Abstract
Background:
During ultrasonic scaling, the harbored microorganisms in the oral cavity get aerosolized, which have important impacts on air quality and can cause a serious health threat to the clinician, patients, and the surroundings. Therefore, this study was conducted to evaluate whether preprocedural mouth rinse has any effect on bacterial load in aerosols generated during ultrasonic scaling.
Materials and Methods:
A total of 80 subjects with chronic periodontitis were selected and randomly grouped into four comprising twenty in each. The groups were based on the use of preprocedural mouth rinse: no rinse group (control) (A), and test groups with preprocedural mouth rinse with water (B), 0.2% Chlorhexidine gluconate (C), and herbal mouthwash (D). The aerosol produced during ultrasonic scaling was collected on blood agar plates positioned at the chest area of patients, operators, and assistants. Aerosol collected in the operatory before the procedure was considered as baseline. Colonies on the blood agar plates were counted after incubating at 37°C for 24 h. Pairwise comparisons involving positions and mouth rinses on microbial colonies were conducted using independent sample t-test and Tukey's test for post hoc analysis considering 0.05 as the significance level.
Results:
Microbial colonies were significantly reduced with chlorhexidine gluconate compared to that of others (P < 0.001), followed by herbal mouthwash and water. Again, microbial colonies were highest at the chest area of the operator and lowest at the chest area of the assistant.
Conclusions:
0.2% Chlorhexidine gluconate is superior in reducing the microbial load in aerosols produced during ultrasonic scaling.
Keywords: Aerosol, chlorhexidine gluconate, herbal mouthwash, microbial colonies, preprocedural mouth wash, ultrasonic scaling
INTRODUCTION
Due to a uniquely moist and warm environment, the oral cavity harbors millions of bacteria and viruses from the respiratory tract, saliva, and dental plaque. These microorganisms get aerosolized when they come in contact with the dental equipment, particularly the high-speed dental drills and ultrasonic scalers.[1,2] The ultrasonic scaler uses ultrasound to remove calculus deposits from the teeth effectively. It oscillates (move forward and backward) a typically blunt metal tip at a high-frequency producing mechanical vibratory, cavitational, and acoustic microstreaming forces in the associated cooling water that remove/disrupt the deposits. However, the water used as a coolant is splattered during the vibration of the tip and becomes contaminated when it is mixed with saliva and plaque. The amount of contamination of dental aerosol depends on the quality of saliva, nasal and throat secretion, blood, dental plaque, and any dental infection including periodontal.[3]
The maximum amount of aerosol is produced by ultrasonic scalers compared to that of the other dental equipment.[4,5] Patients and practitioners are regularly exposed to tens of thousands of bacteria per cubic meter generated during procedures,[6,7] and inhalation of this may cause adverse health effects such as common cold, tuberculosis, severe acute respiratory syndrome (SARS), and even transmission of blood-borne pathogens, namely human immunodeficiency Virus, Hepatitis B and C virus.[8] This disease transmission may be bidirectional, i.e., from patient-to-patient, patient-to-clinician, or clinician-to-patient.[2,9,10,11] Notably, the aerosols are not dispersed evenly in the entire operatory room, and the greatest concentration has been shown within two feet of the patient, radiating more toward the chest of the patient or the face of the operator.[11,12,13,14,15] In addition, the aerosolized microorganisms remain suspended in the air for extended periods with greater potentiality.[11,16] Thus, the risk of health threat by aerosols exists even after the completion of the treatment procedure. Oral health professionals should be aware of these invisible dangers in the operatory and should follow the recommended protocols for the prevention of infection before, during, and after patient care.
Harmful effects of the microbial load in aerosols demand the minimization of microbial quantity in the oral cavity before the generation of aerosol/splatter to reduce the risk of cross-infection in the dental environment. Current research suggests that making a patient rinse with antimicrobial mouthwash before the treatment procedures may reduce the number of microorganisms in aerosols,[11,14,17,18,19] though no antimicrobial agent has been identified as a superior prerinse so far. Commonly used mouthwashes in dental practice are chlorhexidine gluconate and herbal mouthwash.
