reactions) | [26,29,107,115,116,129,184] |
| NHS-based design | Imine bonds formed by NHS ester and amino groups in the tissue | [114,174] | |
| Schiff base design | Imine bonds formed by aldehyde and amino groups in the tissue | [60,88,[111], [112], [113]] | |
| Wettability | Hydrophilicity | Water absorption; Concentration effect | [117] |
| Hydrophobicity | Control blood flow; Prevent excessive blood loss; Ease blood clots separation; Wet adhesion | [23,118,120,121,123,124] | |
| Charge stimulation | Positive charge | Blood cell aggregation; Platelet activation; Accelerating fibrin formation | [32,[125], [126], [127]] |
| Negative charge | Activation intrinsic pathway; Thrombin formation; Strengthen fibrin clot | [74,133,134,136] | |
| Functional groups | Amino group | Positive charge (see above) | [60] |
| Carboxyl group | Negative charge (see above) | [74] | |
| Thiol group | Activating extrinsic pathway; Activating TF and platelets | [137] | |
| Aldehyde group | Protein Binding via imine bonds | [138] | |
| Catechol group | Constrict blood vessels; Negative charge; Protein binding | [139] | |
| Alkyl group | Insert into cell membranes and trap them | [124,[143], [144], [145]] | |
| Ions | Fe | Erythrocyte aggregation; Strengthen fibrin clot | [148] |
| Ca | Activating intrinsic and extrinsic pathway | [51,52,75] | |
| Zn | Promoting blood cell aggregation | [149,168] | |
| Mg | Stabilization of the native conformation of factor IX | [150] |
3.1. Component design
3.1.1. Polysaccharide-derived hemostatic material
-
i)
Chitosan-based Material
Derived from partial deacetylation of chitin, chitosan is the only known natural cationic polymer [54]. Owing to their credible biocompatibility, biodegradability, inherent hemostatic and antibacterial capacity, chitosan and its derivatives, e.g., carboxymethyl chitosan (CMCS), hydroxypropyl chitosan, quaternary ammonium chitosan, have been widely adapted in regeneration medicine, drug delivery, hemostasis and other fields [55].
A commercially made chitosan-based hemostatic material, HemCon® (approved by the FDA in 2002), stands out for its excellent hemostatic effect in the battlefield. The HemCon® is a lyophilized film following dissolution in acetic acid. Studies have shown that the strong acidity of HemCon® could trigger a strong inflammatory response after the implantation [56]. Celox®, a chitosan powder approved in 2007, could absorb blood up to 11 times its own weight, has shown be effective in three models for mixed arterio-venous hemorrhages [57]. These have validated the hemostatic effect of chitosan. Studies also showed that the pro-coagulation of chitosan does not depend on the classical coagulation cascade. The hemostatic effect of positively-charged chitosan on erythrocyte aggregation and platelet adhesion have been demonstrated [58,59]. Xu's group has developed an in-situ imine crosslinked CMCS-based liquid bandage (LBA). Excited by ultraviolet light, the materials could crosslink with amino groups on the tissue. Furthermore, owing to the hemostatic activity of the CMCS, the LBA could stimulate the adhesion and aggregation of blood cells, thereby possesses the capacity for effective hemostasis. The synergy of blood clot formation and wound sealing may have contributed to the excellent hemostatic capacity of the LBA [60]. In addition, the research on chitosan modification is also promptly growing. Song et al. have grafted the catechol group onto the chitosan to prepare a hemostatic hydrogel. An excellent hemostatic effect was verified by mouse model for liver incision, tail amputation and foot trauma. This has been attributed to the catechol group and amino group in the chitosan, which have synergistically enhanced the adhesion ability of the erythrocytes and platelets [61]. Furthermore, by composite the chitosan and caffeic acid with mesoporous silicon, the hemostatic performance was synergistically enhanced. The design of the composite material has endowed the mesoporous silicon with the ability for tissue adhesion, coagulation cascade activation, and erythrocyte and platelet aggregation [62].
However, there were different viewpoints, some have suggested that the positive charge of the chitosan may delay the thrombin generation and blood coagulation due to its inhibition of contact activation and inability to significantly promote the activation of non-adherent platelets at the early stage of coagulation [63]. Nevertheless, the hemostatic material design based on chitosan has obviously become a hotspot by modulating the form and combination with other materials and chemical modification.
-
ii)
Cellulose-based Material
Cellulose is the most abundant polysaccharide in nature and accounts for more than 50% of the carbon content in the plants. It has long been used for hemostasis, as in the case of cotton [64,65]. However, the hydrogen bond network and highly crystalline structure of cellulose endow it a very poor solubility, which greatly hinder its application. Oxidation could foster it with solubility and biodegradability. With the oxidation, partial hydroxyl groups in the cellulose can be transformed into carboxyl groups to form oxidized cellulose. Interestingly, oxidized cellulose have recognized antibacterial properties owing to the carboxylic groups create a bactericidal environment by decreasing pH value [66].
Oxidized cellulose has become a most common derivative used for hemostasis. For example, Surgicel® with oxidized regenerated cellulose as the main component have been one of the most clinically applied hemostatic products [10]. Its hemostatic mechanism may be attributed to two aspects. Firstly, upon its contact with the wound, the excess water from the blood is absorbed, it will gelatinize to seal the wound subsequently. Secondly, cellulose can induce platelet activation and aggregation and complex with Fe3+ ions, which will be released from the acidic hemoglobin and accelerate thrombus formation [67,68]. Li et al. have prepared a series of composite cellulose/collagen materials by altering the concentration of oxidized microcrystalline cellulose (OMCC) in a collagen solution. The composite hemostat has shown multiple hemostatic mechanisms. The hydrophilic carboxyl group can combine with the Fe3+ ions in the hemoglobin to form a brownish gel which can seal the wound [69]. And the interaction between the oxidized cellulose and the platelets was preliminarily investigated. Cheng et al. have realized carboxyl functionalization to the cellulose nanocrystal through 2, 2, 6, 6-tetramethylpiperidine-1oxyl (TEMPO). Carboxylated cellulose nanocrystals not only provided cross-linking sites for Ca2+ ions, but also facilitate the attraction and activation of the platelets [70]. A carboxymethyl cellulose (CMC) fibers-reinforced composite was fabricated for the treatment of uncontrollable massive hemorrhage. The introduction of CMC has enhanced the mechanical properties of the composites, including toughness, mechanical strength and resistance to fatigue. More importantly, the dispersed CMC fibers could induce platelet activation through enhanced release and expression of CD61 (GPIIIa) and P-selectin [68]. Taken together, to tap the hemostatic mechanism and make it multifunctional by rational design has become the key for making new cellulose-based hemostats.
-
iii)
Alginate and Hyaluronic Acid-based Material
Alginate is a biopolymer composed of randomly arranged linear unbranched chains of α-l-guluronate (G block) and β-d-mannuronate (M block) residues. Hyaluronic acid (HA) is a macromolecule in extracellular matrix (ECM) and consists of repeated disaccharide units, namely β-d-glucuronic acid and N-acetyl-d-glucosamine. Both the alginate and HA belong to anionic polysaccharides for the abundant carboxyl groups in their backbone [71,72].
Like collagen, glass, and kaolin, the anionic polysaccharide can interact with positively charged amino acids on factor XII through the negative charge conferred by the carboxyl groups, which can activate the intrinsic pathway and trigger the coagulation cascade [33,73]. Wang et al. have designed a HA-polyurethane hybrid cryogel for the wound hemostasis and repair. Through coagulation four indices testing, the authors found that the hydrogel could significantly decrease the APPT, but had no effect on PT, suggesting that the hydrogel could activate the intrinsic pathway owing to the negative charge of HA [74]. Besides, alginate can form an “egg-box” structure with Ca2+ ions. After the contact with the blood, calcium ions in calcium alginate are released in exchange for Na + ions. The released Ca2+ ions could promote a prothrombin activation cascade, which in turn can lead to rapid hemostasis [75]. Based on the ion-exchange mechanism, Wu et al. have designed a calcium alginate-coated chitosan microsphere. The released Ca2+ ions could activate the platelets and coagulation factors, speeding the generation of thrombin by accelerating the intrinsic and extrinsic pathways. However, as noted by the authors, the negatively-charged alginate also reduced the adhesion of erythrocytes [76].
On the whole, the alginate and HA have limited hemostatic effect by their own. Hence, a large number of chemical modification and hybridization strategies have been designed. Serotonin, as a platelet activator, has been conjugated onto the HA to mimic the clotting mechanism of platelets [77]. Besides electrostatic interaction, chemical modification can also endow other hemostatic mechanisms such as tissue adhesion. Yan and colleagues have introduced the catechol and aldehyde group into the alginate. The dual-functionalized alginate could react with hydrazide-modified poly(l-glutamic acid) to form a strong bio-adhesive hydrogel with superior hemostatic performance [78]. Notably, other biological properties of alginate and HA are outstanding. For instance, their moisturizing properties are widely used in cosmetics and pharmaceutical industries. The pro-healing activity of granulation tissue regeneration and fibroblast proliferation may also be utilized for wound repair after hemostasis [18,79].
3.1.2. Polypeptides-derived hemostatic material
-
i)
Collagen-based Material
Collagen is the most abundant protein in mammalian tissues and mainly maintains their structural integrity. Meanwhile, collagen also plays a remarkable role in physiological hemostasis. Subendothelial collagen can bind with the platelet receptors, further promoting platelet adhesion and aggregation and initiating the clotting pathways. As a prerequisite for secondary hemostasis, negatively charged collagen can trigger the intrinsic pathway. To date, collagen is the only ECM protein known to promote platelet activation [80,81].
On the other hand, the heterogeneity and immunogenicity of animal collagens have gradually become a concern. Using a bottom-up design to synthesize engineered collagen by bioengineering technology is a solution. A recombinant hemostatic collagen sponge with enhanced procoagulant effect has been prepared, which could better promote the blood cell adhesion compared with natural collagen sponge [82]. Moreover, Hartgerink's group has developed a nanofiber consist of collagen mimetic peptide capable of forming large-scale nanofibrous hydrogels. The latter has shown a procoagulant activity manifesting as adhesion and activation of platelets (higher P-selectin secretion) similar to animal collagen [83].
Another strategy to address the biosafety of collagen is hydrolysis [18]. Gelatin is a hydrolysate product of collagen featuring reliable biocompatibility, biodegradability, non-immunogenicity and high commercial value. The arginine-glycine-asparagine (RGD) sequence in the gelatin can promote cell adhesion and migration, conferring the gelatin with extensive use in tissue regeneration [84]. Notably, gelatin has shown hemostatic capability by aggregating and activating platelets. The cationic groups of the gelatin can facilitate the entrapment of negatively-charged erythrocytes into the blood clot to form a dense fibrin mesh [85,86]. Several gelatin-based products are available, which included Gelfoam® and Surgifoam®, displaying high application value [25,87]. Many chemical modification and material composite strategy have been used to foster the hemostatic effect of gelatin. For instance, methacrylic anhydride (MA) was modified in gelatin to enhance the bio-adhesion which can facilitate wound sealing and hemostasis [88]. Alginate and genipin are combined with gelatin to stop hemorrhage and resist water, respectively. And the sponge is suitable for postoperative rapid hemostasis and prevention of tumor recurrence [89].
It is also worthy noting that collagen is the main component of ECM. Compared with purified collagen, ECM has retained the physical and chemical signals and biological properties of the tissues. With a bioactive microenvironment, the ECM has an effect on the angiogenesis, immunomodulatory, stem cell recruitment and other aspects to promote tissue regeneration [90,91]. However, its application in the hemostatic materials is scanty.
-
ii)
Silk Fibroin-based Material
Silk fibroin (SF) is a natural protein extracted from silkworm cocoons and consists of a light and a heavy chain connected by disulfide, which is mainly used in fashion textiles and surgical sutures. Owing to its dependable biocompatibility, biodegradation and adjustable mechanical properties, the SF has attracted a growing interest from many biomedical fields [92]. The SF can undergo gelation transformation under certain external conditions, and the mechanism can be explained as follows: under hydrophobic and hydrogen bond interaction, the secondary structure of the SF will change from random coil to physically crosslinked β-sheet structure [93,94]. The rapid gelation is beneficial to wound sealing. Yan and coworkers have fabricated a supramolecular hemostat (CS/TA/SF hydrogel) including SF, chitosan and tannic acid, where tannic acid crosslinks with the others by electrostatic interaction and hydrogen bonding. The CS/TA/SF hydrogels revealed wet adhesion properties, and the SF and tannic acid can accelerate thrombus through direct interaction with the platelets and coagulation factor, showing an effective hemostatic performance for both arterial and visceral bleeding [95]. Except for hydrogels, the SF has also been prepared into various forms to halt hemorrhage. For instance, a microsphere containing the SF and alginate was prepared for rapid hemostasis in virtue of the improved cell-attachment properties of the SF and the rougher surface morphology of the microspheres [96]. Drawing on the distinctive inherent biodegradability and biocompatibility, the SF and chitosan-based cryogel with exudate absorption capacities and compressive elasticity was prepared, which is conducive to the concentration effect [97].
As a typical hemostatic material, the SF's hemostatic mechanism has been explored. Wei et al. have investigated the mechanism of SF and found that the SF can significantly activate the platelets, causing platelet aggregation and adhesion, and strengthened binding of the platelets with fibrinogen. Of note, although some studies have reported the hemostatic effect of the SF, in most cases it was combined with other components, so it is difficult to figure out the effectiveness of the SF itself. Therefore, the thorough hemostatic mechanism of the SF still needs to be comprehensively explored [13,98].
-
iii)
Keratin-based Material
Keratin as a natural material may be found in skin, hair, nails and other parts of the body. Its rich content of cysteine residues allows the formation of disulfide bonds, conferring strong and elastic properties to the keratin tissues. Of note, keratin has an excellent hemostatic effect and has been frequently adapted for the design of hemostatic materials [5,7]. An expandable keratin-based sponge fabricated through radical polymerization has shown a hemostatic effect for penetrating hepatic hemorrhage in rats and femoral artery transection hemorrhage in a pig model [99]. To attain bio-adhesion and enhance the mechanical properties, the strategy of catechin crosslinking and cellulose combination has been adapted to construct a nanocomposite hydrogel for accelerating the blood coagulation [100]. For its handy storage and portability, a powder-type keratin was prepared as a novel hemostat. The nanosizing has conferred the keratin particles with larger surface area, endowing them with better water absorption and film forming properties [101].
