Abstract
Background
Atrial fibrillation (AF) is the most common heart rhythm disorder among adults and leads to substantial morbidity and mortality.
Objectives
The purpose of the study was to provide current estimates on the incremental healthcare utilization and cost burden associated with incident AF diagnosis in the United States.
Methods
Adults with an incident diagnosis of AF (2017–2020) were identified using the Optum Clinformatics database. Propensity matching was employed to match patients with incident AF to a comparator group of non-AF patients on several demographic and clinical characteristics. Outcomes including 12-month all-cause and cardiovascular (CV)-related healthcare utilization, as well as the medical cost associated with health services use, were assessed. Logistic and general linear models were used to examine study outcomes. Sub-analyses were performed to determine the incremental AF burden by specific sex and racial/ethnic categories.
Results
A total of 79,621 patients were identified in each cohort (AF and non-AF). As compared to the non-AF cohort, patients with AF had significantly higher all-cause inpatient visits (relative risk [RR] 1.77; 95% confidence interval [CI] 1.76–1.78), CV-related inpatient visits (RR 2.51; 95% CI 2:49–2:53), and CV-related emergency room visits (RR: 2.41; 95% CI 2:35–2:47). The mean total healthcare cost for patients with AF was $27,896 more (per patient per year) than the non-AF cohort ($63,031 vs $35,135, P < .001).
Conclusion
Medical services utilization and cost were significantly higher among AF patients than non-AF patients. Early treatment is likely to be critical to addressing the considerable disease burden imposed by AF.
Keywords: Atrial fibrillation, Healthcare utilization, Medical costs, Health burden, United States
Key Findings.
-
▪
As the incidence and prevalence of atrial fibrillation (AF) continue to increase owing to the aging population, contemporary data on the incremental burden of AF are needed. Further, information on AF burden among different racial/ethnic and sex categories is lacking.
-
▪
Our study highlights the considerable burden of medical services utilization associated with AF. The magnitude of cost burden among patients with AF is substantial and seems to be greater than past estimates.
-
▪
Though the incremental burden of AF was observed among all racial/ethnic and sex categories, acute-care utilization was especially pronounced among females and Asians, respectively.
Introduction
Atrial fibrillation (AF) is the most common heart rhythm disorder.1 There is a growing body of evidence to suggest that the incidence and prevalence of AF are increasing globally.2, 3, 4 For instance, using data derived from the Global Health Data Exchange database, a comprehensive global inventory of health-related data and statistics, it was found that from 1997 to 2017, the estimated global incidence rate of AF increased by 31%.4 This global trend appears to hold true in the United States.5, 6, 7 In the Framingham Heart Study, an ongoing cardiovascular cohort study that began in Framingham, Massachusetts, in 1948, the age-adjusted period prevalence of AF increased nearly 4-fold from the period 1958–1967 to 1998–2007.3 By 2050, it has been projected that AF will directly impact 6–12 million persons in the United States.8,9 Such evidence of emerging incidence and prevalence taken with future projections is cause for concern as AF continues to demonstrate a wide range of negative consequences, including diminished functional capacity and quality of life10,11 and increased risk for stroke,12,13 as well as cardiovascular comorbidities and events.14
A few studies have shown that the morbidity from AF translates into considerable healthcare utilization and economic burden.15, 16, 17 For example, a large US-based cross-sectional study that used administrative encounter data derived from the Nationwide Inpatient Sample18 and the Healthcare Cost and Utilization Project’s Nationwide Emergency Department Sample found that 450,000 hospitalizations and 600,000 emergency room (ER) visits were directly attributable to AF in 2014.17 The mean total cost per hospitalization and ER visit was found to be $8819 and $4040, respectively.17 Another study by Patel and colleagues18 using the Nationwide Inpatient Sample database showed a 23% increase in AF-related hospitalizations in the decade of 2000. The authors also reported the average cost of AF-related hospitalization to have increased by 24% during their study period.18
Though these studies provide useful information, much of the information on AF healthcare burden is dated. There is limited information about the current association between AF and healthcare utilization and costs among different racial and ethnic groups and sexes.15 This study aimed to provide updated information on the healthcare burden associated with AF.
Methods
Data source
We used the Optum Clinformatics database, which is an administrative claims database for commercially insured (United Healthcare) patients in the United States.19 Optum Clinformatics comprises enrollment data and physician, facility, and pharmacy claims for approximately 13 million private insurance and Medicare Advantage beneficiaries from geographically diverse regions throughout the United States. Optum includes de-identified data only, and The New England Institutional Review Board has determined that studies conducted using this database are exempt from study-specific institutional review board review, as these studies do not involve active human subject participation.
Study population
Patients at least 19 years of age and above with 2 or more medical service visits (any setting) with a primary diagnosis of AF within a period of 90 days between January 1, 2017, and March 31, 2020, were eligible for inclusion in the AF cohort. Our intention of considering at least 2 visits was to reduce the chance of false-positive diagnosis. The first such visit was classified as the index visit. Patients must be continuously enrolled for 12 months pre- and post-index AF visit. Patients were excluded if, in the 12-month pre-index period, they had a medical services visit (any setting) with a primary or secondary diagnosis of AF or had a prescription claim for an antiarrhythmic drug (AAD) (including amiodarone, disopyramide, dofetilide, dronedarone, flecainide, quinidine, propafenone, and sotalol). The above inclusion and exclusion criteria were used to identify newly diagnosed (incident) cases of AF.