Chlorhexidine gluconate is a bisbiguanide antiseptic and is widely used chemical plaque control agent. It is effective against an array of microorganisms and also exhibits substantivity up to 12 h.[20] This makes it the “gold standard of chemical plaque control,” though a number of side effects, such as brownish discoloration of teeth, restorative materials and the dorsum of the tongue, taste perturbation, oral mucosal erosion, etc., have been reported.[21] Considering the wide range of disadvantages of chlorhexidine gluconate mouthwash, alternative antiplaque agents have been developed in recent years using heterogeneous herbal products. Various naturally available herbs have been used either alone or in combination as safe and effective antibacterial agents in the form of mouthwash.[22] A Herbal mouthwash (HiOra® Mouthwash, Himalaya) containing Bibhitaki (Bellirica Myrobatan), Meswak (Salvadora persica), and Betel leaf (Nagavalli) with no sugar or alcohol is available commercially. It possesses a significant antimicrobial (anticaries) and antifungal activity, and thus, offers a safe and effective option without any adverse effects.[23,24]
Considering the potential hazard of cross-contamination from aerosols produced during ultrasonic scaling, this prospective, randomized, double-centered, double-blind study was conducted to assess the effectiveness of chlorhexidine gluconate (0.2%), herbal mouthwash, and water as preprocedural mouth rinse on the bacterial load in aerosols by assessing the number of bacterial colonies formed in blood agar culture plates positioned at various areas of the operating room during ultrasonic scaling.
MATERIALS AND METHODS
It was a randomized, prospective, double-blind clinical trial, carried out in the Department of Periodontics and Oral Implantology in collaboration with the Department of Microbiology in accordance with the ethical guidelines of the Institutional Research and Ethical Committee. A total of 80 subjects with periodontitis were selected from the outpatient department irrespective of sex, religion, and socioeconomic status. Subjects were explained the entire procedure in detail and written consent was obtained from each of them. The subjects were selected based on the following criteria.
Inclusion criteria
Subjects of 20–65 years old with chronic periodontitis with not <20 teeth
Systemically healthy
Subjects received no antibiotics and Periodontal treatment during the last 3 months.
Exclusion criteria
Allergic to mouthwash
Pregnant and lactating mothers
Smokers.
The subjects were randomly categorized into four groups by a single investigator (SJD) using block randomization. The groups were named A (Control), B, C, and D, containing 20 subjects in each. Group A was allotted no preprocedural mouth rinse, Group B, C and D were allotted water, chlorhexidine gluconate (0.2%), and herbal mouthwash as a preprocedural mouth rinse, respectively. The treatment group was not publicized to the patient as well as the operator.
All subjects underwent periodontal examination by a single examiner (SJD). Periodontal status was assessed using plaque index,[25] probing pocket depth, and clinical attachment level.
Nonselective culture medium (Blood agar) was prepared by boiling 40 g of HIMEDIA Blood agar base in 1000 ml of distilled water, which was then cooled to 45°C–50°C and autoclaved. After that 5% sterile defibrinated sheep blood was added. It was then poured into sterile Petri plates, which were stored in the refrigerator at 2°C–8°C for 5–6 days. The agar plates were coded and positioned at four different places prior or during ultrasonic scaling for the collection of aerosols.
Position 1: Operatory room, 4 feet away from the dental chair (baseline count)
Position 2: Chest of the patients during treatment
Position 3: Chest of the operator during treatment
Position 4: Chest of the assistant during treatment.
To determine if there were any aerosolized bacteria present in the operatory room, the blood agar plate was kept at position 1 for 30 min before conducting the ultrasonic scaling. The plates were positioned with the help of double-sided adhesive tape during ultrasonic scaling for Positions 2, 3, and 4. Various phases of the study are shown in Figure 1.
Figure 1.
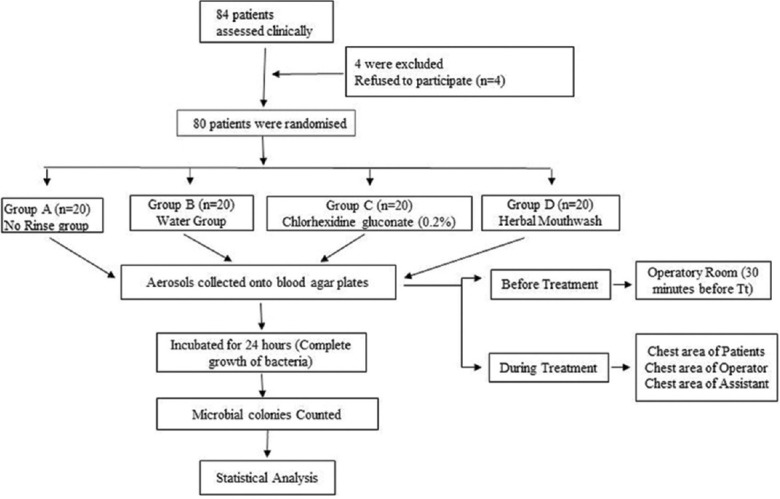
A flowchart representing the study design. n – number of samples
The operatory room was fumigated using formaldehyde (40%) for 15 min on the day before treatment. Only one patient per day was treated to avoid aerosol contamination. The same operatory room was used for all samples. Before each appointment, the operatory surfaces were cleaned and disinfected using 70% ethyl alcohol.
