Abstract
Background:
Patients with idiopathic intracranial hypertension (IIH) have elevated intracranial pressure (ICP) of unclear etiology. This study evaluated the ability of quantitative intracranial Hounsfield unit (HU) histogram analysis to detect pathophysiological changes from elevated ICP in the setting of a normal head CT.
Methods:
Retrospective analysis of non-contrast-enhanced head CT images of IIH patients and matched controls. Following skull stripping, total intracranial CT voxels within the range of 0-70 HU were divided into seven 10 HU bins. A measurement of total intracranial HU was also calculated for each patient. Imaging studies for IIH patients were reviewed for features of IIH including transverse sinus stenosis (TSS). Histogram measures were compared between IIH and control groups and correlated with imaging and clinical data.
Results:
Fourteen IIH patients with CSF opening pressure ≥25 cm water, and 31 age-, sex-, and ethnicity-matched controls were included. Compared to controls, IIH patients had a significantly greater proportion of voxels in the 40-50, 50-60, and 60-70 HU bins (p = 0.003, 0.001, and 0.003, respectively) but similar proportion in the 0-10 HU range. Severity of TSS significantly correlated with total intracranial HU measures. 50-60 HU and 60-70 HU bins demonstrated high AUCs of 0.81 and 0.80, respectively, in differentiating IIH from normal status.
Conclusion:
Idiopathic intracranial hypertension patients have a greater proportion of high intracranial HU voxels representing blood volume, which may be explained by TSS causing venous congestion. The pattern provides further insights into the pathophysiology of IIH and may be useful for detecting elevated ICP in the setting of normal head CT imaging.
Keywords: Intracranial hypertension, histogram analysis, computed tomography, venous sinus stenosis
Introduction
Idiopathic intracranial hypertension (IIH) is a clinical syndrome characterized by increased intracranial pressure (ICP) of unclear etiology. Although the pathophysiology of IIH is not fully understood, main hypotheses include dysregulation of cerebrospinal fluid (CSF) dynamics through excessive secretion, reduced drainage, or both.1-3 There has also been growing interest in the role of dural venous sinus stenosis in the pathophysiology of IIH, with several studies advocating stenting of transverse venous sinus stenosis (TSS) in selected patients. 4 Neuroimaging modalities such as CT, MRI, and MRV have provided valuable insights on imaging findings involving the brain, orbits, skull base, and venous sinuses in IIH. 5 Despite this, much is still unknown regarding the impact of underlying pathophysiologic mechanisms of IIH on the various intracranial components. Blood and CSF compartments may be disproportionately affected by any alterations in intracranial venous and CSF circulations in IIH.
Quantitative whole-brain CT histogram analysis can provide information on how different CT voxels representing different brain tissues change globally, even in the setting of a visually normal appearance. Various histogram analysis studies have been performed with a wide range of goals using different neuroimaging modalities in entities such as brain tumors, multiple sclerosis, and Alzheimer’s disease.6-8 However, the technique has not previously been applied to IIH patients. Investigation of the patterns of histogram change in IIH patients compared to normal brains could further our understanding of the pathophysiology of the disorder and identify features to detect and monitor disease activity, even in the setting of visually normal imaging. In this study, we performed histogram analysis on intracranial compartments from CT images of IIH patients in comparison to a closely demographically matched control group. We also sought to explore the relationship between histogram measures, other imaging features of IIH, and CSF opening pressure.
Methods
Patients
This retrospective study was approved by our institutional review board (Emory University IRB 44740). IIH patients were identified retrospectively from an electronic medical record search of radiology reports for patients aged 18 years and older presenting to Emory University Hospital’s emergency department (ED) between August 2017 and February 2020. Patients were included if they fulfilled diagnostic criteria for IIH, 9 including papilledema confirmed by a neuro-ophthalmologist, and underwent a non-contrast-enhanced head CT on a single specified ED CT scanner without an intracranial process such as hydrocephalus or a mass lesion, followed within 24 h by a lumbar puncture demonstrating CSF opening pressure ≥25 cm water and normal CSF contents. Patients were excluded if they had a previous history of intracranial surgery including placement of a CSF shunt. Patients either had a pre-existing diagnosis of IIH (n = 6) or the diagnosis of IIH was made during the same hospital admission (n = 8). Age, sex, ethnicity, body mass index (BMI), hemoglobin (gm/dL), and hematocrit (%) were recorded in all patients.