Researches on the mechanism of keratin hemostasis are also advancing. Burnett et al. have preliminarily studied the hemostatic mechanism of KeraStat™ (KeraNetics), a keratin-based hydrogel. Platelet adhesion experiments showed that the hemostatic mechanism has involved β1 integrin-mediated platelet adhesion [102]. Moreover, some have pointed out that, as biogenic polypeptides, it is difficult to control the keratin's amino acid composition, batch-to-batch difference, and complex keratin-related proteins. These have stalled the exploration of its use for hemostasis [103]. Synthesis of recombinant keratin through bioengineering methods may ultimately solve the above problems. Guo's group has used Escherichia coli to express two types of human hair keratin and applied them for hemostasis. The results of APTT and PT assays showed that the human hair keratin could participate in both the intrinsic and extrinsic pathways, especially the former, which has enhanced the blood clotting through fibrin clotting [104].
3.2. Design of chemical modification
3.2.1. Adhesion and sealing
Mussel, a marine organism, has excellent wet adhesion properties. Hemostatic materials based on mussel-inspired design have been developed in full bloom in recent years [105]. Studies have shown that the wet adhesion of mussels mainly comes from the rich catechol group. Catechol chemistry is therefore considered a solution to the challenges faced by bio-adhesives and hemostasis. The catechol groups can bind with tissues through physical interactions, e.g., hydrogen bond, metal coordination, π-π, π-cation and hydrophobic interactions, and chemical bonds, e.g., Michael addition reactions and Schiff base reactions [28]. A hemostatic needle with catechol-grafted chitosan coating to prevent bleeding following tissue puncture was developed. The conjugation of catechol achieved enhanced in vivo hemostasis and mucoadhesion [106]. Liu et al. have introduced tannic acid into polyacrylamide gels. Owing to the rich catechol groups on the polyacrylamide gel, the hydrogels showed strong and long-lasting adhesion (up to 500 kPa) on pig skin [107]. Water on the bleeding surface acts as a physical barrier to prevent the adhesive from rapidly adhering to the tissue. In addition, water molecules may interact with adhesive groups in the hydrogels through hydrogen bonds, and ultimately lead to failure of hemostasis [108,109]. Other strategies to address the wet adhesion have also been developed. Li's group has described an ultrasound-mediated strategy to achieve tough bio-adhesion. Ultrasound could push molecules into the tissues, forming strong mechanical interlocking, and enhance the formation of physical interactions with surrounding tissues. Both mechanisms worked together to achieve a strong bio-adhesion, and water molecules were no longer considered an obstacle to adhesion, but acted as a diffuser [110].
In addition to the mussel bionic strategy, Schiff base-based adhesion (i.e., imine connection) has also been adapted by researchers in view of the abundant amino groups on the tissue surface. Hence, aldehyde-functionalization has been validated as an enlightenment for the design of strong bio-adhesion. Guo's group has designed a multifunctional hydrogel based on dynamic Schiff base and copolymer micelle crosslinking. In addition to the self-healing property, formation of the Schiff base between aldehyde groups in the hydrogel and amine groups on tissue surface also endowed the hydrogels with desired adhesive strength [111]. Similarly, aldehyde chitosan could interact with gelatin through Schiff base and hydrogen bond to form a hemostatic bio-adhesive suitable for laparoscopic surgery. They have also demonstrated that this bio-adhesive is thermo-responsive and could peel easily at low temperature (20 °C) [112]. Zhu's group has designed a hydrogel adhesive which suits wet and dynamic environment. The adhesive could adhere to tissues through a rapid S-nitrosylation coupling reaction, which could generate aldehyde group upon 395 nm light irradiation [113]. Moreover, N-hydroxysuccinimide (NHS) ester-based functionalization is also a promising design to achieve bio-adhesion. Active NHS ester could easily crosslink with the tissue through formation of stable amide bonds with the amino groups, and also react with thiol groups to produce thioester, which is less stable compared with amide bonds [15,114].
Adhesion failure can occur under three conditions: i) adhesion failure, failing at the adhesive layer, ii) cohesion failure, failing within the adhesive matrix, and iii) adhesion/cohesion failure, failing at both adhesive layer and adhesive matrix [28]. Bio-adhesives prepared from biopolymer have inadequate mechanical properties and are prone to cohesive failure upon use. Correspondingly, appropriate improvement of the cohesion of the hydrogel is a feasible strategy to foster tissue adhesion [115]. Xie's group has designed a multiple cross-linking hemostatic hydrogel with combined photocuring, dynamic covalent bonding and hydrogen bonding. The photocuring strategy could significantly enhance the cohesion strength and bio-adhesion of the hydrogel [116]. Effective tissue adhesion and wound sealing showed an excellent hemostatic effect. This strategy, independent of physiological coagulation, can facilitate hemostasis in coagulopathy patients.
3.2.2. Wettability
Rapid absorption of water from the blood by the hemostatic material can promote clotting through a concentrating effect. Wang's group has designed a crosslinked graphene sponge (CGS) with hemostatic capacity. The CGS could absorb blood up to 147 times of its own weight, showing a remarkable capability for liquid absorption. Through concentrating effect, vast amount of blood cells and platelets may be enriched on the CQS surface to achieve hemostasis [117].
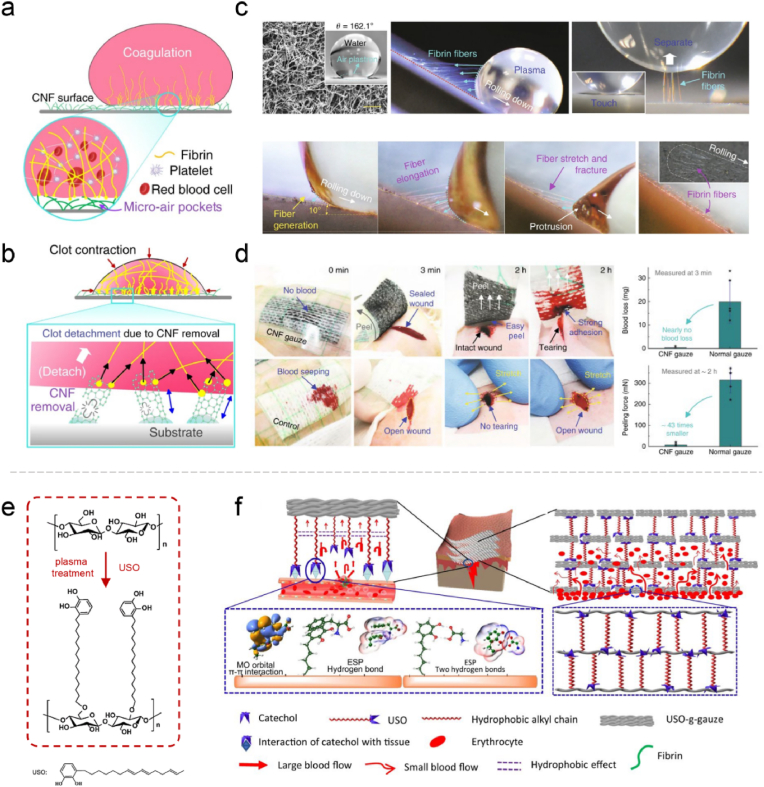
However, in many cases, hydrophilic hemostatic materials will fill the blood and eventually form the blood clots. If the material needs to be taken off after hemostasis, this will increase the difficulty of removal and the risk of secondary bleeding. Therefore, the importance of hydrophobicity in hemostatic materials is recognized [118,119]. Li et al. reported a promising strategy for designing hemostatic patch via hydrophobic and blood-repelling carbon nanofibers (CNFs) surface. CNFs have the hemostatic natural instincts, which is effective in fibrin formation, and because of its superhydrophobic properties, it limits the infiltration to prevent excessive blood loss. Then, minimal contact between the thrombus and CNFs produces self-acting detachment after thrombus contraction. Based on this dexterous design, rapid hemostasis and self-separation of blood clots can be achieved simultaneously in vivo (Fig. 3a–d) [120]. Commonly, the materials can be designed to hydrophobicity by grafting alkyl and siloxane groups. For instance, undecanal-modified chitosan was prepared via sodium cyanoborohydride reduction showing enhanced hydrophobicity and antibacterial properties [121]; Vinyl trimethoxy silane and cellulose nanofiber were combined to prepare a hydrophobic layer [122].
Fig. 3.
Illustration of (a) fibrin fibers formation from blood on a superhydrophobic CNF surface and (b) clot detachment from the superhydrophobic CNF surface. (c) Fibrin fiber generation on the superhydrophobic CNF surface. (d) The in vivo hemostatic experiment of the CNF gauze and control cotton gauze. Reproduced with permission from Ref. [120]. Copyright 2019, Springer Nature. (e) Schematic fabrication of surface USO-grafted cotton cellulose; (f) hemostatic mechanism diagram of USO-g-gauze. Reproduced with permission from Ref. [124]. Copyright 2022, Springer Nature.
As proven by an increasing number of recent researches, modulating the hydrophilic and hydrophobic properties of the materials is feasible to regulate their hemostatic effect. For instance, Janus materials with both properties have attracted much attention [122,123]. A Janus gauze with hydrophobicity on one side and hydrophilicity on the other through a simple paraffin spraying method. By regulating the wettability, this design could effectively control the bleeding and reduce blood loss by more than 50% compared with similar products [23]. Furthermore, Li et al. pointed out that to control blood flow at the gauze-tissue contact surface and inside the gauze is the key in the design of efficient hemostatic gauze. An alkyl chain terminated with the catechol group was introduced onto the cotton fiber, integrating wet adhesiveness and hydrophobicity. The catechol group could quickly anchor to the tissue, prohibiting lateral diffusion of the blood, and the resistance created by the alkyl chains prevented the blood from upward diffusion. Eventually, the moderately hydrophilic fibers promoted concentration of the blood cells and protein to quickly form blood clots (Fig. 3e–f) [124]. As a result, the balance between hydrophilicity and hydrophobicity has been taken into consideration for the design of advanced hemostatic materials.
3.2.3. Charge stimulation
As mentioned above, positive charge can enhance the interaction between blood cells and proteins by electrostatic attraction, thereby inducing blood cell aggregation, platelet activation, and coagulation. As the only cationic polysaccharide, chitosan can effectively halt hemorrhage, during which its positive charge played an important role. Positively charged molecules such as polylysine, polyamine and protamine have shown a hemostatic effect [32]. Owing to its cationic nature, polylysine can affect the coagulation from the following aspects. First, it can enrich and form a complex with fibrinogen; Second, it can promote secondary coagulation by activating factors VII and X [87].
Given the unique nature of positive charge, cationization design of the basal materials is also a strategy to improve the hemostatic performance. Liu and colleagues have designed a cationic starch-based hemostat (CS) by introducing quaternary ammonium groups into the starch. Electropositive CS can confer an adhesion activity to the electronegative erythrocyte, inducing accelerated platelet aggregation and fibrin formation. Other positively charged groups such as sulfanilamide and phosphorus can also be used for the design of hemostatic materials [125,126]. The application of burgeoning short peptides and polypeptides for hemostasis has also been noted. Zhu et al. has conjugated cationic short peptides (amino acid sequence: RRRFRGDK) directly to the hydrogel surface through an amidation reaction, which conferred the hydrogels with enhanced antibacterial and hemostatic properties. Positively charged peptides have shown to induce hemostasis by electrostatic attraction to the erythrocyte, platelet, and plasma fibronectin. Nevertheless, inferior hemocompatibility was noted due to the increased cationic charge [127]. Therefore, studies on the hemostatic effect of electro-positivity focusing on the interaction with blood cells are required.
Negatively charged substances can activate partial coagulation factors to activate the intrinsic pathway. The carboxyl group can provide a negative charge. Anionic polysaccharides, e.g., alginate, sodium hyaluronate, and oxidized cellulose, contain large amount of carboxyl groups. In addition, some substances rich in carboxyl groups have the ability to clot the blood. For instance, graphene oxide (GO) is a two-dimensional material with abundant oxygen-containing groups, e.g., hydroxyl and epoxy groups on the surface, and carboxyl groups on the edges [128,129]. Li et al. have synthesized a GO-polydopamine hemostatic, which showed excellent hemostatic effect owning to its strong activation and aggregation effect on the platelets [130]. Also demonstrated by Zhang et al. through verifying the platelet morphology and CD62p expression, GO could promote platelet adhesion and activation drawing on its oxygen-rich functional groups [131]. Nevertheless, some have proposed that this was due to changing of the charge distribution and hydrophilicity of graphene by the oxygen-containing groups instead the group itself [132]. Moreover, anionic polyphosphate (PolyP), as linear polymer of inorganic phosphates, could accelerate the generation of thrombin and strengthen the fibrin clot. The rate of factor V activation could limit the thrombin generation, whilst the PolyP could enhance it by altering the kinetics of thrombin generation through factors XIa, Xa and thrombin [[133], [134], [135]]. Gu et al. have crosslinked the Polyp onto a collagen scaffold (P-CS), conferring it with improved hemostatic capability. Through a series of experiments, the P-CS has shown to accelerate the coagulation process through the intrinsic pathway, along with platelet activation and thrombin formation [136]. Nevertheless, some have suggested that negatively charged substances and/or groups, such as carboxyl groups, can weaken the adhesion of erythrocyte by electrostatic repulsion [76]. Taken together, to regulate the electric charge of the materials will have a great impact on their hemostatic properties, and the designs based on this may have a wide range of applications.
3.2.4. Procoagulant functional groups and ions
Numerous studies on grafting and chemical modification of the basis material have been carried out to facilitate hemostasis. Alongside with their physical and chemical properties, functional groups can also influence the pro-coagulation of materials. Many functional groups have shown to interact with blood cells, coagulation factors, and can effectively activate the coagulation system. As described above, the positively-charged amino group and negatively-charged carboxyl group can affect the coagulation based on electrostatic interactions and their designs.
The thiol and aldehyde groups can promote the coagulation mainly through direct interaction with proteins and cells in the blood. Wu et al. have modified the thiol group on chitosan, endowing it with efficient hemostatic performance for bleeding from rat liver injury and rabbit vein rupture. The thiol groups could accelerate the extrinsic pathway by activating the TF and platelets, resulting in promoted blood coagulation [137]. The aldehyde groups can be introduced by oxidation of the polysaccharides. Such groups can bind to proteins in the blood and free amino groups on cells by forming imine to initiate the blood clotting [138].