After the baseline sampling and before the treatment procedure, the subjects were instructed to undergo a 30 s preprocedural rinse of 10 ml of chlorhexidine gluconate (0.2%), herbal mouthwash, or water depending upon the group to which the subjects were assigned. Coded agar plates were placed accordingly and stabilized for aerosol collection. The treatment procedure comprising of scaling was carried out for 30 min using a piezoelectric ultrasonic scaler (DTE-D5), with controlled frequency and the pressure of the water coolant was maintained at constant level. A motorized suction was used during the treatment procedure. Immediately after scaling, agar plates were removed and sealed, which were then incubated at 37°C for 24 h in an increased CO2 chamber (BOD Incubator, Chennai, India) and microbial colonies that grew on each plate were counted using a colony counter (INSIF, Haryana, India), where an audible beep confirms the count and digital readout appears on the display.
All the data collected was analyzed statistically using IBM Statistical Package for Social Sciences (SPSS) version 20 (Armonk, New York, US). Analysis of variance (ANOVA) tests was done to compare the mean of four groups by the variance between and within groups ratio with significant inference at P ≤ 0.05. In the case of statistical significance, post hoc pairwise comparisons were done by Tukey's Honestly Significant Difference test. Pair-wise independent sample t-tests were conducted at a 5% level of significance to test the pair-wise difference in the mean values of microbial colonies grown in agar plates position wise. The inferences were drawn with the help of the P ≤ 0.05.
To assure equivalency of groups at baseline, we conducted a one-way ANOVA on baseline values to assess potential differences in airborne bacteria in the ambient air before instrumentation with the ultrasonic scaler. Since no difference in baseline means was found between treatment groups, baseline values were omitted from the subsequent analyses.
RESULTS
The mean plaque index, probing pocket depth and clinical attachment level of the various groups is depicted in Table 1. The mean ± standard deviation of plaque index, probing pocket depth, and clinical attachment level group in Group A, B, C, and D were found to be statistically not significant (P ≥ 0.05), which indicates that all the participants were suffering from periodontitis of equal intensity.
Table 1.
Average plaque index, probing pocket depth and clinical attachment level (mean±standard deviation) in various groups
| Groups | Description | Plaque index | Probing Pocket depth |
Clinical Attachment level |
|---|---|---|---|---|
| A (n=20) | No rinse (control) | 2.17±0.38 | 3.90±0.70 | 4.63±0.78 |
| B (n=20) | Water rinse | 2.23±0.25 | 4.01±0.73 | 4.57±0.98 |
| C (n=20) | Chlorhexidine gluconate rinse | 2.01±0.44 | 3.89±0.44 | 4.71±0.72 |
| D (n=20) | Herbal mouthwash rinse | 2.23±0.26 | 3.67±0.52 | 4.29±0.67 |
| P | 0.09 (NS) | 0.35 (NS) | 0.37 (NS) |
P value was considered significant, when it is<0.05. NS - Not significant; P - Probability value; n - number of samples; Group A - control (no preprocedural mouth rinse); Group B - water; Group C - chlorhexidine gluconate (0.2%); Group D - herbal mouthwash as preprocedural mouth rinses
Microbial colony count at Position 1 was considered as the baseline. The average microbial colony count at this position was found to be 14.91 ± 11.42, being ranged from 13.25 to 16.80 [Table 2].
Table 2.