Controls were identified from a list of consecutive patients presenting to the same ED during the same period as IIH patients, who underwent non-contrast-enhanced head CT on the same specified ED CT scanner with other presentations, including headache, trauma, dizziness, and altered mental status. These patients were selected into the control group if the non-contrast head CT was interpreted as normal, a review of the medical records demonstrated no previous history of IIH, and they were matched with the IIH patients by sex, ethnicity, and age range.
Imaging and lumbar puncture protocol
For IIH patients and controls, helical CT imaging was performed on a single 64-detector row scanner (GE Lightspeed VCT, Milwaukee, Wisconsin) without injection of contrast material using standardized acquisition technique with 0.625-mm collimation and 5-mm axial, coronal, and sagittal slice image reconstructions. IIH patients underwent contrast-enhanced CTV (n = 4) or MRI/MRV (n = 8) within the same hospitalization to exclude venous sinus thrombosis except for two cases where there was already an established diagnosis of IIH. MRI/MRV was performed on a 3T (Tim Trio; Siemens, Erlangen, Germany) or 1.5T unit (Avanto or Espree; Siemens, Erlangen, Germany) and included volumetric post-contrast T1-weighted images for evaluation of the dural venous sinuses. CTV was performed using standard technique following intravenous administration of iodinated contrast.
CT and MRI images were reviewed by an experienced neuroradiologist for the presence or absence of an intracranial mass lesion, hydrocephalus, dural venous sinus thrombosis, or other explanation for elevated ICP. Images were also evaluated for the imaging findings of IIH using previously described methods,10-12 including pituitary grade, cerebellar tonsil position, optic nerve head protrusion, optic nerve head enhancement, scleral flattening, increased peri-optic CSF, optic nerve tortuosity, and transverse sinus stenosis (TSS). Severity of TSS was determined using the method described previously by Riggeal et al. 11 and expressed as an average of the percentage of stenosis of both sides (“average TSS”) or by the largest percentage of stenosis (“maximum TSS”).
Lumbar puncture was performed using fluoroscopic guidance in the prone position using a standardized technique reported previously. 13 Opening pressure was measured using CSF manometry and recorded to the nearest centimeter of water.
CT image analysis
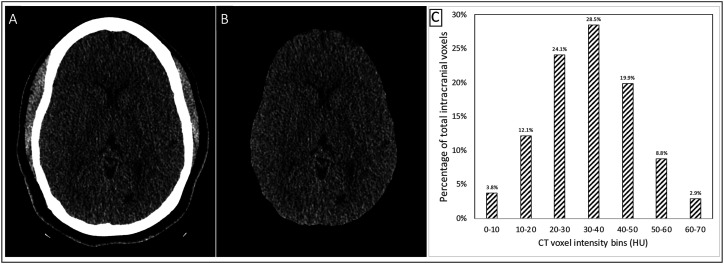
Semi-automated skull stripping was performed to segment the intracranial contents after specifying the level of the foramen magnum (Figure 1(a) and (b)). The 0.625 mm CT images were loaded in 3D Slicer software using the segment editor module. Local Threshold, Threshold, Margin, Islands function, and Paint and Scissor functions were used within the same module for any required final edits of the segmentation. Mask volume function (same module) was used to create the output volume which was then saved as a DICOM series.
Figure 1.
Workflow for histogram analysis: (a). Representative axial non-contrast-enhanced head CT image. (b). Skull-stripped head CT image. (c). Total intracranial histogram analysis for single patient with HU ranging from 0 to 70 in 10 HU bins.