A comparator cohort of non-AF patients who did not have any medical services visit with a primary or secondary diagnosis of AF and without a prescription fill for an AAD during the study period (2017–2020) were identified. To determine an index date for non-AF cohort, we identified their latest year without an AF diagnosis. The first day of that year was then assigned as the index date. Patients in the non-AF cohort were also required to have continuous enrollment in the 12-months pre– and post–index date. Our rationale for having 12-month continuous enrollment pre–index date as a requirement for both AF and non-AF cohort participants was to allow for a better understanding of participant comorbidity status at baseline.
Study outcomes and covariates
Primary outcomes of interest included all-cause, cardiovascular (CV)-related, and AF-related inpatient, outpatient (including office, walk-in retail health clinic, or ambulatory surgery center), ER (including urgent care), and other medical visits (such as pharmacy, ambulance, mobile unit, nursing facility, skilled nursing facility, or residential substance abuse treatment facility); cost associated with medical services use; and total healthcare costs (including costs related to inpatient, outpatient, ER, and other medical visits, and prescription costs). Costs were adjusted for medical inflation and reported in 2021 US dollars. Notably, AF-related visits/costs were defined as any visits/costs with a primary diagnosis of AF. To comprise the definition of an AF diagnosis, the following ICD-9 and ICD-10 diagnosis codes were considered: 427.31 (fibrillation, atrial), I48.0 (paroxysmal atrial fibrillation), I48.1x (persistent atrial fibrillation), I48.2x (chronic atrial fibrillation), and I48.91 (unspecified atrial fibrillation).
Study covariates included age, sex, geographic region (Northeast, Midwest, South, West), race/ethnicity (as assessed during index visit; White, Black, Asian, Hispanic, unknown race), Elixhauser comorbidity score,20 CHA₂DS₂-VASc scores,21 obstructive sleep apnea, hyperthyroidism, and prior history of cardiac surgery.
Statistical analysis
Propensity score matching, with a 1:1 greedy nearest-neighbor matching algorithm with a caliper width of 0.10, was used to match patients with AF to those without AF. The propensity model was derived from a multivariable logistic regression that included all study covariates (ie, age, sex, geographic region, race, year, and clinical characteristics). The balance of covariates among the matched cohort was assessed using standardized mean differences (SMDs). Covariates were considered imbalanced if the SMD was greater than 0.25 or less than -0.25. Consistent with a double-adjustment approach,22 any imbalanced covariates in the matched cohort were adjusted for during regression analyses. As a final step in our primary analysis, we employed several independent sample t tests to compare the mean number of medical services events for the AF and non-AF cohort.
Sub-analyses were performed by sex, race/ethnicity, and age categories. A separate propensity-matched sample was identified for each category, and outcomes were compared in the matched cohort. Logistic regression analysis compared all-cause and CV-related healthcare visits among matched AF vs non-AF patients. A general linear model was used to compare costs among the matched patients. All analyses were conducted using R for Windows, version 4.0.2.23
Results
A total of 79,621 patients met the inclusion criteria for the AF cohort. Supplemental Table 1 shows the attrition steps for incident AF cohort identification. A random sample of approximately 1 million non-AF controls were extracted from the database. After propensity matching, 79,621 patients were identified in each study cohort (AF and non-AF, respectively). The matched cohorts were well balanced with regard to sex, age, geographic region, race, and comorbidity covariates (SMD ≥0.25 or ≤-0.25) (Table 1). Post-matching, the mean age was 74.1 years for the AF cohort and 73.2 years for the non-AF cohort (SMD = 0.084); both groups were approximately 50% male (SMD = 0.012). Approximately three-quarters of patients were White (77.4% AF, 74.2% non-AF), 8.6% (AF) and 10.2% (non-AF) were Black, 8.0% (AF) and 8.7% (non-AF) were Hispanic, and 1.9% (AF) and 2.1% (non-AF) were Asian.
Table 1.