Position wise mean microbial colonies
| Position of agar plate | Rinse group | Mean±SD | Range |
|---|---|---|---|
| Baseline† | Regardless of rinse | 14.91±11.42 | 13.25-16.80 |
| On chest of patients‡ | No rinse | 302.95±74.48 | 105.00-458.00 |
| Water | 232.50±90.33 | 20.00-357.00 | |
| Chlorhexidine gluconate | 70.55±31.78 | 30.00-132.00 | |
| Herbal mouthwash | 129.70±53.35 | 37.00-205.00 | |
| On chest of operator‡ | No rinse | 429.20±62.62 | 298.00-560.00 |
| Water | 378.80±73.94 | 210.00-530.00 | |
| Chlorhexidine gluconate | 121.75±32.37 | 63.00-190.00 | |
| Herbal mouthwash | 206.60±53.12 | 115.00-310.00 | |
| On chest of assistant‡ | No rinse | 193.90±87.92 | 65.00-415.00 |
| Water | 132.55±61.98 | 15.00-282.00 | |
| Chlorhexidine gluconate | 27.50±21.80 | 10.00-90.00 | |
| Herbal mouthwash | 79.00±47.60 | 20.00-200.00 |
4 feet from dental chair 30 min prior to treatment;
During treatment. SD - Standard deviation
In Position 2, the mean number of microbial colonies was 232.50 ± 90.33 (range 20.00–357.00), 70.55 ± 31.78 (range 30.00–132.00) and 129.70 ± 53.35 (range 37.00–205.00) in the groups of preprocedural mouth rinse with water, chlorhexidine gluconate, and herbal mouthwash, respectively. In contrast, the mean number of microbial colonies in preprocedural no-rinse group was 302.95 ± 74.48 (range 105.00–458.00), as shown in [Table 2]. Thus, the numbers of colonies were reduced by 23.10%, 76.69%, and 57.17% with water, chlorhexidine gluconate, and herbal mouthwash, respectively, compared to that of the no-rinse group.
In Position 3, the mean number of microbial colonies was 378.80 ± 73.94 (range 210.00–530.00), 121.75 ± 32.37 (range 63.00–190.00), and 206.60 ± 53.12 (range 115.00–310.00) in the groups of preprocedural mouth rinse with water, chlorhexidine gluconate, and herbal mouthwash, respectively, as depicted in [Table 2]. In contrast, the mean number of microbial colonies in preprocedural no-rinse group was 429.20 ± 62.62 (range 298.00–560.00). Thus, the numbers of colonies were reduced by 11.85%, 71.63%, and 51.86% with water, chlorhexidine gluconate, and herbal mouthwash, respectively, compared to that of the no-rinse group.
In Position 4, the mean number of microbial colonies was 132.55 ± 61.98 (range 15.00–282.00), 27.50 ± 21.80 (range 10.00–90.00), and 79.00 ± 47.60 (range 20.00–200.00) in the groups of preprocedural mouth rinse with water, chlorhexidine gluconate, and herbal mouthwash, respectively, while the mean number of microbial colonies in preprocedural no-rinse group was 193.90 ± 87. 92 (range 65.00–415.00) [Table 2]. Thus, the numbers of colonies were reduced by 31.64%, 85.81%, and 59.25% with water, chlorhexidine, and herbal mouthwash, respectively, compared to that of the no-rinse group.
On position-wise comparison, the lowest number of microbial colony was seen in the operatory room before scaling (14.91 ± 11.42), while the highest number of the microbial colony was observed in the agar plates placed on the operator's chest (Position 3) (284.09 ± 137.78) followed by Position 2 (patient's chest) (183.93 ± 111.38), and Position 4 (assistant's chest) (108.13 ± 85.49) [Table 3]. The number of colonies was observed to be 12.33-, 18.78- and 7.25-times more in Position 2, 3, and 4, respectively, compared to that of position 1 (aerosolized bacteria present in the operatory room). The differences in the mean values of microbial colonies in all four positions were found to be statistically very highly significant (P < 0.001). Microbial colonies in all the four groups at all four positions of blood agar plates with or without preprocedural mouth rinse are schematically shown in Figure 2.
Table 3.
Comparison of microbial colonies at different positions
| Position of agar plates | Mean±SD | Range | Difference in means | ||
|---|---|---|---|---|---|
|
|
|||||
| Position 2 | Position 3 | Position 4 | |||
| 1 | 14.90±11.42 | 13.25-16.80 | 169.01*** | 269.18*** | 93.31*** |
| 2 | 183.93±111.38 | 20.00-357.00 | 100.16*** | 75.80*** | |
| 3 | 284.09±137.78 | 30.00-132.00 | 175.96*** | ||
| 4 | 108.13±85.49 | 37.00-205.00 | |||
Very highly significant (P<0.001). Mean microbial colonies at various positions in different groups are not similar, differ significantly from each other at P (<0.05). SD - Standard deviation; P - Probability value
Figure 2.