CT voxels within the HU range of 0 to 70 HU were included for histogram analysis using MATLAB. The measured HU values were corrected using the background to account for any scanner miscalibration (a median −0.8 HU (range −6.3 to +0.5 HU) correction) based on expected air HU of −1000). Seven bins, each with a width of 10 HU, were defined between the 0 to 70 HU range. For each patient, the total intracranial voxel count (TIVCC, defined by total voxel counts within the range of 0 to 70 HU) was calculated to normalize for intracranial compartment size differences between patients. The total voxel count was calculated for each of the seven 10 HU bins and then divided by the patient’s TIVCC to generate a distribution of the percentage of total intracranial voxels falling within each of the seven HU range bins (Figure 1(c)). An additional measure called total intracranial HU (TICHU) was calculated by multiplying the total voxel counts per bin by their average HU measurement, then summing this value for each of the seven bins. This TICHU value represents a single total measurement of intracranial HU in a patient.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA). The significance level was set at 0.05. Any missing data points were excluded from analysis. For comparison of demographic variables between IIH and control groups, continuous variables were compared using the Student’s t-test, and proportions were compared using chi-squared or Fisher exact tests, as appropriate. For histogram analysis, the percentage of total intracranial voxels within each bin, TICVC, and TICHU were compared using t-test between the IIH and the control groups. Correlation analysis between various variables was performed using Pearson’s correlation. ROC curve analysis was performed for the percentage of total intracranial voxels within each HU range bin and the TICHU as test variables; state variable was set as being in the IIH group. Analysis of covariance (ANCOVA) was performed to assess the contribution of BMI as a covariate variable in the comparison analysis of the HU range proportions across the intracranial pressure status.
Results
Fourteen IIH patients and 31 age-, sex-, and ethnicity-matched controls were included (Table 1). All patients included in this study were female. There was no difference in the mean age or ethnicity between the two groups. IIH patients had a higher BMI of 40.2 kg/m2 compared to controls (28.2 kg/m2; p = 0.001). The mean CSF opening pressure in the IIH group was 36 cm H2O (range, 26–58 cmH2O). No significant differences in hemoglobin level or hematocrit were found between the two groups. Imaging findings of IIH including abnormal sella configuration, posterior scleral flattening, increased peri-optic CSF, and optic nerve tortuosity were found almost universally among the IIH patients on interpretation of the contrast-enhanced CTV or MRI/MRV.
Table 1.
Demographic features of IIH and control patients.
| Demographic | IIH group (n = 14) | Control group (n = 31) | p-value |
|---|---|---|---|
| Age, years | 33.9 ± 10.5 | 30.8 ± 7.0 | 0.24 |
| Sex, female | 14 (100%) | 31 (100%) | 1.0 |
| Ethnicity African-American Other |
12 (86%) 2 (14%) |
23 (74%) 8 (26%) |
0.47 |
| Body mass index, kg/m2 | 40.1 ± 10.7 | 28.2 ± 10.6 | 0.001 |
| Hemoglobin, gm/dL | 13.1 ± 1.06 | 13.2 ± 1.05 | 0.35 |
| Hematocrit, % | 40.6 ± 2.67 | 39.7 ± 2.89 | 0.32 |
All values displayed as mean ± standard deviation or n (%). IIH, idiopathic intracranial hypertension. Continuous variables were compared between groups using the Student’s t-test, and proportions were compared using chi-squared or Fisher exact tests, as appropriate.