Sample characteristics for patients with incident diagnosis of atrial fibrillation and patients without atrial fibrillation
| Pre-match |
Post-match |
|||||
|---|---|---|---|---|---|---|
| AF N = 79,621 |
No-AF N = 917,459 |
SMD† | AF N = 79,621 |
No-AF N = 79,621 |
SMD† | |
| Age, years, mean ± SD | 74.1 ± 10.7 | 53.8 ± 18.9 | 1.325 | 74.1 ± 10.7 | 73.23 ± 10.92 | 0.084 |
| Age, years | 1.331 | 0.046 | ||||
| 19–39 | 833 (1.0) | 252,299 (27.5) | 833 (1.0) | 802 (1.0) | ||
| 40–49 | 1650 (2.1) | 129,210 (14.1) | 1650 (2.1) | 1536 (1.9) | ||
| 50–59 | 5003 (6.3) | 142,227 (15.5) | 5003 (6.3) | 4855 (6.1) | ||
| 60–69 | 13,990 (17.6) | 168,082 (18.3) | 13,990 (17.6) | 15,379 (19.3) | ||
| 70+ | 58,145 (73.0) | 225,641 (24.6) | 58,145 (73.0) | 57,049 (71.7) | ||
| Male | 39,305 (49.4) | 411,014 (44.8) | 0.092 | 39,305 (49.4) | 39,776 (50.0) | 0.012 |
| Race | 0.267 | 0.077 | ||||
| White | 61,597 (77.4) | 609,681 (66.5) | 61,597 (77.4) | 59,087 (74.2) | ||
| Black | 6841 (8.6) | 93,410 (10.2) | 6841 (8.6) | 8121 (10.2) | ||
| Hispanic | 6408 (8.0) | 107,634 (11.7) | 6408 (8.0) | 6897 (8.7) | ||
| Asian | 1549 (1.9) | 41,569 (4.5) | 1549 (1.9) | 1684 (2.1) | ||
| Unknown | 3226 (4.1) | 65,165 (7.1) | 3226 (4.1) | 3832 (4.8) | ||
| Region | 0.095 | 0.111 | ||||
| Northeast | 10,264 (12.9) | 106,141 (11.6) | 10,264 (12.9) | 11,332 (14.2) | ||
| Midwest | 19,234 (24.2) | 224,275 (24.4) | 19,234 (24.2) | 19,608 (24.6) | ||
| South | 31,379 (39.4) | 397,499 (43.3) | 31,379 (39.4) | 33,461 (42.0) | ||
| West | 18,744 (23.5) | 189,544 (20.7) | 18,744 (23.5) | 15,220 (19.1) | ||
| Elixhauser score | 5.057 ± 2.605 | 2.25 ± 2.51 | 1.097 | 5.057 ± 2.605 | 4.98 (3.01) | 0.026 |
| CHA2DS2VASc‡ score 2+ | 3.907 ± 1.842 | 1.95 ± 1.79 | 1.080 | 3.907 ± 1.842 | 3.90 (1.92) | 0.005 |
| Sleep apnea | 11,963 (15.0) | 81,485 (8.9) | 0.190 | 11,963 (15.0) | 12,008 (15.1) | 0.002 |
| Hyperthyroidism | 1156 (1.5) | 7436 (0.8) | 0.061 | 1156 (1.5) | 1135 (1.4) | 0.002 |
| Prior cardiac surgery | 5632 (7.1) | 14,751 (1.6) | 0.271 | 5632 (7.1) | 4618 (5.8) | 0.052 |
| Year§ | 1.361 | 0.061 | ||||
| 2017 | 23,755 (29.8) | 111,650 (12.2) | 23,755 (29.8) | 22,683 (28.5) | ||
| 2018 | 25,018 (31.4) | 122,146 (13.3) | 25,018 (31.4) | 23,743 (29.8) | ||
| 2019 | 25,485 (32.0) | 135,976 (14.8) | 25,485 (32.0) | 27,660 (34.7) | ||
| 2020 | 5363 (6.7) | 547,687 (59.7) | 5363 (6.7) | 5535 (7.0) | ||
Data are n (%) or mean ± standard deviation.
AF = atrial fibrillation; SMD = standardized mean difference.
SMD greater than 0.25 or less than -0.25 indicates an unbalanced covariate.
CHADS2VASc stands for congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age 65–74 years, sex category.
Index year of AF diagnosis for AF-cohort and index year for non-AF cohort.
Incident AF-related healthcare use and costs among AF cases in the 12 months post–index visit are shown in Table 2. Roughly 37% of patients with AF had an AF-related inpatient visit in the 12 months after being diagnosed. Almost 88% of patients with AF had AF-related outpatient visits, 22.5% had other AF-related medical visits, and 8.6% had AF-related ER visits. The average cost per patient was $3530 (95% CI $3328–$3732) for inpatient visits, $3878 (95% CI $3769–$3987) for outpatient visits, and $394 (95% CI $358–$430) for ER visits.
Table 2.
Atrial fibrillation–related healthcare utilization and average per-patient atrial fibrillation–related costs in the 12 month period post incident diagnosis
| AF patients N = 79,621 |
|
|---|---|
| AF-related healthcare utilization | |
| Inpatient visits | 29,564 (37.1) |
| Outpatient visits | 70,099 (88.0) |
| ER visits | 6873 (8.6) |
| Other medical visits† | 17,912 (22.5) |
| AF-related healthcare costs (US dollars) | |
| Inpatient visits | $3530 ($3328–$3732) |
| Outpatient visits | $3878 ($3769–$3987) |
| ER visits | $394 ($358–$430) |
| Other medical visits† | $299 ($284–$314) |
Data are n (%) or mean and 95% CI.
AF = atrial fibrillation; ER = emergency room.
Other medical costs included any medical visits that were not captured with the inpatient, outpatient, or ER visit categories (eg, pharmacy, ambulance, mobile unit, nursing facility, skilled nursing facility, or residential substance abuse treatment facility).