The catechol group has a multiple effect on the promotion of coagulation. Firstly, it can constrict the blood vessels to reduce the blood flow at the bleeding site. Secondly, the phenolic hydroxyl group of the catechol is strongly reactive and can confer a negative charge to the material, triggering a clotting cascade. Furthermore, physical interactions and chemical crosslinks with plasma proteins are produced by the catechol moieties [18,139]. Notably, naturally derived substances rich in ortho-polyphenol moieties like tannic acid can be used as molecular glues to crosslink proteins through hydrogen bond, cation-π interaction, Schiff base reaction and Michael reaction [140,141]. In addition, the catechol group and polyphenols also showed excellent antibacterial, antioxidant and anti-inflammatory bioactivities, which may be considered in the design of new multifunctional hemostatic materials [142].
The alkyl groups have a hemostatic activity by inserting into the cell membrane to trap the blood cells [143]. Dowling et al. have branched the hydrophobic alkyl group on a chitosan backbone with benzene-n-octadecyl and achieved rapid clotting of heparinized human blood. This is attributed to the insertion of the hydrophobes on the modified chitosan into the blood cell membrane [144]. Similarly, Chen et al. have designed a hybrid hydrogel with hemostatic function comprising benzaldehyde-terminated PEG and dodecyl-modified chitosan. The hemostatic mechanism was ascribed to the dodecyl on the modified chitosan which can insert and lock onto the lipid bilayer of cell membrane. In addition to blood cells, the dodecyl can also trap the bacteria in the wound and reduce the risk of infection [145].
Supplement of metal ions, e.g., Fe3+, Ca2+, Zn2+ and Mg2+, may also facilitate blood coagulation [146]. As reported previously, Fe3+ ions could induce generation of hydroxyl radicals, which in turn can induce erythrocyte aggregation and enhance fibrin-erythrocyte interaction. The radicals can cause non-enzymatic formation of fibrinogen aggregates to stabilize the blood clot. Lv et al. have confirmed that the formation of blood clot has increased with the raise of Fe3+ concentration [147,148]. The hemostatic effect of Ca2+ ion as factor IV is self-evident. Moreover, zinc deficiency can result in a bleeding tendency and impaired platelet aggregation. Introduction of ZnO nanoparticles could enhance coagulation ability of chitosan-based nanoparticles through promotion of blood cell aggregation [146,149]. Mg2+ ion is also involved in the blood coagulation cascade by assisting the stabilization of the native conformation of factor IX [150]. Other metals (Ag, Ga and Ce) have also been used for the design of hemostatic materials [86,[151], [152], [153]]. In conclusion, the hemostatic properties may be improved by the virtue of electric charge and metal ions, though the impact of such modifications on the antibacterial ability, hemocompatibility, cell activity of the materials should also be considered.
4. Form design of hemostasis materials
From a macroscopic perspective, for the design of hemostatic materials, researchers need to consider the influence of various forms on the hemostasis. Physical properties of the hemostatic materials such as porosity, absorption, interface interaction, etc. can be adjusted by altering the forms. The hemostatic materials may be classified into four categories based on the form including powder, sponge, gauze and hydrogel.
4.1. Powder-type materials
Powder-type hemostatic materials have a wide application and are propitious to hemostasis in deep and irregular wounds. Some biopolymer-based powders, such as Celox® and NexStat®, have been introduced into military and civilian settings. In recent years, there have been many advanced designs for hemostatic powders with respect to structural regulation and functionalization [154,155].
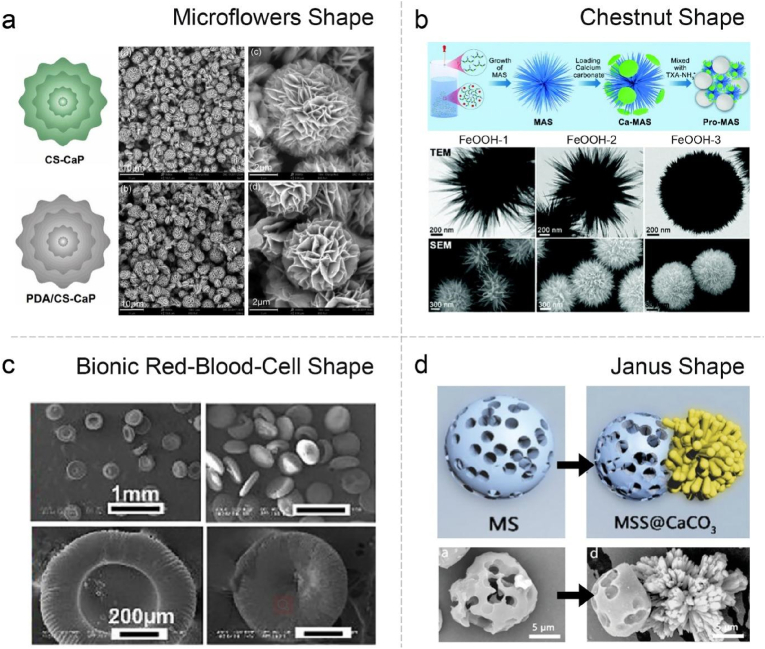
With the development of synthetic technology, the morphology and topological structure of powder materials can be customized, such as micro/nano-morphology, Janus structure and layer-by-layer assembly. For instance, a unique micro-flowers with hierarchical porous structure has been achieved via a facile approach, endowing the powder with high surface roughness and large area (Fig. 4a) [156]. Moreover, a starch/polyphenol composite microparticles with multilayer structure was fabricated through electrostatic interaction. With the optimized outer layer, the microparticles could significantly promote platelet adhesion/activation and erythrocyte aggregation, resulting in satisfying hemostatic performance [157]. Other distinctive morphology was also put into practice for the hemostatic powders such as chestnut and red-blood-cell like shapes (Fig. 4b and c) [158,159]. However, in the face of a deep, narrow and irregular wound, it is still difficult for the powder to reach the bottom of wound. To this end, Lan's group has fabricated a Janus particle. One side of it is phosphate group-modified microporous starch, and the other is flower-like CaCO3. The loaded protonated TXA could produce protons in blood, which can dissolve the CaCO3 to release many bubbles. The results showed that such self-propelling particles could travel upstream to the deep site of wound, and stopped the bleeding of liver and femoral arteries effectively (Fig. 4d) [160].
Fig. 4.
(a) Schematic illustration and SEM images of the CS-CaP and PDA@CS-CaP. Reproduced with permission from Ref. [156]. Copyright 2019, Elsevier. (b) Schematic synthesis process of Pro-MAS, and morphology characterization (SEM and TEM) images of FeOOH-1, 2 and 3. Reproduced with permission from Ref. [158]. Copyright 2021, Royal Society of Chemistry. (c) Typical SEM images of bionic red blood cell-like microspheres. Reproduced with permission from Ref. [159]. Copyright 2022, Elsevier. (d) Schematic illustration and SEM images of the MS and MSS@CaCO3. Reproduced with permission from Ref. [160]. Copyright 2020, John Wiley & Sons. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The functionalization of powder may be firstly achieved by drug carrying and delivery. Powder is an ideal carrier for its large surface area. Targeted delivery of thrombin has been accomplished by a magnetic field guided system (MS@Fe4-BT) which employed microporous starch particles as substrate and Fe3O4 as the driving system. Under the magnetic fields, the MS@Fe4-BT could go deep into narrow and bent wound channels in the liver and femoral arteries to achieve hemostasis instantly [161]. To facilitate the distribution of the drug at the bleeding site, magnetic navigation and gas propelling strategy were jointly used for delivering the drug into vase-type wounds. However, the acidic condition showed certain effect on wound healing, which needs to be further addressed [162]. Gelatinizable hemostatic powders have also been described. Bian and colleagues have designed an ultrafast self-gelling polyethyleneimine/polyacrylic acid/quaternized chitosan (PEI/PAA/QCS) powder, which showed a concentrating effect by adsorbing blood and form a physically crosslinked hydrogel within 4 s in situ. The powder-transformed hydrogel can firmly adhere to the wound surface and attain hemostasis in rat liver, heart and femoral arteries. Meanwhile, it also promoted the healing of full-thickness skin wounds [163]. This transformation allowed the material to combine the advantages of powder and hydrogel, conferring the self-gelling powder with a broad range of applications among hemostatic materials.
4.2. Sponge-type materials
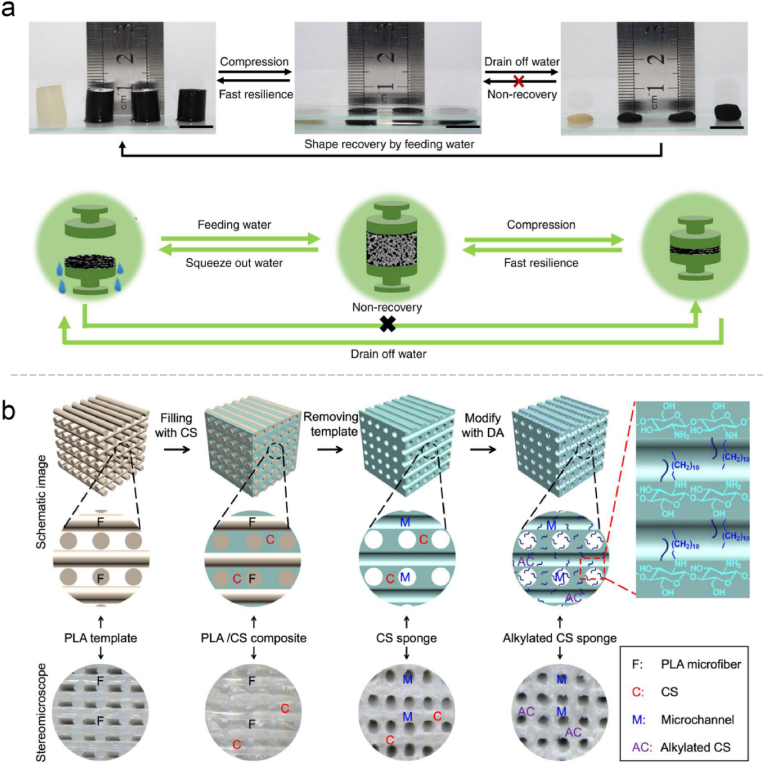
Sponge is one of the most common forms in hemostatic materials with typical structural features including high porosity and large surface area. For its excellent fluid absorption property, it can absorb the outgoing blood and concentrate the procoagulant substances to achieve the purpose of hemostasis [10,164]. Various methods have been used to prepare the sponge, including conventional foaming and freeze drying. But such methods are difficult to obtain a connected aperture to limit the blood flow [18]. The fabrication of cryogels can increase the pore connectivity. Typically, polymers are crosslinked around ice crystals, which conferred unique physical characteristics including large pores and interconnect networks, flexibility, and shape memory property [165]. Guo's group has designed a shape-recovery cryogel using carbon nanotube (CNT) and modified chitosan with enhanced mechanical properties, fast blood-triggered shape recovery, and high blood absorption capacity (Fig. 5a). Compared with gauze and gelatin sponge, it has shown superior ability for blood cells adhesion and activation [166]. Some have suggested that the hemostatic performance of the cryogels was exceeded the hydrogels due to its larger pore structure and swelling ratio with the same composition. This also showed the importance of the form of materials for hemostasis [74]. Moreover, 3D printing technology can ensure the connection of channels and is appropriate for design the parameters such as the size and angle. A micro-channel alkylated chitosan sponge (MACS) was developed and has shown remarkable water/blood absorption and rapid shape memory. By combining 3D-printed microfiber leaching, freeze-drying, and surface modification design, the pore structure of sponge may be customized, while chemical modification can also improve its hemostatic activity and antibacterial ability (Fig. 5b) [167].
Fig. 5.
(a) Fast resilience and macroscopical shape memory property of the cryogels, and schematic representation of the shape memory mechanism. Reproduced with permission from Ref. [166]. Copyright 2018, Springer Nature. (b) Schematic illustration and stereomicroscopic images of the fabrication process of microchannelled alkylated chitosan sponge. Reproduced with permission from Ref. [167]. Copyright 2021, Springer Nature.
Novel materials, such as metal-organic framework (MOF) and MXene, have also been designed and used to stop bleeding. For instance, zeolitic imidazolate framework (ZIF-8) was in situ grown into the chitin sponge to form composite sponge. ZIF-8, with the high porosity and large surface area, can absorb large amounts of water. Additionally, Zn2+ ions could rapidly release from ZIF-8 once exposed to blood, which activate the blood coagulation cascade reactions as outlined above [168]. In the same way, the hemostatic performance of chitin sponge was enhanced by combination with MXene, a booming two dimensional nanomaterials. The mechanism of MXene promoting hemostasis can be divided into two aspects: first, high hydrophilicity contributes to the concentration effect of the composite materials; Second, it contains rich oxygen-containing groups with negative charge, which can induce the internal pathway [169].
Sponge-type hemostatic materials have been developed to become multifunctional, such as pro-healing, antibacterial, postoperative cancer recurrence and so on [170]. The degradation and biosafety of the material are worth considering. However, some problems have remained to be solved. Hemostatic sponge can expand rapidly after absorbing large amount of water, putting pressure on surrounding tissues, which can result in poor blood flow and even compress the nerve in some sites. Furthermore, if sponges need to be removed after hemostasis, secondary damage and bleeding may occur.
4.3. Gauze-type materials
Gauze is an extremely widely used hemostatic material. To meet the requirement of higher emergency and clinical use, it is constantly modified and upgraded through various designs. As mentioned above, hydrophilic and hydrophobic balance can reinforce the hemostatic performance of gauze and avoid excessive blood absorption [23,119,124]. Moreover, the mineralization design of the gauze surface can improve its hemostatic ability. An efficient technique for biomimetic mineralized thrombin was explored, and the results showed that the mineralized thrombin-loaded gauze could stanch the bleeding more effectively compared with gauze only [171]. By taking an on-site template-free growth route, mesoporous single-crystal chabazite zeolite was grown on the surface of cotton fiber in situ to optimize the hemostatic performance, which could reduce the leaking of unfixed kaolin powder into the wound [172].