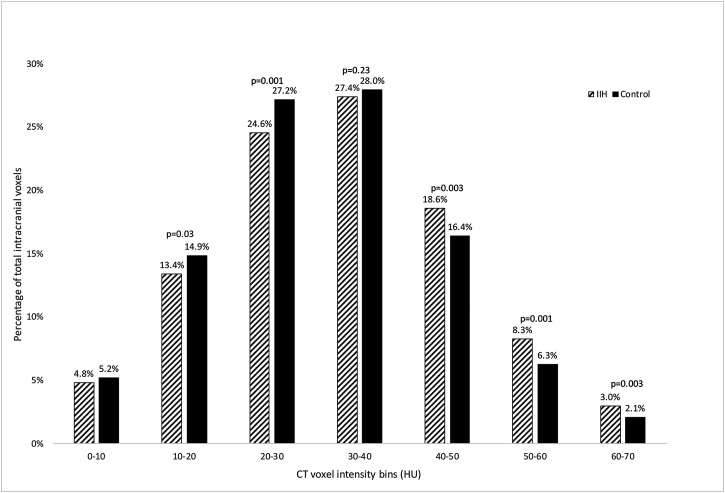
The distribution of brain CT voxels between the range of 0 to 70 HU is illustrated in Figure 2. The proportion of CT voxels within the bins of 40-50, 50-60, and 60-70 HU was significantly higher in IIH patients compared to controls (p = 0.003, 0.001, and 0.003, respectively) and was significantly lower within the bins of 10-20 and 20-30 HU (p = 0.03 and 0.001, respectively). Compared to controls, IIH patients had 33% more blood density voxels (combining the 50-60 and 60-70 range bins into 50-70 HU) [IIH versus controls, 11.2% vs 8.4%, p = 0.002], and a non-significant difference in CSF HU voxels (0 to 10 HU) [IIH versus controls, 18.2% vs 20.1%, p = 0.20]. Mean TICVC and mean TICHU were not significantly different between IIH patients and controls (mean TICVC: 8975453 and 8869849 respectively, p = 0.72; mean TICHU: 294246450 and 277459588 respectively, p = 0.12). Results of the ANCOVA for the bin analysis between IIH and control groups resulted in the group level differences no longer remaining significant, indicating that BMI is a confounder to the group level analysis.
Figure 2.
Total intracranial voxel histogram distributions for IIH patients and controls. Bars represent the proportion of the total intracranial voxels that fall within the bin range of HU for the IIH group (n = 14; diagonal lines) and control group (n = 31; solid). Significantly higher proportions in the IIH group fall within the 40-50 HU, 50-60 HU, and 60-70 HU bins compared to the control group. Significantly lower proportions in the IIH group fall in the 10-20 and 20-30 HU bins compared to the control group. The 0-10 HU and 30-40 HU bins have similar proportions in both groups.
Twelve IIH patients completed CTV or MRV and were included in the TSS analysis. The remaining two had a pre-existing diagnosis of IIH and did not have repeat venography performed. All twelve IIH patients had bilateral TSS with at least 50% stenosis of both transverse venous sinuses, with mean average TSS of 77.1% and maximum TSS of 82.5% (range, 50–95% for both). There was no difference in TSS scores on analysis of the IIH patients according to the status of their IIH diagnosis. The severity of TSS was significantly correlated with total intracranial measures. There was a mildly positive correlation between maximum TSS and TICHU (r = 0.65, p = 0.02). There was also a weakly positive correlation between average TSS and TICHU, which did not reach significance (r = 0.57, p = 0.052). CSF opening pressure did not correlate with TICVC or TICHU measures. ROC analysis was performed for the seven HU range bins and TICHU (Table 2). The higher HU bin ranges had higher area under the curves, with 50-60 HU and 60-70 HU bins having the highest AUCs of 0.81 and 0.80, respectively.
Table 2.
Area under the curve for relative frequency of the 10 HU wide histogram bins and total intracranial HU measure.
| Parameter | AUC |
| 0-10 | 0.442 |
| 10-20 | 0.304 |
| 20-30 | 0.198 |
| 30-40 | 0.394 |
| 40-50 | 0.783 |
| 50-60 | 0.809 |
| 60-70 | 0.804 |
| TICHU | 0.680 |
AUC, area under curve; HU, Hounsfield unit; TICHU, total intracranial Hounsfield units
Discussion
This is the first study to utilize intracranial histogram analysis in IIH patients using standard non-contrast head CT imaging compared to demographically matched controls. Our findings on the change of histogram pattern in IIH patients provide further insights into the pathophysiologic mechanisms that underpin this disease. Our study also provides some preliminary evidence for the usefulness of histogram analyses in the clinical assessment of patients with suspected IIH and normal head CT imaging.