A schematic representation of microbial colonies in all four positions of blood agar plates with or without preprocedural mouth rinse. Note the minimum no of microbial colonies in Position 1
Pairwise comparison involving both the position and preprocedural mouth rinse, the mean number of microbial colonies between the groups at Positions 2, 3, and 4 were analyzed using Tukey's test for post hoc analysis and found to be highly significant statistically.
Regardless of the position of the agar plates, the highest number of microbial colonies were seen in no-rinse group (308.68 ± 75.00), followed by water (247.85 ± 75.41), herbal mouthwash (138.43 ± 51.35), and 0.2% chlorhexidine gluconate (73.26 ± 28.65). The lowest no of microbial colonies was seen in Group 3, where preprocedural mouth rinse was chlorhexidine gluconate (0.2%). As shown in [Table 4], intergroup comparison involving all four groups showed a statistically significant difference.
Table 4.
Microbial colonies (mean±standard deviation) involving all the groups at various positions and pairwise comparison
| Positions | Rinse group | Microbial colony counts | Pairwise mean difference (P) | ||
|---|---|---|---|---|---|
|
|
|||||
| Water | Chlorhexidine gluconate | Herbal mouthwash | |||
| 2 | No rinse | 302.95±74.48 | 70.45**(0.007) | 232.4*** (0.001) | 173.25*** (0.001) |
| Water | 232.50±90.33 | 161.95*** (0.001) | 102.8*** (0.001) | ||
| Chlorhexidine | 70.55±31.78 | 59.15* (0.030) | |||
| Herbal | 129.70±53.35 | ||||
| 3 | No rinse | 429.20±62.62 | 50.4* (0.035) | 307.45*** (0.001) | 222.6*** (0.001) |
| Water | 378.80±73.94 | 257.05*** (0.001) | 172.2*** (0.001) | ||
| Chlorhexidine | 121.75±32.37 | 84.85*** (0.001) | |||
| Herbal | 206.60±53.12 | ||||
| 4 | No rinse | 193.90±87.92 | 61.35** (0.009) | 166.4*** (0.001) | 114.9*** (0.001) |
| Water | 132.55±61.98 | 105.05*** (0.001) | 53.55* (0.029) | ||
| Chlorhexidine | 27.50±21.80 | 51.5* (0.036) | |||
| Herbal | 79.00±47.60 | ||||
| Average | No rinse | 308.68±75.00 | |||
| Water | 247.85±75.41 | ||||
| Chlorhexidine | 73.26±28.65 | ||||
| Herbal | 138.43±51.35 | ||||
Statistically significant (P<0.05);
Highly significant (P<0.01);
Very highly significant (P<0.001). Microbial colony counts are not similar in all groups differ significantly from each other at P (<0.05). SD - Standard deviation; P - Probability value
DISCUSSION
Aerosols produced during the various dental procedures are the potential to spread the infection to dental personnel and other individuals in the dental operatory room. This has long been considered as one of the main concerns in dentistry. It must be emphasized that “layering of protective procedures” is required in reducing the potential danger from dental aerosols. In this procedure, multiple steps are involved in the reduction of the risk of infection; a single step reduces to a certain extent to which another step is added that further reduces the remaining risk until the risk is minimal. It indicates that the dental team should not depend on a single precautionary strategy. Personal protection barriers constitute the first layer of defense, which is upgraded by antiseptic preprocedural mouth rinse (second layer of defense). This is further elevated by the routine use of a high-volume evacuator (HVE), which is further augmented by high-efficiency particulate air (HEPA) filter. The first two layers of defense are inexpensive and should be followed routinely as a part of infection control practices. Furthermore, the maximum amount of contaminated aerosol is observed to be within two feet of the patient,[12] where the dental health professional is usually positioned. This observation reinforces the importance of personal protective barriers such as eye shields and face masks, head cap, glove, and gowns.