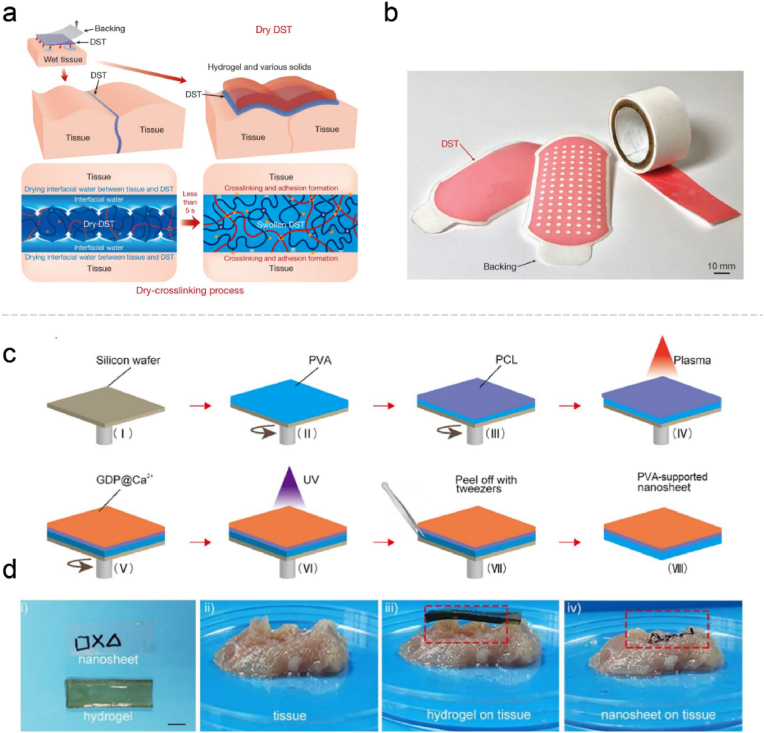
The gauze-type hemostatic materials are not limited to the upgradation and modification of traditional gauze, and more functional films have been designed demonstrating an effective role in hemostasis. Zhao et al. have reported a dry double-sided tape (DST), which could dry the tissue-DST interfacial water in less than 5 s, followed by covalently crosslinking with the amine groups in tissues to accomplish wet adhesion (Fig. 6a and b). The DST worked well in sealing the porcine stomach, lung and heart, and attaching wearable and implantable devices to wet tissues [173]. A series of bio-adhesive polymer films containing commercially made biopolymers (gelatin, sodium alginate or chitosan) and other synthetic polymers were designed, featuring liver and artery hemostasis capacity and real-time physiological monitoring as human-electronic interface material [174]. Moreover, the gauze type also facilitates drug delivery to the wound. For example, a bio-adhesive GelMA/polyurethane patch can not only reduce liver bleeding by approximately 75%, but also facilitate the wound healing with the supplement of l-arginine, which could produce nitric oxygen and ornithine [175].
Fig. 6.
(a) The crosslinking mechanism for the DST integrates the drying of interfacial water by hydration and swelling of the dry DST, temporary crosslinking, and subsequent covalent crosslinking. (b) Various shapes of the DST (colored with a red food dye). Reproduced with permission from Ref. [173]. Copyright 2019, Springer Nature. (c) Preparation of PVA-supported multi-functional nanosheets. (d) The adhesive performances of the hydrogel and nanosheets on a surface with a complicated topography. Reproduced with permission from Ref. [177]. Copyright 2020, Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The physical properties of hemostatic films can also be adjusted in terms of water content, morphology and dimension. An interesting study has introduced pagoda-like hemostatic microneedle patches fabricated by a step-by-step mold replication. The microneedles are firmly fixed to the tissues by physical linkage, which is unaffected by the wet environment and convenient for drug delivery. Dodecyl modified chitosan coating of the microneedles can capture the erythrocytes to promote coagulation [176]. However, such microneedles will require to be pressed to embed into the tissue, which posed a risk to the fragile organs. Furthermore, the nanoscale design also endows different properties to the films. Xuan and coworkers have prepared a flexible nanosheet (approximately 77 nm in thickness) comprising two layers, one is the antibacterial and hemostatic modified gelatin and the another is polycaprolactone with mechanical reinforcement. Compared with bulk materials, the nanosheet with enhanced flexibility could better suit the complicated morphologies via noncovalent interactions such as Van der Waals and hydrogen bonds (Fig. 6c and d) [177].
4.4. Hydrogel-type materials
Hydrogel possessed a three-dimensional network structure which can simulate the ECM of the tissue and is broadly used in biomedicine. The premise of hydrogel hemostasis is to exert bio-adhesion through physical interaction and/or chemical bond [28,109,178]. Following hemostasis, the hydrogel generally dispense with removal and could pro-healing with reasonable design. Many bio-adhesive strategies have been designed, which mainly included mussel inspiration, Schiff base and NHS ester. Many studies have proven that the hydrogels can control errhysis and simple bleeding wounds in liver, kidney and skin and so on [179].
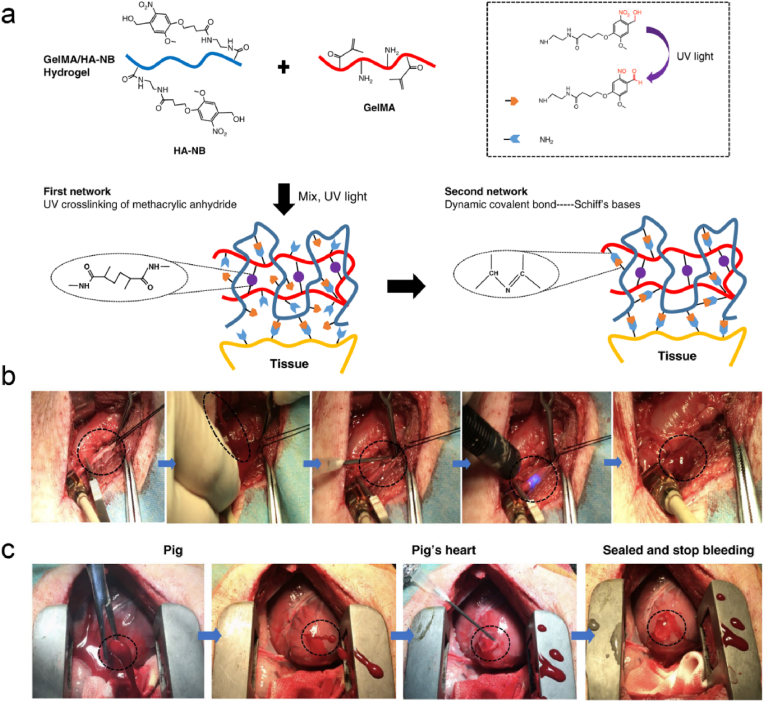
Confronted with severe arteriovenous and cardiac bleeding, most hydrogels will fail to work due to the excessive bleeding and rapid pressurized blood flows, thereby new bio-adhesion strategies and materials need to be developed. Hemostatic materials based on photocuring strategy have attracted increasing attention for their adjustable properties, easy use and shape adaptation ability. Hong et al. have designed an ECM-mimic photoreactive adhesive (GelMA/HA-NB) comprised of methacrylated gelatin and modified hyaluronic acid, which could stop high-pressure bleeding in pig carotid arteries and penetrated heart. Through convenient injection and UV-crosslinking, the GelMA/HA-NB adhesive could rapidly form hydrogel and firmly adhered to the target tissue in an environment full of blood. This effective wet-adhesion property is attributed to the photo-generated aldehyde groups and hydrogel cohesion (Fig. 7) [180]. Furthermore, a borax system was designed to adjust the gelation rate of the amidation reaction. The gelation time can be adjusted from minutes to seconds with different borax concentrations without altering the physical properties of the hydrogel itself. Rapid closure of a penetrating injury to the left ventricle was achieved in a rabbit model [181]. Therefore, rapid adhesion to the bleeding surface and maintenance of matrix stability are key factors for successful hemostasis in the face of the challenging bleeding scenarios.
Fig. 7.
(a) Constituent chemical structures and schematic diagram illustrating the formation of photo-triggered imine-crosslinked matrix hydrogel. Hemostatic properties of the matrix gel in a pig (b) carotid artery damage and (c) cardiac puncture injury model. Reproduced with permission from Ref. [180]. Copyright 2019, Springer Nature.
In recent years, adhesive hydrogels targeting gastrointestinal (GI) bleeding have also been developed [182,183]. Besides hemostasis, post-hemostatic managements, e.g., anti-infection, healing and fibrosis prevention of GI diseases are equally important. For example, a composite hydrogel containing hyaluronic acid and PEG has been designed and stabilized by thiourea-catechol coupling and disulfide bonds, which is unaffected by the pH value. Under an endoscope, the precursor was sprayed onto the wound and crosslinked with oxidant to gelatinize, with which the upper gastrointestinal hemostasis was accomplished in a pig model [184]. Hydrogel-type hemostats are considered to be more advantageous than powders for GI hemostasis. GI hemostasis will need to be assisted by endoscope, and powder materials are inconvenient for delivery and spraying. Moreover, the powders are easy to fall off due to the poor bio-adhesion, which may lead to failure of hemostasis, especially with peristalsis. The novel hydrogels should lay emphasis on adapting to the mechanical properties of tissues in order to achieve effective wet adhesion and an all-in-one design (e.g., hemostasis, antibacterial, antioxidant, promoting healing, and drug delivery).
4.5. Comparison of different forms of hemostatic materials
Various forms of hemostatic materials have been developed in recent years. In addition the abovementioned four forms, with the development and integration of medicine, material science, bioengineering and other disciplines, some novel forms of hemostatic materials have emerged [185]. Nevertheless, so far there is no uniform critique of which form is the best, and it shouldn't be. There is no best, only the most appropriate. Both powder and sponge have large surface area and good water absorption capacity, which enable them to concentrate the blood cells and clotting factors. Powder is suitable for irregular and deep wounds, but poor tissue adhesion and integrity make it prone to be washed away by blood flow. Partial powders may enter the blood circulation, increasing the risk of embolism [18]. Sponge, as a porous blocking material, can absorb blood and expand, exerting pressure on the surrounding tissues. However, for wounds with complex morphology, hemostasis will need to be achieved with the assistance of external force, which may incur some pain to the patients. Moreover, both hydrogel and gauze-type materials tend to achieve hemostasis through bio-adhesion and sealing. The hydrogels are particularly suitable for post-hemostasis management owing to their unique ECM-like structures, though the wet adhesion performance still needs to be improved [28]. Gauze-type hemostatic materials are easy to carry and use, but they are also restricted by wet adhesion. Generally speaking, during the process of hemostasis, the materials tend to fuse with the blood clot, and if it need to be removed, it is easy to cause secondary bleeding [186]. Therefore, different types could be combined to achieve a better hemostatic property.
5. Conclusion and outlooks
In last decades, we has witnessed a rapid advance in biopolymer-based hemostatic materials. Polysaccharides and polypeptides have both shown hemostatic activities, and their physical properties and bioactivities may be improved through chemical modifications. Although various molecular structures and forms have been designed, some limitations still exist with such hemostatic materials as follows:
-
i)
How the materials affect the hemostatic process needs to be further explored, despite many studies have shown the excellent hemostatic effect.
-
ii)
If the material needs to be removed, what is the ultimate in vivo destination of it after the hemostasis?
-
iii)
The clinical transformation of the hemostatic materials falls behind their basic researches. Generally speaking, materials with simple and essential components are more advantageous for transformation, but this has made it more necessary to design them thoughtfully.
The trends for the development of biopolymer-based hemostatic materials concentrating on their chemistry design, advanced forms and broader applications:
-
i)
Procoagulant activity Design:
With the integration of material science and biomedicine, multifarious materials with procoagulant activity have been developed. How hemostasis materials participate in and regulate the physiological hemostasis process should be consideration for their design. In addition, materials mimicking the physiological components of hemostasis, like platelet-mimicking procoagulant nanoparticles [187] and synthetic platelet-like particles [188], have also emerged.
-
ii)
Multi-Form Design:
The strength and weaknesses of various forms of hemostatic materials have been described as above. Firstly, hybrid materials such as combined inorganic and polymer materials or particles and gauzes have exhibited remarkable synergistic effect. Secondly, the transformable hemostatic materials is eye-catching. For instance, the powder-to-hydrogel materials could simultaneously absorb the water in blood and promote the wound healing, and the liquid-to-hydrogel materials can adapt to irregular shape and firmly adhere to the wound.
-
iii)
Inside-out Design:
In the battlefield and clinical settings, bleeding sites may be scattered and hidden, for which current hemostatic materials cannot precisely achieve hemostasis. For this, more smart hemostatic materials need to be designed to target the bleeding sites, achieving inside-out hemostasis.
-
iv)
Prophylactic Application Design:
War and many diseases will increase the risk of bleeding, and surgical procedures also increase the chance present of bleeding due to underlying factors. Such bleeding is like a “time bomb” with uncertainty and danger, and to prevent or reduce the bleeding tendency is necessary. Using micro/nano-materials, to intervene at specific sites which may appear early in bleeding to inhibit further exacerbation may be a potential approach.
-
v)
Multifunction Design:
Hemostasis is no longer the sole ultimate goal. The design of hemostatic materials has become more intelligent and increasingly focused on post-hemostasis management, e.g., tissue regeneration, detection, drug delivery, etc. For instance, after hemostasis, the material should facilitate in situ tissue regeneration, bringing great convenience to surgeons and patients. Moreover, through rational design and interdisciplinarity, the material can monitor key physiological parameters such as the pH value, blood pressure, blood flow rate, etc.
In summary, for the design of hemostatic materials, more attention should be paid to their molecular structures and forms. The interaction between the materials and the coagulation process should be explored in-depth. It is expected that hemostatic materials with proper designs can be developed from bench to bed in a short run.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work has been jointly supported by the National Natural Science Foundation of China (No. 32171351), the “1.3.5” Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC18002), and Med-X Innovation Programme of Med-X Center for Materials, Sichuan University (No. MCM202104). Some elements in the figures are created through BioRender.
Data availability
Data will be made available on request.