The vast majority of the skull-stripped intracranial space voxels fall within the 0-70 HU range, apart from negligible amounts of calcification of the pineal gland and choroid plexus. IIH patients had significantly more voxels in the higher HU bin ranges, and significantly fewer voxels in the lower HU bin ranges. The highest bin ranges of 50-60 and 60-70 HU can be considered large vascular volume including dural venous sinuses, cortical veins, and large arteries that do not suffer from partial volume effects from surrounding brain tissue or CSF. Since patients presenting to the ED may be hemoconcentrated, potentially impacting the observed HU within vascular structures, we assessed hemoglobin and hematocrit at the time of CT imaging and found no significant differences between the two groups. The 40-50 HU and 30-40 HU ranges likely reflect combined brain parenchyma and microvascular volume. The 0-10 HU range can be considered pure CSF volume within the ventricles and subarachnoid space, with the 10-20 HU range perhaps containing some CSF partial volume averaged with adjacent brain parenchyma. In this study we found significantly (approximately 33%) higher large vascular HU (50-70 HU) voxels in IIH, which is felt to represent large venous engorgement due to downstream TSS. The significantly higher proportion of voxels in the 40-50 HU bin in IIH patients also suggests smaller vessel venous engorgement within or adjacent to the gray matter. While the CSF bin (0-10 HU) was decreased on average in IIH patients compared to controls, the magnitude of difference was small and not statistically significant. The shift of the histogram towards voxels that represent blood volume, and similar voxels representing CSF may indicate that the intracranial blood compartment has a greater role in the pathophysiology of IIH rather than the traditionally held view that IIH is primarily a disorder of CSF dysregulation.
We did not control for differences in BMI between the two groups, since we did not wish to generate a control group with similarly high BMI and potentially unrecognized IIH, particularly as the controls did not have lumbar puncture to document lack of elevated ICP. IIH is very highly associated with obesity and therefore it is difficult to separate the effects of obesity and elevated ICP. Prior studies of structural brain differences associated with obesity have demonstrated reductions in gray matter volume and increases in CSF volume;14,15 however, these would be expected to result in comparatively minor and opposite effects on the CT HU histogram as our observed current findings. We therefore consider it unlikely that obesity itself (and not elevated ICP) was the explanation for our findings.
The Monro-Kellie doctrine of homeostatic intracerebral volume regulation gives equal weighting to blood and CSF. 16 However, the slow, steady production of CSF is dwarfed by substantial, continuous blood inflow and outflow. Failure of venous outflow to precisely match arterial flow is recognized to lead to changes in intracranial blood volume and pressure. 16 The increase in blood HU range (50-70 HU) voxels in IIH patients in our study is felt to reflect venous engorgement due to reduced cerebral venous outflow from TSS, which is a near-universal finding in IIH patients and is almost always bilateral.11,17 We found a significant correlation between maximum TSS scores and TICHU, indicating an aggregate shift in intracranial voxels towards higher (intravascular blood range) HU when TSS severity was greater.
Transverse venous sinus stenosis is a highly prevalent finding in IIH, with pooled sensitivity of 84% and pooled specificity of 97% for IIH. 18 The relationship between TSS and ICP is unclear. Obstruction of venous outflow by compression of the jugular veins in Queckenstedt’s maneuver leads to increase in ICP, which can be measured as the CSF opening pressure on lumbar puncture. 19 However, previous studies of IIH patients have not found a correlation between TSS severity, CSF pressure, or clinical outcomes. 11 Additionally, substantial bilateral TSS persists immediately after acute reduction of ICP by lumbar puncture, 20 suggesting a compensatory mechanism such as the development of collateral channels. This is supported by a recent study that found the occipital emissary vein (OEV) was more frequent and larger in IIH patients compared to healthy controls. 21 Emissary veins play an important role in directing cerebral venous blood toward cervical outflow pathways and act as collateral channels.
The pathophysiology of IIH has long been hypothesized to be due to dysregulation of CSF dynamics leading to a net increase in CSF. 3 Many studies have focused on mechanisms that drive increased CSF production such as excess androgen, or reduced CSF absorption due to impaired permeability of the arachnoid granulations. We did not find an increase in CSF voxels in IIH patients to indicate abnormal CSF accumulation. Instead, there was on average a small and nonsignificant reduction in CSF voxels compared to controls. The reduction in CSF voxels could be due to a shift of intracranial CSF into the spinal subarachnoid space. Alternatively, if CSF production is driven by the hydrostatic pressure gradient between blood, choroid plexus epithelial cells, and ventricles, according to Starling’s law of filtration,21,22 then raised ICP may result in a compensatory reduction of CSF production.