The maximum amount of aerosol production is reported during the ultrasonic scaling procedure.[26] By following the American Dental Association protocols dental aerosols may be minimized, though complete elimination is difficult.[11] The most basic and feasible methods to reduce bacterial load in the aerosols is preprocedural rinse suggested by a number of investigators.[11,14,15,27]
In this study, chlorhexidine gluconate, herbal mouthwash and water were used as preprocedural mouth rinses. Chlorhexidine gluconate (0.2%) has a broad-spectrum antimicrobial activity against both Gram-positive and-negative organisms, yeasts, dermatophytes and some lipophilic viruses with a substantivity for 12 h.[11,20] It is an effective antiseptic for free-floating oral bacteria and those loosely adhering to mucous membranes, though not affect bacteria in a biofilm, does not penetrate subgingivally, and is unlikely to affect viruses and bacteria harbored in the nasopharynx. Although herbal mouthwash is accomplished with antimicrobial (anticaries), antifungal and anti-halitosis properties, little evidence is available regarding its efficacy on bacterial load in aerosols when used as a preprocedural rinse.[27]
Blood agar was used to collect the aerosols. This is a valid medium for culturing airborne bacteria.[28] On settle down in the blood agar culture medium, bacteria grows and multiply to form clusters of colonies. In this study, these microbial colonies were counted in the agar plates to evaluate the usefulness of two commonly used mouthwashes in dentistry.
Highest number of microbial colonies was observed in no rinse group (Group A), followed by water (Group B), herbal mouthwash (Group D), and 0.2% chlorhexidine gluconate (Group C) preprocedural mouth rinse. Maximum reduction of microbial colonies was observed with 0.2% chlorhexidine gluconate (Group C). The pairwise comparison between chlorhexidine gluconate (Group C) with water (Group B), herbal mouthwash (Group D), and no-rinse (Group A) showed a statistically significant difference (P < 0.01). This supports the observations of various studies.[11,14,29,30,31] Higher effectiveness of 0.2% chlorhexidine gluconate may be related to its substantivity on oral tissues and its subsequent slow release in an active form. In contrast, Rani et al., (2014) observed more reduction in microbial colonies with herbal mouthwash compared to that of chlorhexidine gluconate, though not significant statistically.[15]
Three plates were kept at different positions, namely the chest of the patient (Position 2), operator (Position 3), and assistant (Position 4) during the scaling procedure to collect aerosols. The highest number of microbial colonies was observed in Position 3, followed by Position 2 and 4. This indicates that Position 3 is closer to the patient's mouth compared to that of position 2. This finding supports the observation made by Rani et al., (2014).[15] However, a large number of studies observed a greater number of microbial colonies in the agar plates placed over the patient's chest than that of the operator, explaining the fact larger salivary droplets generated during dental procedures settle rapidly from the air and would heavily contaminate the agar plates on a patient's chest.[13,14,29,30,31] Greatest concentration of the microorganisms in aerosols was observed within 2 feet of the patient[12] and the number of microbial colonies decreases with increase in distances from the operating area.[11] Bentley et al. (1994) suggested that distribution of bacterially contaminated aerosols and splatter is extremely variable and may be influenced by the type of therapeutic procedure, use of HVE, the position of the subject in the dental chair, position of the tooth in the mouth that affects the position of the operator relative to the subject, levels of the microorganisms in the subject's mouth, etc.[8] The reason of the highest number of colonies at position 3 in this study may be explained based on the fact of the height of the operator that influences the position of the operator. Due to the low height of the operator (DK), the agar plates placed on her chest were probably closer to the patient's mouth than that of the distance between his mouth and chest (Position 2).
This study suggests that 0.2% chlorhexidine gluconate is more effective as a preprocedural mouthwash than that of the herbal mouthwash in reducing microbial load in aerosols produced during ultrasonic scaling. Even preprocedural mouth rinse with water also plays a significant role in reducing microbial load in aerosols. Notably, the observations of this study reinforce the significance of personal protective equipment and validate preprocedural mouth rinsing as an additional barrier to cross-contamination and minimizes the risk of team members and the patients.
The limitation of this study should be considered in interpreting the results. Microbial colonies give a good picture of total airborne bacterial count from a particular procedure, but it does not provide any differentiation between whether the bacteria are relatively benign or a pathogenic species. Any bacteria that require special media or growth conditions, such as mycobacteria or strict anaerobes that are common in periodontal pockets, were not cultured and counted in this study. Furthermore, because they do not grow on the type of media used for bacterial studies, no viral particles such as influenza, rhinoviruses, and SARS coronavirus would be measured. Moreover, the plate count or “fall out” approach used for the collection of the bacteria is subjected to a level of inaccuracy, because bacteria exposed to the air may remain viable, or may lose the ability to form colonies and become nonculturable. Thus, counting only aerobic bacteria gives only a partial picture of the airborne contamination that occurs during dental procedures and underestimates the true extent of bacterial populations in aerosols.[27] Future studies are necessary to investigate the viable pathogenic organisms generated during ultrasonic scaling. To evaluate the levels of airborne bacteria remaining in the operatory room after the ultrasonic scaling procedure, culture plates would have exposed posttherapeutically as well.