All-cause and CV-related healthcare use comparisons among patients with and without AF in the 12 months post–index visit are presented in Table 3. All-cause healthcare use was significantly higher among patients with AF as compared to patients without AF, including all-cause inpatient visits (relative risk [RR] 1.77, 95% confidence interval [CI] 1.76–1.78; P < .001), outpatient visits (RR 1.01, 95% CI 1.01–1.01; P < .001), ER visits (RR 1.23, 95% CI 1.21–1.24; P < .001), and other medical visits (RR 1.13, 95% CI 1.12–1.35; P < .001). In addition, CV-related healthcare use was also higher among patients with AF compared to non-AF controls, including CV-related inpatient visits (RR 2.51, 95% CI 2.49–2.53; P < .001), outpatient visits (RR 1.27, 95% CI 1.27–1.27; P < .001), ER visits (RR 2.41, 95% CI 2.35–2.47; P < .001), and other medical visits (RR 1.67, 95% CI 1.65–1.69; P < .001). Across each individual medical services type, a comparison of the mean number of medical services events for the AF and non-AF cohort revealed significant differences (P < .001).
Table 3.
Medical services utilization in the 12-month period among patients with atrial fibrillation vs those without atrial fibrillation
| AF N = 79,621 |
No AF N = 79,621 |
P value | Absolute risk difference (95% CI) | Relative risk (95% CI) | |
|---|---|---|---|---|---|
| All-cause healthcare use | |||||
| Inpatient visits | 42,475 (53.3) | 19,644 (24.7) | <.001 | 29% (28%–29%) | 1.77 (1.76–1.78) |
| Outpatient visits | 78,809 (99.0) | 77,801 (97.7) | <.001 | 1% (1%–1%) | 1.01 (1.01–1.01) |
| ER visits | 31,417 (39.5) | 25,236 (31.7) | <.001 | 8% (7%–8%) | 1.23 (1.21–1.24) |
| Other medical visits† | 55,307 (69.5) | 48,203 (60.5) | <.001 | 9% (8%–9%) | 1.13 (1.12–1.35) |
| CV-related healthcare use | |||||
| Inpatient visits | 36,658 (46.0) | 9,278 (11.7) | <.001 | 34% (34%–35%) | 2.51 (2.49–2.53) |
| Outpatient visits | 76,575 (96.2) | 46,962 (59.0) | <.001 | 37% (37%–38%) | 1.27 (1.27–1.27) |
| ER visits | 12,612 (15.8) | 4822 (6.1) | <.001 | 10% (9%–10%) | 2.41 (2.35–2.47) |
| Other medical visits† | 32,359 (40.6) | 17,615 (22.1) | <.001 | 19% (18%–19%) | 1.67 (1.65–1.69) |
Data are n (%).
AF = atrial fibrillation; CI = confidence interval; CV = cardiovascular; ER = emergency room.
Other medical visits included any medical visits that were not captured with the inpatient, outpatient, or ER visit categories (eg, pharmacy, ambulance, mobile unit, nursing facility, skilled nursing facility, or residential substance abuse treatment facility).
Average healthcare costs per patient in the 12 months post–index visit are presented in Table 4. The mean all-cause inpatient visit costs were $16,440 higher (P < .001) and outpatient costs were $5638 higher (P < .001) among patients with AF vs patients without AF. All-cause ER visits were $2605 higher (P < .001) and other medical visits were $1562 higher (P < .001) for AF cases vs non-AF controls. Similar results were observed for CV-related medical services utilization. The total healthcare costs were ∼$28,000 (95% CI $26,592–$29,199) higher for patients with AF vs non-AF controls.
Table 4.
Healthcare costs in the 12-month period among patients with atrial fibrillation vs those without atrial fibrillation
| AF mean cost (95% CI) | No AF mean cost (95% CI) | Mean cost difference (95% CI) | P value† | |
|---|---|---|---|---|
| All-cause healthcare cost | ||||
| Inpatient visits | $30,859 ($29,889–$31,829) | $14,419 ($13,874–$14,963) | $16,440 ($15,328–$17,553) | <.001 |
| Outpatient visits | $16,206 ($15,974–$16,438) | $10,568 ($10,366–$10,769) | $5638 ($5331-$5945) | <.001 |
| ER visits | $6538 ($6355–$6721) | $3933 ($3809–$4057) | $2605 ($2384–$2826) | <.001 |
| Other medical visits‡ | $3864 ($3694–$4033) | $2302 ($2175–$2430) | $1562 ($1349–$1773) | <.001 |
| CV-related healthcare costs | ||||
| Inpatient visits | $11,804 ($11,277–$12,330) | $2480 ($2263–$2696) | $9324 ($8754–$9893) | <.001 |
| Outpatient visits | $6883 ($6744–$7022) | $1069 ($1030–$1108) | $5814 ($5670–$5959) | <.001 |
| ER visits | $810 ($758–$861) | $159 ($138–$180) | $651 ($595–$706) | <.001 |
| Other medical visits‡ | $877 ($849–$905) | $288 ($271–$306) | $589 ($555–$622) | <.001 |
| Prescription costs§ | $5565 ($5475–$5654) | $3914 ($3818–$4009) | $1651 ($1520–$1782) | <.001 |
| Total healthcare costs‖ | $63,031 ($61,923–$64,138) | $35,135 ($34,448–$35,823) | $27,896 ($26,592-$29,199) | <.001 |
AF = atrial fibrillation; CI = confidence interval; CV = cardiovascular; ER = emergency room; SD = standard deviation.
From bivariate general linear model.
Other medical visits included any medical visits that were not captured with the inpatient, outpatient, or ER visit categories (eg, pharmacy, ambulance, mobile unit, nursing facility, skilled nursing facility, or residential substance abuse treatment facility).
Prescription costs includes any prescription drug claims present in the database, nonspecific to any diagnosis.