The presence of imaging signs of raised ICP on contrast-enhanced CTV or MRI/MRV was near universal in our IIH group, regardless of whether the IIH diagnosis was pre-existing or made during the same hospitalization. All had active disease at the time of their normal head CT with elevated opening pressure on lumbar puncture performed within 24 h. Intracranial CT histogram analysis could therefore be used in clinical practice to detect features of elevated ICP. However, further studies are required to determine if histogram pattern analysis can be used to distinguish IIH patients with active disease from those with inactive disease, patients with TSS who have normal ICP, and IIH patients who do not have TSS.
There are several limitations to our study. Due to the retrospective nature of the study design, controls were selected based on normal CT imaging and the majority did not undergo MRI or venography to determine the presence of other radiological signs of raised ICP or the prevalence of TSS, and none of the controls underwent lumbar puncture to confirm normal ICP, though inadvertent inclusion of controls with high ICP would have only diminished the magnitude of the observed differences. For similar reasons, we were only able to include 14 patients with IIH as we needed to confirm that all met diagnostic criteria for IIH, including the presence of papilledema and elevated CSF opening pressure. Furthermore, CT imaging is infrequently performed for individuals with suspected IIH due to availability of MRI at our institution. Our study was intentionally limited to a single CT scanner with identical acquisition parameters to limit inter-scanner variation, and the measured HU was corrected by subtraction of background to minimize the effects of minor variations in scanner HU calibration. Further studies will need to address the resultant variation from use of different scanners within and between patient groups.
Conclusions
Contrary to the established view that expansion of the intracranial CSF compartment underpins the pathophysiology of IIH, IIH patients have a greater proportion of high-HU voxels representing intravascular blood on quantitative CT histogram analysis compared to matched controls, and statistically similar low-HU voxels representing CSF. Expansion of the intracranial blood compartment may be explained by TSS restricting cerebral venous outflow. The pattern of changes in the CT histogram of IIH patients provides further insights into the pathophysiology of this condition and may be useful as a tool for differentiating between IIH and normal brains in clinical practice alongside other clinical and radiological markers of disease.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported in part by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine). VB and NJN are consultants for GenSight Biologics. NJN is a consultant for Santhera Pharmaceuticals and Stealth BioTherapeutics.
ORCID iDs
Solmaz Asnafi https://orcid.org/0000-0003-4059-167X
Benson S Chen https://orcid.org/0000-0001-8214-0186
References
- 1.Baykan B, Ekizoglu E, Altiokka Uzun G. An update on the pathophysiology of idiopathic intracranial hypertension alias pseudotumor cerebri. Agri 2015; 27: 63–72. DOI: 10.5505/agri.2015.22599 [DOI] [PubMed] [Google Scholar]
- 2.McGeeney BE, Friedman DI. Pseudotumor cerebri pathophysiology. Headache 2014; 54: 445–458. DOI: 10.1111/head.12291 [DOI] [PubMed] [Google Scholar]
- 3.Donaldson JO. Pathogenesis of pseudotumor cerebri syndromes. Neurology 1981; 31: 877–880. DOI: 10.1212/wnl.31.7.877 [DOI] [PubMed] [Google Scholar]
- 4.Dinkin M, Oliveira C. Men are from mars, idiopathic intracranial hypertension is from venous: the role of venous sinus stenosis and stenting in idiopathic intracranial hypertension. Semin Neurol 2019; 39: 692–703. DOI: 10.1055/s-0039-3399506 [DOI] [PubMed] [Google Scholar]