CONCLUSIONS
In the light of the study carried out, we may conclude that preprocedural mouth rinse could eliminate the majority of bacterial aerosols generated by the ultrasonic scalers. Chlorhexidine gluconate (0.2%) is more effective in reducing the microbial load in aerosols produced during ultrasonic scaling compared to that of herbal mouthwash and water when used as a preprocedural mouth rinse. Again, more microbial colonies are formed on the agar plates placed on the chest area of the operator than that of the plates placed on the chest area of the patients and the assistants. This states the importance of protection for the dentists and dental hygienists, who are the main targets of the microorganisms generated during oral procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl Environ Microbiol. 1995;61:3165–8. doi: 10.1128/aem.61.8.3165-3168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. J Am Dent Assoc. 1998;129:1241–9. doi: 10.14219/jada.archive.1998.0421. [DOI] [PubMed] [Google Scholar]
- 3.Szymańska J. Dental bioaerosol as an occupational hazard in a dentist's workplace. Ann Agric Environ Med. 2007;14:203–7. [PubMed] [Google Scholar]
- 4.Miller RL, Micik RE, Abel C, Ryge G. Studies on dental aerobiology. II. Microbial splatter discharged from the oral cavity of dental patients. J Dent Res. 1971;50:621–5. doi: 10.1177/00220345710500031701. [DOI] [PubMed] [Google Scholar]
- 5.Hinds WC. Aerosol Technology: Properties, Behavior and Measurement of Airborne Particles. 2nd. New York: Wiley; 1999. [Google Scholar]
- 6.Pippin DJ, Verderame RA, Weber KK. Efficacy of face masks in preventing inhalation of airborne contaminants. J Oral Maxillofac Surg. 1987;45:319–23. doi: 10.1016/0278-2391(87)90352-1. [DOI] [PubMed] [Google Scholar]
- 7.Kharbuli D, Das SJ, Deka A, Alam ST, Bora GH. Position wise quantification of microbial load in aerosols produced during ultrasonic scaling. J Med Dent Sci. 2020;2:175–80. [Google Scholar]
- 8.Bentley CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. J Am Dent Assoc. 1994;125:579–84. doi: 10.14219/jada.archive.1994.0093. [DOI] [PubMed] [Google Scholar]
- 9.Smith WH, Davies D, Mason KD, Onions JP. Intraoral and pulmonary tuberculosis following dental treatment. Lancet. 1982;1:842–4. doi: 10.1016/s0140-6736(82)91886-4. [DOI] [PubMed] [Google Scholar]
- 10.Legnani P, Checchi L, Pelliccioni GA, D'Achille C. Atmospheric contamination during dental procedures. Quintessence Int. 1994;25:435–9. [PubMed] [Google Scholar]
- 11.Logothetis DD, Martinez-Welles JM. Reducing bacterial aerosol contamination with a Chlorhexidine gluconate pre-rinse. J Am Dent Assoc. 1995;126:1634–9. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 12.Holbrook WP, Muir KF, Macphee IT, Ross PW. Bacteriological investigation of the aerosol from ultrasonic scalers. Br Dent J. 1978;144:245–7. doi: 10.1038/sj.bdj.4804072. [DOI] [PubMed] [Google Scholar]
- 13.Cochran MA, Miller CH, Sheldrake MA. The efficacy of the rubber dam as a barrier to the spread of microorganisms during dental treatment. J Am Dent Assoc. 1989;119:141–4. doi: 10.14219/jada.archive.1989.0131. [DOI] [PubMed] [Google Scholar]
- 14.Gupta G, Mitra D, Ashok KP, Gupta A, Soni S, Ahmed S, et al. Efficacy of preprocedural mouth rinsing in reducing aerosol contamination produced by ultrasonic scaler: A pilot study. J Periodontol. 2014;85:562–8. doi: 10.1902/jop.2013.120616. [DOI] [PubMed] [Google Scholar]
- 15.Rani KR, Ambati M, Prasanna JS, Pinnamaneni I, Reddy PV, Rajashree D. Chemical vs. herbal formulations as preprocedural mouth rinses to combat aerosol production: A randomized controlled study. J Oral Res Rev. 2014;6:9–13. [Google Scholar]