References
- 1.Albadawi H., Altun I., Hu J., Zhang Z., Panda A., Kim H.J., Khademhosseini A., Oklu R. Nanocomposite hydrogel with tantalum microparticles for rapid endovascular hemostasis. Adv. Sci. 2020;8(1) doi: 10.1002/advs.202003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pourshahrestani S., Kadri N.A., Zeimaran E., Towler M.R. Well-ordered mesoporous silica and bioactive glasses: promise for improved hemostasis. Biomater. Sci. 2018;7(1):31–50. doi: 10.1039/c8bm01041b. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y., Feng L., Zhang Y., He L., Wang C., Xu J., Wu J., Kirk T.B., Guo R., Xue W. Hemostasis mechanism and applications of N-alkylated chitosan sponge. Polym. Adv. Technol. 2017;28(9):1107–1114. doi: 10.1002/pat.4003. [DOI] [Google Scholar]
- 4.Zwinkels R.L.J., Endeman H., Hoeks S.E., de Maat M.P.M., den Hartog D., Stolker R.J. The clinical effect of hemostatic resuscitation in traumatic hemorrhage; a before-after study. J. Crit. Care. 2020;56:288–293. doi: 10.1016/j.jcrc.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Behrens A.M., Sikorski M.J., Kofinas P. Hemostatic strategies for traumatic and surgical bleeding. J. Biomed. Mater. Res. 2014;102(11):4182–4194. doi: 10.1002/jbm.a.35052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruen R.L., Brohi K., Schreiber M., Balogh Z.J., Pitt V., Narayan M., Maier R.V. Haemorrhage control in severely injured patients. Lancet. 2012;380(9847):1099–1108. doi: 10.1016/s0140-6736(12)61224-0. [DOI] [PubMed] [Google Scholar]
- 7.Muir V.G., Burdick J.A. Chemically modified biopolymers for the formation of biomedical hydrogels. Chem. Rev. 2021;121(18):10908–10949. doi: 10.1021/acs.chemrev.0c00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsi K., Kang M., Yang A., Kossard S. Granuloma formation following cyanoacrylate glue injection in peripheral veins and arteriovenous malformation. Phlebology. 2020;35(2):115–123. doi: 10.1177/0268355519856756. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., You X., Dai C., Tong T., Wu J. Hemostatic nanotechnologies for external and internal hemorrhage management. Biomater. Sci. 2020;8(16):4396–4412. doi: 10.1039/d0bm00781a. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., Wu L., Li P., Hao X., Yang X., Xi G., Liu W., Feng Y., He H., Shi C. Polysaccharide based hemostatic strategy for ultrarapid hemostasis. Macromol. Biosci. 2020;20(4) doi: 10.1002/mabi.201900370. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh S., Tripathi A., Gayen P., Sinha Roy R. Peptide-based topical agents and intravenous hemostat for rapid hemostasis. RSC Med. Chem. 2020;11(10):1100–1111. doi: 10.1039/d0md00204f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J., Liu J., Li M., Liu Z., Wang X., Zhang L., Wang Z. Hydrogel-based biomaterials engineered from natural-derived polysaccharides and proteins for hemostasis and wound healing. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.780187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sultan M.T., Hong H., Lee O.J., Ajiteru O., Lee Y.J., Lee J.S., Lee H., Kim S.H., Park C.H. Silk fibroin-based biomaterials for hemostatic applications. Biomolecules. 2022;12(5):660. doi: 10.3390/biom12050660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik A R.F., Shah K.U., Naz S.S., Qaisar S. Hemostatic strategies for uncontrolled bleeding: a comprehensive update. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109(10):1465–1477. doi: 10.1002/jbm.b.34806. [DOI] [PubMed] [Google Scholar]
- 15.Sung Y.K., Lee D.R., Chung D.J. Advances in the development of hemostatic biomaterials for medical application. Biomater. Res. 2021;25(1):37. doi: 10.1186/s40824-021-00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergmeier W., Hynes R.O. Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring Harbor Perspect. Biol. 2012;4(2):a005132. doi: 10.1101/cshperspect.a005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Versteeg H.H., Heemskerk J.W., Levi M., Reitsma P.H. New fundamentals in hemostasis. Physiol. Rev. 2013;93(1):327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 18.Guo B., Dong R., Liang Y., Li M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021;5(11):773–791. doi: 10.1038/s41570-021-00323-z. [DOI] [PubMed] [Google Scholar]
- 19.Pourshahrestani S., Zeimaran E., Kadri N.A., Mutlu N., Boccaccini A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthc. Mater. 2020;9(20) doi: 10.1002/adhm.202000905. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X., Liang Y., Guo B., Yin Z., Zhu D., Han Y. Injectable dry cryogels with excellent blood-sucking expansion and blood clotting to cease hemorrhage for lethal deep-wounds, coagulopathy and tissue regeneration. Chem. Eng. J. 2021;403 doi: 10.1016/j.cej.2020.126329. [DOI] [Google Scholar]
- 21.Bennett B.L., Littlejohn L. Review of new topical hemostatic dressings for combat casualty care. Mil. Med. 2014;179(5):497–514. doi: 10.7205/MILMED-D-13-00199. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.D., Hong S.L., Kim M.J., Kim J.Y., Kim Y.W., Koo S.K., Cho K.S. Effectiveness of hemostatic gelatin sponge as a packing material after septoplasty: a prospective, randomized, multicenter study. Auris Nasus Larynx. 2018;45(2):286–290. doi: 10.1016/j.anl.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Zhu T., Wu J., Zhao N., Cai C., Qian Z., Si F., Luo H., Guo J., Lai X., Shao L., Xu J. Superhydrophobic/Superhydrophilic Janus fabrics reducing blood loss. Adv. Healthc. Mater. 2018;7(7) doi: 10.1002/adhm.201701086. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V.A., Wickremasinghe N.C., Shi S., Hartgerink J.D. Nanofibrous snake venom hemostat. ACS Biomater. Sci. Eng. 2015;1(12):1300–1305. doi: 10.1021/acsbiomaterials.5b00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bal-Ozturk A., Cecen B., Avci-Adali M., Topkaya S.N., Alarcin E., Yasayan G., Ethan Y.C., Bulkurcuoglu B., Akpek A., Avci H., Shi K., Shin S.R., Hassan S. Tissue adhesives: from research to clinical translation. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X.Y., Sun A., Li T., Qian Y.J., Qian H., Ling Y.F., Zhang L.H., Liu Q.Y., Peng T., Qian Z. Mussel-inspired antimicrobial gelatin/chitosan tissue adhesive rapidly activated in situ by H2O2/ascorbic acid for infected wound closure, Carbohydr. Polymer. 2020;247 doi: 10.1016/j.carbpol.2020.116692. [DOI] [PubMed] [Google Scholar]
- 27.Taboada G.M., Yang K., Pereira M.J.N., Liu S.S., Hu Y., Karp J.M., Artzi N., Lee Y. Overcoming the translational barriers of tissue adhesives. Nat. Rev. Mater. 2020;5(4):310–329. doi: 10.1038/s41578-019-0171-7. [DOI] [Google Scholar]
- 28.Nam S., Mooney D. Polymeric tissue adhesives. Chem. Rev. 2021;121(18):11336–11384. doi: 10.1021/acs.chemrev.0c00798. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Z., Bian S., Li Z., Zhang Z., Liu Y., Zhai X., Pan H., Zhao X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020;249 doi: 10.1016/j.carbpol.2020.116826. [DOI] [PubMed] [Google Scholar]
- 30.de la Harpe K.M., Kondiah P.P.D., Choonara Y.E., Marimuthu T., du Toit L.C., Pillay V. The hemocompatibility of nanoparticles: a review of cell-nanoparticle interactions and hemostasis. Cells. 2019;8(10):1209. doi: 10.3390/cells8101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng C., Zeng Q., Pimpi S., Wu W., Han K., Dong K., Lu T. Research status and development potential of composite hemostatic materials. J. Mater. Chem. B. 2020;8(25):5395–5410. doi: 10.1039/d0tb00906g. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J., Feng S., Han S., Zhang X., Chen Y., Zhou X., Wang R., Li X., Hu H., Zhang J. Facile assembly of cost-effective and locally applicable or injectable nanohemostats for hemorrhage control. ACS Nano. 2016;10(11):9957–9973. doi: 10.1021/acsnano.6b04124. [DOI] [PubMed] [Google Scholar]
- 33.Che C., Liu L., Wang X., Zhang X., Luan S., Yin J., Li X., Shi H. Surface-adaptive and on-demand antibacterial sponge for synergistic rapid hemostasis and wound disinfection. ACS Biomater. Sci. Eng. 2020;6(3):1776–1786. doi: 10.1021/acsbiomaterials.0c00069. [DOI] [PubMed] [Google Scholar]
- 34.Pourshahrestani S., Zeimaran E., Djordjevic I., Kadri N.A., Towler M.R. Inorganic hemostats: the state-of-the-art and recent advances. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;58:1255–1268. doi: 10.1016/j.msec.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Sang Y., Roest M., de Laat B., de Groot P.G., Huskens D. Interplay between platelets and coagulation. Blood Rev. 2021;46 doi: 10.1016/j.blre.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H., Zhu Y., Zhang L., Liu H., Liu C., Zhang B., Song Y., Hu Y., Pang Z. Nanoplateletsomes for rapid hemostasis performance. Chin. Chem. Lett. 2022;33(6):2937–2941. doi: 10.1016/j.cclet.2021.12.091. [DOI] [Google Scholar]
- 37.Modery-Pawlowski C.L., Tian L.L., Ravikumar M., Wong T.L., Sen Gupta A. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials. 2013;34(12):3031–3041. doi: 10.1016/j.biomaterials.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 38.Kerris E.W.J., Hoptay C., Calderon T., Freishtat R.J. Platelets and platelet extracellular vesicles in hemostasis and sepsis. J. Invest. Med. 2020;68(4):813–820. doi: 10.1136/jim-2019-001195. [DOI] [PubMed] [Google Scholar]
- 39.Xiao L., Ma Y., Crawford R., Mendhi J., Zhang Y., Lu H., Zhao Q., Cao J., Wu C., Wang X., Xiao Y. The interplay between hemostasis and immune response in biomaterial development for osteogenesis, Mater. Today Off. 2022;54:202–224. doi: 10.1016/j.mattod.2022.02.010. [DOI] [Google Scholar]
- 40.Weisel J.W., Litvinov R.I. Red blood cells: the forgotten player in hemostasis and thrombosis. J. Thromb. Haemostasis. 2019;17(2):271–282. doi: 10.1111/jth.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noubouossie D.F., Henderson M.W., Mooberry M., Ilich A., Ellsworth P., Piegore M., Skinner S.C., Pawlinski R., Welsby I., Renné T., Hoffman M., Monroe D.M., Key N.S. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood. 2020;135(10):755–765. doi: 10.1182/blood.2019001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alamin A.A. The role of red blood cells in hemostasis. Semin. Thromb. Hemost. 2021;47(1):26–31. doi: 10.1055/s-0040-1718889. [DOI] [PubMed] [Google Scholar]
- 43.Guo Y., Wang Y., Zhao X., Li X., Wang Q., Zhong W., Mequanint K., Zhan R., Xing M., Luo G. Snake extract-laden hemostatic bioadhesive gel cross-linked by visible light. Sci. Adv. 2021;7(29) doi: 10.1126/sciadv.abf9635. eabf9635. https://doi.org/doi:10.1126/sciadv.abf9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Litvinov R.I., Khismatullin R.R., Shakirova A.Z., Litvinov T.R., Nagaswami C., Peshkova A.D., Weisel J.W. Morphological signs of intravital contraction (retraction) of pulmonary thrombotic emboli. BioNanoScience. 2017;8(1):428–433. doi: 10.1007/s12668-017-0476-1. [DOI] [Google Scholar]
- 45.Li G., Quan K., Xu C., Deng B., Wang X. Synergy in thrombin-graphene sponge for improved hemostatic efficacy and facile utilization. Colloids Surf. B Biointerfaces. 2018;161:27–34. doi: 10.1016/j.colsurfb.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Beudert M., Gutmann M., Luhmann T., Meinel L. Fibrin sealants: challenges and solutions. ACS Biomater. Sci. Eng. 2022;8(6):2220–2231. doi: 10.1021/acsbiomaterials.1c01437. [DOI] [PubMed] [Google Scholar]
- 47.Girish A., Jolly K., Alsaadi N., de la Fuente M., Recchione A., An R., Disharoon D., Secunda Z., Raghunathan S., Luc N.F., Desai C., Knauss E., Han X., Hu K., Wang H., Sekhon U.D.S., Rohner N., Gurkan U.A., Nieman M., Neal M.D., Sen Gupta A. Platelet-inspired intravenous nanomedicine for injury-targeted direct delivery of thrombin to augment hemostasis in coagulopathies. ACS Nano. 2022 doi: 10.1021/acsnano.2c05306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts I., Ageron F.X. The role of tranexamic acid in trauma -- a life-saving drug with proven benefit. Nat. Rev. Dis. Prim. 2022;8(1):34. doi: 10.1038/s41572-022-00367-5. [DOI] [PubMed] [Google Scholar]
- 49.Su H., Wei S., Chen F., Cui R., Liu C. Tranexamic acid-loaded starch hemostatic microspheres. RSC Adv. 2019;9(11):6245–6253. doi: 10.1039/c8ra06662k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J., Han J., Sun Q., Wang Y., Mu Y., Zhang K., Dou X., Kong M., Chen X., Feng C. Biosynthetic calcium-doped biosilica with multiple hemostatic properties for hemorrhage control. J. Mater. Chem. B. 2018;6(47):7834–7841. doi: 10.1039/c8tb00667a. [DOI] [PubMed] [Google Scholar]