Total healthcare costs include costs of all-cause inpatient visits, all-cause outpatient visits, all-cause ER visits, all-cause other medical visits, and prescription costs.
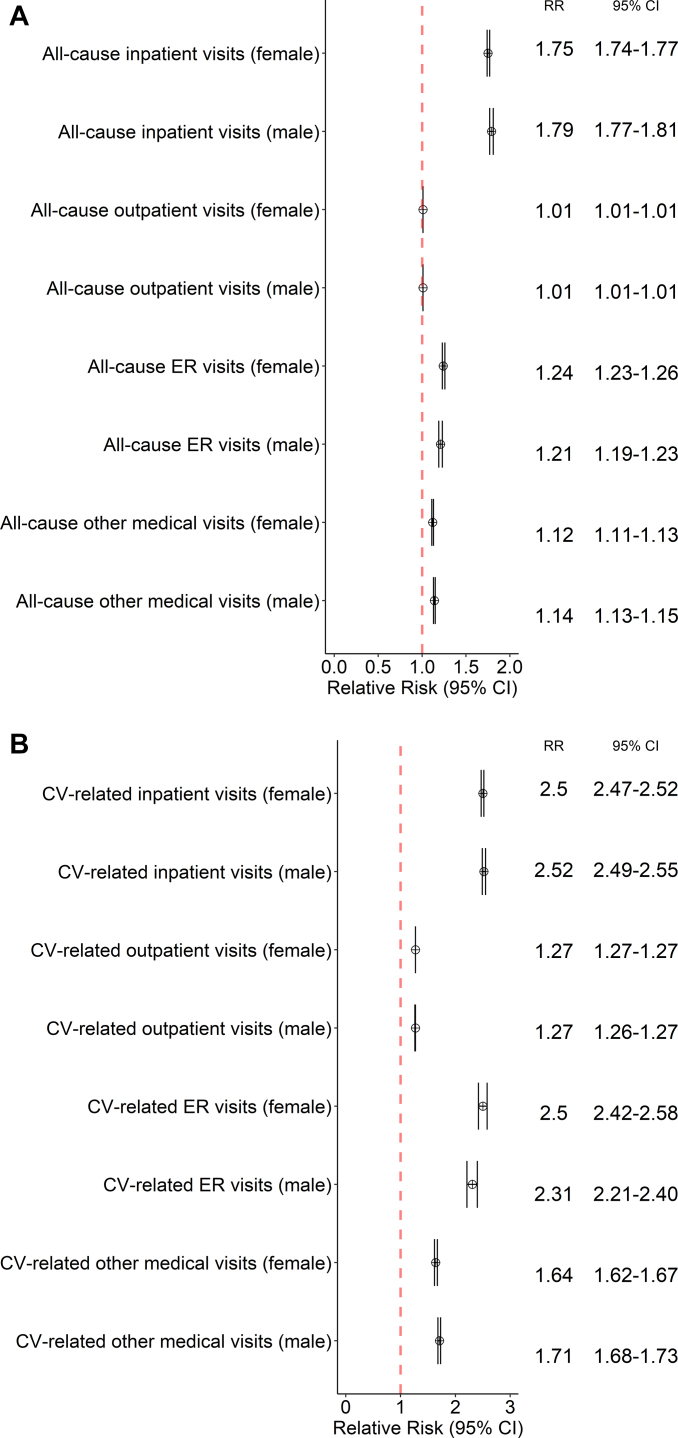
Results of the sub-analysis of healthcare use by sex are shown in Figure 1A and 1B. Both females and males with AF had significantly higher medical services utilization than females and males without AF, respectively. The RR of CV-related ER use was 2.50 (95% CI 2.42–2.58) for females with AF vs females without AF and 2.31 (95% CI 2.21–2.40) for males with AF vs males without AF, respectively.
Figure 1.
Forest plot of relative risk of healthcare utilization among patients with atrial fibrillation (AF) vs non-AF controls, subset by sex, for A: all-cause visits and B: cardiovascular-related visits.