- 16.Reddy S, Prasad MG, Kaul S, Satish K, Kakarala S, Bhowmik N. Efficacy of 0.2% tempered chlorhexidine as a pre-procedural mouth rinse: A clinical study. J Indian Soc Periodontol. 2012;16:213–7. doi: 10.4103/0972-124X.99264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine DH, Mendieta C, Barnett ML, Furgang D, Meyers R, Olshan A, et al. Efficacy of preprocedural rinsing with an antiseptic in reducing viable bacteria in dental aerosols. J Periodontol. 1992;63:821–4. doi: 10.1902/jop.1992.63.10.821. [DOI] [PubMed] [Google Scholar]
- 18.Yamada H, Ishihama K, Yasuda K, Hasumi-Nakayama Y, Shimoji S, Furusawa K. Aerial dispersal of blood-contaminated aerosols during dental procedures. Quintessence Int. 2011;42:399–405. [PubMed] [Google Scholar]
- 19.Sawhney A, Venugopal S, Babu GR, Garg A, Mathew M, Yadav M, et al. Aerosols how dangerous they are in clinical practice. J Clin Diagn Res. 2015;9:C52–7. doi: 10.7860/JCDR/2015/12038.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonesvoll P, Lökken P, Rölla G, Paus PN. Retention of chlorhexidine in the human oral cavity after mouthrinses. Arch Oral Biol. 1974;19:1209–22. doi: 10.1016/0003-9969(74)90263-5. [DOI] [PubMed] [Google Scholar]
- 21.Flötra L, Gjermo P, Rölla G, Waerhaug J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. 1971;79:119–25. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni P, Singh DK, Jalaluddin M, Mandal A. Comparative evaluation of antiplaque efficacy between essential oils with alcohol-based and chlorhexidine with nonalcohol-based mouthrinses. J Int Soc Prev Community Dent. 2017;7:S36–41. doi: 10.4103/jispcd.JISPCD_131_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salam R, Khokon JU, Baidya S, Mussa MT. Effect of neem and betel leaf against oral bacteria. Int J Nat Soc Sci. 2014;1:52–7. [Google Scholar]
- 24.Santhosh S, Siji J, Thangakumaran S, Sasikumar PK, Mahesh J. Miswak an indigenous plant in dentistry – A general outlook. Indian J Med Res Pharm Sci. 2018;5:10–4. [Google Scholar]
- 25.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:l610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 26.Leggat PA, Kedjarune U. Bacterial aerosols in the dental clinic: A review. Int Dent J. 2001;51:39–44. doi: 10.1002/j.1875-595x.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 27.Mamajiwala AS, Sethi KS, Raut CP, Karde PA, Khedkar SU. Comparative evaluation of chlorhexidine and cinnamon extract used in dental unit waterlines to reduce bacterial load in aerosols during ultrasonic scaling. Indian J Dent Res. 2018;29:749–54. doi: 10.4103/ijdr.IJDR_571_17. [DOI] [PubMed] [Google Scholar]
- 28.Johnston JR, Butchart AM, Kgamphe SJ. A comparison of sampling methods for airborne bacteria. Environ Res. 1978;16:279–84. doi: 10.1016/0013-9351(78)90162-7. [DOI] [PubMed] [Google Scholar]
- 29.Rao RM, Shenoy N, Shetty V. Determination of efficacy of pre-procedural mouth rinsing in reducing aerosol contamination produced by ultrasonic scalers. NUJHS. 2015;5:52–6. [Google Scholar]
- 30.Yadav S, Kumar S, Srivastava P, Gupta KK, Gupta J, Khan YS. Comparison of efficacy of three different mouthwashes in reducing aerosol contamination produced by ultrasonic scaler: A pilot study. Indian J Dent Sci. 2018;10:6–10. [Google Scholar]
- 31.Sethi G, Kumar K. A comparative evaluation of efficacy of 0.2% chlorhexidine with a herbal mouthwash as pre-procedural mouthrinse in the reduction of aerosol contamination produced by ultrasonic scaler. Acta Sci Dent Sci. 2018;2:2–6. [Google Scholar]