- 51.Chen F., Cao X., Chen X., Wei J., Liu C. Calcium-modified microporous starch with potent hemostatic efficiency and excellent degradability for hemorrhage control. J. Mater. Chem. B. 2015;3(19):4017–4026. doi: 10.1039/c5tb00250h. [DOI] [PubMed] [Google Scholar]
- 52.Chen J., Qiu L., Li Q., Ai J., Liu H., Chen Q. Rapid hemostasis accompanied by antibacterial action of calcium crosslinking tannic acid-coated mesoporous silica/silver Janus nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;123 doi: 10.1016/j.msec.2021.111958. [DOI] [PubMed] [Google Scholar]
- 53.Muthiah Pillai N.S., Eswar K., Amirthalingam S., Mony U., Kerala Varma P., Jayakumar R. Injectable nano whitlockite incorporated chitosan hydrogel for effective hemostasis. ACS Appl. Bio Mater. 2019;2(2):865–873. doi: 10.1021/acsabm.8b00710. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y., Pan N., Liu Y., Ren X. Novel porous chitosan/N-halamine structure with efficient antibacterial and hemostatic properties, Carbohydr. Polymer. 2021;253 doi: 10.1016/j.carbpol.2020.117205. [DOI] [PubMed] [Google Scholar]
- 55.Hamedi H., Moradi S., Hudson S.M., Tonelli A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: a review. Carbohydr. Polym. 2018;199:445–460. doi: 10.1016/j.carbpol.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 56.Xie H., Lucchesi L., Teach J.S., Virmani R. Long-term outcomes of a chitosan hemostatic dressing in laparoscopic partial nephrectomy. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100(2):432–436. doi: 10.1002/jbm.b.31966. [DOI] [PubMed] [Google Scholar]
- 57.Granville-Chapman J., Jacobs N., Midwinter M.J. Pre-hospital haemostatic dressings: a systematic review. Injury. 2011;42(5):447–459. doi: 10.1016/j.injury.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 58.Hu Z., Zhang D.Y., Lu S.T., Li P.W., Li S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs. 2018;16(8):273. doi: 10.3390/md16080273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan M.A., Mujahid M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019;124:138–147. doi: 10.1016/j.ijbiomac.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 60.Ma Y., Yao J., Liu Q., Han T., Zhao J., Ma X., Tong Y., Jin G., Qu K., Li B., Xu F. Liquid bandage harvests robust adhesive, hemostatic, and antibacterial performances as a first aid tissue adhesive. Adv. Funct. Mater. 2020;30(39):2001820. doi: 10.1002/adfm.202001820. [DOI] [Google Scholar]
- 61.Song F., Kong Y., Shao C., Cheng Y., Lu J., Tao Y., Du J., Wang H. Chitosan-based multifunctional flexible hemostatic bio-hydrogel. Acta Biomater. 2021;136:170–183. doi: 10.1016/j.actbio.2021.09.056. [DOI] [PubMed] [Google Scholar]
- 62.Chen J., Ai J., Chen S., Xu Z., Lin J., Liu H., Chen Q. Synergistic enhancement of hemostatic performance of mesoporous silica by hydrocaffeic acid and chitosan. Int. J. Biol. Macromol. 2019;139:1203–1211. doi: 10.1016/j.ijbiomac.2019.08.091. [DOI] [PubMed] [Google Scholar]
- 63.He Q., Gong K., Ao Q., Ma T., Yan Y., Gong Y., Zhang X. Positive charge of chitosan retards blood coagulation on chitosan films. J. Biomater. Appl. 2013;27(8):1032–1045. doi: 10.1177/0885328211432487. [DOI] [PubMed] [Google Scholar]
- 64.Deng Y., Chen J., Huang J., Yang X., Zhang X., Yuan S., Liao W. Preparation and characterization of cellulose/flaxseed gum composite hydrogel and its hemostatic and wound healing functions evaluation. Cellulose. 2020;27(7):3971–3988. doi: 10.1007/s10570-020-03055-3. [DOI] [Google Scholar]
- 65.Habibi Y., Lucia L.A., Rojas O.J. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem. Rev. 2010;110(6):3479–3500. doi: 10.1021/cr900339w. [DOI] [PubMed] [Google Scholar]
- 66.Aydemir Sezer U., Sahin I., Aru B., Olmez H., Yanikkaya Demirel G., Sezer S. Cytotoxicity, bactericidal and hemostatic evaluation of oxidized cellulose microparticles: structure and oxidation degree approach. Carbohydr. Polym. 2019;219:87–94. doi: 10.1016/j.carbpol.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Zhang S., Li J., Chen S., Zhang X., Ma J., He J. Oxidized cellulose-based hemostatic materials. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115585. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y., Zhao Y., Qiao L., Zou F., Xie Y., Zheng Y., Chao Y., Yang Y., He W., Yang S. Cellulose fibers-reinforced self-expanding porous composite with multiple hemostatic efficacy and shape adaptability for uncontrollable massive hemorrhage treatment. Bioact. Mater. 2021;6(7):2089–2104. doi: 10.1016/j.bioactmat.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H., Cheng W., Liu K., Chen L., Huang Y., Wang X., Lv Z., He J., Li C. Reinforced collagen with oxidized microcrystalline cellulose shows improved hemostatic effects. Carbohydr. Polym. 2017;165:30–38. doi: 10.1016/j.carbpol.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 70.Cheng F., Liu C., Wei X., Yan T., Li H., He J., Huang Y. Preparation and characterization of 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO)-oxidized cellulose nanocrystal/alginate biodegradable composite dressing for hemostasis applications. ACS Sustain. Chem. Eng. 2017;5(5):3819–3828. doi: 10.1021/acssuschemeng.6b02849. [DOI] [Google Scholar]
- 71.Graca M.F.P., Miguel S.P., Cabral C.S.D., Correia I.J. Hyaluronic acid-based wound dressings: a review. Carbohydr. Polym. 2020;241 doi: 10.1016/j.carbpol.2020.116364. [DOI] [PubMed] [Google Scholar]
- 72.Fernando I.P.S., Lee W., Han E.J., Ahn G. Alginate-based nanomaterials: fabrication techniques, properties, and applications. Chem. Eng. J. 2020;391 doi: 10.1016/j.cej.2019.123823. [DOI] [Google Scholar]
- 73.Tang Q., Chen C., Jiang Y., Huang J., Liu Y., Nthumba P.M., Gu G., Wu X., Zhao Y., Ren J. Engineering an adhesive based on photosensitive polymer hydrogels and silver nanoparticles for wound healing. J. Mater. Chem. B. 2020;8(26):5756–5764. doi: 10.1039/D0TB00726A. [DOI] [PubMed] [Google Scholar]
- 74.Wang M., Hu J., Ou Y., He X., Wang Y., Zou C., Jiang Y., Luo F., Lu D., Li Z., Li J., Tan H. Shape-recoverable hyaluronic acid-waterborne polyurethane hybrid cryogel accelerates hemostasis and wound healing. ACS Appl. Mater. Interfaces. 2022;14(15):17093–17108. doi: 10.1021/acsami.2c01310. [DOI] [PubMed] [Google Scholar]
- 75.Ghimire S., Sarkar P., Rigby K., Maan A., Mukherjee S., Crawford K.E., Mukhopadhyay K. Polymeric materials for hemostatic wound healing. Pharmaceutics. 2021;13(12):2127. doi: 10.3390/pharmaceutics13122127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu X., Tang Z., Liao X., Wang Z., Liu H. Fabrication of chitosan@calcium alginate microspheres with porous core and compact shell, and application as a quick traumatic hemostat. Carbohydr. Polym. 2020;247 doi: 10.1016/j.carbpol.2020.116669. [DOI] [PubMed] [Google Scholar]
- 77.An S., Jeon E.J., Jeon J., Cho S.-W. A serotonin-modified hyaluronic acid hydrogel for multifunctional hemostatic adhesives inspired by a platelet coagulation mediator. Mater. Horiz. 2019;6(6):1169–1178. doi: 10.1039/c9mh00157c. [DOI] [Google Scholar]
- 78.Yan S., Wang W., Li X., Ren J., Yun W., Zhang K., Li G., Yin J. Preparation of mussel-inspired injectable hydrogels based on dual-functionalized alginate with improved adhesive, self-healing, and mechanical properties. J. Mater. Chem. B. 2018;6(40):6377–6390. doi: 10.1039/c8tb01928b. [DOI] [PubMed] [Google Scholar]
- 79.Yang H., Song L., Zou Y., Sun D., Wang L., Yu Z., Guo J. Role of hyaluronic acids and potential as regenerative biomaterials in wound healing. ACS Appl. Bio Mater. 2021;4(1):311–324. doi: 10.1021/acsabm.0c01364. [DOI] [PubMed] [Google Scholar]
- 80.Manon-Jensen T., Kjeld N.G., Karsdal M.A. Collagen-mediated hemostasis. J. Thromb. Haemostasis. 2016;14(3):438–448. doi: 10.1111/jth.13249. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y., Gallant R.C., Ni H. Extracellular matrix proteins in the regulation of thrombus formation. Curr. Opin. Hematol. 2016;23(3):280–287. doi: 10.1097/MOH.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 82.He Y., Wang J., Si Y., Wang X., Deng H., Sheng Z., Li Y., Liu J., Zhao J. A novel gene recombinant collagen hemostatic sponge with excellent biocompatibility and hemostatic effect. Int. J. Biol. Macromol. 2021;178:296–305. doi: 10.1016/j.ijbiomac.2021.02.162. [DOI] [PubMed] [Google Scholar]
- 83.Kumar V.A., Taylor N.L., Jalan A.A., Hwang L.K., Wang B.K., Hartgerink J.D. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules. 2014;15(4):1484–1490. doi: 10.1021/bm500091e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han K., Bai Q., Wu W., Sun N., Cui N., Lu T. Gelatin-based adhesive hydrogel with self-healing, hemostasis, and electrical conductivity. Int. J. Biol. Macromol. 2021;183:2142–2151. doi: 10.1016/j.ijbiomac.2021.05.147. [DOI] [PubMed] [Google Scholar]
- 85.Ahmadian Z., Correia A., Hasany M., Figueiredo P., Dobakhti F., Eskandari M.R., Hosseini S.H., Abiri R., Khorshid S., Hirvonen J., Santos H.A., Shahbazi M.A. A hydrogen-bonded extracellular matrix-mimicking bactericidal hydrogel with radical scavenging and hemostatic function for pH-responsive wound healing acceleration. Adv. Healthc. Mater. 2021;10(3) doi: 10.1002/adhm.202001122. [DOI] [PubMed] [Google Scholar]
- 86.Lan G., Li Q., Lu F., Yu K., Lu B., Bao R., Dai F. Improvement of platelet aggregation and rapid induction of hemostasis in chitosan dressing using silver nanoparticles. Cellulose. 2020;27(1):385–400. doi: 10.1007/s10570-019-02795-1. [DOI] [Google Scholar]
- 87.di Lena F. Hemostatic polymers: the concept, state of the art and perspectives. J. Mater. Chem. B. 2014;2(23):3567–3577. doi: 10.1039/c3tb21739f. [DOI] [PubMed] [Google Scholar]
- 88.Chen Z., Wu H., Wang H., Zaldivar-Silva D., Aguero L., Liu Y., Zhang Z., Yin Y., Qiu B., Zhao J., Lu X., Wang S. An injectable anti-microbial and adhesive hydrogel for the effective noncompressible visceral hemostasis and wound repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;129 doi: 10.1016/j.msec.2021.112422. [DOI] [PubMed] [Google Scholar]
- 89.Chen K., Pan H., Yan Z., Li Y., Ji D., Yun K., Su Y., Liu D., Pan W. A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int. J. Biol. Macromol. 2021;182:1339–1350. doi: 10.1016/j.ijbiomac.2021.05.074. [DOI] [PubMed] [Google Scholar]
- 90.Tan J., Zhang Q.Y., Huang L.P., Huang K., Xie H.Q. Decellularized scaffold and its elicited immune response towards the host: the underlying mechanism and means of immunomodulatory modification. Biomater. Sci. 2021;9(14):4803–4820. doi: 10.1039/d1bm00470k. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X., Chen X., Hong H., Hu R., Liu J., Liu C. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z., Yang Z., Jiang J., Shi Z., Mao Y., Qin N., Tao T.H. Silk microneedle patch capable of on-demand multidrug delivery to the brain for glioblastoma treatment. Adv. Mater. 2022;34(1) doi: 10.1002/adma.202106606. [DOI] [PubMed] [Google Scholar]
- 93.Montazerian H., Davoodi E., Baidya A., Baghdasarian S., Sarikhani E., Meyer C.E., Haghniaz R., Badv M., Annabi N., Khademhosseini A., Weiss P.S. Engineered hemostatic biomaterials for sealing wounds. Chem. Rev. 2022;122(15):12864–12903. doi: 10.1021/acs.chemrev.1c01015. [DOI] [PubMed] [Google Scholar]
- 94.Yan S., Han G., Wang Q., Zhang S., You R., Luo Z., Xu A., Li X., Li M., Zhang Q., Kaplan D.L. Directed assembly of robust and biocompatible silk fibroin/hyaluronic acid composite hydrogels. Compos. B Eng. 2019;176 doi: 10.1016/j.compositesb.2019.107204. [DOI] [Google Scholar]
- 95.Qiao Z., Lv X., He S., Bai S., Liu X., Hou L., He J., Tong D., Ruan R., Zhang J., Ding J., Yang H. A mussel-inspired supramolecular hydrogel with robust tissue anchor for rapid hemostasis of arterial and visceral bleedings. Bioact. Mater. 2021;6(9):2829–2840. doi: 10.1016/j.bioactmat.2021.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang X., Fu Q., Deng Y., Wang F., Xia B., Chen Z., Chen G. Surface roughness of silk fibroin/alginate microspheres for rapid hemostasis in vitro and in vivo. Carbohydr. Polym. 2021;253 doi: 10.1016/j.carbpol.2020.117256. [DOI] [PubMed] [Google Scholar]
- 97.Yu Y., Li P., Zhu C., Ning N., Zhang S., Vancso G.J. Multifunctional and recyclable photothermally responsive cryogels as efficient platforms for wound healing. Adv. Funct. Mater. 2019;29(35) doi: 10.1002/adfm.201904402. [DOI] [Google Scholar]