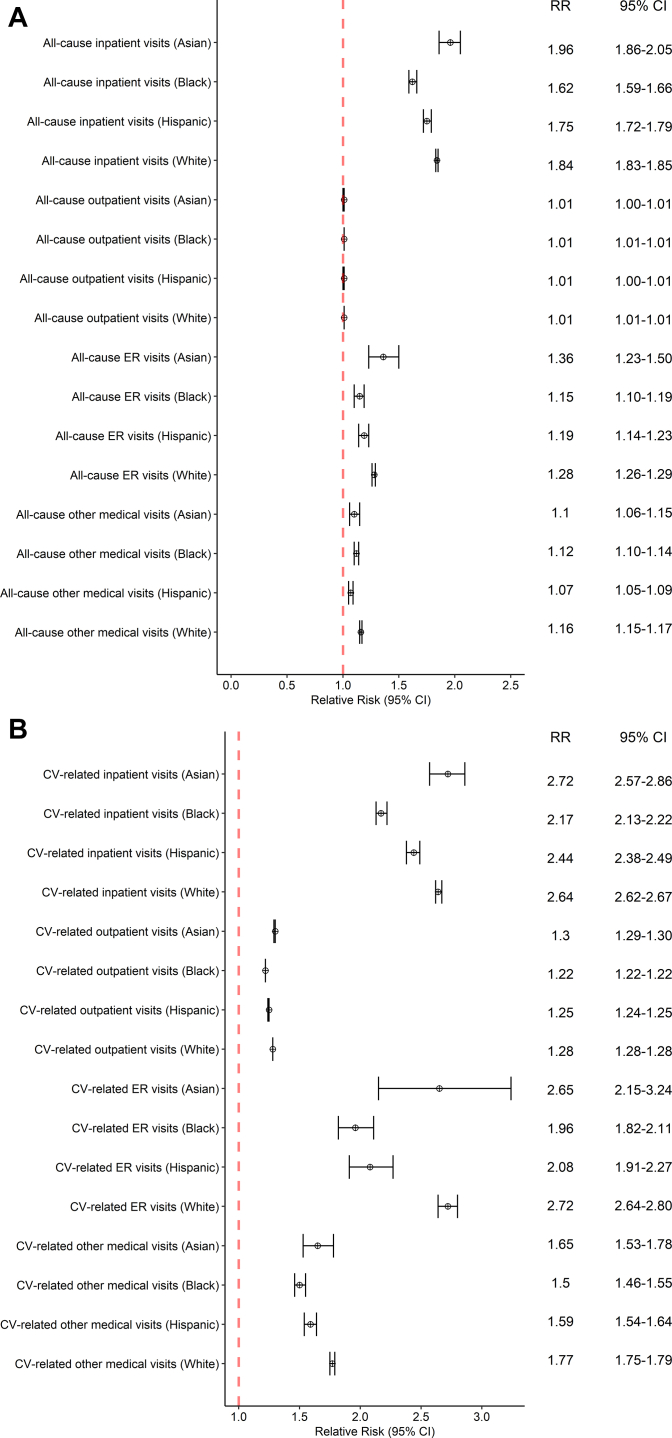
Results of the sub-analysis of healthcare use by race are shown in Figure 2A and 2B. The RR of all-cause inpatient visits for Black patients with AF was 1.62 (95% CI 1.59–1.66), Hispanic patients with AF was 1.75 (95% CI 1.72–1.79), and White patients with AF was 1.84 (95% CI 1.83–1.85), as compared to patients without AF. The RR of CV-related inpatient visits for Asian patients with AF as compared to Asian patients without AF was 2.72 (95% CI 2.57–2.86).
Figure 2.
Forest plot of relative risk of healthcare utilization among patients with atrial fibrillation (AF) vs non-AF controls, subset by race, for A: all-cause visits and B: cardiovascular-related visits.
Results of the age-stratified analysis are shown in Figure 3A and 3B. The RR for CV-related inpatient visits among those 19–64 years of age with AF was 2.91 (95% CI 2.84–2.99), and the RR for CV-related other medical visits was 2.15 (95% CI 2.06–2.24), as compared to individuals in this age bracket without AF. The RR for CV-related inpatient visits for those 65 years of age or greater with AF was 2.47 (95% CI 2.45–2.49), as compared to those 65 years of age or greater without AF.
Figure 3.
Forest plot of relative risk of healthcare utilization among patients with atrial fibrillation (AF) vs non-AF controls, subset by age, for A: all-cause visits and B: cardiovascular-related visits.
Discussion
Our results highlight the following:
-
(1)
AF to be a significant driver of healthcare resource utilization.
-
(2)
Higher all-cause and CV-related healthcare use among patients in the year after an incident AF diagnosis compared to matched patients without AF.
-
(3)
CV-related acute care services and cost of care to be markedly higher for patients with AF compared to those without AF.
Few studies in the past have highlighted the significant medical visits burden and financial burden associated with AF diagnosis. Using data from 1991 to 2009, Bengtson and colleagues15 found that AF patients had 2 times as much outpatient use (rate ratio 2.14; 95% CI 2.00–2.29) and almost 4 times as much inpatient use (rate ratio 3.94; 95% CI 3.29–4.73) compared to patients without AF. AF patients also had 4.58 times (95% CI 3.41–6.16) more CV-related hospitalized days per year than non-AF patients.15 Similarly, using 2004–2006 data, Kim and colleagues16 found significantly higher all-cause hospitalizations (37.5% vs 17.5%, P < .001), mortality during all-cause hospitalizations (2.1% vs 0.1%, P < .001), and CV-related hospitalizations (0.8% vs 0.0%, P < .001) among AF patients compared to those without AF. Combined with these previous studies, our findings confirm that patients with AF have considerably higher healthcare use. The incremental healthcare use was especially prominent for acute care services, placing patients under considerable health burden and straining the healthcare system. Notably, our matched-comparison group of patients without AF were those seeking healthcare for other medical conditions. Prior to matching, the comorbidity burden appeared to be higher among patients with AF vs those without AF. However, after matching, patients in the non-AF group had comorbidity status comparable to those with AF. Our results suggest that patients with AF have higher use and costs even when compared to patients with other comorbidities, further underscoring the incremental medical visits and financial burden associated with AF.