- 5.Bidot S, Saindane AM, Peragallo JH, et al. Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol 2015; 35: 400–411. DOI: 10.1097/WNO.0000000000000303 [DOI] [PubMed] [Google Scholar]
- 6.Nusbaum AO, Tang CY, Wei T, et al. Whole-brain diffusion MR histograms differ between MS subtypes. Neurology 2000; 54: 1421–1427. DOI: 10.1212/wnl.54.7.1421 [DOI] [PubMed] [Google Scholar]
- 7.Ma JH, Kim HS, Rim NJ, et al. Differentiation among glioblastoma multiforme, solitary metastatic tumor, and lymphoma using whole-tumor histogram analysis of the normalized cerebral blood volume in enhancing and perienhancing lesions. AJNR Am J Neuroradiol 2010; 31: 1699–1706. DOI: 10.3174/ajnr.A2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giulietti G, Torso M, Serra L, et al. Whole brain white matter histogram analysis of diffusion tensor imaging data detects microstructural damage in mild cognitive impairment and alzheimer’s disease patients. J Magn Reson Imaging 2018. DOI: 10.1002/jmri.25947 [DOI] [PubMed] [Google Scholar]
- 9.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013; 81: 1159–1165. DOI: 10.1212/WNL.0b013e3182a55f17 [DOI] [PubMed] [Google Scholar]
- 10.Agid R, Farb RI, Willinsky RA, et al. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology 2006; 48: 521–527. DOI: 10.1007/s00234-006-0095-y [DOI] [PubMed] [Google Scholar]
- 11.Riggeal BD, Bruce BB, Saindane AM, et al. Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology 2013; 80: 289–295. DOI: 10.1212/WNL.0b013e31827debd6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuh WT, Zhu M, Taoka T, et al. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging 2000; 12: 808–813. [DOI] [PubMed] [Google Scholar]
- 13.Hu R, Holbrook J, Newman NJ, et al. Cerebrospinal fluid pressure reduction results in dynamic changes in optic nerve angle on magnetic resonance imaging. J Neuroophthalmol 2019; 39: 35–40. DOI: 10.1097/WNO.0000000000000643 [DOI] [PubMed] [Google Scholar]
- 14.Bobb JF, Schwartz BS, Davatzikos C, et al. Cross-sectional and longitudinal association of body mass index and brain volume. Hum Brain Mapp 2014; 35: 75–88. DOI: 10.1002/hbm.22159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK biobank study. Radiology 2019; 291: 763–771. DOI: 10.1148/radiol.2019181012 [DOI] [PubMed] [Google Scholar]
- 16.Wilson MH. Monro-Kellie 2.0: the dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab 2016; 36: 1338–1350. DOI: 10.1177/0271678X16648711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bono F, Giliberto C, Mastrandrea C, et al. Transverse sinus stenoses persist after normalization of the CSF pressure in IIH. Neurology 2005; 65: 1090–1093. DOI: 10.1212/01.wnl.0000178889.63571.e5 [DOI] [PubMed] [Google Scholar]
- 18.Kwee RM, Kwee TC. Systematic review and meta-analysis of MRI signs for diagnosis of idiopathic intracranial hypertension. Eur J Radiol 2019; 116: 106–115. DOI: 10.1016/j.ejrad.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 19.Queckenstedt . Zur Diagnose der Rückenmarkskompression. Deutsche Zeitschrift für Nervenheilkunde 1916; 55: 325–333. DOI: 10.1007/BF01733057 [DOI] [Google Scholar]
- 20.Narayana K, Saindane A, Bruce B, et al. Persistence of transverse sinus stenosis after lumbar puncture in idiopathic intracranial hypertension (IIH). In: Abstract: AAN 69th Annual Meeting Boston; 2017 April 22-28; Boston, United States. Neurology 2017; 88: p3.297. [Google Scholar]
- 21.Hedjoudje A, Piveteau A, Gonzalez-Campo C, et al. The occipital emissary vein: a possible marker for pseudotumor cerebri. AJNR Am J Neuroradiol 2019; 40: 973–978. DOI: 10.3174/ajnr.A6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oreskovic D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev 2010; 64: 241–262. DOI: 10.1016/j.brainresrev.2010.04.006 [DOI] [PubMed] [Google Scholar]