- 98.Wei W., Liu J., Peng Z., Liang M., Wang Y., Wang X. Gellable silk fibroin-polyethylene sponge for hemostasis. Artif. Cell Nanomed. Biotechnol. 2020;48(1):28–36. doi: 10.1080/21691401.2019.1699805. [DOI] [PubMed] [Google Scholar]
- 99.Wang D., Li W., Wang Y., Yin H., Ding Y., Ji J., Wang B., Hao S. Fabrication of an expandable keratin sponge for improved hemostasis in a penetrating trauma. Colloids Surf. B Biointerfaces. 2019;182 doi: 10.1016/j.colsurfb.2019.110367. [DOI] [PubMed] [Google Scholar]
- 100.Sun Z., Chen X., Ma X., Cui X., Yi Z., Li X. Cellulose/keratin-catechin nanocomposite hydrogel for wound hemostasis. J. Mater. Chem. B. 2018;6(38):6133–6141. doi: 10.1039/c8tb01109e. [DOI] [PubMed] [Google Scholar]
- 101.Luo T., Hao S., Chen X., Wang J., Yang Q., Wang Y., Weng Y., Wei H., Zhou J., Wang B. Development and assessment of kerateine nanoparticles for use as a hemostatic agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;63:352–358. doi: 10.1016/j.msec.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 102.Burnett L.R., Richter J.G., Rahmany M.B., Soler R., Steen J.A., Orlando G., Abouswareb T., Van Dyke M.E. Novel keratin (KeraStat™) and polyurethane (Nanosan®-Sorb) biomaterials are hemostatic in a porcine lethal extremity hemorrhage model. J. Biomater. Appl. 2014;28(6):869–879. doi: 10.1177/0885328213484975. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y., Xu Y., Zhang Z., He Y., Hou Z., Zhao Z., Deng J., Qing R., Wang B., Hao S. Rational design of high-performance keratin-based hemostatic agents. Adv. Healthc. Mater. 2022;11(15) doi: 10.1002/adhm.202200290. [DOI] [PubMed] [Google Scholar]
- 104.Guo T., Li W., Wang J., Luo T., Lou D., Wang B., Hao S. Recombinant human hair keratin proteins for halting bleeding. Artif. Cell Nanomed. Biotechnol. 2018;46(2):456–461. doi: 10.1080/21691401.2018.1459633. [DOI] [PubMed] [Google Scholar]
- 105.Liang Y., Li Z., Huang Y., Yu R., Guo B. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano. 2021;15(4):7078–7093. doi: 10.1021/acsnano.1c00204. [DOI] [PubMed] [Google Scholar]
- 106.Shin M., Park S.G., Oh B.C., Kim K., Jo S., Lee M.S., Oh S.S., Hong S.H., Shin E.C., Kim K.S., Kang S.W., Lee H. Complete prevention of blood loss with self-sealing haemostatic needles. Nat. Mater. 2017;16(1):147–152. doi: 10.1038/NMAT4758. [DOI] [PubMed] [Google Scholar]
- 107.Fan X., Wang S., Fang Y., Li P., Zhou W., Wang Z., Chen M., Liu H. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;109 doi: 10.1016/j.msec.2020.110649. [DOI] [PubMed] [Google Scholar]
- 108.Zhang W., Wang R., Sun Z., Zhu X., Zhao Q., Zhang T., Cholewinski A., Yang F.K., Zhao B., Pinnaratip R., Forooshani P.K., Lee B.P. Catechol-functionalized hydrogels: biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020;49(2):433–464. doi: 10.1039/c9cs00285e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cui C., Liu W. Recent advances in wet adhesives: adhesion mechanism, design principle and applications. Prog. Polym. Sci. 2021;116 doi: 10.1016/j.progpolymsci.2021.101388. [DOI] [Google Scholar]
- 110.Ma Z., Bourquard C., Gao Q., Jiang S., De Iure-Grimmel T., Huo R., Li X., He Z., Yang Z., Yang G., Wang Y., Lam E., Gao Z.-h., Supponen O., Li J. Controlled tough bioadhesion mediated by ultrasound. Science. 2022;377(6607):751–755. doi: 10.1126/science.abn8699. https://doi.org/doi:10.1126/science.abn8699. [DOI] [PubMed] [Google Scholar]
- 111.Qu J., Zhao X., Liang Y., Zhang T., Ma P.X., Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 112.Zhou L., Dai C., Fan L., Jiang Y., Liu C., Zhou Z., Guan P., Tian Y., Xing J., Li X., Luo Y., Yu P., Ning C., Tan G. Injectable self healing natural biopolymer based hydrogel adhesive with thermoresponsive reversible adhesion for minimally invasive surgery. Adv. Funct. Mater. 2021;31(14):2007457. doi: 10.1002/adfm.202007457. [DOI] [Google Scholar]
- 113.Zhang W., Bao B., Jiang F., Zhang Y., Zhou R., Lu Y., Lin S., Lin Q., Jiang X., Zhu L. Promoting oral mucosal wound healing with a hydrogel aAdhesive based on a phototriggered S-nitrosylation coupling reaction. Adv. Mater. 2021;33(48) doi: 10.1002/adma.202105667. [DOI] [PubMed] [Google Scholar]
- 114.Wu J., Yuk H., Sarrafian T.L., Guo C.F., Griffiths L.G., Nabzdyk C.S., Zhao X. An off-the-shelf bioadhesive patch for sutureless repair of gastrointestinal defects. Sci. Transl. Med. 2022;14(630) doi: 10.1126/scitranslmed.abh2857. eabh2857. [DOI] [PubMed] [Google Scholar]
- 115.Wang L., Zhang X., Yang K., Fu Y.V., Xu T., Li S., Zhang D., Wang L.N., Lee C.S. A novel double crosslinking double network design for injectable hydrogels with enhanced tissue adhesion and antibacterial capability for wound treatment. Adv. Funct. Mater. 2019;30(1) doi: 10.1002/adfm.201904156. [DOI] [Google Scholar]
- 116.Zou C.-Y., Lei X.-X., Hu J.-J., Jiang Y.-L., Li Q.-J., Song Y.-T., Zhang Q.-Y., Li-Ling, Xie H.-Q. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact. Mater. 2022;16:388–402. doi: 10.1016/j.bioactmat.2022.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quan K., Li G., Luan D., Yuan Q., Tao L., Wang X. Black hemostatic sponge based on facile prepared cross-linked graphene. Colloids Surf. B Biointerfaces. 2015;132:27–33. doi: 10.1016/j.colsurfb.2015.04.067. [DOI] [PubMed] [Google Scholar]
- 118.Sasaki K., Tenjimbayashi M., Manabe K., Shiratori S. Asymmetric superhydrophobic/superhydrophilic cotton fabrics designed by spraying polymer and nanoparticles. ACS Appl. Mater. Interfaces. 2016;8(1):651–659. doi: 10.1021/acsami.5b09782. [DOI] [PubMed] [Google Scholar]
- 119.Chaturvedi A., Dowling M.B., Gustin J.P., Scalea T.M., Raghavan S.R., Pasley J.D., Narayan M. Hydrophobically modified chitosan gauze: a novel topical hemostat. J. Surg. Res. 2017;207:45–52. doi: 10.1016/j.jss.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 120.Li Z., Milionis A., Zheng Y., Yee M., Codispoti L., Tan F., Poulikakos D., Yap C.H. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 2019;10(1):5562. doi: 10.1038/s41467-019-13512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y., Niu H., Wang C., Yang X., Li W., Zhang Y., Ma X., Xu Y., Zheng P., Wang J., Dai K. Bio-inspired, bio-degradable adenosine 5'-diphosphate-modified hyaluronic acid coordinated hydrophobic undecanal-modified chitosan for hemostasis and wound healing. Bioact. Mater. 2022;17:162–177. doi: 10.1016/j.bioactmat.2022.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng H., Xiao D., Tang Y., Wang B., Feng X., Lu M., Vancso G.J., Sui X. Sponges with Janus character from nanocellulose: preparation and applications in the treatment of hemorrhagic wounds. Adv. Healthc. Mater. 2020;9(17) doi: 10.1002/adhm.201901796. [DOI] [PubMed] [Google Scholar]
- 123.Cui C., Wu T., Chen X., Liu Y., Li Y., Xu Z., Fan C., Liu W. A Janus hydrogel wet adhesive for internal tissue repair and anti postoperative adhesion. Adv. Funct. Mater. 2020;30(49):2005689. doi: 10.1002/adfm.202005689. [DOI] [Google Scholar]
- 124.He H., Zhou W., Gao J., Wang F., Wang S., Fang Y., Gao Y., Chen W., Zhang W., Weng Y., Wang Z., Liu H. Efficient, biosafe and tissue adhesive hemostatic cotton gauze with controlled balance of hydrophilicity and hydrophobicity. Nat. Commun. 2022;13(1):552. doi: 10.1038/s41467-022-28209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen F., Cao X., Yu J., Su H., Wei S., Hong H., Liu C. Quaternary ammonium groups modified starch microspheres for instant hemorrhage control. Colloids Surf. B Biointerfaces. 2017;159:937–944. doi: 10.1016/j.colsurfb.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 126.Yin M., Wang Y., Zhang Y., Ren X., Qiu Y., Huang T.S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties, Carbohydr. Polymer. 2020;232 doi: 10.1016/j.carbpol.2019.115823. [DOI] [PubMed] [Google Scholar]
- 127.Zhu J., Han H., Li F., Wang X., Yu J., Qin X., Wu D. Peptide-functionalized amino acid-derived pseudoprotein-based hydrogel with hemorrhage control and antibacterial activity for wound healing. Chem. Mater. 2019;31(12):4436–4450. doi: 10.1021/acs.chemmater.9b00850. [DOI] [Google Scholar]
- 128.Wu B., Du F., W A., Li G., Wang X. Graphene-based hemostatic sponge. Chin. Chem. Lett. 2022;33(2):703–713. doi: 10.1016/j.cclet.2021.06.029. [DOI] [Google Scholar]
- 129.Liang Y., Zhao X., Hu T., Chen B., Yin Z., Ma P.X., Guo B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small. 2019;15(12) doi: 10.1002/smll.201900046. [DOI] [PubMed] [Google Scholar]
- 130.Li G., Liang Y., Xu C., Sun H., Tao L., Wei Y., Wang X. Polydopamine reinforced hemostasis of a graphene oxide sponge via enhanced platelet stimulation. Colloids Surf. B Biointerfaces. 2019;174:35–41. doi: 10.1016/j.colsurfb.2018.10.074. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y., Guan J., Wu J., Ding S., Yang J., Zhang J., Dong A., Deng L. N-alkylated chitosan/graphene oxide porous sponge for rapid and effective hemostasis in emergency situations. Carbohydr. Polym. 2019;219:405–413. doi: 10.1016/j.carbpol.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 132.Singh S.K., Singh M.K., Nayak M.K., Kumari S., Shrivastava S., Grácio J.J.A., Dash D. Thrombus inducing property of atomically thin graphene oxide sheets. ACS Nano. 2011;5(6):4987–4996. doi: 10.1021/nn201092p. [DOI] [PubMed] [Google Scholar]
- 133.Baker C.J., Smith S.A., Morrissey J.H. Polyphosphate in thrombosis, hemostasis, and inflammation. Res. Pract. Thromb. Haemost. 2019;3(1):18–25. doi: 10.1002/rth2.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choi S.H., Smith S.A., Morrissey J.H. Polyphosphate accelerates factor V activation by factor XIa, Thromb. Haemostasis. 2015;113(3):599–604. doi: 10.1160/TH14-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith S.A., Mutch N.J., Baskar D., Rohloff P., Docampo R., Morrissey J.H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA. 2006;103(4):903–908. doi: 10.1073/pnas.0507195103. https://doi.org/doi:10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gu J.T., Jiao K., Li J., Yan J.F., Wang K.Y., Wang F., Liu Y., Tay F.R., Chen J.H., Niu L.N. Polyphosphate-crosslinked collagen scaffolds for hemostasis and alveolar bone regeneration after tooth extraction. Bioact. Mater. 2022;15:68–81. doi: 10.1016/j.bioactmat.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu Z., Zhou W., Deng W., Xu C., Cai Y., Wang X. Antibacterial and hemostatic thiol-modified chitosan-immobilized AgNPs composite sponges. ACS Appl. Mater. Interfaces. 2020;12(18):20307–20320. doi: 10.1021/acsami.0c05430. [DOI] [PubMed] [Google Scholar]
- 138.Bhatia S.K., Arthur S.D., Chenault H.K., Figuly G.D., Kodokian G.K. Polysaccharide-based tissue adhesives for sealing corneal incisions. Curr. Eye Res. 2007;32(12):1045–1050. doi: 10.1080/02713680701767876. [DOI] [PubMed] [Google Scholar]
- 139.Liu C., Yao W., Tian M., Wei J., Song Q., Qiao W. Mussel-inspired degradable antibacterial polydopamine/silica nanoparticle for rapid hemostasis. Biomaterials. 2018;179:83–95. doi: 10.1016/j.biomaterials.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 140.Geng H., Dai Q., Sun H., Zhuang L., Song A., Caruso F., Hao J., Cui J. Injectable and sprayable polyphenol-based hydrogels for controlling hemostasis. ACS Appl. Bio Mater. 2020;3(2):1258–1266. doi: 10.1021/acsabm.9b01138. [DOI] [PubMed] [Google Scholar]
- 141.Wang C., Zhou H., Niu H., Ma X., Yuan Y., Hong H., Liu C. Tannic acid-loaded mesoporous silica for rapid hemostasis and antibacterial activity. Biomater. Sci. 2018;6(12):3318–3331. doi: 10.1039/c8bm00837j. [DOI] [PubMed] [Google Scholar]
- 142.Bigham A., Rahimkhoei V., Abasian P., Delfi M., Naderi J., Ghomi M., Dabbagh Moghaddam F., Waqar T., Nuri Ertas Y., Sharifi S., Rabiee N., Ersoy S., Maleki A., Nazarzadeh Zare E., Sharifi E., Jabbari E., Makvandi P., Akbari A. Advances in tannic acid-incorporated biomaterials: infection treatment, regenerative medicine, cancer therapy, and biosensing. Chem. Eng. J. 2022;432 doi: 10.1016/j.cej.2021.134146. [DOI] [Google Scholar]
- 143.Huang H., Xu R., Ni P., Zhang Z., Sun C., He H., Wang X., Zhang L., Liang Z., Liu H. Water-driven noninvasively detachable wet tissue adhesives for wound closure, Mater. Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dowling M.B., Kumar R., Keibler M.A., Hess J.R., Bochicchio G.V., Raghavan S.R. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials. 2011;32(13):3351–3357. doi: 10.1016/j.biomaterials.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 145.Chen G., Yu Y., Wu X., Wang G., Ren J., Zhao Y. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv. Funct. Mater. 2018;28(33) doi: 10.1002/adfm.201801386. [DOI] [Google Scholar]