In our study, the financial burden associated with AF was of higher magnitude than what was previously reported. Turakhia and colleagues24 found that mean per capita medical spending (in 2014 US$) for working adults with AF was $10,355 higher than for similar patients without AF ($38,861, 95% CI: $35,781–$41,950 vs $28,406, 95% CI: $28,409–$28,603). In an earlier study by Kim and colleagues,16 AF was associated with a total incremental cost of $8705 per patient, and mean annual inpatient costs for AF patients were $5218 higher (P < .001) than for non-AF patients, while outpatient medical costs were $3596 higher (P < .001). In our study, patients with AF had ∼$25,000 higher healthcare costs than patients without AF. Our total cost differentials were more than 2 times higher than these previous studies, indicating that AF continues to result in a considerable and increasing economic burden with substantial financial implications for patients, providers, and payors.
As medical services provision becomes resource-intensive, the medical visits and financial burden for chronic conditions like AF will likely overwhelm the healthcare system. Timely management and treatment could be critical in easing medical visits and financial burden associated with AF. A recent study found that only 7.1% of patients with an incident diagnosis of AF had catheter ablation within the first year of diagnosis, with ∼30% having a prescription fill for AAD.25 The study suggested significant undertreatment among newly diagnosed patients with AF within the first year of diagnosis. When examining differences in healthcare utilization among patients who underwent early ablation (within 6 months of incident AF diagnosis) versus those who had ablation later (post-6 months), D’Angelo and colleagues26 found that the former group had a significantly lower medical visits burden as compared to the latter. As seen in this study, AF places a considerable burden within a year of diagnosis among patients; therefore, providers should consider having conversations with patients on early treatment of AF using recommended treatment modalities.
Our sub-analysis by sex identified a significant burden for acute care services use among females, though the overall healthcare utilization for patients with AF remained considerable for both males and females compared to patients without AF. Studies have found that the cumulative risk of developing AF is higher in males than females over most of the lifespan,27 though females are more likely to be symptomatic than males.28 Higher symptomatology could influence the extent to which females are referred for or seek out CV-related medical care. On the other hand, females appear less likely than males to receive rhythm control therapy, including ablation.28, 29, 30 Less aggressive treatment may result in female patients presenting with more advanced CV-related symptoms and disease needing more acute care, including emergency room visits and inpatient treatment. However, given that females with AF have an increased risk for stroke than males even after adjusting for risk factors,31 it is critical to manage and effectively treat AF at an early stage among females diagnosed with this disease.
When examining the burden of AF by different racial/ethnic groups, our results showed that AF led to significant medical services utilization across all groups. Acute care services appeared to be more prominent among Asians, though it was higher across racial/ethnic groups for patients with AF than those without AF. While previous studies have documented racial and ethnic differences in AF treatment and outcomes,32, 33, 34, 35, 36 limited data is available about racial disparities in overall healthcare use among incident AF patients. Social determinants of health, including financial resources, social support, access to healthcare, residential environment, local language proficiency, and health literacy, could explain some of the observed differences in healthcare use.37 Our results suggest that the burden of AF cuts across different racial/ethnic lines and affects these patients adversely, compared to their peers without the disease.
Limitations
This study has a few limitations. Our study did not assess indirect medical or nonmedical costs associated with AF. The incremental costs reported in this study may underestimate the true economic burden associated with AF. It is also important to consider that we did not explicitly exclude or individually examine specific types of incident AF (eg, temporary AF), which may have varying trajectories of disease burden. Nevertheless, it is unlikely that temporary AF cases were included in our study, as our eligibility criterion of having at least 2 medical services visits with a primary diagnosis of AF diminishes the likelihood of temporary AF case inclusion. The source population in the Optum database is primarily representative of US commercial claims patients (0–65 years old) and some Medicare Advantage patients (65+ years old). Therefore, our results may not generalize to all elderly patients or individuals without commercial insurance. Lastly, coding errors during claims processing could affect study results.
Conclusion
We found a significantly higher medical visits burden among patients with AF than similar patients without AF. The incremental healthcare use associated with AF existed across all racial/ethnic, sex, and age groups. We noted a considerable cost burden associated with AF, indicating the severe strain AF is placing on patients, providers, and payors. Timely intervention and treatment remain critical in minimizing the AF clinical and financial burden.
Acknowledgments
The authors acknowledge Superior Medical Experts for literature research and editorial assistance.
Acknowledgments
Funding Sources
This study was sponsored by Johnson & Johnson.
Disclosures
Abhishek Deshmukh has no conflict of interest to declare. Maximiliano Iglesias, Rahul Khanna, and Tara Beaulieu are Johnson & Johnson employees.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Patient consent was not required for this study.
Ethics Statement
Optum includes de-identified data only, and The New England Institutional Review Board has determined that studies conducted using this database are exempt from study-specific institutional review board review, as these studies do not involve active human subject participation.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2022.07.010.
Appendix. Supplementary data
References
- 1.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 2.Kornej J., Börschel C.S., Benjamin E.J., Schnabel R.B. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. doi: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnabel R.B., Yin X., Gona P., Larson M.G., Beiser A.S., McManus D.D., et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G., Sanchis-Gomar F., Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16:217–221. doi: 10.1177/1747493019897870. [DOI] [PubMed] [Google Scholar]
- 5.Williams B.A., Honushefsky A.M., Berger P.B. Temporal trends in the incidence, prevalence, and survival of patients with atrial fibrillation from 2004 to 2016. Am J Cardiol. 2017;120:1961–1965. doi: 10.1016/j.amjcard.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Wyndham C.R. Atrial fibrillation: the most common arrhythmia. Tex Heart Inst J. 2000;27:257–267. [PMC free article] [PubMed] [Google Scholar]
- 7.Williams B.A., Chamberlain A.M., Blankenship J.C., Hylek E.M., Voyce S. Trends in atrial fibrillation incidence rates within an integrated health care delivery system, 2006 to 2018. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J., et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morillo C.A., Banerjee A., Perel P., Wood D., Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14:195–203. doi: 10.11909/j.issn.1671-5411.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thrall G., Lane D., Carroll D., Lip G.Y. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:448.e1–448.e19. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 11.Westerman S., Wenger N. Gender differences in atrial fibrillation: a review of epidemiology, management, and outcomes. Curr Cardiol Rev. 2019;15:136–144. doi: 10.2174/1573403X15666181205110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin E.J., Wolf P.A., D’Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 14.Anter E., Jessup M., Callans D.J. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 15.Bengtson L.G., Lutsey P.L., Loehr L.R., Kucharska-Newton A., Chen L.Y., Chamberlain A.M., et al. Impact of atrial fibrillation on healthcare utilization in the community: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M.H., Johnston S.S., Chu B.C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 17.Jackson S.L., Tong X., Yin X., George M.G., Ritchey M.D. Emergency department, hospital inpatient, and mortality burden of atrial fibrillation in the United States, 2006 to 2014. Am J Cardiol. 2017;120:1966–1973. doi: 10.1016/j.amjcard.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel N.J., Deshmukh A., Pant S., Singh V., Patel N., Arora S., et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371–2379. doi: 10.1161/CIRCULATIONAHA.114.008201. [DOI] [PubMed] [Google Scholar]
- 19.Optum. Optum Clinformatics™ Data Mart. 2017. https://www.optum.com/content/dam/optum/resources/productSheets/Clinformatics_for_Data_Mart.pdf [Google Scholar]
- 20.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Chen P.C., Lip G.Y., Yeh G., Lin H.J., Chien K.L. Risk of bleeding and stroke with oral anticoagulation and antiplatelet therapy in patients with atrial fibrillation in Taiwan: a nationwide cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T.L., Collins G.S., Spence J., Daurès J.P., Devereaux P.J., Landais P., et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17:78. doi: 10.1186/s12874-017-0338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R . R Foundation for Statistical Computing; Vienna, Austria: 2020. A language and environment for statistical computing. [Google Scholar]
- 24.Turakhia M.P., Shafrin J., Bognar K., Goldman D.P., Mendys P.M., Abdulsattar Y., et al. Economic burden of undiagnosed nonvalvular atrial fibrillation in the United States. Am J Cardiol. 2015;116:733–739. doi: 10.1016/j.amjcard.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 25.D’Angelo R.N., et al. Trends and predictors of early ablation for atrial fibrillation in a nationwide population under age 65: a retrospective observational study. BMC Cardiovasc Disord. 2020;20:161. doi: 10.1186/s12872-020-01446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Angelo R.N., et al. Very early versus early referral for ablation in young patients with newly diagnosed paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2022;45:348–356. doi: 10.1111/pace.14459. [DOI] [PubMed] [Google Scholar]
- 27.Magnussen C., et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe) Circulation. 2017;136:1588–1597. doi: 10.1161/CIRCULATIONAHA.117.028981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnabel R.B., et al. Gender differences in clinical presentation and 1-year outcomes in atrial fibrillation. Heart. 2017;103:1024–1030. doi: 10.1136/heartjnl-2016-310406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko D., et al. Atrial fibrillation in women: treatment. Nat Rev Cardiol. 2017;14:113–124. doi: 10.1038/nrcardio.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel N., et al. Gender, race, and health insurance status in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol. 2016;117:1117–1126. doi: 10.1016/j.amjcard.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Marzona I., et al. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: a systematic review and meta-analysis of 993,600 patients. Int J Cardiol. 2018;269:182–191. doi: 10.1016/j.ijcard.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia S., et al. Racial differences in the prevalence and outcomes of atrial fibrillation in patients hospitalized with heart failure. Am J Cardiol. 2016;117:1468–1473. doi: 10.1016/j.amjcard.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kummer B.R., Bhave P.D., Merkler A.E., Gialdini G., Okin P.M., Kamel H. Demographic differences in catheter ablation after hospital presentation with symptomatic atrial fibrillation. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanda A., Kabra R. Racial differences in atrial fibrillation epidemiology, management, and outcomes. Curr Treat Options Cardiovasc Med. 2019;21:85. doi: 10.1007/s11936-019-0793-5. [DOI] [PubMed] [Google Scholar]
- 35.Tamariz L., Rodriguez A., Palacio A., Li H., Myerburg R. Racial disparities in the use of catheter ablation for atrial fibrillation and flutter. Clin Cardiol. 2014;37:733–737. doi: 10.1002/clc.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhave P.D., Lu X., Girotra S., Kamel H., Vaughan Sarrazin M.S. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–1412. doi: 10.1016/j.hrthm.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essien U.R., Kornej J., Johnson A.E., Schulson L.B., Benjamin E.J., Magnani J.W. Social determinants of atrial fibrillation. Nat Rev Cardiol. 2021;18:763–773. doi: 10.1038/s41569-021-00561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.