- 146.Dalisson B., Barralet J. Bioinorganics and wound healing. Adv. Healthc. Mater. 2019;8(18) doi: 10.1002/adhm.201900764. [DOI] [PubMed] [Google Scholar]
- 147.Lipinski B., Pretorius E., Oberholzer H.M., van der Spuy W.J. Interaction of fibrin with red blood cells: the role of iron. Ultrastruct. Pathol. 2012;36(2):79–84. doi: 10.3109/01913123.2011.627491. [DOI] [PubMed] [Google Scholar]
- 148.Lv C., Li L., Jiao Z., Yan H., Wang Z., Wu Z., Guo M., Wang Y., Zhang P. Improved hemostatic effects by Fe(3+) modified biomimetic PLLA cotton-like mat via sodium alginate grafted with dopamine. Bioact. Mater. 2021;6(8):2346–2359. doi: 10.1016/j.bioactmat.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rao K.M., Suneetha M., Park G.T., Babu A.G., Han S.S. Hemostatic, biocompatible, and antibacterial non-animal fungal mushroom-based carboxymethyl chitosan-ZnO nanocomposite for wound-healing applications. Int. J. Biol. Macromol. 2020;155:71–80. doi: 10.1016/j.ijbiomac.2020.03.170. [DOI] [PubMed] [Google Scholar]
- 150.Campbell J.E., Johnson K.P., Rodriguez A., Cap A.P. Magnesium augments platelet activation and coagulation in citrated blood product. Blood. 2012;120(21) doi: 10.1182/blood.V120.21.3438.3438. 3438-3438. [DOI] [Google Scholar]
- 151.Pourshahrestani S., Zeimaran E., Kadri N.A., Gargiulo N., Jindal H.M., Naveen S.V., Sekaran S.D., Kamarul T., Towler M.R. Potency and cytotoxicity of a novel gallium-containing mesoporous bioactive glass/chitosan composite scaffold as hemostatic agents. ACS Appl. Mater. Interfaces. 2017;9(37):31381–31392. doi: 10.1021/acsami.7b07769. [DOI] [PubMed] [Google Scholar]
- 152.Ma X., Cheng Y., Jian H., Feng Y., Chang Y., Zheng R., Wu X., Wang L., Li X., Zhang H. Hollow, rough, and nitric oxide-releasing cerium oxide nanoparticles for promoting multiple stages of wound healing. Adv. Healthc. Mater. 2019;8(16) doi: 10.1002/adhm.201900256. [DOI] [PubMed] [Google Scholar]
- 153.Kurtuldu F., Mutlu N., Boccaccini A.R., Galusek D. Gallium containing bioactive materials: a review of anticancer, antibacterial, and osteogenic properties. Bioact. Mater. 2022;17:125–146. doi: 10.1016/j.bioactmat.2021.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang L., Hao F., Tian S., Dong H., Nie J., Ma G. Targeting polysaccharides such as chitosan, cellulose, alginate and starch for designing hemostatic dressings. Carbohydr. Polym. 2022;291:119574. doi: 10.1016/j.carbpol.2022.119574. [DOI] [PubMed] [Google Scholar]
- 155.Xu Z., Tian W., Wen C., Ji X., Diao H., Hou Y., Fan J., Liu Z., Ji T., Sun F., Wu D., Zhang J. Cellulose-based cryogel microspheres with nanoporous and controllable wrinkled morphologies for rapid hemostasis. Nano Lett. 2022;22(15):6350–6358. doi: 10.1021/acs.nanolett.2c02144. [DOI] [PubMed] [Google Scholar]
- 156.Liu S., Zheng Z., Wang S., Chen S., Ma J., Liu G., Wang B., Li J. Polydopamine-coated chitosan/calcium pyrophosphate hybrid microflowers as an effective hemostatic agent. Carbohydr. Polym. 2019;224 doi: 10.1016/j.carbpol.2019.115175. [DOI] [PubMed] [Google Scholar]
- 157.Liu J.Y., Hu Y., Li L., Wang C., Wang J., Li Y., Chen D., Ding X., Shen C., Xu F.J. Biomass-derived multilayer-structured microparticles for accelerated hemostasis and bone repair. Adv. Sci. 2020;7(22) doi: 10.1002/advs.202002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu Y., Yu K., Shang S., Xie R., Lu F., Bao R., Lan G., Hu E. Chestnut-like macro-acanthosphere triggered hemostasis: a featured mechanism based on puncturing red blood cells. Nanoscale. 2021;13(21):9843–9852. doi: 10.1039/d1nr01148k. [DOI] [PubMed] [Google Scholar]
- 159.Lv C., Zhou X., Wang P., Wu Z., Jiao Z., Guo M., Wang Z., Wang Y., Wang L., Zhang P. Antibacterial microspheres with a bionic red-blood-cell like hollow structure and superior swelling recovery capacity for efficient traumatic hemostasis. Appl. Mater. Today. 2022;29 doi: 10.1016/j.apmt.2022.101559. [DOI] [Google Scholar]
- 160.Li Q., Hu E., Yu K., Xie R., Lu F., Lu B., Bao R., Zhao T., Dai F., Lan G. Self propelling Janus particles for hemostasis in perforating and irregular wounds with massive hemorrhage. Adv. Funct. Mater. 2020;30(42) doi: 10.1002/adfm.202004153. [DOI] [Google Scholar]
- 161.Shi Z., Lan G., Hu E., Lu F., Qian P., Liu J., Dai F., Xie R. Targeted delivery of hemostats to complex bleeding wounds with magnetic guidance for instant hemostasis. Chem. Eng. J. 2022;427 doi: 10.1016/j.cej.2021.130916. [DOI] [Google Scholar]
- 162.Lu B., Hu E., Xie R., Yu K., Lu F., Bao R., Wang C., Lan G., Dai F. Microcluster colloidosomes for hemostat delivery into complex wounds: a platform inspired by the attack action of torpedoes. Bioact. Mater. 2022;16:372–387. doi: 10.1016/j.bioactmat.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Peng X., Xu X., Deng Y., Xie X., Xu L., Xu X., Yuan W., Yang B., Yang X., Xia X., Duan L., Bian L. Ultrafast self gelling and wet adhesive powder for acute hemostasis and wound healing. Adv. Funct. Mater. 2021;31(33) doi: 10.1002/adfm.202102583. [DOI] [Google Scholar]
- 164.Liu J.Y., Li Y., Hu Y., Cheng G., Ye E., Shen C., Xu F.J. Hemostatic porous sponges of cross-linked hyaluronic acid/cationized dextran by one self-foaming process. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;83:160–168. doi: 10.1016/j.msec.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 165.Fang Y., Xu Y., Wang Z., Zhou W., Yan L., Fan X., Liu H. 3D porous chitin sponge with high absorbency, rapid shape recovery, and excellent antibacterial activities for noncompressible wound. Chem. Eng. J. 2020;388 doi: 10.1016/j.cej.2020.124169. [DOI] [Google Scholar]
- 166.Zhao X., Guo B., Wu H., Liang Y., Ma P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018;9(1):2784. doi: 10.1038/s41467-018-04998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Du X., Wu L., Yan H., Jiang Z., Li S., Li W., Bai Y., Wang H., Cheng Z., Kong D., Wang L., Zhu M. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021;12(1):4733. doi: 10.1038/s41467-021-24972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu Y., Fang Y., Ou Y., Yan L., Chen Q., Sun C., Chen J., Liu H. Zinc metal-organic framework@chitin composite sponge for rapid hemostasis and antibacterial infection. ACS Sustain. Chem. Eng. 2020;8(51):18915–18925. doi: 10.1021/acssuschemeng.0c06044. [DOI] [Google Scholar]
- 169.Li S., Gu B., Li X., Tang S., Zheng L., Ruiz-Hitzky E., Sun Z., Xu C., Wang X. MXene-enhanced chitin composite sponges with antibacterial and hemostatic activity for wound healing. Adv. Healthc. Mater. 2022;11(12) doi: 10.1002/adhm.202102367. [DOI] [PubMed] [Google Scholar]
- 170.Liang Y., Li M., Huang Y., Guo B. An integrated strategy for rapid hemostasis during tumor resection and prevention of postoperative tumor recurrence of hepatocellular carcinoma by antibacterial shape memory cryogel. Small. 2021;17(38) doi: 10.1002/smll.202101356. [DOI] [PubMed] [Google Scholar]
- 171.Shi Y., Ding X., Cao Y., Zhou H., Yu W., Liu M., Yin J., Liu H., Wang J., Huang C., Gong C., Wei H., Zhao G. Preparation and application of quick hemostatic gauze based on biomimetic mineralized thrombin. Biomater. Sci. 2021;9(18):6098–6107. doi: 10.1039/d1bm00917f. [DOI] [PubMed] [Google Scholar]
- 172.Yu L., Shang X., Chen H., Xiao L., Zhu Y., Fan J. A tightly-bonded and flexible mesoporous zeolite-cotton hybrid hemostat. Nat. Commun. 2019;10(1):1932. doi: 10.1038/s41467-019-09849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yuk H., Varela C.E., Nabzdyk C.S., Mao X., Padera R.F., Roche E.T., Zhao X. Dry double-sided tape for adhesion of wet tissues and devices. Nature. 2019;575(7781):169–174. doi: 10.1038/s41586-019-1710-5. [DOI] [PubMed] [Google Scholar]
- 174.Zhang K., Chen X., Xue Y., Lin J., Liang X., Zhang J., Zhang J., Chen G., Cai C., Liu J. Tough hydrogel bioadhesives for sutureless wound sealing, hemostasis and niointerfaces. Adv. Funct. Mater. 2021;32(15) doi: 10.1002/adfm.202111465. [DOI] [Google Scholar]
- 175.Zou F., Wang Y., Zheng Y., Xie Y., Zhang H., Chen J., Hussain M.I., Meng H., Peng J. A novel bioactive polyurethane with controlled degradation and L-Arg release used as strong adhesive tissue patch for hemostasis and promoting wound healing. Bioact. Mater. 2022;17:471–487. doi: 10.1016/j.bioactmat.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pang H., Ma C., Li S., Liu H., Xia C., Li J., Zhang S., Zhang W., Cai L., Huang Z. Tough thermosensitive hydrogel with excellent adhesion to low-energy surface developed via nanoparticle-induced dynamic crosslinking. Appl. Surf. Sci. 2021;560:149935. doi: 10.1016/j.apsusc.2021.149935. [DOI] [Google Scholar]
- 177.Xuan C., Hao L., Liu X., Zhu Y., Yang H., Ren Y., Wang L., Fujie T., Wu H., Chen Y., Shi X., Mao C. Wet-adhesive, haemostatic and antimicrobial bilayered composite nanosheets for sealing and healing soft-tissue bleeding wounds. Biomaterials. 2020;252:120018. doi: 10.1016/j.biomaterials.2020.120018. [DOI] [PubMed] [Google Scholar]
- 178.Mohammadinejad R., Maleki H., Larrañeta E., Fajardo A.R., Nik A.B., Shavandi A., Sheikhi A., Ghorbanpour M., Farokhi M., Govindh P., Cabane E., Azizi S., Aref A.R., Mozafari M., Mehrali M., Thomas S., Mano J.F., Mishra Y.K., Thakur V.K. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today. 2019;16:213–246. doi: 10.1016/j.apmt.2019.04.010. [DOI] [Google Scholar]
- 179.Huang Y., Zhao X., Wang C., Chen J., Liang Y., Li Z., Han Y., Guo B. High-strength anti-bacterial composite cryogel for lethal noncompressible hemorrhage hemostasis: synergistic physical hemostasis and chemical hemostasis. Chem. Eng. J. 2022;427 doi: 10.1016/j.cej.2021.131977. [DOI] [Google Scholar]
- 180.Hong Y., Zhou F., Hua Y., Zhang X., Ni C., Pan D., Zhang Y., Jiang D., Yang L., Lin Q., Zou Y., Yu D., Arnot D.E., Zou X., Zhu L., Zhang S., Ouyang H. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019;10(1):2060. doi: 10.1038/s41467-019-10004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tan H., Jin D., Sun J., Song J., Lu Y., Yin M., Chen X., Qu X., Liu C. Enlisting a traditional Chinese medicine to tune the gelation kinetics of a bioactive tissue adhesive for fast hemostasis or minimally invasive therapy. Bioact. Mater. 2021;6(3):905–917. doi: 10.1016/j.bioactmat.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu W., Wang M., Cheng W., Niu W., Chen M., Luo M., Xie C., Leng T., Zhang L., Lei B. Bioactive antiinflammatory antibacterial hemostatic citrate-based dressing with macrophage polarization regulation for accelerating wound healing and hair follicle neogenesis. Bioact. Mater. 2021;6(3):721–728. doi: 10.1016/j.bioactmat.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen X., Zhang J., Chen G., Xue Y., Zhang J., Liang X., Lei I.M., Lin J., Xu B.B., Liu J. Hydrogel bioadhesives with extreme acid tolerance for gastric perforation repairing. Adv. Funct. Mater. 2022;3 2(29) doi: 10.1002/adfm.202202285. [DOI] [Google Scholar]
- 184.Xia X., Xu X., Wang B., Zhou D., Zhang W., Xie X., Lai H., Xue J., Rai A., Li Z., Peng X., Zhao P., Bian L., Chiu P.W.Y. Adhesive hemostatic hydrogel with ultrafast gelation arrests acute upper gastrointestinal hemorrhage in pigs. Adv. Funct. Mater. 2021;32(16) doi: 10.1002/adfm.202109332. [DOI] [Google Scholar]
- 185.Hickman D.A., Pawlowski C.L., Sekhon U.D.S., Marks J., Gupta A.S. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv. Mater. 2018;30(4) doi: 10.1002/adma.201700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu L., Hu E., Yu K., Xie R., Lu F., Lu B., Bao R., Li Q., Dai F., Lan G. Recent advances in materials for hemostatic management. Biomater. Sci. 2021;9(22):7343–7378. doi: 10.1039/d1bm01293b. [DOI] [PubMed] [Google Scholar]
- 187.Liao G., He F., Li Q., Zhong L., Zhao R., Che H., Gao H., Fang B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020;112 doi: 10.1016/j.pmatsci.2020.100666. [DOI] [Google Scholar]
- 188.Brown A.C., Stabenfeldt S.E., Ahn B., Hannan R.T., Dhada K.S., Herman E.S., Stefanelli V., Guzzetta N., Alexeev A., Lam W.A., Lyon L.A., Barker T.H. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat. Mater. 2014;13(12):1108–1114. doi: 10.1038/nmat4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.